Release Date: June 9, 2023
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
Working with Documents
SVG, AVIF & WebP Rendition Support
Users uploading and managing SVG, AVIF, and WebP files, which are often used in Digital Asset Management, will now see these files rendered in the document viewer. This improves Medical Legal and Regulatory (MLR) review process compliance by ensuring reviewers are able review these files directly within Vault and without needing to download the source files.
Files that include animations are now rendered in Vault as Video Renditions. Vault now also supports Optical Character Recognition (OCR), Overlays, and Signature Pages for these renditions.
Learn more about file type rendering support.
Render Animated GIF Files As Videos
Users that upload GIF files can now see those files rendered as Video Renditions in Vault within the Document Viewer. This will apply to all new uploads, including where files are up-versioned. Existing files will not be updated.
Learn more about file type rendering support.
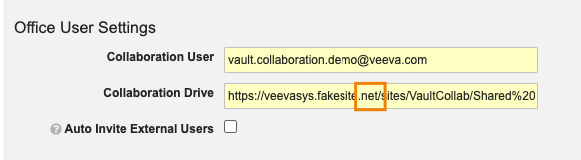
Collaborative Authoring: Expanded URL Compatibility
Collaborative Authoring functionality now supports the use of Sharepoint sites that use URL extensions other than .com, such as .net or .co.uk. Prior to 23R2, customers that leveraged custom Sharepoint sites with URL extensions other than .com would receive a Connection Error when setting up Collaborative Authoring.
Learn more about configuring Collaborative Authoring.
Transparent Handling of Non-Standard PDF Uploads
Restricted PDFs are PDFs that have security settings that prevent changes to the file, but do not have a password or encryption. Vault now respects standard restrictions for Restricted PDFs and PDFs that have certificate-based signatures similar to those applied using solutions such as DocuSign and Adobe Sign. For these types of PDFs, Vault does not perform Optical Character Recognition, merge eSignature pages, apply overlays, or apply PDF protection. This ensures that no modification occurs on these files and makes the behavior for these types of PDFs consistent with the restrictions currently applied to XFA PDFs and Encrypted PDFs.This feature has been moved to a future release.
Vault Objects
Audit All Updates to Document Reference Field Value
The following changes to an object’s document reference field are now included as entries in the audit trail:
- The referring document receives a new version
- The referring document or a version of the referring document is deleted
This ensures the Last Modified Date and the last audit trail entry are in sync. Learn more about document audit events.
Audit Correct User for SDK Trigger Updates
When a user updates a record that executes an SDK trigger, Vault now logs “[SDK User] on behalf of [Current User]” in the audit trail to show that the change was automatic based on the user’s action. Prior to 23R2, the audit trail would show that the user performed the SDK action.
For example, prior to 23R2, the audit trail would show “user@vault.com” performed the action, and with 23R2, the audit trail shows “System on behalf of user@vault.com”.
Learn more about audit events.
Display Format
Admins can now use format masks to configure how Vault should display custom text and number field values. Admins can set a format mask in a new expression editor section when configuring text fields, number fields, formula fields (where the return type is Text or Number), and lookup fields (that are looking up text or number field values). A Preview Tool allows you to see how a value will be displayed prior to saving.
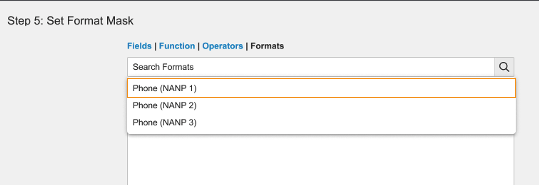
Text fields include standard format mask expressions to display user input as phone numbers (North American Numbering Plan).
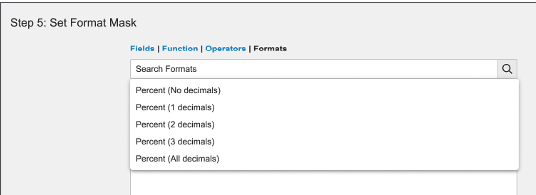
Number fields include standard format mask expressions to display user input as percentages.
Learn more about configuring object fields.
Lifecycle & Workflow
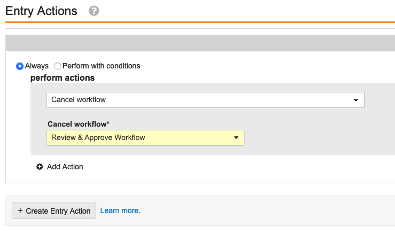
Object Lifecycles: Cancel Workflow Entry Action
Admins can now configure an object lifecycle Cancel Workflow entry action that will automatically cancel any active workflows on the current record.
For instance, in Vault QMS, if a Continuous Improvement is moved to a Canceled state, it is typical that this will automatically move the associated CAPA Action Items, SCARs and Effectiveness Checks to Canceled as well. Prior to 23R2, if there were any active workflows on the related records, Admins had to manually cancel those workflows.
With the new Cancel Workflow entry action, the Canceled state for the related records can automatically cancel the associated workflows.
Learn more about object lifecycle entry actions.
Workflow Custom Actions SDK for Multi Record Object Workflows
Admins can customize and configure custom actions in the Start step participant control for multi-record object workflows and document workflows, and the Task Step of a multi-record object workflow. This enhancement enables customers to use custom actions to automate business processes.
Custom actions are developed by your organization with the external Vault Java SDK. Once deployed to your Vault, Admins can configure the action during workflow configuration.
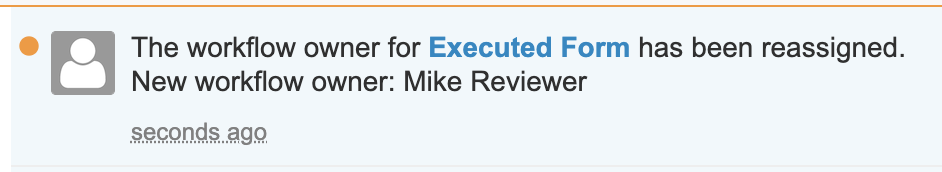
Email Notifications when Workflow Owners are Replaced
Vault will now automatically send a notification when a workflow owner is replaced on an active workflow. The notification is sent to both the previous workflow owner and the new workflow owner.
This enhancement will ensure that both the previous and new workflow owners are aware of a change in ownership without needing to communicate this outside of Vault. A new message template is leveraged for this enhancement with standard language, though Admins may update the template language for these notifications.
This enhancement is not applicable to legacy document workflows.
Learn more about workflows.
Reporting & Dashboards
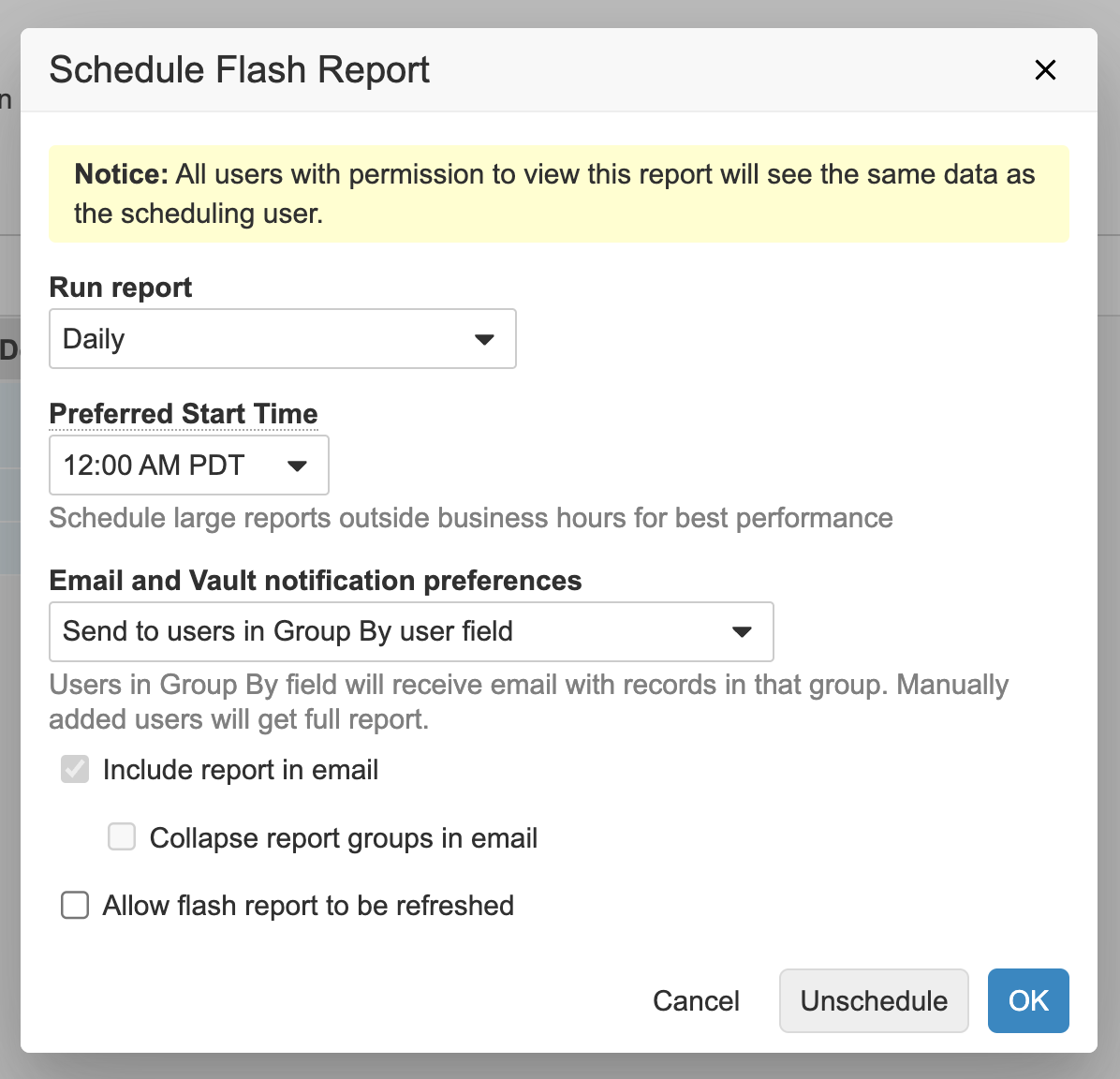
User Reminder Flash Report
Flash reports can now be distributed to users included in the results. The users can only see the rows associated with them. This new option is available for flash reports that are grouped by a user field.
While customers often leverage flash reports to manage processes, defining the right distribution list can be a challenge. If they share the flash report broadly, some users may receive flash report emails that don’t pertain to them, but if they share the flash report too narrowly, such as a flash report for each user or manager, it may be challenging to stay under the flash report limit. This enhancement addresses this challenge by sending results only to the relevant set of users and allowing these users to only access the relevant set of records.
Users with the permission to create flash reports see this as a new option in the Schedule Flash Report dialog:
Learn more about Flash Reports.
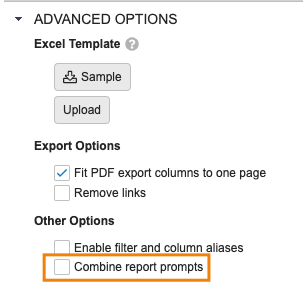
Combine Report Prompts
Users creating reports that have multiple prompts on the same data can now combine those prompts when running the report. The need to have multiple prompts on the same data is a common scenario with Multi-Pass reports in particular, where the reports combine views with overlapping components. The underlying filters should have the same object, data type, name or alias, and filter operator.
When creating reports, users will have a new option under Advanced Options to Combine report prompts:
This option was introduced with Dashboards in a prior release, and extending this to reports will simplify the running of reports for users by ensuring they don’t need to apply the same information in multiple prompts.
Learn more about Vault reporting.
Copy & Paste List of Object Names
Users are now able to copy and paste a list of object names separated by commas in input fields which take multiple objects.
It is common for customers to leverage multi-select filters, such as the In condition, where multiple items can be included. With this feature, when the In condition is on an object field, users will be able to copy and paste the correct object records into the filter from a comma-separated list.
If the names include commas, adding quotation marks around the comma-separated list will allow Vault to recognize the appropriate values. By design, Vault will ignore any duplicates.
Learn more about report filters.
Search
Current User Filter
When a user filters on a field that references a user (such as Created By), Vault now provides the Current User option to dynamically filter search results by the current logged-in user.
This enhancement allows customers to create a single shared view or custom tab that will show each user their own unique information, rather than duplicating for each user. The Current User option is available as a filter value on all user-related fields within documents or records.
For example, if a Vault has a Product Owner field that references users on the Product record, an Admin could create a Saved View that filters on Product Owner = Current User. Then, each user that accesses that view would only see the Products they own.
Learn more about custom views.
Query Limits
Vault Search, VQL queries, and the Vault Java SDK Query Service now enforce limits on search terms to ensure optimal performance:
- 250 characters for individual search terms
- 225,000 characters for the complete query when using Query Service. Learn more in the Developer Release Notes.
Learn more about searching Vault.
Formulas
Advanced Functions in Vault Formulas
Vault Formulas now support the following functions:
- PriorValue
- Regex
- VLookUp
- CurrencyRate
- IsChanged
- IsNew
- Rand
These functions are only available in validation rules, with the exception of CurrencyRate and Rand, which are available in all formula expressions. Additionally, the ID field will now be available in all formula expressions. Prior to 23R2, this field was only available in report formula fields.
Learn more about Vault formula functions.
Checklists
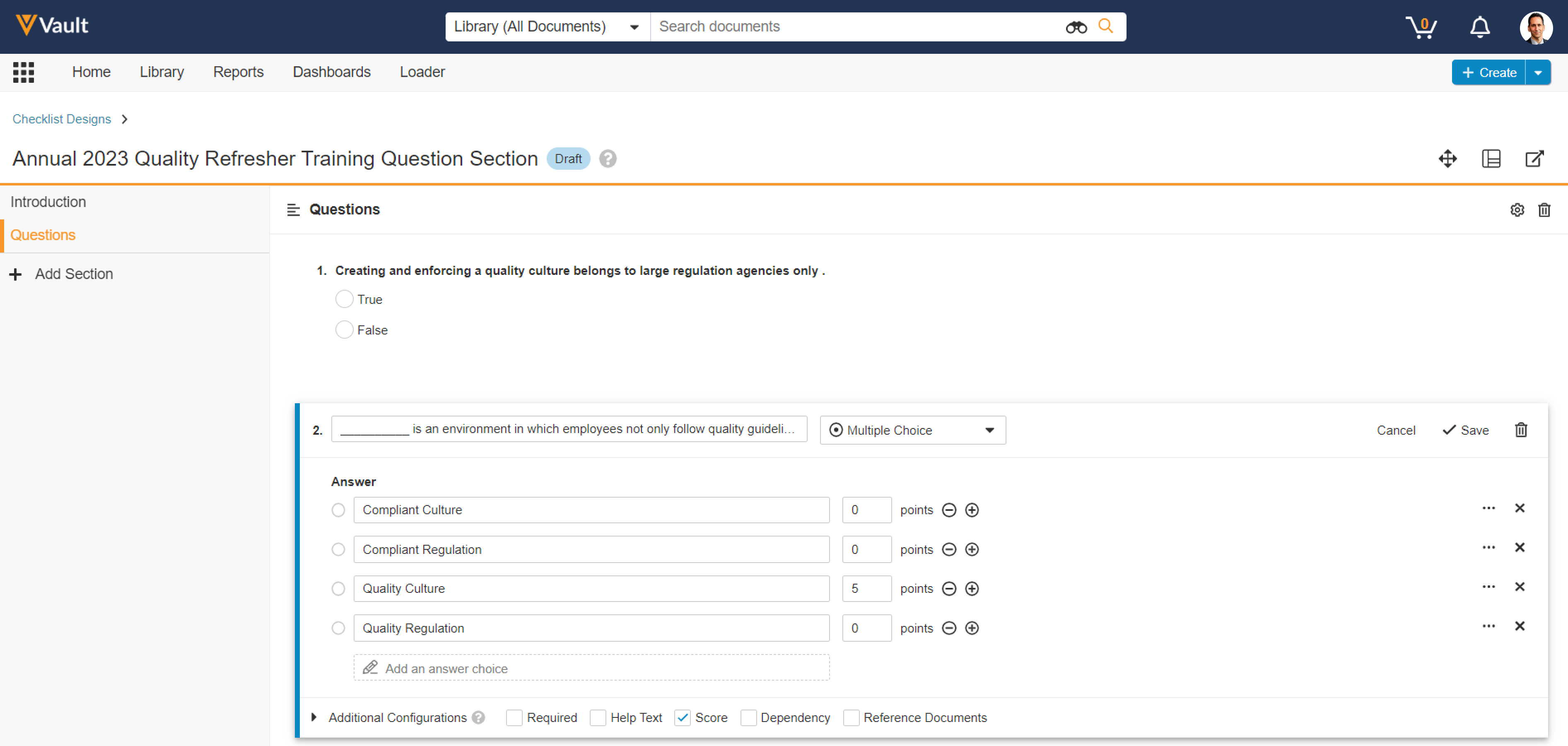
Visual Checklist Designer
Admins now have the ability to design checklists in a new visual user interface similar to what a checklist responder would use. This includes creating, editing, and deleting checklist sections, questions, answers, and dependencies.
This feature significantly increases the efficiency in creating and editing Checklist Designs, without needing to use the Checklist Design Loader. This will particularly benefit Vault Training customers who use checklists for Quizzes, and Vault Study Startup customers who use checklists for site feasibility, though checklists are also commonly leveraged in QMS, QualityOne, and RegulatoryOne Compliance Management.
Admins can launch the new Visual Checklist Designer once a Checklist Design record is created and is in the Draft state.
This feature applies to standard object types for Section Designs, Question Designs, and Available Answer Designs, and is not currently supported for custom object types.
Checklists: Sum Score
Vault will now calculate Sum Scores for checklists to provide a more relevant assessment of the overall score when using both positive and negative scoring. Sum scores will also help provide more meaningful reporting on checklists.
Prior to 23R2, the total checklist score was calculated as a percentage of the total possible score, which provides a less relevant score when negative scoring is in use.
The new Sum Score field will be enabled by default for the following objects: Checklists, Sub-checklists, and Sections.
Vault calculates the Sum Score by adding all Answer scores for multiple-choice answers together, for Sections, Sub-Checklists and the overall Checklists. If the Checklist Design is weighted, the Weight % is taken into account with each answer.
Learn more about checklist scoring and weighting.
UI & Usability Updates
Consistent Default Navigation Behavior
For customers leveraging the Landing Tab or Preferred Tab Collection field on Users, the default navigation behavior has been updated to ensure that these preferences are respected when:
- Using the My Vaults page
- Refreshing a Vault page via the browser
- Clicking the Vault logo in the upper-left
Learn more about Landing Tabs and Tab Collections.
Administration
POD Listed in Domain Information
Admins will now see a new column for POD on the Domain Information page (under Admin > Settings) to ensure that Domain Admins are easily able to identify which POD each of their Vaults is on without needing to log-in to each environment.
Learn more about Vault domains.
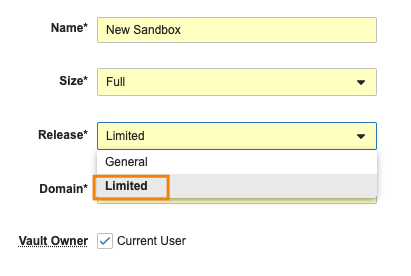
Enable Limited Release Sandbox For All Vaults
Admins are now able to provision limited release sandbox Vaults when creating new sandboxes, without needing to enable a feature flag. Admins will have the choice to select General or Limited as the Release whenever a new sandbox is created.
The ability to provision Limited Release sandboxes has previously been a feature that is only available when the Enable Limited Release Sandbox setting is enabled. This enhancement will remove that setting from Admin > General Settings, and enable this by default in Vaults.
Full Sandbox Refresh Frequency Update
With this release, Admins can now refresh a Full Sandbox once per day, if needed. With the introduction of Sandbox Sizes in the 22R3 release, the refresh limit for Full Sandboxes was once per month. An Admin can also update a Snapshot of a Full Sandbox once a day, increased from the prior frequency of once per month.
We are increasing the frequency allowed to align with the frequency on Large Sandboxes, and to provide Admins with additional flexibility in managing their Sandboxes and Sandbox Snapshots.
Learn more about sandbox administration.
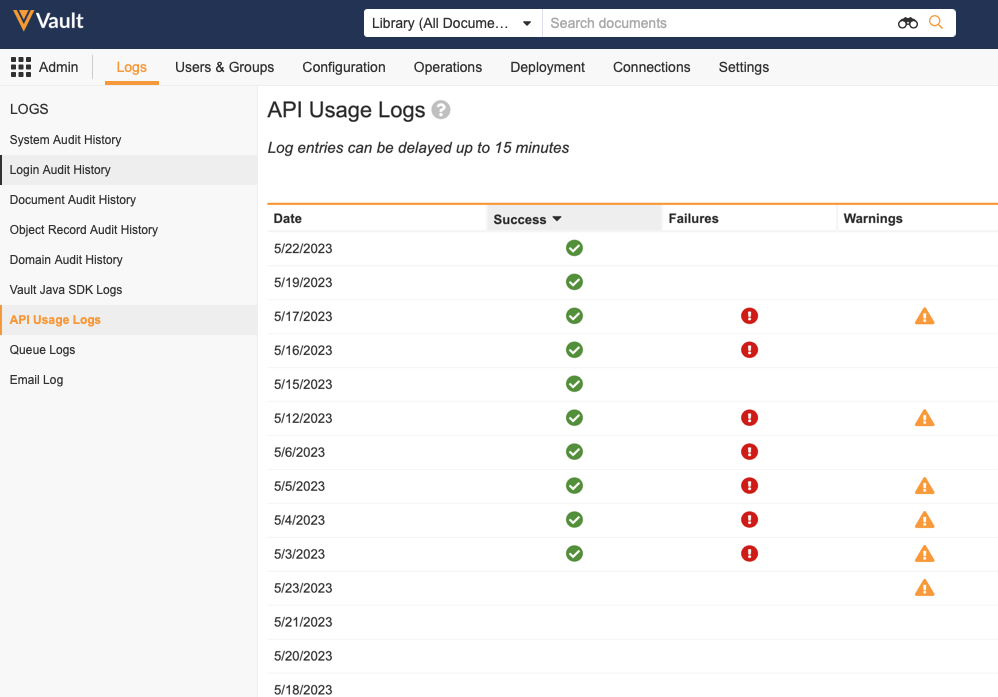
API Response Status Insights
Vault Admins can now see whether daily API Usage Logs contain Success, Failures, and Warnings. This allows Vault Admins to quickly inspect their Vault API usage and know if they need to download the log for further investigation. Insights are only available for API calls made after the release of 23R2.
Vault File Manager
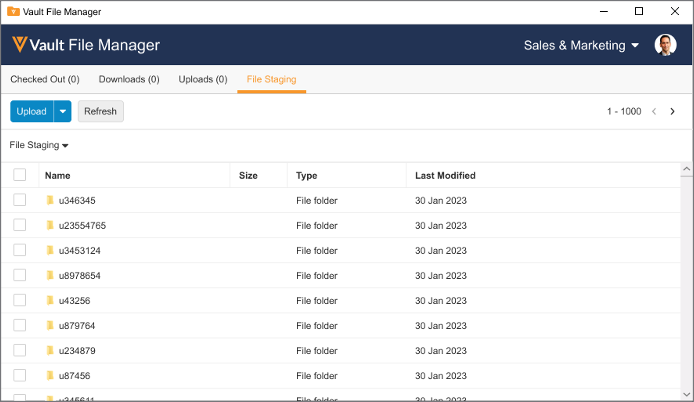
Upload to File Staging
Users can now access the File Staging server to upload documents using Vault File Manager, rather than using a separate File Transfer Protocol Secure (FTPS) client.
Many organizations may have firewalls or security policies in place that are incompatible with the use of FTPS, and this enhancement allows users to use the File Staging server without leveraging FTPS.
Learn more about Vault File Manager.
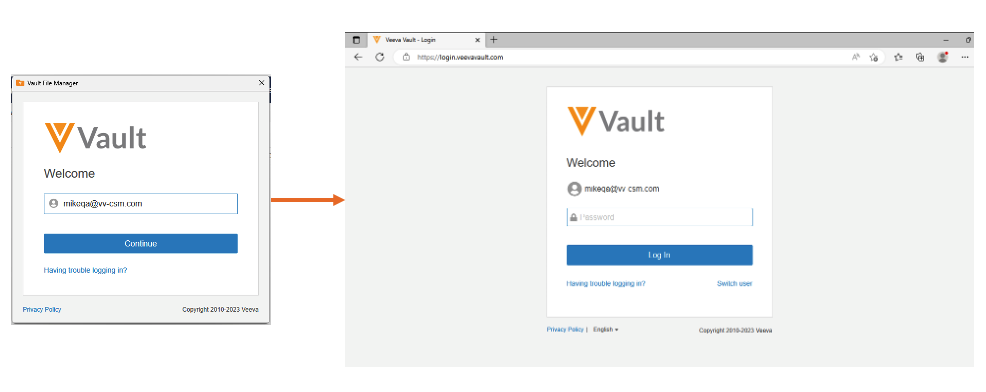
Sign in to Vault File Manager with SAML SSO
With this release, customers that previously configured SAML SSO no longer need to perform additional OAuth profile configuration to leverage Vault File Manager. There is no impact to existing authentication for Vault File Manager customers that previously configured an OAuth profile for Vault File Manager.
This enhancement makes it easier for customers to adopt Vault File Manager by removing the need for additional configuration when leveraging SSO.
With this change, when a user attempts to log-in to Vault File Manager, they are redirected to the browser to authenticate.
Learn more about Vault File Manager.
Vault Java SDK
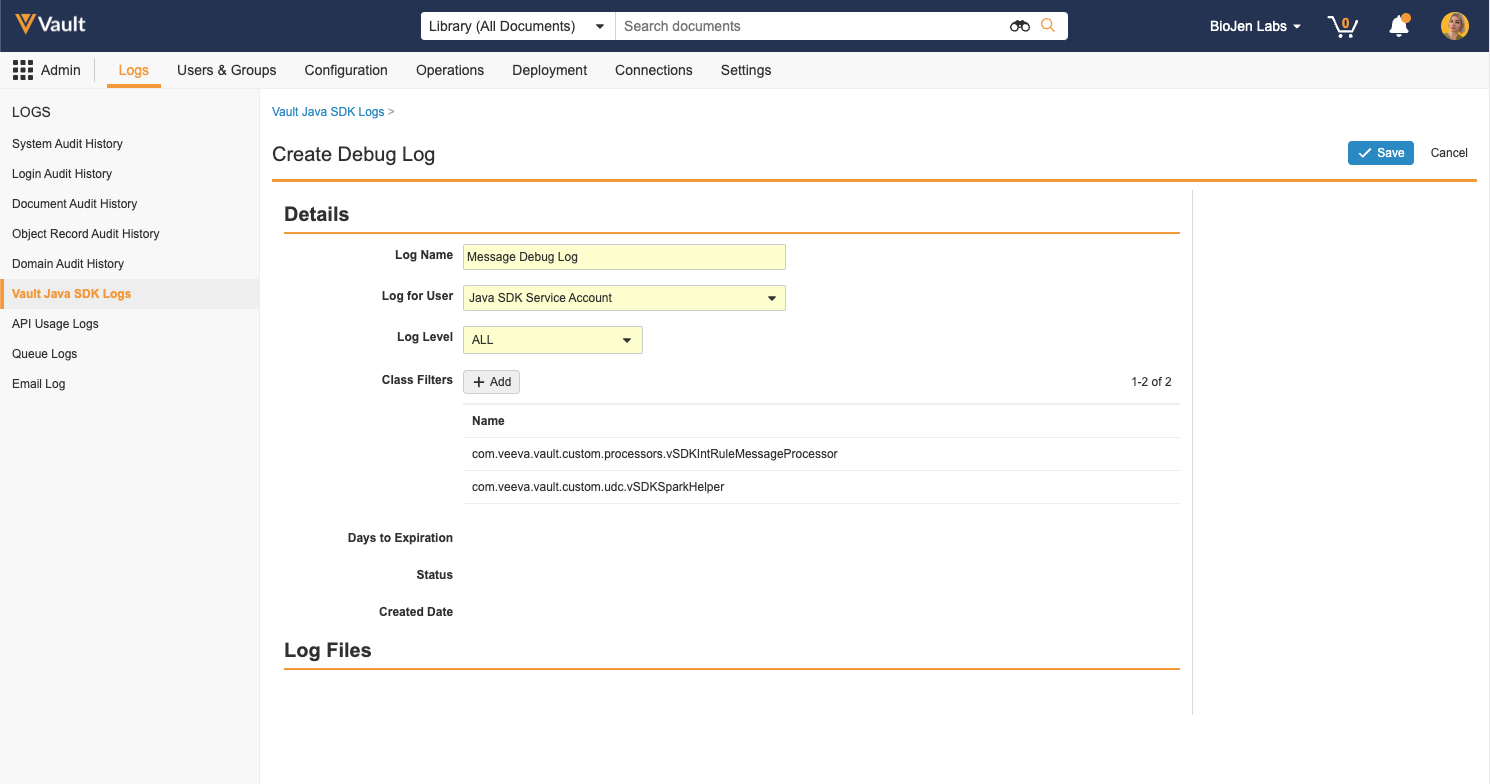
SDK Debug Log Filters
When creating SDK debug logs, Vault Admins and developers can now select additional options including Log Level and Class Filters. This allows for more granular control over what information is included in the logs and helps prevent hitting log limits when debugging. Log Level selection is inclusive of lower levels; for example, when the log level is set to WARN, debug logs will include warnings, errors, and exceptions. The default log level value is ALL. When Class Filters are applied, Vault will only capture log events from the selected class. The maximum number of class filters allowed is 10.
Learn more about Debug Logs.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Platform data model to support new features:
The following changes were made to support the Checklists: Sum Score feature:
- Enabled the Sum Score (
sum_score__sys) field for the following objects:- [target object] Checklist (
[target_object]_checklist__sys) - [target object] Section (
[target_object]_section__sys) - [target object] Subchecklist (
[target_object]_subchecklist__sys)
- [target object] Checklist (
The following changes were made to support the Visual Checklist Designer feature:
- For Question Design (
question_design__sys), Library Question (library_question__sys), Response (response__sys), and [target object] Response ([target object]_response__sys} objects:- Set the following fields to Inactive
- Attachment Allowed (Document) (
doc_attachment_allowed__sys) - Attachment Required (Document) (
doc_attachment_required__sys)
- Attachment Allowed (Document) (
- Enabled the following fields across all object types (if the field exists, primarily in Quality and QualityOne Vaults):
- Documents Allowed (
documents_allowed__sys) - Documents Required (
documents_required__sys)
- Documents Allowed (
- Set the following fields to Inactive
- For records of Question Design (
question_design__sys) and Library Question (library_question__sys) objects:- If Documents Allowed (
documents_allowed__sys) field value is null, copied the values from Attachment Allowed (Document) (doc_attachment_allowed__sys) field to Documents Allowed (documents_allowed__sys) field - If Documents Required (
documents_required__sys) field value is null, copied the equivalent values from Attachment Required (Document) (doc_attachment_required__sys) field to Documents Required (documents_required__sys) field - Note: In Quality and QualityOne Vaults, records of quiz_multiple_choice_question_v object types are excluded from the data copying, as attaching documents in checklists is not officially supported by Quizzes.
- If Documents Allowed (
Connections
Connection Exception Management
To maintain Vault performance, User Exception Messages and User Exception Items have been migrated to HVO objects and no longer support faceting in Vault UI. Additionally, records that have been marked as Inactive or are older than 180 days will be purged from Vault on a nightly basis. Customers who wish to retain this data for longer periods are encouraged to use Scheduled Data Exports or Vault Loader to extract the data for storage outside of Vault.
Learn more about user exceptions.
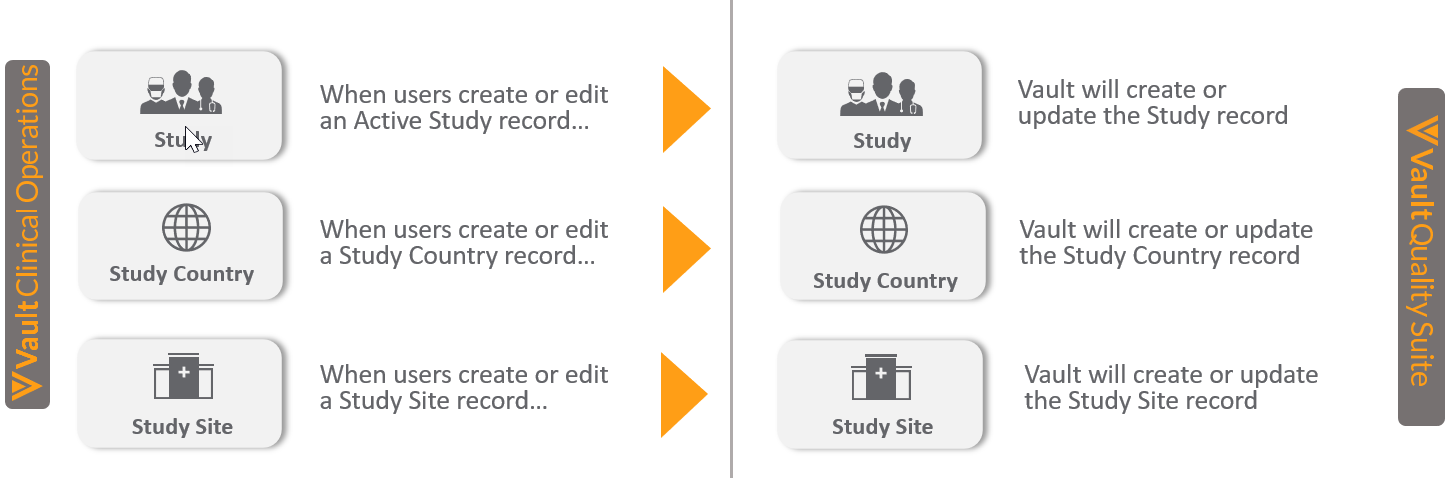
Quality-Clinical Operations: Study Data Connection
Although Clinical Operations and Quality perform different functions within an organization, Clinical Quality teams need access to study information. Study data is maintained in an organization’s Clinical Operations Vault, but Clinical Quality users work in their Quality Vault. Users can manually copy study data, but this is effort-intensive and error-prone. Building and maintaining a custom integration to automate the transfer of study information from Clinical Operations to Quality is expensive.
Vault Connections are Veeva-delivered integrations that seamlessly transfer data between Vaults. The Quality-Clinical Operations Connection automatically shares up-to-the-minute study information with an organization’s Quality Vault. A customer’s Clinical Vault continues to be the master of study information. The connection provides an automated one-way flow of study information, copying active Study, Study Country, and Study Site records to a Quality Vault whenever these records are created or updated in the Clinical Operations Vault. Studies in the Clinical Operations Vault that are in migration mode will not be copied.
This release automatically makes the Quality-Clinical Operations Connection available in every Clinical Operations and Quality Vault, but an Admin must enable the connection in both Vaults. In addition, Quality Vault Admins need to confirm the out-of-box rules that define which active study records are copied, or update the filtering rules to enforce any business constraints. The connection maps the standard Clinical Operations Vault fields in Study, Study Country, and Study Site records to the standard fields for the equivalent records in the Quality Vault. Admins can add or modify the field mappings as needed for their organizations.
This release also includes new standard join objects that track the relationship between Studies and specific Quality processes as follows:
- Quality Events
- Deviations
- Findings
- Audits
- Standalone Quality Processes
These new join objects are useful for showing the connection between studies and quality records. For example, Admins can update the page layout configurations to display a quality record’s related studies, provide new reports that include quality record and study information, and create Search Collections that allow users to search quality record and study information simultaneously.
The Quality-Clinical Operations Connection eliminates the manual effort and data integrity concerns associated with manually copying Study data between Vaults, and eliminates the need to build expensive custom integrations. The connection enables Clinical Quality teams to confidently reference up-to-date Study data in quality processes and documentation.
RIM to Clinical Operations
Improved User Exception Message Processing for Object Transfer
This feature improves how Vault handles User Exception Messages (UEM) when transferring object records from Clinical to RIM by:
- Updating the Last Successful Run Time on successful object transfers or object transfers that encounter an Item Processing Error.
- Inactivate any previously-active User Exception Messages after each job. This allows for only a single User Exception Message to be active for a given integration.
- Rerun only items contained in User Exception Messages, plus any items updated since the Last Successful Run Time for a given integration. This prevents the connection from reprocessing already successful items
Administrators managing the connection between Vault RIM and Vault Clinical are no longer required to review duplicate UEM records on Flash Reports. Learn more about the RIM to Clinical Operations Connection.
Clinical Operations
CTMS
Clinical CRM: Automated Clinical Activities
This feature benefits Vault CTMS customers who plan to use the Clinical Operations to Medical CRM Connection. This connection brings transparency to interactions with Health Care Providers (HCPs) through the bidirectional sharing of information. The connection sends Clinical Activity records from a Clinical Operations Vault to Medical CRM. When the Automated Clinical Activities setting is enabled, Vault automatically creates and updates Clinical Activity records from Monitoring Events and Study Communication Logs, increasing the types of interactions that are transferred to Medical CRM and reducing duplication of effort.
Enablement requires configuration via checkboxes under CTMS Features in Application Settings and provides separate options for automatic creation from Monitoring Events and Study Communication Logs.
Learn more about Clinical CRM.
ClinOps/CDMS - Screening Subjects Outside of CDMS
This feature updates the Clinical Operations to CDMS Connection to support Subject handling in Clinical Operations Vaults prior to Subject creation in CDMS. Previously, if users created Subjects in the Clinical Operations Vault outside of the connection, there was no link to the record in CDMS. Now, the connection uses additional logic to determine if the Subject record in CDMS already exists in the Clinical Operations Vault. If a matching record is found, the Clinical Operations to CDMS Connection links the records. Otherwise, Vault creates a new Subject record in the Clinical Operations Vault. Once the Subject records are linked, the Clinical Operations Vault is updated based on data in CDMS.
Customers who track Subjects in a Clinical Operations Vault, but not in CDMS, prior to enrollment (such as pre-screening/screening) will benefit from this enhancement. It is automatically enabled, but can be disabled in Application Settings.
Learn more about the Clinical Operations to CDMS Vault Connection.
ClinOps/CDMS - Casebook Link for Multiple CDMS Vaults
This feature introduces a new standard EDC Casebook Link field (casebook_url__v) that is available on Subject, Visit, Monitored Subject, and Monitored Visit objects in Clinical Operations Vaults. This standard field does not require manual updates to the field formula and links directly to the relevant review page in the CDMS Vault where the casebook is managed, whether the Clinical Operations Vault is connected to a single or multiple CDMS Vaults.
Vaults with the existing custom Casebook Link field on Subject, Visit, Monitored Subject, or Monitored Visit objects in Clinical Operations Vaults should remove this field from page layouts and reports and use the standard field instead.
Learn more about the Clinical Operations to CDMS Vault Connection.
Subject Transfer History
We’ve enhanced the handling of transferred Subjects in Clinical Operations Vaults, providing a more consistent and efficient approach to subject transfer management during clinical trials. This update ensures that transferred Subjects remain visible at their prior sites. This allows for seamless review during monitoring events and improves overall Subject tracking.
Key improvements encompass the creation of Subject Transfer records, a new user action for updating Subject Sites, and the ability to manually create historical records as needed. This feature will be automatically enabled.
Subject Visit Method
We’re introducing a new feature that incorporates the Visit Method as an additional metadata element on Subject Visit records in Clinical Operations Vaults. This enhancement is a response to the growing trend of trial decentralization, which brings clinical trial activities directly to the patients, thus improving patient experience and speeding up the trial process.
The Visit Method, which can vary from on-site to remote or even video conferencing, plays a crucial role in determining payment amounts in Vault Payments. This flexibility in payment adjustments is vital for sponsors to manage their budgets effectively, especially as the same Visit could generate different payments depending on the Visit Method.
This feature is designed to operate effectively with both new and existing Subject Visits and Fees. As the Subject Visit Method field is populated, the feature will be activated, contributing to improved workflow and data management processes.
Trip Report Question Display Line Breaks
This feature enhances the way Vault displays Trip Report Questions by including the same spacing and line breaks as entered into the Question Text field, improving overall readability. It is automatically enabled in Clinical Operations Vaults.
Study Startup
Feasibility Delighter: Custom Email Senders for Surveys
The Feasibility Surveys functionality within Study Startup now allows for the flexibility of modifying the email sender address and includes customer branding.
Today, surveys are sent from a generic Vault email address which in some cases can be filtered out. This feature allows the customization of the sender email giving sites a more personalized experience while also allowing the recipient to reach out to the sender in case they have any questions. It also includes the ability to add users in copy and blind copy.
An Admin must enable this feature and add new fields on the Study Person object page layout.
eTMF
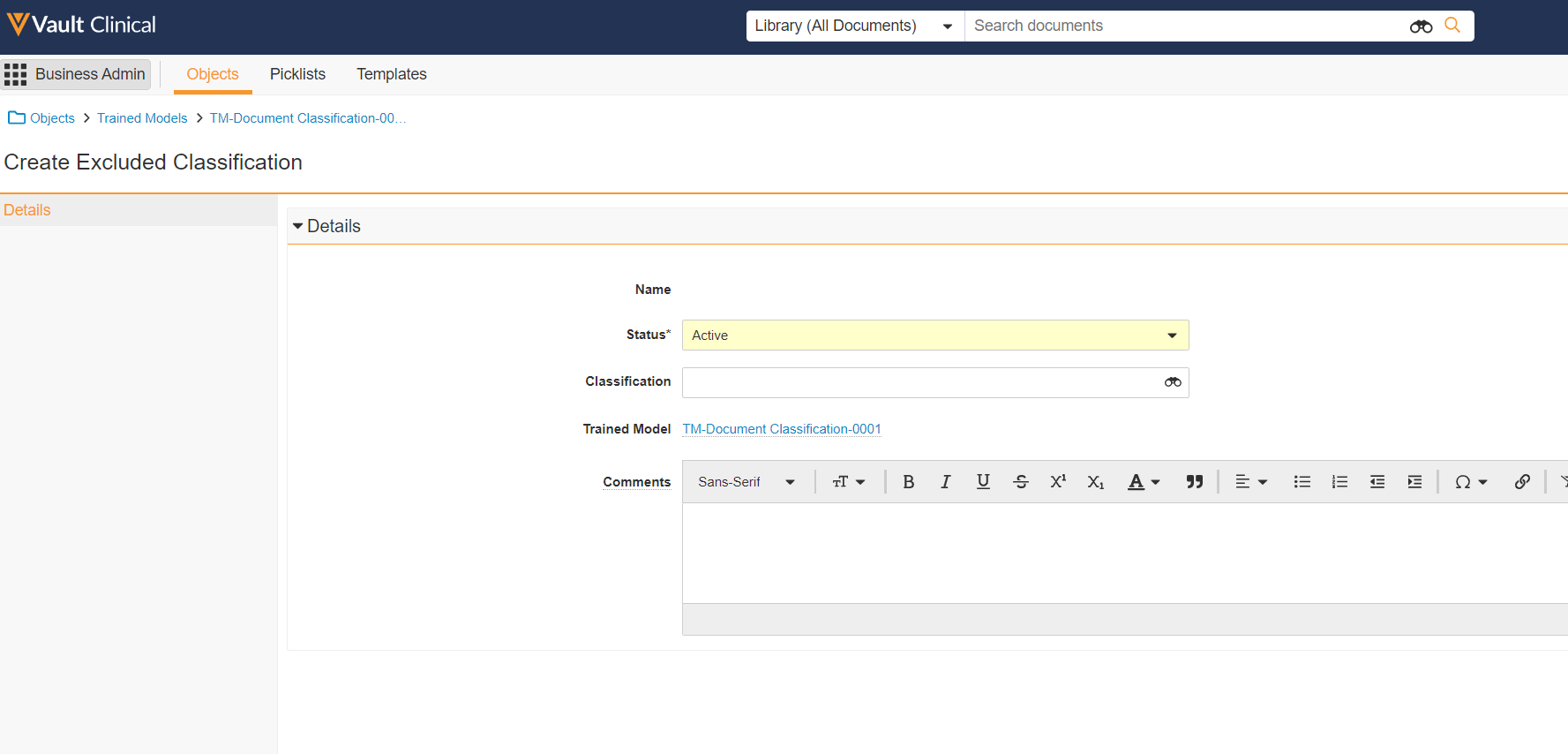
Excluded Classifications for TMF Bot
This feature allows Admins to define classifications that will be excluded from trained models of any type. Specified classifications will be omitted from all extraction, training, and testing during model deployment. Additionally, later predictions by the TMF Bot, made as it processes documents, will not be actioned if a document is in or predicted to be in an excluded classification.
This allows customers to prevent automation, by the TMF Bot, from acting on classifications where it is known or expected to perform poorly, or where a business process requires that humans not rely on automation. For example, for documents that are expected to be authored directly in Vault.
Users can specify excluded classifications before or after a model is trained. If one is added after the model’s training, the model is not automatically re-trained; however the TMF Bot will not take any action against documents of the excluded classifications.
Learn more about TMF Bot auto-classification.
Study Metadata Extraction: Bulk Pipeline Support
This feature enhances the Study Metadata Extraction feature to support documents created via the bulk pipeline, such as through an API or email ingestion. The feature will be especially valuable for customers using one of our clinical email processors, as the TMF Bot can now set the Study field for documents created from email messages or email attachments.
This feature will be auto-on enabled in Vaults that have a Metadata Extraction Trained Model in the deployed state. This ensures that the feature will be readily available for early adopters, and auto-on with future enablement of metadata extraction by other customers.
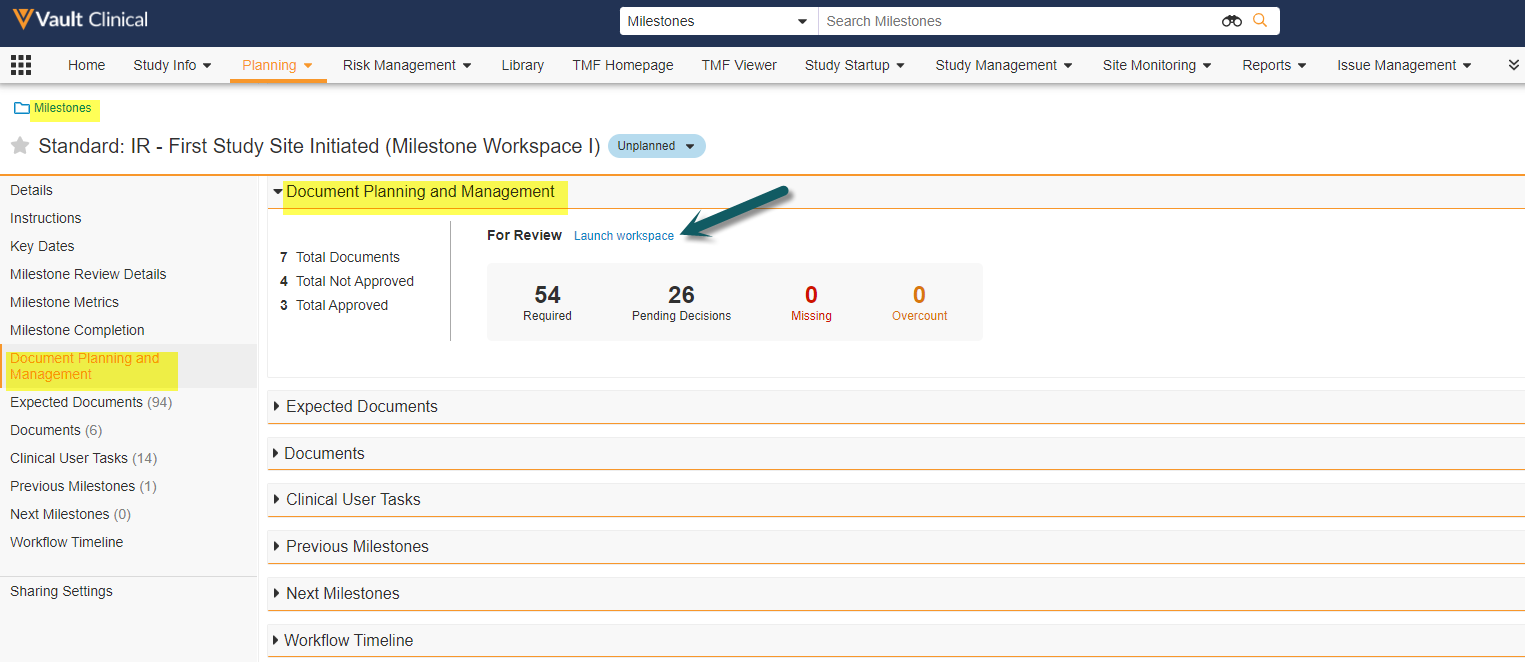
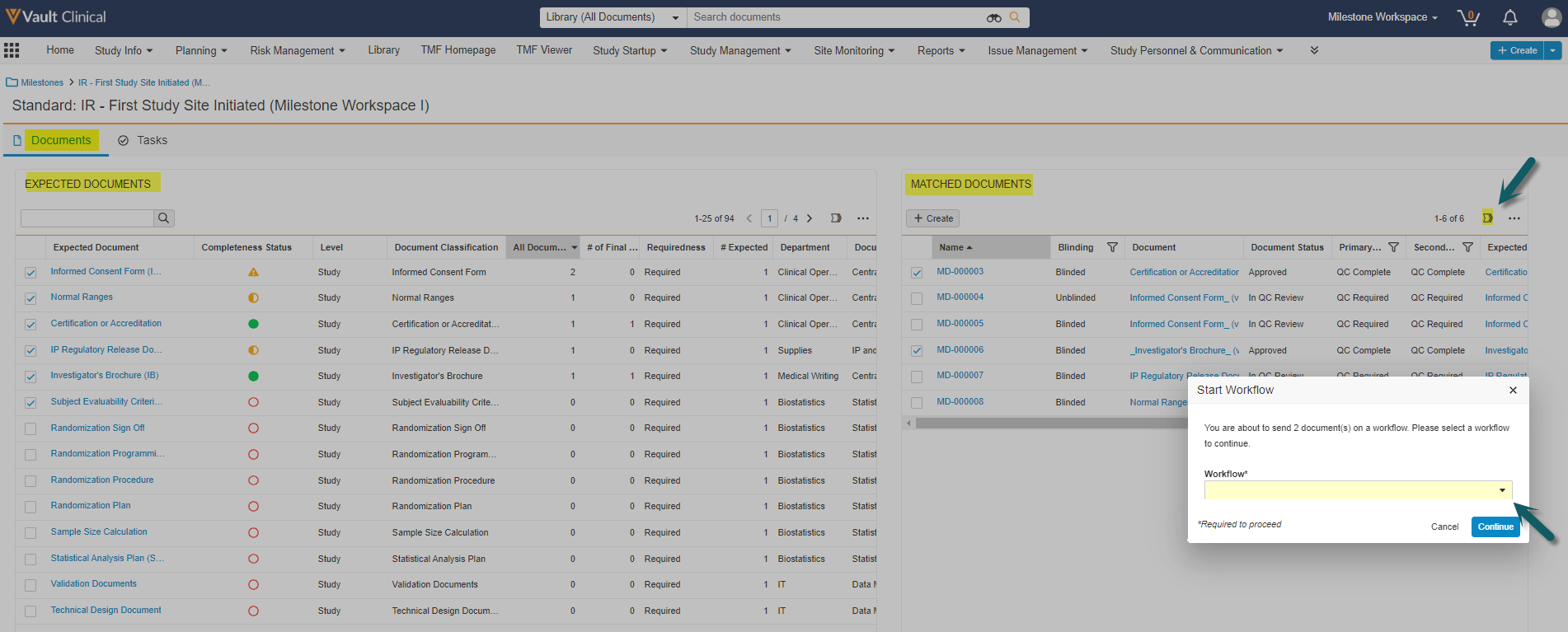
Milestone Workspace
This feature creates a new Milestone section that summarizes the status of a milestone’s related documents and expected documents. This section also includes a link to a workspace page with two data grids (tables) for the milestone’s Expected Documents (at left) and their Matched Documents (at right). These tables both support reviewing, column filtering,updating, and sending on workflows for documents and/or expected documents in a single page and a second page for Tasks.
Study team members will benefit greatly from this feature, as it provides them with a better overview of the milestone activities and allows for a more efficient way to organize, plan and execute document actions and expected document reviews.
Study team members are responsible for managing a large number of expected documents and documents and the assigning of those records, often in workflows, to various functional owners for review. The logical place to organize the management of documents and expected documents is the milestone. Today these users face some of the following limitations:
- Expected documents and documents are related, but located in different sections of the page.
- The large volume of data requires users to navigate different object record lists (such as milestones, expected documents, and clinical tasks) and the library to better view and filter results.
As workarounds to the above constraints, customers today rely on reports, saved views, search collections and excel trackers to manage this process and have a good overview of the milestone activities.
This enhancement will solve these challenges by displaying a unified user friendly view and allowing users to launch multi-document workflows and bulk object record workflows for Expected Documents all from one place:
This feature is Auto-on for all Clinical Operations Vault customers, however to utilize it an Admin must update the Milestone page layout to include the new Document Planning and Management section and make it visible to end-users so they can launch the Milestone Workspace. Additionally, an Admin must also update the permission sets for Milestone Documents and the Milestone Document field on the EDL Item.
Learn more about working with Milestones.
TMF Transfer: Match on Site Name
This feature streamlines the transfer when in-scope Sites already exist in the Target Vault by adding logic to automatically identify and map Study Country and Site name records between Target and Source Vaults. Site matching is based on the combination of Study Country and Site name.
This is useful when sites already exist in the Target Vault, which has previously required customers to manually map records prior to transfer and could result in potential duplication of Site records in the Target Vault.
This feature is automatically enabled. Learn more about TMF Transfer.
Editable Study Country Name
This feature enhances control over your Study Country name field. It provides a balance between manual control and automated consistency for your Study Country data management.
The Enable Study Country System-Managed Name Field setting can be temporarily disabled from Vault Settings, granting end users the flexibility to manually update the Study Country names as needed. This feature is particularly useful when you need to align existing Study Country records with updated country names. When re-enabled, the system resumes managing the Study Country names, ensuring data consistency.
When the system-managed setting is disabled, users will be required to manually populate the name for any new Study Country records.
Learn more about managing Study Countries.
Enhanced Assigned To Control on Quality Issue
This feature enhances the Assigned To field filtering functionality on Quality Issues. When leveraging the app control Assigned To field on Quality Issues, users must have view permission to appear in the drop down.
This enhancement is automatically enabled for customers using the application-controlled Assigned To field.
Learn more about Quality Issues in Clinical Operations.
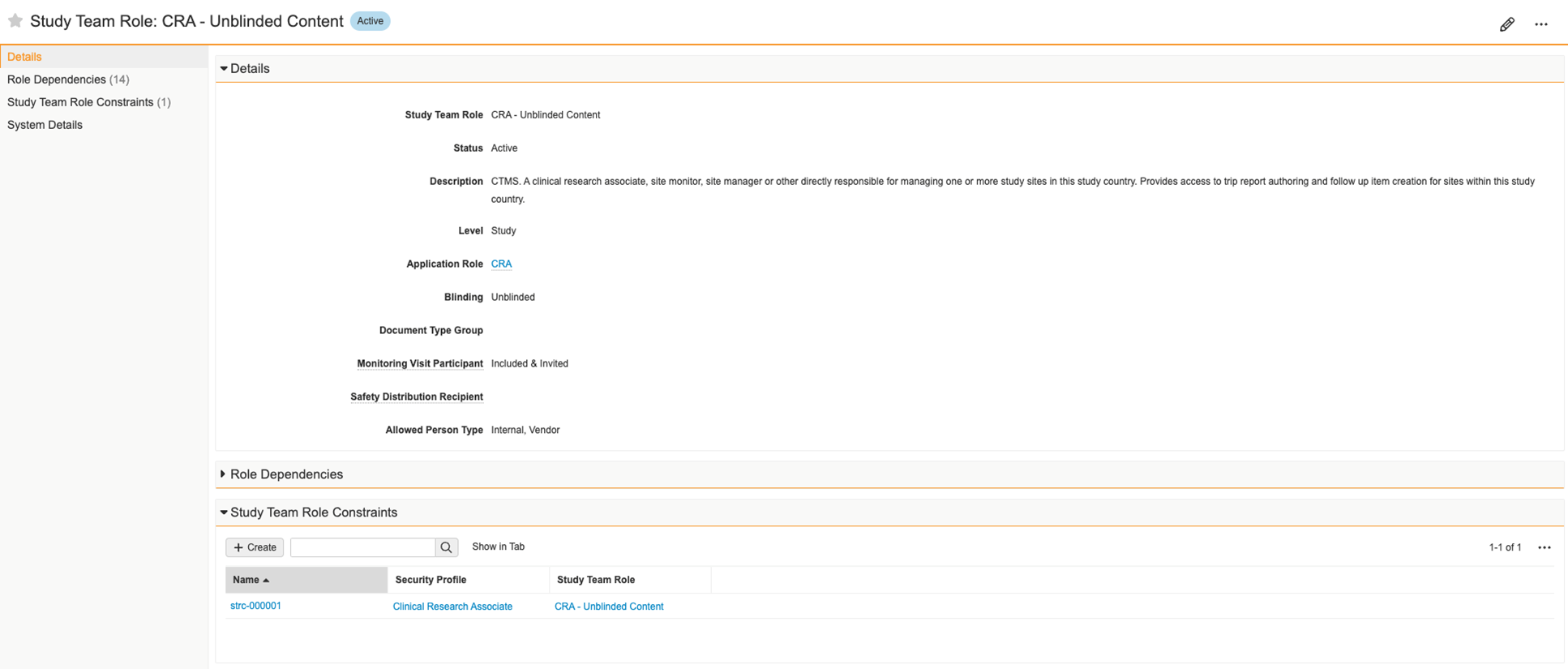
Study Person Role Constraints (User)
In Clinical Operations Vaults, Study Person records are used to track study rosters and to grant users study-specific access. This access is controlled through the assignment of Study Team Role. Providing an incorrect Study Team Role is a compliance risk and could result in inappropriate or insufficient access to Study records.
With this feature, Clinical Operations Vaults now support the restriction of Study Team Role assignment based on Person Type or Security Profile. Validation checks on record save prevent users from creating Study Person records with disallowed combinations. Additionally, a new application-controlled field is available that filters for permitted Study Team Roles during record creation.
When enabled, this feature reduces the risk of providing incorrect Study-specific access. Enablement requires configuration and includes the following:
- A new field Allowed Person Type on the Study Team Role object to restrict based on Person Type
- A new object Study Team Role Constraint on the Study Team Role object to restrict based on Security Profile
- A new application-controlled field for Study Team Role that can replace the existing Study Team Role field on the Study Person object
Learn more about managing Study Person records.
Site Connect
Document Exchange for Additional Vault Clinical Docs
Site Connect customers can now send Relevant External Communications document types to Sites. The configuration for any relevant document types must be updated so that they map to the new Relevant External Communications Vault Clinical Docs artifact.
Learn more about Site Connect.
SiteVault Inviter
With this feature, Sponsors/CROs will be able to invite Sites to sign up for SiteVault, initiating the process from within Clinical Operations Vaults. Within Clinical Operations Vaults, customers will be able to track the status of the signup request from delivery through to its completion and will be notified of the Site’s Universal Site Number (USN) information.
Learn more about Veeva SiteVault.
Clinical Operations Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Clinical Operations data model to support new features:
Commercial
PromoMats
Bulk Generate eCTD Compliance Package
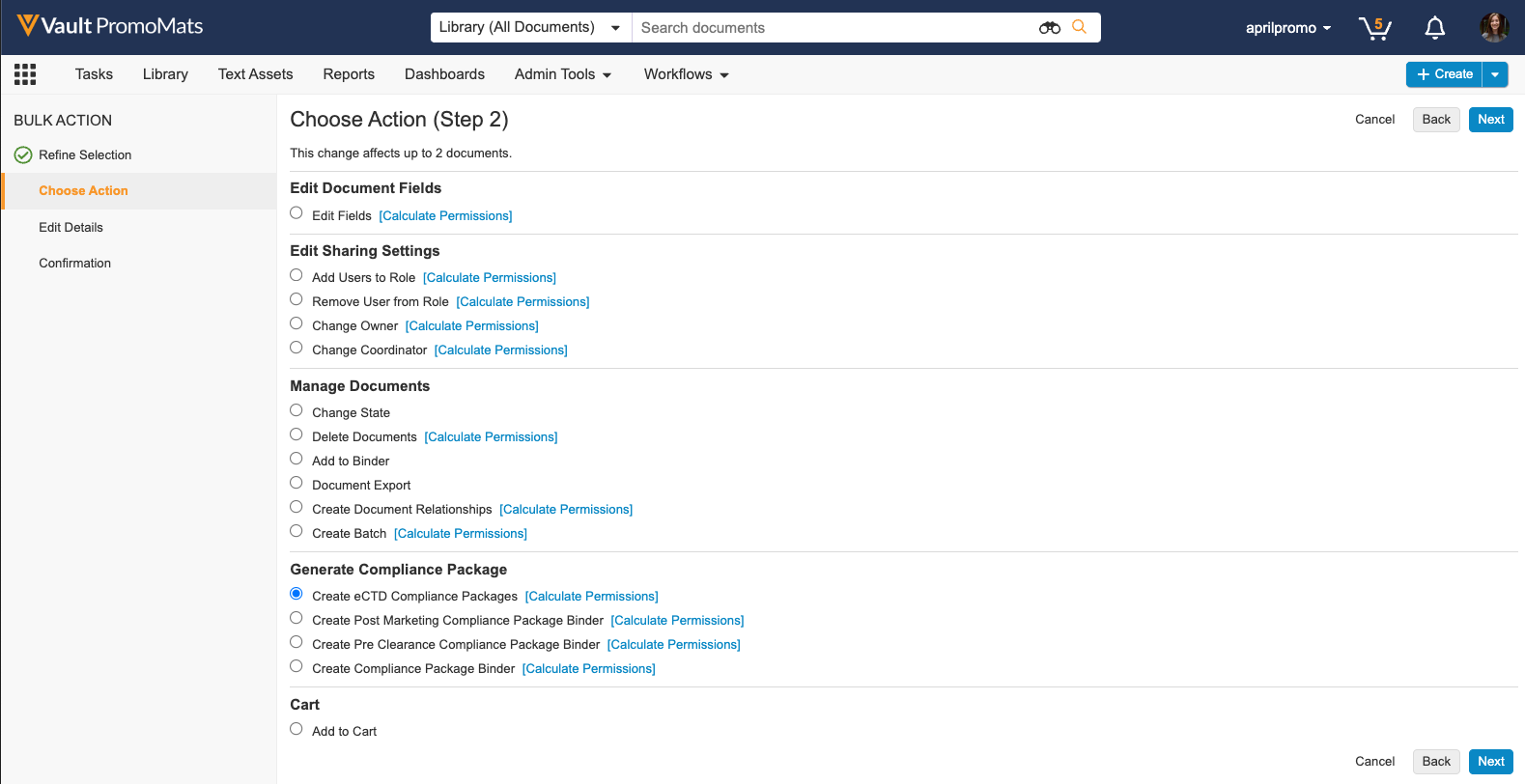
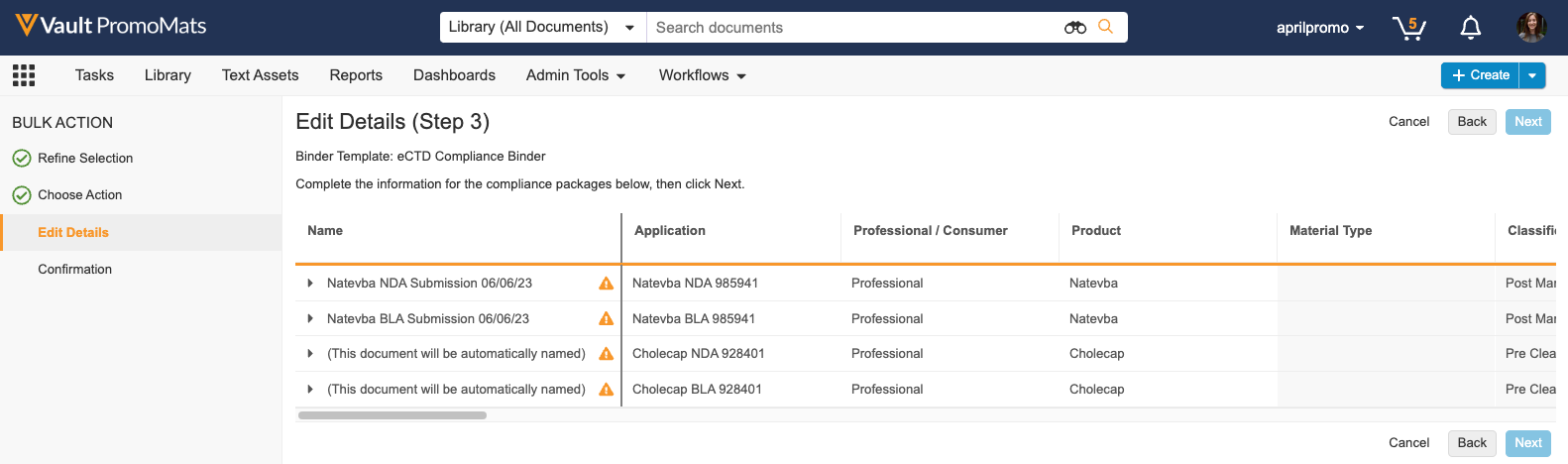
In this release, when performing a bulk action, a new action is available in the ‘Generate Compliance Package’ section to allow users to create multiple eCTD Compliance Packages at once. Vault groups documents and places them into one or more Compliance Packages based on the application, audience, and package type. This feature reduces required manual data entry by automatically populating Compliance Package data where available. Generating multiple eCTD Compliance Packages simultaneously streamlines the submissions process for users who have multiple promotional materials ready for different FDA submissions.
Learn more about configuring eCTD Compliance Packages and generating eCTD Compliance Packages.
Set Match Text Field Length Minimum Value to 5
This feature decreases the minimum number of required characters on the Text Asset object’s Match Text field to five (5). Previously, the Match Text field required a minimum of 20 characters, because a claim is in most cases longer than 20 characters. Now that Text Assets are used for reusable text as well as claims, there was a need to reduce the minimum number of characters required.
Learn more about Text Assets.
AIRs support for SVG and WebP files
Automated Image Renditions (AIRs) functionality now supports SVG and WebP images. You can now use SVG and WebP images as the source file to transpose them to other supported rendition types using AIRs, including WebP. This feature enhances the DAM capabilities within PromoMats to allow content creators to use additional file types that are particularly well suited to web development in Automated Image Renditions.
Learn more about Automated Image Renditions.
Text Asset Substantiation Lifecycle Entry Criteria
In Vault PromoMats, all types of Text Assets require substantiation (reference document or anchor) to allow the record to enter a steady state. Reusable Text, introduced in 22R3, generally does not require substantiation to be added to every record. This functionality introduces flexibility in selecting what types of Text Asset require a reference and at which point in their lifecycle users must link substantiation.
Learn more about Text Assets.
Increase Number of Portal Content filters
Brand Portals allow customers to distribute content efficiently within their teams. With this release, customers can add up to 24 Content Filters to a Portal, providing more flexibility in how the content is curated and improving the user experience for finding content. This increase in content filters is only available on the new Portal User Interface.
Learn more about Portals.
Document Order Support for Portal Document Widgets
The new Portal User Interface, introduced in 23R1, now supports document ordering in custom document widgets. When editing a Portal, Portal Editors now have the option to drag and drop documents to create their desired display order. This gives Portal Editors greater flexibility when curating their content in a Portal.
Learn more about Portals.
Extend File Name Exclusion eCTD
In Vault PromoMats’s eCTD Compliance Package feature, the system creates submission ready documents with FDA-compliant names. The FDA guidance states that document names should include only letters, numbers, hyphens and underscores. This feature ensures submission ready document names only include letters, numbers, hyphens, and underscores by removing any other special characters. This is based on the FDA guidance.
Learn more about generating eCTD Compliance Packages.
Optional Filters in Select Claim Dialog
When using manual Claim Linking, the filters for records (for example, Product or Country) are now optional in the Select Target dialog box. In addition, any custom object reference or picklist filters have become optional as well. The standard filter, Lifecycle State > equals > Approved, remains required. Vault continues to apply record filters by default, but users have the option to remove or modify them when selecting a target.
23R2 Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
The following changes were made to PromoMats Vaults to support the removal of the Vault CRM to PromoMats connection:
- Removed the following components from the data model:
- The Vault CRM to PromoMats connection record
- The PromoMats Document Integration
- The PromoMats Document Outbound integration point
- The Vault CRM to PromoMats Outbound Queue
- The Vault CRM to PromoMats Connection document type group
The following changes were also made to PromoMats Vaults:
- Updated the reference constraint on the Health Authority Decision document field to
id IN (SELECT id FROM constraints2__vr WHERE country__v CONTAINS this.country__v), controlled_vocabulary_type__v = 'health_authority_decision__v', status__v != 'inactive__v'. This allows Admins to build Constraint records based only on Countries. - Added the External ID (
external_id__v) field to the CRM Content Group (crm_content_group__v) object - Added the new inactive CRM Legacy iPad Content Mode (
crm_legacy_ipad_content_mode__v) shared document field - Added the new inactive CRM Slide Notes (
crm_slide_notes__v) shared document field
Medical
MedComms
Set Match Text Field Length Minimum Value to 5
This feature decreases the minimum number of required characters on the Text Asset object’s Match Text field to five (5). Previously, the Match Text field required a minimum of 20 characters, because a scientific statement is in most cases longer than 20 characters. Now that Text Assets are used for reusable text as well as scientific statements, there was a need to reduce the minimum number of characters required.
In Admin, Scientific Statement is the label for the annotation_keywords__sys object in Vault Medical.
Increase Number of Portal Content Filters
Brand Portals allow customers to distribute content efficiently within their teams. With this feature customers are able to add up to 24 Content Filters to a Portal, providing more flexibility in how the content is curated and improving the user experience for finding content. This increase in content filters is only available on the new Portal User Interface.
Learn more about Portals.
Document Order Support for Portal Document Widgets
The new Portal User Interface, introduced in 23R1, now supports document ordering in custom document widgets. When editing a Portal, Portal Editors now have the option to drag and drop documents to create their desired display order. This gives Portal Editors greater flexibility when curating their content in a Portal.
Learn more about Portals.
AIRs Support for SVG & WebP Files
Automated Image Renditions (AIRs) functionality now supports SVG and WebP images. You can now use SVG and WebP images as the source file to transpose them to other supported rendition types using AIRs, including WebP. This feature enhances the DAM capabilities within PromoMats to allow content creators to use additional file types that are particularly well suited to web development in Automated Image Renditions.
Learn more about Automated Image Renditions.
MedInquiry
CRM Data Sharing: Sync Only Required Accounts
This feature introduces the option to pull only relevant Accounts that are related to Inquiries from Veeva CRM. Previously, Vault Medical synchronized and pulled in all Accounts from Veeva CRM, which meant that customers with limited Third Party Agreements could not use the CRM synchronization as it exceeded specified thresholds.
Learn more about configuring CRM Data Sharing.
Frequently Asked Questions for Medical Inquiry
This feature introduces a standard, object-based approach to support Frequently Asked Questions (FAQs). FAQs are Medical Inquiries that are commonly asked by HCPs and patients and usually have a Standard Response that users send in reply.
Today, FAQs are typically stored in an FAQ Document alongside the Standard Response. Previous to this feature the user had to navigate to the FAQ Document, identify the FAQ that had been asked, copy and paste the answer into the Case Response and manually add any fulfillment documents.
By storing FAQs in object records in MedInquiry the user can quickly find the FAQs from the Case Request object. Users can search a library of approved FAQs, typically constrained by the case’s Product, Country, and Language. This allows them to remain on the Case Request while identifying the FAQ, rather than navigating away. It also provides a quick and simple way to apply a Standard Response to the FAQ.
An FAQ typically consists of:
- The question asked
- The Product, Country, and Language of the question
- Related object records for the Standard Responses
Standard Responses for Medical Inquiry
This feature introduces a standard, object-based approach to support Standard Responses. Standard Responses are predefined answers to Medical Inquiries that are commonly asked by HCPs and patients and are typically responses to an FAQ.
When composing a Case Response, the user can select an approved Standard Response. Users can also filter the list of standard responses to show answers to an identified FAQ.
By selecting a Standard Response, Vault Medical can populate the Case Response with the response notes, a response package, and a drafted email. Users can update the response package, edit the email, or simply click to email the response.
A Standard Response typically consists of
- Standard response notes
- Guidelines for use
- The Product, Country and Language of the response
- A package of Fulfillment Documents
- A Case Response Email Template
- The FAQ to which it is an answer
This helps standardize responses and decreases the amount of time it takes to create a response package, allowing users to handle more responses.
23R2 Medical Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Medical data model to support new features:
The following changes were made to MedInquiry Vaults to support the Frequently Asked Questions for Medical Inquiry feature:
- Added the Frequently Asked Question (
frequently_asked_question__v) object - Added the following join objects:
- Frequently Asked Question Country (
frequently_asked_question_country__v) - Frequently Asked Question Product (
frequently_asked_question_product__v) - Frequently Asked Question Standard Resp (
frequently_asked_question_standard_resp__v)
- Frequently Asked Question Country (
- Added the Frequently Asked Question (
frequently_asked_question_lifecycle__v) object lifecycle - Added the Frequently Asked Question (
frequently_asked_question__v) field to the Case Request object
The following changes were made to MedInquiry Vaults to support the Standard Responses for Medical Inquiry feature:
- Added the following objects:
- Standard Response (
standard_response__v) - Standard Response Fulfillment Document (
standard_response_document__v)
- Standard Response (
- Added the following join objects:
- Standard Response Country (
standard_response_country__v) - Standard Response Product (
standard_response_product__v)
- Standard Response Country (
- Added the Standard Response (
standard_response_lifecycle__v) object lifecycle - Added the Standard Response (
standard_response__v) field to the Case Request object
The following changes were also made to Medical Vaults:
- Added the External ID (
external_id__v) field to the CRM Content Group (crm_content_group__v) object - Added the new inactive CRM Legacy iPad Content Mode (
crm_legacy_ipad_content_mode__v) shared document field - Added the new inactive CRM Slide Notes (
crm_slide_notes__v) shared document field
Vault Mobile
Device-Enforced App Access Security Setting
A new security policy setting in Admin > Settings > Security Policies > Vault Mobile Settings called Allow device-enforced access will be visible in 23R1.3 for a future Vault Mobile feature. This new setting is only available for Basic policies, or SSO policies where there is no OAuth profile set with vaultmobile as the client ID. This setting is not applicable until 23R2.0, when the feature will be enabled by default and usable with the 23R2.0 version of the Vault Mobile app.
Learn more about Vault Mobile.
Training
Study Training
In addition to the feature below, Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
Auto Create User Role Setup Security Records
This feature increases efficiency for Admins by reducing administrative overhead. With this feature configured, IT users no longer need to create User Role Setup records manually for each user and study. Instead, Study Training automatically creates the records based on the user’s Learner Role assigned to a Study. This ensures that users, such as CRAs, can see the right information based on their study team role and study.
To use this feature, you need to create Role Dependency records for Learner Roles and Application Roles. The following steps describe the process in which Study Training auto-creates User Role Setup records:
- An Admin creates Role Dependencies for a Learner Role. For example, they could create a dependency with a Target Application Role of Trainee, selecting CRA as its parent role, and selecting a level of Study.
- Users are assigned a Learner Role for a Study. For example, a user could be assigned a CRA Learner Role for the CHLCAP123 study.
- Study Training automatically creates User Role Setup records based on Role Dependency data. In our example, Study Training creates a User Role Setup record for the user specifically for the CRA role in the CHLCAP123 study, automatically limiting their access.
If the dependency is deleted, Study Training automatically deletes any related User Role Setup records. Learn more about setting up Study Training.
Quality
QualityDocs
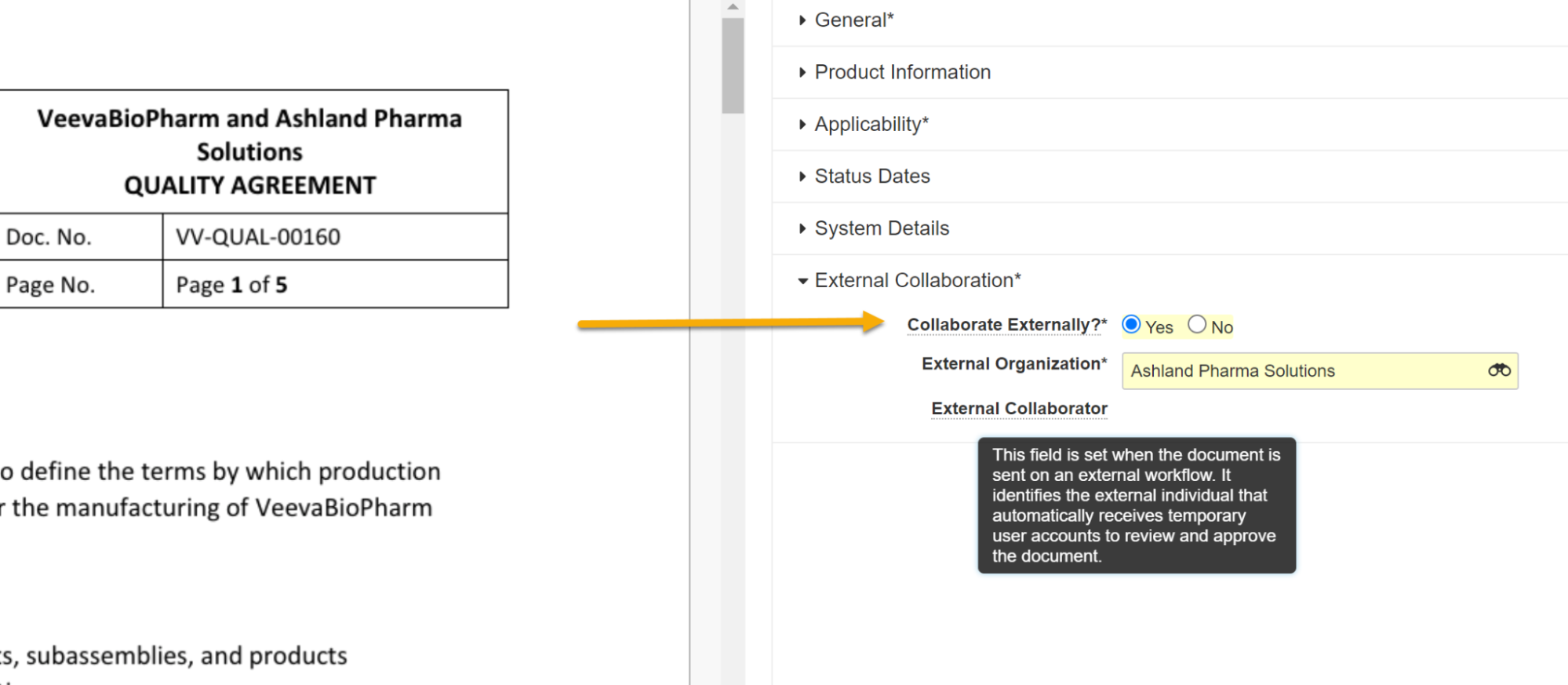
External Collaboration: External Review & Approval of Documents
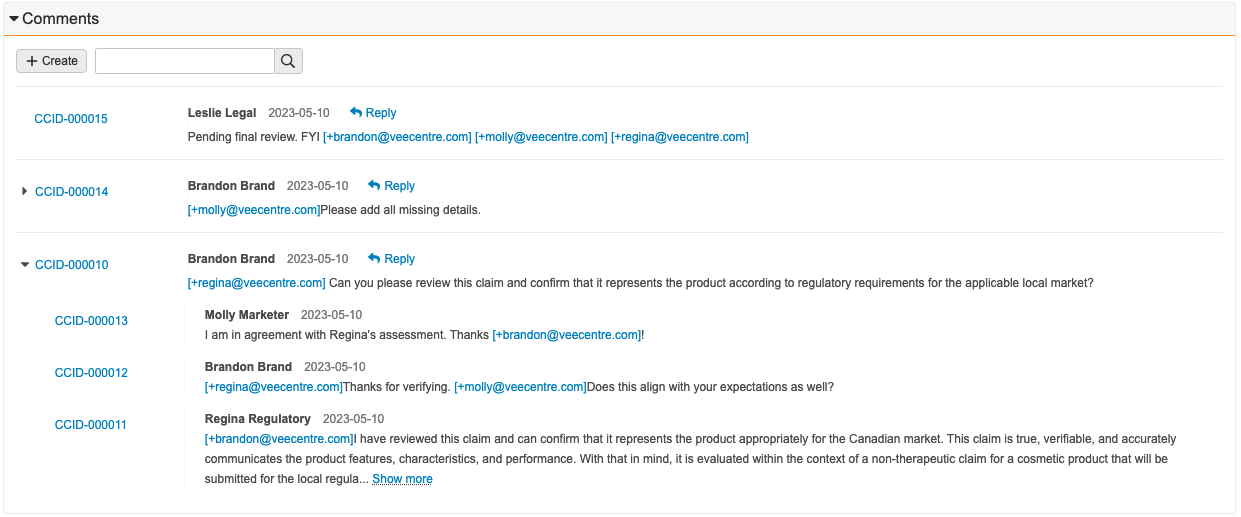
This expansion of the External Collaboration feature, previously available in the QMS application, allows QualityDocs users to collaborate with external stakeholders on document review and approval tasks more efficiently, helping to streamline the collaboration process between organizations and their external stakeholders such as suppliers. This feature can replace less-efficient current collaboration mechanisms such as email, shared drives, Office 365, or others.
Using this feature, document review and approval tasks can be assigned to an external person. Vault creates or activates a user account, provisions appropriate permissions, and claims an external user license. Vault inactivates the user automatically when both the task has been completed and the document reaches its steady state. Then the external user license, utilized while the user was active, becomes available again.
This feature is intended for external stakeholders, such as suppliers, who need infrequent access to review and approve agreements and other documents. An example process may include the following steps:
- A user indicates on the document whether the document will be sent to an external person for review or approval. If yes, the user will select an organization and then from recognized contacts at that organization (Person records in Vault).
- The user then starts a relevant workflow that includes a task to be completed by an external person, for example, Send for Internal / External & QA Approval. The workflow can be configured to include both internal participants and an external participant. The external collaborator is a recognized contact (Person record in Vault) at the selected external organization.
- The system automatically creates and activates a user account from the Person record. The external collaborator receives a Welcome to Vault email (or Welcome Back email if they have collaborated at any point previously) which contains information and login details. They can then access Vault and have access only to the Home tab and their assigned task(s).
- The external collaborator can add annotations to the document if needed, and provide a verdict and eSignature.
- When the external collaborator has completed their open tasks in the system and when the document has reached steady state, the external collaborator receives a Goodbye email and the user account is automatically inactivated.
Learn more about configuring external collaboration for review and approval of documents.
Increase in Default Limits of Visual Hierarchies in Process Navigator
The Adjustable Hierarchy Limits for Process Navigator feature was originally released in 21R3 and allowed default Visual Hierarchy limits in Process Navigator to be increased by Support. The maximum number of base hierarchies increased from three (3) to ten (10) and the number of child processes increased from ten (10) to fifteen (15). In this release, ten (10) base hierarchies and fifteen (15) child processes are now the default limits and no longer have to be enabled through Support.
Search Term Limits for Documents in Process Navigator
With this release, document search terms in the Process Navigator are limited to 4000 characters.This feature was postponed to a future release.
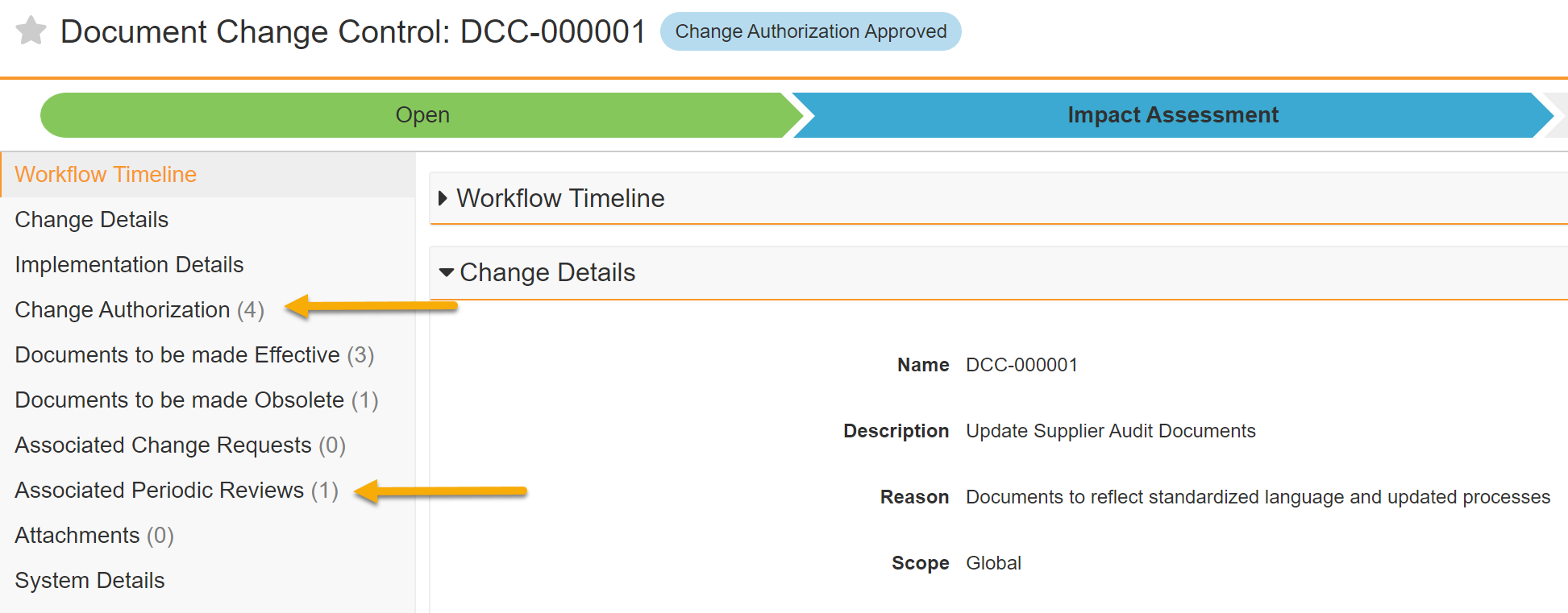
Record Counts Added to the Periodic Review & Change Authorization Sections of Document Change Control
Record counts now appear next to the Periodic Review and Change Authorization Sections of the Document Change Control offering increased visibility, improved usability and consistency with other sections.
Learn more about working with Document Change Controls.
QR Code Generator for Documents & Station Manager
On the manufacturing floor, getting the right content to the right users at the right time is critical. Some organizations use QR codes to enable users with Station Manager to scan the code on a machine or workstation to load the relevant content. However, generating such codes can be cumbersome or difficult. With this release, Vault now supports both the scanning (via Station Manager application or device camera application) and generation of QR codes for use within your organization.
You can generate a QR code via a user action on the Vault document, the Station Document record, or the Station Manager Category record. An Admin must add the action to applicable lifecycle states before it can be used.
You can then download, print, and place the QR codes at desired locations, so users can scan them on a mobile device using the camera app or the Station Manager app.
When scanning a QR code for a Vault document, the latest Steady State version of the document opens in the default mobile browser or the Vault Mobile Application, if configured. When scanning the QR code for a Station Document or Station Manager Category, the relevant document or category opens in the Station Manager app.
Training
Dynamic Enrollment: Automatically Assign Learner Roles to Person
This feature allows Vault Training to automatically assign Learner Roles to a Person, based on structured data available on the Person and Learner Role records. You can select matching fields between Person and Learner Role (such as Department) to drive Learner Role assignment. Additionally, if Person metadata changes, this feature also automatically removes Learner Roles and assigns any newly appropriate Learner Roles.
For example, if a Person has a Department value of “QA”, Vault Training might automatically give them the Learner Role “QA Core”. If that Person then switches to another department, Validation, then Vault Training would remove the “QA Core” Learner Role and may assign a new “Validation Executor Core” Learner Role.
Learner Homepage: Configurable Filter Options
This feature improves search on the Learner Homepage by allowing organizations to configure search fields that can be used to search against open Training Assignments or Training Requirements in the Explore tab.
Learn more about setting up the Learner Homepage.
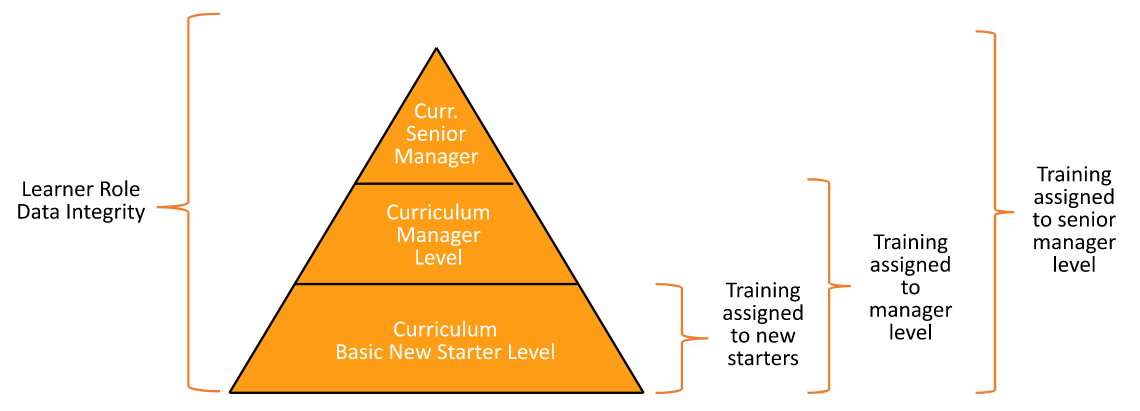
SmartMatch Curricula
This feature adds flexibility to the training matrix and improves automation for the assignment of training to Learners. Using SmartMatch, you can choose which Curricula for a given Learner Role are assigned intelligently: Instead of all Curricula in the Learner Role being assigned for training, you can set up SmartMatch to assign only a subset of Curricula within the Learner Role. Vault Training selects the subset based on matching field values between the Person and Curriculum records. This reduces the need to create large numbers of specific Learner Roles.
For example, assume you have two Learners: A manager for data integrity and a senior manager for data integrity, and you have one Learner Role called Data Integrity covering all training relevant for associated roles. The senior manager needs to be trained on more items than the manager, so the manager only needs to be trained on a subset of the Curricula within Data Integrity. This assignment can be done based on matching fields. In this example we can base it on the senior manager’s Seniority Title field value on their Person record, matched against the Curriculum’s associated Seniority Level field value.
This feature was named SmartMatch during this release and in 23R2. It is updated to Curriculum Matching in 23R3, and the change will be reflected in Vault UI in 24R1.
Assign Curriculum to a Learner’s Training Matrix
This feature allows customers to assign Curricula directly to a Person’s Training Matrix. Unlike direct assignment, these assignments follow all matrix rules such recurrence, up-versioning, etc. This will allow Training Admins to assign specific Curricula to specific Person based on that Person’s specific training needs.
Consolidated Direct Assignment Jobs
In previous releases, Direct Assignments were issued via multiple jobs. In this release, they are now issued via a single job, which makes troubleshooting and access to logs quicker.
Learn more about Direct Assignment.
Ability to Cancel a Classroom Training Assignment Workflow
A new configurable user action allows users to cancel a Classroom Training Assignment Workflow. Previously, the workflow could only be canceled using an entry action. If the entry was not configured properly, this could lead to completed Classroom Training Assignment records with an open workflow task.
Learn more about Instructor-Led Training.
Cancel Instructor Workflow Task
A new configurable user and entry action allows cancelation of a workflow task assigned to an instructor in the improved Instructor Led Training (Classroom Training) experience introduced in 22R3. These actions are useful when a class has already been Published, but needs to be canceled.
Learn more about Instructor-Led Training.
Default Limit for Number of Prerequisite Rule Per Curriculum Increased to 20
We have increased the default limit for the number of prerequisite rules per curriculum to 20 in new Quality Vaults. Existing Quality Vaults will remain at 5 and customers can increase the limit by contacting Support.
Learn more about Prerequisite Training Requirements.
Custom Curriculum and Learner Role Object Types Not Allowed
To enforce appropriate use of Learner Role and Curriculum types, Learner Role or Curricula records in custom object types are not allowed.
Curricula or Learner Role Object Types Usage Restriction Based on Application License
This feature prevents Study Training customers from potentially creating non-Study Curricula and non-Study Learner Roles and Vault Training customers from creating Study Curricula or Study Learner Roles.
To enforce appropriate use of Learner Role and Curriculum types, the following are now enforced:
- Study Training Vaults: Only Study Curricula and Study Learner Roles can be created
- Vault Training Vaults: Only base Curricula and base Learner Roles can be created. Base is
base__vas well ascurricula__vandlearner_role__vobject types.
QMS
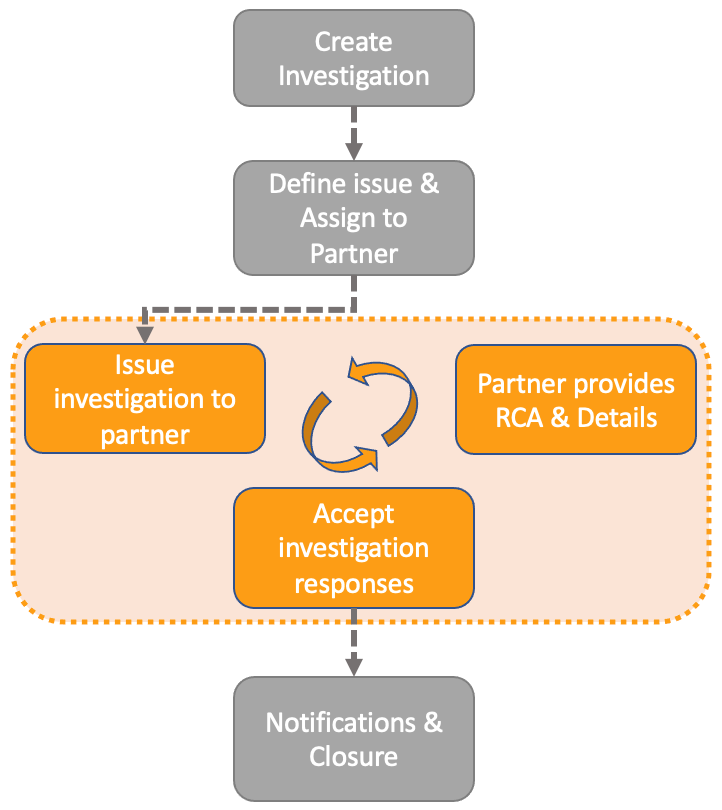
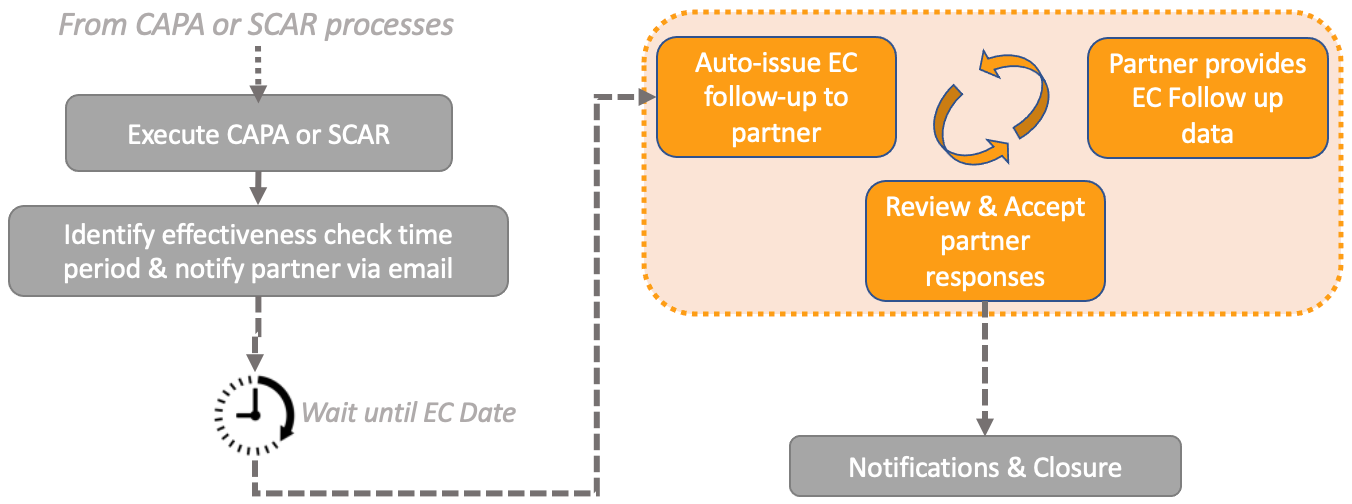
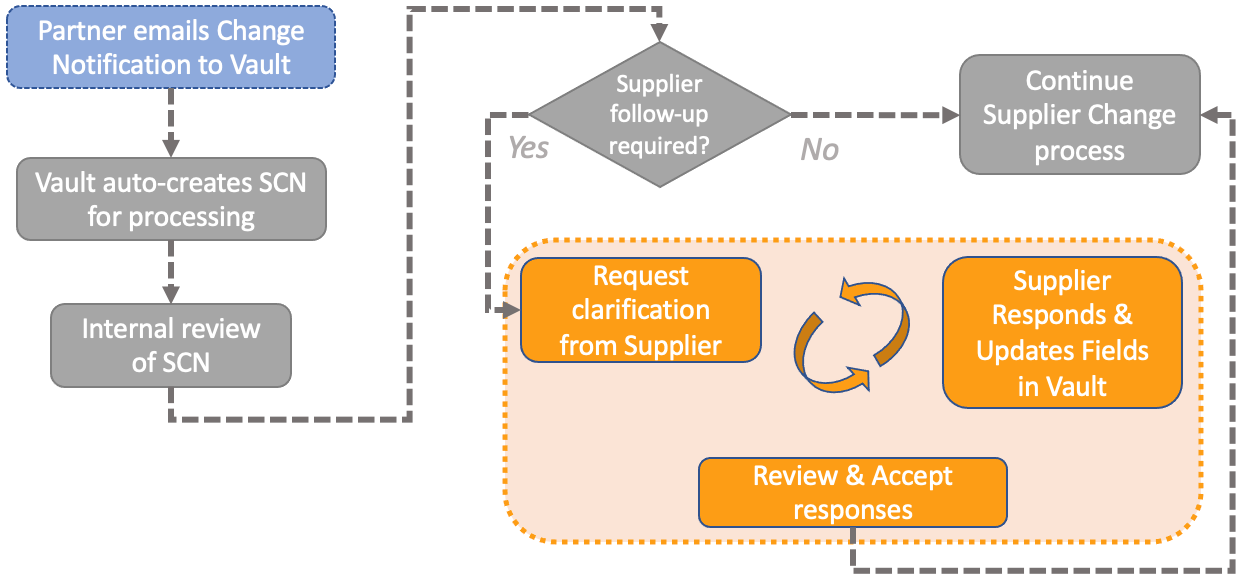
External Collaboration: Support for Investigation, Effectiveness Check, & Supplier Change Notifications
Expanding on the theme of in-Vault collaboration with external organizations, this release introduces External Collaboration functions into the Investigation, Effectiveness Check and Supplier Change Notifications processes. The External Collaboration toolset allows Vault to automatically invite Contacts at your partner organizations to participate temporarily in a Vault process. Vault grants them temporary access to only the records they need to operate on (per the configuration of your Vault) and revokes their access when all collaboration is complete. Dedicated notification templates, specific to the process in which the user is being invited to collaborate, keep them focused and in the loop as to what is expected of them. More about the functional specifics of External Collaboration tools in QMS can be found in Vault Help.
Investigations now supporting External Collaboration means that your organization can now allow external participants to be a part of the Investigation process natively: They can receive tasks with due dates (and the associated notifications), and provide their responses to field prompts directly with the native Vault experience. Put simply, they can now directly enter the Root Cause and Summary of investigations in Vault. This enables faster close-out and mitigates the need for, and risks associated with, manual re-entry of responses received from partners.
Collaborating with external parties on Effectiveness Checks rounds out the SCAR and CAPA processes, allowing for seamless external collaboration from issue identification and assessment to further continue on through action identification, closure and follow-up activities.
Lastly, external collaboration within the Supplier Change Notification process enables organizations to not only receive and immediately begin processing notifications of changes from Suppliers and partners, but to seamlessly interact with their key contacts at those organizations to confirm understanding of the issues. They can triage and assess the impacts of those changes all within Vault, as part of a configured, controlled lifecycle and workflow.
Each process covered with this release includes dedicated Welcome, Welcome Back and Goodbye notification templates which Vault will use when inviting and revoking access for external users. These functions must be configured before external collaboration in any of the identified processes can occur. Configuration and considerations related to External Collaboration may be familiar to organizations who have implemented these functions in other processes. Learn more about configuring external collaboration for QMS processes.
QMS: 5 Whys Root Cause Record Creation Enhancements
Expanding on the 5 Whys Root Cause Analysis features introduced to Vault QMS in 23R1, we’ve introduced additional automation into the creation & management of Root Cause records. This release streamlines the experience for issue investigators, enhancing the automation regarding creating, modifying and deleting Root Cause records during the 5 Whys analysis behind the scenes. This keeps the investigator focused on performing the analysis rather than ensuring the appropriate records are created or deleted in Vault to reflect the outcome of the analysis.
The system behind the scenes has been made smarter with additional configuration options and automation functions. We’ve broadened support for more complex configurations of 5 Whys Root Cause Analysis. When a node in the analysis is set as a Root Cause by a user, Vault QMS is now aware of the possibility of multiple types of Root Causes. The system will now seamlessly prompt the user to select the appropriate type of root cause (defaulting it wherever possible), and then prompt them for the needed fields (which can be specific to the types of Root Causes your organization utilizes) guiding them through the 5 Whys Root Cause Analysis flow per the configuration of your Vault.
Additionally, based on feedback we’ve received, configuration has been opened up for the Cause Type, Category, and Subcategory fields of Root Cause Analysis Items, allowing them to be inactivated as part of the configuration of your process. If the fields are inactivated, they’ll be removed from the 5 Whys Analysis UI if they’re not applicable to the Root Cause as configured.
Lastly, when a Root Cause Analysis enters its lifecycle’s Completed state type, Vault will automatically identify and execute record changes, additions, or removals as needed when a Related Root Cause Analysis Item’s Root Cause field is changed, or the Type of the Related Root Cause Analysis Item is changed during the analysis. This cleans up old or out-dated records left over from prior work within the 5 Whys Root Cause analysis flow automatically.
Some aspects of this feature are automatically enabled for customers who have implemented 5 Whys root cause analysis within their QMS Vault. To take advantage of all of the changes in this release, some configuration may be necessary. Learn more about the new configuration options of and functions within the 5 Whys Root Cause Analysis functions available in Vault QMS.
Create Change Actions from Template Records
Change Controls often define the same Change Actions over and over again. For example, an organization’s manufacturing-related Change Controls might require a routine set of Change Actions for each affected site. If each site regularly creates the same 20 Change Actions, and there are typically 15 manufacturing Change Controls per year, each site recreates the same 20 Change Actions 15 times. Manually creating the same Change Actions over and over again is labor intensive, error prone, and prevents organizations from effectively standardizing repetitive change control processes.
Beginning in this release, Vault provides the ability to add multiple Change Actions to new or existing Change Controls or Change Plans from approved Change Action Templates. Each approved Change Action Template consists of one or more template Change Actions. When Vault creates Change Actions from a template, the field values in the template’s Change Actions are copied to the corresponding fields in the Change Actions. Users that build Change Action Templates can decide whether the copied field values are editable or read-only in the Change Actions.
In order to use this feature, an Admin must configure it. The main configuration options for consideration include:
- The list of approved Change Action Templates available for use depends on the Change Control or Change Plan metadata. Admins specify which fields on a Change Control or Change Plan must have matching values for the equivalent fields in a Change Action Template in order for that template to be available for use.
- Admins configure the ability for users to add Change Actions from matching Change Action Templates at specific states in the Change Control or Change Plan lifecycle.
- Admins can also configure Vault to automatically add Change Actions from a template when a Change Control or Change Plan is created, or when a Change Control or Change Plan enters a particular state.
This feature promotes standardization of repetitive Change Actions, reduces data entry errors, and reduces the effort required to create a Change Control’s Change Actions.
External Notification Usability Enhancements
In this release, the External Notification capability is being enhanced in two ways: A new Distribution Group Label field and and the ability to restrict edit access to the Notification Recipients section of an applicable record.
Organizations often need to notify individuals external to their company about quality management processes occurring within their organization. For example:
- A company wants to notify one of their suppliers at the conclusion of an audit when the audit report is available
- An organization wants to respond to an individual that submitted a product complaint when the complaint is received, and later when the complaint investigation reaches a conclusion
Similarly, it may also be necessary to notify individuals within the organization that are considered “external” because they do not have access to the quality management system. For example, a quality department might want to send a notification to members of the organization’s executive team to alert them to a quality management issue requiring escalation.
The Vault QMS feature known as External Notification enables companies to incorporate notifications to external parties as part of a quality management process such as an Audit, Complaint, Issue Escalation, or any other quality event.
New Distribution Group Label Field for use in Formatted Output Reports
Many customers create a Formatted Output report showing the External Notification recipients associated with a quality process. The Formatted Output report can be downloaded and viewed outside Vault. Configuring the Formatted Output often requires identifying recipients in a particular external notification distribution group.
Prior to this release, the only way to identify an external notification distribution group is by using the Distribution Group field. However, the Distribution Group field identifies a group using a Globally Unique Identifier (GUID) (for example, 128711_OP400000000S003), instead of a human-readable name (for example, Initial Correspondence Group). This is problematic because the GUID for an external notification distribution group changes from Vault to Vault. As a result, the Formatted Output configuration needs to be updated each time it is ported from one Vault to another (for example, from sandbox to production). Maintaining the Formatted Output is more difficult when the configuration refers to a GUID rather than a human-readable name for an external notification distribution group.
This enhancement introduces a new Distribution Group Label field on the objects that store external notification recipients. Distribution Group Label stores the human-readable name of an external notification distribution group. The Distribution Group Label field can be used in Formatted Output configuration to more easily identify the group of recipients to be included in a Formatted Output. Using the Distribution Group Label field will also ensure a Formatted Output configuration can be ported between Vaults without the need to update it in the target Vault. It is important to note that the Distribution Group Label field will be populated from release day forward and will not be updated on historical records.
Refer to the Quality data model updates for more details about this new field.
Security Enhancement: Control Editability of Notification Recipients Section by User Role
When Audit, Complaint, Issue Escalation, or Quality Event utilizes the External Notification capability, their records include a Notification Recipients section. The Notification Recipients section enables a user to identify the individuals (Person records) that receive external notifications for that particular record.
Beginning with this release, customers can configure their quality processes to restrict edit access to the Notification Recipients section based on a user role and the lifecycle state of the Audit, Complaint, Issue Escalation, or Quality Event record.
For example, this allows a customer to permit the owner of a Complaint record to update the Notification Recipients section while a Complaint record is in the Initiated state, and prevent any from updating Notification Recipients once the Complaint is Closed.
Refer to Configuring External Notifications (QMS) for more information on how to enable role and lifecycle state security on a record’s Notification Recipients. Lastly, notification recipient lists will now list full names along with email addresses of the participants, which previously only listed emails and not full names.
QRM: Risk Builder Enhancements
Risk-based decision making is a key component of any organization’s compliance program. Vault QMS allows companies to manage risk associated with enterprise and operational processes. Capturing risk information can be effort intensive. Recognizing this, Veeva introduced the Risk Builder user interface which enables users to easily enter and update Risk data in a Risk Assessment. Risk Builder provides a familiar spreadsheet-like user experience for data entry. This release introduces additional Risk Builder improvements that greatly improve the user experience in the following ways:
Enhancements for Viewing Risks
- Process Step Selector Enhancements: Users can now launch a window to easily search for a Process Step within the Risk Assessment.
- Freeze First Column: The first column in the Risk Builder will now be frozen by default, ensuring that it is never out of view when a user scrolls horizontally to display columns situated on the right side of the Risk Builder matrix.
- See More for Long Text and Rich Text Fields: Risk Builder cells that contain Long Text or Rich Text include a show more link that allows the user to view more of the associated field’s value within a popup window.
- Search Risks: The Risk Builder now includes the ability to search for Risks. A search operation returns Risks that contain at least one field whose value matches the search text provided by the user. Matching text is displayed bold within the search results.
Enhancements for Updating Risks
- Risk Flagging: Users can flag Risk records to identify which ones require further review. A visual indicator is displayed in the Risk Builder, next to a flagged Risk. Users can remove a flag when appropriate. The number of flagged Risk records appears near the top of the Risk Builder.
- Insert Row Above/Below: Users can now add a new row directly above or below the currently selected row in a list of Risks. Previously, users could only add new rows to the bottom of the list.
- Clone / Copy / Paste / Row Actions: The previous release included a Copy Row action that copied the selected row, inserted a new row below the selected row, and pasted the Risk data into the new row. In this release, this action is being re-labeled to Clone Row. A new Copy Row action is now available that simply copies the selected row’s Risk data. The copied data can then be pasted into a selected row using the new Paste Row action.
- Last Saved Timer: Risk Builder now displays approximately how much time has passed since the user saved Risk data.
- Permission Enhancements: Risk Builder will not allow users to add or delete Risk rows, or edit specific Risk fields, unless they have the appropriate permissions.
- Retain Unsaved Data: When Risk Builder saves data and detects incorrect information entered by a user (e.g. ???), a dialog is displayed identifying the issues. After dismissing the dialog, the user will return to Risk Builder in edit mode with the unsaved data retained so corrections can be made. Users can filter the list of Risks to find rows that generated errors.
Other Enhancements
- Keyboard Shortcuts: Risk Builder now supports the following new keyboard shortcuts:
- Ctrl + C: Copy the content of a cell, or copy the selected portion of a cell while in edit mode
- Ctrl + X: Cut the content of a cell, or cut the selected portion of a cell while in edit mode
- Ctrl + V: Overwrite the content of a cell, or paste into the selected portion of a cell while in edit mode
Refer to Vault Help for the full list of keyboard shortcuts available in the current General Release.
These Risk Builder user experience enhancements allow organizations to be more efficient viewing and editing risk data.
Surveillance
23R2: eMDR Enhancements
eMDR safety information submitted to FDA is sourced from several different records within the Vault Product Surveillance application. Organizations must ensure all safety data is thoroughly compiled and reviewed. The 22R1 release addressed this need by providing the ability to automatically assemble data from Product, Person, Contact, MedTech Complaint, and Organization records into a single Adverse Event Report (AER). The AER provides a read-only view of the aggregated safety data related to a complaint so users can review all pertinent information and submit it to FDA.
This feature updates mapping for sections B5, B7, and H10 of the eMDR form.
Surveillance also supports PMA/510(k) number (D4) on both Product and Product Variant level.
Learn more about Vault Product Surveillance adverse event reporting.
EU MIR UI Enhancement: View & Edit Fields on AER
EU MIR safety information submitted to health authorities is sourced from several different records within the Vault Product Surveillance application. Organizations must ensure all Surveillance data is thoroughly compiled and reviewed. The 22R1 release addressed this need by providing the ability to automatically assemble data from Product, Person, Contact, MedTech Complaint, and Organization records into a single Adverse Event Report (AER). The AER provides a read-only view of the aggregated safety data related to a complaint so users can review all pertinent information and submit it to the appropriate health authority.
This feature enhances how users interact with an AER record, allowing them to edit information directly within an AER record. As a result, users no longer need to locate and update the appropriate Product, Person, Contact, MedTech Complaint, and Organization records that represent the source of the information in an AER. When a user updates data on an AER, Vault automatically updates the corresponding fields on the source records.
Allowing users to edit Surveillance data directly in an AER record reduces manual effort and improves data integrity by ensuring the data has a single source of truth.
Learn more about Vault Product Surveillance adverse event reporting.
Validation Management
Deliverable Reference Document Automation Enhancements
This feature enables the system to automatically update the link between a deliverable record and a reference deliverable document to be always up to date until the document version is approved and enters a steady state.
When a document enters the steady state, Admins can configure an entry action on a document lifecycle state that will change the lifecycle state on the Validation Deliverable object record as well as set the value for the specific version of the document as of current. This will allow the approval of a document to mark the Validation Deliverable as “Complete” and keep the version as specific to the event of the approval and completion for the deliverable reference document.
When all associated deliverable records are closed for the deliverable reference document, the document cannot be updated. The deliverable reference document only can be updated when an open deliverable record is associated with it.
Pause, Resume, and Terminate Test
This feature provides the ability to pause, resume, or completely terminate a test script during its execution.
When the actions are configured, they are available in the Execution UI as well as on the record’s details page. Users must provide justifications when pausing, resuming, or terminating a test, but eSignatures can be configured separately via workflow configuration.
When paused, users can see the yellow banner with a message about who paused the test script when and a link to view the justification.
When terminated, users can see the red banner with a message about who paused the test script when and a link to view the justification.
The Execution Change History captures the history of actions and changes for a test script. When a user pauses, resumes, or terminates a test script, the system captures the action type, original state, new state, justification, and test script value in the test script execution change record.
Performance Improvement for Test Authoring & Test Execution
With this release, performance of the custom app pages for test authoring and execution for Authors, Executors, Reviewers, and Approvers has been further enhanced. A section-based pagination control has been introduced in the Test Authoring UI and Test Authoring Review UI to display 25 test step records at a time on a page in the content panel. Similarly, a global pagination control has been introduced in the new UIs that also loads 25 records at a time on a given page in the content panel.
This feature will help ensure various users enjoy authoring and executing test scripts while avoiding long page load times.
Deep Copy Test Script with Test Steps and Linked Requirements
This feature provides the capability for users to copy a test script with its test steps and linked requirements. This allows users to re-run the test script or leverage best practice test scripts to create a clone and re-author to include required changes. Administrators can define which lifecycle state the copied test script will be created in, such as the Pre-Approved state if a new run is needed.
Validation Team
This feature defines how permissions apply across records in the Validation Management application. Validation team members associated with a Validation Inventory Item record will be granted record visibility access rights based on the Inventory Item dimension being applied across all Validation object records.
For global view access users who need access to all validation records, they should have the Validation Viewer role as a User Role.
The team will be selected at the Validation Inventory Item level which will be used to constrain which users can view records and also be assigned as owners for requirements, activities, deliverables, test protocols, test script, test steps, and discrepancies. To give read permission to users, they must be assigned at the Validation Inventory Item level as Validation Contributor at a minimum.
Requirement Owner, Activity Owner, Deliverable Owner, Author, Executor, Witness, and Discrepancy Owner can be defined for each record as a user field. By using the custom UI widget, users can be chosen from a list of Validation Team Members for the Validation Inventory Item.
Role permissions can be defined by the atomic security settings from each object lifecycle. If roles are not added or configured in the object lifecycle, read-only permission is applied by default.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Quality data model to support new features:
Regulatory
RIM
RIM Document Classification Bot
With this release, Vault automatically classifies documents uploaded to the Document Inbox in Vaults with a trained and deployed Document Classification Module. In 23R1, Vault RIM added support for email ingestion, enabling customers to easily import emails. The RIM Document Classification Bot further streamlines this by automatically classifying documents that are added to the Inbox (manually or via the email processor). Users can review the document in the inbox and add additional required metadata, and the system learns each time a user accepts or rejects a classification. Once complete, Vault removes the document from the Inbox.
This feature is Admin-enabled through a checkbox in Application Settings.
Once the RIM Document Classification Bot is enabled, the system automatically provisions the Trained Models object and corresponding Trained Model object lifecycle. The setting also enables the Predictions and Prediction Metric objects, used to track and provide feedback on predictions. The lifecycle includes two user actions, Train Model and Train Model on Production Data, to allow increased accuracy of auto-classification. Users are able to train the model by date (Training Window Start Date) or by attaching a CSV file containing document IDs. Once the training job is complete, the user receives a notification summarizing the training results, and then the model can be deployed.
This feature is part of Vault RIM’s Health Authority Automation journey and increases efficiency and productivity by automating document classification.
Learn more about unclassified documents.
RIM Registrations
EUDAMED Machine-to-Machine UDI Submissions
MedTech companies marketing medical devices in the European Union can now generate and review UDI submissions in Vault Registrations. In previous releases, companies could generate and review UDI submissions in Vault, but had to manually export and submit the UDI data to the EUDAMED database.
To improve the UDI submission management process, Admins can configure the EU EUDAMED UDI Gateway Profile and enable the feature so that business users can directly submit UDI data from Vault Registrations. This eliminates the need for manual export, and users can monitor the submission status to know if it has been accepted or rejected.
Learn more about generating UDI data for EUDAMED.
UPD Submission Data Aggregation
Union Product Database (UPD) is the veterinary product reporting repository for EU animal health medicinal products. In the previous release, Vault Registrations enhanced its data model to include objects and fields required for submission to UPD. This feature creates transactional object records that organize product data in the form and format required for submission. Additionally, the data aggregation algorithm populates these records with source Registration data, transforming where needed to suit health authority requirements. This feature is the next step towards UPD support to enable animal health customers to be compliant with EMA’s UPD regulation. Configuration is required to enable this feature.
Learn more about UPD.
Use for Registrations Field
In this release, we are introducing the Use for Registrations field on the Application, Regulatory Objective, Submission, and Event related records (for example, Event Product). Admins of existing Vaults can configure the field in object page layouts to support the upcoming capability.
In future releases, we will improve the Create Registrations and Manage Registered Details wizards to take into account the Use for Registrations field. This means that:
- When Use for Registrations is set to Yes, Vault uses the Application or Regulatory Objective related record as a source for creating or updating registered details.
- When Use for Registrations is set to No, Vault will not create or update registered details.
This new feature will be particularly useful in situations where users need to add consolidated records, such as All Manufacturers, as related records, but do not want the wizards to create registered details for these records.
Learn more about creating Registrations in bulk and managing registered details.
Automation Enhancements for Labeling Deviations
Labeling teams can now benefit from new automation when managing labeling deviations across dependent markets with the release of Vault’s new labeling deviation trigger enhancements.
When a Labeling Deviation is set to Accepted for Dependent Countries, Vault:
- Sets all labeling deviations for dependent markets into the Reviewed lifecycle state, as additional review is typically not required for dependent markets.
- Populates or updates any blank fields on the dependent labeling deviations each time the reference Labeling Deviation is updated and saved.
The automation is triggered when the new Reference Deviation field is populated on the dependent labeling deviations.
Admins can specify which field values Vault should copy when creating dependent Labeling Deviations, and which fields to update if blank upon subsequent saving.
This feature aims to reflect as much information at the dependent country level as possible to ensure alignment and reduce manual work for labeling teams.
Learn more about Label Concept and Deviation tracking.
Dynamic Affiliate Home Page Title
With this release, Admins can edit the Affiliate Home tab configuration to display a custom label. Previously, Vault displayed this page’s tab and heading with the default Affiliate Home label.
Learn more about the Affiliate Home page.
RIM Publishing
Set Leaf Operation Updates
Vault Submissions Publishing users now have a simpler and more convenient method for setting a document’s eCTD lifecycle operation. An improved interface allows users to select the XML Operation (Replace, Delete, Append) without first having to change the Content Plan Item, reducing the number of clicks. Users will select the document to be lifecycled within the new interface, which has a similar look and feel to the Submissions Archive Viewer. The new interface also aligns with other common publishing actions, creating a more consistent user experience across the application.
Publishing customers will see these immediate improvements:
- A single location to set the XML Operation value
- Leverage the new Submissions Archive Viewer search and its filtering capabilities
- Auto-expansion of the eCTD hierarchy, even if there are repeated sections with the same name
- Ability to Expand All when selecting files in the new interface window
- Full dialog box is visible on any screen size
Learn more about selecting a leaf operation target.
Ukraine eCTD 1.0 Pilot
Ukraine Ministry of Health (MoH) will begin a phased rollout for Marketing Authorisations in eCTD v3.2 format. Their current target for implementation is August 2025. To prepare, Veeva is introducing a pilot for UA eCTD publishing in 23R2, including:
- Ukraine Submission Content Plan Template (UA Module 1)
- UA Regional XML generation, per UA DTD v1.0
- Updated Controlled Vocabularies, Constraints, and picklist values to manage and view Submission Administrative Information
Vault Publishing customers can voluntarily begin generating compliant eCTD submissions after configuration of the above.
RIM Submissions customers using submission content planning can also benefit from configuring and using the new Ukraine Content Plan Template.
Veeva continues to monitor and support all regions that require eCTD, and will address additional UA specification updates (including validation rules) as they are made available.
Learn more about RIM Submissions Publishing.
Pharmaceutical Form Name Override
A new free text field, XML Application Pharmaceutical Form Name, and a new lookup field, XML Submission Pharmaceutical Form Name are available for use in Switzerland (CH) Applications and Submissions.
Previously, the XML was auto-populated with the Application Pharmaceutical Form Name value. If a different name is needed in the CH Module 1 Envelope, publishers can enter it into the XML Application Pharmaceutical Form Name field, and the resulting submission XML contains the override value rather than the default value. These new fields are also available within the Submissions Wizard, when enabled. An Admin will need to add these fields to the appropriate page layouts.
Learn more about working with Pharmaceutical Forms.
Publishing Pre-Validation
For Vault Submissions Publishing to successfully generate a submission, prerequisite actions, fields, and Submission Content Plan (SCP) data must be complete. To better support customers with these manual tasks, Vault now runs pre-validation checks on the most common publishing errors and users when setting the Enable Continuous Publishing field or running the On-Demand Publishing action. These checks are not new criteria for failure, but rather an advance review of existing issues known to cause publishing errors.
Pre-validation checks the following:
- The
published_content_owner__vhas the permission to create documents of the Archive (archive__v) type, and has a Full User Application License - The necessary publishing fields are populated when the Dossier Format is set to eCTD: XML Regional DTD/XSD Version, XML ICH DTD/XSD Version, Validation Criteria Version, XML Sequence ID
- There is only one Content Plan referencing the current Submission
- There are no Submission Clinical Study or Submission Nonclinical Study records related to an active STF Content Plan Item in the current submission with a blank XML Clinical Study Title or XML Nonclinical Study Title
- If there aren’t active STFs, the XML STF DTD XSD Version is not populated
- The Primary Application and Primary Submission are populated on the Content Plan
These new automatic checks will limit time lost to troubleshooting and Support tickets due to known issues or preventable errors.
Learn more about continuous and on-demand publishing validation.
RIM Submissions
Synchronize Content Plans: Iterative & Section-Level Dispatch
Global users managing the Global Content Plan now have the flexibility to initiate multiple dispatches incrementally at a section level, instead of a one-time initial dispatch for the entire Global Content Plan. With this capability, teams can dispatch the modules or sections that are ready as they become finalized as well as changes that are made over time to the Global Content Plan.
Users can quickly review a system generated comparison between the Global Content Plan dispatch and the existing country-specific submission content plan in a new Comparison Viewer which includes new and updated content plan and content plan item records, and new, updated, or removed matched documents. They can then accept or reject all or some of the proposed changes from the Global Content Plan dispatch.
If needed, administrators can set the Mandatory for Dispatch Acceptance field on the Content Plan and/or Content Plan Template records to prevent local affiliates from rejecting specific sections or items.
With each dispatch, Vault creates a new Dispatch Message for each target Activity/Submission so that users can monitor which sections were dispatched and what their current status is per Submission.
See also Synchronize Content Plans: Dispatch Enhancements, a related auto-on feature.
Learn more about Global Content Plans.
Synchronize Content Plans: Dispatch Enhancements
To support the configurable Synchronize Content Plans: Iterative & Section-Level Dispatch feature, several auto-on changes have been made to the existing global content plan dispatch functionality, including:
- When the Copy Relationships option is configured, Vault copies only the relationships within the dispatch scope, instead of all relationships on the Event.
- Dispatch will no longer skip submissions with an existing content plan. Instead, Vault raises an error for the submission if section-level dispatch is not configured.
- Vault will no longer run a general update on the content plan after copying it. Instead, dispatching will only add the specific records which are in the template but missing from the global content plan. This means that Vault will not create repeating sections (based on the template) for relationship records which are on the Submission but not on the Event. If there are additional template or Submission relationship records that are not in the Global Content Plan, the Update Content Plan action must be manually run after each dispatch.
Learn more about Global Content Plans.
RIM Publishing, RIM Submissions
Resolve Embedded Links Based on Source for Published Document Value
Vault Submissions Publishing is improving hyperlink handling when the Source for Published Document is set to a custom rendition. Previously, link data was based on the values for the Viewable Rendition; with this change, Vault retrieves link data from the selected Source for Published Document rendition. This back-end processing will be automatically updated for all Vault Publishing customers.
Permalinks embedded in the source document (for example, Dynamic Links) were previously only resolved if the Source for Published Document value was set to the default Viewable Rendition. These Permalinks will now resolve even when a custom rendition (for example, Imported Rendition) is selected. Vault Link Annotations continue to be resolved only when Viewable Rendition is selected and are not impacted by this change.
Learn more about working with hyperlinks.
RIM Submissions, RIM Submissions Archive
Active Dossier Language Field Update
The document field Vault uses for handling Active Dossier translations has been updated, from Language (language__v) to Language for Submission (language_for_submission__v). This aligns with the document language field used in other RIM applications and features, such as auto-matching and IDMP/XEVMPD.
Learn more about Active Dossiers.
Active Dossier Loader Validations
In this release, we have added two additional validation checks on required and controlling fields to clearly notify users of errors when loading Active Dossier records. Previously, users received an unclear batch error when certain field conditions were not met.
Learn more about loading Active Dossier records.
RIM Submissions Archive
Render XML with no Stylesheet
With this release, Vault Submissions Archive creates a plain text rendition for XMLs when a stylesheet is not available. Previously, these documents were not rendered. This auto-on feature facilitates XML viewing within RIM Submissions Archive.
Learn more about the Submissions Archive Viewer.
Submissions Archive Hierarchy Tree View Expansion Improvement
Submissions Archive Viewer is now further enhanced to support viewing of larger applications. In the redesigned Viewer, UI and back-end limitations did not allow for full expansion of sections in large submissions, such as those with tens of thousands of records. While users were alerted that an expansion limit was reached, they were not provided details on which items failed to load.
Without sacrificing performance, the following Viewer improvements are automatically enabled:
- Increasing the total number of rows exposed in a single view from the current UI limit, and more than doubling the record handling capabilities on the back-end
- Expansion of all nested subsections by depth rather than in a vertical order (for example, all 3.2.P.4 subsections are fully expanded before 3.2.P.5 expands)
- Error messages appear at the site of the expansion limit to clearly identify which items have and have not been loaded in the view
Learn more about the Submissions Archive Viewer.
Submissions Archive: CN eCTD 1.0 Support Update
This auto-on feature updates section labels in Module 1 to align with the latest specification for CN eCTD 1.0.
Learn more about the Submissions Archive Viewer.
23R2 RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model.
Safety
Safety features are targeted for tentative availability on June 15, 2023.
Safety
Multi-Product Selector for Study Products on Inbox Item Configuration
This feature introduces a new action, “Specify Study Products”, to facilitate Study Product entry on manual and E2B imported Inbox Items. Upon selecting the action, Case Processors can add Study Products using a multi-select field. This addition is particularly useful for complex Studies that involve numerous phases or Products, as it allows you to add multiple relevant Study Products to the Inbox Item at once. Vault Safety promotes all Study Product records to Case and as a result, improves Case processing efficiency.
Learn More
- Enablement: Enable the Multi-Product Selector for Study Products on Inbox Items
- User Help: Inbox Item Field Reference: Add Study Products to Inbox Items
Non-E2B Case Identifiers Entry and Duplicate Search Configuration
Vault Safety can now store non-E2B Case Identifiers (Reference Numbers) to use for duplicate search. In addition, Admins can now configure both re-transmittable and non-transmittable Case Identifier types, where only re-transmittable types are included in E2B transmissions. Furthermore, intake users can now enter Case Identifiers on Inbox Items. Lastly, All Case Identifiers will be used for duplicate search.
The ability to include non-E2B Case Identifiers as part of the duplicate search will make the Case Intake process more efficient and give users the flexibility to add Case Identifiers to Inbox Items.
Learn More
- Enablement: Enable Non-E2B Case Identifiers Entry and Duplicate Search
- User Help: Inbox Item Field Reference: Case Identifier(s) fields
Case Compare Limit Increase Auto-on
When creating a Follow-up Case or merging to an In-flight Case, Vault Safety now supports a maximum of 10,000 records for up to 3 objects and 500 records for the remaining objects on Case Compare. Once the limit is reached, the system displays a message and prevents Case promotion from Case Compare. Previously, the Case Compare record limit was capped at 500 records per object.
By increasing the Case Compare record limit, Vault Safety ensures that valuable information is not lost during Case promotion. This feature provides you with an efficient way to carry over new information to Follow-up and In-flight Cases, improving data integrity and streamlining workflows.
Learn More
Fast Verify for Imported Inbox Items Auto-on
Vault Safety now validates the Inbox Item data upon importing a Case to Inbox Item through E2B or API integration. This feature improves Intake processing efficiency by removing the need to manually verify each Inbox Item section before promoting to a Case.
In addition, sections and records will be clearly indicated as Verified or Invalid to facilitate the correction of incorrect data before promotion. Error messages are displayed within the appropriate sections and records. For Case Contact, Product, and Event sections, Vault Safety displays the number of records with invalid data. This is especially important as previously, the system did not identify the invalid records and fields, making it difficult and time-consuming for intake users to correct errors. With this feature, you can easily identify errors, saving valuable time and effort.
Learn More
Automated Case Promotion Action Configuration
This feature introduces significant enhancements to the Automated Case Promotion workflow in Vault Safety.
Admins can configure a new user action to manually trigger Automated Case Promotion for non-Cases (for example, a Case that does not require submission to a Health Authority). They can also configure an entry action to proceed with Automated Case Promotion when a non-Case enters a certain lifecycle state. These configurations streamline the non-Case workflow, which previously was not suitable for Automated Case Promotion.
Learn More
- Enablement: Enable Automated Case Promotion for Non-Cases
- User Help: Automated Case Promotion
Enhanced Product Matching Support for Intake Auto-on
This feature extends all Product matching logic to JSON API import. Previously, Product matching was only supported for E2B import to Inbox Item. Additionally, when a Product Registration Name (Trade Name) is created or updated, Vault Safety automatically creates a Product Alias, if that alias name does not already exist.
Learn More
- User Help: Inbox Item Study and Product Matching
Full Case Data Import to Inbox Item Through API Auto-on
The public JSON Intake API now supports the import of all Case data to Inbox Items. The JSON format will accept any Case-related objects, including Case Medical History, Drug History Test Results, and Case Identifiers. This feature facilitates the integration of Vault Safety Inbox Items with any external source system without the need to generate E2B files.
Company Product Match Verification Configuration
This feature introduces a more advanced coding verification for Product matching upon E2B and JSON import. Admins can configure the Product coding criteria, which include the fields by which the imported Product and library Product must match (Country, Route of Administration, and Dose Form). Admins can also configure the level of confidence needed for Product matching (for example, Confident Match, Likely Match, and more). If the Product coding criteria are met, the system proceeds with Product matching and auto-coding.
This Product matching enhancement improves the accuracy of generic Product matching and increases efficiency during the Case intake process.
Learn More
- Enablement Help: Enable Company Product Match Verification
- Admin Help: Configure Company Product Match Verification
- User Help:
- Inbox Item Study and Product Matching: Company Product Match Verification
- Inbox Item Field Reference: Product Match Confidence and Product Match Criteria
Auto-Generate Serious and Non-Serious Assessments Admin Checkbox
Vault Safety makes Case processing more efficient with automatic generation of Assessments, Assessment Results, and Expectedness records for all serious and non-serious Adverse Events paired with Suspect or Interacting Case Products. Previously, this feature required a user action. Now, auto-generation is available on Case promotion and when adding or editing Case Products and Adverse Events.
Learn More
- Enablement: Enable Auto-Generate Serious and Non-Serious Assessments
- User Help: Generate Assessments
Study Product Expectedness Configuration
To support clinical trial studies with multiple products and different expected Adverse Events, Vault Safety is introducing Study Product Datasheets. Now that Admins can configure datasheets for individual study products, the system generates Expectedness at a more granular level, removing the need for Case Processors to verify Expectedness manually.
In addition, when processing Follow-Up Cases, the system now preserves the Expectedness from the initial Case.
Finally, respecting guidelines for regulatory and aggregate reporting, Expectedness evaluations now consider adverse event onset dates for Clinical Trial Study Cases.
Learn More
- Enablement: Enable Study Product Expectedness
- Admin Help: Manage Datasheets and Auto-Expectedness
PMDA Special Report Classification for Case Processing Configuration
Vault Safety now supports PMDA Special Report Classification for Case Processing. In 23R1, Special Report Classification was introduced on the Inbox Item and Case. With this release, values set on the Inbox Item are promoted when creating Cases or Follow-up Cases or merging to In-flight Cases. This streamlines Case processing and enhances efficiency.
Learn More
Product Masking Selection for Study Products Configuration
For Case Study Products, Studies, and Study Arms, Blinded field values are now consistently Open Label, Unblinded, and Blinded, communicating the masking more clearly.
Learn More
- Enablement: Enable Product Masking Selection for Study Products
- User Help: Enter Case Data: Blinding Details Section
Clinical Trials: Blinded Field and Product Reported Field Updates Auto-on
For Clinical Trial Study Cases, during Case unblinding if all Study Products are unblinded, Case-level blinding is now automatically set to Unblinded. This increases the efficiency of unblinding Study Cases. Additionally, when adding unblinded or open label Study Products to a Case, the Study Product Name is mapped to the Product (Reported) field.
Learn More
- User Help:
Clear Radio Button Selection for Submittable Fields Configuration
Vault Safety now offers a “Clear Selection” button for submittable fields with Yes/No radio buttons on Cases so that users can clear their selection in case of errors or changed information. This feature enhances the usability of data entry and improves the overall efficiency of case processing.
Learn More
Asynchronous Activity Indicators - Validation Results Configuration
Vault Safety now displays an indication to inform users about the status of regulatory conformance validations on Cases and Transmissions. The indication banner also includes an option to refresh the section and view results when they are ready.
This notification will enhance usability by keeping users informed about the progress of generating validation results.
Learn More
Faster Company Product Selection on Inbox Item Configuration
This feature streamlines the Product coding process by introducing a control where you can quickly and efficiently select Products during Case intake. Previously, Product selection was done with a simple dropdown picklist. This is functional for a small number of Products, but selecting a Product from larger Product libraries was cumbersome. This feature introduces two new buttons: Auto-code and Product search (binoculars icon). After selecting a Product, you can hover over the Product field to display information such as the Product Family, Product, Trade Name, Registration, and Country. In addition, selecting the Binoculars icon opens a Product coding browser where you can search, filter, and view additional information related to the Product.
Learn More
- Enablement: Enable Faster Company Product Selection on Inbox Item
- User Help: Auto-Code and Browse Products
Smart MedDRA Coding on Import Auto-on
With this release, Vault Safety extends Smart MedDRA Coding to E2B and JSON import for Inbox Item creation. Our most advanced MedDRA coding option, this feature uses AI and automation to code reported terms that are not an exact match to terms in the active MedDRA dictionary or your MedDRA Synonym list. For example, Smart MedDRA Coding offers suggestions for misspelled terms, abbreviations, ambiguous information, multiple reported terms, and more. This feature could also facilitate Automated Case Promotion by coding high-confidence terms during import or Inbox Item creation.
This feature is Auto-on in Vaults with Smart MedDRA Coding enabled.
Learn More
- Enablement: Enable Smart MedDRA Coding
- User Help:
Rule Engine Destination Evaluation Log Configuration
With this release, Vault Safety creates a CSV file to log the following Rule Set execution information:
- Rule Execution Time
- Destinations Evaluated
- Which Destinations passed a rule
- Which Destinations did not pass a rule
Currently, when running Submission and Distribution rules, Vault Safety creates Transmission messages which describe why each of the submissions was generated. However, if no rule applies, this is not logged, often causing confusion as to why no submission was created.
This feature documents the complete picture and assures users that all Transmission rules were fully evaluated.
Learn More
- Enablement: Enable the Rule Engine Destination Evaluation Log
- User Help: Understand the Reporting Rules Engine
Logically Delete Non-Submittable Transmissions on Subsequent Rules Engine Evaluations Admin Checkbox
The Vault Safety Rule engine re-evaluates reporting obligations on all Case data modifications. It automatically updates previously created Submissions to include only Transmissions that meet reporting obligations. This feature also updates Transmission Due Dates when data is re-evaluated. This prevents over-reporting of Cases without needing to manually remove Submission records.
Learn More
- Enablement: Enable Logical Deletion of Non-Submittable Transmissions on Subsequent Evaluation
- User Help: Transmissions and Subsequent Evaluations
Safety Rule Set Config Report Auto-on
Previously, it was difficult to visualize the Rules and Rule Parameters that configure a Rule Set. Now, Business Administrators can generate a Safety Rule Set Configuration Report for a Rule Set. They can download the report as an XLS file and review the Rule Set’s Rules and Rule Parameters in an easy-to-read format. If required, they can generate Safety Ruleset Configuration Reports in bulk.
Learn More
- User Help: Reporting Rule Sets
IMDRF on FDA MedWatch 3500A Admin Checkbox
Admins can now configure FDA MedWatch 3500A forms to export International Medical Device Regulators Forum (IMDRF) device codes instead of FDA device codes. This provides more flexibility when reporting to the FDA.
Learn More
- Enablement: Enable IMDRF on FDA MedWatch 3500A
- User Help: FDA 3500A Generation Data Mapping
FDA MedWatch 3500A Support for OTC and Additional Device Fields Auto-on
When generating FDA MedWatch 3500A forms, more device-specific fields and Combination Product scenarios are supported. The fields now exported include the following:
- C.7. Is the Product Over-the-Counter?
- G.5. OTC
- H.3. Device Evaluated by Manufacturer
- H.9. If action reported to FDA under 21 USC 360i(f), list correction/removal reporting number
- H.10. Additional Manufacturer Narrative
In addition, when exporting Combination Products to FDA MedWatch 3500A forms, Vault Safety uses the Transmission Product Type to determine whether to export the Product to section C - Suspect Products or section D - Suspect Medical Device and H - Device Manufacturers Only. This feature also introduces OTC Device as a Transmission Product Type.
This feature is Auto-on, however some components require additional configuration.
Learn More
- Enablement: Enable FDA MedWatch 3500A Support for OTC and Additional Device Fields
- User Help: Enter Case Data: Device Information Section
DSUR and PBRER Investigational Product Causality Admin Checkbox
Vault Safety can be configured to only consider Case Assessment Results for the Investigational, Placebo, Active Comparator, and Blinded Study Products when deciding if a Case should be included in the DSUR and PBRER Serious Adverse Reaction listings and tabulations. Previously, Vault Safety considered all assessments for causality and all adverse event counts to determine if a Case should be included in the Serious Adverse Reaction listings.
This feature ensures that Vault Safety only considers causal assessments related to Investigational Products in the DSUR and PBRER.
Learn More
- Enablement: Enable DSUR and PBRER Investigational Product Causality
- User Help:
DSUR and PBRER List All Suspect Products Auto-on
DSUR Line Listings now list all suspect products for a given Case. Previously, only the primary suspect Product was listed. The DSUR and PBRER also now include a Breakdown of Investigative Medicinal Products tabulation for Serious Adverse Events (SAEs) and Serious Adverse Reactions (SARs) from Clinical Trials. This is particularly important for combination therapies and provides enhanced DSUR reporting.
This feature is Auto-on, however an Administrator will need to load two new DSUR templates in your Vault.
Learn More
- Enablement: Enable the DSUR and PBRER to List All Suspect Products
- Admin Help: Configure Aggregate Report Templates
- User Help: Create DSUR Aggregate Reports
DSUR and PBRER Drug Role Filtering Configuration
The DSUR and PBRER now have a Drug Roles to Include report parameter. This report parameter affects the following tabulations:
- Interval Line Listings of Serious Adverse Reactions
- List of Subjects Who Died During the Reporting Period
- Cumulative Tabulation of Serious Adverse Events From Clinical Trials
- Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials.
Previously, these tabulations included all drug roles (Suspect, Drug Not Administered, Interacting, Similar Device, Treatment, and Concomitant). Now you can specify which drug roles to include in these tabulations based on business requirements.
Learn More
- Enablement: Enable Drug Role Filtering for DSUR and PBRER
- User Help:
PMDA Submission Rule Parameter Updates Configuration
In 23R1, Vault Safety introduced support for multiple PMDA Submissions from a single Case, with the plan to update the associated Reporting Rule Parameters in a later release. With 23R2, the PMDA Rule Parameters now leverage the new and changed fields, including Registration Type, Infection, and Special Report Classification. In addition, the new Previously Localized Rule Parameter is also evaluated. These updates support PMDA requirements for calculating due dates.
Learn More
- Enablement: Enable PMDA Submission Rule Parameter Updates
- User Help:
External E2B+ Builder SDK Event Type Configuration
The Vault Safety Java SDK now provides the E2BOutboundEventType. For developers building an external integration with an E2B+ Builder Service, this parameter indicates whether the request type is “Regenerate” or “Submit”.
Feature Enablement Changes
Enablement changes apply to the following features in this release:
| Feature | Previous Enablement | New Enablement |
|---|---|---|
| Generate Assessments Record Action | Support (23R1) | Configuration (23R1.3) |
| Parameter Driven Assessment Selection for Rules Engine | Support (23R1) | Auto-On (23R1.3) |
See the following articles to learn more about these features:
- Generate Assessments Record Action: What’s New in 23R1
- Parameter Driven Assessment Selection for Rules Engine: What’s New in 23R1
SafetyDocs
PSMF Periodic Review Configuration
Vault SafetyDocs can now automatically trigger periodic reviews of PSMF documents. PSMF content must be reviewed on a regular basis for compliance, which can be a repetitive and time-consuming process. With this feature, users can automate periodic reviews and regularly assess if any changes are required. The system can then assign review tasks to users and details about their completion can be tracked. This eliminates the burden of scheduling periodic reviews and helps to maintain compliance through up-to-date PSMF content.
Learn More
- Enablement: Enable PSMF Periodic Reviews
- User Help: Set up PSMF Periodic Reviews
- Admin Help: Configure the PSMF Periodic Review Job
Aggregate Report Authoring Configuration
Vault SafetyDocs now supports the end-to-end drafting of all Aggregate Report components within your Vault.
Authoring tasks can be created for each section of the report and assigned to users in Vault. These tasks can relate to the authoring of the Aggregate Report as a whole or the authoring of a section specific to a particular Aggregate Report Destination. Users can also track the planned and actual submission dates for each Aggregate Report Destination.
This feature allows Aggregate Reports sharing the same type, product, and data periods to be grouped together so their components can apply to multiple markets and health authorities. Supporting the full drafting process in Vault SafetyDocs also provides a validated way of authoring these documents and allows relevant business groups to easily collaborate on them.
Note: Aggregate Report Authoring is a building block for expanding future Aggregate Reporting capabilities in Vault SafetyDocs. This feature will be available for full configuration in an upcoming release. For more information, contact your Veeva representative.
Learn More
- User Help: Author Aggregate Reports
QualityOne
QMS
Product Groups
The Product Groups feature allows customers to create and manage groupings of products based on shared attributes such as usage, brand, material, or other defining characteristics. Product Groups enable customers to build an individualized hierarchy of product groups using a standard self-referencing object, allowing the flexibility to define their product groups according to an organization’s unique business structure and requirements.
Learn more about the QMS shared data model.
COA: Expanded Email Intake Support
This feature builds upon the Automated Email Intake for COA feature by allowing external parties to upload COA files to Vault for analysis without direct interaction with Vault. Expanding this feature allows external parties to easily upload relevant files and create COA Inspections without requiring a supplier Vault login, which helps to speed up the validation, ingestion, and analysis process. Customers must also implement a new Vault Java SDK entry point to allow the Supplier Initiated COA Email Processor email intake type to fully handle incoming emails to Vault.
Learn more about the the expanded Email Intake feature.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram
This feature allows users to create and update a HACCP process flow diagram in an easy-to-use visual interface. Each element on the diagram such as a process step or connection between two steps is saved as a record in Vault, and users with View permission can review a brief summary of each record. Users can lock and unlock the diagram based on the lifecycle state of the HACCP Plan. Process steps identified as CCP or OPRP steps display with the appropriate labels on the diagram.
Learn more about the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Plan Enhancement
The following are some of the improvements to the existing functionality of HACCP in QualityOne.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Ability to Add the Same Process Hazard Analysis to Multiple Process Steps in a HACCP Plan
This feature allows the user to add an existing hazard analysis to multiple steps in the same HACCP Plan. Validation logic is recommended to prevent users from selecting a hazard analysis associated with another HACCP Plan.
Increase in Record Limit for Deep Copy & Populate HACCP Plan Actions
This feature increases the limit of records a user can copy at once to 4000 for the following record actions:
- Create HACCP Plan Design from Design
- Create HACCP Plan from Design
- Populate HACCP Plan
Learn more about HACCP Management.
HSE
Permit to Work
This feature allows users to manage and document Permits to Work in Vault, eliminating the need for manual or paper-based Permit to Work systems. Users can submit Permits to Work for review and approval, and HSE Managers can manage the entire Permit to Work process in Vault.
Learn more about the Permit to Work feature.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on June 20, 2023.
Compliance Management
Formulation Composition Viewer: Freeze Left Columns
This feature allows Admins to freeze up to ten (10) Formulation Composition grid columns in the left side of the Formulation Composition Viewer. This allows the frozen columns to remain visible while users scroll horizontally when performing compliance assessments in the viewer.
Learn more about the Formulation Composition Viewer.
Formulation Composition Viewer: Limit 5 per Page
This feature reduces load times when users open Formulation records by limiting the number of Formulation Composition Viewer sections Admins can add to the Formulation object page layout to five (5).
Learn more about configuring the Formulation Composition Viewer.
Registration & Dossier Management
Registration Item Requirement Hierarchy Viewer
This feature allows Admins to configure a Registration Item Requirement Hierarchy Viewer on the Registration and Registration Item object page layouts to help users visualize the list of Registration Item Requirements in a hierarchical structure. This allows users to easily track the progress of all requirements in a dossier and quickly identify what requirements require attention before exporting a Dossier Binder.
Learn more about the Registration Item Requirement Hierarchy Viewer.
Enable Optimistic Locking on Registration Item Object
This feature turns on optimistic locking on the Registration Item (request__v) object, which prevents users from editing records while another user updates the record and from editing records while Vault updates the record based on user-initiated actions such as the Create Registration and Objective action. This ensures that users don’t accidentally overwrite each other’s updates.
Remove Create Registrations in Bulk Feature: Update
In 23R1.2, we announced Remove the Create Registration in Bulk Feature. This removal is now deferred to 23R3.
Regulatory Documents
Export Binder as Merged PDF
This feature allows users to merge all PDF renditions in a binder into a single downloadable file, allowing them to easily generate a complete file to send to customers and authorities.
Learn more about exporting a binder as a merged PDF.
Enable Optimistic Locking on Registration Object
This feature turns on optimistic locking on the Registration object, which prevents users from editing records while another user updates the record and from editing records while Vault updates the record based on user-initiated actions. This ensures that users don’t accidentally overwrite each other’s updates.
Veeva Claims
Veeva Claims features are targeted for tentative availability on June 20, 2023.
Veeva Claims
Selective Project Automation Enhancements: Actions Run as System
This feature allows Admins to configure new Create Selective Claims and Create Selective Local Adaptations actions. These actions allow users to selectively generate all applicable Claims and Local Adaptations for a project, without any restrictions on which key object fields an Admin has assigned them the Create and Edit permissions.
Learn more about configuring the Create Selective Claims and Create Selective Local Adaptations actions.
Comment Thread Enhancement: Search
This feature allows users to search all comments on a record for specific text in any comment, comment ID, and commenter name in the comment thread section in Project, Claims, Local Adaptations, and Pack Copy pages. This enables users to quickly identify and review relevant comments on an object record.
Learn more about using comments.
Statement Prohibit Join Object Provisioning Update
This feature updates the value format of the Name (name__v) field on the system-managed Statement Prohibit Join (statement_prohibit__v) object to avoid creating duplicate records erroneously.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| SVG, AVIF & WebP Rendition Support | Auto-on | Platform |
| Render Animated GIF Files As Videos | Auto-on | Platform |
| Collaborative Authoring: Expanded URL Compatibility | Auto-on | Platform |
| Vault Objects | ||
| Audit All Updates to Document Reference Field Value | Auto-on | Platform |
| Audit Correct User for SDK Trigger Updates | Auto-on | Platform |
| Display Format | Configuration | Platform |
| Lifecycle & Workflow | ||
| Object Lifecycles: Cancel Workflow Entry Action | Configuration | Platform |
| Workflow Custom Actions SDK for Multi Record Object Workflows | N/A | Platform |
| Email Notifications when Workflow Owners are Replaced | Auto-on | Platform |
| Reporting & Dashboards | ||
| User Reminder Flash Report | Auto-on | Platform |
| Combine Report Prompts | Auto-on | Platform |
| Copy & Paste List of Object Names | Auto-on | Platform |
| Search | ||
| Current User Filter | Auto-on | Platform |
| Query Limits | Auto-on | Platform |
| Formulas | ||
| Advanced Functions in Vault Formulas | Auto-on | Platform |
| Checklists | ||
| Visual Checklist Designer | Auto-on | Platform |
| Checklists: Sum Score | Auto-on | Platform |
| UI & Usability Updates | ||
| Consistent Default Navigation Behavior | Auto-on | Platform |
| Administration | ||
| POD Listed in Domain Information | Auto-on | Platform |
| Enable Limited Release Sandbox For All Vaults | Auto-on | Platform |
| Full Sandbox Refresh Frequency Update | Auto-on | Platform |
| API Response Status Insights | Auto-on | Platform |
| Vault File Manager | ||
| Upload to File Staging | Auto-on | Platform |
| Sign in to Vault File Manager with SAML SSO | Auto-on | Platform |
| Vault Java SDK | ||
| SDK Debug Log Filters | Auto-on | Platform |
| Platform Data Model Changes | Auto-on | Platform |
| Connections | ||
| Connection Exception Management | Auto-on | Platform |
| Quality/ClinOps: Study Data Connection | Configuration | CTMS, Quality |
| Clinical Operations | ||
| Clinical CRM: Automated Clinical Activities | Configuration | CTMS |
| ClinOps/CDMS - Screening subjects outside of CDMS | Auto-on | CTMS<>CDMS |
| ClinOps/CDMS - Casebook Link for Multiple CDMS Vaults | Auto-on | CTMS<>CDMS |
| Subject Transfer History | Auto-on | CTMS |
| Subject Visit Method | Configuration | CTMS, CTMS<>CDMS, Vault Payments |
| Trip Report Question Display Line Breaks | Auto-on | CTMS |
| Feasibility Delighter: Custom Email Senders for Surveys | Configuration | Study Startup |
| Excluded Classifications for TMF Bot | Configuration | Study Startup, eTMF |
| Study Metadata Extraction: Bulk Pipeline Support | Auto-on | eTMF |
| Milestone Workspace | Support | Study Startup, eTMF |
| TMF Transfer: Match on Site Name | Auto-on | Clinical Operations - All |
| Editable Study Country Name | Admin Checkbox | CTMS, eTMF, Study Startup |
| Enhanced Assigned To Control on Quality Issue | Auto-on | CTMS, Study Startup, eTMF |
| Study Person Role Constraints (User) | Configuration | CTMS, Study Startup, Vault Payments, eTMF |
| Document Exchange for Additional Vault Clinical Docs | Configuration | Site Connect |
| Clinical Operations Data Model Changes | Auto-on | CTMS, Study Startup, Vault Payments, eTMF |
| Commercial | ||
| Bulk Generate eCTD Compliance Package | Configuration | PromoMats |
| Set Match Text Field Length Minimum Value to 5 | Auto-on | PromoMats |
| AIRs Support for SVG & WebP Files | Configuration | MedComms, PromoMats |
| Text Asset Substantiation Lifecycle Entry Criteria | Admin Checkbox | PromoMats |
| Increase number of Portal Content filters | Configuration | PromoMats |
| Document Order Support for Portal Document Widgets | Auto-on | PromoMats |
| Extend file name exclusion eCTD | Auto-on | PromoMats |
| Optional Filters in Select Claim Dialog | Auto-on | PromoMats |
| 23R2 Commercial Data Model Changes | Auto-on | MedComms, PromoMats |
| Medical | ||
| Set Match Text Field Length Minimum Value to 5 | Auto-on | MedComms |
| Increase number of Portal Content filters | Configuration | MedComms |
| Document Order Support for Portal Document Widgets | Auto-on | MedComms |
| AIRs support for SVG and WebP files | Configuration | MedComms |
| CRM Data Sharing: Sync Only Required Accounts | Support | MedComms |
| Standard Responses for Medical Inquiry | Configuration | MedComms |
| 23R2 Medical Data Model Changes | Auto-on | MedComms |
| Vault Mobile | ||
| Device-Enforced App Access Security Setting | Admin Checkbox | Vault Mobile |
| Training | ||
| Auto Create User Role Setup Security Records | Configuration | Study Training |
| Quality | ||
| External Collaboration: External Review & Approval of Documents | Configuration | QualityDocs |
| Increase in Default Limits of Visual Hierarchies in Process Navigator | Auto-on | QualityDocs |
| Record Counts Added to the Periodic Review & Change Authorization Sections of Document Change Control | Auto-on | QualityDocs |
| QR Code Generator for Documents & Station Manager | Configuration | QualityDocs, Station Manager |
| Dynamic Enrollment: Automatically Assign Learner Roles to Person | Configuration | Training |
| Learner Homepage: Configurable Filter Options | Configuration | Study Training, Training |
| SmartMatch Curricula | Configuration | Training |
| Assign Curriculum to a Learner's Training Matrix | Configuration | Training |
| Consolidated Direct Assignment Jobs | Auto-on | Study Training, Training |
| Ability to Cancel a Classroom Training Assignment Workflow | Configuration | Study Training, Training |
| Cancel Instructor Workflow Task | Configuration | Training |
| Custom Curriculum and Learner Role Object Types Not Allowed | Auto-on | Study Training, Training |
| Curricula or Learner Role Object Types Usage Restriction Based on Application License | Auto-on | Study Training, Training |
| External Collaboration: Support for Investigation, Effectiveness Check, & Supplier Change Notifications | Configuration | QMS |
| QMS: 5 Whys Root Cause Record Creation Enhancements | Auto-on | QMS |
| Create Change Actions from Template Records | Configuration | QMS |
| External Notification Usability Enhancements | Auto-on | QMS |
| QRM: Risk Builder Enhancements | Auto-on | QMS |
| 23R2: eMDR Enhancements | Auto-on | Surveillance |
| EU MIR UI Enhancement: View & Edit Fields on AER | Configuration | Surveillance |
| Deliverable Reference Document Automation Enhancements | Configuration | Validation Management |
| Pause, Resume, and Terminate Test | Configuration | Validation Management |
| Performance Improvement for Test Authoring & Test Execution | Auto-on | Validation Management |
| Deep Copy Test Scripts with Test Steps and Linked Requirements | Configuration | Validation Management |
| Validation Inventory Application Security | Admin Checkbox | Validation Management |
| Quality Data Model Changes | Auto-on | LIMS, QMS, QualityDocs, Station Manager, Surveillance, Training, Validation Management |
| Regulatory | ||
| EUDAMED Machine-to-Machine UDI Submissions | Configuration | RIM Registrations |
| UPD Submission Data Aggregation | Configuration | RIM Registrations |
| Use for Registrations Field | Configuration | RIM Registrations |
| Automation Enhancements for Labeling Deviations | Configuration | RIM Registrations |
| Dynamic Affiliate Home Page Title | Auto-on | RIM Registrations |
| Set Leaf Operation Updates | Auto-on | RIM Publishing |
| Ukraine eCTD 1.0 Pilot | Configuration | RIM Publishing |
| Pharmaceutical Form Name Override | Configuration | RIM Publishing |
| Publishing Pre-Validation | Auto-on | RIM Publishing |
| Synchronize Content Plans: Iterative & Section-Level Dispatch | Configuration | RIM Submissions |
| Synchronize Content Plans: Dispatch Enhancements | Auto-on | RIM Submissions |
| Resolve Embedded Links Based on Source for Published Document Value | Auto-on | RIM Publishing, RIM Submissions |
| Render XML with no Stylesheet | Auto-on | RIM Submissions Archive |
| Submissions Archive Hierarchy Tree View Expansion Improvement | Auto-on | RIM Submissions Archive |
| Submissions Archive: CN eCTD 1.0 Support Update | Auto-on | RIM Submissions Archive |
| Improved User Exception Message Processing for Object Transfer | Auto-on | ClinOps<>RIM, RIM |
| RIM Document Classification Bot | Admin Checkbox | RIM |
| Active Dossier Language Field Update | Auto-on | RIM Submissions, RIM Submissions Archive |
| Active Dossier Loader Validations | Auto-on | RIM Submissions, RIM Submissions Archive |
| 23R2 RIM Data Model Changes | Auto-on | RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive |
| QualityOne | ||
| Product Groups | Configuration | QualityOne QMS |
| COA: Expanded Email Intake Support | Configuration | QualityOne QMS |
| HACCP Flow Diagram | Configuration | QualityOne QMS |
| 23R2 HACCP Plan Enhancement | Auto-on | QualityOne QMS |
| Ability to Add the Same Process Hazard Analysis to Multiple Process Steps in a HACCP Plan | Auto-on | QualityOne QMS |
| Increase in Record Limit for Deep Copy & Populate HACCP Plan Actions | Auto-on | QualityOne QMS |
| Permit to Work | Auto-on | QualityOne HSE |
| RegulatoryOne | ||
| Formulation Composition Viewer: Freeze Left Columns | Configuration | RegulatoryOne Compliance Management |
| Formulation Composition Viewer: Limit 5 per Page | Configuration | RegulatoryOne Compliance Management |
| Registration Item Requirement Hierarchy Viewer | Configuration | RegulatoryOne Registration & Dossier Management |
| Enable Optimistic Locking on Registration Item Object | Auto-on | RegulatoryOne Registration & Dossier Management |
| Remove Create Registrations in Bulk Feature: Update | N/A | RegulatoryOne Registration & Dossier Management |
| Export Binder as Merged PDF | Configuration | RegulatoryOne Regulatory Documents |
| Enable Optimistic Locking on Registration Object | Auto-on | RegulatoryOne Regulatory Documents |
| Veeva Claims | ||
| Selective Project Automation Enhancements: Actions Run as System | Configuration | Veeva Claims |
| Comment Thread Enhancement: Search | Auto-on | Veeva Claims |
| Statement Prohibit Join Object Provisioning Update | Auto-on | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|