Release Date: April 30, 2021
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Working with Documents
Renditions for RTF Files
With this release, Vault now generates viewable renditions for Rich Text Format (RTF) files. RTF files are a popular cross-platform file type that capture information about font styles, formatting, images, and more.
Reclassified Documents Always Retain Attachments
Starting in this release, Vault always retains attachments when users reclassify documents. Previously, if a user reclassified a document to a document type that did not have attachments enabled, Vault deleted the attachments. Admins still need to enable attachments on each document type to allow users to add new attachments to that document type.
Enable Document Tags on All Vaults
Previously, the Document Tags feature was only available through Veeva Support. With this release, Admins can configure document tags in all Vaults.
Document tags allow Admins to define a set of words or phrases that Vault can automatically detect whenever new document content is added. When any of these words or phrases are found, Vault sets the defined tag on the document metadata. Users can then search and filter on the defined document tags.
Carry Forward All Document Versions
A document version can have document relationships to one or more versions of the same target document. When configured, this feature allows Vault to carry forward all related document versions when there is a new version of the source document, rather than only carrying forward the latest version of each target document.
Disable Office Online on All Vaults
Starting in this release, users can no longer check out documents to Microsoft Office Online. Users can still edit any documents that were previously checked out to Office Online, but these documents must be checked in to Vault before the 21R3 release. In 21R3, the Checkout to Office Online feature will be deprecated entirely, and any edits on documents that are not checked in will be lost.
We have been communicating this change over the past several releases. 21R2 is the last general release in which the legacy Office Online functionality will continue to work. Instead, we recommend using Collaborative Authoring, which allows users to edit documents in Microsoft Office 365 desktop applications and in browsers.
Vault Objects
Related Object Section Criteria VQL Filters
We enhanced Related Object sections of objects to filter based on criteria VQL, similar to Related Document section filters. Admins can now define much more powerful and flexible filters using criteria VQL. Any existing legacy filters will be unchanged and continue to function as before. Vault will automatically convert legacy filters to criteria VQL when an Admin edits the applicable Related Object section.
Admins can also choose to apply the criteria VQL as default when new records are created by setting the Apply on Create checkbox.
Previously, Vault ignored filters that included any fields that a user did not have Read permission on. With this release, Vault applies the criteria VQL to filter the Related Object section, but users do not see any fields that they do not have Read permission on.
User Object Custom Fields Support in Validation Rules
Custom fields on the User object can now be used in Vault validation rules for object records, giving Admins much more flexibility to validate custom information added to their User objects.
Lifecycle & Workflow
Unified Workflow Access
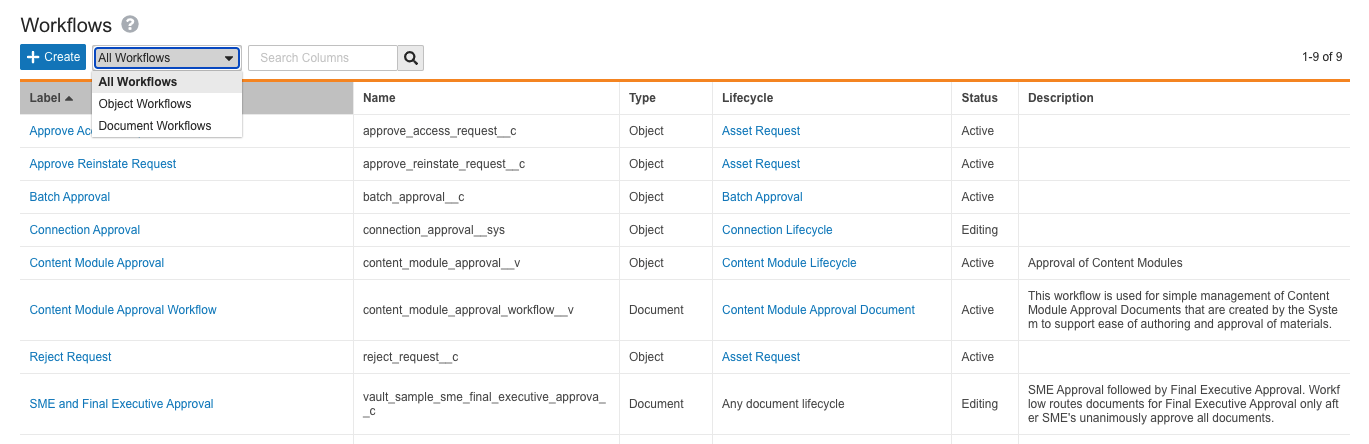
This release brings a major step in the One Workflow initiative to our customers, with the goal of streamlining the Vault workflow creation and configuration experience. To support this initiative, we have unified access to both object and document workflows under a single configuration page located at Admin > Configuration > Workflows. In addition, single-document workflow functionality, now called legacy workflows, no longer appears on document lifecycles that do not have existing legacy workflows. Learn more about Vault workflows.
Vault workflows now fall under the following definitions:
Workflows
Formerly split up into Multi-Document Workflows (MDW) and Object Workflows, workflow creation and configuration is now unified under a single page. Within the page, workflows have two types:
- Document Workflows: Formerly known as Multi-Document Workflows, this is the recommended type for workflows affecting Vault documents, whether there is only one document in the workflow or many.
- Object Workflows: Workflows which affect object records.
On the new Workflows page, Admins can use the search bar, or filter their view by document or object workflow.
Legacy Workflows
Formerly known as single-document workflows or simply document workflows, these workflows are now labelled as legacy workflows. If a document lifecycle does not have any legacy workflows, the Legacy Workflow tab will not appear on that document lifecycle configuration page. In the 21R1.2 release, this also includes any legacy R&U workflows that were shown in the workflow tab.
Restrict Document Workflow to a Single Document
This feature allows workflow administrators to configure a document workflow (formerly multi-document workflow) to only run on one document.
When a document workflow is configured to run on a single document, users can only start the workflow from the document Actions menu using a Start Workflow user action. Users cannot start these workflows from bulk views such as Cart, Library, or Favorites.
Copy Workflow
This feature allows workflow administrators to copy an object or document workflow (formerly multi-document workflow). The copied workflow is created as Inactive, and the workflow administrator must make it Active. The workflow copy has the same type (object or document) and the same lifecycle as the source workflow.
Restrict Workflow Owner from Receiving a Task
This feature allows workflow administrators to restrict workflow owners from receiving an object workflow or document workflow (formerly multi-document workflow) task. When this option is configured on a task, the workflow owner can no longer accept or complete that task, even if the user is part of a broader participant user group that receives the task.
Prompt for Document Fields in Document Workflow Verdicts
Workflow administrators can now configure document fields as part of a document workflow (formerly multi-document workflow) task verdict. Document workflows for specific lifecycles can support all fields available to that lifecycle. Document workflows for any lifecycle can support only Base Document type fields. Fields can be optional or required. Multi-verdict tasks show existing field values for each document verdict.
Prompt for Object Reference Fields in Document Workflows
This feature allows workflow administrators to configure object reference fields inside a document workflow (formerly multi-document workflow) start step, task step, or task verdict. Vault supports both single and multi-value reference fields, and fields can be optional or required.
Support for DAC Lifecycle Role Allowed Group in Document Workflows
Workflow initiators can now select and add users in the Allowed Group of a Dynamic Access Control (DAC) lifecycle role in a specific-lifecycle document workflow (formerly multi-document workflow). This feature must be enabled on the Admin > Settings page and configured for a participant group by a workflow administrator before workflow owners can select users that are part of the allowed user group. Note that Admins cannot disable this feature after enabling it.
In addition, Vault no longer displays errors while trying to add users who are already added to the document lifecycle role using DAC. Instead, Vault simply ignores the selection.
Expression Enhancement for Update Document Field in Lifecycle & Workflow
This feature adds additional functions and operators to allow workflow administrators to build more comprehensive expressions, used for updating document fields inside a lifecycle event action, entry action, or document workflow (formerly multi-document workflow) content action step. New functions include If and Case. New operators include > (Greater Than) and < (Less Than).
Date & DateTime Fields Support in Document Workflow Decisions
Workflow administrators can now configure Date and DateTime fields in a document workflow (formerly multi-document workflow) decision step. Date or DateTime values can be within certain days, weeks, months, or years in the past or in the future.
Task Due Date Audit Updates in Document Audit Trail
This feature adds useful information to the Due Date audit event for object workflows and document workflows (formerly multi-document workflows). If a workflow automatically calculates a due date, it appears in the audit trail as performed by System. If the due date is updated by a user using a workflow action, it is listed in the audit trail as performed by a user. Each update to a user task due date is captured separately in the audit trail.
Reporting & Dashboards
Dashboard Usability Enhancements
This feature makes several aesthetic and usability enhancements to dashboards. When selecting a report, users now see a list of recent reports. When creating a chart, users now select a report prior to selecting a chart type. Additionally, charts no longer include the gray header background, and Vault displays full chart labels.
Consistent Report URLs Across Vaults
This feature allows Admins to define web actions for accessing reports in a source Vault and migrating them successfully to a target Vault without updating the web action’s URL. URLs for the same report can now be consistent across Vaults. Users can still access reports through existing URLs, and Admins can now replace the report ID with the report’s name in URLs.
Standard Reports
This feature will allow Vault applications to introduce standard reports that cannot be deleted and that users can only allow edit in a limited capacity, if at all. Standard reports will include a Veeva tag, a Veeva logo, and the standard Vault namespace (__v) to allow users to easily distinguish them from other reports.
Note that while this feature introduces the ability for Vault applications to add standard reports, this release does not include any new standard reports in customer Vaults.
Search
Expanded Search Enhancements
Expanded search now supports in-line editing, bulk actions, saved views, and exporting from each of the search result sections. The top of each section now displays the total number of results in that section.
Learn more about expanded search results.
Auto Claims Linking
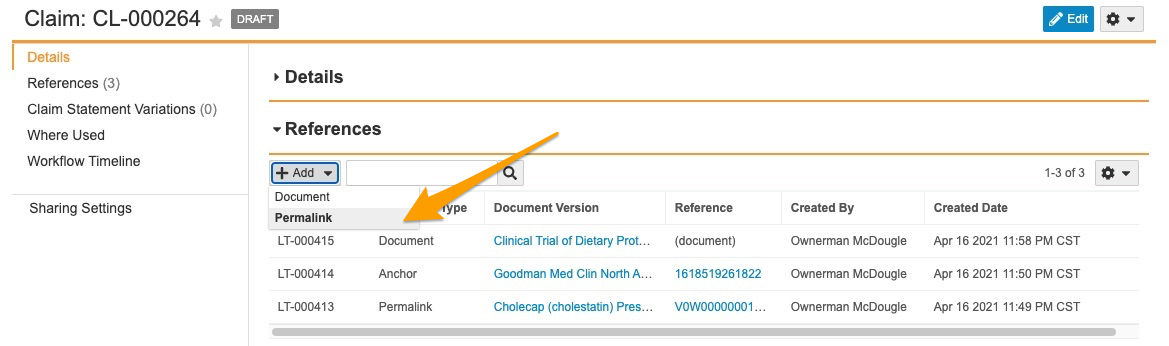
Suggested Links to Permalink Targets
This feature enables Auto Claims Linking to create Suggested Links and Auto Links to permalink targets in addition to version-specific documents and anchors. When enabled, a user adding references to a Claim record can select Permalink as the target type, and add one or more permalink targets as references on the Claim. Once added, the permalink record ID appears in the Reference column within the Claim record’s References section. This link always navigates to the latest version of the permalink target document.
This feature is available when an Admin has enabled all of the following checkboxes in Admin > Settings:
- Enable Copy Link and unlock Permalink Object Type
- Enable linking to permalinks (This checkbox is only available after enabling Copy Link)
- Enable Suggested Links
Vault File Manager
Permission Label Update: Check Out to File Manager
In this release, we have relabeled the Check Out to File Manager permission to Vault File Manager. This permission allows users to access all Vault File Manager functionality, including uploading renditions and importing submission dossiers to RIM Submissions Archive.
Administration
Display the Unclassified Document Type in Admin
This feature was postponed to a future release.
Admins Can Edit All Document Field Labels
Prior to this release, Admins could not edit the Labels on all standard document (__v) fields in the UI, even though this was possible via MDL and through bulk translation tools. This feature allows Admins to edit Labels on all document fields.
Smart Filter Enablement in Search Settings
When enabled, smart filtering automatically converts navigational search terms, such as Document Type or Status, into filters. Before this release, smart filtering was only available through Veeva Support. In this release, Admins can now enable or disable this feature on the Admin > Settings > Search Settings page.
Access Control
Automate Guest Invites to Azure AD from Vault
With this release, Admins can enable automatic invites to external users, allowing users without access to the Admin’s Vault domain to edit and collaborate on Office 365 files. When enabled, Vault automatically adds the external users as Guests using Azure AD.
Enforce Use of HTTPS When Configuring SAML & OAuth 2.0 Profiles
When configuring SAML profiles and OAuth 2.0 profiles, Admins must now use the HTTPS protocol rather than the HTTP protocol. This applies when importing SAML IdP metadata or when uploading OAuth 2.0 Authentication Server metadata. Note that this change only applies when creating new profiles or updating existing profiles.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Platform data model to support new features:
Added the following components to support the Suggested Links to Permalink Targets:
- Added the Permalink (permalink__v) value to the Reference Type (suggestedlink_link_target_type__v) picklist.
Clinical Operations
Quality Issue Assignee Filtering By Object Type
Previously, the presence of custom VQL on the Assigned To field for any Quality Issue object type would prevent the default filtering from applying to the field on other Quality Issue object types. This updated behavior respects custom VQL on an object-type basis.
Commercial
Material ID
This feature adds a Material ID field that generates and adds a unique ID number to the document for submission to a health authority. This number can be automatically updated when the document moves from Steady State to Draft so that there is a unique ID number if a second submission is required. This allows customers to up-version documents past v1.0 while maintaining a unique ID number that can be used for each submission of a specific document.
Modular Content: Rich Text Fields
In 21R1, we added new objects to support the creation and storage of Content Modules, one of the elements of which supports storing text elements. With this release, the text fields use the new Rich Text field type, allowing basic formatting on the text. Existing unformatted text is unaffected and both the plain and rich text are accessible via the API.
eCTD Compliance Package 2253 Form Update
With this release, users can configure Vault to automatically complete line 14 on Form 2253 when generating an eCTD Compliance Package. Once configured, Vault automatically selects “Final” on line 14 of Form 2253 in Post Marketing Compliance Packages when the Center field is set to CBER – APLB. Note that the Center field is now required on the Post Marketing Compliance Package by default and is visible to users without configuration.
Create Fragment Includes Additional CRM Product Fields
With this release, the Create Fragment feature copies any standard CRM Product fields (CRM Org, CRM Product, CRM Product Detail Group) used on a source document to the generated email fragment, as part of the Veeva Approved Email functionality. This reduces the administration required when preparing fragments for use in CRM.
Data Model Change: Multi-Select CRM Product Fields
This data model change lays the foundation for a CRM feature with a planned release in Q3, which will support multiple CRM Products on CLM Presentations and Slides in Vault. With this release, Admins can update the following three (3) fields in PromoMats and MedComms to be multi select or single select:
- CRM Product (crm_product__v)
- CRM Product Group (crm_product_group__v)
- CRM Detail Group (crm_detail_group__v)
These fields are single select by default.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
- CRM Product (crm_product__v)
- CRM Product Group (crm_product_group__v)
- CRM Detail Group (crm_detail_group__v)
The following components have been added to PromoMats Vaults:
- Material ID (material_id__v)
- Revision (revision__v)
- Promotional Material ID (promotional_material_id__v)
- Converted the Text Element Content field on the Content Module Asset object (content_module_asset_v.text_element_content_v) from long text to rich text
Quality
Station Manager: Capture Document Usage
With this release, Vault is now capable of creating activity records to capture the viewing of documents in the Station Manager mobile application. These activity records provide document owners and administrators with insight into the usage of documents, such as SOPs and instructions, in manufacturing and lab environments.
This Vault feature supports a future enhancement to the Station Manager application which will enable activity tracking and sync activity records to Vault. Learn more about setting up Station Manager.
Health Canada Adverse Event Reporting
Customers are now able to leverage Vault Product Surveillance to triage a complaint that is determined to be a reportable adverse event to Health Canada. This feature also adds the ability to generate formatted PDF output of the Health Canada Mandatory Medical Device Problem Reporting Form for Industry.
Customers can use this feature to submit the PDF file manually to Health Canada. This is an extension of Adverse Event Reporting to the US FDA. Learn more about using adverse event reporting.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Quality data model.
The following changes were made to support the Health Canada Adverse Event Reporting feature:
- New object type: Health Canada (health_canada__v)
- New fields added to support Adverse Event Reporting for Health Canada
- Company Identification No (CA) (ca_company_identification__v)
- License No (CA) (ca_license_no__v)
- Medtech Complaint (medtech_complaint__v)
- Type of Assessment (assessment_type__v)
- Severity Outcome (severity_outcome__v)
- Is Reportable? (is_reportable__v)
- Days for Initial Report Due Date (days_reportable__v)
The following changes were made to support the Station Manager: Capture Document Usage feature:
- Added the Station Manager Activity (station_manager_activity__v) object
Regulatory
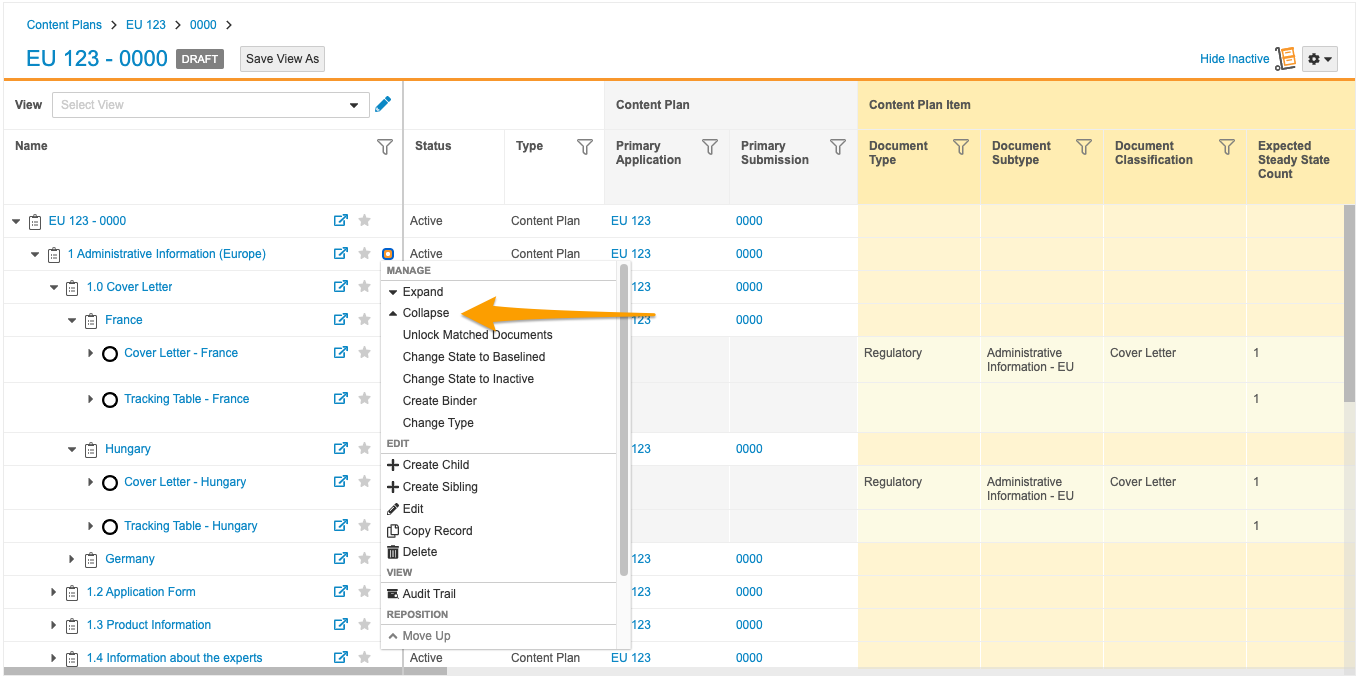
Collapse Action in Content Plan Hierarchy Viewer
With this release, a new Collapse action is available for Content Plan records in the Content Plan Hierarchy Viewer. This action allows users to collapse all descendant Content Plan and Content Plan Item records within a content plan section at once, including matched document rows.
Correspondence in Viewer to Consider Dossier Status
With this release, Vault now takes into account values in the Dossier Status field when displaying correspondence documents in the Submissions Archive Viewer. Submissions Publishing customers with the Correspondence in Viewer feature enabled now see submission-level correspondences in the Viewer for submissions that are not actively being published.
Submissions Archive: Taiwan 1.0
With this release, RIM Submissions Archive Vaults support importing and viewing eCTD submissions based on the Taiwan v1.0 DTD.
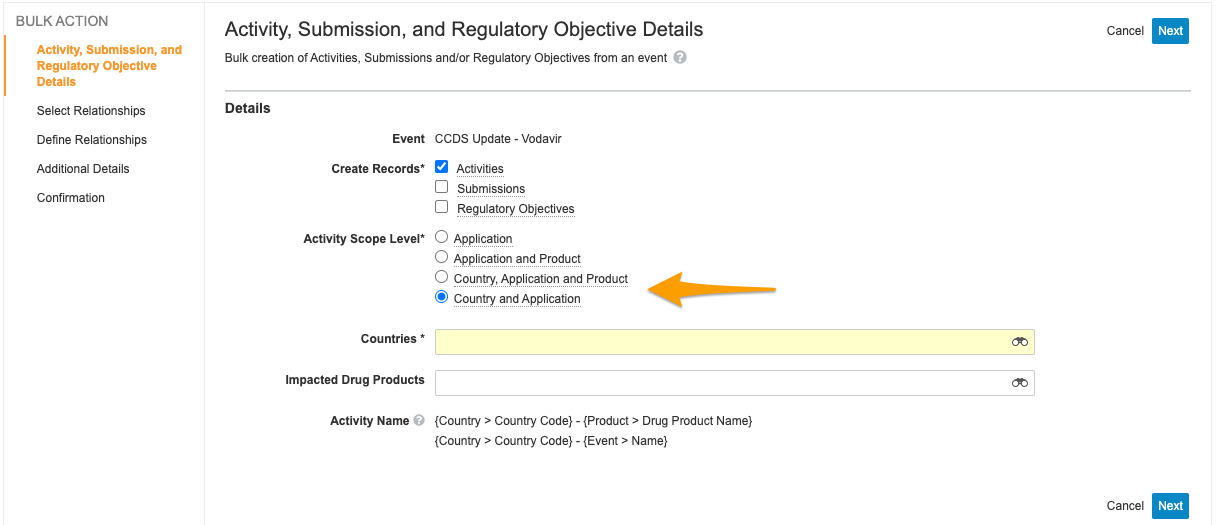
Create Related Records: Application Country Scope
This feature adds an additional level of granularity when users create activities from an Event record using the Create Related Records wizard. The new Activity Scope Level, Country and Application, allows users to create an Activity record for each Country record related to the impacted Application record. This feature also renames the existing Country scope to Country, Application and Product to align with its existing behavior.
Create Related Records: Restrict Submission & Regulatory Objective Joins
When users run the Create Related Records wizard from an Impact Assessment Report, Vault now applies validation logic before creating Submission and Regulatory Objective join records. Validation logic, which is specific to each join record type, prevents the creation of invalid join record relationships based on relevant existing relationships.
IDMP: Registered Packaging to Medicinal Product Registration Mapping
This feature introduces a new action that maps Registered Packaging records to the related Medicinal Product Registration record. Admins can set this up as a user action or an entry action on Registered Packaging object lifecycle states. This feature supports IDMP and extends the IDMP Accelerators feature introduced in the 21R1 release. Learn more about configuring Registered Packaging mapping.
Quality to RIM Vault Connection Data Model Update
With this release, the data model components supporting the Quality to RIM Vault Connection are available in all RIM Vaults. Although Admins can see these components in any RIM Vault, the Quality to RIM Vault Connection works only for RIM Vaults with the Registrations application.
UDI Source Data Model
This feature introduces medical device-related data model changes to allow Vault to capture UDI attributes for EUDAMED and GUDID.
Regulatory Data Model Updates
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model.
Safety
Safety features are targeted for tentative availability on May 6, 2021.
Local to English Language Intake Configuration
Vault Safety now supports case intake from the local language to English through the Inbox Item, provided in the new Inbox. For example, the EMA requires English submissions. If an adverse event is reported from Holland in Dutch, you can enter the Inbox Item in Dutch while translating the data to English before promoting it to a Case.
Note: As of 21R2, AERs will enter a sunset period. No new functionality will be added to AERs. Using the new Inbox (Inbox Item) is optional in 21R2 (August 2021), recommended in 21R3 (December 2021), and mandatory in 22R1 (April 2022), when AERS will become obsolete.
Learn More:
China (NMPA) Local Fields Configuration
Chinese local data elements required by NMPA (China regulatory authority) are now available for data entry.
Learn More:
- Enable Local NMPA (China) Fields
- Prepare a Localized Case
- Configure Controlled Vocabularies: Controlled Vocabulary Types
Multilingual MedDRA Browser and Autocode Auto-on
The Vault Safety MedDRA browser now offers the ability to code Adverse Events in any non-English MedDRA supported language (except Japanese). This capability also extends to auto-coding. The updated browser is available for Inbox Items during intake, and Case Adverse Events during subsequent case processing.
Learn More: Code Multilingual MedDRA Terms
Automatic Intake From Documents With a Viewable PDF Rendition Auto-on
Safety.AI’s automatic PDF form intake feature is now available for all file formats supported in Vault including Word and image files. This will allow users to start the extraction process from any Vault Document with a Viewable PDF Rendition. Upon successful completion, an Inbox Item will be created with the case information extracted from the document text, fields, and checkboxes.
New Checkbox Recognition from Documents Auto-on
Safety.AI will automatically extract additional information from document checkboxes including Outcome, Report Type, Patient Gender, and Age Group. Adverse Event Outcome and Seriousness will be suggested from Case Outcome and Seriousness. The Source Data pane will display this information to facilitate human verification. Safety.AI will also display a value only once in the field’s dropdown menu if it was found multiple times in the source text.
QualityOne
QualityOne Teams
This feature provides a mechanism to manage structured quality teams of participants that are responsible for completing specific processes within workflows assigned within Vault. QualityOne Teams can be configured to help manage change controls, audits, CAPAs, and other quality event-related processes. Learn more about configuring and working with Teams.
Product Hierarchy Data Model for QualityOne
The Consumer and Chemical Product industry has formulations within Quality that can form multilevel hierarchical relationships between the different quality processes. With this release, a shared standard product hierarchy data model is introduced and can be leveraged to store complex hierarchical products and their specification details to reduce reliance on customization. Learn more about QualityOne’s key data model.
Veeva Claims
Note that Veeva Claims features are targeted for tentative availability on May 11th.
Auto-Create Element
Veeva Claims’ Pack Copy module includes a reusable Element library which contains different kinds of packaging elements including Claim-type elements. Claim-type elements are existing Claim records. The current process of populating the element library can be time consuming; after marketers create and approve claims, they have to create related elements manually.
This feature significantly reduces the time needed to populate the packaging Elements library. This feature allows the automatic creation of an Element record after the corresponding Claim reaches a defined lifecycle state. This feature provides Admins the option to configure a record action in a Claim lifecycle or workflow to enable automatic creation of a Claim-type packaging Element. Learn more about configuring Auto-Create Element.
Enablement Details
| Name | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| Renditions for RTF Files | Auto-on | Platform |
| Reclassified Documents Always Retain Attachments | Auto-on | Platform |
| Enable Document Tags on All Vaults | Configuration | Platform |
| Carry Forward All Document Versions | Auto-on | Platform |
| Disable Office Online on All Vaults | Auto-on | Platform |
| Vault Objects | ||
| Related Object Section Criteria VQL Filters | Auto-on | Platform |
| User Object Custom Fields Support in Validation Rules | Configuration | Platform |
| Lifecycle & Workflow | ||
| Unified Workflow Access | Configuration | Platform |
| Restrict Document Workflow to a Single Document | Configuration | Platform |
| Copy Workflow | Auto-on | Platform |
| Restrict Workflow Owner from Receiving a Task | Configuration | Platform |
| Prompt for Document Fields in Document Workflow Verdicts | Configuration | Platform |
| Prompt for Object Reference Fields in Document Workflows | Configuration | Platform |
| Support for DAC Lifecycle Role Allowed Group in Document Workflows | Auto-on | Platform |
| Expression Enhancement for Update Document Field in Lifecycle & Workflow | Auto-on | Platform |
| Date & DateTime Fields Support in Document Workflow Decisions | Auto-on | Platform |
| Task Due Date Audit Updates in Document Audit Trail | Auto-on | Platform |
| Reporting & Dashboards | ||
| Dashboard Usability Enhancements | Auto-on | Platform |
| Consistent Report URLs Across Vaults | Auto-on | Platform |
| Standard Reports | N/A | Platform |
| Search | ||
| Expanded Search Enhancements | Auto-on | Platform |
| Auto Claims Linking | ||
| Suggested Links to Permalink Targets | Auto-on | Platform |
| Vault File Manager | ||
| Permission Label Update: Check Out to File Manager | Auto-on | Platform |
| Administration | ||
| Display the Unclassified Document Type in Admin | Auto-on | Platform |
| Admins Can Edit All Document Field Labels | Auto-on | Platform |
| Smart Filter Enablement in Search Settings | Auto-on | Platform |
| Access Control | ||
| Automate Guest Invites to Azure AD from Vault | Configuration | Platform |
| Enforce Use of HTTPS When Configuring SAML & OAuth 2.0 Profiles | Auto-on | Platform |
| Platform Data Model Changes | ||
| Platform Data Model Changes | Auto-on | Platform |
| Clinical Operations | ||
| Quality Issue Assignee Filtering By Object Type | Auto-on | Study Startup, eTMF |
| Commercial | ||
| Material ID | Configuration | PromoMats |
| Modular Content: Rich Text Fields | Auto-on | PromoMats |
| eCTD Compliance Package 2253 Form Update | Configuration | PromoMats |
| Create Fragment Includes Additional CRM Product Fields | Auto-on | MedComms, PromoMats |
| Data Model Change: Multi-Select CRM Product Fields | Configuration | MedComms, PromoMats |
| Commercial Data Model Changes | Auto-on | MedComms, PromoMats |
| Quality | ||
| Station Manager: Capture Document Usage | Auto-on | Station Manager |
| Health Canada Adverse Event Reporting | Auto-on | Surveillance |
| Quality Data Model Changes | Auto-on |
QMS, QualityDocs, Station Manager, Surveillance, Training
|
| Regulatory | ||
| Collapse Action in Content Plan Hierarchy Viewer | Auto-on | RIM Submissions |
| Correspondence in Viewer to Consider Dossier Status | Auto-on | RIM Submissions Archive |
| Submissions Archive: Taiwan 1.0 | Auto-on | RIM Submissions Archive |
| Create Related Records: Application Country Scope | Auto-on | RIM Registrations |
| Create Related Records: Restrict Submission & Regulatory Objective Joins | Auto-on | RIM Registrations |
| IDMP: Registered Packaging to Medicinal Product Registration Mapping | Configuration | RIM Registrations |
| Quality to RIM Vault Connection Data Model Update | Auto-on |
RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive
|
| UDI Source Data Model | Auto-on | RIM Registrations |
| Regulatory Data Model Changes | Auto-on |
RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive
|
| QualityOne | ||
| QualityOne Teams | Configuration | QualityOne |
| Product Hierarchy Data Model for QualityOne | Auto-on | QualityOne |
| Veeva Claims | ||
| Auto-Create Element | Configuration | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|
Known Issues
Vault Quality (DEV-400085)
In Limited Release Quality Vaults, a non-functional page at Admin > Configuration > Quality Record Check appears. Admins attempting to navigate to this page will encounter a server error. The Quality Record Check feature will be enabled for customers in an upcoming release.