Pre-Release Date: March 27, 2023 | Release Date: April 14, 2023 & April 21, 2023
We are pleased to bring you Vault 23R1. Read about the new features below. You can find information on enabling new features in 23R1 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Platform
Working with Documents
Save Link Type Selection as a User Preference
With this release, when a user creates a link annotation on a document, Vault now pre-selects their most recently selected link type in Vaults where more than one link type is available. Link types can include Document (linking directly to document versions and anchor annotations), Permalink (linking to permalink targets), and Claim (linking to Claim records, PromoMats only). Vault applies this saved user preference across browsers and sessions. Users can change the saved preference by selecting a different link type.
Glossary Usability Enhancements
Vault now includes the user’s name when recording activity in Glossary Event object records. This enables content administrators to follow up with specific users when working to expand and improve the Glossary’s library of definitions. This feature also enables the Glossary on video documents, allowing users to manually search for terms that may appear visually or audibly in video content.
Improved Rendering Status Reporting
Vault now provides more insight into the process and restrictions of creating viewable renditions. If Vault does not return a viewable rendition, it provides users with an explanation and, if applicable, troubleshooting steps.
Prevent Re-Render of Documents with Manual Renditions
Currently, if a document has a manual rendition and is re-rendered, the manual rendition is replaced with a Vault-generated rendition. Users must now delete the manually uploaded rendition before requesting a new rendition via re-render.
Signature Page Generation Enhancements
Previously, signature pages were generated only when viewed for the first time or after a new signature was captured in the user’s language. Now, Vault will also generate signature pages when a document enters its steady state in the set document language. Users can regenerate signature pages through a user action once it has been configured.
File Size Reduction for Viewable Rendition Downloads
With this release, Vault optimizes downloaded viewable renditions configured with overlays by removing duplicate fonts, images, and other elements, resulting in a smaller PDF file.
Manage Document Auto Numbering
With this release, Admins can manage the auto numbering of documents by importing a CSV file containing updates to the auto numbering sequence. The feature can also be used to view the current auto numbers used for all document number formats.
Upload Documents UI Enhancements
With this release, we have updated the icons and typography when uploading documents to clearly indicate to users whether a document uploaded successfully, with warnings, or unsuccessfully.
Binder Display Options for Unbound Documents
The Binder Display Options for Unbound Documents setting allows Admins to configure how unbound documents display when viewing and exporting documents from binders. In this release, Admins can enable this setting through the Admin UI instead of Veeva Support.
Sort Multi-Select Field Values in Alphabetical Order
In this release, Admins can configure the setting to sort multi-select field values in alphabetical order through the Admin UI instead of Veeva Support.
Updated Download Icon for Source File
The Download icon for source files has been updated to an icon that is more intuitive for the action that it represents.
Binder Search with Document Numbers
In addition to searching with document names, the “Search Current Binder” box can now search with document numbers.
Add a File to a Placeholder via Drag & Drop
With this release, you can now add a file to a placeholder via drag & drop.
Lifecycle & Workflow
Object Lifecycle Conditions: Evaluating Related Object Field Values
Admins can now configure conditions in User Actions, Entry Actions, and Entry Criteria to evaluate fields and values on related objects, providing more options to determine when actions and rules are performed on records within states.
Object Lifecycle Entry Criteria: State of Related Record Rule Can Validate Multiple States
The Object Lifecycle Entry Criteria validation rule for validating the state of related records now supports the includes operator, allowing Admins to configure the rule to validate multiple states.
eSignatures: Object Signature User Title & Delegate Capture
Object signatures now capture and store the signing user’s title and delegate user information in the signature record.
Multi-Record Object Workflows: Limiting Roles Allowed to Participate for Workflow Initiator Selected Participant Groups
Admins can now configure start steps in multi-record object workflows to limit the roles allowed to participate when a workflow initiator selects the users for a participant group. This ensures the workflow initiator only selects users from a specific role in sharing settings.
Multi-Record Object Workflow: System Action Step Support for RecordAction SDK
Multi-record workflows now support the execution of Vault Java SDK RecordActions in system action steps.
Multi-Record Object Workflow: Action Step to Automatically Remove Matching Records
Action Steps in multi-record workflows can now be configured to automatically remove records from the workflow based on conditions.
Multi-Record Object Workflows: Action Step for Automatically Removing eSignatures
Admins can now configure action steps in multi-record object workflows to automatically remove eSignatures from the records based on specific task verdicts that were captured during the workflow.
Object Record Lifecycle User Actions Appear in their Configured Order
Object record lifecycle user actions on the object record detail page now appear in their configured order.
Workflow Details on Active Workflows & Timeline
For workflows on the Active Workflows home page, the workflow start date and workflow version are now visible. For object workflows, the workflow version is now visible in the workflow timeline on the object record page. This helps workflow owners and administrators understand which version of the configuration is being used for the active workflow.
Workflow Participant Selection Actions Support Search by Email Address
Start Step participant controls, the add participant action, and the re-assign task action on workflows for documents and objects now support searching users by entering an email address.
Reporting & Dashboards
Advanced Filter Logic for Multi-Pass Reports
This feature allows users to add OR filter logic across different objects and views in a multi-pass chain report, making reports more flexible. Users can also add custom logic expressions between filters.
Object Reference Field in Reports Formulas
This feature adds support for object reference fields in report formula fields. When defining a report formula field, users may now wrap an object reference field in the Text() function to retrieve its label, or the ID() function to retrieve its ID as text. Users may also return an object reference field directly if a function accepts that datatype, such as NetWorkdays().
Search & Filter
Include Search Criteria in Exports to Excel
Administrators now have an option to add search criteria when exporting search results to Excel. When this is enabled, a second sheet will be added to the Excel workbook containing the tab that the results were exported from, all of the applied filters and search terms, and the user who created the export with a timestamp. For most document and object tabs, a clickable link back to the search results in Vault is available.
Later Version Available Indicator
Document search results will now display an icon indicating when a document version that matches their search criteria has progressed to a later version which is visible to them through one of their other roles on the document. Clicking this icon will tell the user which version is the latest available to them and the roles they have on the document that caused the earlier version to be included in the results.
Improved Name Search
Improves the ranking of search results when matching on document name or record name. Vault considers any extra unmatched terms in the name when determining the relevance score. For example, searching for “Deviation Review Process” would rank “Deviation Review Process - Europe” below “Deviation Review Process”. All of the search terms were found in both documents, but the document with one unmatched term appears lower in the results. This helps exact matches to appear above all other matches.
Spanish Stemming Improvements
Users can now search in Spanish without acute accents. For example, searching for “Guia de documentacion” will match “Guía de documentación.” This feature also provides more flexible matching of plural/singular nouns and past, future, and present verbs.
Checklists
Checklists: Batch Saving Responses
This release improves the performance of the checklist respondent UI by batch saving responses instead of saving each response individually.
Checklists: Increased Limit for Maximum Answers per Question
With this release, the maximum number of answers per question is increased to 20. Prior to this release, Vault limited each question to 10 answers.
Usability & UI Updates
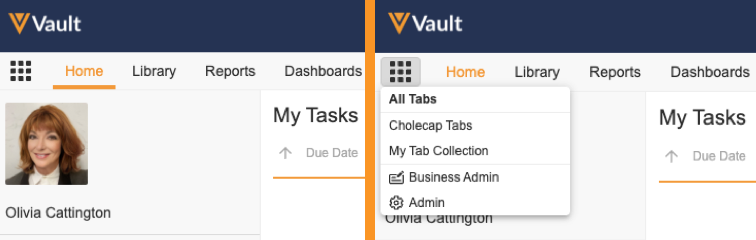
Tab Collection
Admins can create Tab Collections to display relevant tabs based on different use cases, such as tabs for certain roles or tasks. Users click the Tab Collections icon in the navigation bar to select a tab collection to view. Admins can also configure User object records to assign a preferred tab collection that Vault displays when the user logs in. Admins control access to tab collections through permissions assigned through a Security Profile or Application Role.
Learn more about Tab Collections.
Admins can enable the new Hide the All Tabs option in Tab Collections Menu setting from Settings > General Settings > User Interface Options. When enabled, Vault hides the All Tabs item in the Tab Collections menu, so users cannot select and see all tabs available to them. Users see only the tab collections available to them. Before enabling this setting, we strongly recommend configuring and assigning Preferred Tab Collections to users.
This feature increases the limit for the number of custom tabs from 20 to 60. However, the limit on subtabs remains at 20 per menu tab.
Admin & Business Admin Tab Collections
With the introduction of the Tab Collection feature in this release, Business Admin is now separated from the Admin tabs to form a new Business Admin tab collection that contains the Objects, Picklists, and Templates tabs. In addition, the Admin tabs are now placed in the new Admin tab collection. Users with the appropriate Admin permissions can access the Admin and Business Admin tab collections from the new Tab Collections icon, and Vault no longer displays the existing cog icon for viewing Admin tabs.
Learn more about the Business Admin Tab Collection.
Notifications: Add Annotation Replies to Summary Emails
This feature allows Admins to set the email notification preference for annotation replies. By default, Vault sends notifications for annotation replies in Summary emails. Admins can configure Vault to send these notifications on each occurrence or not at all. If this setting is visible on the User Profile page, users can also change this notification preference.
Vault Authentication
New Certificate for SAML SSO & Spark Messaging Connection
Vault is scheduled to rollover the signing certificate used to sign SAML Single Sign-on requests and Spark messaging connections. There is no expected downtime.
Release Dates:
- New Certificate Testing Period: February 3, 2023 7:00 pm PT (February 4, 2023 03:00:00 UTC) - March 3, 2023 6:00 pm PT (March 4, 2023 02:00:00 UTC)
- New Certificate Rollover Event: March 3, 2023 6:00 pm PT (March 4, 2023 02:00:00 UTC)
- Support for New and Old Certificate: March 3, 2023 6:00 pm PT (March 4, 2023 02:00:00 UTC) - March 24, 2023 6:00 pm PT (March 25, 2023 01:00:00 UTC)
- Final Certificate Rollover: March 24, 2023 6:00 pm PT (March 25, 2023 01:00:00 UTC)
Your IT organization must ensure that the new certificate is configured on your Enterprise Identity Provider system prior to the New Certificate Rollover Event on Friday, March 3 2023. Learn more about the action required for the certificate rollover process.
Administration
External Inbound Connections
Vault now supports tracking inbound REST API integrations using External Connections. The URL field is no longer required during configuration to accommodate inbound only connections. Vault Admins can configure Connection Client records that allow developers to identify REST API integration using a unique Client ID.
Vault Java SDK Service Account
In 23R1, Vault will permanently enable the Run Custom Code as Java SDK Service Account feature with the following expected behavior:
- Custom code executes as the Java SDK Service Account, which has Vault Owner access.
- By default, Vault sets the Java SDK Service Account as the owner of object records and documents created by custom SDK code.
- Audit logs entries for documents and object records affected by Vault extensions identify the change with Java SDK Service Account on behalf of the user initiating the request.
This feature is auto-on and cannot be disabled.
Sandbox Snapshots
This feature introduces the concept of snapshots of a sandbox Vault. A sandbox snapshot is a copy of a sandbox Vault that can store configuration and optionally data at a given point of time. Each sandbox Vault can create two (2) Snapshots. Snapshots can be used to create or refresh sandbox Vaults.
Sandbox Sizes
This feature introduces two new sandbox sizes, “Small” and “Full”. Existing sandboxes will be categorized as “Large”. Customers will receive 4 Small sandboxes for every Production Vault in addition to 2 Large and 1 Full sandbox. The size of a sandbox determines the amount of data it can store.
Document Templates Limit in Sandboxing
To prevent sandbox cloning timeouts due to a large number of document templates being included in the clone, Vault no longer includes document templates in the clone when the total size of the document templates exceeds 5GB.
Vault Usage & Performance Statistics: User Activation-Deactivation Performance Statistic
This feature introduces two new performance statistics for a Vault:
- User Activation Count: Number of times an inactive user is activated or a new user is created.
- User Deactivation Count: Number of times an active user is deactivated.
Email Suppression List
With this release, Vault automatically adds email addresses that produce a hard bounce to the Email Suppression List, minimizing the number of emails Vault may send to invalid email addresses. Admins can manually remove an email address from the suppression list. Learn more about the Email Suppression list.
Country Object: Add Code Field
A Code field is now available on the Country object to facilitate easy transfer of Country records in future Vault Connections.
Person Object: Duplicate Person Record Enhancements
When users attempt to create Person records from object reference fields on a document or object record, they can now select an existing record when duplicate records are found.
Jobs: Support DateTimes as Trigger Date
With this release, Admins can leverage DateTime fields as triggers for Date Based Document and Object Operation type jobs.
Lookup Field Value Corrections
Correction to any outstanding lookup fields that were out of sync with its source field, due to a one-off event in the past that led to lookup inconsistencies.
Vault File Manager
VFM Resumable Upload & Download for Check Out & Check In
With this release, if a document check out/in process via Vault File Manager is interrupted, users can resume the process where the interruption occurred. Prior to this release, if a check out/in was interrupted, all progress was lost, and users needed to restart the download process from the beginning.
Support 500GB File Size
Source files up to 500 GB in size can now be uploaded using Vault File Manager. Prior to this release, the source file size limit with Vault File Manager was 100 GB.
Download Source Files using Vault File Manager
When downloading source files from Vault, users can now download using Vault File Manager. Vault File Manager now includes auto-resumable download capabilities, ensuring reliable and successful source file downloads.
Vault Loader
Vault Loader Support for MAXROWS & SKIP
With this release, Vault Loader users can now leverage the MAXROWS and SKIP VQL clauses to page through and retrieve large record sets. This feature is supported in the Vault UI, CLI, and API.
Vault Loader Support for Record Migration Mode Update & Upsert
Users of Vault Loader can now leverage Record Migration Mode to update and upsert records in non-initial state while bypassing reference constraints and validation rules.
Vault Loader Support for Picklist as Unique Field for Upsert
Vault Loader users can now select picklist fields in the Key Field drop-down when updating, upserting, and deleting object records.
Configuration Migration
Configuration Report: Atomic Security Enhancement
Previously, Vault Configuration Reports only included atomic security on fields, controls, actions, and relationships if there are overrides. In this release, Vault Configuration Reports now include non-overridden default values.
Vault Configuration Report: Include all Objects for Metadata Export
With this release, all objects visible in the Admin UI are now included in Vault Configuration Reports with the exception of some system-managed objects. These exceptions have been listed in the documentation.
Platform Data Model Changes
See 23R1 Platform Data Model Changes.
Vault Connections
RIM/Quality: Registered Product for Change Control
The Quality to RIM Vault Connection can now transfer Registered Product records between Vaults to be used for change controls. When users create or edit Product Family, Product or Product Variant object records in their Registrations Vault, Vault creates or updates the Product Family, Product or Product Variant records in the QMS Vault.
RIM/ClinOps: Support Country to Study Country Document Field Rule Mapping
The RIM to Clinical Operations Vault Connection can now transfer Country and Study Country fields on documents when creating CrossLinks. When a user populates a Country field on a RIM document and a CrossLink is created in Clinical Operations, the connection will populate the Study Country field based on the Country field populated in RIM. Alternatively, a document authored in Clinical Operations with a Study Country will populate the Country field on the CrossLink created in RIM.
Additionally, a reference lookup has been added to Clinical Operations that maps the country UUIDs in RIM to the country names in Clinical Operations in order to facilitate the correct country naming between the two applications.
Clinical Operations
CTMS
Clinical Operations to Medical CRM Connection: Study & Study Site Transfer
With this release the connection between Clinical Operations and Medical CRM has been extended to include Studies and Study Sites. Medical Science Leads within Medical CRM and CRAs within Clinical now have visibility into the studies interaction are related.
Trip Report Question Branching: Enhanced Dependent Question Display
This feature improves the user experience of populating Trip Report questions and responses with branching by immediately evaluating display of dependent questions after a controlling question’s response is selected. *Auto-on with Trip Report Question Branching enabled.
Trip Report Question Branching: Review Comments on Dependent Questions
This feature allows Reviewer Comments to be logged on dependent Trip Report questions. *Auto-on with Trip Report Question Branching enabled.
Trip Report Question Branching: Show & Hide Dependent Questions
This feature streamlines the completion of monitoring trip reports by allowing CRAs to toggle whether to display un-editable dependent questions or not. By default un-editable fields are hidden from view. *Auto-on with Trip Report Question Branching enabled.
eTMF
Study Metadata Extraction
The TMF Bot has been extended to not only auto classify documents, but now set the documents study. As documents are loaded to the Inbox in Vault, TMF Bot will inspect the file name and document content to set the value of the study field. This enhancement further streamlines the processing of documents within eTMF. This feature will require enablement by Veeva, and will be turned on only for select early adopters.
Convert Clinical AI Capabilities to Platform
This feature converts certain parts of the TMF Bot data model to platform (__sys) components, copies values in existing records to the new platform fields, and updates functionality to use those platform components going forward. This will allow other Vault applications to take advantage of auto-classification and metadata extraction in the future.
TMF Transfer Improved Error Troubleshooting
To help with troubleshooting transfer errors, Vault now creates Agreement Transfer records and CSV reports whenever a user selects the Retry Failures or Run Associated Rule actions. This provides great visibility into the transfer’s finish time, its contents, and the outcome of each retried item.
CTMS, eTMF
Clinical Operations: Study Training Connection
Customers can now automatically send data from their Clinical Operations Vault to their Study Training Vault. The connection between Study Training and Clinical Operations Vaults simplifies the management of studies and related data and documents. When a Study, Study Country, Study Site, Study Person, or document is created or updated in the Clinical Operations Vault, data is automatically created or updated in the Study Training Vault.
Study Startup, eTMF
Site Candidate Loader
Feasibility teams often get their initial candidate site lists from separate groups, and they need to quickly create those sites in Vault. This feature allows a user to upload a properly formatted CSV to quickly create Study Site records. Vault creates candidate site records and relates them to Organization, Location, and Person records in the global directory based on text matching.
Site Connect
Safety Distribution Gap Pack
With this feature, Site Connect customers can now automatically send designated gap pack Safety Distribution documents when a Study Site moves into a particular lifecycle state (for example, Active). This feature allows customers to easily manage their gap packs across Products and Line Listings so that the appropriate safety documents can automatically be sent to recently onboarded sites.
Safety Distribution Document List Token
With this feature, Site Connect customers can now include a list of the specific safety documents being sent to each site contact in the body of the email. The list contains the documents that are viewable when accessing the special link contained in each safety email. The list can be added to the Safety Distribution Email template using the custom app token ${Custom.safetyDocList}.
Safety Distribution Types
Site Connect customers can now create multiple Safety Distribution Types in order to have multiple sets of distribution defaults per document type. Rather than creating many document types to accommodate the need for a variety of distribution rules, a single document type can now be used in combination with multiple Safety Distribution Types.
Vault Payments
Automate Site Fee Generation
Site reimbursement is often tied to the completion of milestones. For example, a “Site Initiation Fee” is paid when a site is selected, or when the initial monitoring visit is completed. Allowing users to link fees to the completion of milestones streamlines the reimbursement process.
This feature enables the generation of Site Fee records in Vault Payments when milestones are completed.
Users can identify the Milestone that they expect to trigger a Site Fee in the Site Fee Definition and when Milestones with the corresponding Milestone Type value are completed at a Site, corresponding Site Fee records are generated. The Site Fee is linked to the corresponding Milestone record and will update if the completion date or completion status is updated.
Fee Schedule & Template Copy
This feature makes it easier to manage fee schedules; with the ability to both copy fee schedule templates and their associated Fees along with the site-level fee schedules.
Users can now copy a Fee Schedule and related Fees via a single action. This will result in a new Fee Schedule with corresponding record data and a set of matching Fees for the original Fee Schedule.
Users can also now copy a Fee Schedule Template and related Fee Templates via a single action. This will result in a new Fee Schedule Template with corresponding record data and a set of matching Fee Templates for the original Fee Schedule Template.
Site Fee Definition Templates
Vault Payments customers can now create Site Fee Template sets to use for copying to a Study. Templates define a set of Global Site Fee Definitions and allow the association of appropriate Milestones for use with generating Site Fees.
Related Holdback Item Populated with Holdbacks
Vault now automatically populates the Related Holdback Item field when it generates Payable Items for a Fee that includes Holdbacks.
Create Fees From Template Enhancement
The Create Fees From Template user action will now run when Fees exist in a Fee Schedule. When run, any Fee Templates in the approved Fee Schedule Template that do not match an existing Fee name in the Fee Schedule will create additional Fee records in the site Fee Schedule.
Populate Site Fee Definitions
With this feature, Vault Payments customers can now easily create Site Fee Definitions for a study. Customers can create Site Fee Templates and a set of associated Site Fee Definitions as a reference set.
Users can add Site Fee Definitions from either other studies or Site Fee Templates via the Populate Site Fee Definition user action.
Study Migration
With this release, Clinical Operations now offers improved capabilities to support migrating studies for customers who add additional applications.
Vault Administrators can identify individual studies to include in a study migration. Once marked in migration, study-related object data is hidden and uneditable by non-Admin users. New data can be loaded in without executing productized triggers. This allows for faster, easier data load into production and review while users can continue to update documents and enter data for their non-migrating studies.
Additionally, Vault Connections respect the Study Migration flag. Studies in migration do not transfer data to external Vaults until the Study migration is completed.
Support for Tracking EUCT Number & OMS ID in Clinical Operations
This feature introduces text fields to capture EU Clinical Trial (EUCT) Number on Study and Organization Management Service (OMS) ID on Global Directory objects, Organization and Location. Admins will need to configure page layouts to fully utilize these fields.
Track Study Site ISF Location
With the addition of this standard field, Clinical Operations customers can now track where an individual Study Site is storing its Investigator Site File (ISF). This field is beneficial for Site Connect customers looking to better flag which Sites are using SiteVault as their ISF.
Document Task Filter Changes
Study, Study Country and Site have been copied to document tasks to make filtering by these fields more performant. In Vaults where these filters were displayed as a single filter, they will now be available as 3 separate filters so users can filter by Site or Study Country without first filtering by Study. Users will need to select Study Country and Site from the filter options to apply them.
Limit for Study Team Roles
With this release, Vault prevents Admins from adding more than 300 Study Team Role records.
Study Person Field Validation Check
Vault will now prevent the Person, Study Team Role, Study, Study Country, and Study Site fields on Study Person from being edited when the record is used to grant security permissions and Grant Access to Related Records is true/yes.
Clinical Operations Data Model Changes
See 23R1 Clinical Operations Data Model Changes.
Commercial
PromoMats
Brand Portal Refresh for Users & Portal Administrators
This release includes significant improvements to the Portal user interface.
Portal users can experience the new homepage, which provides a modernized look and feel. The Portal refresh provides a more intuitive experience allowing users to find their content quicker. Admins benefit from a refreshed administration interface, allowing multi-document select, rich text support, further customization options, and other subtle yet essential enhancements.
This improvement is currently an opt-in experience and will be auto-on for all customers in 23R3. Learn more about the updated Brand Portal.
Modular Content Document Info Panel
This release introduces the Modular Content Document Info Panel to better inform MLR reviewers of the approved Content Modules used in a document. This doc info panel allows users to visualize where their Content Modules and individual Content Module Assets are located within documents by using a combination of Suggested Link and manual link annotations. The Modular Content Document Info Panel also enables users to find previously approved content within their documents by allowing users to interact with the annotations directly from the panel.
Enhanced Suggest Links within Content Modules
Admins can now configure Enhanced Suggest Links to only match Text Assets contained within Content Modules linked to your content. This action can be configured as both a Lifecycle State Entry Action and a Lifecycle State User Action.
Increased Matching Flexibility for Enhanced Suggest Links
In this release, Enhanced Suggest Links is more flexible in how it searches and matches for Text Assets. This increases the amount of suggested link annotations and matches when performing Enhanced Suggest Links on your content.
CRM Events Management Integration Fields
With this release, Vault has introduced two additional shared picklist fields to support the integration between Vault and CRM Events Management. These fields will be used to support a future feature from CRM Events Management that will allow users to sync Vault documents with CRM Events.
Standard Metrics Track Duration
Document Lifecycle States can now be mapped to standard business process groups on a new Configuration page called Standard Metrics State Mappings. Pulse uses these mappings to determine how long a document spent in each process. The standard processes are: Time in Content Creation, Time in Quality Check, Time in Review and Approval, and Time in Regulatory Submission. This data is not currently available through reports and dashboards.
Retain Vault Digital Publishing Document File Names
With this release, Vault Digital Publishing defaults file names to the name of the file saved in Vault. Prior to this release, Vault named downloaded files with a GUID, which made them difficult to search for and locate.
Product & Country Become Shared Fields
With this release, the standard Product and Country document fields become shared fields. This enhancement provides greater flexibility for document field configuration.
PromoMats & Multichannel as Apps
PromoMats and Multichannel are now independently-licensable applications within the Commercial family. Admins can now specify different license types for different users.
Commercial Data Model Changes
See 23R1 Commercial Data Model Changes.
Medical
Medical Portal Refresh for Users & Portal Administrators
This release includes significant improvements to the Portal user interface.
Portal users can experience the new homepage, which provides a modernized look and feel. The Portal refresh provides a more intuitive experience allowing users to find their content quicker. Admins benefit from a refreshed administration interface, allowing multi-document select, rich text support, further customization options, and other subtle yet essential enhancements.
This improvement is currently an opt-in experience and will be auto-on for all customers in 23R3. Learn more about the updated Medical Portal.
Email Templates for Medical Inquiry Case Responses
When constructing an email response, Medical Inquiry users can now use a predefined template. Via a user action or state change, Vault can populate the email subject and body on the Case Response from the template.
Include Attachments in E2B(R3) Generation in Vault MedInquiry
With this release, users can add files associated with Adverse Events to records as object record attachments, allowing them to share the files with a safety team. Vault then encodes these files when generating an E2B(R3) file.
Response Package: Download Source Document
With this release, Medical Inquiry users can allow HCPs to download source documents when receiving a response to a Medical Inquiry they have made.
Add Country to Cover Letter Generation Action
With this release, when a user runs the Generate Cover Letter action, Vault populates the cover letter’s Country field with the Country from the Case Response object if present. This provides better support to multinational organizations, who require the ability to control which documents a user can see by region.
Retain Vault Digital Publishing Document File Names
With this release, Vault Digital Publishing defaults file names to the name of the file saved in Vault. Prior to this release, Vault named downloaded files with a GUID, which made them difficult to search for and locate.
CRM Events Management Integration Fields
With this release, Vault has introduced two additional shared picklist fields to support the integration between Vault and CRM Events Management. These fields will be used to support a future feature from CRM Events Management that will allow users to sync Vault documents with CRM Events.
MedComms & Multichannel as Apps
With this release, we have separated Multichannel functionality from MedComms. Vaults that were previously Multichannel now appear as Medical Vaults with only Multichannel functionality enabled.
Medical Data Model Changes
See 23R1 Medical Data Model Changes.
Vault Mobile
Saved Views
Users can now see and select saved/custom views from the Library mobile tab to more easily find documents. Only the views that are configured to display in the library__v web tab are available in mobile.
View Related Documents
Users can now view and navigate to related documents when viewing a document on mobile.
Mobile Linking
When accessing a Vault link on a mobile device, the link launches the Vault Mobile app if it is installed on the device. This only applies to a subset of applicable Vault links, such as document, task envelope, and dashboard links.
iOS: Remove PIN Code
On iOS, there is no longer an option to set or use a 4-digit PIN code to open the app.
Display Vault Upgrade Warning
If you are in a Sandbox Vault, Vault Mobile now displays a green SBX indicator next to the Vault name displayed at the top.
Training
Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
Study Training
Study Training: Vault Connections with Clinical Operations
Customers can now automatically send data from their Clinical Operations Vault to their Study Training Vault. The connection between Study Training and Clinical Operations Vaults simplifies the management of studies and related data and documents. When a Study, Study Country, Study Site, Study Person, or document is created or updated in the Clinical Operations Vault, data is automatically created or updated in the Study Training Vault.
Quality
QMS
5 Whys Analysis
Vault QMS now supports the ability for a user to perform and document a 5 Whys Analysis within Vault as a tool to assist in determining the true root cause of a Quality Event or Complaint. For this feature, 5 Whys Analysis will be supported for any standard or custom Quality Event and Complaint object types.
The 5 Whys Analysis is one of several cause-effect techniques that are used to perform root cause analysis as part of a Quality Management System. The general approach is to start with your problem statement and iteratively ask “why” with each answer forming the basis of the new “why” until the root cause is clearly identified. The number 5 has been a general observation on the number of times “why” is asked to get to the true root cause and is a rule of thumb rather than a requirement. Learn more about setting up 5 Whys analysis.
Supplier Change Notifications
QMS now supports the ability for customers to send Supplier Change Notifications that originate via an email to a Vault Email Inbox configured with the new Create Supplier Change Notification email processor.
Once the email is received and processed by the email processor, Vault creates a Supplier Change Notification record, impacted sites can be identified, and impact assessments can be performed to determine if a Change Control is required. If a Change Control is required, it can easily be created directly from the Supplier Change Notification, linking the two records together. Learn more about configuring supplier change notifications.
QRM Risk Builder
This feature adds a purpose built interface to make it easier for users to enter risk data into the system. Users can add new risks by clicking the Add Row button or by pressing the <Ctrl> + <Enter> keys on the last row. Users can also use keyboard short-cuts such as arrow keys, <Tab>, <Shift> + <Tab> to move around the grid. Users will also be able to copy and delete risk rows easily. This feature significantly improves the user experience and usability of Quality Risk Management. Learn more about the Risk Builder tool.
Auto-Create Proposed Audits
When an Audit Program record is created, Vault now provides the ability to automatically create the associated Proposed Audit records from Organization records based on the Planned Start Date and Planned End Date of the Audit Program as well as the configured date field on the applicable Organization records, in addition to specific optional metadata that is configured in the user action. Learn more about setting up Proposed Audit auto-creation.
Generate Document: Add Users to Document Application Role
With this release, the Generate Document actions have been enhanced to automatically assign users that are associated to selected roles on the record to the same roles on the created document. This significantly simplifies security and ensures that only users associated to the record can access the document. In addition, the Generate Document action has been expanded to support all standard objects (with a __qdm or __v namespace) with a lifecycle. Learn more about Quality document generation.
Extend Recurrence Check to Support Custom Object Types on Quality Event
The existing Recurrence Check capability in QMS now supports all custom object types on the Quality Event object. Customers can now configure a recurrence check to identify similar, recurring events for custom object types and drastically reduce the manual effort of identifying recurrences.
QMS <> RIM: Processing Enhancements
This feature updates how messages between the Quality and Regulatory Vaults are processed for the Change Control connection. This release introduces the QMS/RIM Connection Transaction object, which will be created by the source vault whenever a message needs to be sent to the corresponding target Vault. This object has a transaction status (Pending, Complete, Failure, Complete with Errors) that will be updated by the target Vault when processing is complete. If a transaction were to fail, the user on the source Vault has the option to initiate an action to reset the transaction to pending. This will allow for easier recovery in failure scenarios. The transaction object can be added to the page layouts for Change Control and Impact Assessment (in Quality) and Event and Activity (in Regulatory) for more convenient viewing of transaction statuses.
Previously, the QMS RIM: Submission Activity Update integration point only allowed for updating of existing Impacted Country records from Activity records in RIM. In this release, this integration point has been updated to also allow creation of Impacted Countries as well. In order to leverage the create function of this integration point, the Integration Rule QMS RIM: Submission Activity Update will need a field rule for every required field on the Impacted Country object. For example, Country is a required field on Impacted Country. In order to allow creation of Impacted Countries at the QMS RIM: Submission Activity Update integration point, a new field rule will be needed to map the Country field on the Impacted Country record to the Impacted Market field on the Activity record in RIM. The QMS RIM: Completed Regulatory Assessment integration rule can be used as a reference since that integration point creates Impacted Countries as well.
Additionally, a new action has been added to the Activity object in RIM. The ‘Update Impacted Country Details’ action is now available as both a user action and an entry action. This action will create an impacted country record in Quality if one does not exist or will update the connected impacted country if it was previously created.
With the release of this feature, the integration rule QMS RIM: Create RIM Event in the Quality Vault has been deprecated and is no longer run by the connection. Updates to the RIM Event GUID fields in the Change Control and Impact Assessment objects are now handled via the API, called after the Event is created in RIM. All custom field rules configured on this integration rule will no longer be run.
User Exception Items created before the 23R1 release will not be able to be reprocessed using the Resubmit Spark Message action.
Surveillance
eMDR Update: Auto Generate Manufacturer Report Number
This feature automates the generation of Manufacturer Report Number for eMDR. System auto-generates the Manufacturer Report Number as per the FDA’s format when the user generates the required XML file for FDA eMDR submission. The system also automatically restarts the report number sequence to 00001 at the beginning of the new year. Learn more about auto-generating manufacturer report numbers.
VPS: Reporting Enhancements
With this feature, we have made a few enhancements to eMDR and EU MIR reporting. eMDR now supports MDR Contact Office and MDR Contact Information for section G1. It also separates the Manufacturer Report Number for eMDR and EU MIR, so that you can assign different numbers for both health authorities.
Standalone MedTech Objects Data Model
QMS now supports the MedTech Complaints and NonConformance process as a standalone data model separate from Quality Events. This will allow customers to manage their Complaints Lifecycle separate from the Quality Event Lifecycle.
New implementations are encouraged to utilize the standalone MedTech Complaints and NonConformance data model. Customers who are already live or in the process of implementing the existing MedTech Complaints or NonConformance object type on Quality Event are strongly encouraged to continue leveraging their existing configuration. There will be no functional differences between the two models and there is no need or functional requirement to move to the standalone MedTech Complaints or NonConformance data model if already leveraging the existing MedTech Complaints or NonConformance object type on Quality Event.
Standalone Complaints: VPS Support
With this feature, Vault Product Surveillance now supports the Standalone Complaints data model (released in 22R3) VPS supports eMDR and EU MIR reporting on Standalone Complaints process in the 23R1 release.
Standalone MedTech CAPA & Nonconformance Objects
With this feature, QMS now supports the CAPA (for MedTech) and Nonconformance process as a standalone data model separate from Quality Events. This will allow customers to manage their Lifecycle separate from the Quality Event Lifecycle for these processes.
New implementations are encouraged to utilize the standalone CAPA (for MedTech) and Nonconformance data model. Customers who are already live or in the process of implementing the existing CAPA (for MedTech) and Nonconformance object type on Quality Event are strongly encouraged to continue leveraging their existing configuration. There will be no functional differences between the two models and there is no need or functional requirement to move to the standalone CAPA (for MedTech) and Nonconformance data model if already leveraging the existing CAPA (for MedTech) and Nonconformance object type on Quality Event.
QualityDocs
Audit Trail Behavior Update for the Station Documents
This update changes the audit trail behavior of Station Documents and displays the username instead of ‘System’ or ‘System on behalf of <username>’ in the User Name field of the Station Document audit trail when a document is removed from a Station using the Quality Relationships document information panel.
Station Manager
Configurable Document Metadata for Station Manager Apps
With this release, Station Manager documents can now be configured to display an Admin-selected list of fields. Customers can use the Station Document Metadata Admin page to create field configurations and assign them to individual Stations. Once assigned, the document information page on the Station Manager mobile app will display the list of configured fields. Learn more about configuring document metadata.
Certify and Support Station Manager on Android 12 & 13
Beginning this release, Station Manager is now officially being supported on Android 12 & 13. The Station Manager mobile app is now fully compatible with Android devices running Android 12 & 13 and Veeva will continue to develop and test the application on these versions of Android.
Training
Curriculum Sequencing on Learner Homepage
This feature enables Training Admins to suggest an order by which Curricula should be completed by Learners.
Learners are provided with a new grouping option in the Learner Homepage where they can group Training Assignments by Learner Role. Learners can also access a new Learner Role Detail Page, where they get an overview of the selected Learner Role and view all associated Curricula.
Training Admins can now access a new page by which they can reorder Curricula within a specific learner role. The order defined in this page is displayed to Learners via the new Learner Role Detail page.
Tracking Curriculum Completions
This feature provides a new reportable object, Curriculum Completion Status, that records the completion status of every Curriculum in the Training application per Learner and Learner Role. This new object will track the completion percentage of curricula as Learners are assigned and complete training. Fully completed curricula are also marked as “Complete” on the corresponding Curriculum Completion Status record so that Admins can easily determine qualification of Learners. In addition, the system also tracks certain dates to give customers the ability to view a historical record of the curriculum’s completion. The system tracks the dates assignments were first and last assigned, and the dates the curriculum was first and last completed.
To support this feature, we’ve also added a new Training Requirement field on the Assignment Detail object. This field is a lookup to the Training Requirement field on the Training Assignment associated with the Assignment Detail record.
Complete Training Tasks on Vault Mobile
This feature allows the user to complete training tasks from the 23R1 version of the Vault Mobile app. Training tasks assigned to the Learner are displayed in a new My Learning mobile tab. The Learner can view the task, view associated documents, and complete the task. This feature will be available with the 23R1 release of Vault Mobile.
To access their training on the Vault Mobile app, Learners must have Read permissions for the Person object and Read permissions for the User field on the Person object.
Support Checklist Versioning for Quizzes
A quiz can now be versioned using Checklist’s Create New Version action. This allows the user to quickly create a new version of a Quiz, while the action will automatically supersede the previous Quiz.
Learner Homepage Updates
The following updates were made to the Learner Homepage to make it more intuitive for a Learner to use:
- In the Curriculum View, the curriculum card’s View button has been updated to Begin Training. This button now takes the user to the first Training Assignment for the Curriculum.
- Each curriculum card now includes a separate view button to take the user to the Curriculum’s detail page.
- On Curriculum’s Detail Page, the Learner Role is hyperlinked and takes the Learner to the Learner Role’s detail page.
- On Training Assignment cards, the Curriculum is hyperlinked and takes the Learner to the Curriculum’s detail page.
- The Learner’s Homepage now remembers these view preferences when navigating away and back to the page: Group By, Tab, and Card vs. List
Tag Reason for Training Assignment Creation
Training Assignments created via the training matrix after this release now capture the reason in a new picklist field Creation Reason. The possible values are: Matrix Updates, Document Updates, and Recurrence. Previously, this information was only available in a text field, which was not helpful in searching or reporting. Now, the information can be used in trending reports.
Prevent Updates to Person Training Eligibility Field if Job is Queued or Running
With this update, the Training Eligibility field on a Person cannot be modified if the Cancel Training Assignments for Person job is queued or running. This prevents conditions where a Learner is assigned or not assigned Training Assignments.
Add Participant Action Available for All Training Workflows
With this update, the Add Participant workflow action is available for all Training Assignment workflows. This allows an authorized user to add additional participants to a workflow, especially useful in evaluation (on-the-job) workflows where participants may be added at the last minute.
Enable Vault Training Mobile Interface in All Training Vaults
The “Enable Vault Training Mobile Interface” application setting is enabled on all Quality Vaults with the Vault Training or Study Training applications enabled. This allows users to view and complete training on mobile-optimized pages from mobile browser or the Vault Mobile app.
LIMS
Test Steps for Test Execution
Lab Results and Lab Test Inputs can now be grouped into Test Steps. When you complete a Test Step on the Test Execution page, LIMS will validate, calculate, and evaluate the results in that step before proceeding. This feature also includes an update to the collapse/expand control for the procedure document.
Lab Test Reopening, Retesting, Canceling & Rejecting
In the typical laboratory workflow, completed and reviewed sample testing records are sometimes revisited to allow for corrections found by QA or management review. With this release, we add the ability to reopen completed lab tests to allow for controlled and tracked changes to the test data.
Reduced Testing Strategies
Materials can now be scheduled for reduced testing. The sampling protocol now allows you to specify a frequency and duration so you can periodically run full testing on incoming materials, but apply a reduced testing approach to interim batches.
Each sample definition and lab method in your protocol can be flagged to be included or excluded from the reduced testing strategy. By designating who your approved material suppliers are, and whether the supplier has been qualified for reduced testing, the system will automatically monitor the number of incoming batches and the elapsed time since the last full testing and assign the appropriate samples and tests. In the event of a supplier, process or policy change, you can force full testing whenever deemed necessary. Learn more about reduced testing strategies in Vault LIMS.
Capture Instruments and Consumables in Test Execution
With this release, we add the functionality to record instruments and consumables used during test execution. These records are associated with the samples and tests so that it is possible to trace which instruments and consumables were used in preparation and analysis. This can replace the recording of this information in laboratory notebooks.
Lab Hazards & Special Instructions
Samples in laboratories require specific handling and storage precautions for safety and stability reasons.These precautions are based on the material that has been sampled. This applies to samples being tested in the laboratory as well as to reagents and other consumables that are used during the testing process. Capturing this information is a prerequisite step to enabling label design and generation for sample and consumable identification and management purposes.
This feature adds a Precautions object to store a lookup table of common safety, handling and storage precautions, and a join object to connect many Precautions to a single Material, and to use a single Precaution across many Materials.
The join includes an elaboration field which allows the user to specify additional clarifying information that may be material-specific. For example, a precaution might be “Do Not Swallow”; an elaboration for that precaution might be, “In case of ingestion, induce vomiting immediately and seek medical attention.”
Validation Management
Test Authoring with Requirements Burndown
With this release, Vault Validation Management allows authors of test scripts to easily identify and challenge requirements. Available requirements can be dragged and dropped onto a test step which will update the traceability matrix automatically. Included is the ability to easily filter requirements so that they can be linked with test steps based on available attributes such as risk, category, etc.
This feature helps to ensure that all necessary requirements are challenged as part of a validation activity while reducing the burden on validation and qualification professionals that manually maintain the trace matrix today. Learn more about authoring test scripts with the requirements burndown view.
Test Protocol Review
With this release, Vault Validation Management allows executors to view narrative content associated with a test protocol to be accessed from the Test Execution UI and Test Protocol Review UI app pages. Users can access the narrative content from a document mini-browser icon action. Clicking the icon opens the test protocol narrative content linked from QualityDocs.
This feature also supports the approval experience whereby all independently-reviewed test scripts that are part of the protocol will be available for approvers to review, provide their verdict, and complete their final approval task for the test protocol. Learn more about reviewing test protocols.
Quality Data Model Changes
See 23R1 Quality Data Model Changes.
Regulatory
RIM Registrations
IDMP Data Migration
This feature enables users to migrate data from the Registered Site Role to the related registered detail using Vault Loader. The management of manufacturing information was updated to support IDMP per IG v2.1.1. See About the Authorised Pharmaceutical Form & Registered Site Role Data Migrations for more information.
IDMP Data Model Updates
With this release, the Registrations data model is updated to support EU IDMP IG V2.1.1. This includes updates to source and output data points as well as the IDMP data aggregation algorithm. Additionally, a new Pending Withdrawal state type is added to allow the algorithm to skip creation of output records in the lifecycle state assigned to the state type.
FHIR Message Handling in IDMP Viewer
This feature extends the IDMP viewer functionality to render FHIR messages in a human-readable format. Initial support will render FHIR messages generated using HL7 FHIR version 4.6.0 to support viewing the XML output from the eAF form.
Activity Splitting for Labeling
This feature adds a new wizard for users to split Activities while managing Labeling Concepts. The wizard allows outstanding Labeling Concepts that have been deferred to be carried forward while allowing the original Activity to be completed.
Labeling Automation: Logic Update for Labeling Deviation Trigger
This feature modifies how Vault populates fields in labeling records created by a labeling automation trigger: When the trigger runs, Vault sets the Labeling Deviation record’s Deviation Review Disposition field to Accepted for Dependent Countries. Once the field is set, the trigger runs again to create corresponding Labeling Deviation and Activity-Labeling Deviation records for any related dependent Activities.
Multi-Record Registration Verification
This feature updates the Registration Verification workflow to accommodate both single and multi-record workflows.
Submission Type Defaulting with Constraint Records
When creating records in bulk, users can select a global term for the Submission Type field. The selectable global term is mapped to a corresponding local term via Admin-configured Constraint records, and the wizard creates the resulting Submission records with the appropriate local term.
Registration Type Defaulting with Object Type Mapping
This feature allows users to create Registrations in bulk with a default Registration Type value, populated from Admin-configured Object Type Mapping records.
EUDAMED UDI XML Generation Updates
With this release, Vault generates EU UDI submissions using the EUDAMED XML Schema v2.0.6, which increases the maximum length of the EUDAMED Model and Name fields for a Basic UDI or EUDAMED DI to 255 characters.
UPD Data Model Updates
This feature extends the Registrations data model to include data points specific to animal health products, such as species and withdrawal period. These data points are captured in new Registrations object types, but require an Admin to create additional custom object types. See 23R1 RIM Data Model Changes below and UPD Configuration for more information.
RIM Submissions
Copy Report Level Content Plan
With this release, a new Report Level Content Plan workflow system action is available to support creating a Content Plan for a Report Level Content Plan by copying from an existing Report Level Content Plan’s Content Plan. The system action copies both the Content Plan and Content Plan Item records and matched documents. While a new workflow must be configured to enable this feature, a new Copy Content Plan field is automatically added to all Report Level Content Plan object types to support the feature.
Update Content Plan Study Section
With this release, a new Update Content Plan Study Section action is available for the Submission Clinical Study and Submission Nonclinical Study objects. Once configured, the action moves valid study sections related to the Submission Clinical Study or Submission Nonclinical Study within the Submission Content Plan to the new location when the Clinical Study Type or Nonclinical Study Type (or Subtype) fields are updated.
For example, a Submission Clinical Study was originally created in Section 5.3.1.1 of the eCTD content plan with its Clinical Study Type and Clinical Study Subtype values set to Biopharmaceutical and Bioavailability (BA), respectively. To move the record to Section 5.3.1.2 (Comparative BA and Bioequivalence (BE) Study Reports), users can update the Clinical Study Type and Clinical Study Subtype values on the Submission Clinical Study to Comparative BA and Bioequivalence (BE), then run the new action (instead of deleting Section 5.3.1.1 and re-creating in Section 5.3.1.2).
In addition to reparenting the study section, the new action also updates additional fields on the Content Plan and Content Plan Item records in that section, including the Published Output Location, Clinical Study Type, Clinical Study Subtype, Nonclinical Study Type, Nonclinical Study Subtype, Indication, Source Content Plan Template, and Source Content Plan Item Template.
Content Plan and Content Plan Template: Exclude from Auto-Matching
The Content Plan and Content Plan Template field Exclude from Auto-Matching is added in this release to allow auto-matching only for specific sections in a Content Plan Template. Additional configuration is required to leverage this field, and it is intended to be configured as a conditional entry action in the Content Plan object lifecycle, so that Vault only updates the Match Documents field to Yes when Exclude from Auto-Matching is de-selected.
Additional Granularity for Content Plan Template Constraints
This release introduces a new Template Constraint Type picklist field on the Submission, Event, and Constraint objects. The field is leveraged in loading and applying Content Plan Template Constraints at an additional level beyond the Region, Country, Application Type, Submission Type, Submission Subtype, and Supplement Effective Date Type for Submission Content Plans. For Events, the field allows you to set up Content Plan Template Constraints for different types of Global Content Plans.
Increase Limit for Delete Inactive Content Plan Records Job
The limit for the Delete Inactive Content Plans job is raised from 5,000 to 50,000 records deleted per job run.
Start Submission Wizard from Regulatory Objective Update
With this release, when the Submission Wizard is initiated from a Regulatory Objective, selecting a Submission is optional. This allows users to add relationships to a Regulatory Objective without adding to a related Submission.
RIM Submissions, RIM Submissions Archive
Active Dossier Loader
With this release, a new Active Dossier Loader object supports active dossier record migration. When users create an Active Dossier Loader record, Vault creates or updates Active Dossier Structure, Active Dossier Item, and Active Dossier Item Detail records based on the corresponding Active Dossier Loader record metadata.
Active Dossier Template Update
With this release, several new records have been added to the Active Dossier Template and RIM Reference Model objects for all RIM Vaults to add support for 3.2.P.9 Product Interchangeability Equivalence Evidence for ACTD and the granular 3.2.P.2 Pharmaceutical Development components.
RIM Publishing, RIM Submissions
Document Merging on Publish
To support authoring at a lower level of granularity, Vault will merge multiple documents assigned to a single Content Plan Item at the time of publishing. This functionality is available when using Report Level Content Plan publishing or Submission publishing.
Vault Link Annotations: Multiple Target Destination Support
To support document reuse across multiple regions, Vault now supports Vault Link Annotations that target multiple destinations. When multiple destinations are defined, the publishing process determines the destination best suited for the current submission or report. This functionality is available when using Report Level Content Plan publishing or Submission publishing.
RIM Publishing
Support Sequence ID Updates in Continuous Publishing
With this release, Vault Submissions Publishing will trigger continuous publishing when the Sequence ID is updated on the Submission record and Enable Continuous Publishing is enabled. This updates the folder structure without needing to republish all items.
Workgrouping Support for Australia eCTD
Vault Submissions Publishing now supports the ability to add multiple Submission Types and Submission Subtypes to Work-grouping submissions in Australia.
User Logs for Gateway Transmissions
With this release, Vault RIM users are provided with a log file that gives information regarding a submission via the gateway to a Health Authority. The file is added as an Attachment on the Submission record and includes a list of the files that were transmitted and details on any errors that may have occurred.
RIM Submissions Archive
Enforce Submissions Archive User License
The new Submissions Archive Viewer now supports previously-existing functionality enforcing Submissions Archive application licensing, blocking RIM Vault users without a Full User application license from accessing any Submissions Archive functionality.
New Submissions Archive Viewer Automatic Enablement
Submissions Archive Vault users are automatically guided to the new Submissions Archive Viewer as it is the default and only RIM Archive viewer supported by Veeva. As such, the related Submissions Archive Feature (New Submissions Archive Viewer) is removed from Admin > Settings > Application Settings.
See About the New Submissions Archive Viewer for more information.
Save View in the New Submissions Archive Viewer
This feature is a continuation of enhancements to the new Submissions Archive Viewer, providing users with a way to save and share views.
Empty Section Indicator in the New Viewer
With this release, the new Submissions Archive Viewer will support previously-existing functionality of pre-calculating and displaying a visual indicator on empty sections, based on the delete lifecycle operation.
Display Section XML Attributes in the New Viewer
With this feature, section XML attributes for electronically-formatted dossiers are displayed in the new Submissions Archive Viewer grid view.
Display Inactive Applications and Submissions in the New Viewer
With this release, the new Submissions Archive Viewer will support previously-existing functionality of allowing users to select and view inactive Applications and Submissions.
Add Submission Fields as Selectable Columns in the New Viewer
Users navigating the new Submissions Archive Viewer have the option to select, view, and filter on the Submission object fields Lifecycle State, Sequence ID, and Actual Submission Date as part of the final dossier review process.
Submission Based Viewer Filters Automatic Enablement
Users are now automatically able to filter by Submission related objects within the new Submissions Archive Viewer. As such, the related legacy Submissions Archive Viewer settings in Admin > Settings > Application Settings are impacted as follows:
- Enable Submission based Viewer Filter is removed.
- Show eCTD Metadata Mapping to users on submission import is removed from Vaults where Enable Submission based Viewer Filter has been configured.
See About the New Submissions Archive Viewer for more information.
Published Document Display Correction in the New Viewer
Published RIM documents in a steady state are now correctly displayed in the new Submissions Archive Viewer.
Support Filtering for XML Section Attributes
Users are able to filter on XML section attributes for electronically-formatted submissions in the new Submissions Archive Viewer.
Leaf Source File Path Auto-Correction
RIM Vault Admin users are now able to initiate a system process to correct the Submissions Archive metadata of previously imported or published Submissions after the Application folder name is changed.
RIM Email to Vault Processor
With this release, Vault RIM users can forward emails to a predefined email address to create unclassified documents. Once received, Vault automatically extracts attached documents and creates them in the Document Inbox, including a separate document for the email.
23R1 RIM Data Model Changes
See 23R1 Regulatory Data Model Changes.
Safety
Safety features are targeted for tentative availability on April 20, 2023 & April 28, 2023.
Safety
Configurable Inbox Item Sections and Custom Case Fields for Manual IntakeConfiguration
Admins can now configure the list and order of Inbox Item fields for the Details, Patient, Contact, Medical Events, and Products sections. Admins can also hide Medical History and Drug History in the Medical Event and Products sections. Finally, Admins can select custom Case fields for the Inbox Item Details and Patient sections. Users can then manually enter values for the selected fields during intake and the system can copy these values to Case upon promotion.
Learn More
- Admin Help: Customize the Inbox Item Page Layout
Local Case Import to Inbox ItemAdmin Checkbox
Vault Safety can now import local language text to the Inbox Item localized fields to facilitate translation to English in the global fields. When enabled, the system imports the text values to the localized or global fields based on the set Localization.
Learn More
- Enablement: Enable Local Case Import
- User Help: Import an Inbox Item: Local Inbox Item Import
Local Intake Auto-Translation FrameworkConfig
With this release, Vault Safety can now use the Amazon Translate Connection to translate local text fields to English to allow for global Case processing. In other words, you can now use a third-party service to translate Inbox Item text fields from the reporter’s local language to English during Case intake.
Admins can enable translations by configuring a user action. Admins can also configure the localizations, fields, and volume limits for translations.
Learn More
- Enablement: Enable the Local Intake Auto-Translation Framework
- User Help: Send Translation Requests Through the Auto-Translation Framework
Pregnancy Case IntakeConfig
Intake users can now mark an Inbox Item as Pregnancy Case to track pregnancy exposure or to report an Adverse Event suffered by the mother. Upon Case promotion, Child Information placeholders and Case Test Results will be automatically created to facilitate data entry.
Learn More
- Enablement: Enable Pregnancy Case Intake
- User Help: About Pregnancy Case Intake
PII Masking on Potential Matches PageAdmin Checkbox
With this release, Admins can enable personally identifiable information (PII) masking on the Potential Matches page where Atomic Security is not enforced.
Learn More
- Enablement: Enable PHI and PII Masking on the Potential Matches Page
- User Help: Promote to Case: Overview of Duplicate Detection
Indexing of New Migrated Cases for Duplicate SearchAdmin Checkbox
With this release, Admins can initiate indexing of newly migrated Cases to include them in duplicate detection.
Learn More
- Admin Help: Configure the Case Promotion Settings Page: Enable Duplicate Search Options
- User Help: Promote to Case: Overview of Duplicate Detection
Simplified Case Intake for StudiesAuto-on
Inbox Items now support manual intake for unarmed studies. Based on the selected Study, Study Product selection will use either Study Arms or Study Products. In addition, Case Products can now be marked as Blinded, Unblinded, or Open-Label when a blinded Study is selected.
Learn More
- User Help:
New Duplicate Search Algorithm Enablement UpdateAuto-on
With this release, the New Duplicate Search Algorithm feature is Auto-On. This feature was originally introduced in 22R3 with Support-enablement. Although the feature is Auto-On in Vaults with Inbox Items enabled, Admins still control the deep duplicate search options through Case Promotion settings. This feature is available for Inbox Items only, and is not supported for AERs.
Read more about this feature in What’s New in 22R3.
Record Selection Usability Enhancements for Inbox ItemsAuto-on
With this release, searching and selecting records from the Study, Country, and Product Registration dropdown fields during Inbox Item data entry is made easier by displaying recently used values at the top of the list. These fields also now include a binoculars icon for browsing and filtering the available options. In addition, searching and selecting records in picklist fields (for example, Dose Form and Patient RoA) is made easier by displaying exact matches at the top as you type.
Case Access Group SecurityConfig
With Case Access Groups, Vault Safety significantly simplifies security around access to Cases based on Case data, including Sponsor, Report Type, Country, and Market Segment. In addition, this feature provides more granular control over unblinded information and personally identifiable information (PII) at the object and field levels. For example, Admins can manage access to non-sensitive data for specified open and non-Study Products, Drug Roles, and Study Product Roles.
Learn More
- Enablement: Enable Case Access Group Security
- Admin Help: Manage Case Access Group Security
- User Help: Enter Case Data: Details Section
Case Access Groups: Workflow Participants by RoleAuto-on
When using Case Access Groups, Vault Safety can now assign workflow tasks by role on the Case, Localized Case, and Transmission.
Note: This feature is auto-on only after you have enabled Case Access Groups.
Learn More
- Enablement: Enable Case Access Group Security
Generate Assessments Record ActionSupport
Vault Safety now gives Case Processors the ability to generate Assessments, Assessment Results, and Expectedness records for all serious and non-serious Adverse Events on a Case. Completed in a single action, this feature improves Case processing efficiency by removing the need to generate each required record manually.
Learn More
- Enablement: Enable Generate Assessments Record Action
- User Help: Generate Assessments
Record Selection Usability Enhancements for CasesAuto-on
With this release, searching and selecting records from picklist fields during Case data entry is made easier by displaying exact matches at the top as you type.
Domestic Case Edit EnhancementsAuto-on
With this release, global Case Processors can view and edit global information in dual-text app controls, even while the localized Case is still generating.
Case Locking User Interface EnhancementsAuto-on
This feature introduces enhancements to manual and strict Case locking. If another user applies manual or strict Case locking, the relevant user actions will not be displayed or available for you to run in the All Actions menu. This improvement affects only the user interface and contains no functionality changes.
Case Compare: Descriptive Error MessagesAuto-on
This feature introduces more descriptive error messages on the Case Compare page. These enhanced messages help users understand issues that may occur when merging an Inbox Item to an in-flight Case or creating a Follow-Up Case.
Learn More
- User Help: Complete the Inbox Item to Case Compare Page
Automatic MedDRA Hierarchy UpdatesAuto-on
Vault Safety now enables downloading the latest central MedDRA dictionary version as soon as it becomes available. With the download complete, when you update the active version in your Vault, all of the global and localized terms, as well as any hierarchy changes, are automatically applied to MedDRA terms referenced on Cases. This means that you can immediately start the required verifications. In addition, it reduces the manual effort of bulk recoding, enabling MedDRA Admins and Medical Coders to focus on currency status changes, policy, and best practices.
Learn More
- Enablement: Optional: Update the MedDRA Dictionary Page Layout
- Admin Help: Manage the MedDRA Dictionary
- User Help: Code MedDRA Terms
Smart MedDRA CodingConfig
Vault Safety Smart MedDRA Coding offers advanced coding of MedDRA terms that aren’t an exact match to the active dictionary or your synonym list. Using AI and automation, this feature overcomes common problems such as alternate and incorrect spelling, abbreviations, and multiple reported terms. For Case Processors, this means less time spent on manual MedDRA coding.
For Admins, configuring Smart MedDRA Coding reduces the manual effort of maintaining the MedDRA Synonym list. As terms are coded, they are automatically added to the synonym candidate list, making coding more streamlined and consistent over time.
Learn More
- Enablement: Enable Smart MedDRA Coding
- User Help: Code MedDRA Terms
IMDRF Bulk SnapshottingConfig
With this release, when an IMDRF Dictionary version is updated, the system now downloads the full dictionary. Admins can choose when to transition to a new version and receive notifications when updating and downloading are complete.
Learn More
- Admin Help: Manage the IMDRF Dictionary
EDQM Dose Forms and Routes of Administration Enablement UpdateAuto-on
With this release, the EDQM Dose Forms and Routes of Administration feature is Auto-On. This feature was originally introduced in 22R2 with Admin Checkbox-enablement.
Read more about this feature in What’s New in 22R2.
Enhanced Eligible Products Selection for Cross ReportingSupport
With this release, Vault Safety uses improved logic to determine the product driving ICSR reporting requirements in cross reporting scenarios. Contact Veeva Support to enable this feature in your Vault.
Additionally, Admins can modify the behavior of the Product and Study rule parameters when evaluating cross reporting scenarios. Admins can configure this behavior only after Support has enabled the improved logic feature.
Learn More
- User Help:
Parameter Driven Assessment Selection for Rules EngineSupport
Previously, the most conservative product and assessment rule parameter evaluation strictly relied on Case Seriousness, Expectedness, and Relatedness being defined in the Distribution Rules. This can lead to under-reporting for common rule sets. Vault Safety can now employ a more tolerant rule parameter evaluation based on the configuration of relevant reporting rules.
Learn More
Support for New Company Products Type in Safety Data ExchangeConfig
Vault Safety Data Exchange now supports the new Company Product product types, such as Cosmetics and Nutritional. In addition, the Vault Safety rule engine now supports rules based on product type. For example, you can configure FDA Submission rules specific to OTC Drugs so that this product type won’t be subject to the same Submission rules as prescription drugs.
Learn More
- Admin Help: Configure Support for Company Product Type
- User Help:
Generate E2B Formats for Migrated CasesAuto-on
With this release, preview generation of E2B(R3) files and Transmission generation of all E2B formats are now available for migrated Cases.
Learn More
All Product Types Available for FDA 3500A and CIOMS I GenerationSupport
With this release, Vault Safety supports generating MedWatch 3500A and CIOMS I forms for device-only Cases. To accommodate reporting on additional classes of Products (for example, Over the Counter and Cosmetics), exporting Company Product types is also supported.
Learn More
- Admin Help:
- User Help:
MedWatch 3500A and CIOMS I Preview Generation for Open Label Study ArmsAuto-on
With this release, Vault Safety supports generating MedWatch 3500A and CIOMS I preview forms for Cases that use an Open Label Arm from a Blinded Study.
Learn More
- User Help: Blind Protection on Report Previews
CIOMS II Line Listing ExpectednessAuto-on
The CIOMS II standalone report in Vault Safety now supports different expectedness logic for Clinical Trial and Postmarketing Cases in the same report. Clinical Trial expectedness will be based on the Investigational Brochure (IB)/Study datasheets, while Postmarketing Cases will use the Product datasheet.
Learn More
- User Help: Create CIOMS II Reports
PADER Date Filtering Criteria Enablement UpdateAuto-on
With this release, the PADER Date Filtering Criteria feature is Auto-On. This feature was originally introduced in 22R3 with Support-enablement.
Read more about this feature in What’s New in 22R3.
New Info Date for PADER Line ListingsSupport
For PADER Line Listings, previously, the Initial Report Date and a Transmission Date represented the date the FDA first received the Case. With this release, a New Info Date column will be added to the Interval Line Listings and to the Non-Primary Suspect Drug Report, which will be populated from the Case. When you filter the PADER data on the New Info/Receipt Date, you can see what date was used as the inclusion criteria for the report.
The Transmission Date has been added to the log of the 15-Day and Non-15 Day report and the log of the Summary of Adverse Events Report to assist in troubleshooting.
Essentially, all four (4) PADER reports now have both the Receipt/New Info Date and the Transmission Date.
Learn More
- Enablement: Enable PADER Authoring and Table Generation
- User Help:
Partner AS2 Gateway ConnectionsSupport
This release introduces a number of enhancements for Vault Safety gateway connections administration to facilitate managing allowlists and certificates, as well as testing gateway connections.
Learn More
- Admin Help: Configure Custom AS2 Gateways
- User Help: About AS2 Gateway Connections
PMDA Multi-Submission with Localized CaseAuto-on
With this release, Vault Safety supports multiple PMDA Submissions from a single Case.
Learn More
- Enablement: Enable PMDA Multi-Submission with Localized Case
- User Help: Prepare a Localized Case
External Connection SDK Support for Custom E2B ExportConfig
With this release, Vault Safety introduces faster exports of custom E2B formats. This makes generating E2B files for large Cases more efficient.
Learn More
- Admin Help: Custom Agency Report Formats
SafetyDocs
PSMF Binder ApprovalsAuto-on
Vault SafetyDocs can now manifest approval pages on generated PSMF PDFs.
Learn More
- User Help: Review and Approve PSMF Binders
PSMF Binder Table of ContentsConfigurationn
Vault SafetyDocs can now automatically generate a table of contents for PSMF Binders when merging to PDF.
Learn More
- Enablement: PSMF Table of Contents Template
- User Help: PSMF Table of Contents
PSMF Logbook Format EnhancementsAuto-on
The PSMF Logbook in SafetyDocs has been enhanced to have a clearer format. The new Excel template is automatically available in all Vaults. To use this feature, you must first remove any previously generated logbooks in your existing PSMF binders.
Learn More
- User Help: PSMF Logbook
Literature Process ManagementConfiguration
Vault SafetyDocs now supports the management of literature articles and article reviews. This feature includes the data structure and document types necessary for Literature Management. Future enhancements will leverage this data structure.
Learn More
- Enablement: Enable Literature Review Process Management
- User Help: Manage the Literature Review Process
PVA Document and Obligation ManagementConfiguration
Vault SafetyDocs now supports the management of of pharmacovigilance agreements (PVAs) and Safety Data Exchange agreements (SDEAs). This feature includes the data structure and document types necessary for PVA Management. Future enhancements will leverage this data structure.
Learn More
- Enablement: Enable PVA Management
- User Help: Manage Pharmacovigilance Agreements
Data Model Changes
See 23R1 Data Model Changes: Safety.
QualityOne
QualityOne
Independently Licensable Applications in QualityOne
Within Vault QualityOne, three independently licensable applications will be available. Admins can now specify different license types for different users. There is no impact to features or functionality and no action is required.
QMS & Doc Control Applications for QualityOne
With this release users can be independently licensed to QMS and Doc Control. There is no impact to features or functionality and no action is required.
HSE Application for QualityOne
With this release HSE can be licensed independently from QMS and Doc Control. There is no impact to features or functionality and no action is required. Learn more about the HSE application.
Conditional Role Exclusivity for Teams
This feature enables users to define conditional exclusivity for each role within the Team. This allows greater flexibility so that a user can be selected for more than one (1) role on a team while simultaneously providing exclusivity to another role where it is required. Learn more about conditional role exclusivity for Teams.
Displaying Team Assignment Users in Audit Logs
This feature enhances the Audit logs for Team assignments. Previously, the Audit logs would display “System” as the user who performed the Team assignment. Now, Audit logs will display the name of the user who performed the Team assignment with “System” acting on behalf of the user. Learn more about audit logs for Teams.
User Experience & Security Enhancements for Teams
This feature extends the Teams capabilities to enhance security, performance and user experience. Learn more about other Teams enhancements.
Restricting Creation of Teams on Related Objects
Previously, Admins could select the User Task (user_task__v), Related Quality Event (related_quality_event__qdm) objects, and High Volume objects (HVOs) for Team configuration. Since these objects would not require Teams to be enabled, Admins will now be restricted from creating Teams for these objects.
Updating Team Sharing Setting with Workflow Action
Previously, Admins were unable to update Team-governed sharing settings using the Update Sharing Settings workflow action. Now, Admins can update Team-governed sharing settings using this workflow action.
Displaying a Warning when Updating Maximum Users Retroactively
When the Maximum Users field is retroactively updated in the Team Role configuration, users should see a red exclamation mark indicating a warning for an invalid team. This allows the user to update the team assignment according to the warning.
QMS
Generate Documents using Object Record Actions for Audits
This feature allows users to generate a document from a Formatted Output or a Document Template using an entry action or user action on the Audit object record. Learn more about generating documents for Audits.
Deep Copying Inspection Plan Requirement & Acceptable Attributes
This feature extends the Copy Record action to include related records under the Inspection Plan hierarchy. Users can choose to deep copy Inspection Plan Requirement and Acceptable Attribute records whenever an Inspection Plan is copied. Learn more about deep copying Inspection Plan Requirement and Acceptable Attributes.
This feature extends the Application Security design for the Inspection Sample Test Result object to apply record-level access control using the Facility field. Learn more about Application Security.
Inspection Sample Test Results Enhancements
This feature improves the user experience by providing an effective way for presenting test result data. The overall responsiveness of validating test results has been improved, and users can record test result data faster in fewer columns. Learn more about Inspection Sample Test Results enhancements.
Setting Pass / Fail Value for Qualitative Inspection Sample Test Results
With this feature, users can explicitly set a passing or failing test result value for attribute test result data associated to an Inspection Sample Test Result record.
Validating Inspection Sample Test Result Record
As users enter test results of an Inspection Sample Test Result record, its value will be validated against the associated LSL and USL values (quantitative testing), or the pass and fail value (qualitative testing). The existing Analyze Inspection and Analyze Inspection Sample actions will evaluate Inspection Sample Test Results where validation has not been performed.
Recording Inspection Sample Test Result Variable & Attribute Data in a Single Column
Users can record the qualitative (attribute) and quantitative (variable) test result, along with its test result unit and data symbol, in a single test result column. These resulting values will be stored in the existing Inspection Sample Test Result data field for attribute and variable test result data.
Pre-populate Hazards in Material Verification Checklists by Food Category and Sub-category
This feature provides the ability to send a single material verification checklist to a supplier for all the materials that belong to one material category and sub-category. In cases where material verification is requested by a supplier that is supplying multiple materials that belong to the same material category and sub-category, the supplier will only need to submit their response once rather than completing a separate checklist for each material. Learn more about the Material Verification enhancements.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Deep Copy HACCP Plan Enhancement
This feature extends the Copy Record action to include related records under the Inspection Plan hierarchy. Users can choose to deep copy Inspection Plan Requirement and Acceptable Attribute records whenever an Inspection Plan is copied. Learn more about deep copying Inspection Plan Requirement and Acceptable Attributes.
Populate HACCP Plan Enhancement
Enhancements to the Populate HACCP Plan Details feature allow the user to view all the related CCP-Hazard Analysis (ccp_hazard_analysis__v) records on the HACCP Plan page by automatically populating the HACCP Plan (haccp_plan__v) field when the CCP-Hazard Analysis record is created.
Enhancements to the Populate HACCP Plan Details action will also enable the following:
- Admins can configure the objects that will be automatically populated by the system based on the data setup.
- Automation logic will auto populate HACCP Plan Process Step Connection records in addition to the HACCP Plan Process Step records.
- Automation logic is updated to support the standard object type on the Process Step and HACCP Plan Process Step object when automatically populating the HACCP Plan Process Step record.
Learn more about the HACCP Plan enhancements.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Acceptable Attribute Remodel
This feature replaces the existing Acceptable Attribute object and its related objects with a new Attribute Test Result Variant object. This data model change will provide an improved user experience as users can now efficiently capture different variations of qualitative test results found in COA files without having to specify each attribute in the Inspection Plan Requirement record.
This also allows users to view matched text from the COA file that leads to either a “Pass” or a “Fail” verdict. Learn more about the Acceptable Attribute remodel.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
COA Inspection Enhancements
These features improve organizations’ ability to report on overall ingestion accuracy, specifically, the percentage of COA documents that can processed uninterrupted (straight-through processing). Learn more about COA Inspection enhancements.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Analysis Source for Header Fields
With this feature, Admins can report holistically regarding the COA ingestion accuracy. Admins can report on fields that have Matching Rule Variants defined if they were ingested automatically or required manual intervention to be successfully extracted from a COA file.
Automatic Flagging of Problematic COA Formats
With this feature, Vault can automatically flag COA files that were not processed straight-through. Once an Inspection record enters a “Complete” lifecycle state type, Vault assesses whether the document was ingested automatically.
Document Control
Process Navigator: Visual Hierarchy Configuration Page
The Visual Hierarchy Configuration Page will allow customers to configure Process Detail pages to display different types of information about a business process. Enhancements to the Process Navigator feature will also allow users to reparent the Visual Hierarchy records in a business process without losing their associated documents, allowing for more flexibility. Learn more about the Visual Hierarchy configuration page.
Training
QualityOne & Training Vault Connection
This feature enables the synchronization of training documents from a QualityOne Vault to a Training Vault. After the documents are approved (or updated) in the QualityOne Vault, a CrossLink is created (or updated) in the Training Vault so that the Training Admins can create Training Requirements and assign training tasks to users. Learn more about the connection between QualityOne and Training Vaults.
Data Model Changes
See 23R1 Data Model Changes: QualityOne.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on May 2, 2023.
RegulatoryOne
Turn On Audit Tracking for the Organization Object
Audit tracking is now turned on for the Organization (organization__v) object, allowing Admins to view the audit trail of changes made to all Organization records. The Audit data changes in this object attribute cannot be disabled for the Organization object.
Enhanced Configurability for the Organization Object
This enhancement allows Admins to configure the Values must be unique attribute for the Name (name__v) field on the Organization (organization__v) object.
Registration & Dossier Management
Copy Matched Documents
This feature reduces the manual work users must do to customize a Registration Item Requirement by allowing Admins to configure the Customize action to copy matched documents. When configured, Vault includes all matched documents in generated EDL Items that are included in the source EDL Items when users run the Customize action on a target Registration Item Requirement.
Prior to this release, when users ran the Customize action on a target Registration Item Requirement, the generated related EDL items never included any of the documents matched to the corresponding source Registration Item Requirement, which may have been reviewed, auto-matched, manually matched, excluded, or removed after a user ran the Customize action. Learn more about customizing matched documents for shared requirements.
Admin UI to Manage Relational Tokens & Object Mappings
Registration & Dossier Management Vaults use Relational Tokens and Object Mappings to help supported actions, such as Split Registration Items, Generate Requirements, and Generate Registration Data, to determine the action’s behavior. As of this release, a new section is available on the Configuration tab for Admins to manage, create, edit, and delete Relational Tokens, Object Mappings, and Field Mappings. Learn more about defining Relational Tokens and Object Mappings.
Specify Object Type for the Create Registration and Objective Action
This feature allows Admins to specify what Registration object types Vault creates when users run the Create Registration and Objective action. Learn more about configuring the Create Registration and Objective action.
Disable Create Registrations in Bulk Feature
As of this release, the Create Registration in Bulk feature is no longer available for configuration. Existing Vaults with the feature already configured are not impacted. The Create Registration in Bulk feature was released in 21R2 as a temporary solution for users to automate the creation of multiple Registration records, saving time and effort. Since then, we have released several features, such as the Local Impact Assessment feature, which allows users to generate the appropriate Registration records within the registration process, rendering the Create Registration in Bulk feature unnecessary.
Data Model Changes
See 23R1 Data Model Changes: RegulatoryOne.
Veeva Claims
Veeva Claims features are targeted for tentative availability on May 2, 2023.
Veeva Claims
Reuse Element Translations for Different Locales
This feature enables users to reuse element translations in a particular language across different geographical pack copies when that language is mapped to each geographical location. When localizing a Pack Copy, Vault can reuse existing element translations for languages mapped to multiple locations rather than creating a unique element translation for each location. Learn more about translating pack copy elements.
Bulk Add Local Adaptation Comments
This feature enables users to add a Local Adaptation comment to multiple Local Adaptations using a bulk object record action. When configured, users can select Bulk Add Comment after selecting the option to perform a bulk action from the All Actions menu and add the same comment to all selected Local Adaptation records. Users can also mention other users by entering the “@” or “+” keys in the Local Adaptation Comment. Learn more about bulk adding a comment to multiple records.
Project Automation UX Enhancement
This enhancement improves the user experience in record selection dialogs after users run the Create Selective Claims, Create Selective Local Adaptations, and Localize Pack Copy actions. In this release, the Product and Location filters move from the right to the left side of the dialogs. This allows users to work from left to right, preventing the erroneous generation of object records that users were prone to create because they instinctively interacted with the modal from left to right, making the grid checkboxes the focus of their selections. In addition, users must first select a Product or Location in each dialog before selecting Statement, Claim, or Element records.
Product Line Extension Substantiation Copy
This feature enables users to copy over Substantiation records when creating new Claims through the Product Line Extension feature. Users can now specify if they want to copy over Substantiation records to the newly-created Claims by selecting the Copy Related Substantiation Records checkbox after running the Copy Claims to Another Product action. Learn more about Product Line Extensions.
Project & Pack Copy Automation: Record Action Settings
This feature enables Admins to configure the Create Selective Local Adaptations and Localize Pack Copy actions for each lifecycle state of the Project and Pack Copy objects, respectively. For the Generate Local Adaptations action, Admins can define Instructional Text, Grid Columns, and Claims Lifecycle States for each lifecycle state. For the Localize Pack Copy action, Admins can define Instructional Text and Grid Columns for each lifecycle state. Learn more about configuring the Create Selective Local Adaptations and Localize Pack Copy actions.
23R1 Veeva Claims Enhancements
This feature enables users to select multiple Country object records when running the Bulk Create Local Adaptations and Localize Pack Copy actions. In prior releases, Vault relied on a multi-select country picklist for these actions.
Claims Study Object
This feature allows users to capture study results and corresponding formulations in a single Regulatory Study record, which users can easily search for and re-use when creating Substantiation records.
Data Model Changes
See 23R1 Data Model Changes: Veeva Claims.
Enablement Details
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|