Pre-Release Date: July 12, 2021 | Release Date: July 30 & August 6, 2021
We are pleased to bring you Vault 21R2. Read about the new features below. You can find information on enabling new features in 21R2 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Working with Documents
Renditions for RTF Files
With this release, Vault now generates viewable renditions for Rich Text Format (RTF) files. RTF files are a popular cross-platform file type that capture information about font styles, formatting, images, and more.
Reclassified Documents Always Retain Attachments
If a document has attachments, but is then reclassified to a Document Type where Attachments are disabled, Vault will now continue to display the Attachments, regardless. If Attachments are disabled for a Document Type, you cannot add any new Attachments to the document.
Enable Document Tags on All Vaults
Previously, the Document Tags feature was only available through Veeva Support. With this release, Admins can configure document tags in all Vaults.
Document Tags allow Admins to define a set of words or phrases that Vault can automatically detect whenever new document content is added. When any of these words or phrases are found, Vault sets the defined tag on the document metadata. Users can then search and filter on the defined document tags.
Even though this feature is now enabled in all Vaults, you can choose to hide the Tags field using field security in admin, which effectively disables the feature for end users.
Carry Forward All Related Document Versions
For certain document relationship types, the source of a document relationship can point to many versions of the same target document. If that relationship type is also configured with the Carry Forward functionality, when you create a new version of the source document, Vault will now ensure your new version points to every target document version that was present on the prior source document version.
This is a change in behavior, as today the new source document version would only carry forward the latest target document version (as opposed to all target document versions).
This change only applies to relationship types where the source and target are version specific, and where the source has Carry Forward enabled.
Disable Office Online on All Vaults
Starting in this release, users can no longer check out documents to Microsoft Office Online (this is the legacy feature, and not the newer Edit in Office 365 / Collaborative Authoring functionality). Users can still edit any documents that were previously checked out to Office Online, but these documents must be checked in to Vault before the 21R3 release. In 21R3, the Checkout to Office Online feature will be deprecated entirely, and any edits on documents that are not checked in will be lost.
We have been communicating this change over the past several releases. 21R2 is the last general release in which the legacy Office Online functionality will continue to work. We strongly recommend configuring your Vault for Collaborative Authoring, which allows users to edit documents in Microsoft Office 365 desktop applications and in browsers.
Video Annotation: Claim Links
With this release, users can create Claim Links when adding references on video documents by selecting approved Claims in order to define references in the resulting link annotations. This aligns with existing capability on non-video documents.
Share My Inbox Documents
You can now configure your Document Inbox to automatically share any new documents you add to the Inbox, and any documents in your Inbox where you are the Owner. The users and groups you define will be automatically added to the Inbox Editor role on those documents.
This feature is particularly helpful where the user uploading files to the Document Inbox isn’t necessarily the user responsible for completing the document information and classifying it into the Library.
In addition, Admins can define non-removable users and groups that your Inbox Documents will always be shared with.
Learn more about sharing inbox documents.
Relabel the Undefined Document Type to Unclassified
In Vaults created after the 21R2 release, the Undefined document type is relabeled as Unclassified.
Note: In Vaults that use Veeva Snap or custom integrations with the Vault REST API, relabeling the Undefined document type may cause errors when uploading documents. Check with your organization’s developers before updating this label. We recommend that customers experiencing errors change the label back to Undefined until this issue is resolved.
Relabel the Unclassified Lifecycle to Inbox
In Vaults created after the 21R2 release, the Unclassified document lifecycle is relabeled as Inbox.
Note: In Vaults that use Veeva Snap or custom integrations with the Vault REST API, relabeling the Unclassified document lifecycle may cause errors when uploading documents. Check with your organization’s developers before updating this label. We recommend that customers experiencing errors change the label back to Unclassified until this issue is resolved.
Inbox Enhancements
This release introduces features related to the Unclassified document type and Inbox. These enhancements affect the Document Inbox. Users must now have the Create Document permission on the Unclassified document type to view and drag and drop documents into the Document Inbox. Users without this permission without the Create Document permission can only view the Document Inbox if they have the View Document permission on a document using the Inbox lifecycle. Additionally, documents shown in the Document Inbox are now restricted to those assigned to the Inbox lifecycle.
Document Inbox Auto-On for All Vaults
Document Inbox is now enabled for all Vaults. However, now that the Unclassified document type is available in admin, you can control which users and groups have access to the Inbox (and the Classify Later option when creating documents) simply by configuring the Create Document Permission field on the document type.
Navigate From Collapsible Upload Modal to Inbox
With this feature, users can navigate back to the Document Inbox from anywhere in Vault via a new button on the collapsible document upload status dialog.
Attachments for Unclassified Documents
Now the document type Unclassified (previously labeled Undefined) is in admin, you can enable or disable Attachments for this document type.
Prevent Reclassify for Checked-out Documents
A few releases back we started preventing documents from changing state whilst they are checked out. When a document is checked out, its source file is being edited, and it could cause process and security access issues if the state changes before it is checked back in. Reclassification will often affect the available document fields, and can even change the lifecycle state, so we have extended this concept to now prevent reclassification if the document is currently checked out.
Merge Fields Nested Table Rendering
With this release, Vault fully supports nested table rendering. When using Merge Fields for Microsoft Word™ repeaters in a table, Vault now only displays the data relevant to the object record in a table row within its nested tables.
Infinite Document Scrolling
With this release, Vault no longer displays visible tranche navigation on long documents. Users can now scroll seamlessly across all pages. Previously, users needed to navigate from one set or tranche of 25 pages on desktop and 5 pages on mobile. Note that documents viewed in Internet Explorer (IE11) continue to use visible tranche navigation.
Filter on Claim Links & Approved Links
Updates (in 21R1 General Availability release) allow users to show or hide Claim Links by selecting or unselecting the Claims Links filter, and to show or hide Approved Links by selecting or unselecting the Approved Links filter.
Vault Objects
Related Object Section Criteria VQL Filters
We enhanced Related Object sections of objects to filter based on criteria VQL, similar to Related Document section filters. Admins can now define much more powerful and flexible filters using criteria VQL. Any existing legacy filters will be unchanged and continue to function as before. Vault will automatically convert legacy filters to criteria VQL when an Admin edits the applicable Related Object section.
Admins can also choose to apply the criteria VQL as default when new records are created by setting the Apply on Create checkbox.
Previously, Vault ignored filters that included any fields that a user did not have Read permission on. With this release, Vault applies the criteria VQL to filter the Related Object section, but users do not see any fields that they do not have Read permission on.
User Object Custom Fields Support in Validation Rules
Custom fields on the User object can now be used in Vault validation rules for object records, giving Admins much more flexibility to validate custom information added to their User objects.
Create Related Record in Record Create Dialog
In previous releases, users that wished to create a new record within a related record first had to create a new record if it did not already exist. Users can now create a related record directly in the create record dialog.
Hovercard on Record Picker Dialog Fields
Starting this release, users can now hover over fields on the record picker pop-up dialog, and Vault will display a hovercard with up to 1000 characters of text to help users decide which records to choose.
Lifecycle & Workflow
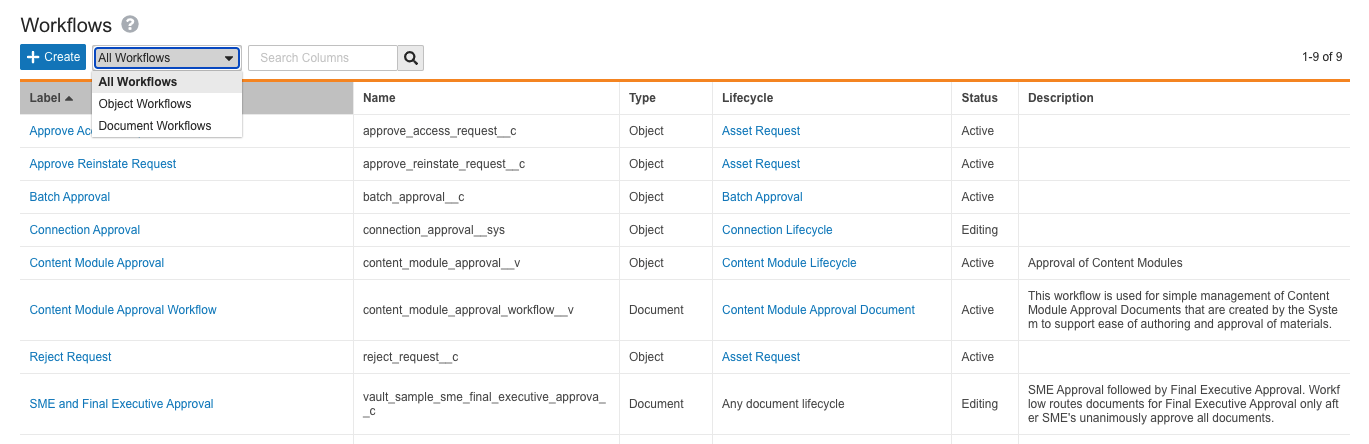
Unified Workflow Access
This release brings a major step in the One Workflow initiative to our customers, with the goal of streamlining the Vault workflow creation and configuration experience. To support this initiative, we have unified access to both object and document workflows under a single configuration page located at Admin > Configuration > Workflows. In addition, single-document workflow functionality, now called legacy workflows, no longer appears on document lifecycles that do not have existing legacy workflows. Learn more about Vault workflows.
Vault workflows now fall under the following definitions:
Workflows
Formerly split up into Multi-Document Workflows (MDW) and Object Workflows, workflow creation and configuration is now unified under a single page. Within the page, workflows have two types:
- Document Workflows: Formerly known as Multi-Document Workflows, this is the recommended type for workflows affecting Vault documents, whether there is only one document in the workflow or many.
- Object Workflows: Workflows which affect object records.
On the new Workflows page, Admins can use the search bar, or filter their view by document or object workflow.
Legacy Workflows
Formerly known as single-document workflows or simply document workflows, these workflows are now labelled as legacy workflows. If a document lifecycle does not have any legacy workflows, the Legacy Workflow tab will not appear on that document lifecycle configuration page. In the 21R1.2 release, this also includes any legacy R&U workflows that were shown in the workflow tab.
Restrict Document Workflow to a Single Document
This feature allows workflow administrators to configure a document workflow (formerly multi-document workflow) to only run on one document.
When a document workflow is configured to run on a single document, users can only start the workflow from the document Actions menu using a Start Workflow user action. Users cannot start these workflows from bulk views such as Cart, Library, or Favorites.
Copy Workflow
This feature allows workflow administrators to copy an object or document workflow (formerly multi-document workflow). The copied workflow is created as Inactive, and the workflow administrator must make it Active. The workflow copy has the same type (object or document) and the same lifecycle as the source workflow.
Restrict Workflow Owner from Receiving a Task
This feature allows workflow administrators to restrict workflow owners from receiving an object workflow or document workflow (formerly multi-document workflow) task. When this option is configured on a task, the workflow owner can no longer accept or complete that task, even if the user is part of a broader participant user group that receives the task.
Prompt for Document Fields in Document Workflow Verdicts
Workflow administrators can now configure document fields as part of a document workflow (formerly multi-document workflow) task verdict. Document workflows for specific lifecycles can support all fields available to that lifecycle. Document workflows for any lifecycle can support only Base Document type fields. Fields can be optional or required. Multi-verdict tasks show existing field values for each document verdict.
Prompt for Object Reference Fields in Document Workflows
This feature allows workflow administrators to configure object reference fields inside a document workflow (formerly multi-document workflow) start step, task step, or task verdict. Vault supports both single and multi-value reference fields, and fields can be optional or required.
Support for DAC Lifecycle Role Allowed Group in Document Workflows
Workflow initiators can now select and add users in the Allowed Group of a Dynamic Access Control (DAC) lifecycle role in a specific-lifecycle document workflow (formerly multi-document workflow). This feature must be enabled on the Admin > Settings page and configured for a participant group by a workflow administrator before workflow owners can select users that are part of the allowed user group. Note that Admins cannot disable this feature after enabling it.
In addition, Vault no longer displays errors while trying to add users who are already added to the document lifecycle role using DAC. Instead, Vault simply ignores the selection.
Expression Enhancement for Update Document Field in Lifecycle & Workflow
This feature adds additional functions and operators to allow workflow administrators to build more comprehensive expressions, used for updating document fields inside a lifecycle event action, entry action, or document workflow (formerly multi-document workflow) content action step. New functions include If and Case. New operators include > (Greater Than) and < (Less Than).
Date & DateTime Fields Support in Document Workflow Decisions
Workflow administrators can now configure Date and DateTime fields in a document workflow (formerly multi-document workflow) decision step. Date or DateTime values can be within certain days, weeks, months, or years in the past or in the future.
Task Due Date Audit Updates in Document Audit Trail
This feature adds useful information to the Due Date audit event for object workflows and document workflows (formerly multi-document workflows). If a workflow automatically calculates a due date, it appears in the audit trail as performed by System. If the due date is updated by a user using a workflow action, it is listed in the audit trail as performed by a user. Each update to a user task due date is captured separately in the audit trail.
Read & Understood Workflows
Admins can now configure document workflows (formerly known as multi-document workflows) as Read & Understood workflows. This new method for creating and using Read & Understood workflows is only available to applications and customers with access to legacy Read & Understood workflows, and leverages the modern document workflow functionality. This includes the ability to start Read & Understood workflows for multiple documents, and when a user clicks into a Read & Understood task, they view the documents in the updated document and multi-document viewer.
Read & Understood workflows are a simplified version of workflows that support read and understood tasks. To create one, an Admin selects the Read & Understood while creating a document workflow.
Read & Understood workflows have a limited set of workflow steps. For example, Content Action steps are not available in Read & Understood workflow configuration. Read & Understood workflow tasks are always required and can only have a single verdict. Read & Understood workflows are only available as specific-lifecycle document workflows. Read & Understood workflow tasks can be made available to External and Read-Only users. Learn more about setting up Read & Understood workflows.
Display Next Workflow Start Dialog When Current Workflow Ends
Admins can now configure a workflow End step to automatically display a workflow start dialog when the current workflow ends. This feature, which was previously available for legacy workflows, is now available for object workflows and document workflows (formerly multi-document workflows).
The start dialog is shown to the last user whose action completes the workflow. Vault displays the dialog only if the user has permissions to start a workflow.
Auto-Removal of Documents from Workflows
In previous releases, documents could only be removed manually from document workflows (formerly known as multi-document workflows). With this release, Admins can now configure document workflows to automatically remove documents as part of a Content Action step.
Removed documents can remain in their current lifecycle state or change back to their start state. This action can only be configured with matching conditions. A Decision step is always recommended before the removal step to avoid situations where all documents could be removed. If every document matches the condition for removal, Vault displays an error and does not remove documents.
Document Workflow on Binders
Document workflows (formerly known as multi-document workflows) can now be started on binders. When a user starts a workflow on a binder, or a set of documents which includes a binder, the workflow addresses the binder itself and not the binder’s document contents. Note that Read & Understood workflows are not available on binders.
Unify Lifecycle Stage Group Configuration
Vault Admins can now create, access, edit and delete object and document lifecycle stage groups from one location at Admin > Configuration > Lifecycle Stage Groups.
This new page contains both document and object lifecycle stage groups. Administrators can search or filter for either type of stage group in this view.
Maximum Character Limit for Instructions
Object workflow and document workflow (formerly known as multi-document workflow) Admins can now enter a maximum of 500 characters for instructions in Start or Task steps. In previous releases, the limit was 200.
Rename Start Multi-Document Workflow to Start Document Workflow
As part of the One Workflow initiative, with this release, the Start Multi-Document Workflow action label has been changed to Start Document Workflow.
VQL for Multi-Document Workflows
This feature allows VQL developers to query for data associated with multi-document workflows. To utilize this feature, users must have the new Application: Workflow: Query permission. By default, all new and existing Vault Owners, System Administrators, and Business Administrators will have this permission.
Reporting & Dashboards
Bulk Actions on Objects in Reports
With this release, users can perform bulk actions on object records in report results. Users can also perform bulk actions on documents when a report is grouped.
Dashboard Usability Enhancements
This feature makes several aesthetic and usability enhancements to dashboards. When selecting a report, users now see a list of recent reports. When creating a chart, users now select a report prior to selecting a chart type. Additionally, charts no longer include the gray header background, and Vault displays full chart labels.
Consistent Report URLs Across Vaults
This feature allows Admins to define web actions for accessing reports in a source Vault and migrating them successfully to a target Vault without updating the web action’s URL. URLs for the same report can now be consistent across Vaults. Users can still access reports through existing URLs, and Admins can now replace the report ID with the report’s name in URLs.
Standard Reports
This feature will allow Vault applications to introduce standard reports that cannot be deleted and that users can only allow edit in a limited capacity, if at all. Standard reports will include a Veeva tag, a Veeva logo, and the standard Vault namespace (__v) to allow users to easily distinguish them from other reports.
Note that while this feature introduces the ability for Vault applications to add standard reports, this release does not include any new standard reports in customer Vaults.
Contains Operator in Reports
This feature introduces the contains operator for report filters and prompts. Users can now filter report results based on whether the defined text can be found within another text or picklist field.
Run Report in Background
This feature provides several enhancements for large reports. Users may now run a report in the background and receive a notification when their report results are ready. Reports run in the background can execute for longer than other reports, allowing them to handle larger volumes of data.
Flash Reports Timeout Behavior
With this release, Vault automatically blocks flash reports that timeout three days in a row. Vault notifies flash report owners, who can then unblock the report by making edits to the report definition.
Reports with Multiple Down Objects in Dashboards
This feature allows users to add reports containing multiple objects with outbound references to the primary reporting object to dashboards.
Search
Expanded Search Enhancements
Expanded search now supports in-line editing, bulk actions, and exporting from each of the search result sections. The top of each section now displays the total number of results in that section.
Learn more about expanded search results.
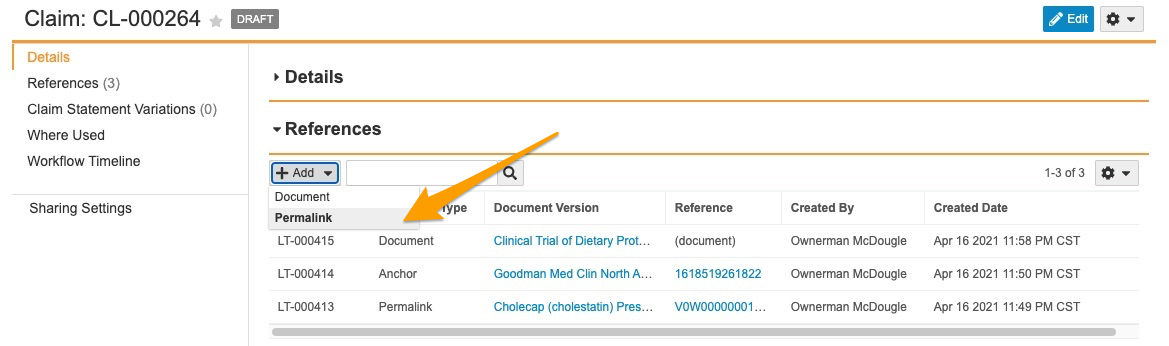
Auto Claims Linking
Suggested Links to Permalink Targets
This feature enables Auto Claims Linking to create Suggested Links and Auto Links to permalink targets in addition to version-specific documents and anchors. When enabled, a user adding references to a Claim record can select Permalink as the target type, and add one or more permalink targets as references on the Claim. Once added, the permalink record ID appears in the Reference column within the Claim record’s References section. This link always navigates to the latest version of the permalink target document.
This feature is available when an Admin has enabled all of the following checkboxes in Admin > Settings:
- Enable Copy Link and unlock Permalink Object Type
- Enable linking to permalinks (This checkbox is only available after enabling Copy Link)
- Enable Suggested Links
Usability & UI Updates
Rename Start MultiDocument Workflow to Start Document Workflow
This feature renames the “Start Multidocument Workflow” action to “Start Document Workflow”.
Notifications: Support for Vault Mobile Push Notifications
This feature enables Vault Mobile to register with Vault for the user to receive mobile push notifications. Vault pushes notifications to Vault Mobile to notify users of new or pending workflow tasks. Users and Admins control whether push notifications are sent from a given Vault through a setting on the user profile. Learn more about mobile app registrations.
This feature supports a future release of Vault Mobile.
Checklists: Welcome Notification Templates
Checklist provisioning now supports welcome notification templates for each Checklist Type. The template includes tokens that reference fields on Checklist Design records, giving non-Admin designers a way to provide Checklist-specific text for welcome notifications sent to respondents.
Checklists: Duplicate Checklist Prevention
With this feature, a user can only create one checklist at a time for a given object record. This prevents users from accidentally creating a duplicate checklist while the first one is still being generated.
Localize Vault to Swedish
The Vault UI now supports Swedish. Users can update the language Vault displays for them by changing the language settings on the User Profile page. Vault also supports setting Swedish translations for labels in user-configurable data such as document types, fields, picklist values, and lifecycle names. Learn more about supported languages and localization settings.
Action UI
Action Bar
The Action Bar continuously learns how each user works and shows their most-used actions right on top and easily accessible with large, recognizable icon buttons. The Action Bar also helps make users more aware of workflow actions they can perform, such as starting available workflows or changing lifecycle states, by dynamically showing a workflow actions button. The Action Bar is available for object records and documents to help users perform important actions easier and faster.
Doc Info UI Enhancements
With this release, the Doc Info pane is now divided into five panels: Information, Relationships, Files, Sharing Settings, and Timeline View. Instead of Mixed View, Content View, and Field View, users can now expand and collapse the entire Doc Info pane. Additionally, users can click and drag to resize the Doc Info pane. Vault maintains this panel width across all documents. Note that, when in Annotation mode, the Doc Info pane is set to 30% width and is not resizable.
Annotate UX Refresh
This enhancement adds a significant number of visual and usability improvements to annotations and document viewing in Vault. These enhancements also apply to video annotations. Updated comment annotations use new, more distinct colors. The number of available colors is reduced from 12 to 6 to ensure more obvious differentiation between each color. All existing annotations will also display the new colors. Each old color is mapped to one of the new colors by the system, and the new color is applied to the annotation when the page is loaded.
Notifications: Enhanced Notification UI
In previous releases, users accessed their notifications on the Notifications page through their Vault home tab. With this release, users access notifications via a new global notification icon, making it easier for Vault users to become aware of documents, records, and tasks that need their attention.
This feature introduces the Notification Bell icon, visible at all times within Vault, which opens a new notification panel and allows access to the new, enhanced notifications page.
This feature also removes the notification icon within Vault’s document viewer, as the new Notification Bell is available everywhere within Vault. Learn more about notifications.
UI Styling Updates
In this release, Vault has a number of visual updates to give it a more pleasing look and feel and a more enjoyable user experience. By applying modern design principles with better spacing, color, highlighting, and icons, many of the visual elements of Vault are improved, helping users stay focused in the areas where they are most engaged. These visual updates do not change any functionality and do not change the locations of common visual elements that existing users are familiar with.
Action Menu Button Icon Change
This feature updates all action menu buttons from a cog icon to ellipsis icon. The ellipsis icon has become a standard symbol for a menu of actions in modern web applications.
Display Start Workflow Actions in Most Frequently Used Actions
Vault administrators can configure Start Workflow user actions to be displayed as Most Frequently Used Actions in the Action Bar in both Document and Object Lifecycles.
By default, all start workflow actions are shown inside the “Workflow Actions” menu, but start workflow actions that are configured to be shown in the Action Bar are shown in the ‘All Actions’ menu.
Enable Action UI Setting
The “Enable Action UI” setting is available in Admin > Settings > User Interface Options. It is also available in advance in the 21R1 release on June 16, 2021 with the setting name: “Enable Action UI for 21R2 Release”. This single setting enables a number of UI enhancements that make using Vault faster and easier. It is enabled by default, but Admins may disable it in advance or any time during 21R2.
Action UI includes the following UI enhancements:
- Action Bar
- Doc Info UI Enhancements
- Annotate UX Refresh
- Notifications: Enhanced Notification UI
- UI Styling Updates
- Action Menu Button Icon Change
- Display Start Workflow Actions in Most Frequently Used Actions
Vault File Manager
Permission Label Update: Check Out to File Manager
In this release, we have relabeled the Check Out to File Manager permission to Vault File Manager. This permission allows users to access all Vault File Manager functionality, including uploading renditions and importing submission dossiers to RIM Submissions Archive.
Install Vault File Manager Without Affecting Checked-Out Files
With this release, manually updating Vault File Manager automatically does not affect your checked out files. All files managed by VFM are now stored in a new Vault File Manager Files folder.
Auto-Logout of Vault File Manager After Inactivity
This feature addresses security concerns and ensures users are logged out of Vault File Manager. Vault File Manager automatically logs users out after eight (8) hours of inactivity.
Vault File Manager Automatically Opens Files
Prior to this release, there was a Vault-wide feature flag to control whether files would automatically open when checked out to Vault File Manager, along with a VFM File Security Policy object that allowed you to define the specific file types that would auto-open. In this release the Vault-wide feature flag has been removed, and you must control auto-open using the object only (if there are no records in this object, no file will auto-open upon checkout to VFM).
Administration
Scheduled Data Exports
With this release, admins can receive an automated daily CSV file export of their Vault object record data and document metadata directly to their Vault File Staging FTP folder or their Amazon S3 Bucket.
Feature Flag Confirmation Dialog in Vault
This feature adds a confirmation message that displays when a Vault Admin enables a feature that cannot be disabled once enabled on the Settings page.
Admins Can Edit All Picklist Labels & Picklist Value Labels
In previous releases, Admins could not edit the labels on all standard (__v) picklists and picklist values, even though this was possible via MDL and the bulk translation tools in Vault. We have removed this constraint.
Limited Release Sandbox Feature Flag
In previous releases, Vault users were required to contact Veeva Support to access the Limited Release Sandbox feature. Starting in 21R1.3, Vault Admins can enable this feature in Admin > Settings > General Settings > Sandbox.
Display the Unclassified Document Type in Admin
With this release, the Unclassified document type, which is labeled as Undefined in existing Vaults, now appears on the Business Admin pages. Admins can assign fields and document type groups to the Unclassified document types and can create document permissions on the document type. Note that Vault does not enforce required fields when a document remains in the Unclassified document type or the Inbox lifecycle.
Additionally, if an Admin assigns a document field to both the Unclassified document type and the Base Document type, it will no longer be directly assigned to Unclassified. All Base Document type fields will appear on the Unclassified document type, aligning it with normal document types.
Admins Can Edit All Document Field Labels
Prior to this release, Admins could not edit the Labels on all standard document (__v) fields in the UI, even though this was possible via MDL and through bulk translation tools. This feature allows Admins to edit Labels on all document fields.
Smart Filter Enablement in Search Settings
When enabled, smart filtering automatically converts navigational search terms, such as Document Type or Status, into filters. Before this release, smart filtering was only available through Veeva Support. In this release, Admins can now enable or disable this feature on the Admin > Settings > Search Settings page.
Promote to Production
This feature introduces a one-time Vault action which allows a Vault owner to promote their initial pre-production Vault to a production Vault. This enhances the self-service model of sandboxing by allowing Vault Admins to fully manage a Vault’s lifecycle from initial to go-live.
Additionally, this feature allows your organization to create their pre-release sandbox from any production, pre-production, or sandbox Vault. Previously, an organization’s pre-release sandbox could only be created from the production Vault.
Infrastructure Release: IP Address Change
Vault IP addresses will change as part of this release.
We recommend all Veeva customers update their DNS caching so that integrations don’t cache the DNS indefinitely. We also recommend that you configure the DNS TTL value to no more than 60 seconds. This ensures that when a Vault resource IP address changes, your integration will be able to receive and use the new IP address by re-querying the DNS.
Learn more about integration DNS caching changes in the Developer Portal.
Security
Automate Guest Invites to Azure AD from Vault for Collaborative Authoring
With this release, Admins can enable automatic invites to external users, allowing users without access to the Admin’s Vault domain to edit and collaborate on Office 365™ files. When enabled, Vault automatically adds the external users as Guests using Azure AD when those users try to start or join a Collaborative Authoring session from Vault.
Enforce Use of HTTPS When Configuring SAML & OAuth 2.0 Profiles
When configuring SAML profiles and OAuth 2.0 profiles, Admins must now use the HTTPS protocol rather than the HTTP protocol. This applies when importing SAML IdP metadata or when uploading OAuth 2.0 Authentication Server metadata. Note that this change only applies when creating new profiles or updating existing profiles.
48 Hour Maximum Session Duration on All Vaults
With this feature, the maximum duration of a Vault session is always 48 hours, even if the session stays active. This feature was enabled in the 21R1 release except on production domains which have at least one Align Vault. This feature will be available on all Vault domains with the 21R1-A release.
Release Account Lockout After 5 Minutes
In previous releases, a user account was locked out after 5 unsuccessful login attempts. Starting in this release, Vault automatically releases the lock out 5 minutes after the last unsuccessful attempt. This is applicable only to security policies with Authentication Type set to Password, and is not applicable to Single Sign-on.
This feature will be available with the 21R2-A release.This feature has been removed from the 21R2 release.
Group Membership Update for Inactive Users
This behavior update addresses a regression issue in the 21R1 release. With this release, group memberships from included security profiles are once again automatically removed when a User becomes inactive. These memberships can be for groups that are either user-managed or system managed.
Domain Admin Support for Cross-Domain Users
In previous releases, cross-domain users could not be Domain Admins. This feature adds support for cross-domain users as Domain Admins, and will be released in 21R2.0a.
Vault Java SDK
JSON Binding Annotations: User-Defined Model & Property
This feature introduces a new Vault Java SDK code type, User-Defined Model, which appears in Admin > Configuration > User-Defined Models.
User-defined models allow developers to serialize a model to JSON and deserialize JSON to a model. Learn more in the Vault Developer Portal release notes.
Audit Enhancements: On Behalf of User
Starting in 21R1.3, System and Application events that make data changes may be logged as System on behalf of {username}, which provides greater visibility into which user’s actions caused data to be created, updated, or deleted. These entries were previously logged simply as System. Existing audit entries will not be altered and only new audit entries will reflect this additional information. Note that not all System actions are included in these changes.
Platform Data Model Changes
See 21R2 Data Model Changes: Platform.
Clinical Operations
TMF Bot: Auto-Classification
This feature delivers the exciting capability for the system to automatically classify documents that have been uploaded to the Document Inbox. This will be done if a Document Classification Trained Model has been trained and deployed within the Vault. Learn more about Using TMF Bot Auto-Classification.
TMF Bot: Model Training
This feature allows administrators to create, train, deploy and archive machine learning models in Clinical Operations Vaults. Initially these trained models will be in support of the TMF Bot: Auto-classification feature, but will be extended to other use-cases in the future. Learn more about Enabling the TMF Bot.
Send Surveys to Non-Users
This feature introduces the option to send, via email, an invitation to complete a survey (Checklist) related to a Site or Outreach Target record. By clicking the link in that email, external respondents can enter Vault and complete the Checklist without having to enter a username and password.
Archive Study From Any State
This feature introduces a new Initiate Study Archival action that Admins can configure on any Study lifecycle state. Admins can enable this feature by inactivating the existing Initiate Study Archival action and then enabling the new Enable Study Archive from Any State setting on the Applications Settings page. Learn more about transitioning to the new Initiate Study Archival action.
Conditional Actions on Trip Report Questions
This feature improves the entry of follow up items and Issues by allowing CRAs to easily see which Trip Report Question Responses require follow up items and Issues. For multiple-choice questions, Admins can specify which answers require users to create or link a related follow up item or Issue record. Learn more about configuring conditional actions on Trip Reports.
Milestone-Specific Expected Documents
This feature updates EDL template configuration to allow Admins to indicate that expected documents are created only for specific types of milestones. Once created from a template, these documents are linked and matched to an Owning Milestone of that specific milestone type while still being linked to additional milestones based on existing behavior. This allows customers to track documents that are truly specific to individual milestones, such as protocol amendments and submissions. Learn more about using EDLs with milestones.
Clinical Budget Tracking
This feature adds support for tracking study-, country-, and site-level planned Budgets and Budget Categories, as well as the ability to track expenses against those Budgets and corresponding Budget Categories. Customers can now track Budget Category values on Fee Templates, Fees, and any Payable Items related to Fees with Budget Categories. Learn more about Study Budgets.
Content Field for Inbox Documents
This feature will give customers the peace of mind that documents within the Document Inbox can be secured based on a document’s Content (blinded/unblinded) value. This feature will work with our Content Field Defaulting feature and will respect field requiredness settings.
Support for Subject Informed Consent Forms
This feature adds support for tracking Subject Informed Consent (IFC) Forms. With the new Subject Informed Consent Form object, users can select a Site Effective Informed Consent Form and a Subject, and then specify the date the Subject signed that ICF in the Signature Date field. A Subject Informed Consent Form field has also been added to the Monitored Informed Consent Form object.
Support for EU Regulatory Agency Issue Notification Tracking
This feature adds support for tracking EU Regulatory Notifications on the Issue (pdv__ctms) object. Within an Issue object record, users can specify a Notification Type (Serious Breach, Unexpected Event, or Urgent Safety Measure) and track when the notification should be reported to the EU/EEA Member States.
Veeva eConsent Authoring in Clinical Operations
This feature allows Veeva eConsent customers to create and maintain Veeva eConsent forms in their Clinical Operations Vault. Vault opens Veeva eConsent forms in the Veeva eConsent editor, where users can edit, save, and check them back into their Vault. Learn more about eConsent Authoring.
Veeva eConsent Transfer with Site Connect
With this feature, Veeva Site Connect customers can exchange Veeva eConsent forms with SiteVault.
Document Reconciliation
This feature provides Veeva Site Connect customers with automated and streamlined document reconciliation between a Sponsor/CRO’s eTMF Vault and a site’s eISF in SiteVault.
Site Connect: Additional Vault Clinical Docs Support
With this release comes support for transferring additional document types via Veeva Site Connect. Document types mapped to the following Vault Clinical Docs artifacts can now be exchanged between Clinical Operations Vaults and SiteVault Vaults:
- Protocol Synopsis
- Protocol Clarification
- Protocol Summary of Changes
- CRF Completion Requirements
- Contractual Agreement
- Regulatory Approval Notification
- Other Approvals
- Indemnity
- Insurance
- Clinical Study Report
CDMS & Clinical Operations Vault Connection: Protocol Deviations
Protocol deviations can now be created in CDMS Vaults and transferred to Clinical Operations Vaults via a new integration available for the CDMS & Clinical Operations Vault Connection. This feature creates Protocol Deviation types of Issues in Clinical Operations Vaults with the corresponding information from the CDMS Vault.
Clinical Operations & Regulatory Vault Connection: Streamlined Reuse Support
This feature selectively ignores streamlined document reuse functionality for CrossLink documents created via the Clinical Operations & RIM Vault Connection. This allows customers to leverage document reuse functionality for any document type, subtype, or classification, even documents within the Clinical Operations & RIM Vault.
Attachments for TMF Transfer
This feature allows customers on eTMF Vaults to include document attachments when transferring documents via TMF Transfer. Any attachments on the documents in the source Vault will now transfer to the target Vault and remain as attachments on the corresponding transferred documents.
Add Yuzu Japanese CTN to the Application Settings Page
This feature allows Admins to enable and disable the Yuzu Enable Japanese CTN Feature from the Application Settings page. It also allows Admins to enable the Create CTN Data for Japanese Study Country feature, which generates CTN Data records for Study Site, Study Person, and Study Organization.
USN Picker Search Improvements
With this feature, Clinical Operations users can now search across multiple criteria in the USN Picker. Searched terms now match across the following columns: Site Name, USN, Country, State/Province, and Address.
New State Types for Soft Deletion
This feature adds the standard Delete Requested, Soft Delete One Version, and Soft Delete All Versions state types to all lifecycles to support workflow configurations for deletion.
Quality Issue Assignee Filtering By Object Type
Previously, the presence of custom VQL on the Assigned To field for any Quality Issue object type would prevent the default filtering from applying to the field on other Quality Issue object types. This updated behavior respects custom VQL on an object-type basis.
Relabel Question Comment Required Object to Answer with Conditional Requirements
The Question Comment Required (question_comment_required__v) object is relabeled to Answer with Conditional Requirements to support the Conditional Actions on Trip Reports feature.
Clinical Operations Data Model Changes
See 21R1 Data Model Changes: Clinical Operations.
Commercial
Material ID
This feature adds a Material ID field that generates and adds a unique ID number to the document for submission to a health authority. This number can be automatically updated when the document moves from Steady State to Draft so that there is a unique ID number if a second submission is required. This allows customers to up-version documents past v1.0, creating a new unique ID number that can be used for a following submission of a specific document.
Modular Content: Rich Text Fields
In 21R1, we added new objects to support the creation and storage of Content Modules, one of the elements of which supports storing text elements. With this release, the text fields use the new Rich Text field type, allowing basic formatting on the text. Existing unformatted text is unaffected and both the plain and rich text are accessible via the API.
eCTD Compliance Package 2253 Form Update
With this release, users can configure Vault to automatically complete line 13 on Form 2253 when generating an eCTD Compliance Package. Once configured, Vault automatically selects “Final” on line 13 of Form 2253 in Post Marketing Compliance Packages when the Center field is set to CBER - APLB.
Create Fragment Includes Additional CRM Product Fields
With this release, the Create Fragment feature copies any standard CRM Product fields (CRM Org, CRM Product, CRM Product Detail Group) used on a source document to the generated email fragment, as part of the Veeva Approved Email functionality. This reduces the administration required when preparing fragments for use in CRM.
Data Model Change: Multi-Select CRM Product Fields
This data model change lays the foundation for a CRM feature with a planned release in Q3, which will support multiple CRM Products on CLM Presentations and Slides in Vault. With this release, Admins can update the following three (3) fields in PromoMats and MedComms to be multi select or single select:
- CRM Product (
crm_product__v) - CRM Product Group (
crm_product_group__v) - CRM Detail Group (
crm_detail_group__v)
These fields are single select by default. They should not be updated to multi-select until CRM releases their feature.
PromoMats & RIM Vault Connection
This feature creates a connection between PromoMats and RIM Submissions Vaults for eCTD Compliance Package submissions to the FDA. The connection enables a seamless flow from Compliance Package generation in PromoMats and planning and tracking in RIM Submissions to the FDA through the gateway via RIM Submissions Publishing. This reduces the time required to get submissions ready for submission to the FDA. Note that the eCTD Compliance Package feature in PromoMats must be configured to use the connector.
Modular Content: Approval Document
This feature leverages objects previously introduced to generate a document based on data in Content Modules. The generated approval document can be used to support the approval process for modules, providing standard document review features.
Support Long Text Request Details field for Medical Inquiry
With this feature, users can map fields in the CRM Data Sharing functionality for Medical Inquiry to the long text type. Long text fields are required to share fields that are longer than a standard text field, which is limited to 1,500 characters.
CRM Approved Links Data Model Change
This data model lays the foundation for a future CRM Approved Links feature. See the list of Commercial Data Model Changes below.
eCTD Compliance Package Generation Documentation
This feature updates validation documentation to fully reflect the impact of the eCTD Compliance Package Generation feature, released in 20R3.
Commercial Data Model Changes
See 21R1 Data Model Changes: Commercial.
Mobile
Recent Documents Enhancements
Manufacturing operators often need to repeatedly switch between multiple procedures and instructions when performing a task on the shop floor. With this Station Manager feature, operators can easily access the most recently viewed documents from within the document/video viewer and flip back and forth between documents without losing their place, increasing efficiency while performing the task at hand. In addition, the Recents tab is now displayed as the second tab after the All tab and is now sortable by the same aspects as the All tab. This feature is available on both platforms:
Recent Documents Enhancements on Android
Recent Documents Enhancements on iOS
Capture Document Usage on Android
With this feature, Station Manager records document views as manufacturing operators are viewing documents on the device. When Station Manager syncs with Vault, document views are written to the Station Manager Activity and Document Usage objects, providing document owners and administrators with insight into the usage of SOPs and instructions in manufacturing and lab environments. This feature is available on both platforms:
Capture Document Usage on Android
Capture Document Usage on iOS
Quality
Station Manager: Capture Document Usage
With this release, Vault is now capable of creating activity records to capture the viewing of documents in the Station Manager mobile application. These activity records provide document owners and administrators with insight into the usage of documents, such as SOPs and instructions, in manufacturing and lab environments.
This Vault feature supports an enhancement to the Station Manager application which enables activity tracking and sync activity records to Vault. Learn more about setting up Station Manager.
Health Canada Adverse Event Reporting
Customers are now able to leverage Vault Product Surveillance to triage a complaint that is determined to be a reportable adverse event to Health Canada. This feature also adds the ability to generate formatted PDF output of the Health Canada Mandatory Medical Device Problem Reporting Form for Industry.
Customers can use this feature to submit the PDF file manually to Health Canada. This is an extension of Adverse Event Reporting to the US FDA. Learn more about using adverse event reporting.
Enhanced Periodic Review for Documents
This Vault QualityDocs feature enhances the existing periodic review process by adding auto-start functionality. A new job type, Document Periodic Review, allows administrators to configure a job that identifies documents due for periodic review. When the job runs, Vault creates a Periodic Review object record for each document, and an Admin can configure a document workflow to automatically start a periodic review document workflow. This feature does not support legacy document workflows.
Once a user provides a periodic review verdict, an Admin can configure follow-up tasks to ensure that the proper steps have been taken based on the periodic review verdict. In addition, a new Associated Periodic Review section is available on Document Change Control (DCC), allowing users to add associated periodic reviews to a DCC for documents that are part of a DCC. This additional section provides full visibility of the periodic reviews that may have originated the need for a DCC. Learn more about setting up enhanced periodic reviews.
Process Navigator: Navigation Drawer & Favorites
This Vault QualityDocs feature enhances the Process Navigator with the following additions:
- The Navigation Drawer allows an end user to navigate process hierarchies using a left-hand navigation drawer. Instead of clicking through into each process hierarchy individually, the navigation drawer allows users to expand available hierarchies and jump directly to a specific process without having to traverse the entire tree. Users can navigate to different hierarchies of the same type using the navigation drawer without returning to the Process Navigator landing page.
- The Favorites capability allows users to favorite hierarchy records and documents by clicking on the Favorites star within the Process Navigator user interface. This allows users quick access to favorited hierarchy records and documents. Favorited hierarchy records and documents are displayed and searchable by clicking on the Favorites tab when the navigation drawer is expanded. Clicking on a favorited hierarchy record will take the user to the detail page of the record, while clicking on a favorited document will open the document in a pop-out viewer.
Learn more about using the Process Navigator.
On the Job Training Type
This Vault Training feature allows customers to electronically track qualification, certification and other skill or task-based assignments associated with On the Job Training (OJT) where an evaluator must verify the learner’s ability in a given area.
A checklist captures the hands-on learning actions and performance outcomes for a defined job position and task. As part of the evaluation process, the checklist captures, in real time, the step-by-step process followed, comments, and a pass/fail outcome. The checklist is then stored as part of a Learner’s Training Assignment.
Assign Effective Version for Training
With this Vault Training feature, if a Training Requirement references an Issued document, Vault Training can also assign an additional Training Assignment to the Learner containing the Effective version of the document. This allows the Learner to be trained on the Effective version of the document, in addition to the Issued version. Learn more about configuring Vault Document Training Requirements.
Allow Learner Choice For Substitute Training
This Vault Training feature allows a Learner to choose an appropriate substitute training from a list of available training requirements. A Training Admin can configure Substitute Training Rules with an option to allow the Learner to make a substitute selection. Once configured, the Learner has the option of selecting a substitute training or continuing with the primary training assignment. Learn more about configuring Learner choice for substitute training.
Generate Quality Document from Object Record Action
This Vault QMS feature allows administrators to configure User Actions or Entry Actions which create a document based on a Quality Event or Audit record. Documents created in this way are created from Formatted Output templates, allowing complex configurations and data from multiple related records to be brought into the generated document. Documents created by this function inherit Vault Document metadata from the starting record, are saved into the Document Library, and are linked to the record from which they were generated. This powerful feature enables automation for the generation of key artifacts such as Audit introduction letters, Audit summaries or Inspection copies for completed or closed Quality Events. Once created in the Library, these documents can be configured to support lifecycles or workflows for reviews or approvals, allowing robust processes to be linked together between your process records and generated artifacts.
Since quality document artifacts can include large amounts of data from many records, this operation can take some time to complete. We encourage careful consideration of where in your processes’ lifecycles to invoke or allow these actions.
This first release supports document creation via Formatted Output templates for Quality Events and Audit objects, and we’ll be investigating additional use cases in future releases. Learn more about setting up Quality document generation.
Relationship Automation for Quality Objects: Quality Events, Audits, CAPAs
With this Vault QMS feature, Vault Admins have a new tool to configure certain complex relationship objects to be automatically bi-directional once established. This feature is targeted at relationship objects which link two records of the same object together, like the Related Quality Event object in QMS. Additionally for these types of relationship objects, this feature allows such relationships to describe the nature in which the two records are linked. This allows for simple relationships, in which no nature is captured, or described relationships which capture the linking nature. This streamlines the way in which users identify related records, reduces the amount of effort required to link records of the same object together, and means that there is one page section to look at when reviewing all links between such records.
Sometimes links between records of the same type can contain information about how the two records are related. If the new Relationship Natures picklist field is used on Related Quality Event or Related Audit records, Vault allows Admins the ability to map “opposites” to describe the directionality of a relationship. For example, if a user wishes to link a deviation record DEV-0023 to DEV-9942 because investigation of DEV-0023 discovered DEV-9942, Admins could add values to the Relationship Natures picklist such as Testing Discovered and Discovered While Testing, configuring Vault to recognize these as opposite values. Then, when a user creates a new Related Quality Event record to link DEV-0023 to DEV-9942, and selects Testing Discovered as the nature of that link, Vault automatically creates an additional Related Quality Event record with a relationship nature indicating that DEV-9942 was Discovered While Testing DEV-0023. Any user who views either DEV-0023 or DEV-9942 would be able to understand that the two deviations are linked and how they are linked by looking at the same related record section on each. In previous releases, approximating this behavior required two page layout sections on Deviations, one showing inbound relationships, and one showing outbound relationships, as well as manually creating two Related Quality Event records to indicate both directions of the link.
This feature requires configuration to be used. This feature also introduces a new standard object called Related CAPA (related_capa__v). With this first release, the Related Quality Event, Related Audit standard objects, as well as the new standard object Related CAPA are supported. Learn more about setting up Quality Relationship Automation configurations.
Batch Release Process Support
In this release, Vault QMS customers will notice new objects and related components available in their Vaults to support a GMP Batch Release / Disposition process. Vault provides objects which can be configured to allow batches to have associated disposition assessments, acting as a workspace to identify, collect and track content and tasks needed to arrive at a GMP disposition for a given lot or batch of product.
This initial release introduces the object model which can be configured and extended to support these business processes. We’ll be looking to further expand upon this area in future releases.
Reportability Assessment Management
This Vault Product Surveillance feature allows Admins to set up questions to determine the Severity of a complaint and thus determine the Reportability of the Adverse Event to health authorities. The Severity and Reportability are determined based on the answers provided by Complaint handling users. This feature also automates various scenarios of Reassessment by automatically creating, updating, or canceling Reportable Event records when the Severity of the incident changes.
Adverse Event Reporting Enhancements
This release brings the following enhancements to Adverse Event Reporting in Vault Product Surveillance:
- Follow-up submissions: Users can generate follow-up reports if new information comes to light after an initial report has already been submitted to the health authority.
- On-demand validation: Users can perform on-demand validation for a Reportable Event against the validation format of the given health authority.
- Notification for truncated fields:With this feature, users are notified if there is any truncation of information when it is compiled from reference objects while generating the Reportable Event XML/PDF.
Enablement Change: Extensible Controlled Copy
With this release, Vault QualityDocs Admins can enable the Extensible Controlled Copy feature through an Admin checkbox. Enabling this feature immediately disables legacy controlled copy functions and new legacy controlled copies can no longer be generated. The Distribution report is still enabled and allows existing legacy controlled copies to be reconciled during transition to Extensible Controlled Copy. Enabling this feature allows Admins to configure the Extensible Controlled Copy feature, which requires additional configuration before being utilized by an end-user. Learn more about configuring Extensible Controlled Copy.
Quality Teams: Restore Memberships UI Update
In accordance with the updated Vault interface, the restore team role membership gesture has been updated with an icon to replace the previous Restore text link.
Rename Reportable Event to Adverse Event Report
In Vault Product Surveillance Vaults, the Reportable Event (reportable_event__v) object has been renamed to Adverse Event Report (adverse_event_report__v) to better reflect the object’s context.
Quality Data Model Changes
See 21R1 Data Model Changes: Quality.
Regulatory
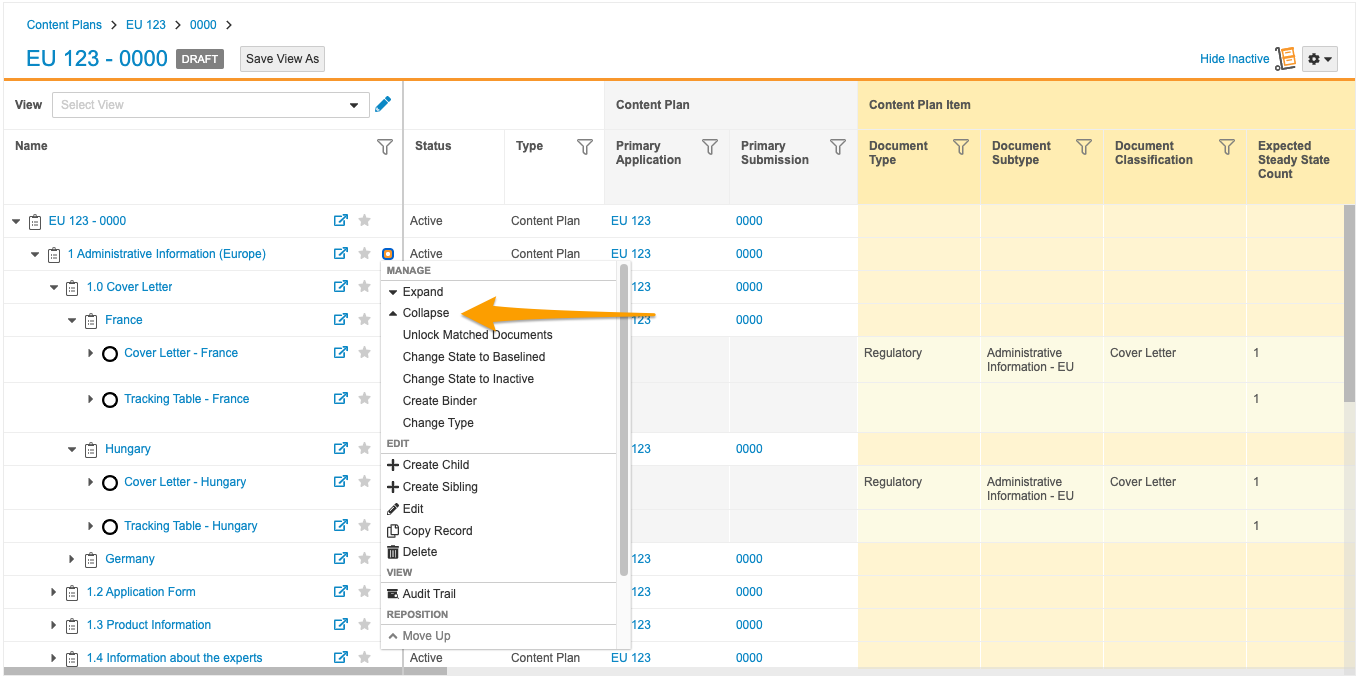
Collapse Action in Content Plan Hierarchy Viewer
With this release, a new Collapse action is available for Content Plan records in the Content Plan Hierarchy Viewer. This action allows users to collapse all descendant Content Plan and Content Plan Item records within a content plan section at once, including matched document rows.
Correspondence in Viewer to Consider Dossier Status
With this release, Vault now takes into account values in the Dossier Status field when displaying correspondence documents in the Submissions Archive Viewer. Submissions Publishing customers with the Correspondence in Viewer feature enabled now see submission-level correspondences in the Viewer for submissions that are not actively being published.
Submissions Archive: Taiwan 1.0
With this release, RIM Submissions Archive Vaults support importing and viewing eCTD submissions based on the Taiwan v1.0 DTD.
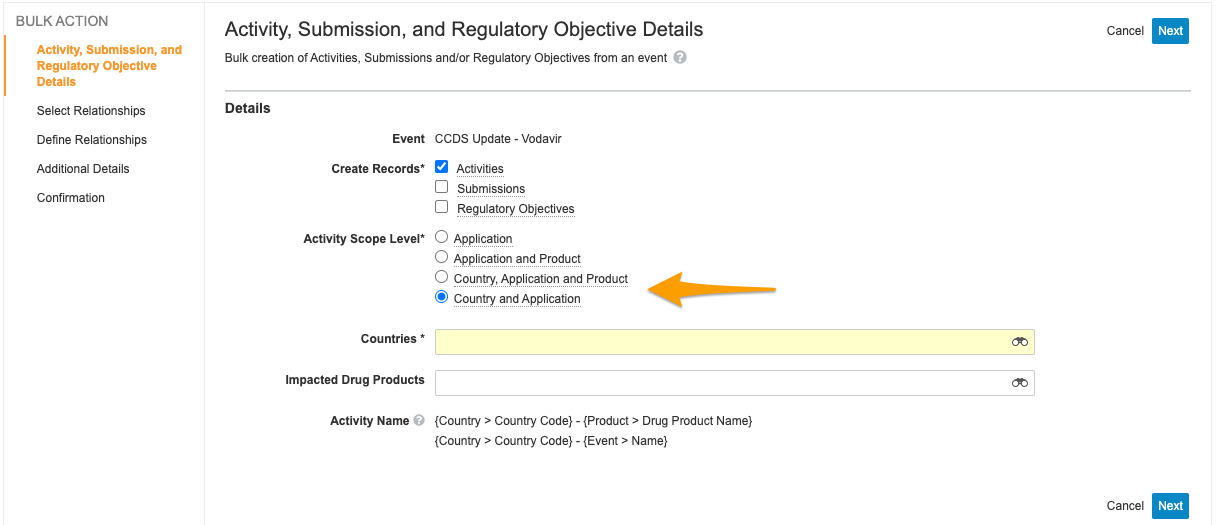
Create Related Records: Application Country Scope
This feature adds an additional level of granularity when users create activities from an Event record using the Create Related Records wizard. The new Activity Scope Level, Country and Application, allows users to create an Activity record for each Country record related to the impacted Application record. This feature also renames the existing Country scope to Country, Application and Product to align with its existing behavior.
Create Related Records: Restrict Submission & Regulatory Objective Joins
When users run the Create Related Records wizard from an Impact Assessment Report, Vault now applies validation logic before creating Submission and Regulatory Objective join records. Validation logic, which is specific to each join record type, prevents the creation of invalid join record relationships based on relevant existing relationships.
IDMP: Registered Packaging to Medicinal Product Registration Mapping
This feature introduces a new action that maps Registered Packaging records to the related Medicinal Product Registration record. Admins can set this up as a user action or an entry action on Registered Packaging object lifecycle states. This feature supports IDMP and extends the IDMP Accelerators feature introduced in the 21R1 release. Learn more about configuring Registered Packaging mapping.
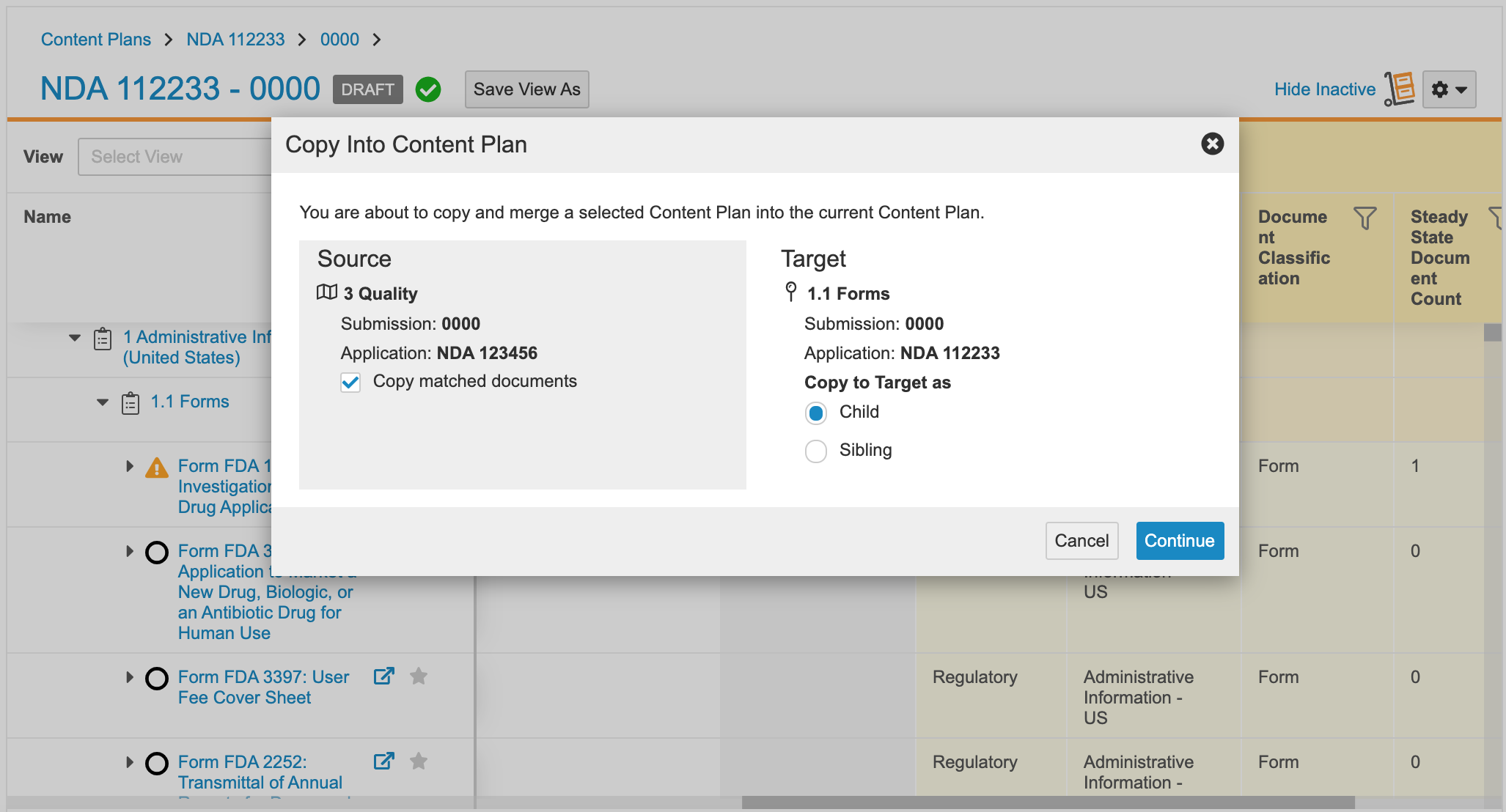
Copy Into Content Plans
This feature allows users to copy a content plan section or content plan item from one content plan to another. When enabled, users working in the Content Plan Hierarchy Viewer can drag and drop a Content Plan or Content Plan Item record to another content plan when that content plan is open in the viewer in a separate browser window.
When users drag and drop a Content Plan Item record, Vault copies only that record to the target location and performs the copy automatically. When users drag and drop a Content Plan record, Vault copies that section, including its descendant Content Plan and Content Plan Item records and any missing submission join records, to the target location asynchronously. When the asynchronous copy is complete, Vault sends the user a notification with results. Learn more about copying into content plans.
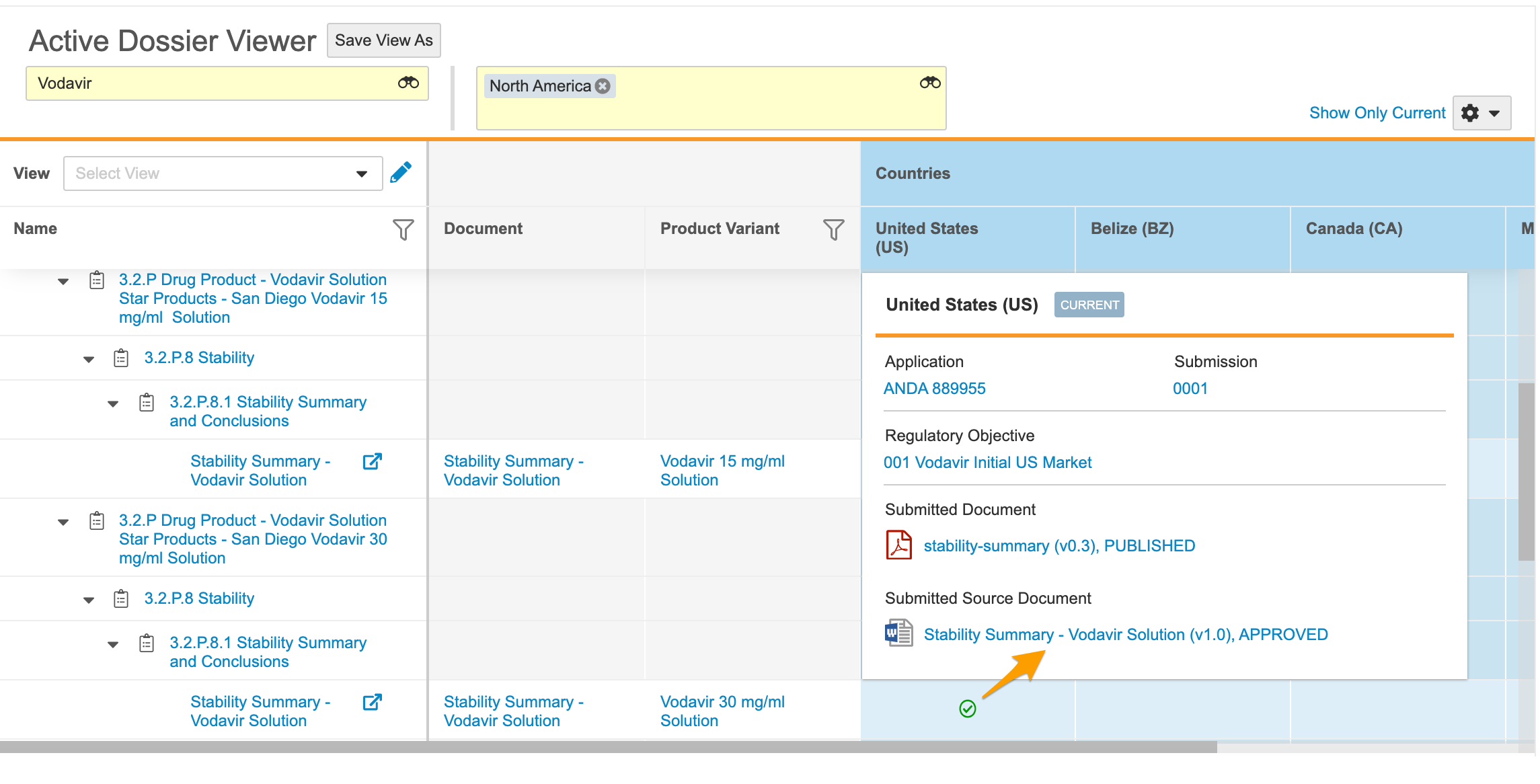
Active Dossier
RIM customers with RIM Submissions and Submissions Archive can configure Vault to track and manage documents that are active for a product in a given market. Leveraging the Submission and Regulatory Objective object lifecycles, Vault can populate the Active Dossier and subsequently calculate which documents are currently active based on the eCTD lifecycle or document versions. From the Submission or Regulatory Objective records, users can view or edit the Active Dossier with contextual filters applied based on the record’s relationships. Users can also view the Active Dossier directly with Product Family and Region filtering applied. Learn more about viewing and editing the Active Dossier.
RIM Reference Model
This release introduces a standard hierarchy reference model for RIM document types. Two new objects, RIM Reference Model and RIM Reference Model Mapping, are available with Vault-provisioned records. The RIM Reference Model Mapping object is used for the creation of CrossLink documents in RIM by the inbound Vault Clinical Operations connection and for the mapping of document types to the Active Dossier. Learn more about setting up the RIM reference model.
Auto-Create Commitment Application Joins
This release introduces a new setting on the Application Settings page that enables Vault to create Commitment Application join object records automatically when users create or update the Related Application field on Commitment records and click Save. Learn more about automatic record creation in RIM Vaults.
Sequential Pagination of TOC
For Report Level Content Plans, users can now generate a Table of Contents (TOC) using a new user action. Vault generates the TOC utilizing Publishing Elements records configured by an Admin. Additionally, users can now create a TOC with overall page numbering when merging and publishing the Report Level Content Plan. Learn more about configuring and using TOC publishing for report level content plans.
Extend Content Plan Duplicate Submission Join Detection
This feature enhances the submission join duplicate detection that Vault uses in the C opy Content Plan action. Vault uses this duplicate detection to determine whether to use an existing submission join record on the target submission rather than copying the join record from the source submission. The duplicate detection now considers the XML fields on the submission join records.
Dynamically Adjust Content Plan Row Height to Fit Text
In the Content Plan Hierarchy, the row height for Content Plan rows now adjust dynamically to fit text so that cell text is never cut off vertically when wrapped. As a result, users can no longer manually adjust row height, since Vault will resize the row height automatically.
Submissions Archive Documents Created as System
With this release, users no longer need Create permission for binders and documents on the Submissions Archive document type. The document audit trail reflects the creation by the System user, and Vault assigns the document Owner role to the user who initiated the import.
Propagate Fields from Source to Archived Documents
With this release, Vault can populate any fields configured within a RIM Submissions Archive Vault when a submission is published as long as the source document contains fields with the same namespace.
Archived Document Security in RIM Submissions
With this release, Vault does not include archived documents in published outputs as part of the Submissions Publishing or Report Level Content Plan Publishing processes.
US FDA eCTD Validation Criteria v4.1 Support
With this release, RIM Submissions Publishing Vaults now supports the US FDA eCTD Validation Criteria v4.1.
Registration Verification Improvements
This feature provides new configuration options to enhance the registration verification workflow process. Admins can choose how to allocate workflow tasks and in which states the verification workflow should run. Additionally, Vault can now support verification when users edit Registration records or registered details directly. Previously, Vault only supported registration verification after users completed work in the Manage Registered Details wizard. Learn more about registration verification.
Activity Country Dependencies
This feature allows Admins to define country-to-country dependencies for planned changes. When Activity records are created, Vault will automatically create the applicable dependency records to ensure that the country dependencies are visible to country-level users. When a reference country activity reaches its approval or submission milestone, the owners of the dependent country activity will be notified that they can proceed with implementing the change in their market. Learn more about configuring country dependencies.
IDMP Process Automation
This feature introduces automation into the IDMP process. When users generate IDMP data from a Regulatory Objective record, Vault identifies applicable medicinal products, creates the product data submission for each applicable medicinal product, and generates the IDMP elements.
Learn more about IDMP configuration and generating IDMP data.
Updates to IDMP Accelerators to Support UDI
This feature splits the Automatically Generate Registered Details Records setting on the Application Settings page into two separate settings for Registered Site Role records and Registered Packaged Product records. Vault now generates Registered Packaged Product records with the same object type as their source Registered Packaging records if there is a matching object type name. These changes will allow Vault to create Registered Packaged Medicinal Product records for IDMP and Registered Packaged Device Product records for UDI.
XEVMPD Attachment-Only Submission
This feature allows for the creation of attachment-only XEVMPD submissions. When the attachment-only submission is generated, Vault automatically submits it to the EMA. The EMA can then assign an EV Code to the document in the attachment-only submission, which can be used in subsequent product submissions.
Learn more about enabling and using attachment-only submissions.
XEVMPD Procedure Number
This feature updates the XEVMPD data aggregation algorithm to populate the MRP/DCP/EMA Number from the IDMP Procedure Number field on the Registration record.
PromoMats & RIM Vault Connection
With this release, admins can create a connection between PromoMats and RIM for eCTD Compliance Package submissions to the FDA. The connection enables a seamless flow from Compliance Package generation in PromoMats to Content Plan creation in RIM Submissions, and, if licensed, to the FDA through the gateway via RIM Submissions Publishing. This reduces the time required to submit the compliance package to the FDA.
Asynchronous Content Plan Actions: Locking & Indicator
This release introduces locking on asynchronous Content Plan actions to prevent an action from being initiated if a conflicting action is already processing on the same content plan, minimizing data concurrency issues. The locking is controlled at the Content Plan root level and the locked actions include the Content Plan update, split Content Plan Item, asynchronous state change, and copy into Content Plan.
Additionally, this feature adds a new status icon to the header of the Content Plan Hierarchy Viewer to inform users if Vault is processing one of these asynchronous actions within the current Content Plan.
Quality to RIM Vault Connection Data Model Update
With this release, the data model components supporting the Quality to RIM Vault Connection are available in all RIM Vaults. Although Admins can see these components in any RIM Vault, the Quality to RIM Vault Connection works only for RIM Vaults with the Registrations application.
UDI Source Data Model
This feature introduces medical device-related data model changes to allow Vault to capture UDI attributes for EUDAMED and GUDID.
21R2 Regulatory Data Model Changes
See 21R2 Data Model Changes: Regulatory.
Safety
Safety features are targeted for tentative availability on August 5, 2021 & August 13, 2021.
Vault UI Refresh Changes Auto-on
Vault Safety now includes icons for all system actions that can be displayed in the Action UI. The application has been updated to reflect Vault Platform’s new look.
Learn More: About the Action Bar and All Actions Menu
Local to English Language Intake Configuration
Vault Safety now supports case intake from the local language to English through the Inbox Item, provided in the new Inbox. For example, the EMA requires English submissions. If an adverse event is reported from Holland in Dutch, you can enter the Inbox Item in Dutch while translating the data to English before promoting it to a Case.
Note: As of 21R2, AERs will enter a sunset period. No new functionality will be added to AERs. Using the new Inbox (Inbox Item) is optional in 21R2 (August 2021), recommended in 21R3 (December 2021), and mandatory by 22R3 (December 2022).
Learn More:
E2B R2 and R3 Import to Inbox Item Configuration
Vault Safety now supports the ability to import single-case and multi-case XML files in E2B(R2) and (R3) formats to Inbox Items. The system performs an E2B import upon receiving an AS2 gateway transmission, or when a user runs the import action from an E2B document in the vault library. The Inbox Item displays the mapped E2B source data for the Case, Patient, Reporter, Adverse Events, Case Products, and Medical and Drug History in the Source Data pane. This feature also benefits from Vault Safety’s existing import to AER capabilities, including Study and Product matching, data mapping, and literature and attachments import.
Note: As of 21R2, AERs will enter a sunset period. No new functionality will be added to AERs. Using the new Inbox (Inbox Item) is optional in 21R2 (August 2021), recommended in 21R3 (December 2021), and mandatory by 22R3 (December 2022).
Note: This feature is auto-on for Safety.AI customers who are already using the Create Inbox Item action released in 21R1 for form intake.
Learn More:
- Enable E2B Import to Inbox Item
- Case Intake Overview: Case Intake Using an Inbox Item
- Import an Inbox Item
- E2B Case Import Data Mapping
E2B Imported Case Naming and Versioning Auto-on
Imported Cases created from E2B are now named by their Unique Identifier (UID). This will map from C.1.1 in E2B(R3). Newly created Imported Cases will default to version 0.1. Existing Imported Cases will not be modified.
Learn More: Configure Object Naming Conventions: Naming Conventions for Imported Cases
China (NMPA) Local Fields Configuration
Chinese local data elements required by NMPA (China regulatory authority) are now available for data entry.
Learn More:
Provision Additional EMA Supported Units of Measurement Auto-on
Vault Safety now includes a full list of units of measurement supported by EMA E2B(R3).
Learn More: About the Case Page: Units of Measurement
Support Substance Decimal Places Auto-on
Case Product Substance Strength now supports up to 4 decimal places of precision. E2B export will truncate at the appropriate number of characters.
Multilingual MedDRA Browser and Autocode Configuration
The Vault Safety MedDRA browser now offers the ability to code Adverse Events in any non-English MedDRA supported language. This capability also extends to auto-coding. The updated browser is available for Inbox Items during intake, and Case Adverse Events during subsequent case processing.
Learn More:
Multilingual MedDRA: Japanese Configuration
Vault Safety Inbox Items now support the ability to browse, search, and auto-code MedDRA terms in Japanese. When the MedDRA control language is set to Japanese, the MedDRA browser filters out non-current Japanese terms by default.
Learn More:
E2B Import: Match Non-Current MedDRA in Central Auto-on
Vault Safety now supports coding non-current terms using central MedDRA when importing E2B files that contain non-current terms. A ‘Recode’ badge is displayed to remind users when a coded term is non-current. Also, the Case MedDRA Version of promoted cases from AER or Inbox Item is now set by the Active MedDRA version on the user’s vault.
Note: This feature is auto-on for customers who are using Central MedDRA.
Learn More: Import an Inbox Item: Auto-Code Non-Current MedDRA Terms
DSUR — Extended Definition of Death Auto-on
In Vault Safety, the List of Subjects Who Died During the Reporting Period Appendix now lists only the latest version of a fatal case received or approved during the reporting period, based on the value selected in the DSUR “Filter Case by” field. The appendix table constraints have also been updated to consider additional criteria for more accurate fatal case identification.
Note: This feature is auto-on for customers who have DSUR appendices enabled. Previously generated DSURs need to be regenerated to see the update.
Learn More: DSUR Table Data Mapping: List of Subjects Who Died During the Reporting Period
Default Sender User by Destination Configuration
Administrators can now configure a default sender user on all Transmission Profiles to be populated on system generated transmissions. For example, this allows defaulting different QPPVs for EMA and MHRA.
Learn More: Enable Default Sender User on Transmissions from a Transmission Profile
EMA Certification: Name Parts and RoA Prioritization Auto-on
Vault Safety now supports the import and export of EMA Product Name Parts. New fields for Product Name Parts are now available during Case Product data entry. EMA E2B(R3) file exports also prioritizes structured G.k.4.r.10.2a/b data over unstructured G.k.4.r.10.1 data for the Route of Administration.
Learn More:
- Enable EMA Certification Enhancements (Name Parts and RoA Prioritization)
- Enter Case Data: Case Product Details Section
- Enter Case Data: Case Drug History Section
- EMA E2B Generation Data Mapping
- E2B Case Import Data Mapping
Case Level Regional Validation for VAERS and EMA/MHRA E2B R3 Auto-on
Users can evaluate and review regional FDA VAERS and EMA E2B(R3) Validation Results at the Case level. The FDA VAERS and EMA regional rules are now evaluated when the Evaluate Regulatory Conformance action runs on the Case. Regional Validation Results are displayed on the Case, alongside the ICH (global) Validation Results.
Note: This feature is auto-on for customers who have the Evaluate Regulatory Conformance user action enabled for the Case object. An admin must configure the action on the appropriate lifecycle state for the Case object.
Learn More: Case and Submission Validation
Replace UTF Smart Quotes with ASCII Straight Quotes in FDA E2B(R2) Auto-on
Vault Safety now converts smart quote characters to straight quote characters when generating FDA E2B(R2) submissions. This will ensure the generated ICSR continues to be ISO 8859-1 compliant, as ISO is the only encoding supported for FDA E2B(R2) submissions. Related to this, Health Canada E2B(R2) submissions will now generate in UTF instead of ISO encoding (both are supported by Health Canada) to circumvent the aforementioned issue.
Generate E2B Message ID for Manually Created Transmission Auto-on
Vault Safety will now auto-generate an E2B Message ID for a manually-created E2B submission / distribution. This identifier maps to a mandatory E2B field that facilitates the subsequent receipt of acknowledgments. Failure to submit an E2B with this identifier may result in submission failure. Now, users will no longer need to remember to enter this identifier for a manually created transmission.
Note: This feature is auto-on for customers submitting E2Bs to an AS2 gateway.
FDA E2B R2 — Support “FDA Safety Report Type” (A.1.FDA.16) Field For Post Market Support
Vault Safety now supports adding the FDA Safety Report Type (A.1.FDA.16) element to FDA E2B(R2) Transmissions. This feature only impacts the Transmission file format FDA E2B(R2). This feature only supports postmarket submissions (E2B Code=3). Other IND Safety Report tags are not yet supported. This field will only be populated for post market cases and will not be present for study Cases.
Learn More:
New Safety Intake API Auto-on
The Vault Safety API has been extended with new endpoints to receive E2B files and import to either Imported Cases or Inbox Items. New endpoints have also been introduced for retrieving import status and ACKs.
Learn More: Vault Developer Portal: Safety Intake
E2B (Plus) Import SDK Extension for Custom Import Formats Configuration
Vault Safety now allows developers to extend existing E2B XML formats or create their own XML data transfer formats for inbound ICSRs. Different Inbound and Outbound E2B formats can be configured on AS2 and System Gateways.
Feature Enablement Changes
Features in this section have an enablement change in 21R2.
| Feature | Previous Enablement | New Enablement (21R2) |
|---|---|---|
| Configurable Back Reporting | Support (21R1) | Auto-On |
| Multi-Case E2B Import via API/AS2 | Support (21R1) | Auto-On |
| VAERS Certification Enhancements | Support (21R1) | Auto-On |
Automatic Intake From Documents With a Viewable PDF Rendition Auto-on
Safety.AI’s automatic PDF form intake feature is now available for all file formats supported in Vault including Word and image files. This will allow users to start the extraction process from any Vault Document with a Viewable PDF Rendition. Upon successful completion, an Inbox Item will be created with the case information extracted from the document text, fields, and checkboxes.
New Checkbox Recognition from Documents Auto-on
Safety.AI will automatically extract additional information from document checkboxes including Outcome, Report Type, Patient Gender, and Age Group. Adverse Event Outcome and Seriousness will be suggested from Case Outcome and Seriousness. The Source Data pane will display this information to facilitate human verification. Safety.AI will also display a value only once in the field’s dropdown menu if it was found multiple times in the source text.
Deprecate Create AER Action Auto-on
The Create AER user action was replaced in 20R2 by Create Case to allow users to promote an Inbox Item directly to Case without creating an AER record. The Create AER action is being deprecated in 21R2.
AI Service with no Vault Connections Auto-on
Configuration is no longer required to connect to the AI Service when setting up a new Vault with Safety.AI. Previously, an administrator had to create an External Vault Connection.
SiteVault
Set Context in Vault Selector
With this feature, all SiteVault users will see their research organization and sites in the Vault Selector. Users with appropriate permissions can select a research organization to view data across all sites associated with the research organization.
Single Source of Truth for Person Profiles
This feature ensures that each person in a research organization only needs to manage one set of profile documents no matter how many sites they are associated with in SiteVault.
Prompt for Fields on Upload to Document Inbox
When uploading documents to the Document Inbox, users are now prompted to add values for common fields such as Study, Participant, and Organization.
Expanded Language Support
This feature provides additional support for users to view a translated version of SiteVault. See the About Supported Languages page for more information on the languages that are available.
Optional State Change for Send Document via Clinical Network Action
With this feature, Admins can configure an automatic document state change when a document is sent to a sponsor or CRO for a Connected Study.
New Exchangeable Document Types on Connected Studies
This feature adds support to transfer the following new document types from a sponsor or CRO’s Vault for a Connected Study:
-
Ancillary Committee Response
-
Clinical Study Report
-
Contract
-
Insurance
-
Protocol Clarification
-
Procedure Manual
-
Protocol Summary of Changes
-
Protocol Synopsis
-
Regulatory Authority Response
Veeva eConsent Early Adopter Feedback & Enhancements
This feature includes the following enhancements to Veeva eConsent in SiteVault:
- Site users receive a notification when a participant declines an eConsent form in MyVeeva for Patients, and users can cancel or resend the declined form as needed.
- Site users can view, search, filter, and report on eConsent form responses collected from participants in MyVeeva for Patients during the eConsent process.
- Site users can view and share a preview of the eConsent form as it would appear to a participant during the consent process.
Veeva eConsent: Patient Declines Consent
With this feature, sites using Veeva eConsent can receive a notification in SiteVault when a participant declines an eConsent form in MyVeeva for Patients. Moreover, site users can cancel a declined form or resend the form if the participant declined in error or changed their mind.
Veeva eConsent: Form Responses
This feature enables sites using Veeva eConsent to view, search, filter, and report on eConsent form responses collected from participants in MyVeeva for Patients during the eConsent process.
Veeva eConsent: Shareable eConsent Preview
This feature enables sites using Veeva eConsent to create a URL of an eConsent form. This enables users to share a preview of the eConsent as it would appear to a participant during the consent process with an external third party such as an internal review board (IRB).
Digital Delegation Log Updates
This feature provides a digital and compliant way to record all of the significant study-related duties that have been delegated to study staff by the principal investigator as part of the Delegation of Authority log. This feature can only be configured in Vaults with the Extensible SiteVault Permissions feature enabled.
SiteVault Settings Framework
This feature enables users with appropriate permissions to enable and disable SiteVault features for a research organization, site, or study.
Extensible SiteVault Permissions
This feature updates how user permissions are managed in SiteVault. To provide additional granularity in user permissions, additional access can be defined at the level of the research organization, site, and study.
Language Field Alignment
This feature standardizes and consolidates document language under a new Language (translated_language__v) document field. The new field is added to the following document types:
- Advertisement for Recruitment (subject_advertisement__v)
- Diary (blank) (subject_diary_blank__v)
- Informed Consent Form (blank) (informed_consent_form_blank__v)
- Participant Information Sheet (subject_information_sheet__v)
- Participant Materials - Other (other_subject_information__v)
- Participant Questionnaire (blank) (subject_questionnaire_blank__v)
- Study Participant Card (participation_card__v)
- Subject Diary (blank) (subject_diary_blank__c)
- Subject Questionnaire (blank) (subject_questionnaire_blank__c)
Edit Checks for Patient & Participant Data Quality
This feature ensures that the information captured in SiteVault about patients and study participants is appropriately formatted and assigned, reducing the likelihood of errors. This includes validating the format of email addresses and phone numbers and that patients are only assigned to a study once.
Data Model Updates
See 21R2 Data Model Changes: SiteVault.
QualityOne
QualityOne Teams
This feature provides a mechanism to manage structured quality teams of participants that are responsible for completing specific processes within workflows assigned within Vault. QualityOne Teams can be configured to help manage change controls, audits, CAPAs, and other quality event-related processes. Learn more about configuring and working with Teams.
Product Hierarchy Data Model for QualityOne
The Consumer and Chemical Product industry has formulations within Quality that can form multilevel hierarchical relationships between the different quality processes. With this release, a shared standard product hierarchy data model is introduced and can be leveraged to store complex hierarchical products and their specification details to reduce reliance on customization. Learn more about QualityOne’s key data model.
Training Management Phase 3
With the 21R1.3 release, we’ve improved QualityOne Training Management in the following ways:
- External Training Type:This release introduces the External Training type, which allows Learners to complete training on content or topics that occurred outside of Vault. For example, an organization may require that Learners complete a third-party online course or receive an external certification.
- Manager Access to Training Records: In many organizations, managers are responsible for the training completion status of the employees that report to them. This release introduces the ability for a Learner’s direct manager to have visibility into Learners’ Training Assignment records, as well as other training-related records. The Manager Groups feature must be enabled before configuring this feature in Training Management.
- Improvements to Learner Task Page: The Learner Task Page user interface is enhanced for Training Assignments containing multiple documents. Learners will arrive at a default landing page, requiring them to select a document. Vault now always displays the Learner Task Page for completed Training Assignments, along with a link to View Quiz.
- Direct Assignment to Multiple Learners: In previous releases, the Training Direct Assignment feature could issue a Training Assignment to only one Learner at a time. With this release, Direct Assignment now supports issuing a Direct Assignment Training Assignment to multiple Learners with a single request.
- Direct Assignment to Curriculum: With this release, the Direct Assignment action can also be performed on a Curriculum. This allows users to issue Training Assignments for that Curriculum to the Learners selected.
- Complete Training Assignment Workflow Visible in Admin: Previously, the Complete Training Assignment Workflow, which is used for Vault Document Training Assignment, was hidden in Vault Admin. In this release, the workflow is now visible. This allows Notification and Task Reminders to be configured for the workflow. Note: Modifying this workflow can cause issues delivering Training to Learners. Only certain workflow elements can be updated.
- Person Object - Remove User Restrictions When Adding a Learner Role: In previous releases, when a Learner Role was added to a Person record, Vault required an active User reference, regardless of the value of the Training Eligibility field. With this release, an active User reference is only required if Training Eligibility is set to Eligible.
- Training Requirement Object Type Limitation: To ensure that the Training feature continues to function as intended as we continue to add new features, Training Requirement record’s object type cannot be changed once the record is saved.
- Training Requirement ZIP File Limitation: To ensure Training Requirements function as intended, ZIP files cannot be added to the Related Training Materials page section for Training Requirements, regardless of object type.
- E-Learning Technology Standards Support: Many organizations include e-learning content in their training implementations. This format of training content can be dynamic, engaging, and easier for learners to absorb than other formats. With this Training Management release, we have introduced support for the e-learning technology standards AICC and SCORM. Content published in AICC or SCORM can be uploaded into a Vault, just like any other document. Once uploaded into the Vault, training admins can associate the content to a Training Requirement as part of a training matrix.
- Substitute Training: This feature enables the Training feature to assign an alternative, or substitute, Training Assignment to a Learner in place of a required Training Assignment using Training Admin-defined substitution rules. When the Learner completes the Substitute Training Assignment, the Learner automatically gets completion credit for the required Training Assignment.
- Facilitated Training:This feature enables a Training Admin, or a user with proper access, to give completion credit for Training Assignments on behalf of Learners. This allows completion of Training Assignments for Learners that may not have access to Vault. For example, in some organizations, users could be contractors, external employees, temporary employees, or otherwise outside the primary organization.
- Training Nightly Job Performance & Robustness Improvements:This feature improves the performance and robustness of the Update Training Assignments job which drives Training Management automation. With this release, the job now continues to issue Training Assignment records even if an issue is encountered, skipping paths which have issues.
- Power Delete Action for the Training Assignment Object: During Training configuration and testing, you may need to delete a Training Assignment record and all of its related records. By default, you must delete each record individually. With this release, we have introduced the Power Delete Training Assignment Records record action, which completely deletes a Training Assignment record and its related records.
- Assignment Details Lifecycle State No Longer Updated: To improve accurate reporting and searching, the Assignment Details lifecycle state is no longer updated. In previous releases, the Assignment Details lifecycle state changed when the Training Assignment entered the Assigned, Completed, or Cancelled states. With this release, there is now a lookup field on Assignment Details records that references the parent Training Assignment record’s lifecycle state. This provides a real-time indication of the Training Assignment lifecycle state. The Assignment Details lifecycle state is no longer in sync with the Training Assignment’s lifecycle state.
- Classroom Training: Usability Enhancements: With this release, Classroom Training Assignments now have an associated auto-start workflow called View Classroom Training Assignment that provides easy access to the Classroom Training Assignment record for Learners. Once an Admin activates the View Classroom Training Assignment workflow, it is automatically started when a Classroom Training Assignment enters the Assigned state. When an Instructor changes a Classroom Training Assignment record’s state to Completed, the open Training Assignment workflow is automatically canceled with a configurable entry action.
- Training Assignment Lifecycle User Actions: With this release, Admins can now edit user actions on the Training Assignment Lifecycle. This feature is only intended to support the Download Formatted Output user action.
- Updated Training Requirement Document Picker Dialog: The dialog box when selecting documents in a Training Requirement or Training Requirement Impact Assessment has been updated to match the checkbox-style document picker.
- Learner Homepage: This release introduces the Learner Homepage, an intuitive, easy-to-use dashboard that provides the Learner with an overview of the Learner’s open assignments. Assignment cards display key information such as the related Curriculum, estimated completion time, due date, categories, and more. In addition, the Learner can access their completed Training Assignments.
- Mobile Training Task Completion: This release allows Learners to view and complete Vault Document Training Assignments from mobile device browsers. When a Learner accesses a Vault Document Training Assignment from a phone or a tablet browser, Vault displays the assignment in a mobile-friendly way that’s appropriate based on the device size. In the mobile interface, the Learner can view the document or documents, scroll through the document pages, view key document metadata, and complete the workflow task with e-signature from their mobile device.
- Hide Training Report from Document Actions Menu: This feature hides the Training Report from the document Actions menu for customers who do not utilize Read & Understood functionality in QualityOne Vaults. This feature is enabled in Admin > Settings > Application Settings, and the Training Report can be made available again through the document Actions menu if a customer later chooses to utilize Read & Understood capabilities.
Learn more about Training Management features.
Inspection & NCR Product Model Compatibility
This feature ensures that customers using the new Product Hierarchy data model from 21R1 are able to use Inspection and Mobile NCR functionality.
QualityOne Entry Action: Create Related Record
QualityOne adds a new entry action configuration that will automate creation of related records when the parent record enters in a particular lifecycle state. The Create Related Record action will run as an entry action for the admin-configured components available through the Related Record Setup tab. Learn more about setting up related record automation.
QualityOne Mobile for iOS - Local Storage Support
This feature allows items to be stored locally on a device when attempting to submit an Incident or NCR when there is no active network connection. Users may attempt to resubmit manually when they move into an area with network coverage. This supports users in their work environment as network connectivity may not be present throughout the operation. Learn more about local storage support.
Note that QualityOne Mobile features are targeted for tentative availability on August 17th.
QualityOne Mobile for iOS - Run User Actions on Submit
This feature allows users to run configured User Actions on NCR & HSE Incidents when submitting a record to Vault via QualityOne for iOS. This supports users by reducing the need to triage incoming NCRs and HSE Events, running initial actions from QualityOne Mobile instead of Vault. Learn more about running User Actions on submit.
Note that QualityOne Mobile features are targeted for tentative availability on August 17th.
QualityOne Mobile for iPadOS - Audit Checklist UI Enhancements
This feature updates the QualityOne for iPadOS user interface (UI) of the Audit Checklist application.
Note that QualityOne Mobile features are targeted for tentative availability on August 17th.
HACCP Data Model
This feature offers an additional data model which allows organizations to manage Hazard Analysis and Critical Control Points (HACCP) to ensure food safety from biological, chemical, and physical hazards during production. Learn more about HACCP Management.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Incident Management - Track Labor Information
This feature extends the existing Incident Management object model with a new Labor Information object that enables tracking of labor hours and headcount for employees and contractors. Learn more about Track Labor Information.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
COA Textract Response File Download
This feature is an enhancement to the location-based header matching rule introduced in 21R1. Business Admins can create location-based header matching rules easily by removing dependencies of extracting the OCR response from AWS Textract console and storing the response in Vault. Once the response is stored in Vault, the file can be downloaded for OCR response extraction. Learn more about COA Textract Response File Download.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Support Localized Number Separator for COA
This COA enhancement extends the support to ingest various types of decimal and grouping separators found in a COA document such as period, comma, and single quotation. For example, Vault now identifies these number formats - “123.456”, “123,456”, “123’456”.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Data Model Changes
See 21R2 Data Model Changes: QualityOne.
RegulatoryOne
Note that RegulatoryOne features are targeted for tentative availability on August 17th.
Dynamic Template to Create Requirements
When configured, this feature provides users with the ability to automatically create a list of registration item requirements from an Admin-defined template. Requirements can be dynamic in the sense that Admins can configure criteria to include or exclude a requirement based on information specific to what is being registered. Admins can define tokens that describe a registration item’s type of information and leverage those tokens in requirement records. This feature provides Regulatory users with an efficient way to define and track what needs to be completed for a registration request. Learn more about configuring a Dynamic Template to Create Requirements.
Create Registrations in Bulk
When configured, this feature provides users with the ability to automatically create Registration Objectives for one or more Registration Items on an Event. Users can apply filters to find and select the appropriate Registration Items and either choose to create unique Registration Objectives for each Registration Item or group Registration Items so they have the same Registration Objective. When configuring, Admins can specify which columns to display to the user and have the ability to hide the option that allows Registration Items to share the same Registration Objective. This feature allows Regulatory Affairs users to work more efficiently during the product registration process. Learn more about configuring bulk registration creation.
Regulatory Request Management
When configured, this feature provides the users with the ability to mitigate non-compliance risks by accessing accurate, up to date, and consistent information to include in responses to requests for information related to the regulatory compliance of a product or formulation. Requests can be external or internal. For each request, users can quickly create a request from predefined types and include relevant details, and then submit for review. Regulatory users can provide responses, associate related documents, view similar requests to ensure consistency in responses, and create any required secondary requests. Learn more about Regulatory Request Management.
Data Model Changes
See 21R2 Data Model Changes: RegulatoryOne.
Veeva Claims
Note that RegulatoryOne features are targeted for tentative availability on August 17th.
Auto-Localize Pack Copy Enhancements
A localized Pack Copy is a specification document that brand marketers create in order to deliver a localized version of the packaging brief to the creative teams. As a common use case, users need to generate a Local Pack Copy from an existing Global Pack copy which pulls in all of the associated localized Elements in an automated fashion. The existing Auto-Populate Local Pack Copy feature enables users to generate auto-populated Local Pack Copies from a Global Pack Copy for a single country. The auto-generated Local Pack Copy has the same Pack Copy hierarchy as the Global Pack Copy and associated Local Elements.
This enhancement allows users to check if any Global Elements are missing localizations and then enables users to select which of those Global Elements to create localized versions for each specified country. When the action runs, Vault then automatically generates translation placeholders or localized elements based on Admin configuration.
The feature allows Admins to define if a Global Element should be Localized or Translated. The Localization process can be different for different element types; for example, users currently translate only a subset of packaging elements in multiple languages and adapt the rest in one or no language. Based on Admin configuration, the action creates a new Local Pack Copy record for each country selected with existing translations and localizations and creates any new Local Elements. Learn more about configuring auto-localize pack copy.
Multi-Country Pack Copy Localization
This feature allows users to localize a Global Pack Copy by selecting multiple countries before running a single action. Previously, users could create a local Pack Copy from a Global Pack Copy for only one country at a time.
Selective Creation of Local Adaptations from a Project
The feature allows users to create multiple Local Adaptations for the Claims and Countries of their choice by enabling them to selectively control which countries to automatically create local adaptations for through a new user action.
This feature reduces extra clicks caused by unwanted Local Adaptations generated when users can’t specify which Claim and Country combinations to create Local Adaptations for. When configured, users can now view the new action on the Project record(s) if there is at least one Claim and one Country in the project. After clicking the action, users can select the Claims under the Project in a new dialog for All or specific countries. After users complete their selection, clicking Continue creates new Local Adaptations for the Claims and Countries selected and links them to the existing Project. If Local Adaptations already exist for any of the Claims, the system will not generate duplicate records but will link the existing Local Adaptation record to the Project. Learn more about Selective Creation of Local Adaptations from a Project.
Auto-Create Element
Veeva Claims’ Pack Copy module includes a reusable Element library which contains different kinds of packaging elements including Claim-type elements. Claim-type elements are existing Claim records. The current process of populating the element library can be time consuming; after marketers create and approve claims, they have to create related elements manually.
This feature significantly reduces the time needed to populate the packaging Elements library. This feature allows the automatic creation of an Element record after the corresponding Claim reaches a defined lifecycle state. This feature provides Admins the option to configure a record action in a Claim lifecycle or workflow to enable automatic creation of a Claim-type packaging Element. Learn more about configuring Auto-Create Element.
Data Model Changes
See See 21R2 Data Model Changes: Veeva Claims.
Enablement Details
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|