Release Date: May 5, 2023
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
Working with Documents
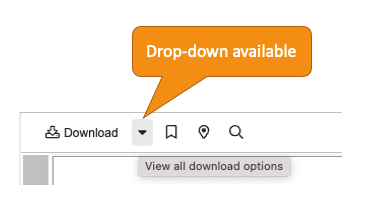
Download All Option for External Viewer
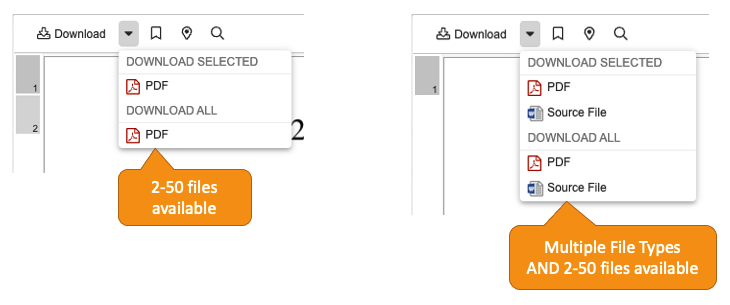
When viewing up to 50 documents in the External Viewer at one time, users now have a Download All option. Users automatically receive a ZIP file that contains the viewable renditions or source files of their documents.
The External Viewer is accessed via direct URL and is often used to send documents to non-Vault users. Common uses for sending multiple documents via a direct URL to the External Viewer include:
- Sending Response Packages in MedInquiry
- Approved Email
- Safety Distribution via SiteConnect (for sites that do not have an active Agreement)
Learn more about the external viewer.
Impose Limits on Create & Edit Annotations
When users are working with Annotations, they may encounter character limits enforced by Vault. The limits vary depending upon the type of annotation:
- For anchor annotations, Name (Title) is limited to 140 characters
- For note, link, and anchor annotations, Subject (in Header) is limited to 32,000 characters
- For note, link, line, and reply annotations, Comment is limited to 32,000 characters
- For external links, URL Length is limited to 32,000 characters
- For line annotations, Placemark is limited to 50 lines
These limits are enforced when creating and editing annotations in Vault UI, as well as when using Import Annotations functionality. These limits are not enforced on existing annotations unless users edit the annotation.
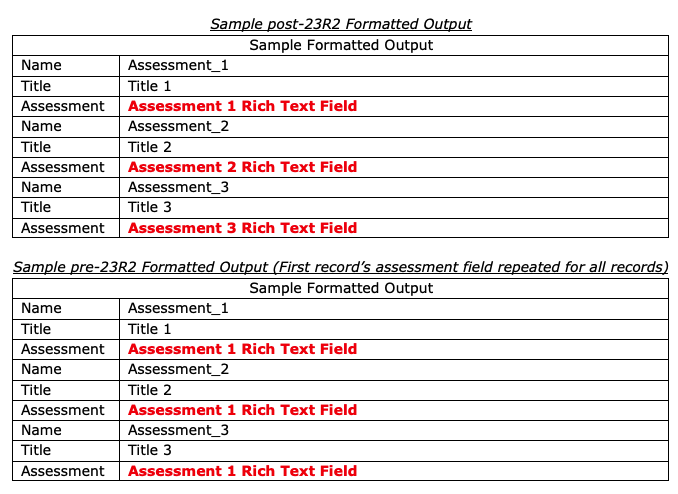
Rich Text Support for Related Objects in Formatted Outputs
Vaults that leverage formatted output templates as well as Rich Text fields will now be able to display Rich Text fields from records that repeat in tables on the downloaded formatted output. Formatted outputs are often used in regulatory agency audits, and this enhancement ensures data integrity in the downloaded formatted output provided to auditors.
Prior to this release, if a table in a formatted output referenced records that would repeat in the table, Vault repeated the Rich Text metadata from the first record in the table. Customers commonly addressed this by changing the formatted output field to plain text. This provided the appropriate information, but removed any prior Rich Text formatting.
With this release, the Rich Text format is respected and where records are repeated, users will always see the correct Rich Text metadata on each line in the table.
In this screenshot, there is an example of how this example will display after 23R2, as well as how this example displays before 23R2.
Learn more about formatted outputs and Rich Text fields.
SVG, AVIF and WebP Rendition Support
Users that are uploading and managing SVG, AVIF and WebP files, which are often used in Digital Asset Management, now see those files rendered in the Document Viewer within Vault. This improves the compliance of the Medical Legal and Regulatory (MLR) review process by ensuring reviewers are able review these files directly within Vault and without needing to download the source files.
Files that include animations are now rendered in Vault as Video Renditions. Vault now also supports Optical Character Recognition (OCR), Overlays, and Signature Pages for these renditions.
You can see more information about all file types that Vault supports rendering for here.
This feature has been moved to a later release.
Render Animated GIF files as Videos
Users that upload GIF files can now see those files rendered as Video Renditions in Vault within the Document Viewer. A new icon has been added to distinguish these file types in Vault, which is visible when documents are uploaded, in the Library list, and in the Document Viewer.
You can see more information about all file types for which Vault supports rendering here.
Picklist Value Label Increased from 128 Characters to 256
Vault now supports picklist values with labels up to 256 characters, doubling the amount of characters that can be used from the prior limit of 128 characters.
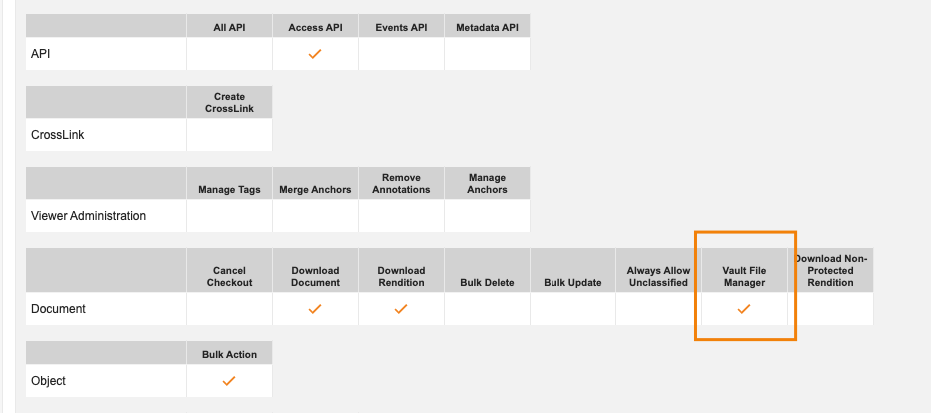
Require Vault File Manager Permission to Check in Documents
Users leveraging Vault File Manager now require the Application: Document: Vault File Manager permission to both check out and check in documents via Vault File Manager. Prior to this release, a user only needed this permission to check out. We have updated this to ensure consistency and avoid confusion going forward.
Learn more about Vault File Manager here.
Default Maximum Number of Attachments Allowed is 100
The default maximum number of of attachments for documents has been increased from 50 to 100.
Vault Objects
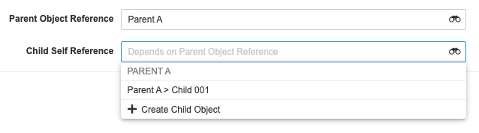
Self References for Child Objects
Admins can now configure Object reference fields on Child Objects that reference themselves. Admins have the option to choose the existing Parent Object reference as the controlling field or a new Object reference to the Parent Object.
For example, given Product (Parent) and Product Specification (Child), a “Related Spec” field may be needed on the Product Specification object to relate two records (Spec 2.1 is related to Spec 2).
This screenshot shows an example where an Admin has configured a self-reference field for their Child Object, and used Parent Object Reference as the controlling field so that they can only choose other records from the same parent:
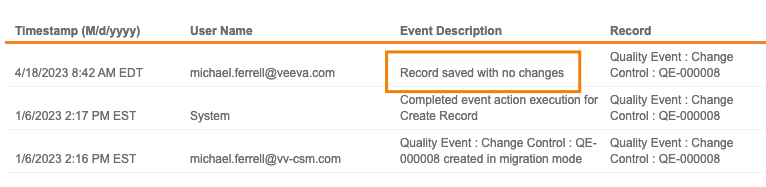
Intelligent Record Update
Vault now only updates object records if any changes have been made. Prior to this release, if a record was saved without any changes, Vault updated the Last Modified Date and added an audit trail entry with an Event Description of “Record saved with no changes”.
For customers with integrations or custom SDK code, this could have resulted in an unnecessarily large volume of audit trail entries. In 23R2, this will no longer be an issue.
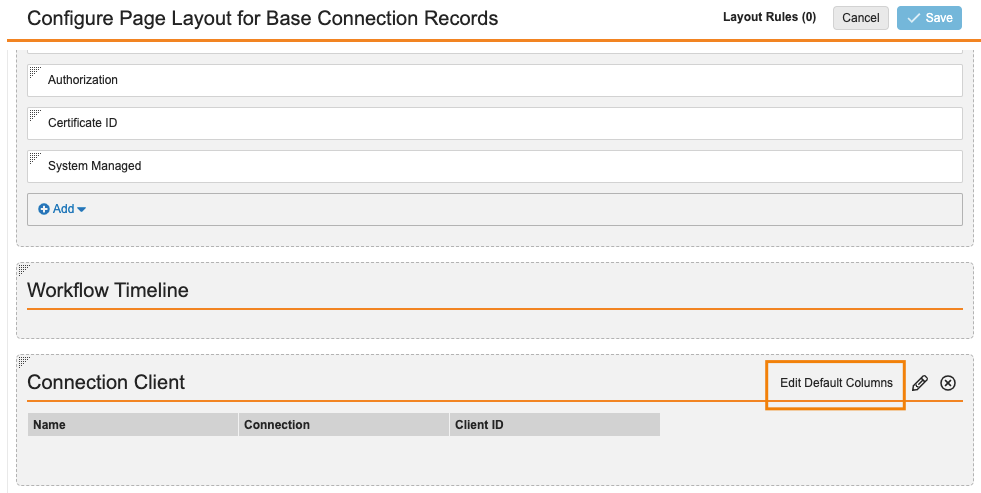
Update “Edit Column” Label in Page Layout Configuration
When Admins configure page layouts, they can define which columns Vault displays by default in related object sections. With this release, that option is being relabeled to “Edit Default Columns” rather than “Edit Columns” to more clearly articulate to an Admin that they are editing the Default layout. Users can adjust for themselves what columns they want to see when viewing records.
Lifecycle & Workflow
Override Checkout option for State Change Document Operation Jobs
This feature provides an option to allow a State Change Document Operation Job to successfully change the state of a document even when a minor version of the document is checked out. This feature is only available if the configured Current State is the steady state type of the selected lifecycle and the configured Destination State is the obsolete state type of the lifecycle.
This feature is especially valuable for customers who maintain compliance, such as PromoMats and Medical customers. For example, if a document is in a steady state but has a new minor version that is checked out, this feature allows the job configuration to correctly expire the steady state version when the job runs.
Prevent Deletion of Document Templates Used in a Workflow
With this release, Vault will now prevent deletion of document templates that are used in a workflow to ensure that processes are not inadvertently impacted by a user who may be unaware of the use of a given document template in workflows. This would apply to both Basic and Controlled Document Templates, in any instance where a workflow is configured to generate a document based off of a given template.
Object Lifecycle Entry Criteria: State & State Type of Related Records can be Validated with Conditions
Customers may now prevent state transitions for object records by using Entry Criteria to validate against multiple lifecycle states of related records. This ensures that the current record can only transition into the next state when its related records are in one of multiple lifecycle states. Previously, only one state could be validated for related records.
For example, a Quality Change Control record can only enter the Implementation Complete state when all related (child) Change Action records are in the Approved state. However, if one of those records is Canceled, the validation on the related objects fails. Now, customers can configure the Entry Criteria to allow for the Canceled state in addition to Approved.
Reporting & Dashboards
Union Report Type
This feature adds a new report type to Vault: Union-All. This report type allows customers to combine objects in a single report. Unlike a join, which places objects side by side and returns rows based on matching field values, a union combines the objects into a single object with rows stacked on top of each in the same columns.
This is particularly useful in any case where customers need to report across objects as if they were a single object. For example, in Vault Quality, if a customer is moving Batch information from the Context object to the Batch object in order to leverage LIMS, the Union-All Report Type allows reporting on both objects as if they were a single object.
Currency Support in Dashboards
Customers can now view local and corporate currency fields with the proper currency format in Vault Dashboard charts. Prior to this release, the currency field numbers were displayed with six decimal places. It is important for CTMS Payment and Commercial application users to view currency fields formatted properly.
Report Formula Fields Support Long Text
Customers can now use Long Text fields in report formulas by wrapping the fields in the Left() function. This function will convert the Long Text field to a normal text field which can then be used with additional formula functions. Users may return up to 250 characters when using the Left() function on a Long Text field in reports.
This feature enables full reporting capabilities on objects such as Checklists. As a result, the Checklist Response field is now available for grouping, filtering, sorting, and formula use.
Include Report Owner in Report MDL
Customers can now see the report owner column in the Configuration Report. This field will be populated for all the reports that are created or edited after 23R1.2 for those customers on Limited Release Vaults and 23R2 for all others.
Formulas
New Functions in Vault Formulas
Customers now have a number of useful new functions available for use in Vault Formulas. Among those that customers might find most useful are:
Contains(): This function compares two arguments, such as the value of a text field and a specific string or word, and returns TRUE if the first argument contains the second argument. Otherwise, it returns FALSE. For example,CONTAINS(Product_Type__c, "part"). If the word “part” appears anywhere in the Product Type field, the formula will return TRUE.Find(): This function now supports a third parameter(start_num)to identify where in the search text the function should begin looking for the second parameter, just as it does in Microsoft Excel.PicklistCount(): This function returns the number of selected values in a multi-values picklist. This allows you to quickly identify records where more than one value is selected in a specific list.Rand(): This function allows you to default a field to a random number, as it does in Excel, which is particularly useful when a business process dictates that you need to pull a random sampling of records. For example, you could use this function to default a hidden field upon creation or assign the random number using Entry Actions at a particular state. Once records have been assigned random numbers, you can then use filtering in a Report to pull records within a range of random numbers.BlankValue(): This function allows you to quickly use a substitute expression if an expression returns blank.
For a full listing of new functions and their use, visit Vault Help.
Enhanced Text Formatting
The Text function can now be used to format numbers to include special characters like dashes and brackets. For example, you can format phone numbers by passing Text(9254526500, "(###) ###-####") , which returns “(925) 452-6500”.
Administration
Vault Platform HTTP/2 Support
Starting with Vault release 23R1.2, Vault will use HTTP/2 as the default protocol to optimize network performance. This update will enable faster page loads, but end-users are not required to upgrade or make changes. To take advantage of this update, we advise reviewing and updating your network configurations and browser policies to ensure HTTP/2.0 is allowed.
Customer-Supported Languages
Vault now allows customers to support 12 additional languages:
- Bulgarian
- Czech
- Danish
- Finnish
- Greek
- Indonesian
- Latvian
- Romanian
- Slovak
- Spanish (Mexico)
- Ukrainian
- Vietnamese
An Admin must activate any additional languages and import all translations for these languages via the Bulk Translation tool. Vault does not provide translations for these languages.
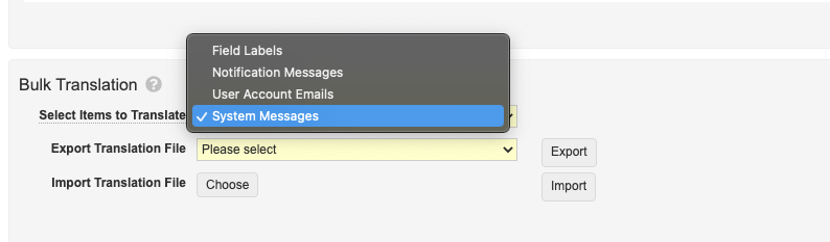
Bulk Translation Support for System Messages
Admins can now use the Bulk Translation tool to load translations for System Messages (i.e. static messages such as the “Save + Create” button label (button_save_and_create)).
Scheduled Data Export Performance Enhancements
This feature improves the performance of the Scheduled Data Exports job by bypassing field level security, allowing Vault Owners to export all relevant data.
EDLs: EDL option visible in Admin UI for all Vaults
The option to enable the Expected Document Lists (EDLs) feature from the Admin UI is now available for all Vaults. Previously, only newer Vaults had this option visible. Having the option visible for all Vaults allows for more efficient implementations of EDLs, enabling all customers to turn on this feature themselves instead of contacting Veeva Support.
Access Control
Improved Permission Set Sorting for Security Profile Details Page
Permission sets are now sorted by Name on the Security Profile details page to improve searchability.
Vault Loader
Vault Loader Command Line Support for Record Migration Mode Update & Upsert
Vault Loader Command Line users can now leverage Record Migration Mode to update and upsert records to any state while bypassing reference constraints, validation rules, entry actions, and entry criteria.
Learn more about using Record Migration Mode with the Vault Loader Command Line.
Clinical Operations
Study Startup
Redacted Document Field
Transparency in clinical trials is increasingly requested by regulatory authorities (e.g. EU CTR) as it increases patient knowledge of available medications as well as potential treatment in development. That said, Sponsors still have the obligation to protect personal data and commercial confidential information (CCI). Consequently, they may have to share a “for publication” document version with authorities where personal data & CCI are expunged, and a “not for publication” version restricted to authorities. Several Customers have decided to store both document versions in their Vault.
To better support Sponsor transparency processes, Vault Clinical Operations now provides a redacted document field (yes/no). It helps the clinical study teams to clearly track and identify document copies where the personal data and CCI have been redacted. The field could be leveraged for reporting and used to filter documents.
Site Connect
Protect Universal Site Number (USN) Changes
Vault Clinical Operations will now prevent users from making changes to the Universal Site Number (USN) on an Organization if that Study Site has a Connected Study Invitation and is in the Pending, Paused or Active lifecycle state. Additionally, users will be unable to edit the Organization field on Study Sites that have a Connected Study Invitation in the Pending, Paused, or Active lifecycle state. The user will encounter an error message if they try to update these fields in the above cases. These new guardrails will ensure that updates to the Organization and Universal Site Number (USN) do not lead to a mismatch between the connected Study Site and the data being tracked in Clinical Operations.
Learn more about Site Connect.
eTMF
Study Metadata Extraction: Test Model from Production
Customers will now have the ability to test a Metadata Extraction model using their Production data from a Sandbox or Pre-release environment, similar to how they can currently train an Auto-classification model. To facilitate this, an action called Test Model from Production will be made available, which will allow customers to test the model using a training window start date or a CSV file containing a list of document IDs.
This will allow customers to:
- evaluate a Metadata Extraction model in a sandbox environment prior to its deployment in the production environment
- gauge the model’s effectiveness without the need to directly train a model in a production environment
Prediction Metrics for Study Metadata Extraction
The Prediction Metric records provide a summary of predictions made by a deployed TMF Bot Trained Model to monitor the model’s performance. This feature aims to enhance the current Prediction Metrics to display the Metadata Extraction model’s performance data.
In order to distinguish between the different Trained Model types (Auto-classification and Metadata Extraction) a new field Trained Model Type will be added. Additionally, to make the Prediction Metrics less specific to the Auto-classification Trained Model, the field Auto-classification Success Rate will be inactivated and a new field will be added called Success Rate. The previous values for the Auto-classification Success Rate will be copied to the new field Success Rate.
Moreover, each combination of Trained Model and Field Extracted will have one set of Metadata Extraction prediction records. This means that in a Vault with both an Auto-classification Trained Model and a Metadata Extraction Trained Model, prediction metrics will be calculated for Auto-classification, Study, Study Country and Site.
These changes will enable the Prediction Model to be utilized for future enhancements to the TMF Bot capabilities.
TMF Transfer
TMF Transfer: Match on Country Code
When doing a TMF Transfer between a source and target Vault, errors may occur due to the differences in each Vault’s country__v records. TMF Transfer creates Study Country records in the target Vault and must set the country__v field on these records upon creation. This field is set based on the source Vault record’s value but must use target country__v records to populate the field. Transfer failures may occur due to differences in country__v records between Vaults.
Customers currently resolve this by either renaming countries or mapping country__v records between source and target Vaults and prolong transfer timelines activities. In many cases, target and source Vaults will have different Country record names, (e.g. Russia instead of The Russian Federation) which would trigger an error in the transfer or require prior customer mapping.
This TMF Transfer enhancement will mitigate this by looking at the Country Code (code__sys) where it is the same both in the target and source Vaults, regardless of what the actual Country record name is in both Vaults.
TMF Viewer UI Enhancements
Two changes will give users greater flexibility to quickly review documents in the TMF Viewer. First, users will now be able to display up to 15 columns at once, where previously they were limited to 9. Secondly, when text within a cell exceeds column width there will now be an action under the action menu to Wrap Text, just like in the Library.
Vault Payments
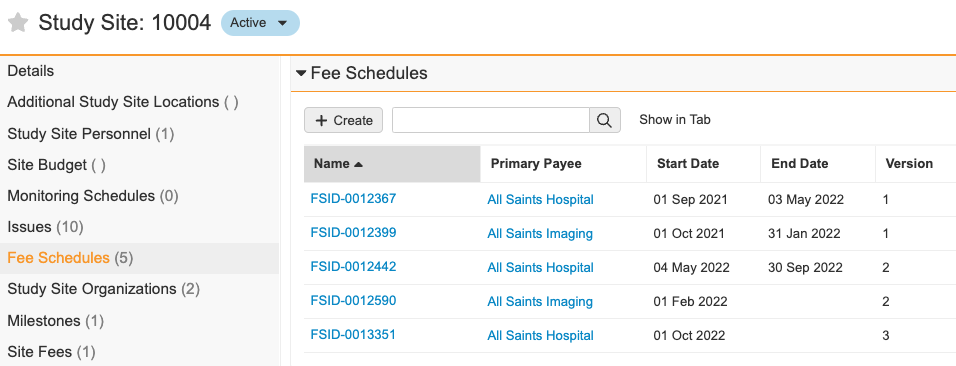
Fee Schedules for Study Organizations
With Vault Payments, customers can now efficiently manage different Fee Schedules for multiple Payees on the same Site. This enhancement addresses the need to divide fees between various organizations at a site when there are separate contracts for main hospitals, labs, or pharmacies in some countries. Previously, customers had to define the Payee split individually for each Fee.
The new Fee Schedules for Organizations feature allows users to manage entire Fee Schedules for multiple payees, each with its own effective dates. This provides greater flexibility and reduces administrative burden. Additionally, the existing Payee Overrides functionality can be utilized as needed for further flexibility.
Clinical Operations to CDMS Vault Connection
Clinical Operations/CDMS - Enhanced Job Labels
This feature will provide more useful information for inbound jobs in the Clinical Operations Vault. The inbound jobs will contain additional details about the connection and the integration which will simplify troubleshooting and monitoring for administrators. The job label will now include information about which connection and integration is impacted. This feature will be particularly useful for customers who connect with multiple CDMS Vaults.
Task Widget Optimization
This feature refactors the Clinical Operations homepage task widgets to improve performance and include Envelope (One Workflow) tasks for multi-documents workflows. With the addition of the Envelope tasks, users of Clinical Operations homepages will be able to see all tasks requiring attention in one place and can take action on those directly from the homepage, saving navigation time.
Clinical Operations Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Clinical Operations data model to support new features:
The following change was made to Clinical Operations Vaults to support the Clinical Operations/CDMS - Screening Subjects Outside of CDMS feature:
- Added the new RTSM ID (
rtsm_id__v) field on the Subject object
The following changes were made to Clinical Operations Vaults to support the Fee Schedules for Study Organizations feature:
- Added the new Primary Organization (
primary_organization__v) field to the Fee Schedule object - Added the new Primary Payee (
primary_payee__v) field to the Fee Schedule object
The following changes were made to Clinical Operations Vaults to support the Prediction Metrics for Metadata Extraction feature:
- Added the following new fields to the Prediction Metrics object:
- Field Extracted (
field_extracted__sys) - Incorrect Predictions Above Threshold (
incorrect_predictions_above_threshold__sys) - Trained Model Type (
trained_model_type__sys) - Partial Predictions Above Threshold (
partial_predictions_above_threshold__sys)
- Field Extracted (
- Added Layout Rules to the Prediction Metrics page layout
- Updated the Auto-classification Success Rate (
autoclassification_success_rate__sys) field to the new Success Rate (sucess_rate__sys) field
The following change was made to Clinical Operations Vaults to support the Redacted Document Field feature:
- Added the Redacted (
redacted__v) document field
Commercial
PromoMats
Controlled Vocabularies for PromoMats
Two new objects are available called Controlled Vocabularies and Constraints. When combined with field reference constraints, customers can use these objects to restrict the available options in a dropdown with respect to document or object metadata. This functionality is similar to that of field dependencies but can be applied in OneWorkflow task prompts.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
The following changes were made to PromoMats Vaults to support the Controlled Vocabularies for PromoMats feature:
- Added the following document fields:
- Health Authority Submission
- Health Authority Decision
- Added the following objects:
- Controlled Vocabulary (
controlled_vocabulary__v) - Constraints (
constraint__v)
- Controlled Vocabulary (
- Added the picklist Controlled Vocabulary Type (
controlled_vocabulary_type) with the following values:- Health Authority Submission
- Health Authority Decision
The following changes were also made to PromoMats Vaults:
- Field dependencies are now allowed on the Actual Submitted Date (
actual_submitted_date__v) and Professional / Consumer (professional_consumer__v) document fields
Medical
MedInquiry
Medical Inquiry UI: Incremental Save
This feature allows users to save information incrementally as they are capturing the inquiry in the Medical Inquiry User Interface. Previously, users would commit all the information in one go, and upon clicking Save would be navigated away from the page. Users can now save the information that they have entered so far, then continue working on the same page.
This feature also helps mitigate the risk of data loss from interruptions, such as when a user has not saved information yet and they experience a network connection issue.
The feature operates in two ways:
- A new Save icon in the UI allows the user to save what they have entered so far then continue working on the same page.
- When a user attempts to add a new object record (such as an Adverse Event), Vault can be configured to automatically save all of the information that has been entered so far before they begin working on the new object record.
- Admins must configure the objects that will trigger the Enforced Save when a new object record is created.
Medical Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Medical data model to support new features:
The following changes were made to Medical Vaults:
- Increased the character limit on the External ID (
external_id__v) field on the Key Messages (key_message__v) to 255 characters
Training
Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
Quality
QualityDocs
Update Controlled Copy Distribution Package Naming Convention to be Unique
The Job Instance ID has been added to the Controlled Copy Distribution Package naming convention to ensure that there are no duplications in the auto-generated file names. The file naming format is now:
{label of the configured action} * {job instance id} * {current date/time}
Learn more about Controlled Copies.
QMS
Duplicate Check: Edit Fields & Copy Attachments
We have made additional functionality and usability enhancements to the Duplicate Check for Complaints feature, introduced in 22R3. The key enhancements being introduced are:
- A Summary Page configuration component, allowing Admins to define which fields can be edited by end users on the summary page and whether attachments can be copied from one record to another
- The ability to edit fields in the Original record panel of the summary page, based on the configuration defined, and save those edits on completion
- The ability to view attachments on the comparison page
- The ability to configure an asynchronous job to copy any attachments from records identified as a duplicate or follow-up to the record identified as original upon completion of the Duplicate Check workflow
- Improved Admin interface and Pages to provide efficiency and ease of use
Learn more about configuring Duplicate Check.
Training
Default Limit for Number of Prerequisite Rule Per Curriculum Increased to 20
We have increased the default limit for the number of prerequisite rules per curriculum to 20 in new Quality Vaults. Existing Quality Vaults will remain at 5 and customers can increase the limit by contacting Support.
Learn more about Prerequisite Training Requirements.
Surveillance
Standalone Complaints: Health Canada Support
With this release, Surveillance now supports Adverse Event Report generation and data validation for submission to Health Canada using the standalone Complaint object.
Training
Always Show Attachment Section on External Training Assignments
The Attachments section on an External Training Assignment is now always shown regardless of the “proof required” value. This change makes it so the Learner has the option to upload a file, even if proof is not required.
The section message text was updated to differentiate between required and optional attachments.
Validation Management
Copy Screenshots from Clipboard & Paste to Test Step
With this release, test executors can now copy a source image from screen capture software and attach it to an execution step using a system shortcut (CMD + V or CTRL + V). One or more files can also be copied from a file location and attached as well. A new attachment limit of five (5) attachments per test step has been introduced in 23R2.
This greatly enhances the productivity of executors that need to collect objective evidence using screenshots from screen capture software. Previously, screenshots were first saved to a file location and then uploaded using the upload action and upload dialog or by dragging the files and dropping them onto the upload control. Learn more about executing tests.
Regulatory
RIM
Embedded Links in Documents to External URLs Open in a Separate Tab
External links embedded in RIM documents will open in a new browser tab. Primarily, RIM users will experience this behavior in RIM Submissions Archive when clicking on external links in the Submissions Archive Viewer, and in RIM Submissions Publishing when reviewing hyperlinks within eCTD submissions.
EDL & Manual Matching Enabled in All RIM Vaults
The Expected Document Lists (EDL) feature and Manual Matching are now both enabled in all RIM Vaults, particularly older Vaults that were previously not using this functionality. Customers who already enabled these features and customers whose Vaults were provisioned after the EDL and Manual Matching features were released are not impacted. This enablement provides consistency across all RIM environments and provides existing customers with the most up-to-date EDL settings.
Auto-On Enablement of Submissions Settings
Four Content Plan-related settings were automatically enabled and removed from the Vault Admin UI. These enablement settings were originally introduced to allow customers to adopt them on their own timelines. Now that the features are standard in all RIM Vaults, the enablement checkboxes are unnecessary, and will be fully deprecated in a future release. The auto-enabled settings include:
- Enable actions on binder content (Application Settings)
- Enable Submission Content Planning (General Settings)
- Enable Copy Into Content Plan (Application Settings)
- Enable Tree Grid (Support-enabled setting)
Additionally, the RIM Dynamic Linking setting has been moved from Submissions Features to RIM Settings within Admin > Settings > Application Settings.
These cleanup efforts make the Settings page easier for Admins to manage, and easier for Veeva to deliver updates consistently in future releases.
Active Dossier Support for Product Family Level Documents
Active Dossier now supports documents only associated with a Product Family (and not a Product, Active Substance, or Inactive Ingredient). This is needed to support documents in the Active Dossier that are written on a Product Family level, for example documents for Section 3.2.R - Regional Information.
Active Dossier Viewer/Editor Performance Improvement
This feature significantly improves the loading time and overall performance of the Active Dossier Viewer/Editor, especially when the Submission and Regulatory Objective have many relationship records or countries.
Add to Active Dossier Dialog Update
New functionality for the Active Dossier Editor allows users to populate Application, Regulatory Objective, and Submission fields on manually-created Active Dossier Item Detail records. Users will see these new options in the Add to Active Dossier dialog box when in Edit mode. When a user opens the Active Dossier Editor action from a Regulatory Objective or Submission record, Vault automatically populates the field with the current record value.
Active Dossier Loader Validations
To provide users with specific error messages when loading Active Dossier records, the underlying SDK trigger logic is updated to include validation of required and controlling fields. Additionally, the trigger is reordered to run before some platform validations.
This feature has been moved to a later release.
RIM Registrations
Global-to-Local Mappings for Submission Subtypes and Site Roles
With this release, users can select a global term for the Submission Subtype field in the Create Related Records wizard, and Vault maps the global term to the corresponding local Submission Subtype value. Additionally, when using the wizard to create Regulatory Objectives and/or Submissions with relationships that specify Manufacturing Site Role (for example, Submission Product or Submission Packaging), Vault maps the global term to local values.
This greatly enhances the wizard’s efficiency and improves data quality, as users are no longer required to manually enter local Submission Subtype values on each Submission record, nor Manufacturing Site Role values on each relevant Regulatory Objective and Submission relationship record.
Detailed Activity and Regulatory Objective Bundling Settings
Administrators can now view and manage the configuration of Activity and Regulatory Objective Bundling capability in Admin > Settings > Application Settings.
Previously, Admins were required to contact Veeva Support to request the enablement or an update. Now, Admins have the flexibility to perform the following actions for bundling from the Admin UI:
- Limit bundling of Activities and Regulatory Objectives to selected lifecycle states
- Configure bundling to set the Activity and the Regulatory Objective records as Inactive
- Set default filters
- Define naming patterns for Activities and Regulatory Objectives
- Restrict Submissions copying by Worksharing or Submissions creation for US Grouping
Once the Activity and Regulatory Bundling feature is enabled, Admins can easily update the detailed settings to fit their organization’s needs.
Create Registrations from a Regulatory Objective
Regulatory teams can now create and update registrations and their registered details from the same location, a Regulatory Objective, using the regulatory objective’s details as the source for the registered details.
This feature enhances the process of creating registrations, particularly in cases where submissions are not required to approve a product on the market, such as for low-risk medical devices.
Renaming of DADI Settings and Action to eAF
In this release, all Application Settings previously labeled “DADI” are updated to eAF to reflect rebranding.
RIM Publishing
Support for CH Submissions with Multiple Application Numbers
Swissmedic guidance allows for multiple variations for the same medicinal product to be submitted jointly, as a multiple application. This could include variations of the same type or different types (IA, IB, II, extension).
To address these use cases, in 23R2 Vault Publishing will allow publishers to include additional Application Number(s) for Swiss (CH) submissions. After configuration, publishers will be able to:
- Include additional Application Number(s) with a new Add Override action
- Bring forward Overrides to subsequent sequences without affecting any previous sequences
- Delete Overrides for current or future sequences without affecting any previous sequences
When Vault generates the XML, a new line for each Application Number is generated in the envelope.
These changes will not impact any existing CH submissions with a single Application Number.
Local Connection to Run Publishing as Vault App Owner
To enhance Publishing speed and performance, Admins will see a RIM Local Connection under the Admin > Connections tab. This infrastructure improvement will be automatically deployed as Active to leverage Vault Platform functionality and prevent any publishing errors when the Source of Published Documents is set to Source.
While the Publishing end-user experience will not change, users will benefit from quicker published output generation when Source is chosen as the Source of Published Documents.
RIM Submissions
Support Multi-Select on Document Set
For customers working with Global Content Plans, this feature allows for greater flexibility when creating document sets for the dispatch to local affiliates. Previously, the Document Set field value on the Activity, Application, Country, and Content Plan Item objects was single-select. By updating the Document Set field on these objects to be multi-select:
- Less effort is required to create Global Content Plans. For Content Plan Items with document(s) that apply to more than one document set, users can select all applicable document sets.
- There is greater flexibility when assigning documents sets to Activities, as users can assign more than one document set based on the different areas and dossier sections.
Update to Duplicate Detection Criteria for Drag and Drop from Desktop to Content Plan Item
Drag and Drop from Desktop to Content Plan Item was originally released in 23R1. With this release, the duplicate detection criteria for documents dropped to the Content Plan Item are updated to exclude Submissions Archive documents so that only documents from the Library that are not Submissions Archive documents are considered in duplicate detection.
As Vault does not support Submissions Archive document publishing, this update prevents Submission Archive documents from being matched to the content plan and getting published.
Update to Inactive Content Plan Records Cleanup Job Deletion Criteria
The deletion criteria for the Inactive Content Plan Records Cleanup Job is updated to improve the Content Plan Item deletion scope. The job now deletes records where the All Document Count field is blank in addition to 0. This allows Content Plan Item records that are created as Inactive to be properly deleted A new Cleanup Status picklist field has also been added to the Content Plan. This status field is automatically set by Vault to reflect the cleanup status, but can also be set by Admins to skip or reprocess a Content Plan in the next job run.
Content Plan Viewer Icon Updates
The icon for Content Plan sections is updated to align with the icons in the new Submissions Archive Viewer, with a closed folder icon when the section is collapsed and an open folder when the section is expanded. This is more intuitive for users than the previous clipboard icon.
Add Pages column to Content Plan Viewer
The selectable columns for matched documents in the Content Plan Hierarchy viewer are extended to show the document page count from the document metadata. Users can now include the column Pages in the Content Plan Hierarchy viewer and export this information with the Content Plan export.
RIM Submissions Archive
Submissions Archive Correspondence Document Handling Improvement
This feature resolves an issue in the Submission Archive Viewer when a Correspondence document, tagged to a specific Application and/or Submission, was imported and then immediately approved. Previously, the Viewer did not display these documents.
Submissions Archive Viewer Performance Optimization
Submissions Archive Viewer performance is improved to display additional rows when users select Expand All at the module level.
Submissions Archive: Ukraine eCTD 1.0
Users can now import, view, and export Ukrainian dossiers that use the UA 1.0 DTD for eCTD submissions.
Expand All for Single Submission in the Viewer
When users filter on a single submission in the Submissions Archive Viewer, they have the option to Expand All from the root. This expands all child nodes down to the document level for the selected submission. Note: The Correspondence documents are not expanded.
Additional Fields Selectable as Viewer Grid Columns
In the Submission Archive Viewer, users can now choose to display four additional fields: Clinical Study and Clinical Site (from the Submission Metadata object), and Submission Type and Submission Subtype (from the related Submission record). The new fields can also be used for filtering.
Redacted Document Field and Relationship
To support EU Clinical Trial Regulation (CTR) and the growing industry trend towards clinical trial transparency, a new Redacted Yes/No document field and Redaction document relationship data model changes are added in this release. With Admin configuration on the desired document type, subtype, and/or classification, the Redacted field is available for authors or publishers to identify whether a document contains redactions, such as to support EU CTR or for required or voluntary public disclosure. Additionally, the Redaction document relationship (automatically available in the Doc Info panel) allows these users to establish a relationship between redacted documents and their unredacted source files.
See also RIM Data Model Changes below.
23R2 RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model:
- Updated the following components to support the Global-to-Local Mappings for Submission Subtypes and Site Roles feature:
- Added the following values to the Controlled Vocabulary Type (
controlled_vocabulary_type__rim) picklist:- Global Submission Subtype (
global_submission_subtype__v) - Global Site Role (
global_site_role__v)
- Global Submission Subtype (
- Relabeled the Controlled Vocabulary Type picklist value Manufacturing Site Role to Local Manufacturing Site Role (
manufacturing_site_role__v). - Added the Domain (
domain__v) field to the Constraint (constraint__rim) object and its following object types:- Global to Local Submission Type (
global_to_local_submission_type__rim) - Site Role (
manufacturing_site_role__v)
- Global to Local Submission Type (
- Added the following values to the Controlled Vocabulary Type (
- Added the Cleanup Status (
cleanup_status__v) picklist and field to the Content Plan (edl__v) object to support the Update to Inactive Content Plan Records Cleanup Job Deletion Criteria feature. - Updated the following components to support the Active Dossier Support for Product Family Level Documents feature:
- Added the Product Family (
product_family__v) field to the following objects:- Active Dossier Item (
active_dossier_item__v) - Active Dossier Item Detail (
active_dossier_item_detail__v)
- Active Dossier Item (
- Activated the Product Family (
prodct_family__v) field within the Active Dossier Loader (active_dossier_loader__v) object.
- Added the Product Family (
- Added the following components to support the Redacted Document Field and Relationship feature:
- Redacted (
redacted__v) document field - Redaction (
redaction__v) document relationship type
- Redacted (
- Updated the Document Set (
submission_grouping__v) field to be multi-select on the following objects for the Support Multi-Select on Document Set feature:- Activity (
activity__rim) - Application (
application__v) - Content Plan Item (
edl_item__v) - Country (
country__v)
- Activity (
Safety
Safety features are targeted for tentative availability on May 11, 2023.
Safety
Study Site Reporter on Inbox Item Support
Admins can now configure Study Contacts to use as Site Reporters during the initial intake of Inbox Item Study Cases. This feature was previously only available for AERs, but now Intake users can select the appropriate Site Reporter during Inbox Item intake. Upon Case promotion, the system populates all required details of the Site Reporter on the Case, increasing data entry efficiency and accuracy. This new feature is particularly important because it streamlines the Case intake process, reduces the risk of errors, and ensures compliance with regulatory requirements. The ability to configure Study Contacts for use as Site Reporters facilitates identifying and selecting the appropriate person for the role, saving Intake users valuable time and effort.
Learn More
- Enablement: Enable Study Site Reporter: (23R1) Add the Site Reporter Field to the Inbox Item Page Layout
- Admin Help: Manage Studies: Set Up Study Site Reporters
- User Help: Inbox Item Field Reference: Site Reporter
New Substance Name Field in Product Library Configuration
To match E2B guidance, the Vault Safety Product Library includes a new Substance Name field that supports up to 250 characters. The increase from 128 characters means more accurate substance descriptions can be used, improving compliance on E2B Transmissions. When generating Substance Names on Cases, the system downloads values from the new field, if populated, or will continue to download from the existing field. This logic supports setting up longer Substance Names only when required.
Learn More
- Enablement: Enable the New Substance Name Field in the Product Library
- Admin Help: Manage Products: Add Substances
Custom Rulesets and Rules Configuration Configuration
Vault Admins can now configure Custom Reporting Rulesets and Rules for global Agencies and Trading Partners without having to request approval by raising a Support Ticket. This provides the flexibility to configure Safety rulesets based on your business needs.
Learn More
- User Help: Create Custom Safety Rule Sets
Prioritize Seriousness for Downgrades Auto-on
To ensure more accurate transmission creation for Downgrade and Upgrade reports, when Vault Safety calculates the Most Conservative Product for Submission Rules, Serious Adverse Events are always prioritized over Non-Serious Adverse Events as shown in the following table:
| Summary | Seriousness | Expectedness | Relatedness | Ranking |
|---|---|---|---|---|
| Fatal/LT SUSAR |
|
Unexpected | Related | 1 (Most Conservative) |
| SUSAR | Serious | Unexpected | Related | 2 |
| SU | Serious | Unexpected | Unrelated | 3 |
| SESAR | Serious | Expected | Related | 4 |
| SE | Serious | Expected | Unrelated | 5 |
| NSUR | Non-Serious | Unexpected | Related | 6 |
| NSU | Non-Serious | Unexpected | Unrelated | 7 |
| NSER | Non-Serious | Expected | Related | 8 |
| NSE | Non-Serious | Expected | Unrelated | 9 (Least Conservative) |
| 1. If a case contains both a Life Threatening SUSAR and a Fatal SUSAR, the tiebreaker for Most Conservative Assessment will be the Fatal SUSAR. | ||||
Learn More
- User Help:
OTC Carton Attachment Export for FDA E2B(R2) Auto-on
To adhere to FDA guidelines for reporting cases with Over-the-Counter (OTC) products, Vault Safety now supports attaching images of OTC cartons when sending FDA E2B(R2) Transmissions through an AS2 Gateway.
Learn More
- User Help: Enter Case Data: Documents Section
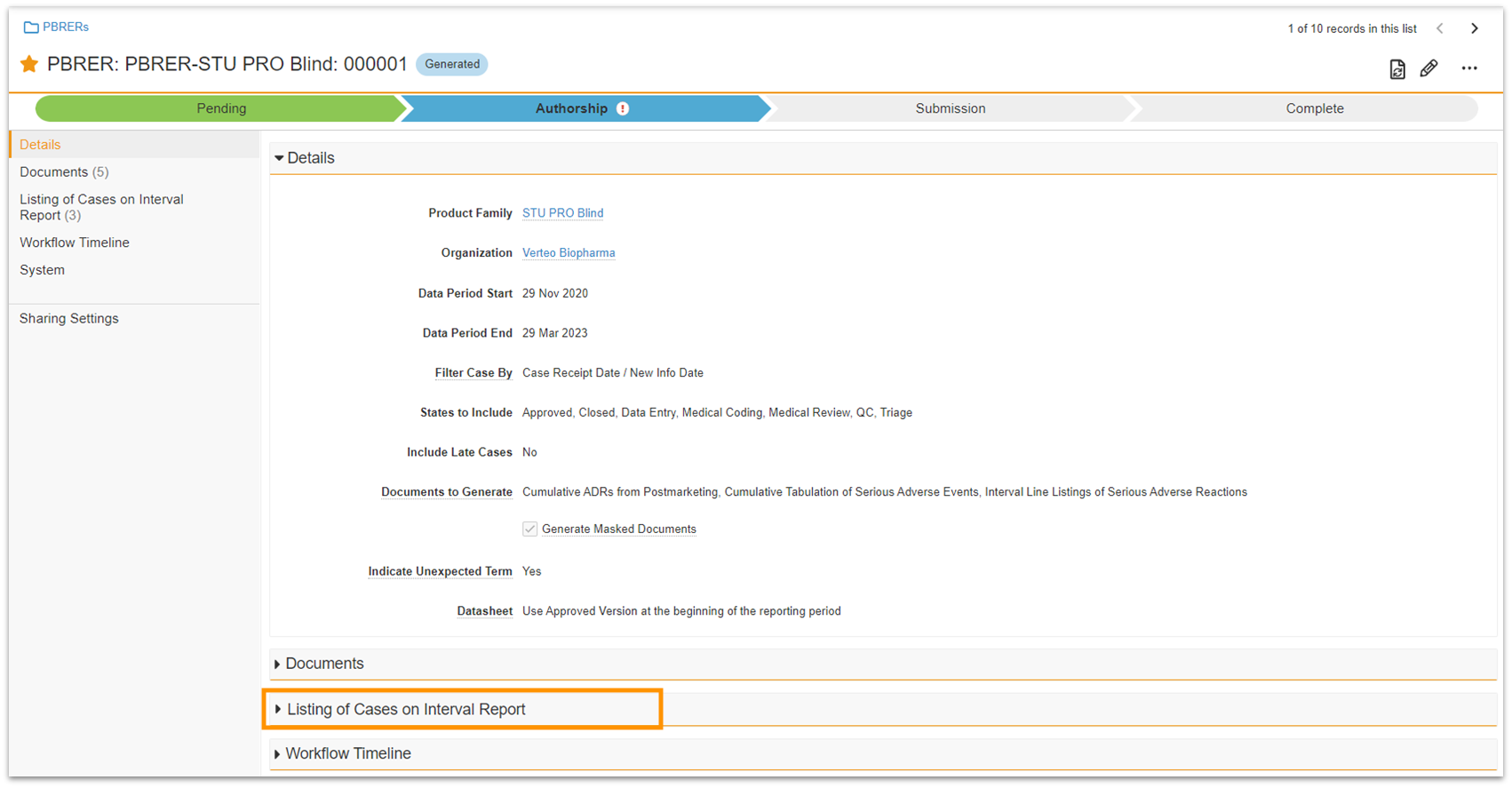
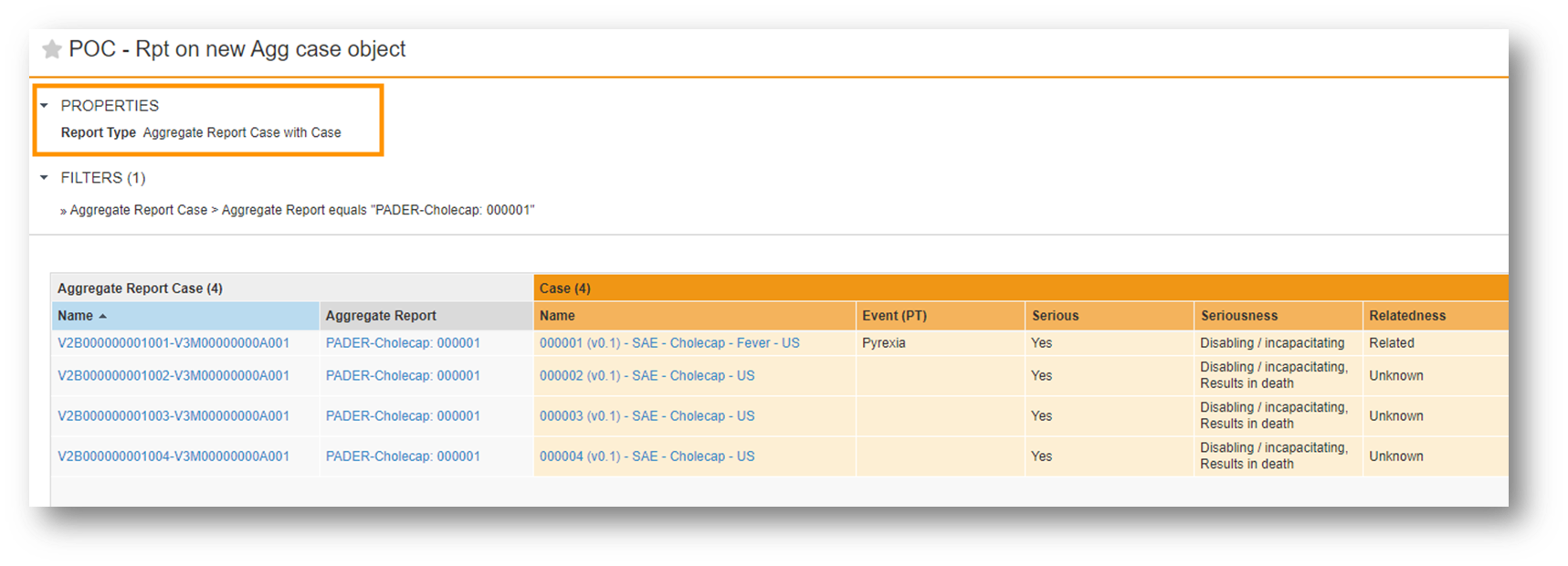
Aggregate Report Interval Case Listing Configuration
Vault Safety now provides in-system access to a comprehensive listing of all Cases included in the interval report for a specific Aggregate Report (DSUR, PBRER, PADER, PSUR, CIOMS II) with this aggregate report Case linking feature.
You can incorporate the new Aggregate Report Case object into your analytics reports to verify that all relevant Cases are captured.
This feature provides greater insight and control over the regulatory reporting process.
Learn More
- Admin Help: Enable Interval Case Listing for Aggregate Reports
- User Help:
PMDA Local Receipt Date Overrides Configuration
For PMDA E2B(R3) Submissions, local Case Processors can now enter values for Receipt Date (C.1.4) and New Info Date (C.1.5), overriding the dates from related Global Cases. This feature introduces two (2) new fields on Localized Cases, Local Initial Receipt Date and Local New Info Date, offering greater alignment with PMDA and NMPA guidelines.
Learn More
- Enablement: Enable Local Receipt Date Overrides
- User Help: Prepare a Localized Case: Details Section
Domestic Local Awareness Date Synchronization Auto-on
To ensure dates remain consistent, reduce manual data entry, and support on-time Case Submissions, the Local Awareness Date on Domestic Localized Cases is now populated with the New Info Date from Global Cases.
Learn More
- User Help: Prepare a Localized Case: Details Section
Manual Transmission Creation Automation Auto-on
To increase efficiency for business users when manually creating Submission or Distribution records, Vault Safety now populates the following fields on a transmission based on the selected Transmission Profile:
- Origin
- Destination
- Outbound Format
- Additional Output Format
- Sender User
- Message Subject (Including Tokens)
- Message Body (Including Tokens)
- Cover Letter Template
Learn More
Feature Enablement Changes
Enablement changes apply to the following features in this release:
| Feature | Previous Enablement | New Enablement |
|---|---|---|
| Enhanced Eligible Products Selection for Cross Reporting | Support (23R1) | Auto-On (23R1.2) |
| All Product Types Available for FDA 3500A and CIOMS I Generation | Support (23R1) | Configuration (23R1.2) |
See the following articles to learn more about these features:
- Enhanced Eligible Products Selection for Cross Reporting: What’s New in 23R1
- All Product Types Available for FDA 3500A and CIOMS I Generation: What’s New in 23R1
SafetyDocs
PSMF Version Binding Configuration
Vault SafetyDocs now automatically selects the correct PSMF document versions when creating a draft of the PSMF binder and when reviewing and approving PSMF binders. Previously, users had to manually edit the binder and select which version of the documents to include.
This feature improves the efficiency of the review process, reduces the risk of errors, and ensures compliance with regulatory requirements.
Learn More
- Enablement: Enable PSMF Version Binding
- User Help: Manage PSMF Binders and Logbooks
QualityOne
QMS
Generate Documents for Standard QMS Processes
This feature extends the Generate Documents using Object Record Actions for Audits feature introduced in 23R1 by adding nine (9) additional objects.
- CAR
- Change Control
- Complaints
- Formulation (to generate Specification document)
- NCR
- Packaging (to generate Specification document)
- Product (to generate Specification document)
- Risk Register
- Risk Study
This feature allows users to generate a document from a Formatted Output or a Document Template using an Admin-configured entry or user action. By generating documents, users can leverage Vault’s full Document Management functionalities, removing dependency on manually configuring and uploading documents to the Vault Library. Generating documents using a 1-click solution also automatically generates a link between the object record and the document in the Vault Library, ensuring users retain the context of the generated document. Learn more about generating documents for the standard QMS processes.
Lab Investigations
Lab Investigations helps in the management and investigation of Out of Specification (OOS) or Out of Trend (OOT) laboratory errors. Customers can document, standardize, and track lab investigations using a phased investigation approach to establish if the product is OOS or OOT, or if there was a potential laboratory error that resulted in the product being outside of the acceptable limits and specifications. Learn more about the Lab Investigations feature.
QMS & HSE
Risk Management: Support for 2D or 3D Risk Matrices
This feature allows customers to choose between two-dimensional or three-dimensional risk matrices at the time of new Vault provisioning. Customers can also change from two-dimensional to three-dimensional matrices in an existing Vault, but cannot revert to two-dimensional once three-dimensional matrices have been enabled. This enables organizations to decide if they want to measure Detectability as a dimension of risk for a more detailed risk assessment. Learn more about working with risk matrices.
Document Control
Recall Controlled Copies
In previous releases, if a document utilizing Extensible Controlled Copy functionality was superseded or obsolesced, users would need to manually review the associated Controlled Copy Trace records to determine if a recall process was necessary for any currently-disbursed copies.
With this feature, customers can configure their Vault to find all controlled copies of a document that has been superseded or obsolesced and change their lifecycle state to In Recall. Customers can also configure a workflow to auto-start on all issued controlled copies when the associated document version is superseded or made obsolete. This workflow can be configured to issue a task to the responsible party for each issued copy, automatically launching a recall process which is done manually in Document Control today. Learn more about the Recall Controlled Copies action.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on May 16, 2023.
RegulatoryOne
EDL & Manual Matching Enabled in All RegulatoryOne Vaults
The Expected Document Lists (EDL) feature and Manual Matching are now both enabled in all RegulatoryOne Vaults. Customers who already enabled these features and customers whose Vaults were provisioned after the EDL and Manual Matching features were released are not impacted. This enablement provides consistency across all RegulatoryOne environments and provides existing customers with the most up-to-date EDL settings.
Compliance Management
Bulk Create Formulation Questionnaires
This feature allows users to create up to 1,000 Formulation Questionnaires records in bulk so that regulatory teams can save time when they need to send questionnaires to many suppliers at the same time. In previous releases, users could only create a single Formulation Questionnaire at a time. Users can then use the records to generate and send related FQ Checklists to suppliers, helping prevent a backlog of RMQs. Learn more about bulk creating Formulation Questionnaires.
Registration & Dossier Management
Remove Create Registrations in Bulk Feature
As of this release, we have removed the Create Registrations in Bulk feature, which we no longer support. We have replaced this functionality with the Local Impact Assessment feature (released in 22R1), which allows users to generate the appropriate Registration records within the registration process and makes the Create Registrations in Bulk feature unnecessary.
Veeva Claims
Veeva Claims features are targeted for tentative availability on May 16, 2023.
Veeva Claims
Expanded Statement Uniqueness to Consider Statement Language
This feature allows users to create the same statement translation for two (2) different languages while enforcing uniqueness criteria on a combination of statement text and language. This allows organizations to, for example, use the same translated statement variation for both Finnish and Swedish even while enforcing uniqueness. This is an enhancement of the existing Statement Uniqueness field, which only considered statement text in previous releases and didn’t allow matching statements for different languages. As part of this feature, customers can migrate their existing Statement records to utilize this new uniqueness criteria. Learn more about configuring statement uniqueness.
Translator View Enhancement: Freeze Header & Global Element Columns
This feature improves the usability of the Translator View section on Pack Copy records by freezing the header row and the global element columns so that Translators can scroll horizontally across different language columns while viewing the global element columns. Learn more about translating pack copy elements.
23R2 Veeva Claims Enhancements
This feature improves the usability of several previously released features. Users can now hover over a cell to view the content in Selectively Create Claims, Generate Local Adaptations, and Localize Pack Copy dialogs, allowing them to see any previously truncated text. The Statements, Claims, and Elements listed in those dialogs now default sorts by record Name fields to ensure users see a logical order of records. This feature also adds the Expand the section by default option to the Comment sections for the Project, Claim, Local Adaptation, and Pack Copy objects so that Admins can configure whether these sections are open or closed when users open records with comment threads, allowing Admins to have greater control over the user experience.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| Download All Option for External Viewer | Auto-on | Platform |
| Impose Limits on Create & Edit Annotations | Auto-on | Platform |
| Rich Text Support for Related Objects in Formatted Outputs | Auto-on | Platform |
| SVG, AVIF and WebP Rendition Support | Auto-on | Platform |
| Render Animated GIF files as Videos | Auto-on | Platform |
| Picklist Value Label Increased from 128 Characters to 256 | Auto-on | Platform |
| Require Vault File Manager Permission to Check in Documents | Auto-on | Platform |
| Vault Objects | ||
| Self References for Child Objects | Configuration | Platform |
| Intelligent Record Update | Auto-on | Platform |
| Update "Edit Column" Label in Page Layout Configuration | Auto-on | Platform |
| Lifecycle & Workflow | ||
| Override checkout option for State Change Document Operation Jobs | Configuration | Platform |
| Prevent Deletion of Document Templates Used in a Workflow | Auto-on | Platform |
| Object Lifecycle Entry Criteria: State & State Type of Related Records can be Validated with Conditions | Configuration | Platform |
| Reporting & Dashboards | ||
| Union-All Report Type | Auto-on | Platform |
| Currency Support in Dashboards | Configuration | Platform |
| Report Formula Fields Support Long Text | Auto-on | Platform |
| Include Report Owner in Report MDL | Auto-on | Platform |
| Formulas | ||
| New Functions in Vault Formulas | Auto-on | Platform |
| Enhanced Text Formatting | Configuration | Platform |
| Administration | ||
| Vault Platform HTTP/2 Support | N/A | Platform |
| Customer-Supported Languages | Configuration | Platform |
| Bulk Translation Support for System Messages | Configuration | Platform |
| Scheduled Data Export Performance Enhancements | Auto-on | Platform |
| EDLs: EDL option visible in Admin UI for all Vaults | Admin Checkbox | Platform |
| Access Control | ||
| Improved Permission Set Sorting for Security Profile Details Page | Auto-on | Platform |
| Vault Loader | ||
| Vault Loader Command Line Support for Record Migration Mode Update & Upsert | Auto-on | Platform |
| Clinical Operations | ||
| Redacted Document Field | Auto-on | CTMS, Study Startup |
| Study Metadata Extraction: Test Model from Production | Auto-on | eTMF |
| Prediction Metrics for Study Metadata Extraction | Auto-on | eTMF |
| TMF Transfer: Match on Country Code | Auto-on | eTMF |
| TMF Viewer UI Enhancements | Auto-on | eTMF |
| Fee Schedules for Study Organizations | Configuration | Vault Payments |
| ClinOps/CDMS - Enhanced Job Labels | Auto-on | CTMS<>CDMS |
| Task Widget Optimization | Auto-on | Clinical Operations - All, Study Startup, eTMF |
| Clinical Operations Data Model Changes | Auto-on | CTMS, Study Startup, Vault Payments, eTMF |
| Commercial | ||
| Controlled Vocabularies for PromoMats | Configuration | PromoMats |
| 23R2 Commercial Data Model Changes | Auto-on | MedComms, PromoMats |
| Medical | ||
| Medical Inquiry UI: Incremental Save | Auto-on | MedComms |
| 23R2 Medical Data Model Changes | Auto-on | MedComms |
| Quality | ||
| Duplicate Check Enhancements: Edit Fields & Copy Attachments | Configuration | QMS |
| Standalone Complaints: Health Canada Support | Auto-on | Surveillance |
| Always Show Attachment Section on External Training Assignments | Auto-on | Study Training, Training |
| Copy Screenshots from Clipboard and Paste to Test Step | Auto-on | Validation Management |
| Regulatory | ||
| Embedded Links in Documents to External URLs Open in a Separate Tab | Auto-on | RIM Submissions Archive, RIM, RIM Publishing |
| EDL & Manual Matching Enabled in All RIM Vaults | Auto-on | Platform |
| Active Dossier Support for Product Family Level Documents | Auto-on | RIM Submissions, RIM Submissions Archive |
| Active Dossier Viewer/Editor Performance Improvement | Auto-on | RIM Submissions, RIM Submissions Archive |
| Active Dossier Loader Validations | Auto-on | RIM Submissions, RIM Submissions Archive |
| Global-to-Local Mappings for Submission Subtypes and Site Roles | Admin Checkbox | RIM Registrations |
| Detailed Activity and Regulatory Objective Bundling Settings | Auto-on | RIM Registrations |
| Create Registrations from a Regulatory Objective | Configuration | RIM Registrations |
| Renaming of DADI Settings and Action to eAF | Auto-on | RIM Registrations |
| Support for CH Submissions with Multiple Application Numbers | Auto-on | RIM Publishing |
| Local Connection to Run Publishing as Vault App Owner | Auto-on | RIM Publishing |
| Support Multi-Select on Document Set | Configuration | RIM Submissions |
| Update to Duplicate Detection Criteria for Drag and Drop from Desktop to Content Plan Item | Auto-on | RIM Submissions |
| Update to Inactive Content Plan Records Cleanup Job Deletion Criteria | Auto-on | RIM Submissions |
| Content Plan Viewer Icon Updates | Auto-on | RIM Submissions |
| Add Pages column to Content Plan Viewer | Auto-on | RIM Submissions |
| Submissions Archive Correspondence Document Handling Improvement | Auto-on | RIM Submissions Archive |
| Submissions Archive Viewer Performance Optimization | Auto-on | RIM Submissions Archive |
| Submissions Archive: Ukraine eCTD 1.0 | Auto-on | RIM Submissions Archive |
| Expand All for Single Submission in the Viewer | Auto-on | RIM Submissions Archive |
| Additional Fields Selectable as Viewer Grid Columns | Auto-on | RIM Submissions Archive |
| Redacted Document Field and Relationship | Auto-on | RIM Registrations, RIM, RIM Publishing, RIM Submissions, RIM Submissions Archive |
| QualityOne | ||
| Generate Documents for Standard QMS Processes | Configuration | QualityOne QMS |
| Lab Investigations | Auto-on | QualityOne QMS |
| Risk Management: Support for 2D or 3D Risk Matrices | Configuration | QualityOne HSE, QualityOne QMS |
| Recall Controlled Copies | Configuration | QualityOne Document Control |
| RegulatoryOne | ||
| EDL & Manual Matching Enabled in All RegulatoryOne Vaults | Auto-on | RegulatoryOne Compliance Management, RegulatoryOne Registration & Dossier Management, RegulatoryOne Regulatory Documents, Veeva Claims |
| Bulk Create Formulation Questionnaires | Configuration | RegulatoryOne Compliance Management |
| Remove Create Registrations in Bulk Feature | Auto-on | RegulatoryOne Registration & Dossier Management |
| Veeva Claims | ||
| Expanded Statement Uniqueness to Consider Statement Language | Configuration | Veeva Claims |
| Translator View Enhancement: Freeze Header & Global Element Columns | Auto-on | Veeva Claims |
| 23R2 Veeva Claims Enhancements | Configuration | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|