Release Date: December 9, 2022 Postponed to December 15, 2022
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
Working with Documents
Manage Document Auto Numbering
With this release, Admins can import a CSV file to update the database tables for document auto-numbering. This tool can also be used to view the current numbers used for all document number formats.
Reporting & Dashboards
Advanced Filter Logic for Multi-Pass Reports
This feature allows users to add OR filter logic across different objects and views in a multi-pass chain report, making reports more flexible. Users can also add custom logic expressions between filters.
Vault Objects
Allow Picklist as Unique Field for Upsert
This enhancement allows Admins to select picklist fields in the Key Field drop-down when upserting object records. Because the Vault Loader UI always uses the most recent API version, this change also affects Loader jobs created in the Vault UI in General Release Vaults on version 22R3.0.
Lifecycle & Workflow
Multi-Record Workflow: Action Step Automatically Remove Matching Records
Action Steps in multi-record workflows can now be configured to automatically remove records from the workflow based on conditions.
eSignatures: Object Signature User Title & Delegate Capture
Object signatures now capture and store the signing user’s title and delegate user information in the signature record.
Multi-Record Workflow System Action Step Support for RecordAction SDK
Multi-record workflows now support the execution of Vault Java SDK RecordActions in system action steps.
UI & Usability Updates
Tab Collection
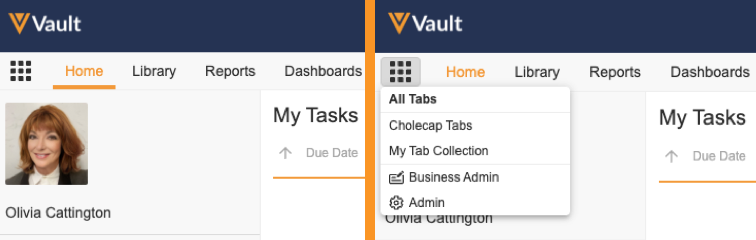
Admins can create Tab Collections to display relevant tabs based on different use cases, such as tabs for certain roles or tasks. Users click the Tab Collections icon in the navigation bar to select a tab collection to view. Admins can also configure User object records to assign a preferred tab collection that Vault displays when the user logs in. Admins control access to tab collections through permissions assigned through a Security Profile or Application Role.
Learn more about Tab Collections.
Admin Tabs & Business Admin Tabs
With the introduction of the Tab Collection feature in this release, Business Admin is now separated from the Admin tabs to form a new Business Admin tab collection that contains the Objects, Picklists, and Templates tabs. In addition, the Admin tabs are now placed in the new Admin tab collection. Users with the appropriate Admin permissions can access the Admin and Business Admin tab collections from the new Tab Collections icon, and Vault no longer displays the existing cog icon for viewing Admin tabs.
Learn more about the Business Admin Tab Collection.
Administration
Sandbox Snapshots
This feature introduces the concept of snapshots of a sandbox Vault. A sandbox snapshot is a copy of a sandbox Vault that can store configuration and optionally data at a given point of time. Each sandbox Vault can create two (2) Snapshots. Snapshots can be used to create or refresh sandbox Vaults.
Vault Usage & Performance Statistics: User Activation-Deactivation Performance Statistic
This feature introduces two new performance statistics for a Vault:
- User Activation Count: Number of times an inactive user is activated or a new user is created.
- User Deactivation Count: Number of times an active user is deactivated.
Sandbox Sizes
This feature introduces two new sandbox sizes, “Small” and “Full”. Existing sandboxes will be categorized as “Large”. Customers will receive 4 Small sandboxes for every Production Vault in addition to 2 Large and 1 Full sandbox. The size of a sandbox determines the amount of data it can store.
External Inbound Connections
Vault now supports tracking inbound REST API integrations using External Connections. The URL field is no longer required during configuration to accommodate inbound only connections. Vault Admins can configure Connection Client records that allow developers to identify REST API integration using a unique Client ID.
Manual Pagination Rules for Query Governor
Query Governor now returns a response status of WARNING when it detects manual pagination and FAILURE when manually paginating more than 10k records. Manual pagination is the use of PAGEOFFSET in the query string. Instead of manual pagination, developers should use the next_page and previous_page URLs returned in the responseDetails of the query response.
Jobs: Support DateTimes as Trigger Date
With this release, Admins can select DateTime fields from the Trigger Date dropdown when configuring Date Based Document Operation and Date Based Object Operation type Job definitions.
Configuration Migration
Vault Configuration Report: Include all Objects for Metadata Export
With this release, all objects visible in the Admin UI are now included in Vault Configuration Reports with the exception of some system-managed objects. These exceptions have been listed in the documentation.
Vault Loader
Vault Loader Support for MAXROWS & SKIP
With this release, Vault Loader users can now leverage the MAXROWS and SKIP VQL clauses to page through and retrieve large record sets. This feature is supported in the Vault UI, CLI, and API.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Platform data model to support new features:
- Added the Tab Collection component type
- Added the Preferred Tab Collection (
preferred_tab_col__sys) field to the _User _object
The following fields were added to signature system objects (objects generated when eSignatures are enabled) to support the eSignatures: Object Signature User Title and Delegate Capture feature:
- Signature Title (
signature_title__sys) - Delegate Title (
delegate_title__sys) - Delegate Name (
delegate_name__sys) - Delegate User (
delegate_user__sys)
Vault Connections
Vault Connections as System Managed Connections
Standard Vault Connections now have their System Managed field enabled by default, meaning they can no longer be referenced in custom Java SDK code. This change is not visible in connection record audit trails in Vaults cloned from existing Vaults. Previously, standard connections could be referenced in custom SDK code, providing inappropriate access to a remote Vault.
Clinical Operations
CTMS
Trip Report Question Branching: Enhanced Dependent Question Display
This feature improves the user experience of populating Trip Report questions and responses with branching by immediately evaluating display of dependent questions after a controlling question’s response is selected.
Commercial
Multichannel & PromoMats
PromoMats and Multichannel as Apps
PromoMats and Multichannel are now independently-licensable applications within the Commercial family. Admins can now specify different license types for different users.
PromoMats
CRM Events Management Integration Fields
With this release, Vault has introduced two additional shared picklist fields to support the integration between Vault and CRM Events Management. These fields will be used to support a future feature from CRM Events Management that will allow users to sync Vault documents with CRM Events.
Retain Vault Digital Publishing Document File Names
With this release, Vault Digital Publishing defaults file names to the name of the file saved in Vault. Prior to this release, Vault named downloaded files with a GUID, which made them difficult to search for and locate.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
The following changes were made to PromoMats Vaults to support the CRM Events Management Integration Fields feature:
- Added the CRM EM Catalog Type (
crm_em_catalog_type__v) and CRM EM Presentation Type (crm_em_presentation_type__v) picklists - Added the CRM EM Catalog Type (
crm_em_catalog_type__v) and CRM EM Presentation Type (crm_em_presentation_type__v) shared document fields
The following changes were also made to PromoMats Vaults:
- To create a many-to-many relationship between the Product (
product__v) and Application (pm_application__v) objects, we’ve added the new join object Application Product (application_product__v) with the following fields:- Name (
name__v) - Application (
application__v) - Product (
product__v) - Created By (
created_by__v) - Created Date (
created_date__v) - Last Modified By (
modified_by__v) - Last Modified Date (
modified_date__v) - Status (
status__v) - Global ID (
global_id__sys) - ID (
id) - Link (
link_sys)
- Name (
- Added the following fields to the Application (
pm_application__v) object:- Responsible Official (
responsible_official__v) - CBER BLA Supplement Number (
cber_bla_supplement_number__v)
- Responsible Official (
- Added the Application (
application__v) field to the Submission (submission__v) object - Updated the Submission (
submission__v) object to be a child of the Application (pm_application__v) object
Medical
MedComms
CRM Events Management Integration Fields
With this release, Vault has introduced two additional shared picklist fields to support the integration between Vault and CRM Events Management. These fields will be used to support a future feature from CRM Events Management that will allow users to sync Vault documents with CRM Events.
Retain Vault Digital Publishing Document File Names
With this release, Vault Digital Publishing defaults file names to the name of the file saved in Vault. Prior to this release, Vault named downloaded files with a GUID, which made them difficult to search for and locate.
Medical Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Medical data model to support new features:
The following change was made to MedComms Vaults to support the CRM Events Management Integration Fields feature:
- Added the CRM EM Catalog Type (
crm_em_catalog_type__v) and CRM EM Presentation Type (crm_em_presentation_type__v) picklists - Added the CRM EM Catalog Type (
crm_em_catalog_type__v) and CRM EM Presentation Type (crm_em_presentation_type__v) shared document fields
Quality
QMS
Generate Document: Add Users to Document Application Role
With this release, the Generate Document actions have been enhanced to automatically assign users that are associated to selected roles on the record to the same roles on the created document. This significantly simplifies security and ensures that only users associated to the record can access the document. In addition, the Generate Document action has been expanded to support all standard objects (with a __qdm or __v namespace) with a lifecycle. Learn more about Quality document generation.
Surveillance
Standalone MedTech Objects Data Model
QMS now supports the MedTech Complaints and NonConformance process as a standalone data model separate from Quality Events. This will allow customers to manage their Complaints Lifecycle separate from the Quality Event Lifecycle.
New implementations are encouraged to utilize the standalone MedTech Complaints and NonConformance data model. Customers who are already live or in the process of implementing the existing MedTech Complaints or NonConformance object type on Quality Event are strongly encouraged to continue leveraging their existing configuration. There will be no functional differences between the two models and there is no need or functional requirement to move to the standalone MedTech Complaints or NonConformance data model if already leveraging the existing MedTech Complaints or NonConformance object type on Quality Event.
Training
Prevent Updates to Person Training Eligibility If Job is Queued or Running
With this update, the Training Eligibility field on a Person cannot be modified if the Cancel Training Assignments for Person job is queued or running. This prevents conditions where a Learner is assigned or not assigned Training Assignments.
Support Checklist Versioning for Quizzes
A quiz can now be versioned using Checklist’s Create New Version action. This allows the user to quickly create a new version of a Quiz, while the action will automatically supersede the previous Quiz.
LIMS
Test Steps for Test Execution
Lab Results and Lab Test Inputs can now be grouped into Test Steps. When you complete a Test Step on the Test Execution page, LIMS will validate, calculate, and evaluate the results in that step before proceeding. This feature also includes an update to the collapse/expand control for the procedure document.
Reduced Testing Strategies
Materials can now be scheduled for reduced testing. The sampling protocol now allows you to specify a frequency and duration so you can periodically run full testing on incoming materials, but apply a reduced testing approach to interim batches.
Each sample definition and lab method in your protocol can be flagged to be included or excluded from the reduced testing strategy. By designating who your approved material suppliers are, and whether the supplier has been qualified for reduced testing, the system will automatically monitor the number of incoming batches and the elapsed time since the last full testing and assign the appropriate samples and tests. In the event of a supplier, process or policy change, you can force full testing whenever deemed necessary. Learn more about reduced testing strategies in Vault LIMS.
Station Manager
Configurable Document Metadata for Station Manager Apps
With this release, Station Manager documents can now be configured to display an Admin-selected list of fields. Customers can use the Station Document Metadata Admin page to create field configurations and assign them to individual Stations. Once assigned, the document information page on the Station Manager mobile app will display the list of configured fields. Learn more about configuring document metadata.
This enhancement supports future versions of the Station Manager mobile application.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Quality data model to support new features:
Made the following changes to support the Standalone MedTech Objects Data Model feature:
- Added the Complaint (
vps_complaint__v) field to the following objects:- VPS: Reportability Assessment (
reportability_assessment__v) - Adverse Event Report (
adverse_event_report__v) - Adverse Event Problem Code (
ae_problem_code__v) - Relevant Test (
relevant_test__v) - Concomitant Product and Therapy Date (
concomitant_therapy__v)
- VPS: Reportability Assessment (
- Added the Complaint (
complaint__v) field to the following objects:- MedTech Complaint Details (
mt_complaint_details__v) - Product Returns (
product_return__v) - SCAR (
qms_scar__v)
- MedTech Complaint Details (
- Added the Nonconformance (
nonconformance__v) object and added the Nonconformance (nonconformance__v) object reference field to the following objects:- Containment (
containment__v) - Corrections (
correction__v) - Investigations (
investigation__qdm) - Root Cause (
root_cause__qdm) - Related Event (
related_event__v) - Extension Request (
extension_request__qdm)
- Containment (
Added the following fields to the Test Step object to support the Test Steps for Test Execution feature:
- Evaluate to Proceed (
evaluate_to_proceed__v) - Completed (
completed__v)
Regulatory
RIM Submissions
Increase Limit for Delete Inactive Content Plan Records Job
The limit for the Delete Inactive Content Plans job is raised from 5,000 to 50,000 records deleted per job run.
Start Submission Wizard from Regulatory Objective Update
With this release, when the Submission Wizard is initiated from a Regulatory Objective, selecting a Submission is optional. This allows users to add relationships to a Regulatory Objective without adding to a related Submission.
RIM Registrations
EUDAMED UDI XML Generation Updates
With this release, Vault generates EU UDI submissions using the EUDAMED XML Schema v2.0.6, which increases the maximum length of the EUDAMED Model and Name fields for a Basic UDI or EUDAMED DI to 255 characters.
Submission Type Defaulting with Constraint Records
When creating records in bulk, users can select a global term for the Submission Type field. The selectable global term is mapped to a corresponding local term via Admin-configured Constraint records, and the wizard creates the resulting Submission records with the appropriate local term.
Activity Splitting for Labeling
This feature adds a new wizard for users to split Activities while managing Labeling Concepts. The wizard allows outstanding Labeling Concepts that have been deferred to be carried forward while allowing the original Activity to be completed.
Registration Type Defaulting with Object Type Mapping
This feature allows users to create Registrations in bulk with a default Registration Type value, populated from Admin-configured Object Type Mapping records.
RIM Email to Vault Processor
With this release, Vault RIM users can forward emails to a predefined email address to create unclassified documents. Once received, Vault automatically extracts attached documents and creates them in the Document Inbox, including a separate document for the email.
RIM Submissions Publishing
User Logs for Gateway Transmissions
With this release, Vault RIM users are provided with a log file that gives information regarding a submission via the gateway to a Health Authority. The file is added as an Attachment on the Submission record and includes a list of the files that were transmitted and details on any errors that may have occurred.
RIM Submissions Archive
Published Document Display Correction in the New Viewer
Published RIM documents in a steady state are now correctly displayed in the new Submissions Archive Viewer.
Display Section XML Attributes in the New Viewer
With this feature, section XML attributes for electronically-formatted dossiers are displayed in the new Submissions Archive Viewer grid view.
Support Filtering for XML Section Attributes
Users are able to filter on XML section attributes for electronically-formatted submissions in the new Submissions Archive Viewer.
23R1 RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added or updated the following components to the Regulatory data model:
- Enabled the Values must be unique configuration for the below fields (listed by object):
- Content Plan Template Constraint (
content_plan_template_constraint__rim) object: External ID (external_id__v) - Registered Packaged Product (
registered_packaged_medicinal_product__v) object: PCID (pcid__v)
- Content Plan Template Constraint (
- Enabled the User must always enter a value (required) configuration for the below fields (listed by object):
- Contact (
contact__rim) object:- Email Address (
email_address__rim) - Telephone Number (
telephone_number__rim)
- Email Address (
- Indication Grouping (
indication_grouping__v) object: Intended Effect (intended_effect1__v) - Medicinal Product Full Name (
medicinal_product_full_name__rim) object:- Country (
country__rim) - Language (
language__rim)
- Country (
- Medicinal Product Registration (
medicinal_product_registration__v) object:- Country (
country__v) - Language (
language__v)
- Country (
- Contact (
- Enabled hierarchical copy on the following fields (listed by object):
- Activity Registration (
activity_registration__rim) object: Registration (registration__rim) - Indication Grouping (
indication_grouping__v) object: Full Indication Text (full_indication_text__v) - Medicinal Product Registration (
medicinal_product_registration__v) object: Medicinal Product (medicinal_product__v) - Registered Active Substance (
registered_active_ingredient__rim) object: Registration (registration__rim) - Registered Authorization (
registered_authorization__v) object: Registration (registration__v) - Registered Clinical Study (
registered_clinical_study__v) object: Registration (registration__v) - Registered Indication (
registered_indication__rim) object: Registration (registration__rim) - Registered Packaged Product (
registered_packaged_medicinal_product__v) object: Registration (registration__v) - Registered Packaging (
registration_packaging__rim) object: Registration (registration__rim) - Registered Product (
registered_drug_product__rim) object: Registration (registration__rim) - Registered Regulatory Text (
registered_regulatory_text__v) object: Registration (registration__v) - Registration Regulatory Objective (
registration_regulatory_objective__rim) object: Registration (registration__rim)
- Activity Registration (
- Enabled the System manages field value (read-only) configuration for the Name (
name__v) field in the following objects:- Product Variant (
product_detail__v) (Note: Thename__vfield label is Product Variant.) - Product Variant Description (
product_variant_description__v) - Report Level Content Plan (
report_level_content_plan__v)
- Product Variant (
- Enabled the Audit data changes in this object configuration on the following objects:
- Administered Product Description (
admin_product_description__v) - IDMP Manufactured Item Description (
idmp_manufactured_item_description__v) - IDMP Pack Size (
idmp_pack_size__v) - IDMP Pharmaceutical Product Description (
idmp_pharmaceutical_product_description__v) - Pack Size (
pack_size__v) - Product Variant Description (
product_variant_description__v)
- Administered Product Description (
- Added the Created from email (
created_from_email__v) shared document field to support the RIM Email to Vault Processor feature. - Increased the Device Model or Version (
device_model_or_version__v) field length to 255 characters (from 120) in the following objects to support the EUDAMED UDI XML Generation Updates feature:- Application Product Characteristic (
application_product_characteristic__v) - Event Product Characteristic (
event_product_characteristic__v) - Product (
drug_product__v) - Reg Objective Product Characteristic (
reg_objective_product_characteristic__v) - Registered Product Characteristic (
registered_product_characteristic__v) - Submission Product Characteristic (
submission_product_characteristic__v)
- Application Product Characteristic (
- Added the following components to support the Submission Type Defaulting with Object Type Mapping feature:
- Global Submission Type (
global_submission_type__rim) value added to the Controlled Vocabulary Type (controlled_vocabulary_type__rim) picklist. - Global to Local Submission Type (
global_to_local_submission_type__rim) object type added to the Constraint (constraint__rim) object.
- Global Submission Type (
Safety
Safety features are targeted for tentative availability on December 19, 2022.
Safety
CIOMS II Line Listing Expectedness Auto-on
The CIOMS II standalone report in Vault Safety now supports different expectedness logic for Clinical Trial and Postmarketing Cases in the same report. Clinical Trial expectedness will be based on the Investigational Brochure (IB)/Study datasheets, while Postmarketing Cases will use the Product datasheet.
Learn More
- User Help: Create CIOMS II Reports
Feature Enablement Changes
Note the following feature with enablement changes in this release:
| Feature | Previous Enablement | New Enablement |
|---|---|---|
| PADER Date Filtering Criteria | Support (22R3) | Auto-on (22R3.2) |
Read more about this feature in What’s New in 22R3.
QualityOne
QMS
Deep Copy HACCP Plan Enhancement
This feature enhances the existing functionality of deep copying HACCP Plans by including the HACCP Plan-Process Step connections and their related validation and verification actions of critical control as part of the cloning process. It also introduces two (2) new object types for the HACCP Plan object: HACCP Plan and HACCP Plan Design.
This functionality tracks the source of the copied record and includes the following abilities:
- Create a draft factory HACCP Plan from a reference HACCP Plan.
- The new Create HACCP Plan from Design object action creates a factory HACCP Plan by duplicating a HACCP Plan design template to use as a reference.
- Create a reference HACCP Plan from another reference HACCP Plan.
- The Deep Copy HACCP Plan object action is renamed to Create HACCP Plan Design from Design. This action duplicates a designed HACCP Plan template to use as a reference design template.
Learn more about the HACCP Plan enhancement.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on December 20, 2022.
RegulatoryOne
Enhanced Configurability for the Organization Object
This enhancement allows Admins to configure the Values must be unique attribute for the Name (name__v) field on the Organization (organization__v) object.
Registration & Dossier Management
Copy Matched Documents
This feature reduces the manual work users must do to customize a Registration Item Requirement by allowing Admins to configure the Customize action to copy matched documents. When configured, Vault includes all matched documents in generated EDL Items that are included in the source EDL Items when users run the Customize action on a target Registration Item Requirement.
Prior to this release, when users ran the Customize action on a target Registration Item Requirement, the generated related EDL items never included any of the documents matched to the corresponding source Registration Item Requirement, which may have been reviewed, auto-matched, manually matched, excluded, or removed after a user ran the Customize action. Learn more about customizing matched documents for shared requirements.
Disable Create Registrations in Bulk Feature
As of this release, the Create Registration in Bulk feature is no longer available for configuration. Existing Vaults with the feature already configured are not impacted. The Create Registration in Bulk feature was released in 21R2 as a temporary solution for users to automate the creation of multiple Registration records, saving time and effort. Since then, we have released several features, such as the Local Impact Assessment feature, which allows users to generate the appropriate Registration records within the registration process, rendering the Create Registration in Bulk feature unnecessary.
Admin UI to Manage Relational Tokens & Object Mappings
Registration & Dossier Management Vaults use Relational Tokens and Object Mappings to help supported actions, such as Split Registration Items, Generate Requirements, and Generate Registration Data, to determine the action’s behavior. As of this release, a new section is available on the Configuration tab for Admins to manage, create, edit, and delete Relational Tokens, Object Mappings, and Field Mappings. Learn more about defining Relational Tokens and Object Mappings.
Veeva Claims
Veeva Claims features are targeted for tentative availability on December 20, 2022.
Veeva Claims
Product Line Extension Substantiation Copy
This feature enables users to copy over Substantiation records when creating new Claims through the Product Line Extension feature. Users can now specify if they want to copy over Substantiation records to the newly-created Claims by selecting the Copy Related Substantiation Records checkbox after running the Copy Claims to Another Product action. Learn more about Product Line Extensions.
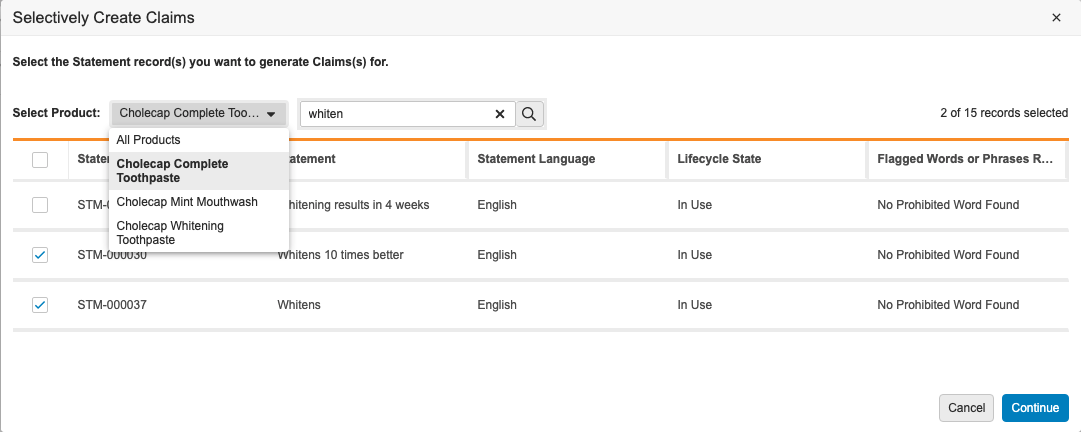
Project Automation UX Enhancement
This enhancement improves the user experience in record selection dialogs after users run the Create Selective Claims, Create Selective Local Adaptations, and Localize Pack Copy actions. In this release, the Product and Location filters move from the right to the left side of the dialogs. This allows users to work from left to right, preventing the erroneous generation of object records that users were prone to create because they instinctively interacted with the modal from left to right, making the grid checkboxes the focus of their selections. In addition, users must first select a Product or Location in each dialog before selecting Statement, Claim, or Element records.
Claims Study Object
This feature allows users to capture study results and corresponding formulations in a single Regulatory Study record, which users can easily search for and re-use when creating Substantiation records.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| Manage Document Auto Numbering | Auto-on | Platform |
| Reporting & Dashboards | ||
| Advanced Filter Logic for Multi-Pass Chain Reports | Auto-on | Platform |
| Vault Objects | ||
| Allow Picklist as Unique Field for Upsert | Auto-on | Platform |
| Lifecycle & Workflow | ||
| Multi-Record Workflow: Action Step to Automatically Remove Matching Records | Configuration | Platform |
| eSignatures: Object Signature User Title and Delegate Capture | Auto-on | Platform |
| Multi-Record Workflow System Action Step Support for RecordAction SDK | Configuration | Platform |
| Usability & UI Updates | ||
| Tab Collection | Configuration | Platform |
| Admin Tabs & Business Admin Tabs | Auto-on | Platform |
| Administration | ||
| Sandbox Snapshots | Auto-on | Platform |
| Vault Usage & Performance Statistics: User Activation-Deactivation Performance Statistic | Auto-on | Platform |
| Sandbox Sizes | Auto-on | Platform |
| External Inbound Connections | Configuration | Platform |
| Manual Pagination Rules for Query Governor | Auto-on | Platform |
| Jobs: Support DateTimes as Trigger Date | Auto-on | Platform |
| Configuration Migration | ||
| Vault Configuration Report: Include all Objects for Metadata Export | Auto-on | Platform |
| Vault Loader | ||
| Vault Loader Support for MAXROWS & SKIP | Auto-on | Platform |
| Platform Data Model Changes | ||
| Platform Data Model Changes | Auto-on | Platform |
| Vault Connections | ||
| Vault Connections as System Managed Connections | Auto-on | Platform |
| Clinical Operations | ||
| Trip Report Question Branching: Enhanced Dependent Question Display | Auto-on | CTMS |
| Commercial | ||
| CRM Events Management Integration Fields | Auto-on | PromoMats |
| Retain Vault Digital Publishing Document File Names | Auto-on | PromoMats |
| Commercial Data Model Changes | Auto-on | PromoMats |
| Medical | ||
| CRM Events Management Integration Fields | Auto-on | MedComms |
| Retain Vault Digital Publishing Document File Names | Auto-on | MedComms |
| Medical Data Model Changes | Auto-on | MedComms |
| Quality | ||
| Generate Document: Add Users to Document Application Role | Configuration | QMS |
| Standalone MedTech Objects Data Model | Configuration | QMS, Surveillance |
| Prevent Updates to Person Training Eligibility If Job is Queued or Running | Auto-on | Training |
| Support Checklist Versioning for Quizzes | Auto-on | Training |
| Test Steps for Test Execution | Auto-on | LIMS |
| Reduced Testing Strategies | Auto-on | LIMS |
| Configurable Document Metadata for Station Manager Apps | Configuration | Station Manager |
| Quality Data Model Changes | Auto-on | LIMS, QMS, QualityDocs, Station Manager, Surveillance, Training, Validation Management |
| Regulatory | ||
| Increase Limit for Delete Inactive Content Plan Records Job | Auto-on | RIM Submissions |
| Start Submission Wizard from Regulatory Objective Update | Auto-on | RIM Submissions |
| EUDAMED UDI XML Generation Updates | Auto-on | RIM Registrations |
| Submission Type Defaulting with Constraint Records | Configuration | RIM Registrations |
| Activity Splitting for Labeling | Configuration | RIM Registrations |
| Registration Type Defaulting with Object Type Mapping | Configuration | RIM Registrations |
| RIM Email to Vault Processor | Configuration | RIM |
| User Logs for Gateway Transmissions | Auto-on | RIM Publishing |
| 23R1 RIM Data Model Changes | Auto-on | RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive |
| QualityOne | ||
| Deep Copy HACCP Plan Enhancement | Configuration | QualityOne |
| RegulatoryOne | ||
| Enhanced Configurability for the Organization Object | Auto-on | RegulatoryOne Compliance Management, RegulatoryOne Registration & Dossier Management, RegulatoryOne Regulatory Documents, Veeva Claims |
| Copy Matched Documents | Configuration | RegulatoryOne Registration & Dossier Management |
| Disable Create Registrations in Bulk Feature | Auto-on | RegulatoryOne Registration & Dossier Management |
| Admin UI to Manage Relational Tokens & Object Mappings | Auto-on | RegulatoryOne Registration & Dossier Management |
| Veeva Claims | ||
| Product Line Extension Substantiation Copy | Configuration | Veeva Claims |
| Project Automation UX Enhancement | Configuration | Veeva Claims |
| Claims Study Object | Configuration | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|