Release Date: June 25, 2021
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Working with Documents
Merge Fields Nested Table Rendering
With this release, Vault fully supports nested table rendering. When using Merge Fields for Microsoft Word repeaters in a table, Vault now only displays the data relevant to the object record in a table row within its nested tables.
Infinite Document Scrolling
With this release, Vault no longer displays visible tranche navigation on long documents. Users can now scroll seamlessly across all pages. Previously, users needed to navigate from one set or tranche of 25 pages on desktop and 5 pages on mobile. Note that documents viewed in Internet Explorer (IE11) continue to use visible tranche navigation.
Navigate From Collapsible Upload Modal to Inbox
With this feature, users can navigate back to the Document Inbox from anywhere in Vault via a new button on the collapsible document upload status dialog.
Reporting & Dashboards
Bulk Actions on Objects in Reports
With this release, users can perform bulk actions on object records in report results. Users can also perform bulk actions on documents when a report is grouped.
Action UI
Action Bar
The Action Bar continuously learns how each user works and shows their most-used actions right on top and easily accessible with large, recognizable icon buttons. The Action Bar also helps make users more aware of workflow actions they can perform, such as starting available workflows or changing lifecycle states, by dynamically showing a workflow actions button. The Action Bar is available for object records and documents to help users perform important actions easier and faster.
Doc Info UI Enhancements
With this release, the Doc Info pane is now divided into five panels: Information, Relationships, Files, Sharing Settings, and Timeline View. Instead of Mixed View, Content View, and Field View, users can now expand and collapse the entire Doc Info pane. Additionally, users can click and drag to resize the Doc Info pane. Vault maintains this panel width across all documents. Note that, when in Annotation mode, the Doc Info pane is set to 30% width and is not resizable.
Annotate UX Refresh
This enhancement adds a significant number of visual and usability improvements to annotations and document viewing in Vault. These enhancements also apply to video annotations.
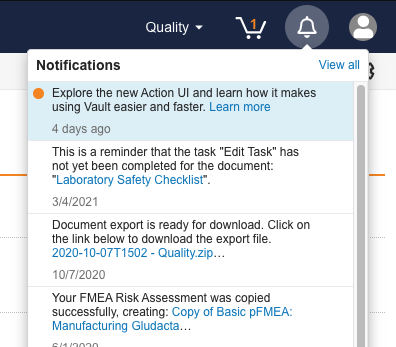
Notifications: Enhanced Notification UI
In previous releases, users accessed their notifications on the Notifications page through their Vault home tab. With this release, users access notifications via a new global notification icon, making it easier for Vault users to become aware of documents, records, and tasks that need their attention.
This feature introduces the Notification Bell icon, visible at all times within Vault, which opens a new notification panel and allows access to the new, enhanced notifications page.
This feature also removes the notification icon within Vault’s document viewer, as the new Notification Bell is available everywhere within Vault. Learn more about notifications.
UI Styling Updates
In this release, Vault has a number of visual updates to give it a more pleasing look and feel and a more enjoyable user experience. By applying modern design principles with better spacing, color, highlighting, and icons, many of the visual elements of Vault are improved, helping users stay focused in the areas where they are most engaged. These visual updates do not change any functionality and do not change the locations of common visual elements that existing users are familiar with.
Action Menu Button Icon Change
This feature updates all action menu buttons from a cog icon to ellipsis icon. The ellipsis icon has become a standard symbol for a menu of actions in modern web applications.
Display All Actions Enhancement: Action Bar
Vault administrators can configure Start Workflow user actions to be displayed as Most Frequently Used Actions in the Action Bar in both Document and Object Lifecycles.
By default, all start workflow actions are shown inside the “Workflow Actions” menu, but start workflow actions that are configured to be shown in the Action Bar are shown in the ‘All Actions’ menu.
Administration
Promote to Production
This feature introduces a one-time vault action which allows a Vault owner to promote their initial pre-production vault to a production vault. This enhances the self-service model of sandboxing by allowing Vault Admins to fully manage a vault’s lifecycle from initial to go-live.
Additionally, this feature allows your organization to create their pre-release sandbox from any production, pre-production, or sandbox vault. Previously, an organization’s pre-release sandbox could only be created from the production vault.
Infrastructure Release: IP Address Change
Vault IP addresses will change as part of this release.
We recommend all Veeva customers update their DNS caching so that integrations don’t cache the DNS indefinitely. We also recommend that you configure the DNS TTL value to no more than 60 seconds. This ensures that when a Vault resource IP address changes, your integration will be able to receive and use the new IP address by re-querying the DNS.
Learn more about integration DNS caching changes in the Developer Portal.
Clinical Operations
Milestone-Specific Expected Documents
This feature updates EDL template configuration to allow Admins to indicate that expected documents are created only for specific types of milestones. Once created from a template, these documents are linked and matched to an Owning Milestone of that specific milestone type while still being linked to additional milestones based on existing behavior. This allows customers to track documents that are truly specific to individual milestones, such as protocol amendments and submissions. Learn more about using EDLs with milestones.
TMF Bot: Auto-Classification
This feature delivers the exciting capability for the system to automatically classify documents that have been uploaded to the Document Inbox. This will be done if a Document Classification Trained Model has been trained and deployed within the Vault.
TMF Bot: Model Training
This feature allows administrators to create, train, deploy and archive machine learning models in Vault Clinical. Initially these trained models will be in support of the TMF Bot: Auto-classification feature, but will be extended to other use-cases in the future.
Document Reconciliation
This feature provides Veeva Site Connect customers with automated and streamlined document reconciliation between a Sponsor/CRO’s eTMF vault and a site’s eISF in SiteVault.
Send Surveys to Non-Users
This feature introduces the option to send, via email, an invitation to complete a survey (Checklist) related to a Site or Outreach Target record. By clicking the link in that email, an external user can enter Vault and complete the Checklist without having to enter a username and password.
Content Field for Inbox Documents
This feature will give customers the peace of mind that documents within the Document Inbox can be secured based on a document’s Content (blinded/unblinded) value. This feature will work with our Content Field Defaulting feature and will respect field requiredness settings.
Support for Subject Informed Consent Forms
This feature adds support for tracking Subject Informed Consent (IFC) Forms. With the new Subject Informed Consent Form object, users can select a Site Effective Informed Consent Form and a Subject, and then specify the date the Subject signed that ICF in the Signature Date field. A Subject Informed Consent Form field has also been added to the Monitored Informed Consent Form object.
Commercial
CRM Approved Links Data Model Change
This data model lays the foundation for a future CRM Approved Links feature. See the list of Commercial Data Model Changes below.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
The following components have been added to MedComms and PromoMats vaults to support the CRM Approved Links feature:
- Added new picklist value Approved Links(approved_links__v) to the Distribution Channel Type (distribution_channel_type__v) picklist
- Added new object record Approved Links_to the _Website(website__v) object
- Added new field layout section Approved Links (approved_links__v)
- Added the following shared document fields:
- Shareable as an Approved Link (shareable_as_an_approved_link__v)
- Message (message__v)
- Link Label (link_label__v)
Quality
Enablement Change: Extensible Controlled Copy
With this release, Vault QualityDocs Admins can enable the Extensible Controlled Copy feature through an Admin checkbox. Enabling this feature immediately disables legacy controlled copy functions and new legacy controlled copies can no longer be generated. The Distribution report is still enabled and allows existing legacy controlled copies to be reconciled during transition to Extensible Controlled Copy. Enabling this feature allows Admins to configure the Extensible Controlled Copy feature, which requires additional configuration before being utilized by an end-user. Learn more about configuring Extensible Controlled Copy.
Quality Teams: Restore Memberships UI Update
In accordance with the updated Vault interface, the restore team role membership gesture has been updated with an icon to replace the previous Restore text link.
Rename Reportable Event to Adverse Event Report
In Vault Product Surveillance vaults, the Reportable Event (reportable_event__v) object has been renamed to Adverse Event Report (adverse_event_report__v) to better reflect the object’s context.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve modified the following components in the Quality data model to support new features:
In QualityDocs vaults, the Do Not Copy attribute was set to True on the Release Change Control (release_change_control__v) and Obsolete Change Control (obsolete_change_control__v) document fields.
Regulatory
PromoMats & RIM Vault Connection
With this release, admins can create a connection between PromoMats and RIM for eCTD Compliance Package submissions to the FDA. The connection enables a seamless flow from Compliance Package generation in PromoMats to Content Plan creation in RIM Submissions, and, if licensed, to the FDA through the gateway via RIM Submissions Publishing. This reduces the time required to submit the compliance package to the FDA.
Asynchronous Content Plan Actions: Locking & Indicator
This release introduces locking on asynchronous Content Plan actions to prevent an action from being initiated if a conflicting action is already processing on the same content plan, minimizing data concurrency issues. The locking is controlled at the Content Plan root level and the locked actions include the Content Plan update, split Content Plan Item, asynchronous state change, and copy into Content Plan.
Additionally, this feature adds a new status icon to the header of the Content Plan Hierarchy Viewer to inform users if Vault is processing one of these asynchronous actions within the current Content Plan.
21R2 Regulatory Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model.
Added the following components to support the PromoMats & RIM Connection feature:
- Added document type group RIM to PromoMats Connection
- Added field Related Compliance Package ID to the Submission (submission__v) object
- Added object state type Baselined
Safety
Safety features are targeted for tentative availability on June 30, 2021.
E2B Imported Case Naming and Versioning Auto-on
Imported Cases created from E2B are now named by their Unique Identifier (UID). This will map from C.1.1 in E2B(R3). Newly created Imported Cases will default to version 0.1.
Learn More: Configure Object Naming Conventions: Naming Conventions for Imported Cases
Multilingual MedDRA: Japanese Configuration
Vault Safety Inbox Items now support the ability to browse, search, and auto-code MedDRA terms in Japanese. When the MedDRA control language is set to Japanese, the MedDRA browser filters out non-current Japanese terms by default.
Learn More: Code MedDRA Terms: Use the Multilingual MedDRA Browser
E2B Import: Match Non-Current MedDRA in Central Auto-on
Vault Safety now supports coding non-current terms using central MedDRA when importing E2B files that contain non-current terms. A ‘Recode’ badge is displayed to remind users when a coded term is non-current. Also, the Case MedDRA Version of promoted cases from AER or Inbox Item is now set by the Active MedDRA version on the user’s vault.
Note: This feature is auto-on for customers who are using Central MedDRA.
Learn More: Import an Inbox Item: Auto-Code Non-Current MedDRA Terms
Support Substance Decimal Places Auto-on
Case Product Substance Strength now supports up to 4 decimal places of precision. E2B export will truncate at the appropriate number of characters.
EMA Certification: Name Parts and RoA Prioritization Auto-on
Vault Safety now supports the import and export of EMA Product Name Parts. New fields for Product Name Parts are now available during Case Product data entry. EMA E2B(R3) file exports also prioritizes structured G.k.4.r.10.2a/b data over unstructured G.k.4.r.10.1 data for the Route of Administration.
Learn More:
- E2B Generation Data Mapping: G.k.2.2 Medicinal Product Name as Reported by the Primary Source
- Enter Case Data: Case Product Details Section
Generate E2B Message ID for Manually Created Transmission Auto-on
Vault Safety will now auto-generate an E2B Message ID for a manually-created E2B submission / distribution. This identifier maps to a mandatory E2B field that facilitates the subsequent receipt of acknowledgments. Failure to submit an E2B with this identifier may result in submission failure. Now, users will no longer need to remember to enter this identifier for a manually created transmission.
Note: This feature is auto-on for customers submitting E2Bs to an AS2 gateway.
Case Level Regional Validation for VAERS and EMA/MHRA E2B R3 Auto-on
Users can evaluate and review regional FDA VAERS and EMA E2B(R3) Validation Results at the Case level. The FDA VAERS and EMA regional rules are now evaluated when the Evaluate Regulatory Conformance action runs on the Case. Regional Validation Results are displayed on the Case, alongside the ICH (global) Validation Results.
Note: This feature is auto-on for customers who have the Evaluate Regulatory Conformance user action enabled for the Case object. An admin must configure the action on the appropriate lifecycle state for the Case object.
Learn More: Case and Submission Validation
Replace UTF Smart Quotes with ASCII Straight Quotes in FDA E2B(R2) Auto-on
Vault Safety now converts smart quote characters to straight quote characters when generating FDA E2B(R2) submissions. This will ensure the generated ICSR continues to be ISO 8859-1 compliant, as ISO is the only encoding supported for FDA E2B(R2) submissions. Related to this, Health Canada E2B(R2) submissions will now generate in UTF instead of ISO encoding (both are supported by Health Canada) to circumvent the aforementioned issue.
FDA E2B R2 - Support “FDA Safety Report Type” (A.1.FDA.16) Field For Post Market Support
Vault Safety now supports adding the FDA Safety Report Type (A.1.FDA.16) element to FDA E2B(R2) Transmissions. This feature only impacts the Transmission file format FDA E2B(R2). This feature only supports postmarket submissions (E2B Code=3). Other IND Safety Report tags are not yet supported. This field will only be populated fo
New Safety Intake API Auto-on
The Vault Safety API has been extended with new endpoints to receive E2B files and import to either Imported Cases or Inbox Items. New endpoints have also been introduced for retrieving import status and ACKs.
See the Vault Developer Portal for more information.
E2B (Plus) Import SDK Extension for Custom Import Formats Configuration
Vault Safety now allows developers to extend existing E2B XML formats or create their own XML data transfer formats for inbound ICSRs. Different Inbound and Outbound E2B formats can be configured on AS2 and System Gateways.
Vault UI Refresh Changes Auto-on
Vault Safety now includes icons for all system actions that can be displayed in the Action UI. The application has been updated to reflect Vault Platform’s new look.
Learn More: About the Action Bar and All Actions Menu
AI Service with no Vault Connections Auto-on
Configuration is no longer required to connect to the AI Service when setting up a new Vault with Safety.AI. Previously, an administrator had to create an External Vault Connection.
SiteVault
Digital Delegation Log Updates
This feature provides a digital and compliant way to record all of the significant study-related duties that have been delegated to study staff by the principal investigator as part of the Delegation of Authority log. This feature can only be configured in vaults with the Extensible SiteVault Permissions feature enabled.
SiteVault Settings Framework
This feature enables users with appropriate permissions to enable and disable SiteVault features for a research organization, site, or study.
Extensible SiteVault Permissions
This feature updates how user permissions are managed in SiteVault. To provide additional granularity in user permissions, additional access can be defined at the level of the research organization, site, and study.
This feature was postponed to a future release.
Language Field Alignment
This feature standardizes and consolidates document language under a new Language(translated_language__v) document field. The new field is added to the following document types:
- ~~ Advertisement for Recruitment (subject_advertisement__v)~~
Diary (blank) (subject_diary_blank__v)Informed Consent Form (blank) (informed_consent_form_blank__v)Participant Information Sheet (subject_information_sheet__v)Participant Materials - Other (other_subject_information__v)Participant Questionnaire (blank) (subject_questionnaire_blank__v)Study Participant Card (participation_card__v)Subject Diary (blank) (subject_diary_blank__c)Subject Questionnaire (blank) (subject_questionnaire_blank__c)
This feature was postponed to a future release.
Edit Checks for Patient & Participant Data Quality
This feature ensures that the information captured in SiteVault about patients and study participants is appropriately formatted and assigned, reducing the likelihood of errors. This includes validating the format of email addresses and phone numbers and that patients are only assigned to a study once.
This feature was postponed to a future release.
Products in the Agreement Wizard
When reviewing a Connected Study Agreement, Regulatory users are now prompted to reconcile the products on the study. This enables users to choose whether to merge the study agreement product with an existing product in SiteVault or to create a new product if it does not already exist.
This feature was postponed to a future release.
QualityOne
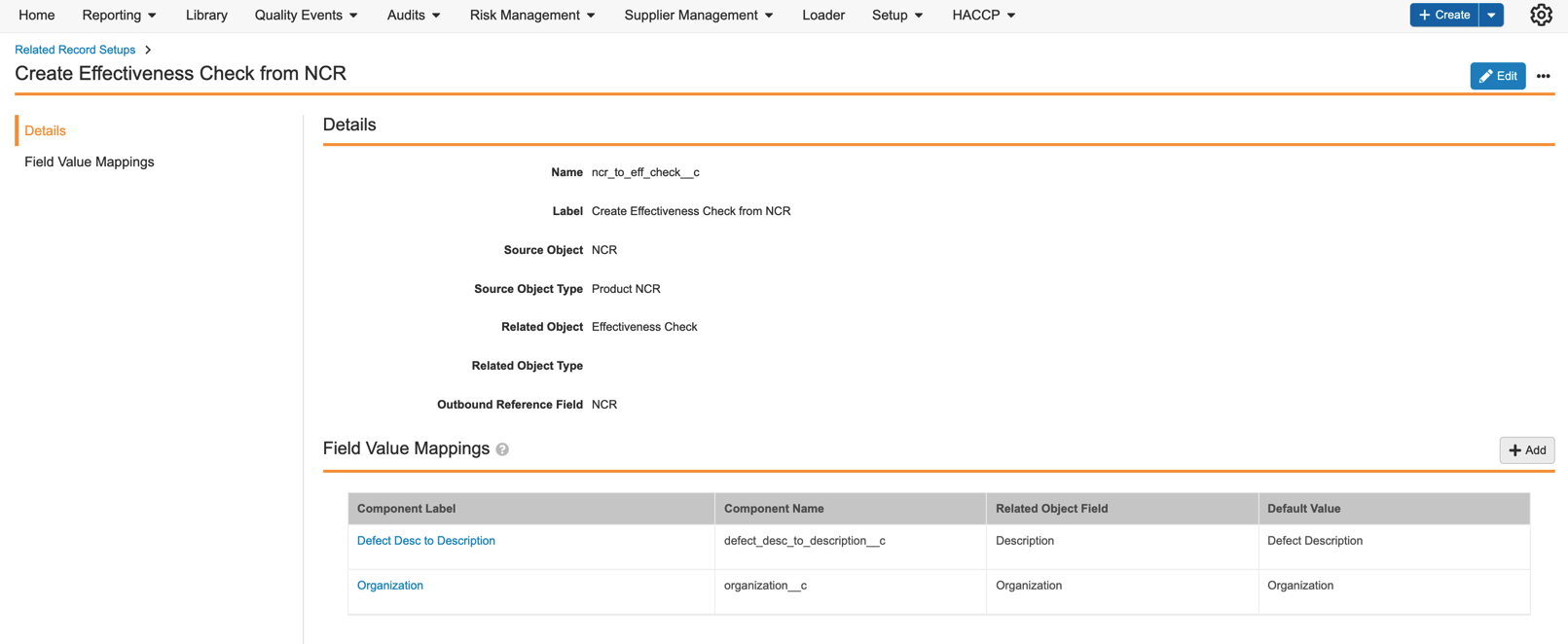
QualityOne Entry Action: Create Related Record
QualityOne adds a new entry action configuration that will automate creation of related records when the parent record enters in a particular lifecycle state. The Create Related Record action will run as an entry action for the admin-configured components available through the Related Record Setup tab. Learn more about setting up related record automation.
RegulatoryOne
Note that RegulatoryOne features are targeted for tentative availability on July 6th.
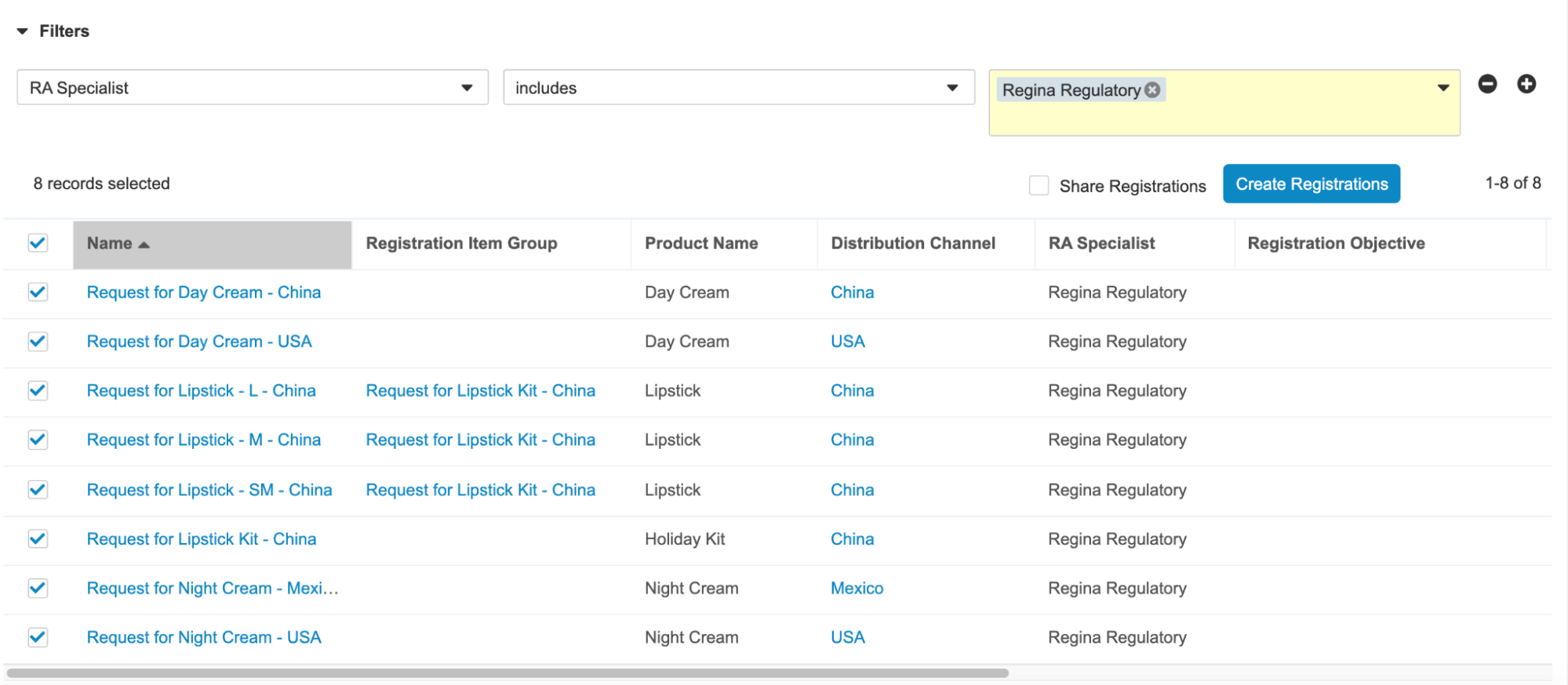
Create Registrations in Bulk
When configured, this feature provides users with the ability to automatically create Registration Objectives for one or more Registration Items on an Event. Users can apply filters to find and select the appropriate Registration Items and either choose to create unique Registration Objectives for each Registration Item or group Registration Items so they have the same Registration Objective. When configuring, Admins can specify which columns to display to the user and have the ability to hide the option that allows Registration Items to share the same Registration Objective. This feature allows Regulatory Affairs users to work more efficiently during the product registration process. Learn more about configuring bulk registration creation.
Veeva Claims
Note that Veeva Claims features are targeted for tentative availability on July 6th.
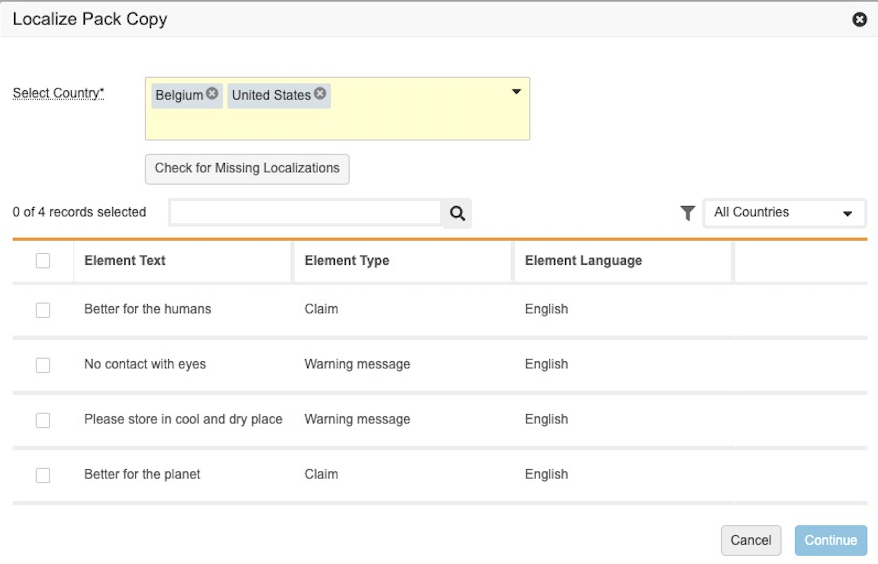
Multi-Country Pack Copy Localization
This feature allows users to localize a Global Pack Copy by selecting multiple countries before running a single action. Previously, users could create a local Pack Copy from a Global Pack Copy for only one country at a time.
Auto-Localize Pack Copy Enhancements
A localized Pack Copy is a specification document that brand marketers create in order to deliver a localized version of the packaging brief to the creative teams. As a common use case, users need to generate a Local Pack Copy from an existing Global Pack copy which pulls in all of the associated localized Elements in an automated fashion. The existing Auto-Populate Local Pack Copy feature enables users to generate auto-populated Local Pack Copies from a Global Pack Copy for a single country. The auto-generated Local Pack Copy has the same Pack Copy hierarchy as the Global Pack Copy and associated Local Elements.
This enhancement allows users to check if any Global Elements are missing localizations and then enables users to select which of those Global Elements to create localized versions for each specified country. When the action runs, Vault then automatically generates translation placeholders or localized elements based on Admin configuration.
The feature allows Admins to define if a Global Element should be Localized or Translated. The Localization process can be different for different element types; for example, users currently translate only a subset of packaging elements in multiple languages and adapt the rest in one or no language. Based on Admin configuration, the action creates a new Local Pack Copy record for each country selected with existing translations and localizations and creates any new Local Elements. Learn more about configuring auto-localize pack copy.
Enablement Details
| Name | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| Merge Fields Nested Table Rendering | Auto-on | Platform |
| Infinite Document Scrolling | Auto-on | Platform |
| Navigate From Collapsible Upload Modal to Inbox | Auto-on | Platform |
| Reporting & Dashboards | ||
| Bulk Actions on Objects in Reports | Auto-on | Platform |
| Action UI | ||
| Action Bar | Auto-on | Platform |
| Doc Info UI Enhancements | Auto-on | Platform |
| Annotate UX Refresh | Auto-on | Platform |
| UI Styling Updates | Auto-on | Platform |
| Action Menu Button Icon Change | Auto-on | Platform |
| Display Start Workflow Actions in Most Frequently Used Actions | Configuration | Platform |
| Administration | ||
| Promote to Production | Auto-on | Platform |
| Infrastructure Release: IP Address Change | Auto-on | Platform |
| Clinical Operations | ||
|
Milestone-Specific Expected Documents |
Configuration |
CTMS, Study Startup, eTMF |
|
TMF Bot: Auto-Classification |
Configuration |
eTMF |
|
TMF Bot: Model Training |
Auto-on |
eTMF |
|
Document Reconciliation |
Configuration |
SiteConnect |
|
Send Surveys to Non-Users |
Configuration |
Study Startup |
|
Content Field for Inbox Documents |
Configuration |
eTMF |
|
Support for Subject Informed Consent Forms |
Auto-on |
CTMS |
| Clinical Operations Data Model Changes | Auto-on |
CTMS, SiteConnect, Study Startup, Vault Payments, eTMF |
| Commercial | ||
| CRM Approved Links Data Model Change | Auto-on |
MedComms, PromoMats |
| Commercial Data Model Changes | Auto-on |
MedComms, PromoMats |
| Quality | ||
| Enablement Change: Extensible Controlled Copy | Admin Checkbox | QualityDocs |
| Quality Teams: Restore Memberships UI Update | Auto-on | QMS |
| Rename Reportable Event to Adverse Event Report | Auto-on | Surveillance |
| Quality Data Model Changes | Auto-on |
QMS, QualityDocs, Station Manager, Surveillance, Training |
| Regulatory | ||
| PromoMats & RIM Vault Connection | Configuration | RIM |
| Asynchronous Content Plan Actions: Locking & Indicator | Auto-on | RIM Submissions |
| 21R2 Regulatory Data Model Changes | Auto-on |
RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive |
| SiteVault | ||
| Digital Delegation Log Updates | Support |
SiteVault Enterprise, SiteVault Free |
| Extensible SiteVault Permissions | Configuration |
SiteVault Enterprise, SiteVault Free |
| SiteVault Settings Framework | Configuration |
SiteVault Enterprise, SiteVault Free |
| Language Field Alignment | Auto-on |
SiteVault Enterprise, SiteVault Free |
| Edit Checks for Patient & Participant Data Quality | Auto-on |
SiteVault Enterprise, SiteVault Free |
|
|
|
|
| QualityOne | ||
| QualityOne Entry Action: Create Related Record | Configuration | QualityOne |
| RegulatoryOne | ||
| Create Registrations in Bulk | Configuration |
RegulatoryOne Registration & Dossier Management |
| Veeva Claims | ||
| Auto-Localize Pack Copy Enhancements | Configuration | Veeva Claims |
| Multi-Country Pack Copy Localization | Configuration | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|