Release Date: June 30, 2023
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
Platform Data Model Changes
In addition to the data model changes done for Visual Checklist Designer in 22R1.3, the following changes were also made to ensure that, where applicable, checklist respondents are still allowed or required to attach a document for active checklists that are not completed yet:
-
For [target object] Response (
[target object]_response__sys) objects, if the record is a child record of a checklist that is not in the Completed (completed_state__sys) or Inactive (inactive_state__sys) state:- If Documents Allowed (
documents_allowed__sys) field value is null, copied the values from Attachment Allowed (Document) (doc_attachment_allowed__sys) field to Documents Allowed (documents_allowed__sys) field. - If Documents Required (
documents_required__sys) field value is null, copied the equivalent values from Attachment Required (Document) (doc_attachment_required__sys) field to Documents Required (documents_required__sys) field.
- If Documents Allowed (
-
Note: In Quality and QualityOne Vaults, TA Response (
ta_response__sys) records for Quizzes are excluded from the data copying, as attaching documents in checklists is not officially supported by Quizzes.
Clinical Operations
Clinical EDL Template Overrides
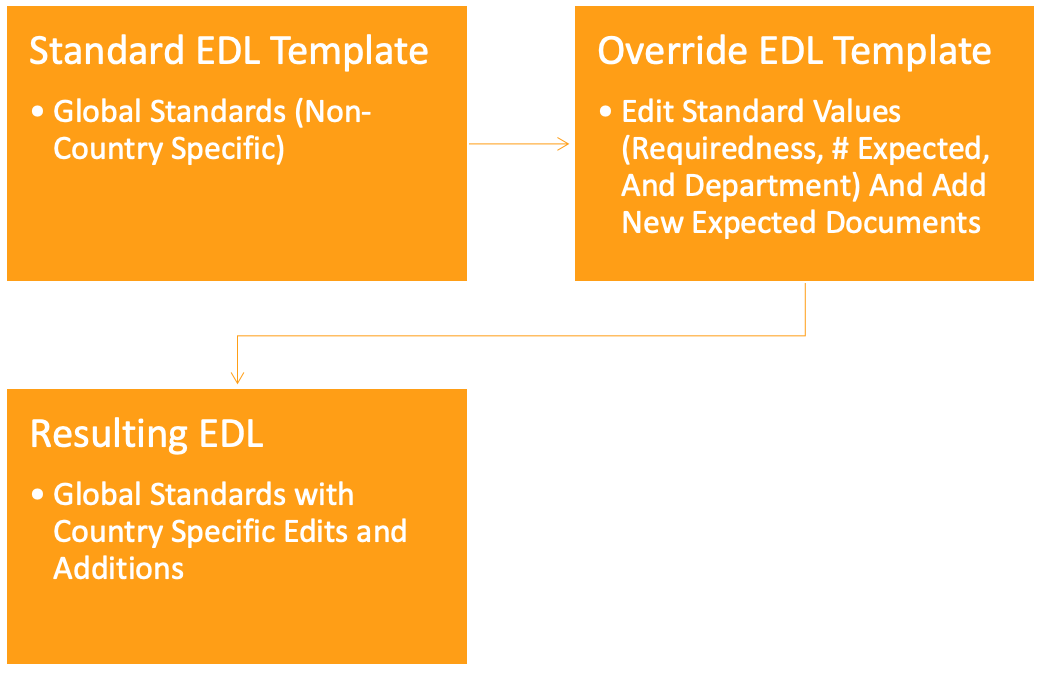
This feature introduces a more streamlined approach to managing country intelligence. It significantly reduces the need for duplicate expected document templates and provides greater flexibility to countries to define milestone types for Study-level expected documents, making it a valuable tool for enhancing global operations and addressing the diverse regional filing practices.
The key innovation is the introduction of Override Expected Document List (EDL) templates. These templates allow for greater customization, addressing the unique challenges faced by multinational customers, especially when country-specific ethics submissions require a select number of Study-level expected documents.
The following diagram describes the way this feature works. The process begins with a standard EDL Template that applies non-country-specific global standards. It then incorporates an Override EDL Template for country-specific modifications and additions, resulting in a final EDL that combines global standards with country-specific elements.
Learn more about EDL Administration.
ePRO Monitoring Exports from MyVeeva
Previously, Users could only access Site ePRO data by either requesting the data directly from the site, which can cause delays, or by having access to the ePRO Module, which can cause security concerns. With this feature, Users can now download Site ePRO data directly from Vault Clinical using a new user action, Generate ePRO Survey Report, that is available on Study Sites and Monitoring Events. This action creates a zip file containing the following:
- Full site survey data
- Full site adherence data
- Aggregated compliance issues report
Notifications alert Users when the ePRO data export is ready for download or if errors were encountered. This feature is enabled through support and is available in Vaults where ePRO is enabled. Studies that have ePRO as a Connected Study Type, have the ePRO code populated, and have sites with Connected Study Agreements will have the user action visible when enabled. Users with all Object Action permissions for Study Site and Monitoring Event can run the Generate ePRO Survey Report action, but this configuration can be updated as needed.
This feature saves time by eliminating the need to request ePRO data from sites and provides more meaningful information on survey completion and compliance.
Clinical Operations Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Clinical Operations data model to support new features:
The following changes were made to Clinical Operations Vaults to support the Clinical EDL Template Overrides feature:
- Added the Override Template (
override_template__v) field to the EDL Template (edl_template__v) object
Commercial
Modular Content Document Info Panel: Manual Annotation Creation
With this release, users are able to create a link annotation to a Text Asset (Claim/Reusable Text) and/or document directly from the Modular Content Document Info Panel. Content Module Assets not identified with Enhanced Suggest Links now have an ‘Add Link’ action next to them that brings the user directly into the add link annotation mode. This simplifies the process of linking Content Module Assets to areas within the promotional material that were not automatically linked, and is particularly useful when linking Content Module Assets to images on promotional material.
PPTX Video Support for CLM Auto-Publishing
CLM Auto-Publishing allows customers to manage the distribution, creation, versioning, and withdrawal of multichannel content directly from an original MLR document. Previously, when a PowerPoint slide containing a video was pushed to CLM via auto-publishing, Vault displayed a single slide showing the first frame of a video and could not play the video in CLM. As a result, Brand teams had to use the manual Create Presentation feature instead, which required the creation of a multichannel binder and slides which added to the review and approval timeline.
This new enhancement allows Brand teams to include embedded videos in presentations delivered to CLM via the Auto-Publish feature. Reps can now play videos in CLM on auto-published presentations. This significantly streamlines the review process for getting content to CLM and reduces the duplication of content as the Create Presentation feature is no longer needed for this use case.
While PPTX files generate a playable video, Vault does not support legacy PPT files and continues to generate the first frame PNG slide of the video.
23R2 Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
The following changes were made to PromoMats Vaults:
-
Removed the CRM Message Template Translation (
crm_message_template_translation__v) object - Updated the CRM Message Template (
crm_message_template__v) object as follows:- Renamed the Global Content Template (
global_content_template__v) object type to Universal Content Template (universal_content_template__v) - Made Universal Content Template the default object type
- Removed the Product Content Template (
product_content_template__v) object type - Added the Country (
country__v), Language (language__v), and Text (text__v) object fields - Added the Country (
country__v), Language (language__v), and Text (text__v) fields to the Manual Assign Content Template (manual_assign_content_template__v) object type
- Renamed the Global Content Template (
-
Removed the CRM Message Template CRM Product (
crm_message_template_crm_product__v) object -
Removed the CRM Message Template CRM Product Group (
crm_message_template_crm_product_group__v) object - Removed the CRM Message Template Translations Lifecycle (
crm_message_template_translations__v) and added the CRM Message Template Lifecycle (crm_message_template_lifecycle__v)
Medical
MedComms
Standard Responses for Medical Inquiry
This feature introduces a standard, object-based approach to support Standard Responses. Standard Responses are predefined answers to Medical Inquiries commonly asked by HCPs and Patients. They are typically the response to an FAQ.
When composing a Case Response, users can select an approved Standard Response. The list of standard responses can be filtered to show answers to an identified FAQ.
After users select a Standard Response, Vault can populate the Case Response with the response notes, a response package, and a drafted email. Users can choose to update the response package or edit the email, or simply click to email the response.
A Standard Response typically consists of:
- Standard Response Notes
- Guidelines for Use
- The Product, Country, and Language of the response
- A package of Fulfillment Documents
- A Case Response Email Template
- The FAQ to which it is an answer
This feature helps standardize responses and lessens the time required to create a response package, allowing Medical Inquiry users to manage more responses.
PPTX Video support for CLM Auto-Publishing
CLM Auto-Publishing allows customers to manage the distribution, creation, versioning, and withdrawal of multichannel content directly from an original MLR document. Previously, when a PowerPoint slide containing a video was pushed to CLM via auto-publishing, Vault displayed a single slide showing the first frame of a video and could not play the video in CLM. As a result, Brand teams had to use the manual Create Presentation feature instead, which required the creation of a multichannel binder and slides which added to the review and approval timeline.
This new enhancement allows Brand teams to include embedded videos in presentations delivered to CLM via the Auto-Publish feature. Reps can now play videos in CLM on auto-published presentations. This significantly streamlines the review process for getting content to CLM and reduces the duplication of content as the Create Presentation feature is no longer needed for this use case.
While PPTX files generate a playable video, Vault does not support legacy PPT files and continues to generate the first frame PNG slide of the video.
23R2 Medical Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Medical data model to support new features:
The following changes were made to Medical Vaults:
-
Removed the CRM Message Template Translation (
crm_message_template_translation__v) object -
Updated the CRM Message Template (
crm_message_template__v) object as follows:- Renamed the Global Content Template (
global_content_template__v) object type to Universal Content Template (universal_content_template__v) - Made Universal Content Template the default object type
- Removed the Product Content Template (
product_content_template__v) object type - Added the Country (
country__v), Language (language__v), and Text (text__v) object fields - Added the Country (
country__v), Language (language__v), and Text (text__v) fields to the Manual Assign Content Template (manual_assign_content_template__v) object type
- Renamed the Global Content Template (
-
Removed the CRM Message Template CRM Product (
crm_message_template_crm_product__v) object -
Removed the CRM Message Template CRM Product Group (
crm_message_template_crm_product_group__v) object -
Removed the CRM Message Template Translations Lifecycle (
crm_message_template_translations__v) and added the CRM Message Template Lifecycle (crm_message_template_lifecycle__v)
Quality
QMS
Standalone Quality Events Data Model & Feature Parity
With this release, the Vault QMS data model provides standalone data models for Change Controls, Deviations, Findings, Lab Investigations, and Continuous Improvements. These objects were previously managed as object types on the Quality Event object. Due to this broad utilization of the Quality Event object, some customers were running into limit issues which impacted performance and feature expansion. The following features will be supported for the new standalone objects:
- Recurrence Checks
- Relationship Automation
- Generate Document Actions
- External Collaboration
- Effectiveness Checks
- Cycle Time Metrics
- External Notifications
Once this feature is available, all new implementations will utilize the new standalone data model. There is no impact or action needed for customers using the current Quality Event data model.
Create Change Actions from Template Records (Standalone Change Control and Change Plan)
The Create Change Actions from Template Records feature was introduced in the 23R1.3 release with support for the Quality Event object. With 23R1.4, this functionality now extends to the standalone Change Control and Change Plan objects.
LIMS
Test Execution & Exception Summary Enhancements
In the Test Execution and Exception Summary interfaces, there is now better contrast between the Out of Specification and Previously Out of Specification icons, an improved user flow for making Result changes, clearer error text for invalid Test Input entries, and users can now see the outcome of calculations and evaluations for the final Test Step before proceeding.
LIMS Result Exceptions Enhancements
Reopened tests show as exceptions in the Exception Summary widget, and we addressed a rare OOS scenario that could mean the user was not aware of OOS until after test completion.
LIMS Static Data Enhancements
Check Syntax formula errors are no longer included in the formula text itself.
LIMS Automation Enhancements
We have improved the notifications for initiating Batches and Samples, notifying you only in the application and not via email, and have included better support for manually progressing Lab Tests.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Quality data model to support new features:
Safety
Safety features are targeted for tentative availability on July 6, 2023.
Safety
Simplified Case Access Group Security Setup Configuration
In 23R1, Vault Safety introduced Case Access Group Security to provide a more straightforward way to manage access to Cases. The feature matches groups to Cases based on data such as Sponsor, Report Type, Country, and Market Segment. It also provides more granular control over unblinded and protected information.
With this release, setting up Case Access Groups is made even simpler. Optimized matching logic and the addition of a Country field on User Access Group Assignment records make setup more flexible, reducing the administrative burden of creating many Case Access Group Assignment records.
Learn More
- Enablement: Enable Case Access Group Security: 23R2 Update
- Admin Help: Manage Case Access Group Security
PMDA Local Case Processing Automations Auto-on
To streamline reporting and reduce manual effort, Case Product Registrations and Case Assessments are now automatically populated for Domestic and Localized Cases that are reportable to Japan.
PMDA Automated Reportable Product Registration Retrieval
This supports the auto-population of reportable Case Product Registrations, expediting data entry for the J2 data elements of PMDA E2B(R3) reports.
PMDA Local Assessments and Expectedness
Auto-populated Case Product Registrations enable local processors to assess Expectedness more efficiently. Comprehensive Product and Assessment data supports more informed decision-making for both Domestic and Localized Cases.
Learn More
- Enablement: Enable PMDA Local Case Processing Automations
- Admin Help: Set Up the Localized Business Admin Library: Local Assessments and Expectedness
- User Help:
Upgrade/Downgrade Transmission State Evaluation Support
Vault Safety has the option to ignore the Transmission Lifecycle State when considering if a Case qualifies as an Upgrade or Downgrade report to a specific destination. Previously, the Upgrade and Downgrade parameters did not consider if the previous Case version was submitted. As a result, the Downgrade rule might be executed even though the initial Case was not reportable.
Learn More
- User Help:
Gateway Connection IP Management Support
Along with enhanced troubleshooting capabilities, Vault Safety Administrators can now create and delete entries in the IP Allowed list for AS2 Gateway Connections.
Learn More
- Admin Help: About AS2 Gateway Connections
- User Help: Configure Custom AS2 Gateways
SafetyDocs
PSMF Automated Vault Report Annexes Configuration
Vault SafetyDocs now leverages Vault Reports to automatically generate PSMF Documents. These reports can contain data such as information about marketed products, studies, and delegated tasks. Previously, every time changes were applied to a specific document, users had to manually run the relevant report, download it, upload it as a document to the relevant binder, and then approve it. This feature allows documents to be updated during PSMF generation or periodic review, making the process more efficient. Additionally, this automated process reduces the risk of errors and ensures compliance with regulatory requirements.
Learn More
- Enablement: Enable Automatic Generation of PSMF Documents from Vault Reports
- User Help: Generate PSMF Documents from Vault Reports
Signal Management Configuration
Signal Management is now supported in Vault SafetyDocs. This new area introduces capabilities for conducting Signal Investigations, tracking emerging signals (as Product-Event combinations), and managing the overall lifecycle based on GVP Module IX.
Once configured, users are able to:
- Track Product-Event combinations and set dispositions
- Perform Safety Investigations using lifecycle states and workflows based on GVP Module IX
- Link one or more Product-Events to a Safety Investigation
- Set final verdicts and dispositions on a Safety Investigation
- Link documents to a Product-Event combination or Safety Investigation
Using this feature will ensure compliance to Safety standards and increase user productivity.
Learn More
- Enablement: Enable Signal Management
- User Help: Signal Management
QualityOne
QMS
Supporting Multi-Batch Identification for COA
This feature provides Vault with additional capabilities to determine the number of batches in a single COA file during ingestion and analysis. Based on the number of identified batches, Vault will create one (1) COA Inspection record per batch. Identifying multi-batch COA files allows customers to ingest a wider range of COA formats.
Learn more about analyzing multi-batch COA files.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Training
QualityOne Training Connector: eLearning Data Model Provisioning
This feature extends the existing QualityOne Training Connector with new fields, and an integration and integration point to support E-Learning content synchronization between QualityOne and Training Vault. New fields in the Training Vault capture details from the E-Learning Source Document, while the new integration and integration point aids in the transferring of E-Learning content between the two (2) Vaults.
Learn more about the QualityOne to Training Connection.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| Platform | ||
| Platform Data Model Changes | Auto-on | Platform |
| Clinical Operations | ||
| Clinical EDL Template Overrides | Auto-on | Study Startup, eTMF |
| ePRO Monitoring Exports from MyVeeva | Auto-on | CTMS, ePRO, eTMF |
| Commercial | ||
| Modular Content Document Info Panel: Manual Annotation Creation | Auto-on | PromoMats |
| PPTX Video Support for CLM Auto-Publishing | Auto-on | Multichannel |
| Medical | ||
| Standard Responses for Medical Inquiry | Configuration | MedInquiry |
| PPTX Video support for CLM Auto-Publishing | Auto-on | Multichannel |
| Quality | ||
| Standalone Quality Events Data Model & Feature Parity | Auto-on | QMS |
| Create Change Actions from Template Records (Standalone Change Control and Change Plan) | Configuration | QMS |
| QualityOne | ||
| QualityOne Training Connector: eLearning Data Model Provisioning | Auto-on | QualityOne<>Training |
| Supporting Multi-Batch Identification for COA | Configuration | QualityOne QMS |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|