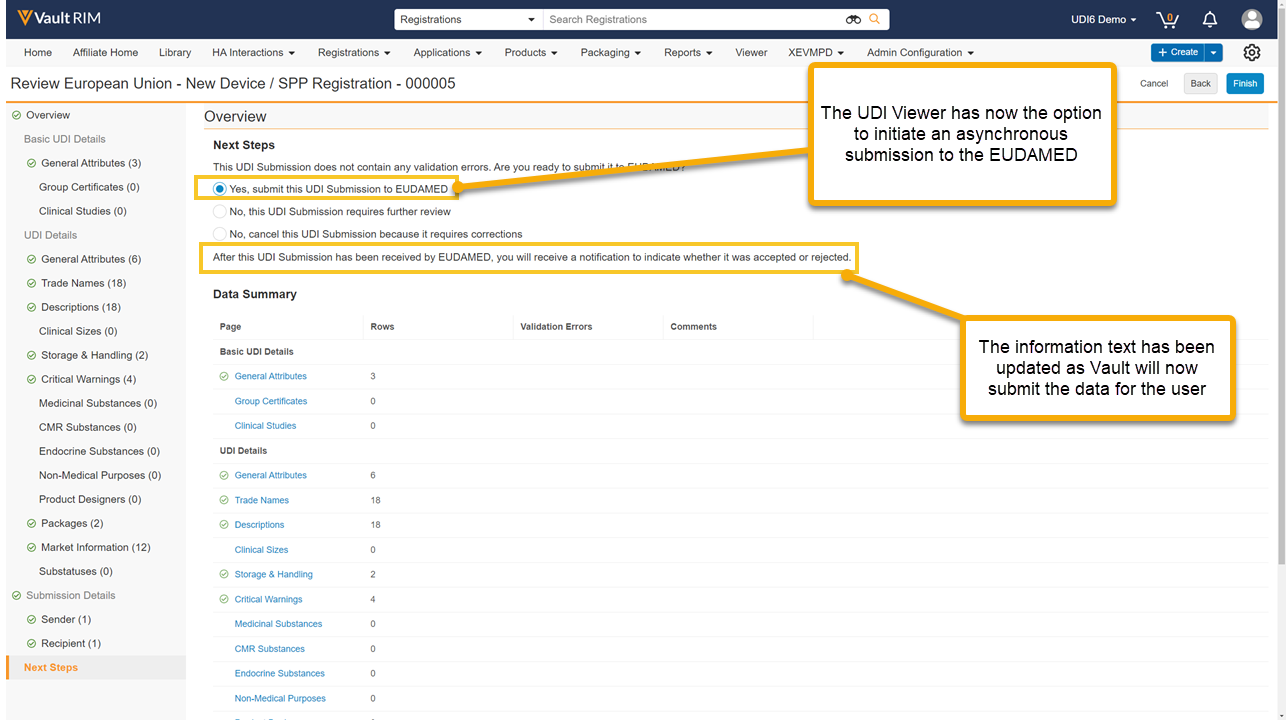
Prerelease Date: July 17, 2023 | Release Date: August 4, 2023 & August 11, 2023
The following applications may have different release dates: Safety, QualityOne Client Applications, RegulatoryOne, and Veeva Claims.
We are pleased to bring you Vault 23R2. Read about the new features below. You can find information on enabling new features in 23R2 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Platform
Working with Documents
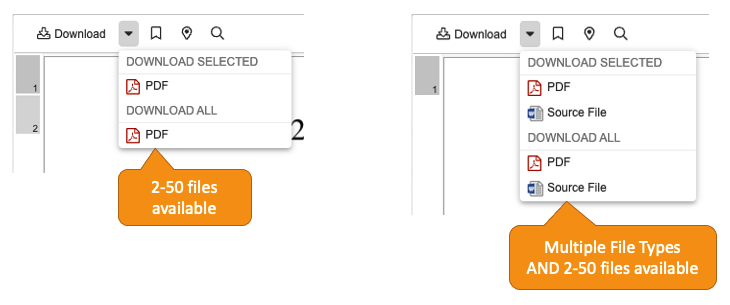
Download All Option for External Viewer
When viewing up to 50 documents in the External Viewer at one time, users now have a Download All option, if enabled. When Download All is performed, users automatically receive a ZIP file that contains the viewable renditions or source files of their documents.
The External Viewer is accessed via direct URL and is often used to send documents to non-Vault users. Common uses for sending multiple documents via a direct URL to the External Viewer include:
- Sending Response Packages in MedInquiry
- Approved Email in Medical and PromoMats
- Safety Distribution via SiteConnect (for sites that do not have an active Agreement)
Learn more about the external viewer.
Embedded Links in Documents to External URLs Open in a Separate Tab
External links embedded in documents will open in a new browser tab. With this enhancement, Vault opens all qualifying embedded links that target URLs in a new tab, instead of in a pop-up window. A qualifying link is one where the target URL is absolute (contains the full path) and uses a whitelisted protocol (http, https, or mailto).
For example, RIM users will experience this behavior in RIM Submissions Archive when clicking on external links in the Submissions Archive Viewer, and in RIM Submissions Publishing when reviewing hyperlinks within eCTD submissions.
SVG, AVIF & WebP Rendition Support
Users uploading and managing SVG, AVIF, and WebP files, which are often used in Digital Asset Management, will now see these files rendered in the document viewer. This is useful for PromoMats and MedComms Vaults as it improves Medical, Legal, and Regulatory (MLR) review process compliance by ensuring reviewers are able review these files directly within Vault and without needing to download the source files.
Files that include animations are now rendered in Vault as Video Renditions. Vault now also supports Optical Character Recognition (OCR), Overlays, and Signature Pages for these renditions.
Learn more about file type rendering support.
Render Animated GIF Files As Videos
Users that upload GIF files can now see those files rendered as Video Renditions in Vault within the Document Viewer. This will apply to all new uploads, including where files are up-versioned. Existing files will not be updated.
Learn more about file type rendering support.
Limits on Create & Edit Annotations
When users are working with Annotations, they may encounter character limits enforced by Vault. The limits vary depending upon the type of annotation:
- For anchor annotations, Name (Title) is limited to 140 characters
- For note, link, and anchor annotations, Subject (in Header) is limited to 32,000 characters
- For note, link, line, and reply annotations, Comment is limited to 32,000 characters
- For external links, URL Length is limited to 32,000 characters
- For line annotations, Placemark is limited to 50 lines
These limits are enforced when creating and editing annotations in Vault UI, as well as when using Import Annotations functionality. These limits are not enforced on existing annotations unless users edit the annotation.
Picklist Value Label Increased from 128 Characters to 256
Vault now supports picklist values with labels up to 256 characters, doubling the amount of characters that can be used from the prior limit of 128 characters.
Default Maximum Number of Attachments Allowed is 100
The maximum number of attachments for documents has been increased from 50 to 100.
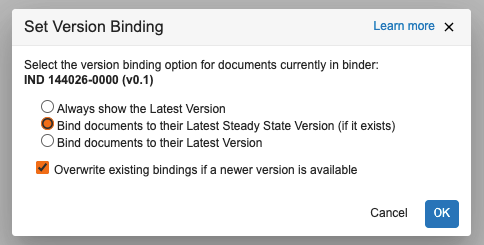
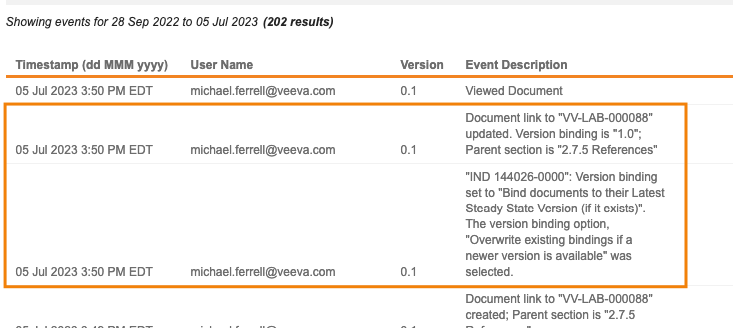
Binder Audit Trail: Audit Logs for Binding Rules
Vault now logs changes that were made to a binder’s binding rules in the binder’s audit trail. This ensures a more complete history of changes to a binder.
Learn more about Version Binding.
Standardized Deletion of Documents with Lifted Legal Holds
Users with Power Delete permission will be able to delete documents that were previously under Legal Hold once the Legal Hold is Lifted. This has previously been the intended behavior, though there have been inconsistencies in how this was handled by Vault depending upon the actions taken by the user with Power Delete permission.
With this enhancement, this ability will be consistently applied to single document deletion, single version deletion, and multiple document or multiple document version deletions, and will apply to deletions performed via the User Interface, API or Vault Java SDK.
Power Delete permission is available by default in Vault Owner permission sets.
Learn more about Legal Hold.
Vault Objects
Display Format
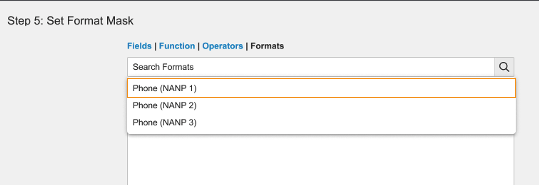
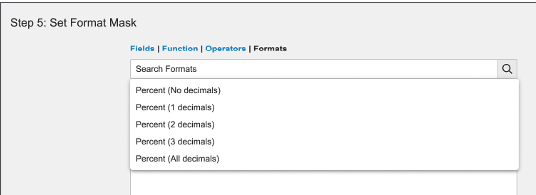
Admins can now use format masks to configure how Vault should display custom (__c) text and number field values. Format masks for standard (__v) text and number field values will become configurable in 23R3.
Admins can set a format mask in a new expression editor section when configuring text fields, number fields, formula fields (where the return type is Text or Number), and lookup fields (that are looking up text or number field values).
Text fields include standard format mask expressions to display user input as phone numbers (North American Numbering Plan).
Number fields include standard format mask expressions to display user input as percentages.
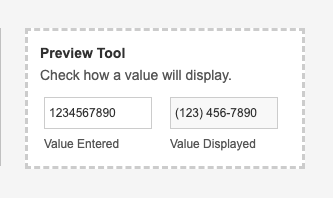
A Preview Tool allows you to see how a value will be displayed prior to saving, after checking syntax.
Learn more about configuring object fields.
Object Framework Limit Increases
In this release, we increased the following maximum limits related to Vault’s object framework:
- 500 custom objects
- 10 unique custom fields per object
- 10 lookup field relationships per object
- 20 lookup fields per relationship
- 50 inbound relationships to a document
- 500 custom fields per object
- 25 formula fields per object
- 30 custom object-level validation rules
- 20 custom object type-level validation rules
- 50 custom layout rules per page layout
- 30 custom outbound relationships
- 50 custom inbound relationships
- 80 custom inbound relationships for the User object
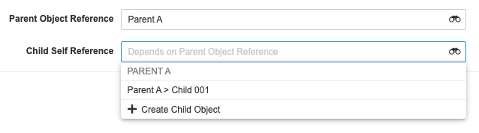
Self References for Child Objects
Admins can now configure Object reference fields on Child Objects that reference themselves. Admins have the option to choose the existing Parent Object reference as the controlling field or a new Object reference to the Parent Object.
For example, given Product (Parent) and Product Specification (Child), a “Related Spec” field may be needed on the Product Specification object to relate two records (Spec 2.1 is related to Spec 2).
This screenshot shows an example where an Admin has configured a self-reference field for their Child Object, and used Parent Object Reference as the controlling field so that they can only choose other records from the same parent:
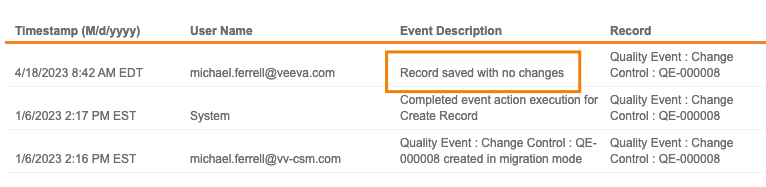
Intelligent Record Update
Vault now only updates object records if any changes have been made. Prior to this release, if a record was saved without any changes, Vault updated the Last Modified Date and added an audit trail entry with an Event Description of “Record saved with no changes”.
For customers with integrations or custom SDK code, this often resulted in a large volume of unnecessary audit trail entries.
Audit All Updates to Document Reference Field Value
The following changes to an object’s document reference field are now included as entries in the audit trail:
- The referred document receives a new version
- The referred document or a version of the referring document is deleted
This ensures the Last Modified Date and the last audit trail entry are in sync. Learn more about document audit events.
Audit Accurate User for SDK Trigger Updates
When a user updates a record that executes an SDK trigger, Vault now logs “[SDK User] on behalf of [Current User]” in the audit trail to show that the change was automatic based on the user’s action. Prior to 23R2, the audit trail would show that the user performed the SDK action.
For example, prior to 23R2, the audit trail would show “user@vault.com” performed the action, and with 23R2, the audit trail shows “System on behalf of user@vault.com”.
This feature is available with the 23R2.0 B release and is not available in pre-release or limited release Vaults.
Learn more about audit events.
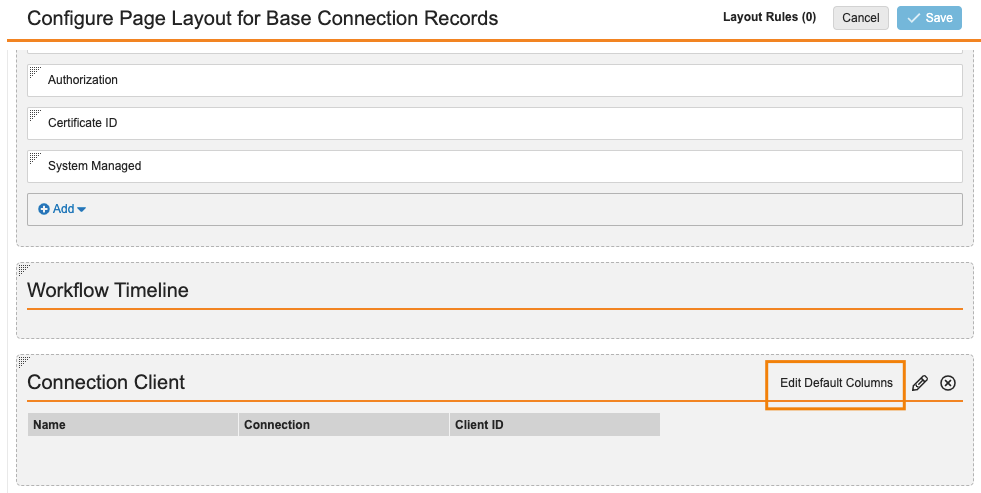
Update “Edit Column” Label in Page Layout Configuration
When Admins configure page layouts, they can define which columns Vault displays by default in related object sections. With this release, that option is being relabeled to “Edit Default Columns” rather than “Edit Columns” to more clearly articulate to an Admin that they are editing the Default layout. Users can adjust for themselves what columns they want to see when viewing records.
Rich Text Support for Related Objects in Formatted Outputs
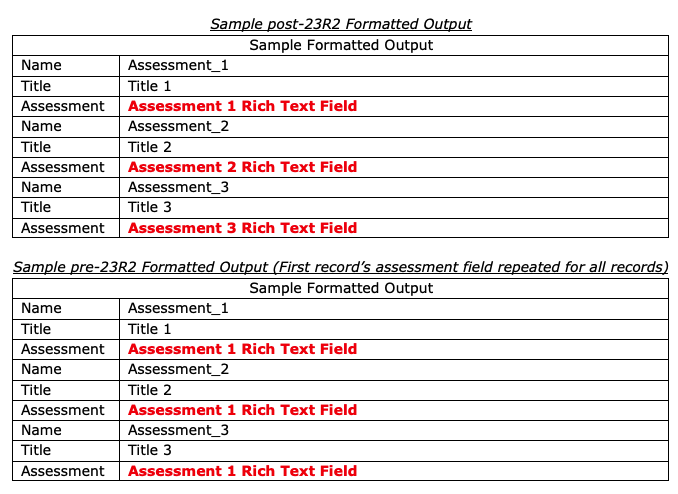
Vaults that leverage formatted output templates as well as Rich Text fields will now be able to display Rich Text fields from records that repeat in tables on the downloaded formatted output. Formatted outputs are often used in regulatory agency audits, and this enhancement ensures data integrity in the downloaded formatted output provided to auditors.
Prior to this release, if a table in a formatted output referenced records that would repeat in the table, Vault repeated the Rich Text metadata from the first record in the table. Customers commonly addressed this by changing the formatted output field to plain text. This provided the appropriate information, but removed any prior Rich Text formatting.
With this release, the Rich Text format is respected and where records are repeated, users will always see the correct Rich Text metadata on each line in the table.
In this screenshot, there is an example of how this example will display after 23R2, as well as how this example displays before 23R2.
Learn more about formatted outputs and Rich Text fields.
Lifecycle & Workflow
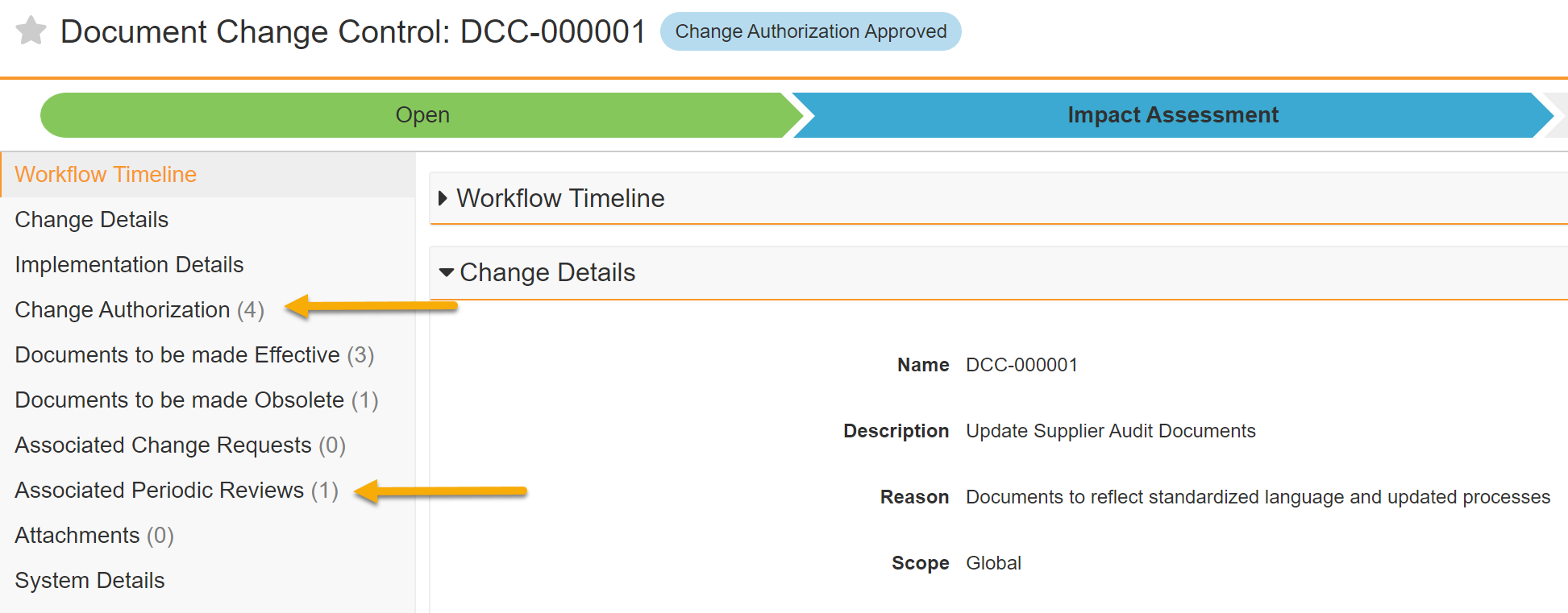
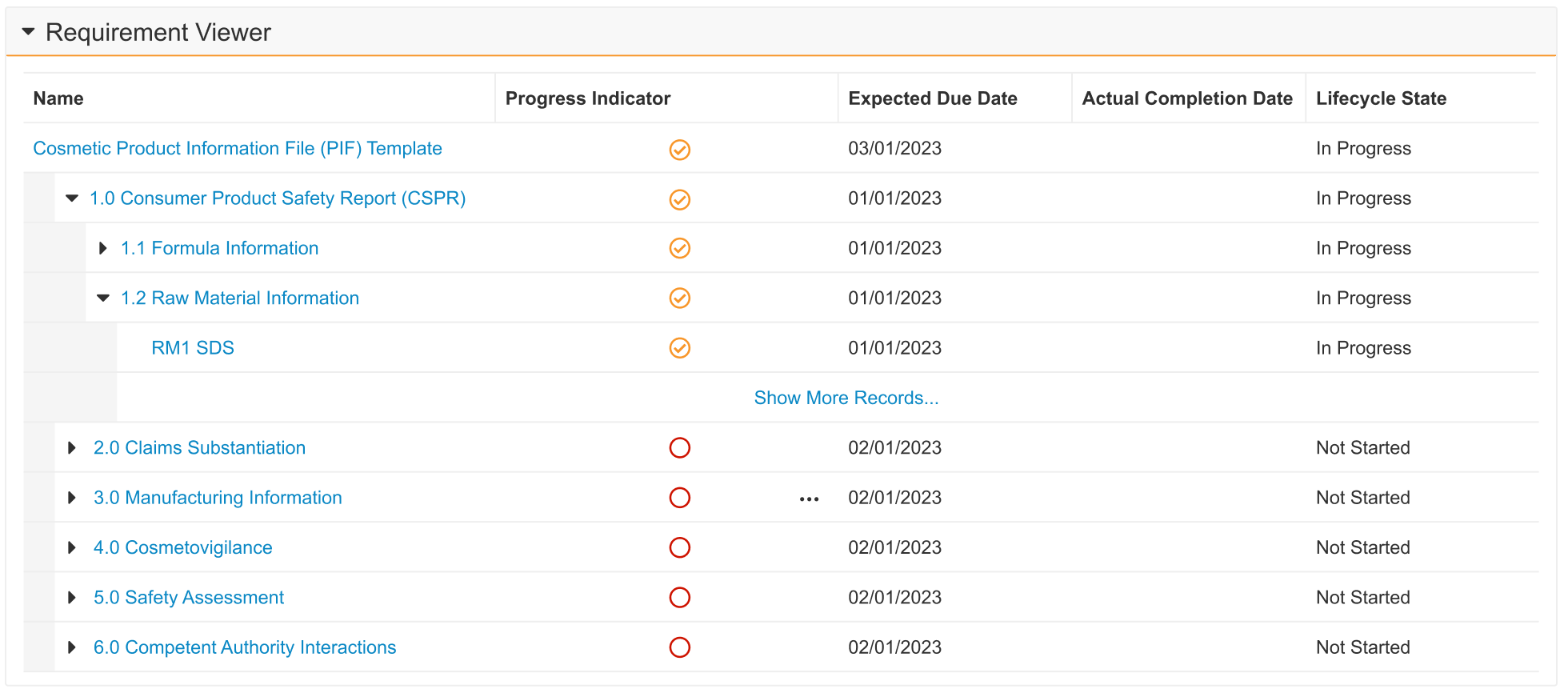
Object Lifecycle Entry Criteria: State & State Type of Related Records can be Validated with Conditions
Customers may now prevent state transitions for object records by using Entry Criteria to validate against multiple lifecycle states of related records. This ensures that the current record can only transition into the next state when its related records are in one of multiple lifecycle states. Previously, only one state could be validated for related records.
For example, a Quality Change Control record can only enter the Implementation Complete state when all related (child) Change Action records are in the Approved state. However, if one of those records is Canceled, the validation on the related objects fails. Now, customers can configure the Entry Criteria to allow for the Canceled state in addition to Approved.
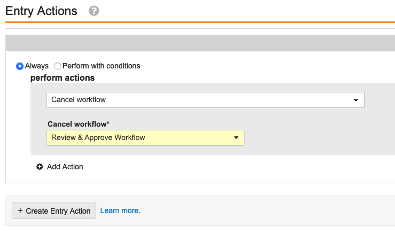
Object Lifecycles: Cancel Workflow Entry Action
Admins can now configure an object lifecycle Cancel Workflow entry action that will automatically cancel any active workflows on the current record.
For instance, in Vault QMS, if a Continuous Improvement is moved to a Canceled state, it is typical that this will automatically move the associated CAPA Action Items, SCARs and Effectiveness Checks to Canceled as well. Prior to 23R2, if there were any active workflows on the related records, Admins had to manually cancel those workflows.
With the new Cancel Workflow entry action, the Canceled state for the related records can automatically cancel the associated workflows.
Learn more about object lifecycle entry actions.
Object Lifecycle Entry Criteria: State & State Type of Related Records Can Be Validated with Conditions/Object Lifecycles: Cancel Workflow Entry Action
Prevent Deletion of Document Templates Used in a Workflow
With this release, Vault will now prevent deletion of document templates that are used in a workflow to ensure that processes are not inadvertently impacted by a user who may be unaware of the use of a given document template in workflows. This would apply to both Basic and Controlled Document Templates, in any instance where a workflow is configured to generate a document based off of a given template.
Override Checkout option for State Change Document Operation Jobs
This feature provides an option to allow a State Change Document Operation Job to successfully change the state of a document even when a minor version of the document is checked out. This feature is only available if the configured Current State is the steady state type of the selected lifecycle and the configured Destination State is the obsolete state type of the lifecycle.
This feature is especially valuable for customers who maintain compliance, such as PromoMats and Medical customers. For example, if a document is in a steady state but has a new minor version that is checked out, this feature allows the job configuration to correctly expire the steady state version when the job runs.
Workflow Custom Actions SDK Updates
Admins can customize and configure custom actions in the Start step participant control for multi-record object workflows and document workflows, and the Task Step of a multi-record object workflow. This enhancement enables customers to use custom actions to automate business processes.
Custom actions are developed by your organization with the external Vault Java SDK. Once deployed to your Vault, Admins can configure the action during workflow configuration.
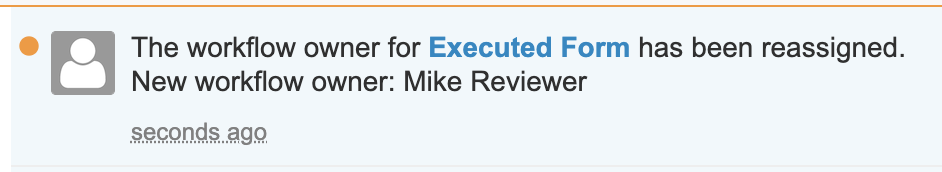
Email Notifications when Workflow Owners are Replaced
Vault will now automatically send a notification when a workflow owner is replaced on an active workflow. The notification is sent to both the previous workflow owner and the new workflow owner.
This enhancement will ensure that both the previous and new workflow owners are aware of a change in ownership without needing to communicate this outside of Vault. A new message template is leveraged for this enhancement with standard language, though Admins may update the template language for these notifications.
This enhancement is not applicable to legacy document workflows.
Learn more about workflows.
Reporting & Dashboards
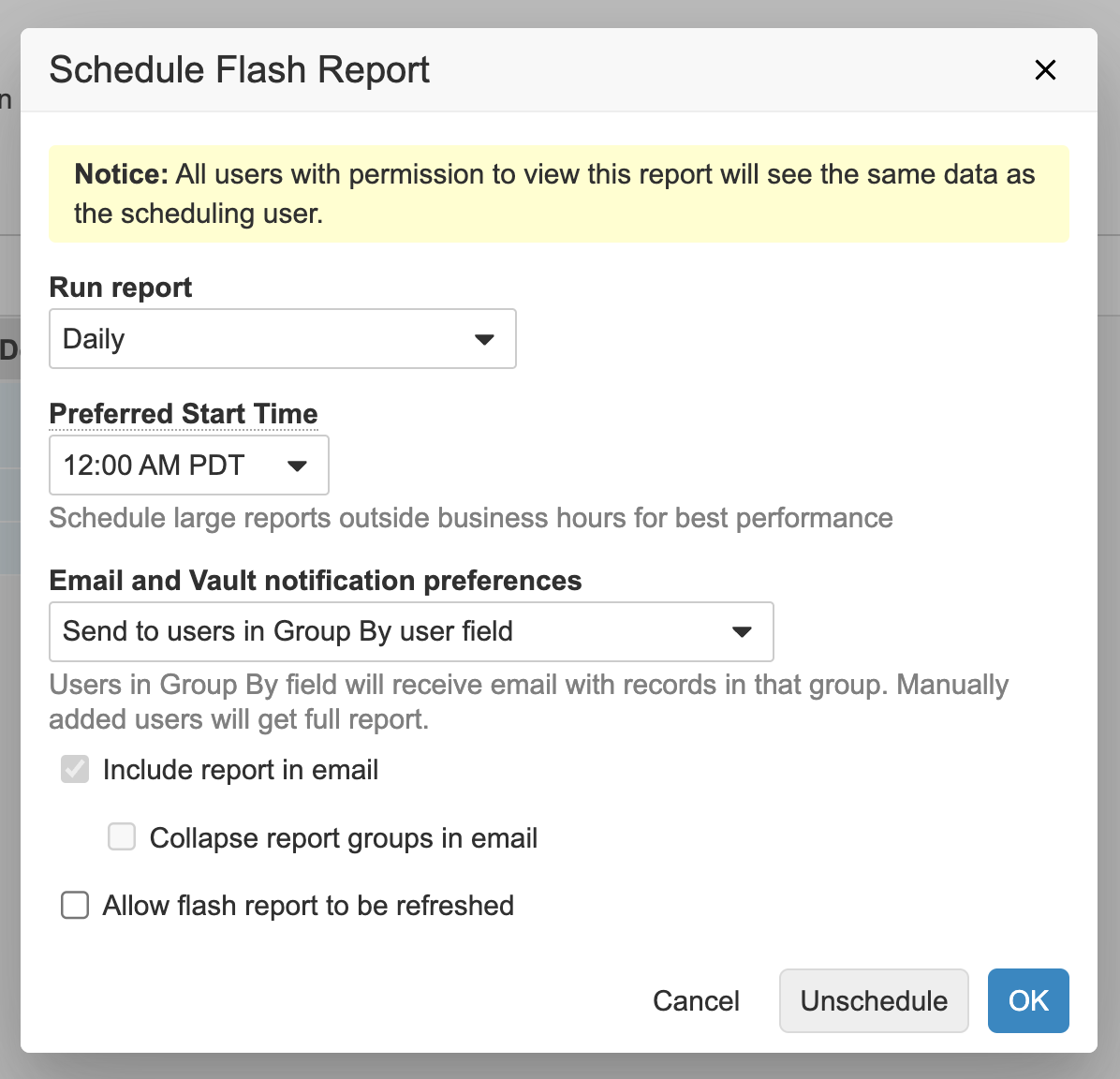
Dynamic Distribution Flash Reports
Flash reports can now be distributed to users included in the results. The users can only see the rows associated with them. This new option is available for flash reports that are grouped by a user field.
While customers often leverage flash reports to manage processes, defining the right distribution list can be a challenge. If they share the flash report broadly, some users may receive flash report emails that don’t pertain to them, but if they share the flash report too narrowly, such as a flash report for each user or manager, it may be challenging to stay under the flash report limit. This enhancement addresses this challenge by sending results only to the relevant set of users and allowing these users to only access the relevant set of records.
Users with the permission to create flash reports see this as a new option in the Schedule Flash Report dialog:
Learn more about Flash Reports.
Dynamic Distribution Flash Reports
Union-All Report Type
This feature adds a new report type to Vault: Union-All. This report type allows customers to combine objects in a single report. Unlike a join, which places objects side by side and returns rows based on matching field values, a union combines the objects into a single object with rows stacked on top of each in the same columns.
This is particularly useful in any case where customers need to report across objects as if they were a single object. For example, in Vault Quality, if a customer is moving Batch information from the Context object to the Batch object in order to leverage LIMS, the Union-All Report Type allows reporting on both objects as if they were a single object.
Union Report Type
Currency Support in Dashboards
Customers can now view local and corporate currency fields with the proper currency format in Vault Dashboard charts. Prior to this release, the currency field numbers were displayed with six decimal places. It is important for CTMS Payments and Commercial application users to view currency fields formatted properly.
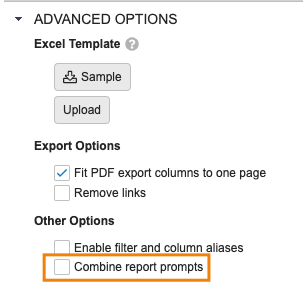
Combine Report Prompts
Users creating reports that have multiple prompts on the same data can now combine those prompts when running the report. The need to have multiple prompts on the same data is a common scenario with Multi-Pass reports in particular, where the reports combine views with overlapping components. The underlying filters should have the same object, data type, name or alias, and filter operator.
When creating reports, users will have a new option under Advanced Options to Combine report prompts:
This option was introduced with Dashboards in a prior release, and extending this to reports will simplify the running of reports for users by ensuring they don’t need to apply the same information in multiple prompts.
Learn more about Vault reporting.
Copy & Paste List of Object Names in Reports
Users are now able to copy and paste a list of object names separated by commas in input fields which take multiple objects.
It is common for customers to leverage multi-select filters, such as the In condition, where multiple items can be included. With this feature, when the In condition is on an object field, users will be able to copy and paste the correct object records into the filter from a comma-separated list.
If the names include commas, adding quotation marks around the comma-separated list will allow Vault to recognize the appropriate values. By design, Vault will ignore any duplicates.
Learn more about report filters.
Report Formula Fields Support Long Text
Customers can now use Long Text fields in report formulas by wrapping the fields in the Left() function. This function will convert the Long Text field to a normal text field which can then be used with additional formula functions. Users may return up to 250 characters when using the Left() function on a Long Text field in reports.
This feature enables full reporting capabilities on objects such as Checklists. As a result, the Checklist Response field is now available for grouping, filtering, sorting, and formula use.
Additional Columns in Report Type List Page
Customers can now view Created By, Created Date, Last Modified By, Modified Date columns which will help them to sort and find the report types.
Include Report Owner in Report MDL
Customers can now see the report owner column in the Configuration Report. This field will be populated for all the reports that are created or edited after 23R1.2 for those customers on Limited Release Vaults and 23R2 for all others.
Search & Filtering
Current User Filter
When a user filters on a field that references a user (such as Created By), Vault now provides the Current User option to dynamically filter search results by the current logged-in user.
This enhancement allows customers to create views and tabs that only include results relevant to the user performing the search. The Current User option is available as a filter value on all user-related fields within documents or records.
For example, if a Vault has a Product Owner field that references users on the Product record, an Admin could create a Saved View that filters on Product Owner = Current User. Then, each user who accesses that view would only see the Products they own.
Learn more about custom views.
Query Limits
Vault Search, VQL queries, and the Vault Java SDK Query Service now enforce limits on search terms to ensure optimal performance:
- 250 characters for individual search terms
- 225,000 characters for the complete query when using Query Service. Learn more in the Developer Release Notes.
Learn more about searching Vault.
Formulas
New Functions in Vault Formulas
Customers now have a number of useful new functions available for use in Vault Formulas. Among those that customers might find most useful are:
Contains(): This function compares two arguments, such as the value of a text field and a specific string or word, and returns TRUE if the first argument contains the second argument. Otherwise, it returns FALSE. For example,CONTAINS(Product_Type__c, "part"). If the word “part” appears anywhere in the Product Type field, the formula will return TRUE.Find(): This function now supports a third parameter(start_num)to identify where in the search text the function should begin looking for the second parameter, just as it does in Microsoft Excel.PicklistCount(): This function returns the number of selected values in a multi-values picklist. This allows you to quickly identify records where more than one value is selected in a specific list.Rand(): This function allows you to default a field to a random number, as it does in Excel, which is particularly useful when a business process dictates that you need to pull a random sampling of records. For example, you could use this function to default a hidden field upon creation or assign the random number using Entry Actions at a particular state. Once records have been assigned random numbers, you can then use filtering in a Report to pull records within a range of random numbers.BlankValue(): This function allows you to quickly use a substitute expression if an expression returns blank.
For a full listing of new functions and their use, visit Vault Help.
Advanced Functions in Vault Formulas
Vault Formulas now support the following functions:
- PriorValue
- Regex
- VLookUp
- CurrencyRate
- IsChanged
- IsNew
These functions are only available in validation rules, with the exception of CurrencyRate, which is available in all formula expressions. Additionally, the ID field will now be available in all formula expressions. Prior to 23R2, this field was only available in report formula fields.
Learn more about Vault formula functions.
Enhanced Text Formatting
The Text function can now be used to format numbers to include special characters like dashes and brackets. For example, you can format phone numbers by passing Text(9254526500, "(###) ###-####") , which returns “(925) 452-6500”.
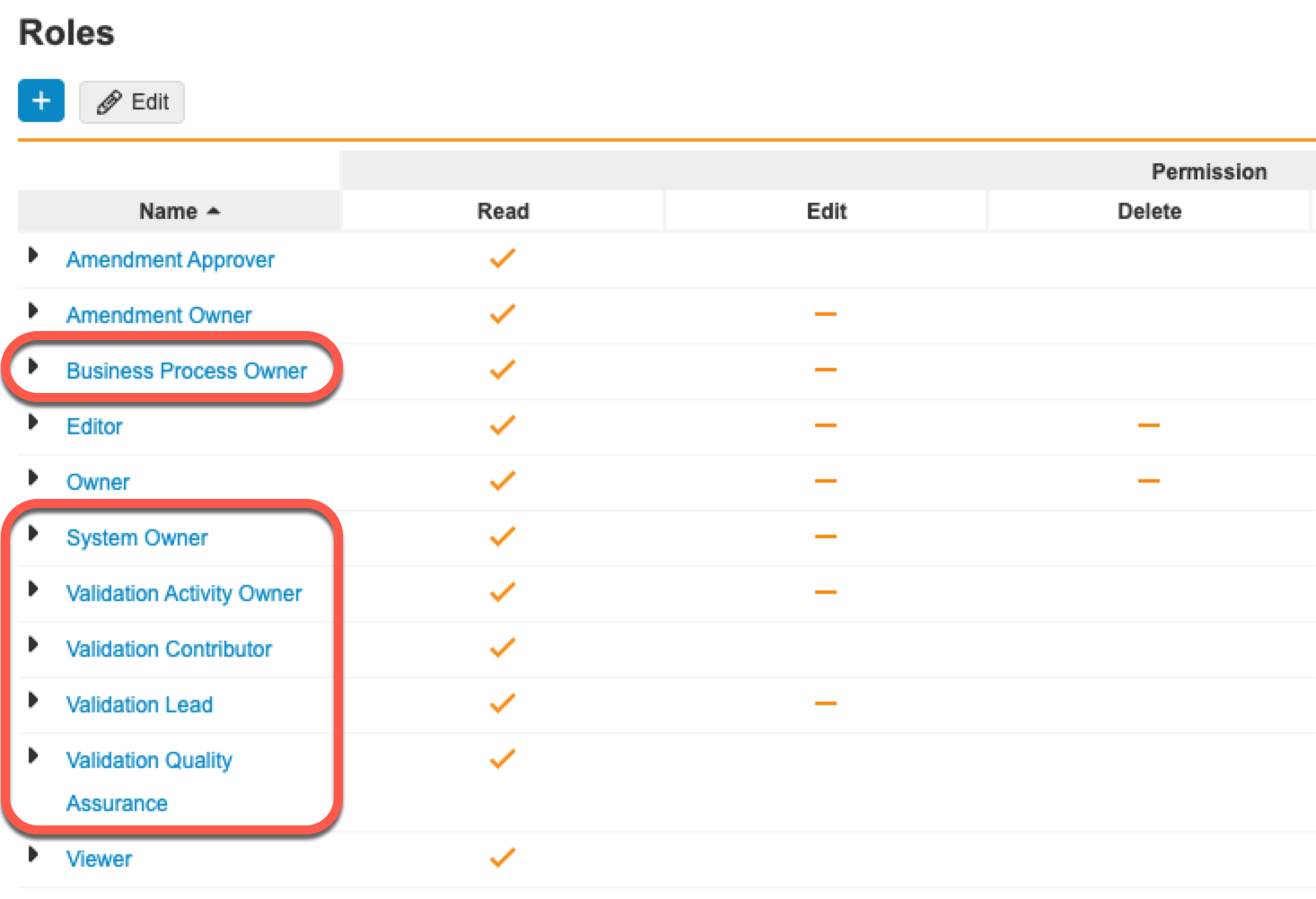
Access Control
Additional access control features are available for Vault Mobile and Vault File Manager.
Improved Permission Set Sorting for Security Profile Details Page
Permission sets are now sorted by Name on the Security Profile details page to improve searchability.
Administration
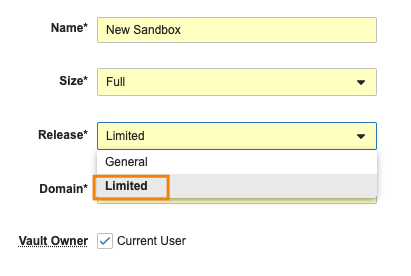
Enable Limited Release Sandbox For All Vaults
Admins are now able to provision limited release sandbox Vaults when creating new sandboxes, without needing to enable a feature flag. Admins will have the choice to select General or Limited as the Release whenever a new sandbox is created.
The ability to provision Limited Release sandboxes has previously been a feature that is only available when the Enable Limited Release Sandbox setting is enabled. This enhancement will remove that setting from Admin > General Settings, and enable this by default in Vaults.
Full Sandbox Refresh Frequency Update
With this release, Admins can now refresh a Full Sandbox once per day, if needed. With the introduction of Sandbox Sizes in the 22R3 release, the refresh limit for Full Sandboxes was once per month. An Admin can also update a Snapshot of a Full Sandbox once a day, increased from the prior frequency of once per month.
We are increasing the frequency allowed to align with the frequency on Large Sandboxes, and to provide Admins with additional flexibility in managing their Sandboxes and Sandbox Snapshots.
Learn more about sandbox administration.
Vault Platform HTTP/2 Support
Starting with Vault release 23R1.2, Vault will use HTTP/2 as the default protocol to optimize network performance. This update will enable faster page loads, but end-users are not required to upgrade or make changes. To take advantage of this update, we advise reviewing and updating your network configurations and browser policies to ensure HTTP/2.0 is allowed.
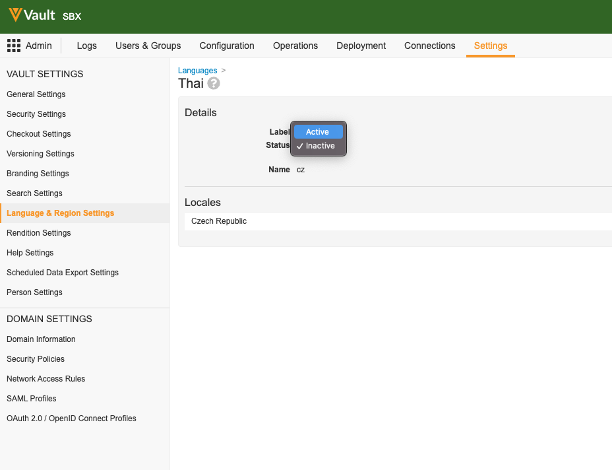
Customer-Supported Languages
Vault now allows customers to support 12 additional languages:
- Bulgarian
- Czech
- Danish
- Finnish
- Greek
- Indonesian
- Latvian
- Romanian
- Slovak
- Spanish (Mexico)
- Ukrainian
- Vietnamese
An Admin must activate any additional languages and import all translations for these languages via the Bulk Translation tool. Vault does not provide translations for these languages.
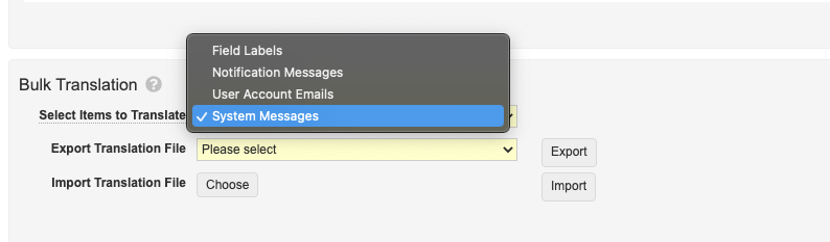
Bulk Translation Support for System Messages
Admins can now use the Bulk Translation tool to load translations for System Messages (i.e. static messages such as the “Save + Create” button label (button_save_and_create)).
EDLs: EDL Option Visible in Admin UI for All Vaults
The option to enable the Expected Document Lists (EDLs) feature from the Admin UI is now available for all Vaults. Previously, only newer Vaults had this option visible. Having the option visible for all Vaults allows for more efficient implementations of EDLs, enabling all customers to turn on this feature themselves instead of contacting Veeva Support.
Connection Exception Management
To maintain Vault performance, User Exception Messages and User Exception Items have been migrated to HVO objects and no longer support faceting in Vault UI. Additionally, records that have been marked as Inactive or are older than 180 days will be purged from Vault on a nightly basis. Customers who wish to retain this data for longer periods are encouraged to use Scheduled Data Exports or Vault Loader to extract the data for storage outside of Vault.
Learn more about user exceptions.
POD Listed in Domain Information
Admins will now see a new column for POD on the Domain Information page (under Admin > Settings) to ensure that Domain Admins are easily able to identify which POD each of their Vaults is on without needing to log-in to each environment.
Learn more about Vault domains.
Scheduled Data Export Performance Enhancements
This feature improves the performance of the Scheduled Data Exports. Vault Owners’ scheduled data exports will now include data from all fields including those hidden by document field level security.
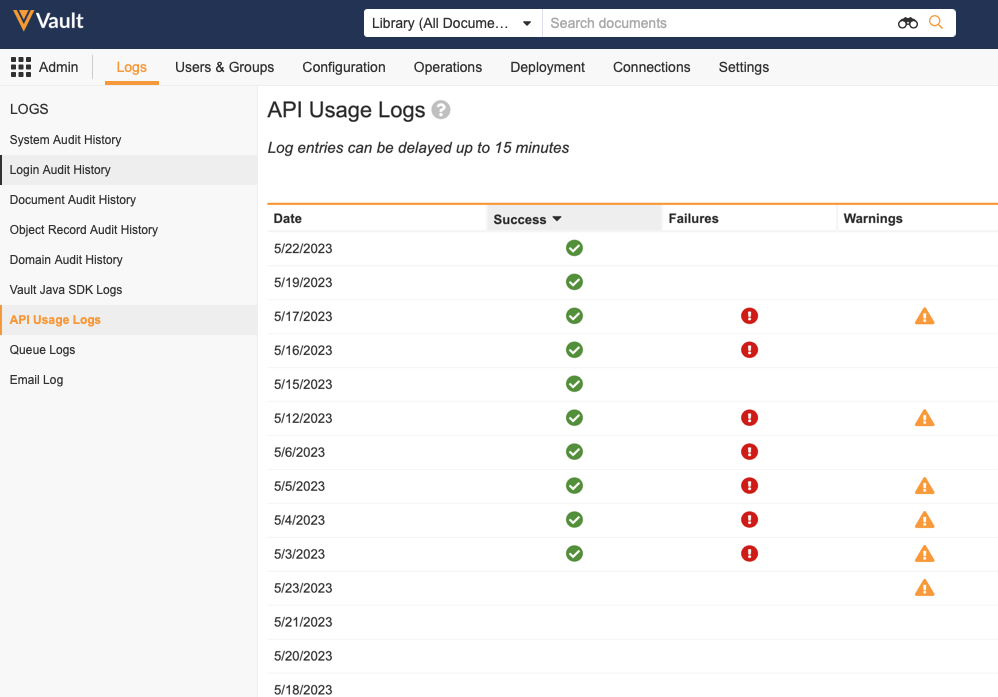
API Response Status Insights
Vault Admins can now see whether daily API Usage Logs contain Success, Failures, and Warnings. This allows Vault Admins to quickly inspect their Vault API usage and know if they need to download the log for further investigation. Insights are only available for API calls made after the release of 23R2.
Checklists
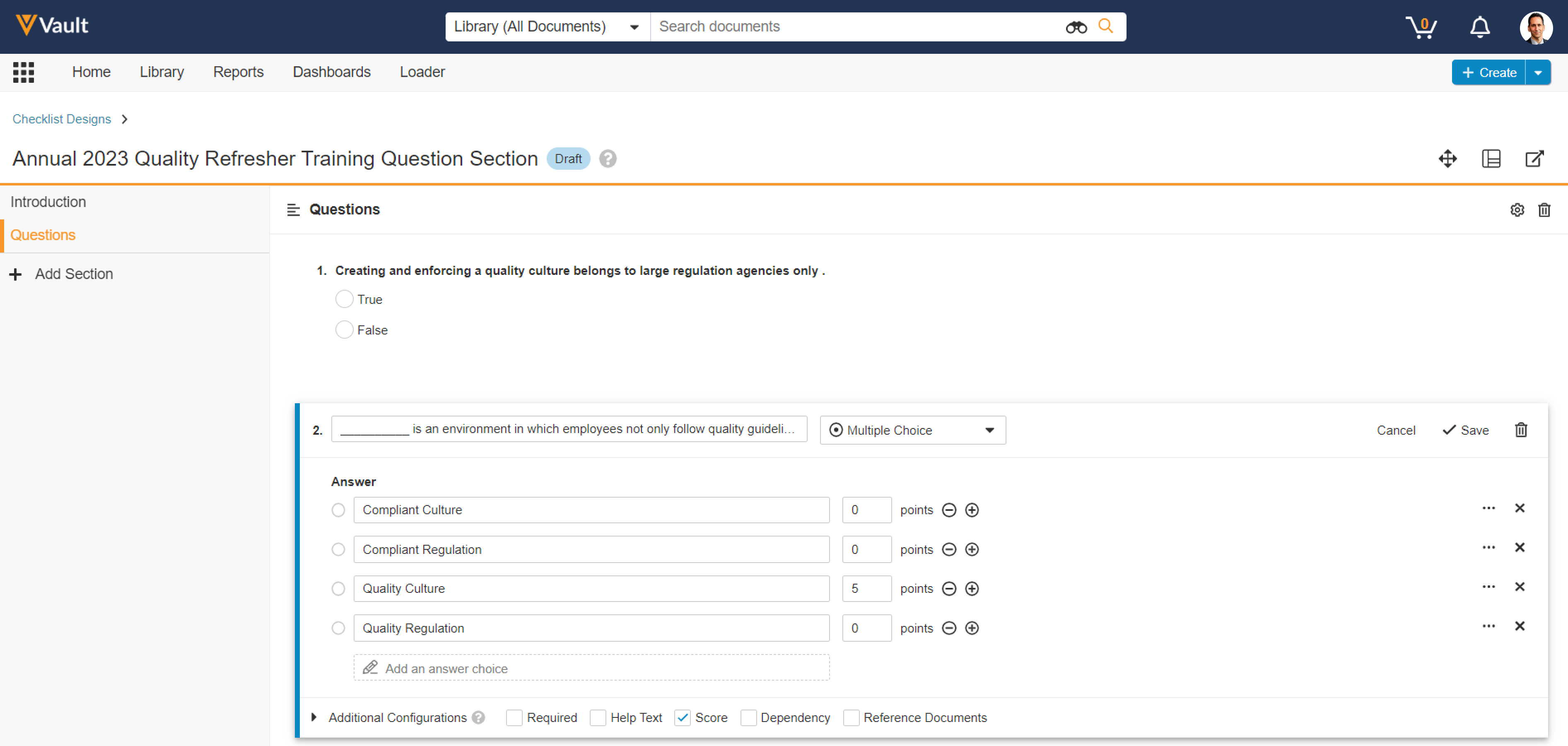
Visual Checklist Designer
Admins now have the ability to design checklists in a new visual user interface similar to what a checklist responder would use. This includes creating, editing, and deleting checklist sections, questions, answers, and dependencies.
This feature significantly increases the efficiency in creating and editing Checklist Designs, without needing to use the Checklist Design Loader. This feature particularly benefits Vault Training customers who use checklists for Quizzes, and Vault Study Startup customers who use checklists for site feasibility, though checklists are also commonly leveraged in QMS, QualityOne, and RegulatoryOne Compliance Management.
Admins can launch the new Visual Checklist Designer once a Checklist Design record is created and is in the Draft state.
This feature applies to standard object types for Section Designs, Question Designs, and Available Answer Designs, and is not currently supported for custom object types.
Visual Checklist Designer
Checklists: Sum Score
Vault will now calculate Sum Scores for checklists to provide a more relevant assessment of the overall score when using both positive and negative scoring. Sum scores will also help provide more meaningful reporting on checklists.
Prior to 23R2, the total checklist score was calculated as a percentage of the total possible score, which provides a less relevant score when negative scoring is in use.
The new Sum Score field will be enabled by default for the following objects: Checklists, Sub-checklists, and Sections.
Vault calculates the Sum Score by adding all Answer scores for multiple-choice answers together, for Sections, Sub-Checklists and the overall Checklists. If the Checklist Design is weighted, the Weight % is taken into account with each answer.
Learn more about checklist scoring and weighting.
Usability Updates
Consistent Default Navigation Behavior
For customers leveraging the Landing Tab or Preferred Tab Collection field on Users, the default navigation behavior has been updated to ensure that these preferences are respected when:
- Using the My Vaults page
- Clicking the Vault logo in the upper-left
Learn more about Landing Tabs and Tab Collections.
Vault File Manager
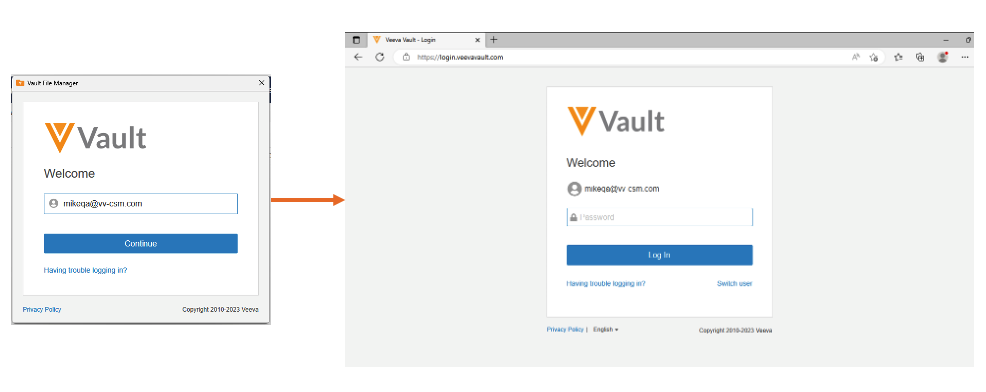
Sign in to Vault File Manager with SAML SSO
With this release, customers that previously configured SAML SSO no longer need to perform additional OAuth profile configuration to leverage Vault File Manager. There is no impact to existing authentication for Vault File Manager customers that previously configured an OAuth profile for Vault File Manager.
This enhancement makes it easier for customers to adopt Vault File Manager by removing the need for additional configuration when leveraging SSO.
With this change, when a user attempts to log-in to Vault File Manager, they are redirected to the browser to authenticate.
Learn more about Vault File Manager.
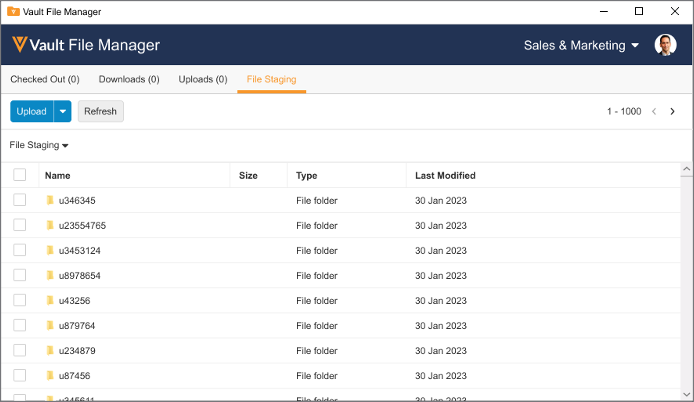
Upload to File Staging with VFM
Users can now access the File Staging server to upload documents using Vault File Manager, rather than using a separate File Transfer Protocol Secure (FTPS) client.
Many organizations may have firewalls or security policies in place that are incompatible with the use of FTPS, and this enhancement allows users to use the File Staging server without leveraging FTPS.
Learn more about Vault File Manager.
Sign in to Vault File Manager with SAML SSO & Upload to File Staging
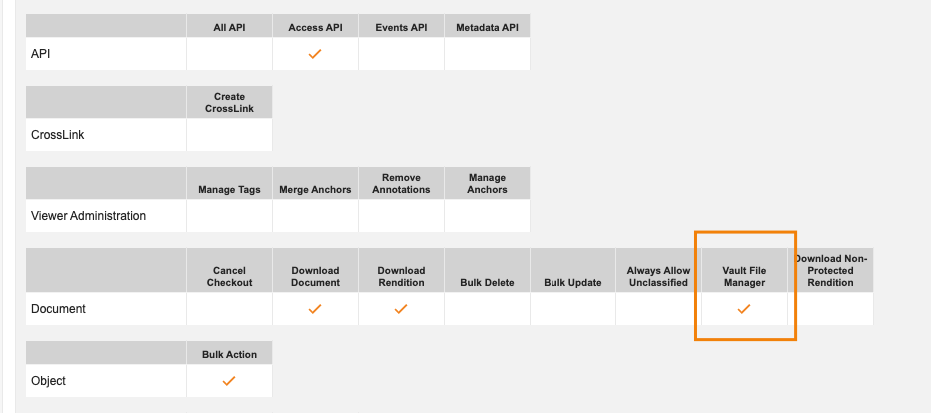
Require Vault File Manager Permission to Check in Documents
Users leveraging Vault File Manager now require the Application: Document: Vault File Manager permission to both check out and check in documents via Vault File Manager. Prior to this release, a user only needed this permission to check out. We have updated this to ensure consistency and avoid confusion going forward.
Learn more about Vault File Manager here.
Automatic Deletion of Local File Upon Check In with Vault File Manager
When a file is checked in with Vault File Manager, the local file is automatically deleted upon completion of the check in. The 23R1 feature VFM Resumable Upload & Download for Check Out & Check In updated removal of files to be only based on manual user action. We have reverted this change to match the behavior prior to 23R1.
Vault Loader
Vault Loader Command Line Support for Record Migration Mode Update & Upsert
Vault Loader Command Line users can now leverage Record Migration Mode to update and upsert records to any state while bypassing reference constraints, validation rules, entry actions, and entry criteria.
Learn more about using Record Migration Mode with the Vault Loader Command Line.
Vault Java SDK
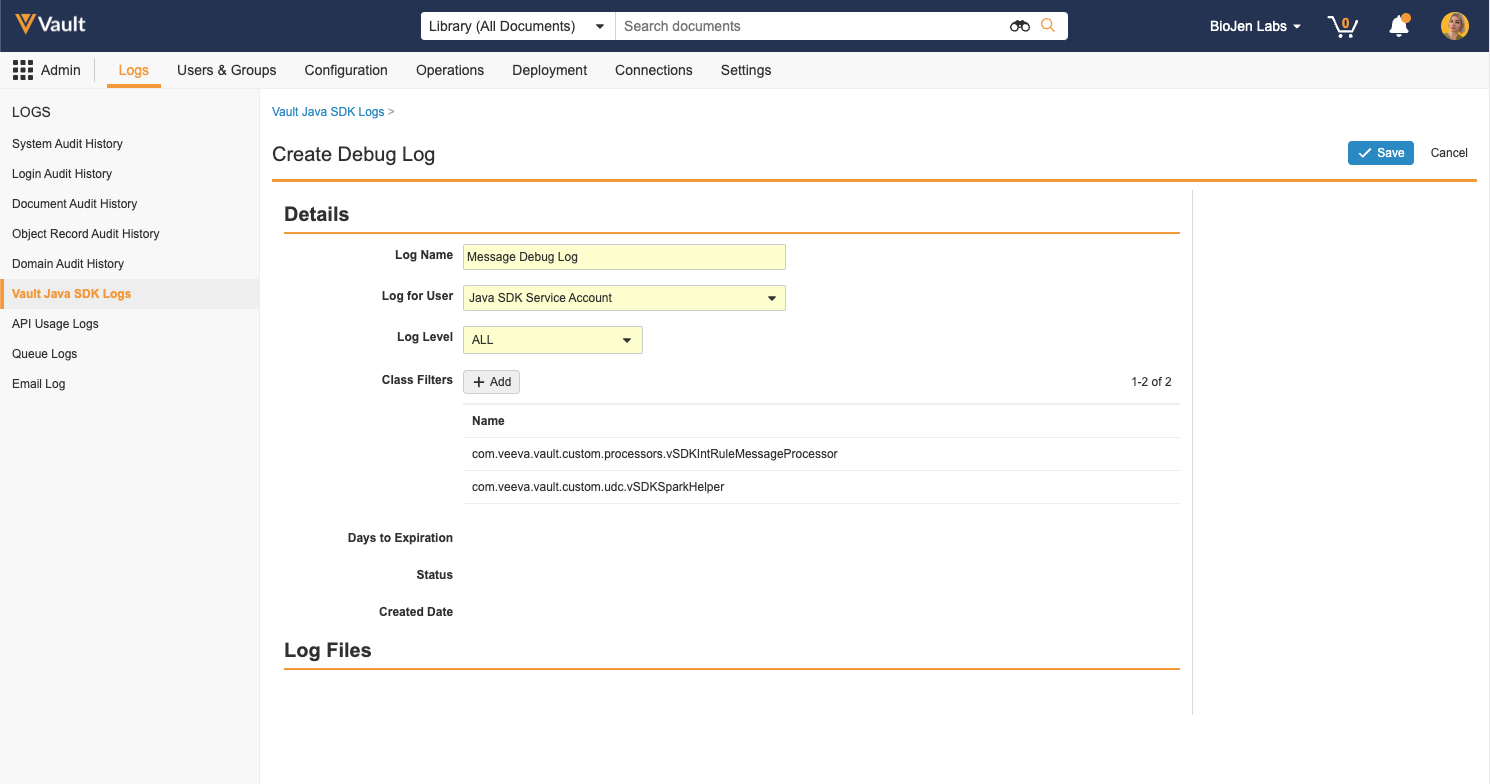
SDK Debug Log Filters
When creating SDK debug logs, Vault Admins and developers can now select additional options including Log Level and Class Filters. This allows for more granular control over what information is included in the logs and helps prevent hitting log limits when debugging. Log Level selection is inclusive of lower levels; for example, when the log level is set to WARN, debug logs will include warnings, errors, and exceptions. The default log level value is ALL. When Class Filters are applied, Vault will only capture log events from the selected class. The maximum number of class filters allowed is 10.
Learn more about Debug Logs.
Platform Data Model Changes
See 23R2 Platform Data Model Changes.
Vault Connections
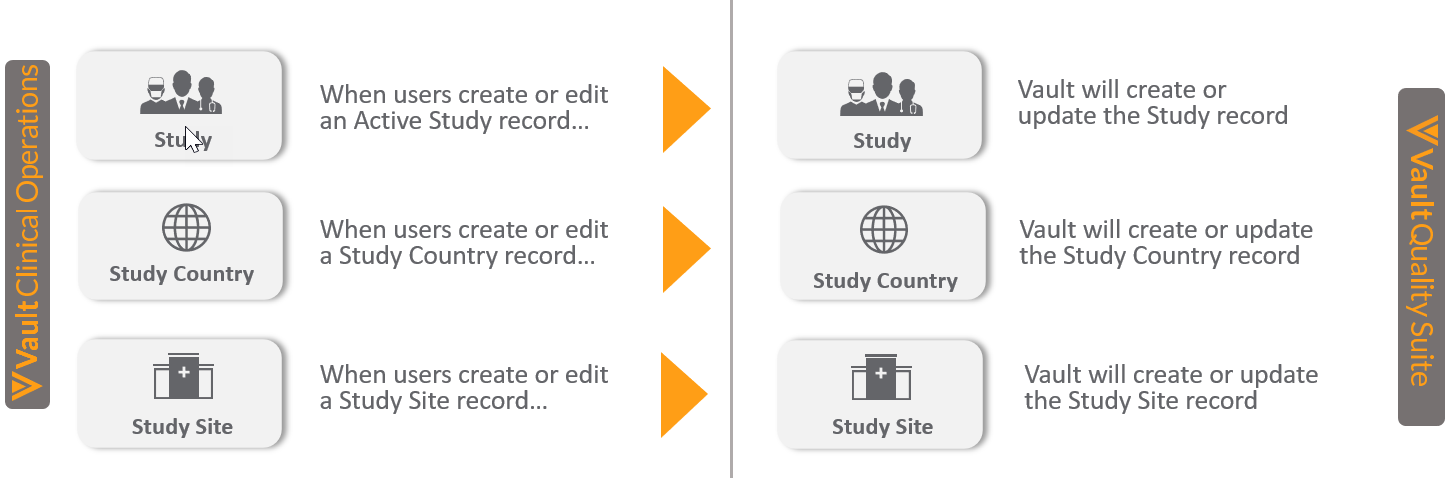
Quality-Clinical Operations: Study Data Connection
Although Clinical Operations and Quality perform different functions within an organization, Clinical Quality teams need access to study information. Study data is maintained in an organization’s Clinical Operations Vault, but Clinical Quality users work in their Quality Vault. Users can manually copy study data, but this is effort-intensive and error-prone. Building and maintaining a custom integration to automate the transfer of study information from Clinical Operations to Quality is expensive.
Vault Connections are Veeva-delivered integrations that seamlessly transfer data between Vaults. The Quality-Clinical Operations Connection automatically shares up-to-the-minute study information with an organization’s Quality Vault. A customer’s Clinical Vault continues to be the master of study information. The connection provides an automated one-way flow of study information, copying active Study, Study Country, and Study Site records to a Quality Vault whenever these records are created or updated in the Clinical Operations Vault. Studies in the Clinical Operations Vault that are in migration mode will not be copied.
This release automatically makes the Quality-Clinical Operations Connection available in every Clinical Operations and Quality Vault, but an Admin must enable the connection in both Vaults. In addition, Quality Vault Admins need to confirm the out-of-box rules that define which active study records are copied, or update the filtering rules to enforce any business constraints. The connection maps the standard Clinical Operations Vault fields in Study, Study Country, and Study Site records to the standard fields for the equivalent records in the Quality Vault. Admins can add or modify the field mappings as needed for their organizations.
This release also includes new standard join objects that track the relationship between Studies and specific Quality processes as follows:
- Quality Events
- Deviations
- Findings
- Audits
- Standalone Quality Processes
These new join objects are useful for showing the connection between studies and quality records. For example, Admins can update the page layout configurations to display a quality record’s related studies, provide new reports that include quality record and study information, and create Search Collections that allow users to search quality record and study information simultaneously.
The Quality-Clinical Operations Connection eliminates the manual effort and data integrity concerns associated with manually copying Study data between Vaults, and eliminates the need to build expensive custom integrations. The connection enables Clinical Quality teams to confidently reference up-to-date Study data in quality processes and documentation.
Clinical Operations
CTMS
Clinical CRM: Automated Clinical Activities
This feature benefits Vault CTMS customers who plan to use the Clinical Operations to Medical CRM Connection. This connection brings transparency to interactions with Health Care Providers (HCPs) through the bidirectional sharing of information. From Vault Clinical, the connection sends Clinical Activities to Medical CRM so that Medical Science Liaisons (MSLs) have insight into clinical interactions among study teams. When this feature is enabled, and the Generate Clinical Activities job is activated, Vault automatically creates and updates Clinical Activity records from Monitoring Events and/or Study Communication Logs, increasing the types of interactions that are transferred to Medical CRM and reducing duplication of effort.
This is a configurable feature. Enablement can be done separately for Monitoring Events and Study Communication Logs, providing flexibility in the type of automation performed.
Learn more about Clinical CRM.
Subject History
We’ve enhanced the handling of transferred Subjects in Clinical Operations Vaults, providing a more consistent and efficient approach to subject management during clinical trials. This update ensures that transferred Subjects prior sites are recorded in Subject History records. This allows for seamless review during monitoring events and improves overall Subject tracking.
Key improvements encompass the creation of Subject History records, a new user action for Subjects to easily update Subject Sites, and the ability to manually create historical records as needed. This feature will be automatically enabled.
Trip Report Question Display Line Breaks
This feature enhances the way Vault displays Trip Report Questions by including the same spacing and line breaks as entered into the Question Text field, improving overall readability. It is automatically enabled in Clinical Operations Vaults.
Clinical Operations to CDMS Connection: Support for Screening Subjects Outside of CDMS
This feature updates the Clinical Operations to CDMS Connection to support Subject handling in Clinical Operations Vaults prior to Subject creation in CDMS. Previously, if users created Subjects in the Clinical Operations Vault outside of the connection, there was no link to the record in CDMS. Now, the connection uses additional logic to determine if the Subject record in CDMS already exists in the Clinical Operations Vault. If a matching record is found, the Clinical Operations to CDMS Connection links the records. Otherwise, Vault creates a new Subject record in the Clinical Operations Vault. Once the Subject records are linked, the Clinical Operations Vault is updated based on data in CDMS.
Customers who track Subjects in a Clinical Operations Vault, but not in CDMS, prior to enrollment (such as pre-screening/screening) will benefit from this enhancement. It is automatically enabled, but can be disabled in Application Settings.
Learn more about the Clinical Operations to CDMS Vault Connection.
Clinical Operations to CDMS Connection: Single-Click Navigation to EDC Casebooks
This feature introduces a new standard EDC Casebook Link field (casebook_url__v) that is available on Subject, Visit, Monitored Subject, and Monitored Visit objects in Clinical Operations Vaults. This standard field does not require manual updates to the field formula and links directly to the relevant review page in the CDMS Vault where the casebook is managed, whether the Clinical Operations Vault is connected to a single or multiple CDMS Vaults.
Vaults with the existing custom Casebook Link field on Subject, Visit, Monitored Subject, or Monitored Visit objects in Clinical Operations Vaults should remove this field from page layouts and reports and use the standard field instead.
Learn more about the Clinical Operations to CDMS Vault Connection.
Clinical Operations to CDMS Connection: Enhanced Job Labels
This feature will provide more useful information for inbound jobs in the Clinical Operations Vault. The inbound jobs will contain additional details about the connection and the integration which will simplify troubleshooting and monitoring for administrators. The job label will now include information about which connection and integration is impacted. This feature will be particularly useful for customers who connect with multiple CDMS Vaults.
CTMS, Vault Payments
Subject Visit Method
We’re introducing a new feature that incorporates the Visit Method as an additional metadata element on Subject Visit records in Clinical Operations Vaults. This enhancement is a response to the growing trend of trial decentralization, which brings clinical trial activities directly to the patients, thus improving patient experience and speeding up the trial process.
The Visit Method, which can vary from on-site to remote or even video conferencing, plays a crucial role in determining payment amounts in Vault Payments. This flexibility in payment adjustments is vital for sponsors to manage their budgets effectively, especially as the same Visit could generate different payments depending on the Visit Method.
This feature is designed to operate effectively with both new and existing Subject Visits and Fees. As the Subject Visit Method field is populated, the feature will be activated, contributing to improved workflow and data management processes.
eTMF
TMF Transfer: Match on Site Name
This feature streamlines the transfer when in-scope Sites already exist in the Target Vault by adding logic to automatically identify and map Study Country and Site name records between Target and Source Vaults. Site matching is based on the combination of Study Country and Site name.
This is useful when sites already exist in the Target Vault prior to transfer. Previously, this required manual mapping of records prior to transfer and could result in potential duplication of Site records in the Target Vault.
This feature is automatically enabled. Learn more about TMF Transfer.
TMF Transfer: Match on Country Code
When doing a TMF Transfer between a source and target Vault, errors may occur due to the differences in each Vault’s country__v records. TMF Transfer creates Study Country records in the target Vault and must set the country__v field on these records upon creation. This field is set based on the source Vault record’s value but must use target country__v records to populate the field. Transfer failures may occur due to differences in country__v records between Vaults.
Previously, this was resolved by either renaming countries or mapping country__v records between source and target Vaults which prolonged transfer timelines. In many cases, target and source Vaults will have different Country record names, (e.g. Russia instead of The Russian Federation) which would trigger an error in the transfer or require prior customer mapping.
This TMF Transfer enhancement will mitigate this by looking at the Country Code (code__sys). Countries with a matching Country Code will be used regardless of what the actual Country record name is in both Vaults.
Study Metadata Extraction: Test Model from Production
Customers will now have the ability to test a Metadata Extraction model using their Production data from a Sandbox or Pre-release environment, similar to how they can currently train an Auto-classification model. To facilitate this, an action called Test Model from Production will be made available, which will allow customers to test the model using a training window start date or a CSV file containing a list of document IDs.
This will allow customers to:
- Evaluate a Metadata Extraction model in a sandbox environment prior to its deployment in the production environment
- Gauge the model’s effectiveness without the need to directly train a model in a production environment
Study Metadata Extraction: Bulk Pipeline Support
This feature enhances the Study Metadata Extraction feature to support documents created via the bulk pipeline, such as through an API or email ingestion. The feature will be especially valuable for customers using one of our clinical email processors, as the TMF Bot can now set the Study field for documents created from email messages or email attachments.
This feature will be auto-on enabled in Vaults that have a Metadata Extraction Trained Model in the deployed state. This ensures that the feature will be readily available for early adopters, and auto-on with future enablement of metadata extraction by other customers.
Prediction Metrics for Study Metadata Extraction
The Prediction Metric records provide a summary of predictions made by a deployed TMF Bot Trained Model to monitor the model’s performance. This feature aims to enhance the current Prediction Metrics to display the Metadata Extraction model’s performance data.
In order to distinguish between the different Trained Model types (Auto-classification and Metadata Extraction) a new field Trained Model Type will be added. Additionally, to make the Prediction Metrics less specific to the Auto-classification Trained Model, the field Auto-classification Success Rate will be inactivated and a new field will be added called Success Rate. The previous values for the Auto-classification Success Rate will be copied to the new field Success Rate.
Moreover, each combination of Trained Model and Field Extracted will have one set of Metadata Extraction prediction records. This means that in a Vault with both an Auto-classification Trained Model and a Metadata Extraction Trained Model, prediction metrics will be calculated for Auto-classification and Study extraction.
These changes will enable the Prediction Model to be utilized for future enhancements to the TMF Bot capabilities.
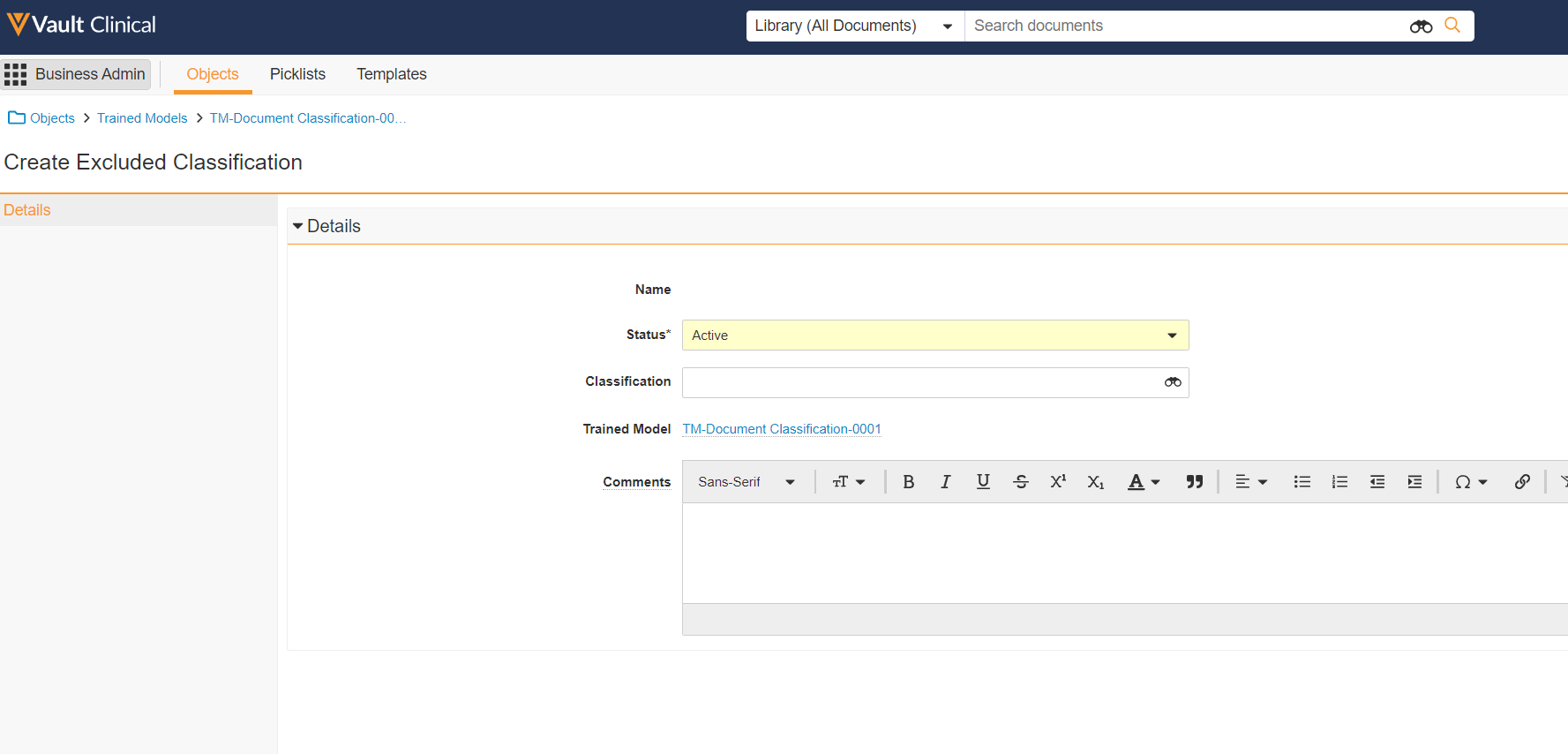
Excluded Classifications for Trained Models
This feature allows Admins to define classifications that will be excluded from trained models of any type. Specified classifications will be omitted from all extraction, training, and testing during model deployment. Additionally, later predictions by the TMF Bot, made as it processes documents, will not be actioned if a document is in or predicted to be in an excluded classification.
This allows customers to prevent automation, by the TMF Bot, from acting on classifications where it is known or expected to perform poorly, or where a business process requires that humans not rely on automation. For example, for documents that are expected to be authored directly in Vault.
Users can specify excluded classifications before or after a model is trained. If one is added after the model’s training, the model is not automatically re-trained; however the TMF Bot will not take any action against documents of the excluded classifications.
Learn more about TMF Bot auto-classification.
TMF Viewer UI Enhancements
Two changes will give users greater flexibility to quickly review documents in the TMF Viewer. First, users will now be able to display up to 15 columns at once, where previously they were limited to 9. Secondly, when text within a cell exceeds column width there will now be an action under the action menu to Wrap Text, just like in the Library.
Study Training
The Study Training application, released in 22R2, enables organizations to manage training for sponsors, CROs, and site staff. See the Training section for information on new Study Training functionality in this release.
Study Startup
Custom Email Senders for Surveys
The Feasibility Surveys functionality within Study Startup now allows for the flexibility of modifying the email sender address and includes customer branding.
Today, surveys are sent from a generic Vault email address which in some cases can be filtered out. This feature allows the customization of the sender email giving sites a more personalized experience while also allowing the recipient to reach out to the sender in case they have any questions. It also includes the ability to add users in copy and blind copy.
An Admin must enable this feature and add new fields on the Study Person object page layout.
Study Startup, eTMF
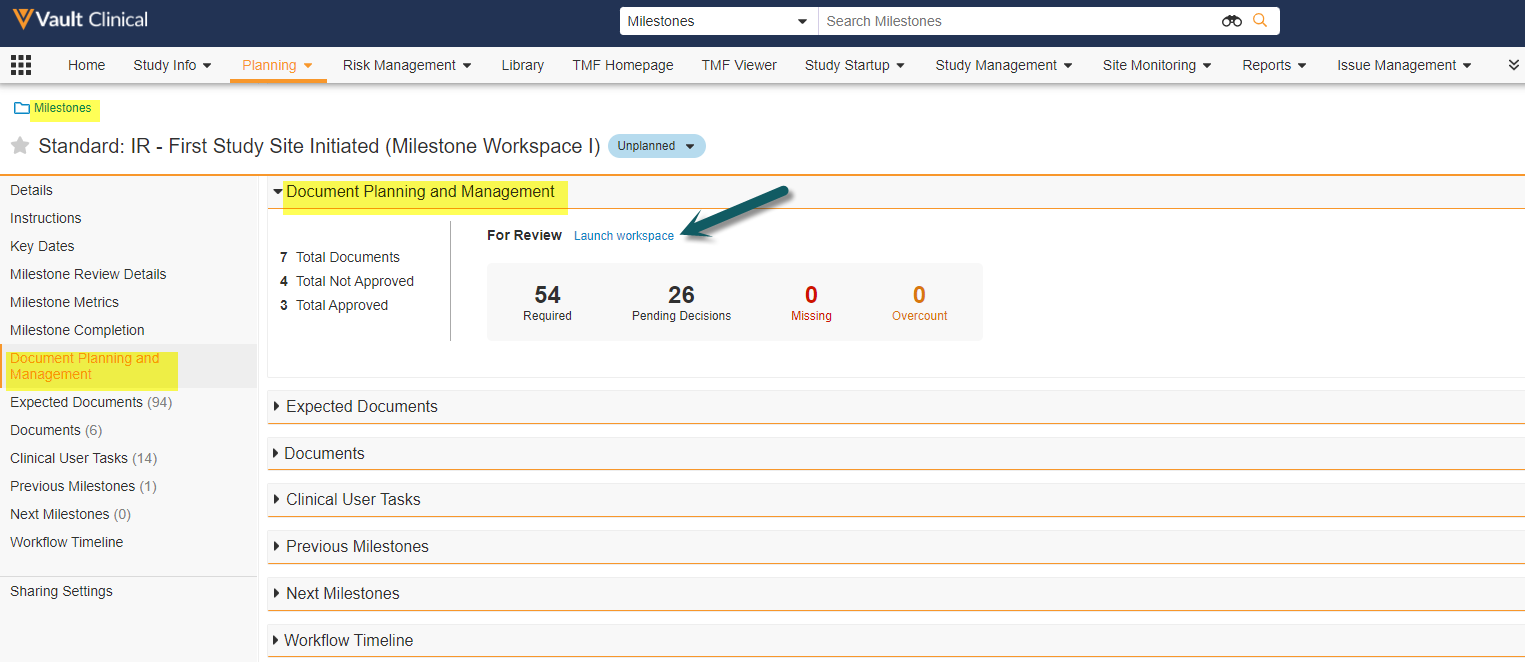
Milestone Workspace
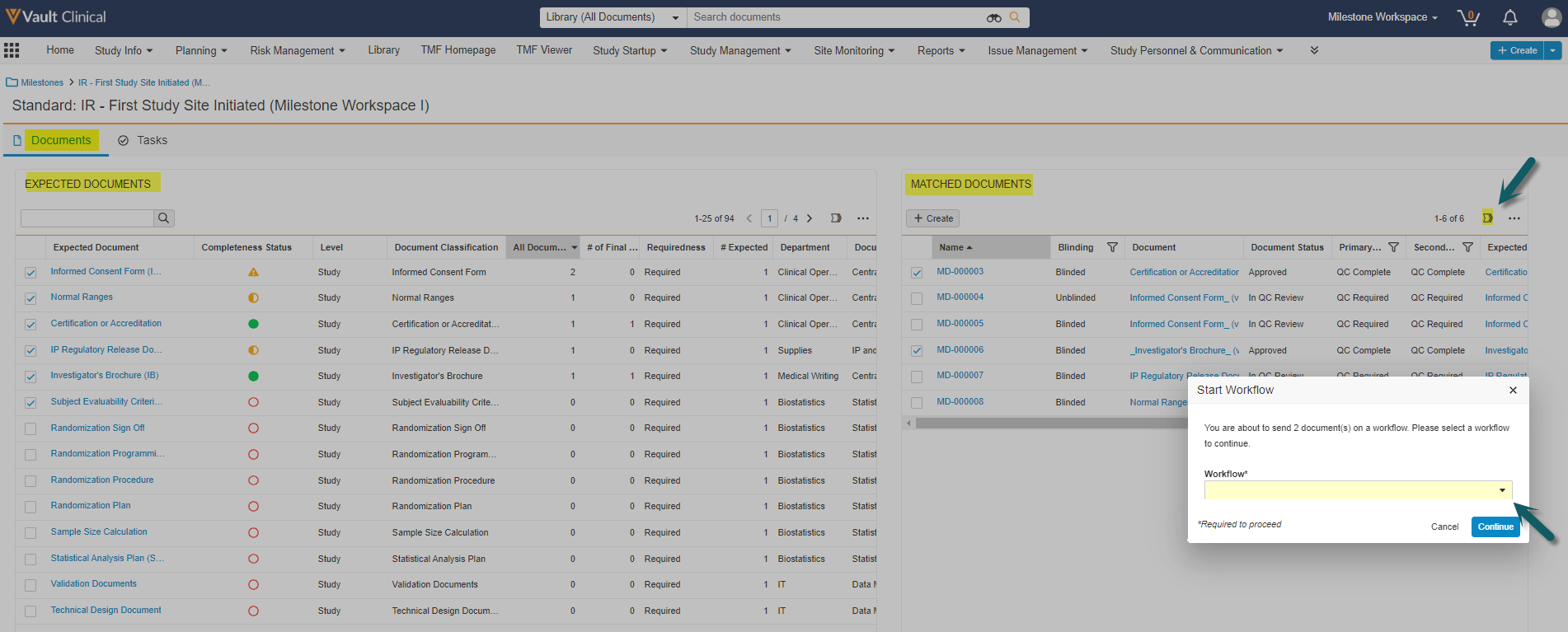
This feature creates a new section on the Milestone page layout. The section, called Document Planning and Management, summarizes the status of a milestone’s related documents and expected documents. This section also includes a link to a workspace page with two data grids (tables) for the milestone’s Expected Documents (at left) and their Matched Documents (at right). These tables support searching, column filtering, reviewing, and updating records, and also allow you to send documents and/or expected documents on workflows from a single page. A second page lets you view and work with Clinical User Tasks.
Study team members will benefit greatly from this feature, as it provides them with a better overview of the milestone activities and allows for a more efficient way to organize, plan and execute document actions and expected document reviews.
Study team members are responsible for managing a large number of expected documents and documents and the assigning of those records, often in workflows, to various functional owners for review. The logical place to organize the management of documents and expected documents is the milestone. Today these users face some of the following limitations:
- Expected documents and documents are related, but located in different sections of the page.
- The large volume of data requires users to navigate different object record lists (such as milestones, expected documents, and clinical tasks) and the library to better view and filter results.
As workarounds to the above constraints, customers today rely on reports, saved views, search collections and excel trackers to manage this process and have a good overview of the milestone activities.
This enhancement will solve these challenges by displaying a unified user friendly view and allowing users to launch multi-document workflows and bulk object record workflows for Expected Documents all from one place:
This feature is Auto-on for all Clinical Operations Vault customers, however to utilize it an Admin must update the Milestone page layout to include the new Document Planning and Management section and make it visible to end-users so they can launch the Milestone Workspace. Additionally, an Admin must also update the permission sets for Milestone Documents and the Milestone Document field on the EDL Item.
Learn more about working with Milestones.
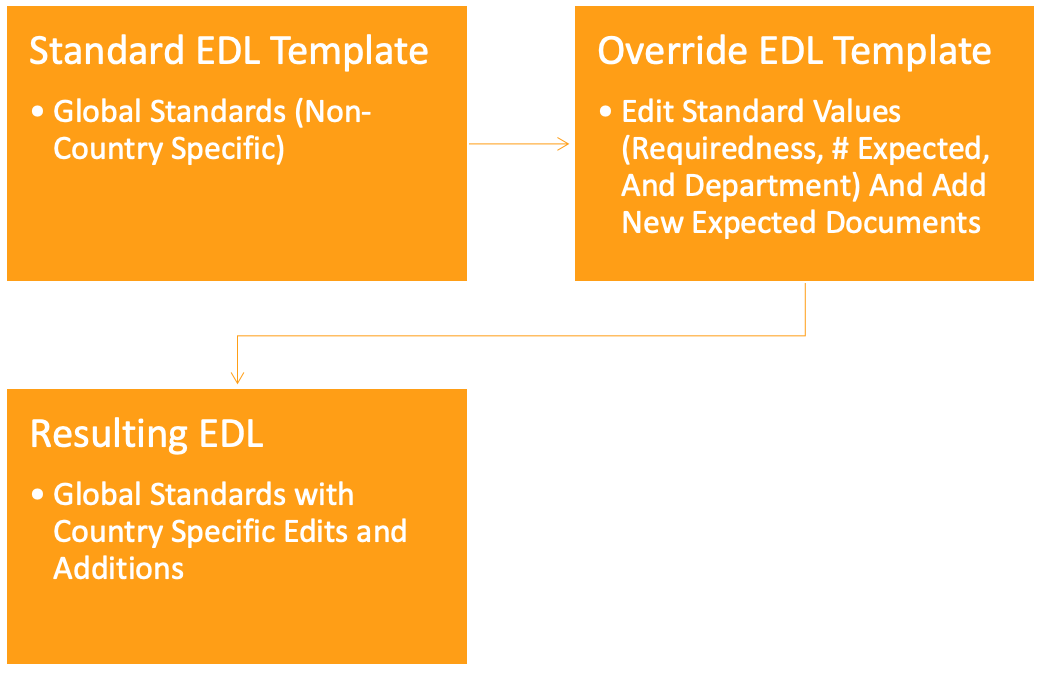
Clinical EDL Template Overrides
This feature introduces a more streamlined approach to managing country intelligence. It significantly reduces the need for duplicate expected document templates and provides greater flexibility to countries to define milestone types for Study-level expected documents, making it a valuable tool for enhancing global operations and addressing the diverse regional filing practices.
The key innovation is the introduction of Override Expected Document List (EDL) templates. These templates allow for greater customization, addressing the unique challenges faced by multinational customers, especially when country-specific ethics submissions require a select number of Study-level expected documents.
The following diagram describes the way this feature works. The process begins with a standard EDL Template that applies non-country-specific global standards. It then incorporates an Override EDL Template for country-specific modifications and additions, resulting in a final EDL that combines global standards with country-specific elements.
Learn more about EDL Administration.
Vault Payments
Fee Schedules for Study Organizations
With Vault Payments, customers can now efficiently manage different Fee Schedules for multiple Payees on the same Site. This enhancement addresses the need to divide fees between various organizations at a site when there are separate contracts for main hospitals, labs, or pharmacies in some countries. Previously, customers had to define the Payee split individually for each Fee.
The new Fee Schedules for Organizations feature allows users to manage entire Fee Schedules for multiple payees, each with its own effective dates. This provides greater flexibility and reduces administrative burden. Additionally, the existing Payee Overrides functionality can be utilized as needed for further flexibility.
Site Connect
Features in this section are included in the Digital Trials Platform Release Impact Assessment.
Document Exchange for Additional Vault Clinical Docs
Site Connect customers can now send Relevant External Communications document types to Sites. The configuration for any relevant document types must be updated so that they map to the new Relevant External Communications Vault Clinical Docs artifact.
Learn more about Site Connect.
Protect Universal Site Number (USN) Changes
Vault Clinical Operations will now prevent users from making changes to the Universal Site Number (USN) on an Organization if that Study Site has a Connected Study Invitation and is in the Pending, Paused or Active lifecycle state. Additionally, users will be unable to edit the Organization field on Study Sites that have a Connected Study Invitation in the Pending, Paused, or Active lifecycle state. The user will encounter an error message if they try to update these fields in the above cases. These new guardrails will ensure that updates to the Organization and Universal Site Number (USN) do not lead to a mismatch between the connected Study Site and the data being tracked in Clinical Operations.
Learn more about Site Connect.
SiteVault Inviter
With this feature, Sponsors/CROs will be able to invite Sites to sign up for SiteVault, initiating the process from within Clinical Operations Vaults. Within Clinical Operations Vaults, customers will be able to track the status of the signup request from delivery through to its completion and will be notified of the Site’s Universal Site Number (USN) information.
Learn more about Veeva SiteVault.
ePRO Survey Report for Monitors
Previously, Users could only access Site ePRO data by either requesting the data directly from the site, which can cause delays, or by having access to the MyVeeva Studio, which can cause security concerns. With this feature, Users can now download Site ePRO data directly from Vault Clinical using a new user action, Generate ePRO Survey Report, that is available on Study Sites and Monitoring Events. This action creates a zip file containing the following:
- Full site survey data
- Full site adherence data
- Full site audit data
- Aggregated compliance data
- Site access report
Notifications alert Users when the ePRO data export is ready for download or if errors were encountered. This feature is automatically available in Vaults where ePRO is enabled. Studies that have ePRO as a Connected Study Type, have the ePRO code populated, and have sites with Connected Study Agreements will have the user action visible when enabled. Users with all Object Action permissions for Study Site and Monitoring Event can run the Generate ePRO Monitor Export action, but this configuration can be updated as needed.
This feature saves time by eliminating the need to request ePRO data from sites and provides more meaningful information on survey completion and compliance.
Display Envelope Tasks in Clinical Operations Homepages
This feature refactors the Clinical Operations homepage task widgets to improve performance and include Envelope (One Workflow) tasks for multi-documents workflows. With the addition of the Envelope tasks, users of Clinical Operations homepages will be able to see all tasks requiring attention in one place and can take action on those directly from the homepage, saving navigation time.
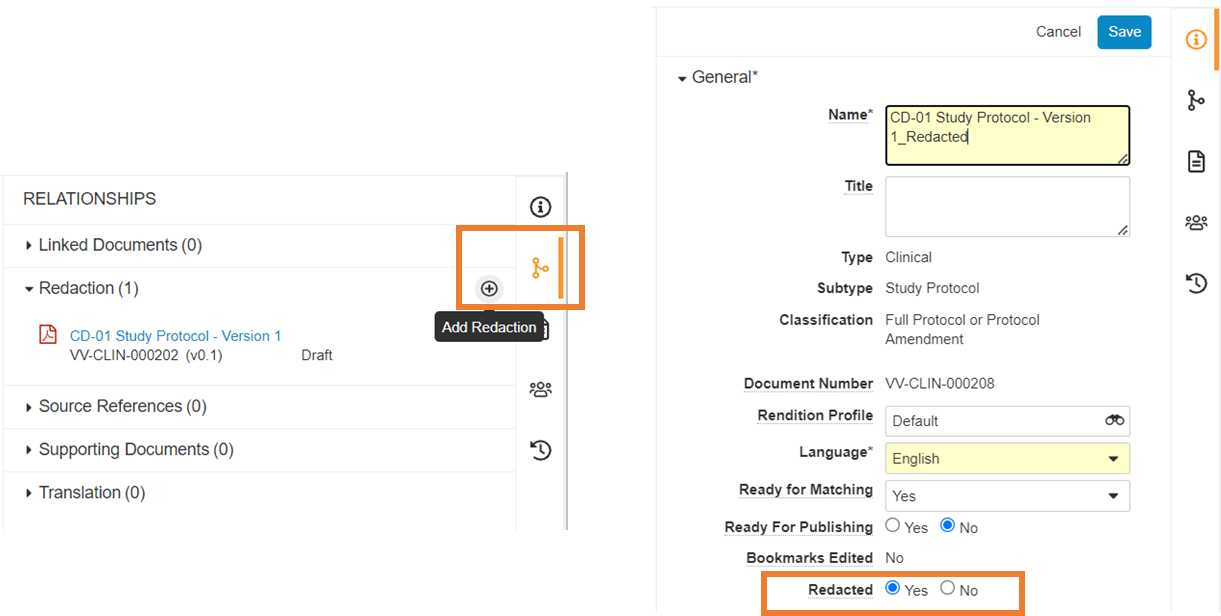
Redacted Document Field
Transparency in clinical trials is increasingly requested by regulatory authorities (e.g. EU CTR) as it increases patient knowledge of available medications as well as potential treatment in development. That said, Sponsors still have the obligation to protect personal data and commercial confidential information (CCI). Consequently, they may have to share a “for publication” document version with authorities where personal data & CCI are expunged, and a “not for publication” version restricted to authorities. Several Customers have decided to store both document versions in their Vault.
To better support Sponsor transparency processes, Vault Clinical Operations now provides a redacted document field (yes/no). It helps the clinical study teams to clearly track and identify document copies where the personal data and CCI have been redacted. The field could be leveraged for reporting and used to filter documents.
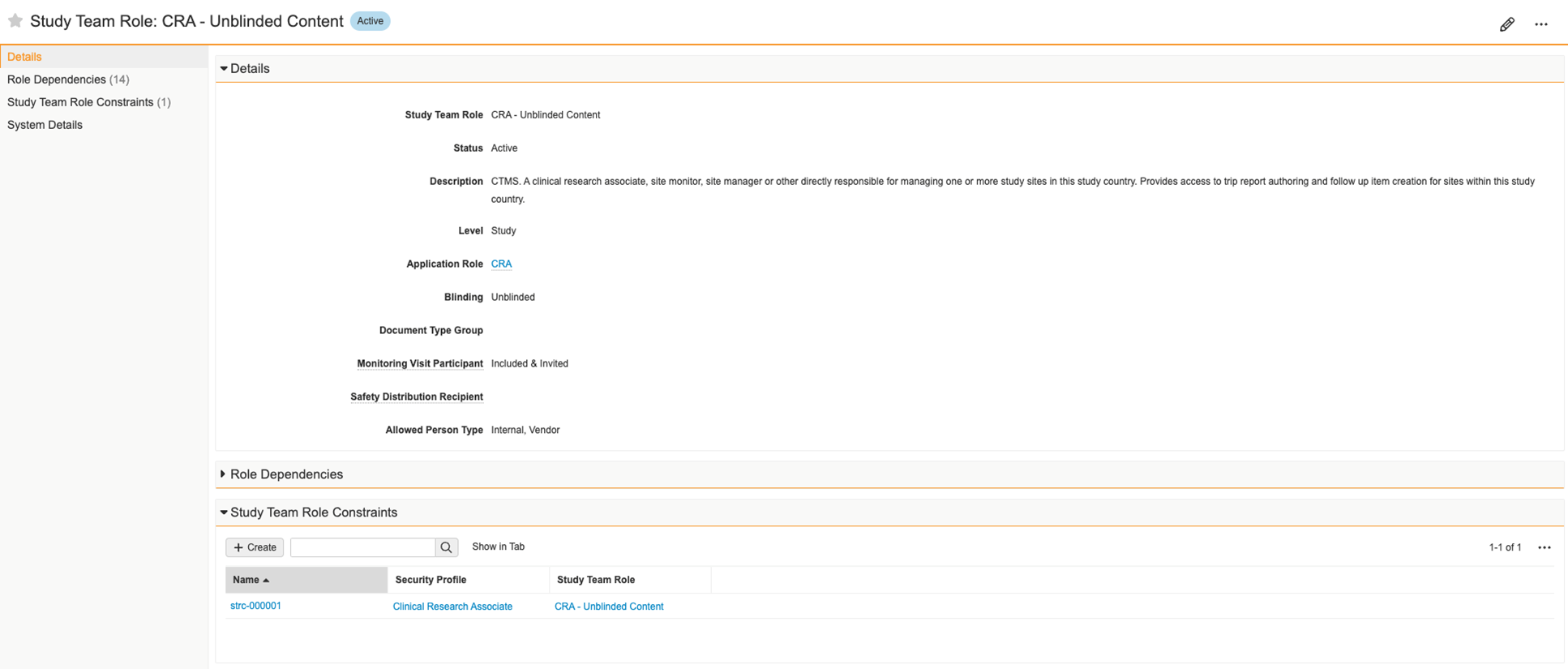
Study Person Role Constraints
In Clinical Operations Vaults, Study Person records are used to track study rosters and to grant users study-specific access. This access is controlled through the assignment of Study Team Role. Providing an incorrect Study Team Role is a compliance risk and could result in inappropriate or insufficient access to Study records.
With this feature, Clinical Operations Vaults now support the restriction of Study Team Role assignment based on Person Type or Security Profile. Validation checks on record save prevent users from creating Study Person records with disallowed combinations. Additionally, a new application-controlled field is available that filters for permitted Study Team Roles during record creation.
When enabled, this feature reduces the risk of providing incorrect Study-specific access. Enablement requires configuration and includes the following:
- A new field Allowed Person Type on the Study Team Role object to restrict based on Person Type
- A new object Study Team Role Constraint on the Study Team Role object to restrict based on Security Profile
- A new application-controlled field for Study Team Role that can replace the existing Study Team Role field on the Study Person object
Learn more about managing Study Person records.
Enhanced Assigned To Control on Quality Issue
This feature enhances the Assigned To field filtering functionality on Quality Issues. When leveraging the app control Assigned To field on Quality Issues, users must have view permission to appear in the drop down.
This enhancement is automatically enabled for customers using the application-controlled Assigned To field.
Learn more about Quality Issues in Clinical Operations.
Editable Study Country Name
This feature enhances control over your Study Country name field. It provides a balance between manual control and automated consistency for your Study Country data management.
The Enable Study Country System-Managed Name Field setting can be temporarily disabled from Vault Settings, granting end users the flexibility to manually update the Study Country names as needed. This feature is particularly useful when you need to align existing Study Country records with updated country names. When re-enabled, the system resumes managing the Study Country names, ensuring data consistency.
When the system-managed setting is disabled, users will be required to manually populate the name for any new Study Country records.
Learn more about managing Study Countries.
Clinical Operations Data Model Changes
See 23R2 Clinical Operations Data Model Changes.
Commercial
PromoMats
Modular Content Document Info Panel: Annotation Creation
With this release, users are able to create a link annotation to a Text Asset (Claim/Reusable Text) and/or document directly from the Modular Content Document Info Panel. Content Module Assets not identified with Enhanced Suggest Links now have an ‘Add Link’ action next to them that brings the user directly into the add link annotation mode. This simplifies the process of linking Content Module Assets to areas within the promotional material that were not automatically linked, and is particularly useful when linking Content Module Assets to images on promotional material.
Modular Content Document Info Panel: Annotation Creation
Text Asset Substantiation Lifecycle Entry Criteria
In Vault PromoMats, all types of Text Assets require substantiation (reference document or anchor) to allow the record to enter a steady state. Reusable Text, introduced in 22R3, generally does not require substantiation to be added to every record. This functionality introduces flexibility in selecting what types of Text Asset require a reference and at which point in their lifecycle users must link substantiation.
Learn more about Text Assets.
Text Asset Substantiation Lifecycle Entry Criteria
Optional Filters in Select Claim Dialog
When using manual Claim Linking, the filters for records (for example, Product or Country) are now optional in the Select Target dialog box. In addition, any custom object reference or picklist filters have become optional as well. The standard filter, Lifecycle State > equals > Approved, remains required. Vault continues to apply record filters by default, but users have the option to remove or modify them when selecting a target.
Optional Filters in Select Claims Dialog
Set Match Text Field Length Minimum Value to 5
This feature decreases the minimum number of required characters on the Text Asset object’s Match Text field to five (5). Previously, the Match Text field required a minimum of 20 characters, because a claim is in most cases longer than 20 characters. Now that Text Assets are used for reusable text as well as claims, there was a need to reduce the minimum number of characters required.
Learn more about Text Assets.
Document Order Support for Portal Document Widgets
The new Portal User Interface, introduced in 23R1, now supports document ordering in custom document widgets. When editing a Portal, Portal Editors now have the option to drag and drop documents to create their desired display order. This gives Portal Editors greater flexibility when curating their content in a Portal.
Learn more about Portals.
Increase Number of Portal Content filters
Brand Portals allow customers to distribute content efficiently within their teams. With this release, customers can add up to 24 Content Filters to a Portal, providing more flexibility in how the content is curated and improving the user experience for finding content. This increase in content filters is only available on the new Portal User Interface.
Increase Number of Portal Content Filters
Learn more about Portals.
AIRs Support for SVG & WebP Files
Automated Image Renditions (AIRs) functionality now supports SVG and WebP images. You can now use SVG and WebP images as the source file to transpose them to other supported rendition types using AIRs, including WebP. This feature enhances the DAM capabilities within PromoMats to allow content creators to use additional file types that are particularly well suited to web development in Automated Image Renditions.
Learn more about Automated Image Renditions.
Controlled Vocabularies for PromoMats
Two new objects are available called Controlled Vocabularies and Constraints. When combined with field reference constraints, customers can use these objects to restrict the available options in a dropdown with respect to document or object metadata. This functionality is similar to that of field dependencies but can be applied in OneWorkflow task prompts.
Controlled Vocabularies for PromoMats: Feature Demo
Controlled Vocabularies for PromoMats: Configuration
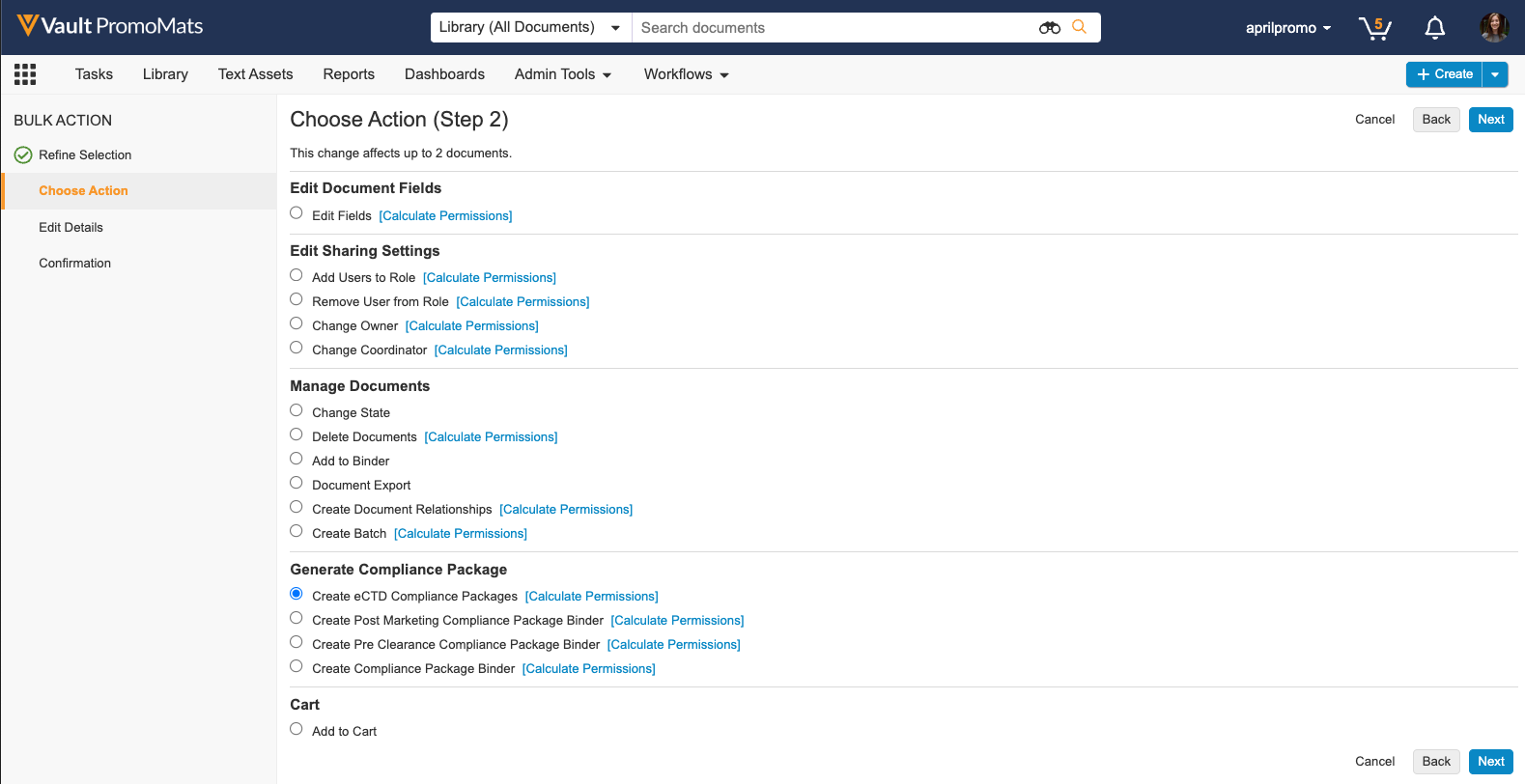
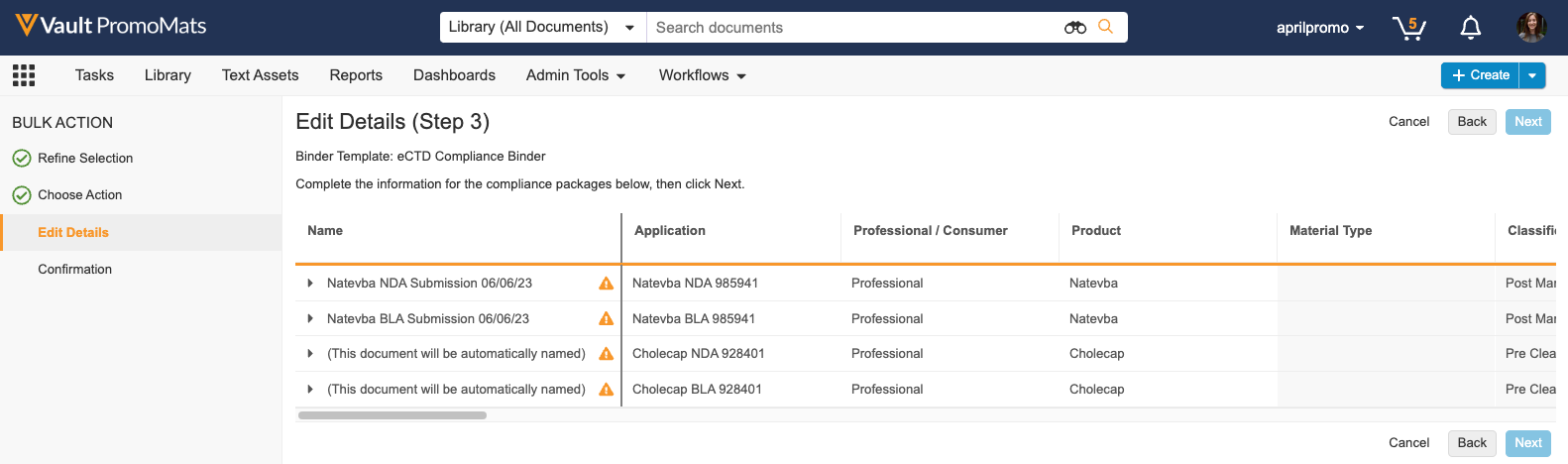
Bulk Generate eCTD Compliance Package
In this release, when performing a bulk action, a new action is available in the ‘Generate Compliance Package’ section to allow users to create multiple eCTD Compliance Packages at once. Vault groups documents and places them into one or more Compliance Packages based on the application, audience, and package type. This feature reduces required manual data entry by automatically populating Compliance Package data where available. Generating multiple eCTD Compliance Packages simultaneously streamlines the submissions process for users who have multiple promotional materials ready for different FDA submissions.
Bulk Generate eCTD Compliance Package
Learn more about configuring eCTD Compliance Packages and generating eCTD Compliance Packages.
Exclude All Special Characters in eCTD Submission Ready Copies
In Vault PromoMats’s eCTD Compliance Package feature, the system creates submission ready documents with FDA-compliant names. The FDA guidance states that document names should include only letters, numbers, hyphens and underscores. This feature ensures submission ready document names only include letters, numbers, hyphens, and underscores by removing any other special characters. This is based on the FDA guidance.
Learn more about generating eCTD Compliance Packages.
Multichannel
PPTX Video Support for CLM Auto-Publishing
CLM Auto-Publishing allows customers to manage the distribution, creation, versioning, and withdrawal of multichannel content directly from an original MLR document. Previously, when a PowerPoint slide containing a video was pushed to CLM via auto-publishing, Vault displayed a single slide showing the first frame of a video and could not play the video in CLM. As a result, Brand teams had to use the manual Create Presentation feature instead, which required the creation of a multichannel binder and slides which added to the review and approval timeline.
This new enhancement allows Brand teams to include embedded videos in presentations delivered to CLM via the Auto-Publish feature. Reps can now play videos in CLM on auto-published presentations. This significantly streamlines the review process for getting content to CLM and reduces the duplication of content as the Create Presentation feature is no longer needed for this use case.
While PPTX files generate a playable video, Vault does not support legacy PPT files and continues to generate the first frame PNG slide of the video.
Learn more about CLM Auto-Publishing.
Multichannel Events Management Enablement
With this release the Multichannel Events Management feature will automatically be enabled for use in Commercial and Medical Vaults that are licensed for the Multichannel app and no longer require support to enable the feature.
23R2 Commercial Data Model Changes
See 23R2 Commercial Data Model Changes.
Medical
MedInquiry
Frequently Asked Questions for Medical Inquiry
This feature introduces a standard, object-based approach to support Frequently Asked Questions (FAQs). FAQs are Medical Inquiries that are commonly asked by HCPs and patients and usually have a Standard Response that users send in reply.
Today, FAQs are typically stored in an FAQ Document alongside the Standard Response. Previous to this feature the user had to navigate to the FAQ Document, identify the FAQ that had been asked, copy and paste the answer into the Case Response and manually add any fulfillment documents.
By storing FAQs in object records in MedInquiry the user can quickly find the FAQs from the Case Request object. Users can search a library of approved FAQs, typically constrained by the case’s Product, Country, and Language. This allows them to remain on the Case Request while identifying the FAQ, rather than navigating away. It also provides a quick and simple way to apply a Standard Response to the FAQ.
An FAQ typically consists of:
- The question asked
- The Product, Country, and Language of the question
- Related object records for the Standard Responses
Standard Responses for Medical Inquiry
This feature introduces a standard, object-based approach to support Standard Responses. Standard Responses are predefined answers to Medical Inquiries commonly asked by HCPs and Patients. They are typically the response to an FAQ.
When composing a Case Response, users can select an approved Standard Response. The list of standard responses can be filtered to show answers to an identified FAQ.
After users select a Standard Response, Vault can populate the Case Response with the response notes, a response package, and a drafted email. Users can choose to update the response package or edit the email, or simply click to email the response.
A Standard Response typically consists of:
- Standard Response Notes
- Guidelines for Use
- The Product, Country, and Language of the response
- A package of Fulfillment Documents
- A Case Response Email Template
- The FAQ to which it is an answer
This feature helps standardize responses and lessens the time required to create a response package, allowing Medical Inquiry users to manage more responses.
CRM Data Sharing: Sync Only Required Accounts
This feature introduces the option to pull only relevant Accounts that are related to Inquiries from Veeva CRM. Previously, Vault Medical synchronized and pulled in all Accounts from Veeva CRM, which meant that customers with limited Third Party Agreements could not use the CRM synchronization as it exceeded specified thresholds.
Medical Inquiry UI: Incremental Save
This feature allows users to save information incrementally as they are capturing the inquiry in the Medical Inquiry User Interface. Previously, users would commit all the information in one go, and upon clicking Save would be navigated away from the page. Users can now save the information that they have entered so far, then continue working on the same page.
This feature also helps mitigate the risk of data loss from interruptions, such as when a user has not saved information yet and they experience a network connection issue.
The feature operates in two ways:
- A new Save icon in the UI allows the user to save what they have entered so far then continue working on the same page.
- When a user attempts to add a new object record (such as an Adverse Event), Vault can be configured to automatically save all of the information that has been entered so far before they begin working on the new object record.
- Admins must configure the objects that will trigger the Enforced Save when a new object record is created.
MedComms
AIRs Support for SVG & WebP Files
Automated Image Renditions (AIRs) functionality now supports SVG and WebP images. You can now use SVG and WebP images as the source file to transpose them to other supported rendition types using AIRs, including WebP. This feature enhances the DAM capabilities within PromoMats to allow content creators to use additional file types that are particularly well suited to web development in Automated Image Renditions.
Set Match Text Field Length Minimum Value to 5
This feature decreases the minimum number of required characters on the Text Asset object’s Match Text field to five (5). Previously, the Match Text field required a minimum of 20 characters, because a scientific statement is in most cases longer than 20 characters. Now that Text Assets are used for reusable text as well as scientific statements, there was a need to reduce the minimum number of characters required.
In Admin, Scientific Statement is the label for the annotation_keywords__sys object in Vault Medical.
Document Order Support for Portal Document Widgets
The new Portal User Interface, introduced in 23R1, now supports document ordering in custom document widgets. When editing a Portal, Portal Editors now have the option to drag and drop documents to create their desired display order. This gives Portal Editors greater flexibility when curating their content in a Portal.
Learn more about Portals.
Increase Number of Portal Content Filters
Brand Portals allow customers to distribute content efficiently within their teams. With this feature customers are able to add up to 24 Content Filters to a Portal, providing more flexibility in how the content is curated and improving the user experience for finding content. This increase in content filters is only available on the new Portal User Interface.
Learn more about Portals.
Vault Medical Feature Enablement
Admins may now enable the following features from Admin.
Suggested Links & Scientific Statements
Medical content needs to be substantiated by references, which are linked to content within the document viewer. Links may be created manually or automatically. In order to automate link creation, customers need to have Scientific Statements defined in MedComms, and configure the system to run Suggested Links.
This demo shows how Scientific Statements can save users time and manual effort.
Medical Inquiry User Interface
This feature provides a modern and configurable user interface dedicated to Medical Inquiry that allows users to capture all the information related to the inquiry in one place. As well as core details about the case, the user captures multiple requests, adverse events, and product quality complaints, alongside each of which one or more responses may be logged. Doing so in a single screen drives speed and efficiency for Medical Information users.
OpenData Account Search
For eligible customers, this connection allows users capturing Medical Inquiries to search OpenData for reliable, accurate HCP reference data when creating a Case Contact.
“Reply to” with Email Responses
This feature allows the user creating the case to add an alternative email address to send to the HCP if needed.
Multichannel
PPTX Video support for CLM Auto-Publishing
CLM Auto-Publishing allows customers to manage the distribution, creation, versioning, and withdrawal of multichannel content directly from an original document. Previously, when a PowerPoint slide containing a video was pushed to CRM via auto-publishing, Vault displayed a single slide showing the first frame of a video and could not play the video. As a result, Medical Communication teams had to use the manual Create Presentation feature instead, which required the creation of a multichannel binder and slides which added to the review and approval timeline.
This new enhancement allows Medical Communication teams to include embedded videos in presentations delivered to CRM via the Auto-Publish feature. Field teams can now play videos on auto-published presentations. This significantly streamlines the review process for getting content delivered to the field teams and reduces the duplication of content as the Create Presentation feature is no longer needed for this use case.
While PPTX files generate a playable video, this new feature does not support legacy PPT files and continues to generate the first frame PNG slide of the video.
Learn more about Scientific Communications Auto-Publishing.
Multichannel Events Management Enablement
With this release the Multichannel Events Management feature will automatically be enabled for use in Commercial & Medical Vaults that are licensed for the Multichannel App and no longer require support to enable the feature.
Learn more about Events Management.
23R2 Medical Data Model Changes
See 23R2 Medical Data Model Changes.
Mobile
Vault Mobile
SSO Login Support
With 23R2, Vault Mobile will now be accessible to customers and users using Single Sign-On with no additional configuration required. When an SSO user attempts to login to Vault Mobile, their authentication will re-route temporarily to a browser in order to leverage the existing SAML configuration.
For customers who have already configured OAuth profiles for Vault Mobile, there will be no change in behavior for SSO users. Vault defaults to the SAML method only when an OAuth profile does not already exist.
For Basic/Password users, the login flow will be slightly different as they will also be temporarily redirected to a browser to complete their authentication.
Device-Enforced App Access
This enhancement allows users to re-authenticate into Vault Mobile using their device’s biometrics up to a configurable length of time, while still respecting your domain’s session timeout configuration. This new feature setting will be visible in the Security Policy and will be defaulted to four (4) weeks, though customers may change the length of time or disable this feature. After the configurable length of time has passed, users will need to re-enter their credentials.
This feature does not apply for customers leveraging an OAuth profile for Vault Mobile. It only applies to Basic/Password Security Policies and SSO Security Policies that do not have an OAuth profile associated.
Vault Mobile Pre-Release Build
With 23R2, customers will now be able to download pre-release versions of Vault Mobile during the typical pre-release window, for both iOS and Android. This allows customers the ability to assess and test new Vault Mobile features ahead of General Releases, as they would with other Vault features.
Mobile User Interface Enhancements
Adds several minor UI enhancements for improved usability. Main changes include:
- The ability to view Dashboards in landscape mode
- The ability to search the list of Vaults in the Vault Selector
- The ability to expand notification text to view more in the Notifications mobile tab
Large Document Limit
Document renditions that are over 100 MB in size will no longer be rendered in Vault Mobile. This limit is to reduce load times within Vault Mobile, improving the overall performance and user experience. Users will have the option to open a document that is over the size limit in an in-app browser, if needed.
Veeva Snap
Veeva Snap: End of Support Warning
With the 23R3 General Release, Veeva Snap will be deprecated and removed from app stores. After this time, Veeva Snap will continue to function as is for users with it installed, but without further support from Veeva. In 24R1, users will no longer be able to scan and upload documents from Veeva Snap.
With 23R2, when users log into Veeva Snap, they will see an alert warning them of the future Snap deprecation.
Current users of Veeva Snap are encouraged to move to Vault Mobile. Vault Mobile includes all the functionality of Veeva Snap, as well as many other additional features, and is available for both iOS and Android.
Training
Study Training receives data model updates in parallel with the Quality Suite: Vault Training application as well as functionality updates from the following features:
- Learner Homepage: Configurable Filter Options
- Ability to Cancel a Classroom Training Assignment Workflow
- Cancel Instructor Workflow Task
- Curricula or Learner Role Object Types Usage Restriction Based on Application License
- Records In Custom Curriculum & Learner Role Object Types Not Allowed
- Consolidated Direct Assignment Jobs
- Allow Optional Attachments for External Training Assignments
- Default Limit for Number of Prerequisite Rule Per Curriculum Increased to 20
Study Training
Auto Create User Role Setup Security Records
This feature increases efficiency for Admins by reducing administrative overhead. With this feature configured, IT users no longer need to create User Role Setup records manually for each user and study. Instead, Study Training automatically creates the records based on the user’s Learner Role assigned to a Study. This ensures that users, such as CRAs, can see the right information based on their study team role and study.
Learn more about setting up Study Training.
Study Check Before Crosslink Creation in Study Training
For a Crosslink to be created in Study Training, at least one (1) Study reference must exist in the Study Training Vault.
Quality
QualityDocs
External Collaboration: External Review & Approval of Documents
This expansion of the External Collaboration feature, previously available in the QMS application, allows QualityDocs users to collaborate with external stakeholders on document review and approval tasks more efficiently, helping to streamline the collaboration process between organizations and their external stakeholders such as suppliers. This feature can replace less-efficient current collaboration mechanisms such as email, shared drives, Office 365, or others.
Using this feature, document review and approval tasks can be assigned to an external person. Vault creates or activates a user account, provisions appropriate permissions, and claims an external user license. Vault inactivates the user automatically when both the task has been completed and the document reaches its steady state. Then the external user license, utilized while the user was active, becomes available again.
This feature is intended for external stakeholders who need infrequent access to review and approve agreements and other documents. An example process may include the following steps:
- A user indicates on the document whether the document will be sent to an external person for review or approval. If yes, the user will select an organization and then from recognized contacts at that organization (Person records in Vault).
- The user then starts a relevant workflow that includes a task to be completed by an external person, for example, Send for Internal / External & QA Approval. The workflow can be configured to include both internal participants and an external participant. The external collaborator is a recognized contact (Person record in Vault) at the selected external organization.
- The system automatically creates and activates a user account from the Person record. The external collaborator receives a Welcome to Vault email (or Welcome Back email if they have collaborated at any point previously) which contains information and login details. They can then access Vault and have access only to the Home tab and their assigned task(s).
- The external collaborator can add annotations to the document if needed, and provide a verdict and eSignature.
- When the external collaborator has completed their open tasks in the system and when the document has reached steady state, the external collaborator receives a Goodbye email and the user account is automatically inactivated.
Learn more about configuring external collaboration for review and approval of documents.
External Collaboration: External Review and Approval of Documents
Increase in Default Limits of Visual Hierarchies in Process Navigator
The Adjustable Hierarchy Limits for Process Navigator feature was originally released in 21R3 and allowed default Visual Hierarchy limits in Process Navigator to be increased by Support. The maximum number of base hierarchies increased from three (3) to ten (10) and the number of child processes increased from ten (10) to fifteen (15). In this release, ten (10) base hierarchies and fifteen (15) child processes are now the default limits and no longer have to be enabled through Support.
Periodic Review & Change Authorization Record Counts
Record counts now appear next to the Periodic Review and Change Authorization Sections of the Document Change Control offering increased visibility, improved usability and consistency with other sections.
Learn more about working with Document Change Controls.
Controlled Copy Distribution Package Unique Naming
The Job Instance ID has been added to the Controlled Copy Distribution Package naming convention to ensure that there are no duplications in the auto-generated file names. The file naming format is now:
{label of the configured action} * {job instance id} * {current date/time}
Learn more about Controlled Copies.
QR Code Generator for Documents & Station Manager
On the manufacturing floor, getting the right content to the right users at the right time is critical. Some organizations use QR codes to enable users with Station Manager to scan the code on a machine or workstation to load the relevant content. However, generating such codes can be cumbersome or difficult. With this release, Vault now supports both the scanning (via Station Manager application or device camera application) and generation of QR codes for use within your organization.
You can generate a QR code via a user action on the Vault document, the Station Document record, or the Station Manager Category record. An Admin must add the action to applicable lifecycle states before it can be used.
You can then download, print, and place the QR codes at desired locations, so users can scan them on a mobile device using the camera app or the Station Manager app.
When scanning a QR code for a Vault document, the latest Steady State version of the document opens in the default mobile browser. When scanning the QR code for a Station Document or Station Manager Category, the relevant document or category opens in the Station Manager app.
QR Code Generator for Documents and Station Manager
Station Manager
Station Manager Sync Warning Enhancement
Station Manager sync warning messages alert a user that the station manager is not synchronized. This feature enhances the warning messages in Station Manager with a configuration option controlling the time before the appearance of the warning message since the device was last synchronized.
Previously, a red triangle was visible after 15 minutes and the warning message was displayed if the Station Manager app was not synchronized after a critical time of the day. The warning message can now also be displayed in case the Station Manager application has not successfully synchronized within a defined period of time, which can be set via.EMM configuration
Vault Station Manager iOS & Android Operating System Support
The Android version of the Vault Station Manager Application will not be certified or supported on Android Operating System (OS) version 8.x as of the 23R2 release. Additionally, as of the 23R2 release, the Android version of the Station Manager Application will only be supported on the latest Android OS version through two (2) previous major versions. The iOS version of the Station Manager Application will continue to only be supported on the latest iOS version through one (1) previous major version.
Training
Dynamic Enrollment: Automatically Assign Learner Roles to Person
This feature allows Vault Training to automatically assign Learner Roles to a Person, based on structured data available on the Person and Learner Role records. You can select matching fields between Person and Learner Role (such as Department) to drive Learner Role assignment. Additionally, if Person metadata changes, this feature also automatically removes Learner Roles and assigns any newly appropriate Learner Roles.
For example, if a Person has a Department value of “QA”, Vault Training might automatically give them the Learner Role “QA Core”. If that Person then switches to another department, Validation, then Vault Training would remove the “QA Core” Learner Role and may assign a new “Validation Executor Core” Learner Role.
Dynamic Enrollment: Automatically Assign Learner Roles to Person
SmartMatch Curricula
This feature adds flexibility to the training matrix and improves automation for the assignment of training to Learners. Using SmartMatch, you can choose which Curricula for a given Learner Role are assigned intelligently: Instead of all Curricula in the Learner Role being assigned for training, you can set up SmartMatch to assign only a subset of Curricula within the Learner Role. Vault Training selects the subset based on matching field values between the Person and Curriculum records. This reduces the need to create large numbers of specific Learner Roles.
For example, assume you have two Learners: A manager for data integrity and a senior manager for data integrity, and you have one Learner Role called Data Integrity covering all training relevant for associated roles. The senior manager needs to be trained on more items than the manager, so the manager only needs to be trained on a subset of the Curricula within Data Integrity. This assignment can be done based on matching fields. In this example we can base it on the senior manager’s Seniority Title field value on their Person record, matched against the Curriculum’s associated Seniority Level field value.
This feature was named SmartMatch at the time of its release. It is updated to Curriculum Matching in 23R3, and the change will be reflected in Vault UI in 24R1.
SmartMatch Curricula
Assign Curriculum to a Learner’s Training Matrix
This feature allows customers to assign Curricula directly to a Person’s Training Matrix. Unlike direct assignment, these assignments follow all matrix rules such recurrence, up-versioning, etc. This will allow Training Admins to assign specific Curricula to specific Person based on that Person’s specific training needs.
Assign Curriculum to a Learner Training Matrix
Learner Homepage: Configurable Filter Options
This feature improves search on the Learner Homepage by allowing organizations to configure search fields that can be used to search against open Training Assignments in the Open tab or Training Requirements in the Explore tab.
Learn more about setting up the Learner Homepage.
Allow Optional Attachments for External Training Assignments
The Attachments section on an External Training Assignment is now always shown regardless of the “proof required” value. This change makes it so the Learner has the option to upload a file, even if proof is not required.
The section message text was also updated to differentiate between required and optional attachments.
Ability to Cancel a Classroom Training Assignment Workflow
A new configurable user action allows users to cancel a Classroom Training Assignment Workflow. Previously, the workflow could only be canceled using an entry action. If the entry was not configured properly, this could lead to completed Classroom Training Assignment records with an open workflow task.
Learn more about Instructor-Led Training.
Records In Custom Curriculum & Learner Role Object Types Not Allowed
To enforce appropriate use of Learner Role and Curriculum types, Learner Role or Curricula records in custom object types are not allowed.
Curricula or Learner Role Object Types Usage Restriction Based on Application License
To ensure appropriate use of Learner Role and Curriculum types, the following are now enforced:
- Study Training Vaults: Only Study Curricula and Study Learner Roles can be created
- Vault Training Vaults: Only base Curricula and base Learner Roles can be created. Base is
base__vas well ascurricula__vandlearner_role__vobject types.
Default Limit for Number of Prerequisite Rule Per Curriculum Increased to 20
We have increased the default limit for the number of prerequisite rules per curriculum to 20 in new Quality Vaults. Existing Quality Vaults will remain at 5 and customers can increase the limit by contacting Support.
Learn more about Prerequisite Training Requirements.
Cancel Instructor Workflow Task
A new configurable user and entry action allows cancelation of a workflow task assigned to an instructor in the improved Instructor Led Training (Classroom Training) experience introduced in 22R3. These actions are useful when a class has already been Published, but needs to be canceled.
Learn more about Instructor-Led Training.
Consolidated Direct Assignment Jobs
In previous releases, Direct Assignments were issued via multiple jobs. In this release, they are now issued via a single job, which makes troubleshooting and access to logs quicker.
Learn more about Direct Assignment.
QMS
External Collaboration: Support for Investigation, Effectiveness Check, & Supplier Change Notifications
Expanding on the theme of in-Vault collaboration with external organizations, this release introduces External Collaboration functions into the Investigation, Effectiveness Check and Supplier Change Notifications processes. The External Collaboration toolset allows Vault to automatically invite Contacts at your partner organizations to participate temporarily in a Vault process. Vault grants them temporary access to only the records they need to operate on (per the configuration of your Vault) and revokes their access when all collaboration is complete. Dedicated notification templates, specific to the process in which the user is being invited to collaborate, keep them focused and in the loop as to what is expected of them. More about the functional specifics of External Collaboration tools in QMS can be found in Vault Help.
Investigations now supporting External Collaboration means that your organization can now allow external participants to be a part of the Investigation process natively: They can receive tasks with due dates (and the associated notifications), and provide their responses to field prompts directly with the native Vault experience. Put simply, they can now directly enter the Root Cause and Summary of investigations in Vault. This enables faster close-out and mitigates the need for, and risks associated with, manual re-entry of responses received from partners.
Collaborating with external parties on Effectiveness Checks rounds out the SCAR and CAPA processes, allowing for seamless external collaboration from issue identification and assessment to further continue on through action identification, closure and follow-up activities.
Lastly, external collaboration within the Supplier Change Notification process enables organizations to not only receive and immediately begin processing notifications of changes from Suppliers and partners, but to seamlessly interact with their key contacts at those organizations to confirm understanding of the issues. They can triage and assess the impacts of those changes all within Vault, as part of a configured, controlled lifecycle and workflow.
Each process covered with this release includes dedicated Welcome, Welcome Back and Goodbye notification templates which Vault will use when inviting and revoking access for external users. These functions must be configured before external collaboration in any of the identified processes can occur. Configuration and considerations related to External Collaboration may be familiar to organizations who have implemented these functions in other processes. Learn more about configuring external collaboration for QMS processes.
Standalone Quality Events Data Model & Feature Parity
With this release, the Vault QMS data model provides standalone data models for Change Controls, Deviations, Findings, Lab Investigations, and Continuous Improvements. These objects were previously managed as object types on the Quality Event object. Due to this broad utilization of the Quality Event object, some customers were running into limit issues which impacted performance and feature expansion. The following features will be supported for the new standalone objects:
- Recurrence Checks
- Relationship Automation
- Generate Document Actions
- External Collaboration (Deviation, Change Control, and Finding only)
- Effectiveness Checks (Deviation, Change Control, Finding, and Continuous Improvement only)
- Cycle Time Metrics
- External Notifications
Once this feature is available, all new implementations will utilize the new standalone data model. There is no impact or action needed for customers using the current Quality Event data model.
Duplicate Check Enhancements: Edit Fields & Copy Attachments
We have made additional functionality and usability enhancements to the Duplicate Check for Complaints feature, introduced in 22R3. The key enhancements being introduced are:
- A Summary Page configuration component, allowing Admins to define which fields can be edited by end users on the summary page and whether attachments can be copied from one record to another
- The ability to edit fields in the Original record panel of the summary page, based on the configuration defined, and save those edits on completion
- The ability to view attachments on the comparison and summary pages
- The ability to configure an asynchronous job to copy any attachments from records identified as a duplicate or follow-up to the record identified as original upon completion of the Duplicate Check workflow
- Improved Admin interface and Pages to provide efficiency and ease of use
Learn more about configuring Duplicate Check.
Create Change Actions from Template Records
Change Controls often define the same Change Actions over and over again. For example, an organization’s manufacturing-related Change Controls might require a routine set of Change Actions for each affected site. If each site regularly creates the same 20 Change Actions, and there are typically 15 manufacturing Change Controls per year, each site recreates the same 20 Change Actions 15 times. Manually creating the same Change Actions over and over again is labor intensive, error prone, and prevents organizations from effectively standardizing repetitive change control processes.
Beginning in this release, Vault provides the ability to add multiple Change Actions to new or existing Change Controls or Change Plans from approved Change Action Templates. Each approved Change Action Template consists of one or more template Change Actions. When Vault creates Change Actions from a template, the field values in the template’s Change Actions are copied to the corresponding fields in the Change Actions. Users that build Change Action Templates can decide whether the copied field values are editable or read-only in the Change Actions.
In order to use this feature, an Admin must configure it. The main configuration options for consideration include:
- The list of approved Change Action Templates available for use depends on the Change Control or Change Plan metadata. Admins specify which fields on a Change Control or Change Plan must have matching values for the equivalent fields in a Change Action Template in order for that template to be available for use.
- Admins configure the ability for users to add Change Actions from matching Change Action Templates at specific states in the Change Control or Change Plan lifecycle.
- Admins can also configure Vault to automatically add Change Actions from a template when a Change Control or Change Plan is created, or when a Change Control or Change Plan enters a particular state.
This feature promotes standardization of repetitive Change Actions, reduces data entry errors, and reduces the effort required to create a Change Control’s Change Actions.
Create Change Actions from Template Record
External Notification Usability Enhancements
In this release, the External Notification capability is being enhanced in two ways: A new Distribution Group Label field and and the ability to restrict edit access to the Notification Recipients section of an applicable record.
Organizations often need to notify individuals external to their company about quality management processes occurring within their organization. For example:
- A company wants to notify one of their suppliers at the conclusion of an audit when the audit report is available
- An organization wants to respond to an individual that submitted a product complaint when the complaint is received, and later when the complaint investigation reaches a conclusion
Similarly, it may also be necessary to notify individuals within the organization that are considered “external” because they do not have access to the quality management system. For example, a quality department might want to send a notification to members of the organization’s executive team to alert them to a quality management issue requiring escalation.
The Vault QMS feature known as External Notification enables companies to incorporate notifications to external parties as part of a quality management process such as an Audit, Complaint, Issue Escalation, or any other quality event.
New Distribution Group Label Field for use in Formatted Output Reports
Many customers create a Formatted Output report showing the External Notification recipients associated with a quality process. The Formatted Output report can be downloaded and viewed outside Vault. Configuring the Formatted Output often requires identifying recipients in a particular external notification distribution group.
Prior to this release, the only way to identify an external notification distribution group is by using the Distribution Group field. However, the Distribution Group field identifies a group using a Globally Unique Identifier (GUID) (for example, 128711_OP400000000S003), instead of a human-readable name (for example, Initial Correspondence Group). This is problematic because the GUID for an external notification distribution group changes from Vault to Vault. As a result, the Formatted Output configuration needs to be updated each time it is ported from one Vault to another (for example, from sandbox to production). Maintaining the Formatted Output is more difficult when the configuration refers to a GUID rather than a human-readable name for an external notification distribution group.
This enhancement introduces a new Distribution Group Label field on the objects that store external notification recipients. Distribution Group Label stores the human-readable name of an external notification distribution group. The Distribution Group Label field can be used in Formatted Output configuration to more easily identify the group of recipients to be included in a Formatted Output. Using the Distribution Group Label field will also ensure a Formatted Output configuration can be ported between Vaults without the need to update it in the target Vault. It is important to note that the Distribution Group Label field will be populated from release day forward and will not be updated on historical records.
Security Enhancement: Control Editability of Notification Recipients Section by User Role
When Audit, Complaint, Issue Escalation, or Quality Event utilizes the External Notification capability, their records include a Notification Recipients section. The Notification Recipients section enables a user to identify the individuals (Person records) that receive external notifications for that particular record.
Beginning with this release, customers can configure their quality processes to restrict edit access to the Notification Recipients section based on a user role and the lifecycle state of the Audit, Complaint, Issue Escalation, or Quality Event record.
For example, this allows a customer to permit the owner of a Complaint record to update the Notification Recipients section while a Complaint record is in the Initiated state, and prevent any from updating Notification Recipients once the Complaint is Closed.
Refer to Configuring External Notifications (QMS) for more information on how to enable role and lifecycle state security on a record’s Notification Recipients. Lastly, notification recipient lists will now list full names along with email addresses of the participants, which previously only listed emails and not full names.
QRM: Risk Builder Enhancements
Risk-based decision making is a key component of any organization’s compliance program. Vault QMS allows companies to manage risk associated with enterprise and operational processes. Capturing risk information can be effort intensive. Recognizing this, Veeva introduced the Risk Builder user interface which enables users to easily enter and update Risk data in a Risk Assessment. Risk Builder provides a familiar spreadsheet-like user experience for data entry. This release introduces additional Risk Builder improvements that greatly improve the user experience in the following ways:
Enhancements for Viewing Risks
- Process Step Selector Enhancements: Users can now launch a window to easily search for a Process Step within the Risk Assessment.
- Freeze First Column: The first column in the Risk Builder will now be frozen by default, ensuring that it is never out of view when a user scrolls horizontally to display columns situated on the right side of the Risk Builder matrix.
- See More for Long Text and Rich Text Fields: Risk Builder cells that contain Long Text or Rich Text include a show more link that allows the user to view more of the associated field’s value within a popup window.
- Search Risks: The Risk Builder now includes the ability to search for Risks. A search operation returns Risks that contain at least one field whose value matches the search text provided by the user. Matching text is displayed bold within the search results.
Enhancements for Updating Risks
- Risk Flagging: Users can flag Risk records to identify which ones require further review. A visual indicator is displayed in the Risk Builder, next to a flagged Risk. Users can remove a flag when appropriate. The number of flagged Risk records appears near the top of the Risk Builder.
- Insert Row Above/Below: Users can now add a new row directly above or below the currently selected row in a list of Risks. Previously, users could only add new rows to the bottom of the list.
- Clone / Copy / Paste / Row Actions: The previous release included a Copy Row action that copied the selected row, inserted a new row below the selected row, and pasted the Risk data into the new row. In this release, this action is being re-labeled to Clone Row. A new Copy Row action is now available that simply copies the selected row’s Risk data. The copied data can then be pasted into a selected row using the new Paste Row action.
- Last Saved Timer: Risk Builder now displays approximately how much time has passed since the user saved Risk data.
- Permission Enhancements: Risk Builder will not allow users to add or delete Risk rows, or edit specific Risk fields, unless they have the appropriate permissions.
- Retain Unsaved Data: When Risk Builder saves data and detects incorrect information entered by a user (e.g. ???), a dialog is displayed identifying the issues. After dismissing the dialog, the user will return to Risk Builder in edit mode with the unsaved data retained so corrections can be made. Users can filter the list of Risks to find rows that generated errors.
Other Enhancements
- Keyboard Shortcuts: Risk Builder now supports the following new keyboard shortcuts:
- Ctrl + C: Copy the content of a cell, or copy the selected portion of a cell while in edit mode
- Ctrl + X: Cut the content of a cell, or cut the selected portion of a cell while in edit mode
- Ctrl + V: Overwrite the content of a cell, or paste into the selected portion of a cell while in edit mode
Refer to Vault Help for the full list of keyboard shortcuts available in the current General Release.
These Risk Builder user experience enhancements allow organizations to be more efficient viewing and editing risk data.
QMS: 5 Whys Root Cause Record Creation Enhancements
Expanding on the 5 Whys Root Cause Analysis features introduced to Vault QMS in 23R1, we’ve introduced additional automation into the creation & management of Root Cause records. This release streamlines the experience for issue investigators, enhancing the automation regarding creating, modifying and deleting Root Cause records during the 5 Whys analysis behind the scenes. This keeps the investigator focused on performing the analysis rather than ensuring the appropriate records are created or deleted in Vault to reflect the outcome of the analysis.
The system behind the scenes has been made smarter with additional configuration options and automation functions. We’ve broadened support for more complex configurations of 5 Whys Root Cause Analysis. When a node in the analysis is set as a Root Cause by a user, Vault QMS is now aware of the possibility of multiple types of Root Causes. The system will now seamlessly prompt the user to select the appropriate type of root cause (defaulting it wherever possible), and then prompt them for the needed fields (which can be specific to the types of Root Causes your organization utilizes) guiding them through the 5 Whys Root Cause Analysis flow per the configuration of your Vault.
Additionally, based on feedback we’ve received, configuration has been opened up for the Cause Type, Category, and Subcategory fields of Root Cause Analysis Items, allowing them to be inactivated as part of the configuration of your process. If the fields are inactivated, they’ll be removed from the 5 Whys Analysis UI if they’re not applicable to the Root Cause as configured.
Lastly, when a Root Cause Analysis enters its lifecycle’s Completed state type, Vault will automatically identify and execute record changes, additions, or removals as needed when a Related Root Cause Analysis Item’s Root Cause field is changed, or the Type of the Related Root Cause Analysis Item is changed during the analysis. This cleans up old or out-dated records left over from prior work within the 5 Whys Root Cause analysis flow automatically.
Some aspects of this feature are automatically enabled for customers who have implemented 5 Whys root cause analysis within their QMS Vault. To take advantage of all of the changes in this release, some configuration may be necessary. Learn more about the new configuration options of and functions within the 5 Whys Root Cause Analysis functions available in Vault QMS.
Surveillance
eMDR Data Mapping Enhancements
eMDR safety information submitted to FDA is sourced from several different records within the Vault Product Surveillance application. Organizations must ensure all safety data is thoroughly compiled and reviewed. The 22R1 release addressed this need by providing the ability to automatically assemble data from Product, Person, Contact, MedTech Complaint, and Organization records into a single Adverse Event Report (AER). The AER provides a read-only view of the aggregated safety data related to a complaint so users can review all pertinent information and submit it to FDA.
This feature updates mapping for sections B5, B7, and H10 of the eMDR form.
Surveillance also supports PMA/510(k) number (D4) on both Product and Product Variant level.
Learn more about Vault Product Surveillance adverse event reporting.
EU MIR UI Enhancement: View & Edit Fields on AER
EU MIR safety information submitted to health authorities is sourced from several different records within the Vault Product Surveillance application. Organizations must ensure all Surveillance data is thoroughly compiled and reviewed. The 22R1 release addressed this need by providing the ability to automatically assemble data from Product, Person, Contact, MedTech Complaint, and Organization records into a single Adverse Event Report (AER). The AER provides a read-only view of the aggregated safety data related to a complaint so users can review all pertinent information and submit it to the appropriate health authority.
This feature enhances how users interact with an AER record, allowing them to edit information directly within an AER record. As a result, users no longer need to locate and update the appropriate Product, Person, Contact, MedTech Complaint, and Organization records that represent the source of the information in an AER. When a user updates data on an AER, Vault automatically updates the corresponding fields on the source records.
Allowing users to edit Surveillance data directly in an AER record reduces manual effort and improves data integrity by ensuring the data has a single source of truth.
Learn more about Vault Product Surveillance adverse event reporting.
Standalone Complaints: Health Canada Support
With this release, Surveillance now supports Adverse Event Report generation and data validation for submission to Health Canada using the standalone Complaint object.
Validation Management
Copy Screenshots from Clipboard & Paste to Test Step
With this release, test executors can now copy a source image from screen capture software and attach it to an execution step using a system shortcut (CMD + V or CTRL + V). One or more files can also be copied from a file location and attached as well. A new attachment limit of five (5) attachments per test step has been introduced in 23R2.
This greatly enhances the productivity of executors that need to collect objective evidence using screenshots from screen capture software. Previously, screenshots were first saved to a file location and then uploaded using the upload action and upload dialog or by dragging the files and dropping them onto the upload control. Learn more about executing tests.
Validation Team
This feature defines how permissions apply across records in the Validation Management application. Validation team members associated with a Validation Inventory Item record will be granted record visibility access rights based on the Inventory Item dimension being applied across all Validation object records.
For global view access users who need access to all validation records, they should have the Validation Viewer role as a User Role.
The team will be selected at the Validation Inventory Item level which will be used to constrain which users can view records and also be assigned as owners for requirements, activities, deliverables, test protocols, test script, test steps, and discrepancies. To give read permission to users, they must be assigned at the Validation Inventory Item level as Validation Contributor at a minimum.
Requirement Owner, Activity Owner, Deliverable Owner, Author, Executor, Witness, and Discrepancy Owner can be defined for each record as a user field. By using the custom UI widget, users can be chosen from a list of Validation Team Members for the Validation Inventory Item.
Role permissions can be defined by the atomic security settings from each object lifecycle. If roles are not added or configured in the object lifecycle, read-only permission is applied by default.
Pause, Resume, & Terminate Test
This feature provides the ability to pause, resume, or completely terminate a test script during its execution.
When the actions are configured, they are available in the Execution UI as well as on the record’s details page. Users must provide justifications when pausing, resuming, or terminating a test, but eSignatures can be configured separately via workflow configuration.
When paused, users can see the yellow banner with a message about who paused the test script when and a link to view the justification.
When terminated, users can see the red banner with a message about who paused the test script when and a link to view the justification.
The Execution Change History captures the history of actions and changes for a test script. When a user pauses, resumes, or terminates a test script, the system captures the action type, original state, new state, justification, and test script value in the test script execution change record.
Deep Copy Test Scripts with Test Steps & Linked Requirements
This feature provides the capability for users to copy a test script with its test steps and linked requirements. This allows users to re-run the test script or leverage best practice test scripts to create a clone and re-author to include required changes. Administrators can define which lifecycle state the copied test script will be created in, such as the Pre-Approved state if a new run is needed.
Deliverable Reference Document Automation Enhancements
This feature enables the system to automatically update the link between a deliverable record and a reference deliverable document to be always up to date until the document version is approved and enters a steady state.
When a document enters the steady state, Admins can configure an entry action on a document lifecycle state that will change the lifecycle state on the Validation Deliverable object record as well as set the value for the specific version of the document as of current. This will allow the approval of a document to mark the Validation Deliverable as “Complete” and keep the version as specific to the event of the approval and completion for the deliverable reference document.
When all associated deliverable records are closed for the deliverable reference document, the document cannot be updated. The deliverable reference document only can be updated when an open deliverable record is associated with it.
Performance Improvement for Test Authoring & Test Execution
With this release, performance of the custom app pages for test authoring and execution for Authors, Executors, Reviewers, and Approvers has been further enhanced. A section-based pagination control has been introduced in the Test Authoring UI and Test Authoring Review UI to display 25 test step records at a time on a page in the content panel. Similarly, a global pagination control has been introduced in the new UIs that also loads 25 records at a time on a given page in the content panel.
This feature will help ensure various users enjoy authoring and executing test scripts while avoiding long page load times.
LIMS
Test Execution & Exception Summary Enhancements
In the Test Execution and Exception Summary interfaces, there is now better contrast between the Out of Specification and Previously Out of Specification icons, an improved user flow for making Result changes, clearer error text for invalid Test Input entries, and users can now see the outcome of calculations and evaluations for the final Test Step before proceeding.
LIMS Result Exceptions Enhancements
Reopened tests show as exceptions in the Exception Summary widget, and we addressed a rare OOS scenario that could mean the user was not aware of OOS until after test completion.
LIMS Static Data Enhancements
Check Syntax formula errors are no longer included in the formula text itself.
LIMS Automation Enhancements
We have improved the notifications for initiating Batches and Samples, notifying you only in the application and not via email, and have included better support for manually progressing Lab Tests.
Quality Data Model Changes
See 23R2 Quality Data Model Changes.
Regulatory
RIM Registrations
EUDAMED Machine-to-Machine UDI Submissions
MedTech companies marketing medical devices in the European Union can now submit their UDI data directly to EUDAMED from Vault Registrations. In addition to generating, validating, and reviewing UDI submissions, Vault now streamlines the process of submitting UDI data and handles any responses from EUDAMED.
Admins can configure an EU EUDAMED UDI gateway profile for each EUDAMED Actor that will be submitting UDI data to EUDAMED. This eliminates the need for manually exporting the XML for a UDI submission from Vault and manually uploading it to EUDAMED, and users can monitor the submission status to know if it has been accepted or rejected.
Learn more about generating UDI data for EUDAMED.
EUDAMED Machine to Machine UDI Submissions
Automation Enhancements for Labeling Deviations
Labeling teams can now benefit from new automation when managing labeling deviations across dependent markets with the release of Vault’s new labeling deviation trigger enhancements.
When a Labeling Deviation is set to Accepted for Dependent Countries, Vault:
- Sets all labeling deviations for dependent markets into the Reviewed lifecycle state, as additional review is typically not required for dependent markets.
- Populates or updates any blank fields on the dependent labeling deviations each time the reference Labeling Deviation is updated and saved.
The automation is triggered when the new Reference Deviation field is populated on the dependent labeling deviations.
Admins can specify which field values Vault should copy when creating dependent Labeling Deviations, and which fields to update if blank upon subsequent saving.
This feature aims to reflect as much information at the dependent country level as possible to ensure alignment and reduce manual work for labeling teams.
Learn more about Label Concept and Deviation tracking.
Automation Enhancements for Labeling Deviations
Create Registrations from a Regulatory Objective
Regulatory teams can now create and update registrations and their registered details from the same location, a Regulatory Objective, using the regulatory objective’s details as the source for the registered details.
This feature enhances the process of creating registrations, particularly in cases where submissions are not required to approve a product on the market, such as for low-risk medical devices.
Create a Registration from a Regulatory Objective
Detailed Activity & Regulatory Objective Bundling Settings
Administrators can now view and manage the configuration of Activity and Regulatory Objective Bundling capability in Admin > Settings > Application Settings.
Previously, Admins were required to contact Veeva Support to request the enablement. Now, Admins have the flexibility to enable Activity and Regulatory Objective Bundling and perform the following actions for bundling from the Admin UI:
- Limit bundling of Activities and Regulatory Objectives to selected lifecycle states
- Configure bundling to set the Activity and the Regulatory Objective records as Inactive
- Set default filters
- Define naming patterns for Activities and Regulatory Objectives
- Restrict Submissions copying by Worksharing or Submissions creation for US Grouping
Once the Activity and Regulatory Bundling feature is enabled, Admins can easily update the detailed settings to fit their organization’s needs.
Global-to-Local Mappings for Submission Subtypes & Site Roles
With this release, users can select a global term for the Submission Subtype field in the Create Related Records wizard, which Vault will map to the corresponding local Submission Subtype value. Additionally, when using the wizard to create Regulatory Objectives and/or Submissions with relationships that specify a global Manufacturing Site Role (for example, Submission Product or Submission Packaging), Vault will map the global term on the Event relationship records to the appropriate local values on the resulting Regulatory Objective and/or Submission relationship records.
This greatly enhances the wizard’s efficiency and improves data quality, as users are no longer required to manually enter local Submission Subtype values on each Submission record, nor Manufacturing Site Role values on each relevant Regulatory Objective and Submission relationship record.
UPD Submission Data Aggregation
Union Product Database (UPD) is the veterinary product reporting repository for EU animal health medicinal products. In the previous release, Vault Registrations enhanced its data model to include objects and fields required for submission to UPD. This feature creates transactional object records that organize product data in the form and format required for submission. Additionally, the data aggregation algorithm populates these records with source Registration data, transforming where needed to suit health authority requirements. This feature is the next step towards UPD support to enable animal health customers to be compliant with EMA’s UPD regulation. Configuration is required to enable this feature.
Learn more about UPD.
Dynamic Affiliate Home Page Title
With this release, the label configured by Admins for the Affiliate Home tab will also be used as the label for that page’s heading. Previously, Vault always displayed this page’s heading as Affiliate Homepage.
Learn more about the Affiliate Home page.
Use for Registrations Field
In this release, we are introducing the Use for Registrations field on the Application, Regulatory Objective, Submission, and Event related records (for example, Event Product). Admins of existing Vaults can configure the field in object page layouts to support the upcoming capability.
In future releases, we will enhance the Create Registrations and Manage Registered Details wizards to take into account the Use for Registrations field. When these enhancements become available:
- When Use for Registrations is set to Yes, Vault will use the Submission or Regulatory Objective related record as a source for creating or updating registered details.
- When Use for Registrations is set to No, Vault will not create or update registered details.
This new feature will be particularly useful in situations where users need to add consolidated records, such as All Manufacturers, as related records, but do not want the wizards to create registered details for these records.
Learn more about creating Registrations in bulk and managing registered details.
DADI Settings & Action Renamed to eAF
In this release, all Application Settings previously labeled “DADI” are updated to eAF to reflect EMA’s rebranding.
RIM Submissions
Add Pages Column to Content Plan Viewer
The selectable columns for matched documents in the Content Plan Hierarchy viewer are extended to show the document page count from the document metadata. Users can now include the column Pages in the Content Plan Hierarchy viewer and export this information with the Content Plan export.
Content Plan Items: Drag & Drop Duplicate Detection Update
Drag and Drop from Desktop to Content Plan Item was originally released in 23R1. With this release, the duplicate detection criteria for documents dropped to the Content Plan Item are updated to exclude Submissions Archive documents so that only documents from the Library that are not Submissions Archive documents are considered in duplicate detection.
As Vault does not support Submissions Archive document publishing, this update prevents Submission Archive documents from being matched to the content plan and getting published.
Content Plan Viewer Icon Updates
The icon for Content Plan sections is updated to align with the icons in the new Submissions Archive Viewer, with a closed folder icon when the section is collapsed and an open folder when the section is expanded. This is more intuitive for users than the previous clipboard icon.
Redacted Document Field & Relationship
To support EU Clinical Trial Regulation (CTR) and the growing industry trend towards clinical trial transparency, a new Redacted Yes/No document field and Redaction document relationship type have been added. With Admin configuration on the desired document type, subtype, and/or classification, the Redacted field is available for authors or publishers to identify whether a document contains redactions. Additionally, the Redaction document relationship (automatically available in the Doc Info panel) allows these users to establish a relationship between redacted documents and their unredacted source files.
Update to Inactive Content Plan Records Cleanup Job
The deletion criteria for the Inactive Content Plan Records Cleanup Job is updated to improve the Content Plan Item deletion scope. The job now deletes records where the All Document Count field is blank in addition to 0. This allows Content Plan Item records that are created as Inactive to be properly deleted A new Cleanup Status picklist field has also been added to the Content Plan. This status field is automatically set by Vault to reflect the cleanup status, but can also be set by Admins to skip or reprocess a Content Plan in the next job run.
RIM Publishing
Publishing Pre-Validation
For Vault Submissions Publishing to successfully generate a submission, prerequisite actions, fields, and Submission Content Plan (SCP) data must be complete. To better support customers with these manual tasks, Vault now runs pre-validation checks on the most common publishing errors and users when setting the Enable Continuous Publishing field or running the On-Demand Publishing action. These checks are not new criteria for failure, but rather an advance review of existing issues known to cause publishing errors.
Pre-validation checks the following:
- The
published_content_owner__vhas the permission to create documents of the Archive (archive__v) type, and has a Full User Application License - The necessary publishing fields are populated when the Dossier Format is set to eCTD: XML Regional DTD/XSD Version, XML ICH DTD/XSD Version, Validation Criteria Version, XML Sequence ID
- There is only one Content Plan referencing the current Submission
- There are no Submission Clinical Study or Submission Nonclinical Study records related to an active STF Content Plan Item in the current submission with a blank XML Clinical Study Title or XML Nonclinical Study Title
- If there aren’t active STFs, the XML STF DTD XSD Version is not populated
- The Primary Application and Primary Submission are populated on the Content Plan
These new automatic checks will limit time lost to troubleshooting and Support tickets due to known issues or preventable errors.
Learn more about continuous and on-demand publishing validation.
Set Leaf Operation Updates
Vault Submissions Publishing users now have a simpler and more convenient method for setting a document’s eCTD lifecycle operation. An improved interface allows users to select the XML Operation (Replace, Delete, Append) without first having to change the Content Plan Item, reducing the number of clicks. Users will select the document to be lifecycled within the new interface, which has a similar look and feel to the Submissions Archive Viewer. The new interface also aligns with other common publishing actions, creating a more consistent user experience across the application.
Publishing customers will see these immediate improvements:
- A single location to set the XML Operation value
- Leverage the new Submissions Archive Viewer search and its filtering capabilities
- Auto-expansion of the eCTD hierarchy, even if there are repeated sections with the same name
- Ability to Expand All when selecting files in the new interface window
- Full dialog box is visible on any screen size
Learn more about selecting a leaf operation target.
Set Leaf Operation
Pharmaceutical Form Name Override
A new free text field, XML Application Pharmaceutical Form Name, and a new lookup field, XML Submission Pharmaceutical Form Name are available for use in Switzerland (CH) Applications and Submissions.
Previously, the XML was auto-populated with the Application Pharmaceutical Form Name value. If a different name is needed in the CH Module 1 Envelope, publishers can enter it into the XML Application Pharmaceutical Form Name field, and the resulting submission XML contains the override value rather than the default value. These new fields are also available within the Submissions Wizard, when enabled. An Admin will need to add these fields to the appropriate page layouts.
Learn more about working with Pharmaceutical Forms.
Support for CH Submissions with Multiple Application Numbers
Swissmedic guidance allows for multiple variations for the same medicinal product to be submitted jointly, as a multiple application. This could include variations of the same type or different types (IA, IB, II, extension).
To address these use cases, in 23R2 Vault Publishing will allow publishers to include additional Application Number(s) for Swiss (CH) submissions. After configuration, publishers will be able to:
- Include additional Application Number(s) with a new Add Override action
- Bring forward Overrides to subsequent sequences without affecting any previous sequences
- Delete Overrides for current or future sequences without affecting any previous sequences
When Vault generates the XML, a new line for each Application Number is generated in the envelope.
These changes will not impact any existing CH submissions with a single Application Number.
Local Connection to Run Publishing as Vault App Owner
To enhance Publishing speed and performance, Admins will see a RIM Local Connection under the Admin > Connections tab. This infrastructure improvement will be automatically deployed as Active to leverage Vault Platform functionality and prevent any publishing errors when the Source of Published Documents is set to Source.
While the Publishing end-user experience will not change, users will benefit from quicker published output generation when Source is chosen as the Source of Published Documents.
Ukraine eCTD 1.0 Pilot
Ukraine Ministry of Health (MoH) will begin a phased rollout for Marketing Authorisations in eCTD v3.2 format. Their current target for implementation is August 2025. To prepare, Veeva is introducing a pilot for UA eCTD publishing in 23R2, including:
- Ukraine Submission Content Plan Template (UA Module 1)
- UA Regional XML generation, per UA DTD v1.0
- Updated Controlled Vocabularies, Constraints, and picklist values to manage and view Submission Administrative Information
Vault Publishing customers can voluntarily begin generating compliant eCTD submissions after configuration of the above.
RIM Submissions customers using submission content planning can also benefit from configuring and using the new Ukraine Content Plan Template.
Veeva continues to monitor and support all regions that require eCTD, and will address additional UA specification updates (including validation rules) as they are made available.
Learn more about RIM Submissions Publishing.
RIM Submissions Archive
Submissions Archive: Ukraine eCTD 1.0
Users can now import, view, and export Ukrainian dossiers that use the UA 1.0 DTD for eCTD submissions.
Additional Fields Selectable as Viewer Grid Columns
In the Submissions Archive Viewer, users can now choose to display four additional fields: Clinical Study and Clinical Site (from the Submission Metadata object), and Submission Type and Submission Subtype (from the related Submission record). The new fields can also be used for filtering.
Expand All for Single Submission in the Viewer
When users filter on a single submission in the Submissions Archive Viewer, they have the option to Expand All from the root. This expands all child nodes down to the document level for the selected submission. Note: The Correspondence documents are not expanded.
Render XML with no Stylesheet
With this release, Vault Submissions Archive creates a plain text rendition for XMLs when a stylesheet is not available. Previously, these documents were not rendered. This auto-on feature facilitates XML viewing within RIM Submissions Archive.
Learn more about the Submissions Archive Viewer.
Submissions Archive Correspondence Document Handling Improvement
This feature resolves an issue in the Submission Archive Viewer when a Correspondence document, tagged to a specific Application and/or Submission, was imported and then immediately approved. Previously, the Viewer did not display these documents.
Submissions Archive Hierarchy Tree View Expansion Improvement
Submissions Archive Viewer is now further enhanced to support viewing of larger applications. In the redesigned Viewer, UI and back-end limitations did not allow for full expansion of sections in large submissions, such as those with tens of thousands of records. While users were alerted that an expansion limit was reached, they were not provided details on which items failed to load.
Without sacrificing performance, the following Viewer improvements are automatically enabled:
- Increasing the total number of rows exposed in a single view from the current UI limit, and more than doubling the record handling capabilities on the back-end
- Expansion of all nested subsections by depth rather than in a vertical order (for example, all 3.2.P.4 subsections are fully expanded before 3.2.P.5 expands)
- Error messages appear at the site of the expansion limit to clearly identify which items have and have not been loaded in the view
Learn more about the Submissions Archive Viewer.
Submissions Archive Viewer Performance Optimization
Submissions Archive Viewer performance is improved to display additional rows when users select Expand All at the module level.
Submissions Archive: CN eCTD 1.0 Support Update
This auto-on feature updates section labels in Module 1 to align with the latest specification for China (CN) eCTD 1.0.
Learn more about the Submissions Archive Viewer.
RIM Registrations, RIM Submissions
Synchronize Content Plans: Iterative & Section-Level Dispatch
Global users managing the Global Content Plan now have the flexibility to initiate multiple dispatches incrementally at a section level, instead of a one-time initial dispatch for the entire Global Content Plan. With this capability, teams can dispatch the modules or sections that are ready as they become finalized as well as changes that are made over time to the Global Content Plan.
Users can quickly review a system generated comparison between the Global Content Plan dispatch and the existing country-specific submission content plan in a new Comparison Viewer which includes new and updated content plan and content plan item records, and new, updated, or removed matched documents. They can then accept or reject all or some of the proposed changes from the Global Content Plan dispatch.
If needed, administrators can set the Mandatory for Dispatch Acceptance field on the Content Plan and/or Content Plan Template records to prevent local affiliates from rejecting specific sections or items.
With each dispatch, Vault creates a new Dispatch Message for each target Activity/Submission so that users can monitor which sections were dispatched and what their current status is per Submission.
Content Plan: Iterative & Section Level Dispatch
See also Synchronize Content Plans: Dispatch Enhancements, a related auto-on feature.
Learn more about Global Content Plans.
Synchronize Content Plans: Dispatch Enhancements
To support the configurable Synchronize Content Plans: Iterative & Section-Level Dispatch feature, several auto-on changes have been made to the existing global content plan dispatch functionality, including:
- When the Copy Relationships option is configured, Vault copies only the relationships within the dispatch scope, instead of all relationships on the Event.
- Dispatch will no longer skip submissions with an existing content plan. Instead, Vault raises an error for the submission if section-level dispatch is not configured.
- Vault will no longer run a general update on the content plan after copying it. Instead, dispatching will only add the specific records which are in the template but missing from the global content plan. This means that Vault will not create repeating sections (based on the template) for relationship records which are on the Submission but not on the Event. If there are additional template or Submission relationship records that are not in the Global Content Plan, the Update Content Plan action must be manually run after each dispatch.
Learn more about Global Content Plans.
Support Multi-Select on Document Set
For customers working with Global Content Plans, this feature allows for greater flexibility when creating document sets for the dispatch to local affiliates. Previously, the Document Set field value on the Activity, Application, Country, and Content Plan Item objects was single-select. By updating the Document Set field on these objects to be multi-select:
- Less effort is required to create Global Content Plans. For Content Plan Items with document(s) that apply to more than one document set, users can select all applicable document sets.
- There is greater flexibility when assigning documents sets to Activities, as users can assign more than one document set based on the different areas and dossier sections.
RIM Submissions, RIM Publishing
Resolve Embedded Links Based on Source for Published Document Value
Vault Submissions Publishing is improving hyperlink handling when the Source for Published Document is set to a custom rendition. Previously, link data was based on the values for the Viewable Rendition; with this change, Vault retrieves link data from the selected Source for Published Document rendition. This back-end processing will be automatically updated for all Vault Publishing customers.
Permalinks embedded in the source document (for example, Dynamic Links) were previously only resolved if the Source for Published Document value was set to the default Viewable Rendition. These Permalinks will now resolve even when a custom rendition (for example, Imported Rendition) is selected. Vault Link Annotations continue to be resolved only when Viewable Rendition is selected and are not impacted by this change.
Learn more about working with hyperlinks.
RIM Submissions, RIM Submissions Archive
Active Dossier Support for Product Family Level Documents
Active Dossier now supports documents only associated with a Product Family (and not a Product, Active Substance, or Inactive Ingredient). This is needed to support documents in the Active Dossier that are written on a Product Family level, for example documents for Section 3.2.R - Regional Information.
Active Dossier Viewer/Editor Performance Improvement
This feature significantly improves the loading time and overall performance of the Active Dossier Viewer/Editor, especially when the Submission and Regulatory Objective have many relationship records or countries.
Add to Active Dossier Dialog Update
New functionality for the Active Dossier Editor allows users to populate Application, Regulatory Objective, and Submission fields on manually-created Active Dossier Item Detail records. Users will see these new options in the Add to Active Dossier dialog box when in Edit mode. When a user opens the Active Dossier Editor action from a Regulatory Objective or Submission record, Vault automatically populates the field with the current record value.
Active Dossier Loader Validations
In this release, we have added two additional validation checks on required and controlling fields to clearly notify users of errors when loading Active Dossier records. Previously, users received an unclear batch error when certain field conditions were not met.
Learn more about loading Active Dossier records.
Active Dossier Language Field Update
The document field Vault uses for handling Active Dossier translations has been updated, from Language (language__v) to Language for Submission (language_for_submission__v). This aligns with the document language field used in other RIM applications and features, such as auto-matching and IDMP/XEVMPD.
Learn more about Active Dossiers.
RIM Document Classification Bot
With this release, Vault automatically classifies documents uploaded to the Document Inbox in Vaults with a trained and deployed Document Classification Module. In 23R1, Vault RIM added support for email ingestion, enabling customers to easily import emails. The RIM Document Classification Bot further streamlines this by automatically classifying documents that are added to the Inbox (manually or via the email processor). Users can review the document in the inbox and add additional required metadata, and the system learns each time a user accepts or rejects a classification. Once complete, Vault removes the document from the Inbox.
This feature is Admin-enabled through a checkbox in Application Settings.
RIM Classification Bot Enablement
Once the RIM Document Classification Bot is enabled, the system automatically provisions the Trained Models object and corresponding Trained Model object lifecycle. The setting also enables the Predictions and Prediction Metric objects, used to track and provide feedback on predictions. The lifecycle includes two user actions, Train Model and Train Model on Production Data, to allow increased accuracy of auto-classification. Users are able to train the model by date (Training Window Start Date) or by attaching a CSV file containing document IDs. Once the training job is complete, the user receives a notification summarizing the training results, and then the model can be deployed.
This feature is part of Vault RIM’s Health Authority Automation journey and increases efficiency and productivity by automating document classification.
RIM Classification Bot
Learn more about unclassified documents.
Auto-On Enablement of Submissions Settings
Four Content Plan-related settings were automatically enabled and removed from the Vault Admin UI. These enablement settings were originally introduced to allow customers to adopt them on their own timelines. Now that the features are standard in all RIM Vaults, the enablement checkboxes are unnecessary, and will be fully deprecated in a future release. The auto-enabled settings include:
- Enable actions on binder content (Application Settings)
- Enable Submission Content Planning (General Settings)
- Enable Copy Into Content Plan (Application Settings)
- Enable Tree Grid (Support-enabled setting)
Additionally, the RIM Dynamic Linking setting has been moved from Submissions Features to RIM Settings within Admin > Settings > Application Settings.
These cleanup efforts make the Settings page easier for Admins to manage, and easier for Veeva to deliver updates consistently in future releases.
EDL & Manual Matching Enabled in All RIM Vaults
The Expected Document Lists (EDL) feature and Manual Matching are now both enabled in all RIM Vaults, particularly older Vaults that were previously not using this functionality. Customers who already enabled these features and customers whose Vaults were provisioned after the EDL and Manual Matching features were released are not impacted. This enablement provides consistency across all RIM environments and provides existing customers with the most up-to-date EDL settings.
Improved User Exception Message Processing for Object Transfer
This feature improves how Vault handles User Exception Messages (UEM) when transferring object records from Clinical to RIM by:
- Updating the Last Successful Run Time on successful object transfers or object transfers that encounter an Item Processing Error.
- Inactivate any previously-active User Exception Messages after each job. This allows for only a single User Exception Message to be active for a given integration.
- Rerun only items contained in User Exception Messages, plus any items updated since the Last Successful Run Time for a given integration. This prevents the connection from reprocessing already successful items
Administrators managing the connection between Vault RIM and Vault Clinical are no longer required to review duplicate UEM records on Flash Reports. Learn more about the RIM to Clinical Operations Connection.
23R2 RIM Data Model Changes
See 23R2 Data Model Changes: Regulatory.
Safety
The Safety 23R2 release, including all Platform features, is targeted for tentative availability on August 17, 2023 & August 25, 2023.
Safety
Multi-Product Selector for Study Products on Inbox Item
This feature introduces a new action, “Specify Study Products”, to facilitate Study Product entry on manual and E2B imported Inbox Items. Upon selecting the action, Case Processors can add Study Products using a multi-select field. This addition is particularly useful for complex Studies that involve numerous phases or Products, as it allows you to add multiple relevant Study Products to the Inbox Item at once. Vault Safety promotes all Study Product records to Case and as a result, improves Case processing efficiency.
Multi-Product Selector for Study Products on Inbox Item
Learn More:
- Enablement: Enable the Multi-Product Selector for Study Products on Inbox Items
- User Help: Inbox Item Field Reference: Add Study Products to Inbox Items
Study Site Reporter on Inbox Item
Admins can now configure Study Contacts to use as Site Reporters during the initial intake of Inbox Item Study Cases. This feature was previously only available for AERs, but now Intake users can select the appropriate Site Reporter during Inbox Item intake. Upon Case promotion, the system populates all required details of the Site Reporter on the Case, increasing data entry efficiency and accuracy. This new feature is particularly important because it streamlines the Case intake process, reduces the risk of errors, and ensures compliance with regulatory requirements. The ability to configure Study Contacts for use as Site Reporters facilitates identifying and selecting the appropriate person for the role, saving Intake users valuable time and effort.
The following video provides a demo of using Site Reporters for Inbox Items:
Study Site Reporter on Inbox Item
Learn More:
- Enablement: Enable Study Site Reporter: (23R1) Add the Site Reporter Field to the Inbox Item Page Layout
- Admin Help: Manage Studies: Set Up Study Site Reporters
- User Help: Inbox Item Field Reference: Site Reporter
Non-E2B Case Identifiers Entry and Duplicate Search
Vault Safety can now store non-E2B Case Identifiers (Reference Numbers) to use for duplicate search. In addition, Admins can now configure both re-transmittable and non-transmittable Case Identifier types, where only re-transmittable types are included in E2B transmissions. Furthermore, intake users can now enter Case Identifiers on Inbox Items. Lastly, All Case Identifiers will be used for duplicate search.
The ability to include non-E2B Case Identifiers as part of the duplicate search will make the Case Intake process more efficient and give users the flexibility to add Case Identifiers to Inbox Items.
Non-E2B Case Identifiers Entry and Duplicate Search
Learn More:
- Enablement: Enable Non-E2B Case Identifiers Entry and Duplicate Search
- User Help: Inbox Item Field Reference
Case Compare Limit Increase
When creating a Follow-up Case or merging to an In-flight Case, Vault Safety now supports a maximum of 10,000 records for up to 3 objects and 500 records for the remaining objects on Case Compare. Once the limit is reached, the system displays a message and prevents Case promotion from Case Compare. Previously, the Case Compare record limit was capped at 500 records per object.
By increasing the Case Compare record limit, Vault Safety ensures that valuable information is not lost during Case promotion. This feature provides you with an efficient way to carry over new information to Follow-up and In-flight Cases, improving data integrity and streamlining workflows.
Learn More:
Fast Verify for Imported Inbox Items
Vault Safety now validates the Inbox Item data upon importing a Case to Inbox Item through E2B or API integration. This feature improves Intake processing efficiency by removing the need to manually verify each Inbox Item section before promoting to a Case.
In addition, sections and records will be clearly indicated as Verified or Invalid to facilitate the correction of incorrect data before promotion. Error messages are displayed within the appropriate sections and records. For Case Contact, Product, and Event sections, Vault Safety displays the number of records with invalid data. This is especially important as previously, the system did not identify the invalid records and fields, making it difficult and time-consuming for intake users to correct errors. With this feature, you can easily identify errors, saving valuable time and effort.
Fast Verify for Imported Inbox Items
Learn More:
Automated Case Promotion Action
This feature introduces significant enhancements to the Automated Case Promotion workflow in Vault Safety.
Admins can configure a new user action to manually trigger Automated Case Promotion for non-Cases (for example, a Case that does not require submission to a Health Authority). They can also configure an entry action to proceed with Automated Case Promotion when a non-Case enters a certain lifecycle state. These configurations streamline the non-Case workflow, which previously was not suitable for Automated Case Promotion.
Learn More:
- Enablement: Enable Automated Case Promotion for Non-Cases
- User Help: Automated Case Promotion
Enhanced Product Matching Support for Intake
This feature extends all Product matching logic to JSON API import. Previously, Product matching was only supported for E2B import to Inbox Item. Additionally, when a Product Registration Name (Trade Name) is created or updated, Vault Safety automatically creates a Product Alias, if that alias name does not already exist.
Learn More:
- User Help: Inbox Item Study and Product Matching
Full Case Data Import to Inbox Item Through API
The public JSON Intake API now supports the import of all Case data to Inbox Items. The JSON format will accept any Case-related objects, including Case Medical History, Drug History Test Results, and Case Identifiers. This feature facilitates the integration of Vault Safety Inbox Items with any external source system without the need to generate E2B files.
Company Product Match Verification
This feature introduces a more advanced coding verification for Product matching upon E2B and JSON import. Admins can configure the Product coding criteria, which include the fields by which the imported Product and library Product must match (Country, Route of Administration, and Dose Form). Admins can also configure the level of confidence needed for Product matching (for example, Confident Match, Likely Match, and more). If the Product coding criteria are met, the system proceeds with Product matching and auto-coding.
This Product matching enhancement improves the accuracy of generic Product matching and increases efficiency during the Case intake process.
Company Product Match Verification
Learn More:
- Enablement Help: Enable Company Product Match Verification
- Admin Help: Configure Company Product Match Verification
- User Help:
- Inbox Item Study and Product Matching
- Inbox Item Field Reference: Product Match Confidence and Product Match Criteria
Simplified Case Access Group Security Setup
In 23R1, Vault Safety introduced Case Access Group Security to provide a more straightforward way to manage access to Cases. The feature matches groups to Cases based on data such as Sponsor, Report Type, Country, and Market Segment. It also provides more granular control over unblinded and protected information.
With this release, setting up Case Access Groups is made even simpler. Optimized matching logic and the addition of a Country field on User Access Group Assignment records make setup more flexible, reducing the administrative burden of creating many Case Access Group Assignment records.
Learn More:
- Enablement: Enable Case Access Group Security: 23R2 Update
- Admin Help: Manage Case Access Group Security
PMDA Special Report Classification for Case Processing
Vault Safety now supports PMDA Special Report Classification for Case Processing. In 23R1, Special Report Classification was introduced on the Inbox Item and Case. With this release, values set on the Inbox Item are promoted when creating Cases or Follow-up Cases or merging to In-flight Cases. This streamlines Case processing and enhances efficiency.
Learn More:
PMDA Local Case Processing Automations
To streamline reporting and reduce manual effort, Case Product Registrations and Case Assessments are now automatically populated for Domestic and Localized Cases that are reportable to Japan.
PMDA Automated Reportable Product Registration Retrieval
This supports the auto-population of reportable Case Product Registrations, expediting data entry for the J2 data elements of PMDA E2B(R3) reports.
PMDA Local Assessments and Expectedness
Auto-populated Case Product Registrations enable local processors to assess Expectedness more efficiently. Comprehensive Product and Assessment data supports more informed decision-making for both Domestic and Localized Cases.
Learn More:
- Enablement: Enable PMDA Local Case Processing Automations
- Admin Help: Set Up the Localized Business Admin Library
- User Help:
Auto-Generate Serious and Non-Serious Assessments
Vault Safety makes Case processing more efficient with automatic generation of Assessments, Assessment Results, and Expectedness records for all serious and non-serious Adverse Events paired with Suspect or Interacting Case Products. Previously, this feature required a user action. Now, auto-generation is available on Case promotion and when adding or editing Case Products and Adverse Events.
Auto-Generate Serious and Non-Serious Assessments
Learn More:
- Enablement: Enable Auto-Generate Serious and Non-Serious Assessments
- User Help: Enter Case Data: Assessments Section
Study Product Expectedness
To support clinical trial studies with multiple products and different expected Adverse Events, Vault Safety is introducing Study Product Datasheets. Now that Admins can configure datasheets for individual study products, the system generates Expectedness at a more granular level, removing the need for Case Processors to verify Expectedness manually.
In addition, when processing Follow-Up Cases, the system now preserves the Expectedness from the initial Case.
Finally, respecting guidelines for regulatory and aggregate reporting, Expectedness evaluations now consider adverse event onset dates for Clinical Trial Study Cases.
Learn More:
- Enablement: Enable Study Product Expectedness
- Admin Help: Manage Datasheets and Auto-Expectedness: Datasheet Types
Product Masking Selection for Study Products
For Case Study Products, Studies, and Study Arms, Blinded field values are now consistently Open Label, Unblinded, and Blinded, communicating the masking more clearly.
Learn More:
- Enablement: Enable Product Masking Selection for Study Products
- User Help: Enter Case Data: Blinding Details Section
Clinical Trials: Blinded Field and Product Reported Field Updates
For Clinical Trial Study Cases, during Case unblinding if all Study Products are unblinded, Case-level blinding is now automatically set to Unblinded. This increases the efficiency of unblinding Study Cases. Additionally, when adding unblinded or open label Study Products to a Case, the Study Product Name is mapped to the Product (Reported) field.
Learn More:
- User Help:
New Substance Name Field in Product Library
To match E2B guidance, the Vault Safety Product Library includes a new Substance Name field that supports up to 250 characters. The increase from 128 characters means more accurate substance descriptions can be used, improving compliance on E2B Transmissions. When generating Substance Names on Cases, the system downloads values from the new field, if populated, or will continue to download from the existing field. This logic supports setting up longer Substance Names only when required.
Learn More:
- Enablement: Enable the New Substance Name Field in the Product Library
- Admin Help: Manage Products: Add Substances
Clear Radio Button Selection for Submittable Fields
Vault Safety now offers a “Clear Selection” button for submittable fields with Yes/No radio buttons on Cases so that users can clear their selection in case of errors or changed information. This feature enhances the usability of data entry and improves the overall efficiency of case processing.
Learn More
Asynchronous Activity Indicators - Validation Results
Vault Safety now displays an indication to inform users about the status of regulatory conformance validations on Cases and Transmissions. The indication banner also includes an option to refresh the section and view results when they are ready.
This notification will enhance usability by keeping users informed about the progress of generating validation results.
Learn More
Faster Company Product Selection on Inbox Item
This feature streamlines the Product coding process by introducing a control where you can quickly and efficiently select Products during Case intake. Previously, Product selection was done with a simple dropdown picklist. This is functional for a small number of Products, but selecting a Product from larger Product libraries was cumbersome. This feature introduces two new buttons: Auto-code and Product search (binoculars icon). After selecting a Product, you can hover over the Product field to display information such as the Product Family, Product, Trade Name, Registration, and Country. In addition, selecting the Binoculars icon opens a Product coding browser where you can search, filter, and view additional information related to the Product.
Faster Company Product Selection on Inbox Item
Learn More
- Enablement: Enable Faster Company Product Selection on Inbox Item
- User Help: Auto-Code and Browse Products
Smart MedDRA Coding on Import
With this release, Vault Safety extends Smart MedDRA Coding to E2B and JSON import for Inbox Item creation. Our most advanced MedDRA coding option, this feature uses AI and automation to code reported terms that are not an exact match to terms in the active MedDRA dictionary or your MedDRA Synonym list. For example, Smart MedDRA Coding offers suggestions for misspelled terms, abbreviations, ambiguous information, multiple reported terms, and more. This feature could also facilitate Automated Case Promotion by coding high-confidence terms during import or Inbox Item creation.
This feature is Auto-on in Vaults with Smart MedDRA Coding enabled.
Learn More
- Enablement: Enable Smart MedDRA Coding
- User Help:
Rule Engine Destination Evaluation Log
With this release, Vault Safety creates a CSV file to log the following Rule Set execution information:
- Rule Execution Time
- Destinations Evaluated
- Which Destinations passed a rule
- Which Destinations did not pass a rule
Currently, when running Submission and Distribution rules, Vault Safety creates Transmission messages which describe why each of the submissions was generated. However, if no rule applies, this is not logged, often causing confusion as to why no submission was created.
This feature documents the complete picture and assures users that all Transmission rules were fully evaluated.
Learn More
- Enablement: Enable the Rule Engine Destination Evaluation Log
- User Help: Understand the Reporting Rules Engine: Download the Submission Rule Log for a Case
Logically Delete Non-Submittable Transmissions on Subsequent Rules Engine Evaluations
The Vault Safety Rule engine re-evaluates reporting obligations on all Case data modifications. It automatically updates previously created Submissions to include only Transmissions that meet reporting obligations. This feature also updates Transmission Due Dates when data is re-evaluated. This prevents over-reporting of Cases without needing to manually remove Submission records.
Learn More
- Enablement: Enable Logical Deletion of Non-Submittable Transmissions on Subsequent Evaluation
- User Help: Submissions and Distributions Overview: Transmissions and Subsequent Evaluations
Safety Rule Set Config Report
Previously, it was difficult to visualize the Rules and Rule Parameters that configure a Rule Set. Now, Business Administrators can generate a Safety Rule Set Configuration Report for a Rule Set. They can download the report as an XLS file and review the Rule Set’s Rules and Rule Parameters in an easy-to-read format. If required, they can generate Safety Ruleset Configuration Reports in bulk.
Safety Rule Set Config Report
Learn More
Custom Rulesets and Rules Configuration
Vault Admins can now configure Custom Reporting Rulesets and Rules for global Agencies and Trading Partners without having to request approval by raising a Support Ticket. This provides the flexibility to configure Safety rulesets based on your business needs.
Learn More
- User Help: Create Custom Safety Rule Sets
Prioritize Seriousness for Downgrades
To ensure more accurate transmission creation for Downgrade and Upgrade reports, when Vault Safety calculates the Most Conservative Product for Submission Rules, Serious Adverse Events are always prioritized over Non-Serious Adverse Events as shown in the following table:
| Summary | Seriousness | Expectedness | Relatedness | Ranking |
|---|---|---|---|---|
| Fatal/LT SUSAR |
|
Unexpected | Related | 1 (Most Conservative) |
| SUSAR | Serious | Unexpected | Related | 2 |
| SU | Serious | Unexpected | Unrelated | 3 |
| SESAR | Serious | Expected | Related | 4 |
| SE | Serious | Expected | Unrelated | 5 |
| NSUR | Non-Serious | Unexpected | Related | 6 |
| NSU | Non-Serious | Unexpected | Unrelated | 7 |
| NSER | Non-Serious | Expected | Related | 8 |
| NSE | Non-Serious | Expected | Unrelated | 9 (Least Conservative) |
| 1. If a case contains both a Life Threatening SUSAR and a Fatal SUSAR, the tiebreaker for Most Conservative Assessment will be the Fatal SUSAR. | ||||
Learn More
- User Help:
Upgrade/Downgrade Transmission State Evaluation
Vault Safety has the option to ignore the Transmission Lifecycle State when considering if a Case qualifies as an Upgrade or Downgrade report to a specific destination. Previously, the Upgrade and Downgrade parameters did not consider if the previous Case version was submitted. As a result, the Downgrade rule might be executed even though the initial Case was not reportable.
Learn More
- User Help:
One Last Time Reporting for Reportability Changes
Previously, the evaluation of One Last Time (OLT) Transmissions used a “Rule Set by Rule Set” approach. Consequently, if a Rule Set was executed in a previous version of a Case, it would not be executed in any subsequent follow-up versions where the Case Product, Drug Role, or Study changed. This resulted in under-reporting to health authorities.
With this release, the OLT reporting logic automatically assesses all reporting destinations that include Transmissions in the selected states, regardless of the rule set configuration.
Learn More:
- Enablement: Enable One Last Time Reporting for All Destinations
- User Help: One Last Time Reporting
IMDRF on FDA MedWatch 3500A
Admins can now configure FDA MedWatch 3500A forms to export International Medical Device Regulators Forum (IMDRF) device codes instead of FDA device codes. This provides more flexibility when reporting to the FDA.
Learn More
- Enablement: Enable IMDRF on FDA MedWatch 3500A
- User Help: FDA 3500A Generation Data Mapping
FDA MedWatch 3500A Support for OTC and Additional Device Fields
When generating FDA MedWatch 3500A forms, more device-specific fields and Combination Product scenarios are supported. The fields now exported include the following:
- C.7. Is the Product Over-the-Counter?
- G.5. OTC
- H.3. Device Evaluated by Manufacturer
- H.9. If action reported to FDA under 21 USC 360i(f), list correction/removal reporting number
- H.10. Additional Manufacturer Narrative
In addition, when exporting Combination Products to FDA MedWatch 3500A forms, Vault Safety uses the Transmission Product Type to determine whether to export the Product to section C - Suspect Products or section D - Suspect Medical Device and H - Device Manufacturers Only. This feature also introduces OTC Device as a Transmission Product Type.
This feature is Auto-on, however some components require additional configuration.
Learn More
- Enablement: Enable FDA MedWatch 3500A Support for OTC and Additional Device Fields
- User Help: Enter Case Data: Device Information Section
OTC Carton Attachment Export for FDA E2B(R2)
To adhere to FDA guidelines for reporting cases with Over-the-Counter (OTC) products, Vault Safety now supports attaching images of OTC cartons when sending FDA E2B(R2) Transmissions through an AS2 Gateway.
Learn More
- User Help: Enter Case Data: Documents Section
Aggregate Report Interval Case Listing
Vault Safety now provides in-system access to a comprehensive listing of all Cases included in the interval report for a specific Aggregate Report (DSUR, PBRER, PADER, PSUR, CIOMS II) with this aggregate report Case linking feature.
You can incorporate the new Aggregate Report Case object into your analytics reports to verify that all relevant Cases are captured.
This feature provides greater insight and control over the regulatory reporting process.
Learn More
- Admin Help: Enable Interval Case Listing for Aggregate Reports
- User Help:
DSUR and PBRER List All Suspect Products
DSUR Line Listings now list all suspect products for a given Case. Previously, only the primary suspect Product was listed. The DSUR and PBRER also now include a Breakdown of Investigative Medicinal Products tabulation for Serious Adverse Events (SAEs) and Serious Adverse Reactions (SARs) from Clinical Trials. This is particularly important for combination therapies and provides enhanced DSUR reporting.
This feature is Auto-on, however an Administrator will need to load two new DSUR templates in your Vault.
Learn More
- Enablement: Enable the DSUR and PBRER to List All Suspect Products
- Admin Help: Configure Aggregate Report Templates: Standard Table Templates
- User Help: Create DSUR Aggregate Reports
DSUR and PBRER Drug Role Filtering
The DSUR and PBRER now have a Drug Roles to Include report parameter. This report parameter affects the following tabulations:
- Interval Line Listings of Serious Adverse Reactions
- List of Subjects Who Died During the Reporting Period
- Cumulative Tabulation of Serious Adverse Events From Clinical Trials
- Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials.
Previously, these tabulations included all drug roles (Suspect, Drug Not Administered, Interacting, Similar Device, Treatment, and Concomitant). Now you can specify which drug roles to include in these tabulations based on business requirements.
Learn More
- Enablement: Enable Drug Role Filtering for DSUR and PBRER
- User Help:
DSUR and PBRER Investigational Product Causality
Vault Safety can be configured to only consider Case Assessment Results for the Investigational, Placebo, Active Comparator, and Blinded Study Products when deciding if a Case should be included in the DSUR and PBRER Serious Adverse Reaction listings and tabulations. Previously, Vault Safety considered all assessments for causality and all adverse event counts to determine if a Case should be included in the Serious Adverse Reaction listings.
This feature ensures that Vault Safety only considers causal assessments related to Investigational Products in the DSUR and PBRER.
Learn More
- Enablement: Enable DSUR and PBRER Investigational Product Causality
- User Help:
23R2 Aggregate Report Features
PMDA Submission Rule Parameter Updates
In 23R1, Vault Safety introduced support for multiple PMDA Submissions from a single Case, with the plan to update the associated Reporting Rule Parameters in a later release. With 23R2, the PMDA Rule Parameters now leverage the new and changed fields, including Registration Type, Infection, and Special Report Classification. In addition, the new Previously Localized Rule Parameter is also evaluated. These updates support PMDA requirements for calculating due dates.
Learn More
- Enablement: Enable PMDA Submission Rule Parameter Updates
- User Help:
PMDA Local Receipt Date Overrides
For PMDA E2B(R3) Submissions, local Case Processors can now enter values for Receipt Date (C.1.4) and New Info Date (C.1.5), overriding the dates from related Global Cases. This feature introduces two (2) new fields on Localized Cases, Local Initial Receipt Date and Local New Info Date, offering greater alignment with PMDA and NMPA guidelines.
Learn More
- Enablement: Enable Local Receipt Date Overrides
- User Help: Prepare a Localized Case: Details Section
Domestic Local Awareness Date Synchronization
To ensure dates remain consistent, reduce manual data entry, and support on-time Case Submissions, the Local Awareness Date on Domestic Localized Cases is now populated with the New Info Date from Global Cases.
Learn More
- User Help: Prepare a Localized Case: Details Section
Manual Transmission Creation Automation
To increase efficiency for business users when manually creating Submission or Distribution records, Vault Safety now populates the following fields on a transmission based on the selected Transmission Profile:
- Origin
- Destination
- Outbound Format
- Additional Output Format
- Sender User
- Message Subject (Including Tokens)
- Message Body (Including Tokens)
- Cover Letter Template
Learn More
Gateway Connection IP Management
Along with enhanced troubleshooting capabilities, Vault Safety Administrators can now create and delete entries in the IP Allowed list for AS2 Gateway Connections.
External E2B+ Builder SDK Event Type
The Vault Safety Java SDK now provides the E2BOutboundEventType. For developers building an external integration with an E2B+ Builder Service, this parameter indicates whether the request type is “Regenerate” or “Submit”.
Feature Enablement Changes
Enablement changes apply to the following features in this release:
| Feature | Previous Enablement | New Enablement |
|---|---|---|
| Generate Assessments Record Action | Support (23R1) | Configuration (23R2) |
| Parameter Driven Assessment Selection for Rules Engine | Support (23R1) | Auto-On (23R2) |
| Enhanced Eligible Products Selection for Cross Reporting | Support (23R1) | Auto-On (23R2) |
| All Product Types Available for FDA 3500A and CIOMS I Generation | Support (23R1) | Configuration (23R2) |
See the following articles to learn more about these features:
- Generate Assessments Record Action: What’s New in 23R1
- Parameter Driven Assessment Selection for Rules Engine: What’s New in 23R1
- Enhanced Eligible Products Selection for Cross Reporting: What’s New in 23R1
- All Product Types Available for FDA 3500A and CIOMS I Generation: What’s New in 23R1
SafetyDocs
PSMF Periodic Review
Vault SafetyDocs can now automatically trigger periodic reviews of PSMF documents. PSMF content must be reviewed on a regular basis for compliance, which can be a repetitive and time-consuming process. With this feature, users can automate periodic reviews and regularly assess if any changes are required. The system can then assign review tasks to users and details about their completion can be tracked. This eliminates the burden of scheduling periodic reviews and helps to maintain compliance through up-to-date PSMF content.
PSMF Periodic Review
Learn More
- Enablement: Enable PSMF Periodic Reviews
- User Help: Set up PSMF Periodic Reviews
- Admin Help: Configure the PSMF Periodic Review Job
PSMF Version Binding
Vault SafetyDocs now automatically selects the correct PSMF document versions when creating a draft of the PSMF binder and when reviewing and approving PSMF binders. Previously, users had to manually edit the binder and select which version of the documents to include.
This feature improves the efficiency of the review process, reduces the risk of errors, and ensures compliance with regulatory requirements.
Learn More
- Enablement: Enable PSMF Version Binding
- User Help: Manage PSMF Binders and Logbooks
PSMF Automated Vault Report Annexes
Vault SafetyDocs now leverages Vault Reports to automatically generate PSMF Documents. These reports can contain data such as information about marketed products, studies, and delegated tasks. Previously, every time changes were applied to a specific document, users had to manually run the relevant report, download it, upload it as a document to the relevant binder, and then approve it. This feature allows documents to be updated during PSMF generation or periodic review, making the process more efficient. Additionally, this automated process reduces the risk of errors and ensures compliance with regulatory requirements.
Learn More
- Enablement: Enable Automatic Generation of PSMF Documents from Vault Reports
- User Help: Generate PSMF Documents from Vault Reports
Signal Management
Signal Management is now supported in Vault SafetyDocs. This new area introduces capabilities for conducting Signal Investigations, tracking emerging signals (as Product-Event combinations), and managing the overall lifecycle based on GVP Module IX.
Once configured, users are able to:
- Track Product-Event combinations and set dispositions
- Perform Safety Investigations using lifecycle states and workflows based on GVP Module IX
- Link one or more Product-Events to a Safety Investigation
- Set final verdicts and dispositions on a Safety Investigation
- Link documents to a Product-Event combination or Safety Investigation
Using this feature will ensure compliance to Safety standards and increase user productivity.
Signal Management
Learn More
- Enablement: Enable Signal Management
- User Help: Signal Management
Aggregate Report Authoring
Vault SafetyDocs now supports the end-to-end drafting of all Aggregate Report components within your Vault.
Authoring tasks can be created for each section of the report and assigned to users in Vault. These tasks can relate to the authoring of the Aggregate Report as a whole or the authoring of a section specific to a particular Aggregate Report Destination. Users can also track the planned and actual submission dates for each Aggregate Report Destination.
This feature allows Aggregate Reports sharing the same type, product, and data periods to be grouped together so their components can apply to multiple markets and health authorities. Supporting the full drafting process in Vault SafetyDocs also provides a validated way of authoring these documents and allows relevant business groups to easily collaborate on them.
Note: Aggregate Report Authoring is a building block for expanding future Aggregate Reporting capabilities in Vault SafetyDocs. This feature will be available for full configuration in an upcoming release. For more information, contact your Veeva representative.
Learn More
- User Help: Author Aggregate Reports
Data Model Changes
See 23R2 Data Model Changes: Safety.
QualityOne
QMS
Product Groups
The Product Groups feature allows customers to create and manage groupings of products based on shared attributes such as usage, brand, material, or other defining characteristics. Product Groups enable customers to build an individualized hierarchy of product groups using a standard self-referencing object, allowing the flexibility to define their product groups according to an organization’s unique business structure and requirements.
Lab Investigations
Lab Investigations helps in the management and investigation of Out of Specification (OOS) or Out of Trend (OOT) laboratory errors. Customers can document, standardize, and track lab investigations using a phased investigation approach to establish if the product is OOS or OOT, or if there was a potential laboratory error that resulted in the product being outside of the acceptable limits and specifications.
Learn more about the Lab Investigations feature.
Lab Investigations
Generate Documents for Standard QMS Processes
This feature extends the Generate Documents using Object Record Actions for Audits feature introduced in 23R1 by adding nine (9) additional objects.
- CAR
- Change Control
- Complaints
- Formulation (to generate Specification document)
- NCR
- Packaging (to generate Specification document)
- Product (to generate Specification document)
- Risk Register
- Risk Study
This feature allows users to generate a document from a Formatted Output or a Document Template using an Admin-configured entry or user action. By generating documents, users can leverage Vault’s full Document Management functionalities, removing dependency on manually configuring and uploading documents to the Vault Library. Generating documents using a 1-click solution also automatically generates a link between the object record and the document in the Vault Library, ensuring users retain the context of the generated document.
Learn more about generating documents for the standard QMS processes.
Generate Documents for Standard QMS Processes
QualityOne Audit Checklist on Android
This feature supports Audit Checklists performed on the Android operating system, allowing respondents to perform an Audit via a checklist on an Android device for an audit checklist configured in Vault. This Android application works in partially-connected environments, where many audits are performed in locations where cellular or WiFi connectivity is either inconsistent or not permitted.
The QualityOne Audit Checklist Mobile 23R2 release is targeted for tentative availability on August 22, 2023.
Learn more about Android support for audit checklists.
COA: Expanded Email Intake Support
This feature builds upon the Automated Email Intake for COA feature by allowing external parties to upload COA files to Vault for analysis without direct interaction with Vault. Expanding this feature allows external parties to easily upload relevant files and create COA Inspections without requiring a supplier Vault login, which helps to speed up the validation, ingestion, and analysis process. Customers must also implement a new Vault Java SDK entry point to allow the Supplier Initiated COA Email Processor email intake type to fully handle incoming emails to Vault.
Learn more about the the expanded Email Intake feature.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Supporting Multi-Batch Identification for COA
This feature provides Vault with additional capabilities to determine the number of batches in a single COA file during ingestion and analysis. Based on the number of identified batches, Vault will create one (1) COA Inspection record per batch. Identifying multi-batch COA files allows customers to ingest a wider range of COA formats.
Learn more about analyzing multi-batch COA files.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram
This feature allows users to create and update a HACCP process flow diagram in an easy-to-use visual interface. Each element on the diagram such as a process step or connection between two steps is saved as a record in Vault, and users with View permission can review a brief summary of each record. Users can lock and unlock the diagram based on the lifecycle state of the HACCP Plan. Process steps identified as CCP or OPRP steps display with the appropriate labels on the diagram.
Learn more about the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Plan Enhancement
The following are some of the improvements to the existing functionality of HACCP in QualityOne.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Ability to Add the Same Process Hazard Analysis to Multiple Process Steps in a HACCP Plan
This feature allows the user to add an existing hazard analysis to multiple steps in the same HACCP Plan. Validation logic is recommended to prevent users from selecting a hazard analysis associated with another HACCP Plan.
Increase in Record Limit for Deep Copy & Populate HACCP Plan Actions
This feature increases the limit of records a user can copy at once to 4000 for the following record actions:
- Create HACCP Plan Design from Design
- Create HACCP Plan from Design
- Populate HACCP Plan
Learn more about HACCP Management.
QMS & HSE
Risk Management: Support for 2D or 3D Risk Matrices
This feature allows customers to choose between two-dimensional or three-dimensional risk matrices at the time of new Vault provisioning. Customers can also change from two-dimensional to three-dimensional matrices in an existing Vault, but cannot revert to two-dimensional once three-dimensional matrices have been enabled. This enables organizations to decide if they want to measure Detectability as a dimension of risk for a more detailed risk assessment.
Learn more about working with risk matrices.
Document Control
Recall Controlled Copies
In previous releases, if a document utilizing Extensible Controlled Copy functionality was superseded or obsolesced, users would need to manually review the associated Controlled Copy Trace records to determine if a recall process was necessary for any currently-disbursed copies.
With this feature, customers can configure their Vault to find all controlled copies of a document that has been superseded or obsolesced and change their lifecycle state to In Recall. Customers can also configure a workflow to auto-start on all issued controlled copies when the associated document version is superseded or made obsolete. This workflow can be configured to issue a task to the responsible party for each issued copy, automatically launching a recall process which is done manually in Document Control today.
Learn more about the Recall Controlled Copies action.
Recall Controlled Copies
HSE
Permit to Work
This feature allows users to manage and document Permits to Work in Vault, eliminating the need for manual or paper-based Permit to Work systems. Users can submit Permits to Work for review and approval, and HSE Managers can manage the entire Permit to Work process in Vault.
Learn more about the Permit to Work feature.
Training
QualityOne Training Connector: eLearning Content Management
This feature extends the capabilities of the QualityOne and Training connection to include synchronization of E-Learning content. Users can upload, preview, and approve E-Learning files in QualityOne and the Vault Connector synchronizes the E-learning files to Training Vault. Once the files are added to the Training Vault as documents, the E-Learning documents are ready to be used for Training Requirements and Training Assignments.
Learn more about the E-Learning content management.
Data Model Changes
See 23R2 Data Model Changes: QualityOne.
RegulatoryOne
The RegulatoryOne 23R2 release, including all Platform features, is scheduled for tentative availability on August 22, 2023.
RegulatoryOne
EDL & Manual Matching Enabled in All RegulatoryOne Vaults
The Expected Document Lists (EDL) feature and Manual Matching are now both enabled in all RegulatoryOne Vaults. Customers who already enabled these features and customers whose Vaults were provisioned after the EDL and Manual Matching features were released are not impacted. This enablement provides consistency across all RegulatoryOne environments and provides existing customers with the most up-to-date EDL settings.
Regulatory Documents
Export Binder as Merged PDF
This feature allows users to merge all PDF renditions in a binder into a single downloadable file, allowing them to easily generate a complete file to send to customers and authorities.
Learn more about exporting a binder as a merged PDF.
Export Binder as Merged PDF
Enable Optimistic Locking on Registration Object
This feature turns on optimistic locking on the Registration object, which prevents users from editing records while another user updates the record and from editing records while Vault updates the record based on user-initiated actions. This ensures that users don’t accidentally overwrite each other’s updates.
Compliance Management
Bulk Create Formulation Questionnaires
This feature allows users to create up to 1,000 Formulation Questionnaires records in bulk so that regulatory teams can save time when they need to send questionnaires to many suppliers at the same time. In previous releases, users could only create a single Formulation Questionnaire at a time. Users can then use the records to generate and send related FQ Checklists to suppliers, helping prevent a backlog of RMQs.
Learn more about bulk creating Formulation Questionnaires.
Bulk Create Formulation Questionnaires
Formulation Composition Viewer: Freeze Left Columns
This feature allows Admins to freeze up to ten (10) Formulation Composition grid columns in the left side of the Formulation Composition Viewer. This allows the frozen columns to remain visible while users scroll horizontally when performing compliance assessments in the viewer.
Learn more about the Formulation Composition Viewer.
Formulation Composition Viewer: Limit 5 per Page
This feature reduces load times when users open Formulation records by limiting the number of Formulation Composition Viewer sections Admins can add to the Formulation object page layout to five (5).
Learn more about configuring the Formulation Composition Viewer.
Registration & Dossier Management
Registration Item Requirement Hierarchy Viewer
This feature allows Admins to configure a Registration Item Requirement Hierarchy Viewer on the Registration and Registration Item object page layouts to help users visualize the list of Registration Item Requirements in a hierarchical structure. This allows users to easily track the progress of all requirements in a dossier and quickly identify what requirements require attention before exporting a Dossier Binder.
Learn more about the Registration Item Requirement Hierarchy Viewer.
Registration Item Requirement Hierarchy Viewer
Enable Optimistic Locking on Registration Item Object
This feature turns on optimistic locking on the Registration Item (request__v) object, which prevents users from editing records while another user updates the record and from editing records while Vault updates the record based on user-initiated actions such as the Create Registration and Objective action. This ensures that users don’t accidentally overwrite each other’s updates.
Data Model Changes
See 23R2 Data Model Changes: RegulatoryOne.
Veeva Claims
The Veeva Claims 23R2 release, including all Platform features, is targeted for tentative availability on August 22, 2023.
Veeva Claims
Comment Thread Enhancement: Search
This feature allows users to search all comments on a record for specific text in any comment, comment ID, and commenter name in the comment thread section in Project, Claims, Local Adaptations, and Pack Copy pages. This enables users to quickly identify and review relevant comments on an object record.
Learn more about using comments.
Selective Project Automation Enhancements: Actions Run as System
This feature allows Admins to configure new Create Selective Claims and Create Selective Local Adaptations actions. These actions allow users to selectively generate all applicable Claims and Local Adaptations for a project, without any restrictions on which key object fields an Admin has assigned them the Create and Edit permissions.
Learn more about configuring the Create Selective Claims and Create Selective Local Adaptations actions.
Expanded Statement Uniqueness to Consider Statement Language
This feature allows users to create the same statement translation for two (2) different languages while enforcing uniqueness criteria on a combination of statement text and language. This allows organizations to, for example, use the same translated statement variation for both Finnish and Swedish even while enforcing uniqueness. This is an enhancement of the existing Statement Uniqueness field, which only considered statement text in previous releases and didn’t allow matching statements for different languages. As part of this feature, customers can migrate their existing Statement records to utilize this new uniqueness criteria.
Learn more about configuring statement uniqueness.
23R2 Veeva Claims Enhancements
This feature improves the usability of several previously released features. Users can now hover over a cell to view the content in Selectively Create Claims, Generate Local Adaptations, and Localize Pack Copy dialogs, allowing them to see any previously truncated text. The Statements, Claims, and Elements listed in those dialogs now default sorts by record Name fields to ensure users see a logical order of records. This feature also adds the Expand the section by default option to the Comment sections for the Project, Claim, Local Adaptation, and Pack Copy objects so that Admins can configure whether these sections are open or closed when users open records with comment threads, allowing Admins to have greater control over the user experience.
Translator View Enhancement: Freeze Header & Global Element Columns
This feature improves the usability of the Translator View section on Pack Copy records by freezing the header row and the global element columns so that Translators can scroll horizontally across different language columns while viewing the global element columns. Learn more about translating pack copy elements.
Statement Prohibit Join Object Provisioning Update
This feature updates the value format of the Name (name__v) field on the system-managed Statement Prohibit Join (statement_prohibit__v) object to avoid creating duplicate records erroneously.