Release Date: February 11, 2022
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Working with Documents
Caption Bookmark Exclusions
This feature builds on Vault’s ability to generate bookmarks in a PDF Viewable Rendition based on captions in Microsoft Word documents. With this release, Admins can exclude specific captions when generating bookmarks and can control generation of “Lists of” bookmark sections based on captions, which previously required enablement from Veeva Support.
Annotate Usability Improvements
With this release, we have made the following enhancements to improve the user’s ability to interact with documents and annotations:
- Annotation filters: Filter by Author includes reply authors, and the Links section differentiates document links from permalinks.
- Permalinks have the distinct document field “Annotations (Permalinks),” which is available in Library view, Reporting, and other locations.
- Rotate Page is replaced by Rotate Page and Rotate All Pages, allowing users to temporarily rotate all pages during a viewing session.
- Zoom, Fit, and Full Screen are applied with buttons instead of menus.
- Toolbar buttons that previously dismissed unsaved annotations are visibly disabled while composing annotations.
- Users can now scroll, zoom, apply fullscreen, fit width, and height while composing annotations.
- Admins can opt into a new “strict” permission requirement for Copy Text functionality.
Text Indexing & Combination Enhancements
This enhancement improves Vault’s ability to correctly index, find, and highlight text in documents that contain specific kinds of punctuation that serve as word separating characters, including periods, commas, semicolons, and dashes. This enhancement also improves its ability to handle formatting variations of single and double quotation marks. Users see better results when performing certain actions on documents, including Find in Document, Create annotation, and Suggest Links (PromoMats).
Collaborative Authoring Troubleshooting Page
This feature introduces a logs page that allows users to view all Microsoft Graph API errors that occur when using Collaborative Authoring with Microsoft Office. This provides users with a self-service method for troubleshooting issues with Collaborative Authoring. Learn more about the Collaborative Authoring Error Log.
Google Drive Integration: Create New Document
This enhancement allows users to browse for and select files from their Google Drive when creating a new document in Vault.
Enhanced Bulk Document Update: Validation Behavior Changes
This feature introduces several changes to bulk document update validation:
- For any UI errors which did not previously have a defined error message, Vault now displays a generic error message.
- Vault now displays an error if a user attempts to update a read-only field on a document.
Legacy Feature Enablement Updates
With this feature:
- The Enable checkout to Office Online feature is now disabled on all Vaults.
- The Searchable document type picker and Sort multi-select field values in alphabetical order features are now enabled on all Vaults.
EDLs: Configurable States for Document Version Locking
Customers can configure which document lifecycle states support document version locking for EDL matched items, allowing customers to lock document versions in states other than Steady or Superseded. The EDL Match Job will take a little longer to complete the first time it runs after this update due to the data model updates. The completion time for subsequent runs of the EDL Match Job will return to their normal run time.
Vault Objects
High Volume Object Multi-Value Picklist Field
This feature provides support for the multi-value picklist field in high volume objects. You can create up to two (2) multi-value picklist fields per high volume object.
Lifecycle & Workflow
Consistent Steps Across Workflows
This feature makes workflow steps consistent across Workflows on Documents and Objects. The Action step now combines the former object workflow State Change, Update Record Field, and Update Related Record Field steps, and allows users to perform them conditionally. Additionally, state changes performed within an Action step no longer check entry criteria or fire entry actions when the target state is the same as the existing state. Learn more about configuring workflows on documents and objects.
Populate Object Reference Fields with Workflows & Lifecycles
This feature provides the ability to update object reference fields in lifecycle or workflow actions using expressions. Users may return specific records using the new RecordByLabel() function.
Enhanced Stages Chevron Card for Workflows
In previous releases, users had to navigate to the timeline view to see actions on open tasks. With this release, lifecycle stage chevron cards now display actions on open tasks as well as additional workflow information, saving users clicks and providing easier access to this info.
Show More on Tasks in Home Pages
This feature makes it easier for users to manage their tasks by providing additional task and workflow information on the homepage. It also enables Admins to configure whether or not users may complete a task directly from the homepage.
Remove Default Task Link in Label
This feature allows Admins to turn off the default link provided with the task label.
Bulk Actions on Record Actions
Applications and customers may now expose SDK Record Actions within Vault’s bulk actions. Admins will now see a Record Actions option within the bulk action interface for objects. Learn more about bulk object record actions.
Reporting & Dashboards
In Operator for Name Report Filters
With this release, users can view and select the names of the object or document records and add them as filter values in a report. This feature also adds the conditional operator “in” to the report filters for the Name fields. Users can then view record values before adding them to the filter, and can select up to 100 values in a single filter. This user experience is similar to the object reference field selector and is supported for both standard and HVO objects.
Optional Prompts
With this release, report creators can make prompts optional, allowing users to run a report without adding a value to the report prompt. Users may view all the records or a subset of the records from the same report. Reports are more flexible, allowing one report to solve the use cases of two reports previously. Users can also filter on a null or blank value.
Additional Document Field Support in Report
With this release, Vault supports the Checked Out By, Checked Out Date, Checked Out, and Description fields on Document reports.
Usability & UI Updates
Breadcrumb Enhancements for Object Tabs
As users view data in an Object tab, each click to view details of a record or drilldown to view a related record or document is tracked by a breadcrumb. Users can mouseover the breadcrumb to display a hovercard that identifies the previously viewed record before navigating back to that page. Previously bookmarked Object Record Detail pages no longer display the breadcrumb generated when the page was originally bookmarked. Such breadcrumbs are stale and prone to errors as data changes over time.
Notifications: View Full Content in Panel
Users can now expand the content of longer notifications directly within the notification panel to view up to 25 lines of text without opening the notification page. This allows users to view longer notifications without having to navigate away from their current task. For notifications longer than 25 lines of text, the notification includes a hyperlink to navigate directly to the full text on the notification page.
Notifications: Email Burst Threshold
With this feature, Admins can set a burst threshold for the number of emails Vault sends for the same notification template in a 30-minute period. Once the burst threshold is reached, Vault throttles the sending of additional email notifications for that template. The burst threshold is a guardrail for customers whose email provider or infrastructure cannot handle a large influx of emails. Learn more about email administration.
Email to Vault: Create Records for Bounced Emails
With this feature, when Vault bounces an inbound email because it is considered spam or because the sender fails SPF or DKIM authenticity checks, it now creates an Email object record in the Bounced lifecycle state. This provides Admins with the ability to report on bounced emails, inspect these emails, and have Vault process them if deemed legitimate.
Updated UI Style on Users & Groups Pages
In this release, we have updated the Security Profiles, Permission Sets, and Groups list views (Admin > Users & Groups) with our new UI style (Create button, filter, and search UI element placement). There is no functionality change. This feature was added in 21R3.2.
Workflow Action Menu for Documents: Drop-Down Behavior Update
With this release, clicking on a chevron button with only one workflow action available displays a drop-down instead of initiating that workflow action. Users can then click on the action label in the drop-down to initiate it, or click elsewhere to cancel. This enhancement helps prevent users from accidentally triggering workflow actions.
Rich Text/Long Text Pop-up Window Size Update
This change makes the Rich Text and Long Text field pop-up window size bigger when viewing a record so that it is the same size as when editing the record. This change provides a consistent experience between viewing and editing Rich Text and Long Text fields.
Auditing
Audit Trail Enhancements to Track Lookup Field Changes in Object Records
This enhancement allows the Audit Trail to log an entry on object records with lookup fields whenever the values change due to source field changes, reference object changes, and changes caused by lookup field initializations.
Administration
Scheduled Data Exports: Initial Full Data Export
This feature provides users with the ability to export all of their Vault object data and document metadata records in an initial load of Vault data into their data lakes and data warehouses.
Scheduled Data Exports: Escaping characters in CSV Output
Currently, when Vault exports picklist fields with Scheduled Data Exports, commas contained within picklist items are escaped by an extra comma. For example, ["value1a,,value1b"]. With this release, commas contained within picklist values are escaped by two pairs of double quotes. For example, ["""value1a, value1b"", value2"] for multi-value and ["value1a, value1b"] for single value.
Additionally, empty values for multi-value object reference fields no longer contain commas in the output, and the exported field value is empty.
Finally, field values with backslashes no longer include an additional backslash.
Scheduled Data Exports: Including Inactive Document Fields by Default
With this release, Vault automatically includes inactive document fields in exports when users select the Document entity in a scheduled data export. Prior to this release, Vault only exported active document fields in Document exports.
Scheduled Data Exports: Increased precision on field values
With this release, currency fields exported in Vault Objects now have an extra decimal place. For example, 1.0 now exports as 1.00. Additionally, DateTime fields on Document and Document Version exports now include seconds in the timestamp.
Scheduled Data Exports: Uploading CSV Files to S3 with Bucket Owner Full Control ACL
With this release, customers using a self-managed AWS S3 bucket as a storage option for Scheduled Data Exports can be the S3 object owner for every export file uploaded to the bucket. Additional AWS S3 configuration may be required. This feature is Auto-on in Vaults configured for Scheduled Data Exports.
Object and Document Field Types for Integration Field Rules
This feature allows Vault Admins to configure the field type for an object or document Query field when creating field rules within Spark integration rules.
Configuration Management
Configuration Management: Allow Outbound & Inbound Packages enabled by default on Align Vaults
Align Vaults will now have Allow Outbound Packages and Allow Inbound Packages enabled by default. These settings can be found under Admin > General Settings > Configuration Management.
Data VPK: Validate VQL in Dataset Filter
This feature allows users to validate Criteria VQL prior to saving a dataset filter.
Access Control
Object Control Security
Object Controls are interface controls delivered by Vault Applications, allowing Admins to put them in the Object Record Detail Page layout to provide a tailored experience. For instance, in Vault Safety, users see object controls when viewing or editing a case object record.
This feature allows Admins to configure which object controls Vault displays at runtime. Admins can configure security profiles and permission sets to control the visibility of these controls at the object level (View permission). They can also use Atomic Security for fine-grained control of visibility on the object record details page, based on the object record state and role assigned to users.
Search
Expanded Search with Related Records
You can now configure Search Collections to include relationships between objects. This allows expanded searches to return results in one object section because of their relationship to records matching the search criteria in another object section. For example, if there is a relationship between Product and Country to manage the list of countries in which each product is marketed, you could set up a Collection to return all of the country records related to the search results from Products. As you filter down Product results, the Country results update as well.
Field Centric Strict Matching
To facilitate multi-term synonym searches, we’ve changed Vault searches with more than one term to allow a search like “myocardial infarction” to appear as a phrase in the thesaurus and match to other phrases like “heart attack.” This update also makes the Strict matching option more field-centric, meaning that the minimum required matching terms must be in the same field rather than split between multiple fields. Strict matching is optional, so Admins can enable or disable it at any time under Admin > Settings > Search Settings.
Checklists
Checklists: Add Reference Documents to Questions
Checklist designers can link one or more Vault documents to their questions as reference material for checklist respondents to review when providing answers in the checklist.
Checklists: Dependent Sections
Multiple-choice question responses can now control the appearance of one or more sections in a checklist.
Checklists: Hide Unseen Questions on Review Page
The checklist review page no longer displays dependent questions that the respondent did not see when completing the checklist.
Auto Claims Linking
Auto Claims Linking: View Claim Details from Select Claims Dialog
With this release, when manually linking to Claims, users can click to view the Claim record in detail from the Select Claim dialog. This enables a more informed selection, and replicates the existing experience when linking to documents and anchors.
Auto Claims Linking: Ignore Superscript & Subscript Characters When Matching to Claims
This feature allows Vault to ignore superscript and subscript characters at the end of words in documents when determining matches to approved Claim records. This eliminates the need for a majority of wildcards in match text values, which were previously necessary to account for variable footnote and endnote references in content.
Vault Formulas
Workdays & Holidays
This feature allows users to perform calculations in expressions that factor out weekends and holidays, making timeliness metrics and due dates more accurate. The new function NetWorkdays() returns the difference between two dates excluding weekends and optionally holidays. Workday() returns a date that is N days in the future excluding weekends and optionally holidays. Admins can define Holiday Schedules and their children Holidays, and can select a Holiday Schedule record for each user.
RecordByLabel() Function in Expressions
This feature allows users to return object references based on object record labels in expressions. The RecordByLabel() function accepts object labels and returns object references which can then be used to populate fields. This feature is only available in workflow and lifecycle actions.
Significant Figures in Expressions
This feature modifies the Round function to accept an optional parameter that can either be “significant” or “significant-astm”. This enables users to round their numbers down to the appropriate number of significant figures based on two common methodologies.
Vault File Manager
VFM Download Rendition Usability Enhancements
This feature provides usability enhancements when downloading rendition files using Vault File Manager, including: providing additional error messages when a file fails to download; including Vault Overlays on files downloaded with VFM; and displaying a dialog to users with VFM permissions suggesting they download using VFM. Vault provides this suggestion when applicable users attempt to download a rendition file larger than 4GB in their browser from the Library Action Bar, Document Info Action bar, and Document All Actions menu.
Pause & Resume Uploads & Downloads with Vault File Manager
This feature allows users to pause and resume in progress uploads and downloads in the Vault File Manager application. Users can also sort files on the Uploading and Downloading tabs by the order in which they will be uploaded and downloaded.
Vault Loader
Vault Loader: Return Updated Roles in Success Log
With this release, when updating document roles using Vault Loader, the success log now contains the updated role IDs. *Auto-on with Vault Loader enabled
Vault Loader: Log Skipped Lines in Failure Log
With this release, Vault Loader failure logs now include any input CSV lines that were skipped due to errors. *Auto-on with Vault Loader enabled
Vault Loader: Disabling Workflow System Objects from Load or Extract Requests
With this release, the envelope__sys and envelope_content__sys system objects are no longer available for load or extract using Vault Loader. These objects drive the behavior of Vault workflows, and excluding them from Vault Loader ensures data integrity within these objects. *Auto-on with Vault Loader enabled
Vault Java SDK
Enhancements to Reference Lookups
With this feature, we’ve introduced a new Reference Lookup Type, Generic, which enables Administrators to configure lookups for mismatched data types. Additionally, the new Generic option provides support for any-to-any mapping for all single-value fields which were previously unsupported, such as Boolean. Multi-value fields such as multi-value picklists are not supported.
Object and Document Field Types for Integration Field Rules
This feature allows Vault Admins to configure the field type for an Object or Document Query Field in Field Rules. Additionally, developers can access this value in Vault Java SDK and use the value to create call-back VQL queries with the necessary VQL functions such as LONGTEXT(), RICHTEXT, and TONAME().
SDK Runtime Logs
This feature provides Vault Admins and developers runtime logging for SDK requests. Daily logs are available via the Vault UI and Vault REST API, and log entries include SDK exceptions and custom code usages of LogService. Vault Admins can configure the current log level as DISABLED, EXCEPTIONS (default), ERROR, WARN, and INFO. SDK Runtime Log entries are available 15 minutes after the request has completed and can be accessed for 30 days. Logging is limited to 10KB for Exceptions and 40KB for LogService entries.
Vault Tokens
This feature introduces a new component type, Vault token, and adds the new Vault Tokens permission type. Admins with the Vault Tokens: Create permission can configure up to ten (10) Vault-wide tokens using MDL. Learn more in the Vault Developer Portal.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Platform data model to support new features:
Vault Connections
PromoMats/RIM: Conditional Transfer Support Using Query Object Rules (QORs)
This feature supports Query Object Rules for RIM Vaults using the PromoMats/RIM Connection, adding the ability to filter out the documents and records transferred in an Integration Rule. Query Object Rules are already supported in PromoMats Vaults.
Clinical Operations
Milestone Roll Up Enhancements
This feature delivers improvements to Milestone Rollup behavior. When the Milestone Rollup Enhancements setting is enabled and Rollup (max) and/or Rollup (min) Milestone Dependencies are configured, Vault will reassess rolled-up dates when a previous Milestone’s date is set to null, updated, or a related Milestone Dependency is inactivated. This feature supports “First” and “Last” Site and Subject-related Milestones tracked in Vault Clinical.
Support to Identify Non-enrolling Sites
This feature identifies Sites that did not enroll any Subjects when the Enable Automated Enrollment Milestones setting is enabled, and the “No New Subjects” field is checked for a Site, allowing users to quickly inactivate Subject-related Milestones for the non-enrolling Sites with additional configuration.
Non-User Viewing of Documents in Surveys
This feature extends the Clinical capabilities on Checklists to allow non-users to view a Vault document in the context of completing a Checklist in Vault.
Restrict Doc Study to Single Value
This feature introduces a Vault-wide setting that prevents non-system-managed users from populating more than one Study on a document. Documents cannot be limited to a single Study if you have configured Vault for streamlined document reuse.
Automate Milestone State Changes: Additional Date Field Triggers
This feature introduces additional triggers that fire following the update of a milestone’s planned start, planned finish, and actual start dates; and which updates other milestone date fields, metrics, or state without relying on lifecycle actions.
CTMS/CDMS: Single Study Refresh
This feature adds support for re-syncing CTMS data under a single study. This feature replaces the “full refresh” functionality that was available via the “refresh inbound” action.
Site Document Exchange Section
This feature introduces a new section to the Study Site page layout that increases visibility into documents sent and received from Sites, condensing the five existing sections currently used to view this information into a single section.
Monitoring Schedule Planning
This feature allows for the triggered and ongoing creation of Monitoring Events in Vault CTMS based on updates to milestones in a CTMS Vault. This functionality automates the creation of future Monitoring Events and allows users to assess the timeliness of created monitoring visits. Vault can create monitoring Schedule Templates at a Study- or Study Country- level to define the expected schedule.
Site Payment Holdbacks
This feature extends support for Payments to allow for holdback and overhead tracking in the delivered application. Fees defined as holdback fees generate two payable items to track accrued costs and make the initial payment to the Site. A new system action allows updating holdback items in bulk at the Site-, Study Country-, or Study- level if the matching payment criteria are still valid.
Additional Site Payment Rules
This feature allows Vault Payments customers to add additional Payment Rules to their Fees. You can add Subject Status as a required criterion for a Visit or Procedure fee, and use Source Data Verification status as a criterion for a Visit fee.
Vault Payments Overhead
Vault Payments can now define whether Fee and Fee Templates should expect the addition of Overhead. If added, Vault Payments will calculate the Total Amount field value with the matching Overhead Percentage.
Transfer CSV Reports for TMF Transfer
This feature provides greater visibility into TMF Transfer activity on both the sending and receiving Vaults. Vault provides two CSV reports to each Agreement Transfer record to specify the Names and Global IDs of each transferred item:
- Compare CSV compares the items that have been transferred from Source to Target over the life of the Agreement.
- Transferred Items CSV shows the specific items transmitted by TMF Transfer as part of a specific transfer action.
TMF Bot : Auto-classification Customer Feedback (22R1)
This feature incorporates several changes derived from feedback received from customers, including changing auto-on models to .95 Prediction Confidence, and excluding Documents with null pages when training the auto-classification model.
Site Connect: Additional Vault Clinical Docs Support
This feature adds support to transfer the following new document artifacts via Veeva Site Connect.
- IP Storage Condition Excursion Documentation
- Additional Monitoring Activity
- Trial Initiation Monitoring Report
Site Information on Documents
Site Connect customers can now see additional details regarding documents sent and received over Site Connect. A new Site Information section is available on documents to display the below items directly within the document metadata.
- Sent to Site(s)
- Initial Sent to Site(s) Date
- Last Sent to Site(s) Date
- Received from Site Date
Cancel Site Connect Agreement Invitation
This feature allows Site Connect customers to cancel Pending Site Connect Study Invitations, deactivating the invitation and canceling the workflow task in SiteVault.
Mark Site Connect document as Rescinded
With this feature, Site Connect customers can now take a Mark Rescinded user action on documents sent over Site Connect. Taking this action will update the Marked Rescinded by Sponsor/CRO field to Yes on the corresponding document in SiteVault.
Site Connect Details User Action
Site Connect customers can now access more details regarding a document’s distribution to Sites via a Site Connect Details user action. This action launches a dialog to display tracking information and the document’s comment history exchanged with a site.
Clinical Operations Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Clinical Operations data model to support new features:
Commercial
Document Notifications: Support for “Based On” Relationship
This feature provides the ability to notify the Document Owner of a document that is related to a document via the “Based On” Relationship. Admins can configure the “Send Notification to Based On Content” action on a state entry action or user action. The action sends a notification to the owners of documents created from a source document via the Make A Copy action. Learn more about parent document notifications.
Modular Content Approval Document Enhancements
This feature expands the functionality and generation of the Content Module Approval Documents to support CJK languages, increase rich text formatting options, and support text background colors.
External Viewer: Number Of Views
This feature allows users to report on the number of views through an external viewer per document. Users can also report on which external viewer was used, such as which CRM Org or which Public Content Distribution channel, including SharePoint or Website. This assists marketing managers in understanding the effectiveness of content viewed through the external viewer. Learn more about the external viewer.
Medical Inquiry UI: Admin Page
The dedicated Medical Inquiry user interface was added to MedComms in 21R3. This feature updates the user interface configuration screen to provide a better user experience for Admins. The screen is used for both the initial setup of the user interface and ongoing maintenance. Admins can define the objects, fields, and user interface layout that medical inquiry users see.
Medical Inquiry UI: Case Contact Section
This feature adds a section to the Medical Inquiry user interface containing information on the Case Contact. If the contact already exists, Medical Inquiry users can modify the Case Contact record during the interaction. If the contact is new, users can capture and save their details as a new Case Contact record. This streamlines the process of capturing Case Contact details and collects all Case and Case Contact information in one place, rather than across multiple screens.
Allow Case Response Emails to be Deleted
Medical Inquiry allows users to respond to requests for medical information via email directly from MedComms. Vault stores this email content in the system as a referenceable, auditable record of what was sent to the contact. This feature allows users with the required permissions to permanently delete these records from the system.
CRM Data Sharing: Support Merged CRM Accounts
Today, duplicate accounts in Veeva CRM can be merged together. This feature adds support to the standard integration between MedComms and Veeva CRM for sharing Medical Inquiry data to reflect this in MedComms, maintaining consistency across the two products.
Automated Image Rendition: Include Source Document Name
For Automated Image Rendition types, Admins now have the option to concatenate the document name with the rendition type. Vault applies the prepended name when creating the rendition, and the name is visible on downloads.
eCTD Binder Generation Failure Notifications
With this release, Vault provides more detailed notifications when eCTD binder generation failures occur due to insufficient permissions or other configuration related causes.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
Quality
Send Email to Non-Vault Users
Organizations often need to send emails to internal and external recipients for various processes in QMS. With this release, customers can define distribution groups, notification templates, send emails and share documents for the following processes: Audits, Complaints, and Issue Escalation. This new functionality addresses several scenarios that organizations may encounter:
- When an Audit report is completed, that report often needs to be sent to an external recipient, such as the supplier contact, as well as persons associated with the Audit or supplier.
- In Complaint processes, customers often need to send correspondence letters and forms to the complainant or the reporter associated with the Complaint at various times during the Complaint lifecycle.
- If a serious issue is encountered, such as a critical deviation, upper management external to Vault may need to be notified of the serious event.
In addition, when using this feature with Complaint records, the sender of the email will be notified if the email was not successfully delivered to the recipient’s email address. Learn more about configuring external notifications.
APQR/QMR
With this release, Vault QMS now supports management of APQR and QMR processes.
The APQR process is an annual evaluation of the quality standard of a drug product to determine the need for adjustments in drug product specifications, manufacturing, and control procedures. It is a collaborative effort to generate the final APQR report, which is a compilation of data from multiple data sources, the summarized results, and the recommendations from distinct SMEs.
The QMR process is very similar to the APQR process but does not require the generation of a final summary report, but does require the capturing of meeting notes, meeting attendees, and post-meeting action items.
Both of these processes share common challenges which include but are not limited to:
- Data extraction and identification
- Scheduling, reminder, and progress updates
- Data and records which come from disparate systems
- Involvement of many different inputs from various stakeholders
With the APQR/QMR feature, customers will now be able to:
- Manage both the APQR and QMR process within QMS
- Create APQR and QMR templates for known documents that need to be collected
- Once those templates have been defined, APQR or QMR records can be created from the templates and provide a consistent starting point based on various scopes, such as Country, Product, or Facility
- This feature is designed to work in conjunction with the Generate Document from Report feature, which allows reports to be defined to extract key data that already resides in QMS
- Once all documents have been collected, an APQR Binder can be created from the APQR record and the Generate Merged PDF document action can then be leveraged to generate the final APQR report.
Generate Document from Report: Support for APQR Item & QMR Item Lifecycles
A large portion of the data that needs to be collected for APQR final reports and QMR presentations already resides in QMS. In previous releases, the process of extracting this data was highly manual and time-consuming.
With this release, the Generate Document from Report action allows users to run a report, export that report to a predefined export format, create a document, and then associate it to the APQR Item or QMR Item record with a single user action. This greatly reduces the number of user clicks and leverages the power of Vault Reporting to easily extract this data.
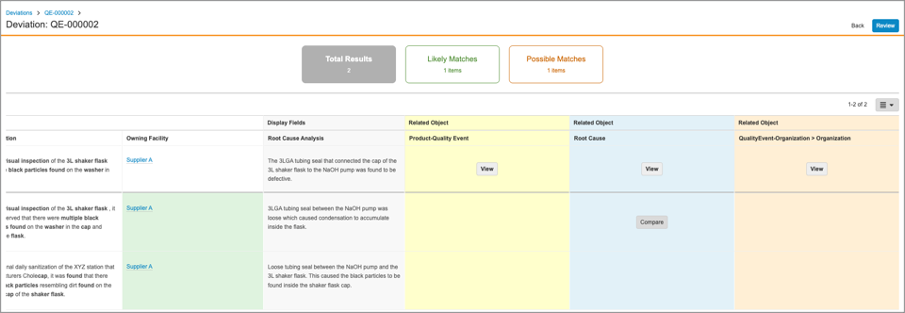
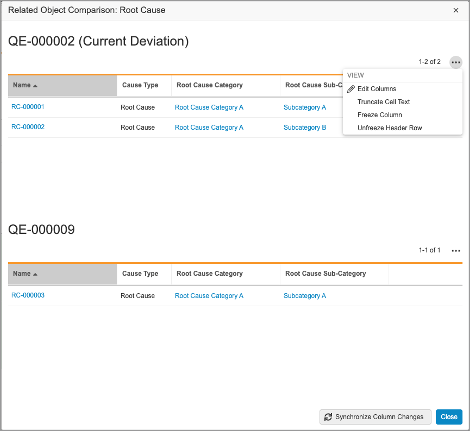
Recurrence Check: UI Enhancements
With this release, the Recurrence Check interface has been improved. Once potential recurrences are returned, the Comparison and Grid views have been enhanced to allow users to view related object data referenced by the record check for both the current record and the potential duplicate. Users can click on the Compare button which to compare related object data in an easily-accessible user interface.
Generate Merged PDF Document Action
As part of the APQR process, customers are often required to produce a final report which combines summary, recommendation, and detailed report documents into a single document. In previous releases, the creation of this single document was often done manually, outside of Vault.
With this release, users can upload the individual documents into an APQR Binder and, using the Generate Merged PDF Document action, all of the documents in the APQR Binder are merged together into a single document in their binder order. The resulting document is attached to the APQR Binder through the APQR Reports document relationship and can be reviewed and approved like any other Vault document.
If any document in the APQR Binder is revised, the action can be run again. The existing Final Report document and the relationship linking it to the binder will be up-versioned and the report will include the latest content.
Generate Document for Standard QMS Processes
This feature enhances the Generate Document actions to allow execution on additional processes within QMS, and can now be configured to allow a user to generate a document from a list of available document templates, formatted outputs, or reports. This is useful when a default document is typically utilized but a different template may need to be generated based on the Country or Product of the record. Learn more about using and configuring Quality Document Generation.
Standard Cycle Time Metrics: Audit Object & Quality Event Custom Object Type Support
With this release, Vault QMS extends the capability of Standard Cycle Time Metrics to include support for all custom Quality Event object types and to automatically capture the following cycle time metrics for all standard and custom Audit object types:
- Cycle Time: Duration in number of days from initiation to completion of the Audit
- Audit Preparation Cycle Time: Numbers of days an Audit record is in preparation prior to the Actual Start Date.
- Audit Reporting/Response Cycle Time: Number of days from the Actual End Date until completion of all post-audit or inspection activities, such as reporting, finding issuance, response, or others
- Planned vs. Actual Audit Start Delta: Number of days the Actual Start Date for the Audit is before (early) or after (late) the Scheduled Start Date
- Planned vs. Actual Audit Duration Delta: Actual duration of the Audit (Actual Start Date to Actual End Date) compared to planned duration (Scheduled Start Date to Scheduled End Date)
Learn more about standard cycle time metrics.
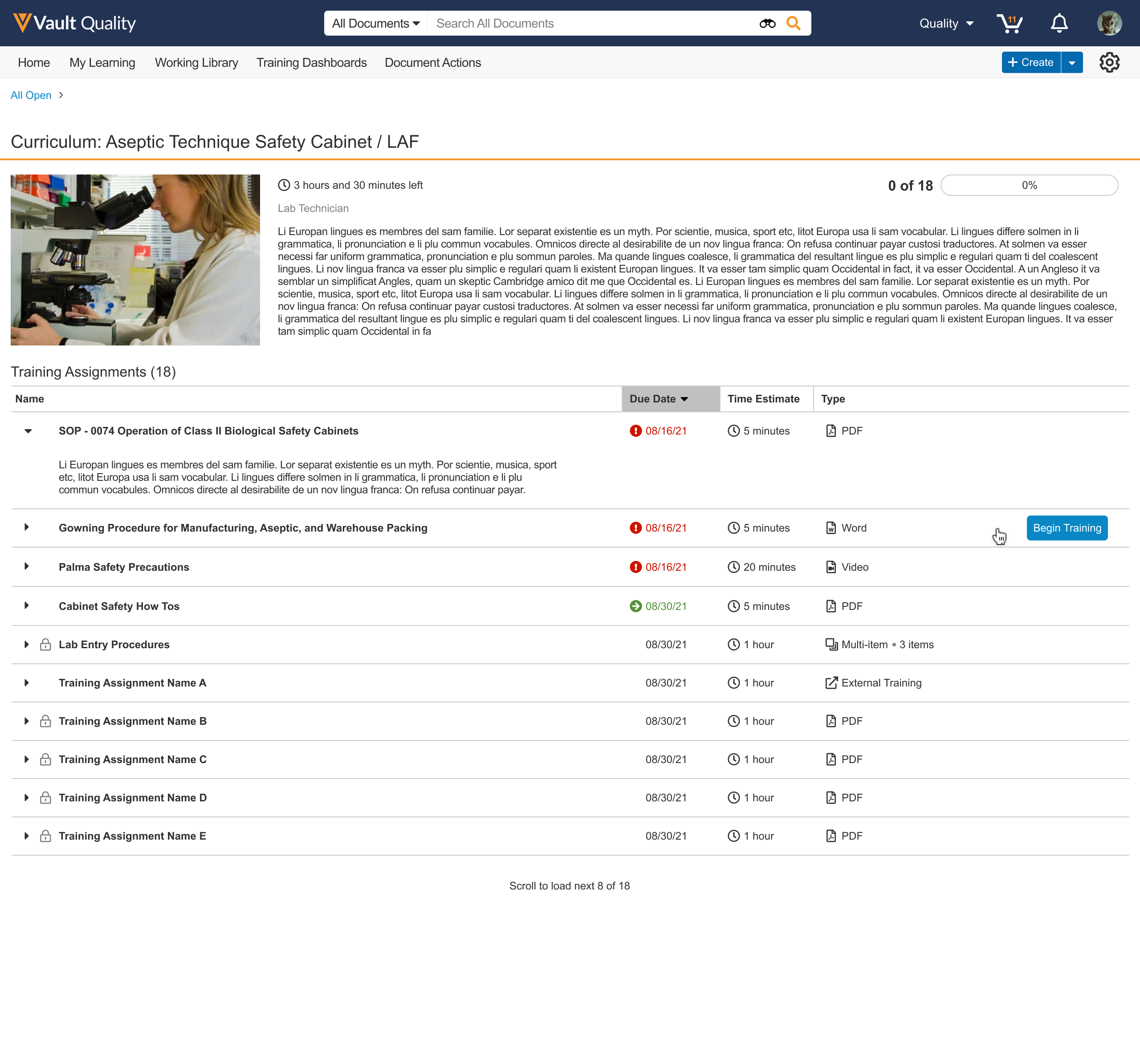
Curriculum View On Learner Homepage
Training Assignments on the Learner Homepage can now be grouped by Curriculum allowing Learners to prioritize training by Curriculum. When grouped, each Curriculum is represented as a card. The Curriculum card shows the number of Training Assignments assigned, Curriculum description, and other helpful information for Learners. From a Curriculum card, a user can navigate to a curriculum details page, which shows all Training Assignments assigned in the Curriculum, allowing the Learner to focus on a targeted list of Training Assignments. Learn more about the Learner Homepage.
Admin Alerts: Training Materials, Prerequisite Rules, & Substitute Rules
Admin Alerts inform the Training Administrator when they are about to make a change to a Training Requirement that could impact Learners’ Training Assignments. This feature explains what the impact of a change will be and how many users could be impacted. This set of auto-on alerts appear when changing Training Materials or modifying Prerequisite or Substitute rule configurations. For Training Materials, the feature also allows the Admin to adjust the settings that control impact directly from the warning message.
Admin Alerts: Quiz & Recurrence
Admin Alerts inform the Training Administrator when they are about to make a change to a Training Requirement that could impact Learners’ Training Assignments. This feature explains what the impact of a change will be and how many users could be impacted. This set of configuration-enabled alerts can appear for Quiz and Recurrence configuration changes.
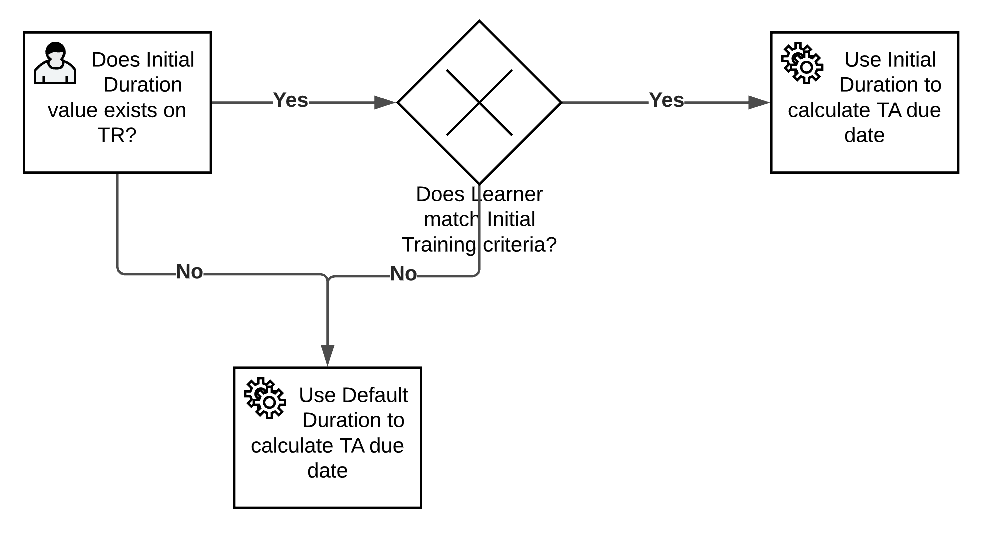
Initial Training Due Date
This feature allows an Admin to set a duration for initial training that is different from the general duration. This means that new hires or new transfers receiving this training for the first time can have due dates that are different from a Learner who has received this training before. Learners who have received this training before (for example, those receiving the assignment via recurrence) will be given assignments with the default due date. Learn more about configuring initial training due dates.
Training Jobs: New Jobs to Process Training Assignments
This feature optimizes the performance of the Update Training Assignment job by introducing child jobs that perform the following functions alongside the parent Vault Training job:
- Transition the state of Training Assignments to the target state
- Cancel all Training Assignments for a Person in the Ineligible state
The child jobs allow Vault Training to assign Training Assignment records quickly and efficiently. Status and logs for the child jobs are available in a new Training Job Status page available at Admin > Configuration > Training Job Status. In addition, Training Assignments created or updated after this release have the originating Vault Training job ID written to a new field for quick troubleshooting. Learn more about Vault Training automation.
Imported Training Assignments: Sharing Settings Not Updated
The Update Training Assignments job will no longer add the Learner and Direct Manager, if none exist, to Sharing Settings for the most recent imported Training Assignment for a Learner or Training Requirement. This change improves the overall Update Training Assignment job performance.
Updates to Cancel Training Assignments Job
The Cancel Training Assignments job, which is executed from a document lifecycle’s Cancel Training Assignments entry action, now logs the following information:
- Whether Training Assignments were cancelled
- Training Assignments that were cancelled
- Error information, if there was an error during the job execution
Training Requirement Impact Assessment: Optional Task Assignee
The Assign Task To input for the Create Training Requirement Impact Assessment and Retire or Assess Impact on Training Requirements document entry actions is now optional. This allows configuration of workflow task participants at the Training Requirement Impact Assessment object level.
Training Requirement Impact Assessment: Action to Re-evaluate Records
This feature provides the ability for a user to re-evaluate a Training Requirement Impact Assessment record in the event that the record cannot enter the Completed state. This allows a quick way to update the record, without requiring a configuration change.
Assignment Details Creation Allowed for Imported Records
Assignment Details, a child record of Training Assignment, can now be created for imported Training Assignments. This allows historical Training Assignments to have Assignment Details, enabling reporting on Curriculum and Learner Role for historical Training Assignments.
Training Materials: Prevent Duplicate Documents
With this release, Training Admins are prevented from adding the same document multiple times to the Training Materials for a Training Requirement, regardless of whether they are attempting to add different versions of the document.
Learner Application Role Updates in Sharing Settings
This feature allows updates to be made to the Learner Application Role in Sharing Settings for Training Assignment, Assignment Details, and other Training object records. Learners can then be added to the Learner Application Role in Sharing Settings using the Vault API.
Prevent Duplicate Assignment Detail Records
This update prevents a user or Vault from creating duplicate Assignment Details records for a given Training Assignment. A duplicate Assignment Details record is considered the same Curriculum and Learner Role for a given Training Assignment.
HVO Migration Preparation: Field Configuration Limitations
In an upcoming release, Vault Training will migrate some objects’ Data Store setting to High Volume. To prepare for this, the following fields can no longer be created on the Training Assignment or Assignment Details objects:
- Field types that support field-level encryption
- More than two (2) multi-value picklist fields
eMDR: Usability Enhancements
This Vault Product Surveillance feature allows Admins to add custom sections to the Adverse Event Report object page layout for the eMDR object type. It allows the users to view the information in the exact same format as it would be submitted to FDA by aggregating information from various objects and transforming it into the required format.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Quality data model to support new features:
Regulatory
Matched Document Ordering
This feature allows users to set a specific order for matched documents in the Content Plan hierarchy viewer when multiple documents are matched to a single Content Plan Item. By default, matched documents are sorted in ascending alphanumeric name order on a Content Plan Item when no specific order is set.
Users can also drag and drop documents to match to a specific matched document position under the Content Plan Item. Existing content plan actions including splitting, binder creation, copy content plan, and starting workflow from hierarchy viewer are also updated to utilize the matched document order.
Submission Wizard
A new wizard is available from the Application, Regulatory Objective, and Submission to simplify creation of Submissions, Regulatory Objectives, and their relationships as well as improve data consistency and integrity.
Users can select from a controlled list of relationships within the wizard, which are defined using new Application relationship objects to manage metadata at the application level. New options are available in the RIM Maintenance tab to extract and load a zip of loader sheets for suggested Application relationships based on the current set of Submission relationships in the Vault.
Global Content Plan
With this release, users can create and update a Content Plan directly from an Event record, where Vault generates a Global Submission and builds the Global Content Plan structure based on the Event’s Activities and other relationships. Users can also view the Global Content Plan directly from the Event or Activity, where viewing from the Activity filters the Global Content Plan based on the content relevant to the Activity. Once finalized, the Global Content Plan can be copied in bulk to multiple submissions using a new dispatch action.
Select Section in Add to Active Dossier Dialog
When adding a single document to Active Dossier in the Active Dossier Editor, users are now able to select and change the section in which to add the document via the Add to Active Dossier dialog.
Show Only Pending Toggle in Active Dossier Editor
With this release, a new toggle between ‘Show Only Pending’ and ‘Show All’ in the Active Dossier Editor allows users to quickly switch between viewing all Active Dossier Item Detail records and those with a Pending-type status, for example Pending Current.
Pull Objective Data Enhancements
With this release, the Pull Objective Data action can create Submission details with a mix of object types when the internal names of the object types on the source and target objects match. Pull Objective Data also adds the following details to Regulatory Objectives and Submissions: Authorizations such as certificates, Regulatory Text such as device trade names and descriptions, and Product Organizations such as authorised representatives. Support is also added for copying additional standard fields that were added to the data model in previous releases.
Create Related Records Enhancements
With this release, Create Related Records can create Regulatory Objective and Submission details with a mix of object types when the internal names of the object types on the source and target objects match. Create Related Records also adds the following details to Regulatory Objectives and Submissions: Authorizations such as certificates, Regulatory Text such as device trade names and descriptions, and Product Organizations such as authorised representatives. Support is also added for copying additional standard fields that were added to the data model in previous releases. Finally, Create Related Records will now always create relationships between the source Event and any resulting Submissions and Regulatory Objectives.
China XSD 1.0 eCTD Publishing & Validation
With this release, RIM Submissions Publishing supports the CN NMPA v1.0 (XSD 1.0) specification. Users can now create Content Plans and generate submissions that are compliant with CN NMPA v1.0 specification. Vault validates these submissions based on the corresponding CN NMPA v1.0 Validation Criteria Version.
Asynchronous FDA Gateway Support
With this release, Admins can configure a Gateway profile for US FDA submissions to send the Mail Delivery Notification (MDN) asynchronously.
Resubmission of Previously Transmitted Submissions
With this release, users can resubmit submissions that were already sent to the Health Authority and a technical rejection was received.
Providing Additional Data in the Publishing Progress Indicator
With this release, additional information is added to the Publishing Progress Indictor’s download CSV to enable better understanding and troubleshooting of publishing-related issues.
Content Plan Creation & Copy Notification Token
A new token, ${templateConstraints}, is now supported for the Content Plan Creation and Copy notifications, providing end-users visibility into whether records were created as Inactive or Excluded due to template constraints.
Generic Token Support for Submission Pharmaceutical Form
With this release, content plans now support tokens for text and object reference fields within the Submission Pharmaceutical Form object.
RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model:
Safety
Safety features are targeted for tentative availability on February 16, 2022.
Automated Case Promotion to Initial and Follow-Up Case Configuration
Vault Safety now supports automated Inbox Item promotion to an Initial or Follow-Up Case for E2B transmissions received via AS2 Gateway from external systems that have their own case processing workflows. The system verifies whether Case promotion is valid, leverages Case Identifier matching, and has selectable merging methods based on seriousness.
Learn More
- Enablement: Enable Automated Case Promotion
- Admin Help: Configure the Case Promotion Settings Page
- User Help: Automated Case Promotion
- Developer Documentation:
Merge Inbox Item to In-Flight Case Admin Checkbox
Vault Safety now supports merging information received in Inbox Item into the latest Case version. When merged, source documents will also be added to the latest Case version. To facilitate parallel processing, Intake users can now alert the case processor that there is new case information by marking an Inbox Item as a follow-up to the latest case. Inbox Items marked as follow-up can either be merged into the latest Case version or can be used to create a Follow-Up Case.
Learn More
- Enablement: Enable Merge to In-Flight Case
- Admin Help: Configure the Case Promotion Settings Page
- User Help: Merge to In-Flight Case
Promote to Multiple Cases Configuration
Vault Safety can now promote a single Inbox Item to up to 100 cases. The cases will be related and contain all the information from the Inbox Item, including source documents. This functionality is primarily intended for the intake of literature and legal cases. This feature is not supported for E2B imported Inbox Items.
Learn More
- Enablement: Promote an Inbox Item to Multiple Cases
- User Help: Promote to Multiple Cases
Follow-Up with Any Matching UID Configuration
Admins can now configure Vault Safety to allow Inbox Items to be promoted to a Follow-Up Case when the Worldwide UID does not match but matches another UID, such as an External System UID or a Case Identifier.
Learn More
- Admin Help: Configure the Case Promotion Settings Page: Enable Duplicate Detection for Mismatching WWUID
Import Product Matching by Company Product Substance, Trade Name, and Alias Configuration
This release enhances product matching when importing Inbox Items or AERs from E2B and JSON files by supporting product generic names, trade names, product aliases, and substance aliases.
Learn More
- Admin Help: Manage Products: Create Product and Substance Aliases
- User Help: Inbox Item Study and Product Matching: E2B Import Study and Product Matching
Conditional Expectedness Configuration
This feature brings multiple enhancements to the configuration and evaluation of Datasheets for auto-expectedness. When configuring Datasheets, administrators can now specify seriousness criteria conditions, which define when a listed term is unexpected. Seriousness criteria can be configured at both the Datasheet and MedDRA Criteria (listed term) level. Admins can also now configure precise expectedness on Datasheets, which prevents the system from marking terms as unexpected if they are not listed on the Datasheet. Additionally, a new Expectedness MedDRA Criteria setting allows you to specify unexpected terms on a Datasheet.
Learn More
- Enablement: Enable Conditional Expectedness
- Admin Help: Manage Datasheets and Auto-Expectedness
Case Nullification Configuration
Vault Safety now supports the ability to void a case, including the option to nullify previous Transmissions using a configurable user action at the Case-level. You must specify a reason for nullification when you run the action, which will auto-populate in new system-generated transmission records related to the case. Additionally, upon initiating the voiding process, the system automatically cancels any in-progress workflows.
Learn More
- Enablement: Enable Case Nullification
- User Help:
Case Validations: Control Workflow Transitions Configuration
Vault Safety can now calculate the worst validation result for a case and transmission to be used in entry criteria and prevent lifecycle state changes. The system will also prevent gateway submissions for any transmissions with Hard Failures.
Learn More
- Enablement: Enable Control Workflow Transitions for Case Validations
- User Help: Enter Case Data (Validation Status field)
MedDRA Hierarchy for CMQ and Vault Reporting Configuration
Admins can now download the full active MedDRA version from the central dictionary to your vault instead of uploading dictionary .zip files. Admins can also perform a deep copy of MedDRA queries and associated records, as well as update the MedDRA hierarchy with higher-level terms associated with all LLT terms in your vault.
Additionally, Vault Safety now supports creating Custom MedDRA Queries (CMQs) using any level of the MedDRA hierarchy (SOCs, HLGTs, HLTs, PTs, and LLTs). Previously, MedDRA Queries could only be constructed using LLTs. The MedDRA Queries with non-LLTs will be supported through standard Vault Reporting and Dashboarding.
Learn More
- Enablement: Enable MedDRA Hierarchy Updates for CMQ and Vault Reporting
- Admin Help:
Precise Inclusion of Age-Related and Medically Confirmed E2B Elements Auto-on
This release includes enhancements to E2B generation for precise inclusion of age-related elements (B.1 / D.2) and medical confirmation by a health professional (A.1.14 / E.i.8). This change impacts all standard E2B(R2) and (R3) formats that Vault Safety generates. The following table describes the impacted data elements:
| Element Name | E2B(R2) | E2B(R3) | Changes |
|---|---|---|---|
| Medically Confirmed by Healthcare Professional | A.1.14 | E.i.8 | Now only transmitted when the primary Reporter is not a health professional. |
| Patient Date of Birth | B.1.2.1 | D.2.1 | Now only transmitted when a full date is entered in the corresponding Vault Safety field (CCYYMMDD), except for PMDA where the Date of Birth is always masked if provided. |
| Date of Birth of Parent | B.1.10.2.1 | D.10.2.1 | Now only transmitted when a full date is entered in the corresponding Vault Safety field (CCYYMMDD). |
| Age at Time of Onset | B.1.2.2 | D.2.2 | Now only transmitted if the Date of Birth (D.2.1 / B.1.2.1) is not transmitted. For example, Date of Birth is partial or Masked. |
| Age Group | B.1.2.3 | D.2.3 | Now only transmitted when both the Patient Date of Birth (B.1.2.1 / D.2.1) and Age at Onset (B.1.2.2 / D.2.2) are not transmitted. For example, Date of Birth is partial or Masked. |
Learn More
- User Help: E2B Generation Data Mapping
E2B(R2) Enhancements Auto-on
This release includes the following E2B(R2) enhancements for all E2B(R2) report formats (ICH, FDA, HC):
- When “Drug Not Administered” is selected for a Product Drug Role, include drug characterization with “Suspect” (B.4.k.1)
- Importing and exporting the
< safetyreportversion >tag - Collating seriousness from all Case Adverse Events when transmitting Case Seriousness criteria (A.1.5.2)
- Masking E2B(R2) elements with “PRIVACY” for masked distributions
Learn More
- Enablement: Enable Safety Report Version Customization
- User Help:
E2B(R2) Partial Dates for Combination Product Expiration Date Support
Vault Safety now accepts partial dates in the Case Product “Expiration Date” field. For FDA E2B(R2) combination product reports, partial dates are exported to Expiration Date elements B.4.k.2.4.FDA.1a-b using the appropriate date format code.
Learn More
- Enablement: Contact Veeva Support to enable this feature. Once enabled, an admin must replace the existing “Expiration Date” field with the new Expiration Date control on the Product and Case Product page layouts. Note: If you enable this feature, you must manually migrate any existing Expiration Date data after adding the new control.
- User Help:
Reporter Masking for Post-Market Non-Literature Cases Admin Checkbox
Vault Safety provides a system-level setting to support masking reporter information in all outbound Submissions and Distribution for post-market non-literature Cases (E2B(R2), E2B(R3), CIOMS I, MedWatch3500A). If an admin enables this setting, reporter masking applies to all post-market non-literature Cases.
Learn More
- Enablement: Enable Reporter Masking System Setting
- User Help: Reporter Masking for Postmarket Non-Literature Cases
Transmission Product Types Configuration
This feature enables products to be registered as different product types in different markets. For example, a product may be registered as a combination product with the FDA, and a drug with the EMA. This feature is controlled by a new Transmission Product Type setting, which can be set on a Transmission or at the Product or Study Registration level by an admin.
One scenario this feature supports is omitting device constituents from combination product E2B Reports. Because certain jurisdictions do not accept combination product submissions, you can exclude Device-type product constituents from E2B files generated for Combination Product reports. Unless this feature is enabled, E2B files generated for a Combination Product Case continue to include both Device-type and Drug-type Product Constituents.
Learn More
- Enablement: Exclude Device Constituents from E2B Exports: Prerequisites
- Admin Help: Manage Combination Products: Exclude Device Constituents from E2B Exports
- User Help: Create a Submission
Health Canada Transmission Profiles and Message Types Auto-on
Vault Safety now supports the Health Canada gateway for electronic submissions of clinical and post-market cases. A valid Health Canada E2B(R2) file with correct header information is also generated.
Learn More
- Enablement: Configure Health Canada Gateway: Prerequisites
- Admin Help: Configure Health Canada Gateway
- User Help:
PHI Masking on Foreign Submissions Configuration
Vault Safety can now generate safety reports with PHI masking for Submissions. Masking can be configured at the reporting rule level to apply to all cases or only foreign cases.
Learn More
- Enablement:
- To allow users to manually select masking for a Submission, an admin can add the “Patient Content Protection” and “Exceptions to Patient Content Protection” fields to the Submission Page Layout.
- To automatically apply masking with reporting rules, an admin can configure the Mask PII rule parameter.
- Admin Help: Reporting Rule Parameter Reference
- User Help:
Configurable Validation Criteria Configuration
Vault Safety now supports additional custom validation criteria using the standard validation criteria syntax, which will run as part of the Case validations. In addition, the Vault Safety validation engine now supports E2B(R2) formats.
Learn More
For assistance in configuring custom validation criteria, contact Veeva Services.
Safety Rule Version Management Admin Checkbox
Admins can now configure an Active Version of the system-provided standard rulesets. When new rules are introduced into a standard ruleset (for example, FDA, EMA, and PMDA) as part of a release, admins can set the rule version to allow for adoption of the most recent version of a standard ruleset on a configurable basis.
Learn More
- Enablement: Manage Reporting Rule Versions: Prerequisites
- Admin Help: Manage Reporting Rule Versions
Automated Cross Reporting Configuration
In this release, Vault Safety extends the evaluation of reporting obligations to include cross-reporting (investigational to marketing registration) scenarios. Vault Safety evaluates the investigational and marketing registrations of study products, then identifies any additional reporting destinations for products and studies. Administrators can also specify destination overrides on product licenses.
Learn More
- Enablement: Enable Automated Cross Reporting
- Admin Help:
Identifiable Patient Definition Ruleset Parameter Configuration
In this release, Vault Safety allows submission rules to evaluate the Identifiable Patient Definition ruleset parameter to determine whether or not a case contains an Identifiable Patient as defined by E2D (ICH standard), or less strict “Patient Known to Exists” - a newly introduced case field. This allows greater specificity in submission to health authority and partners with less strict definitions of patient (i.e. FDA) while minimizing the potential for over reporting to destinations that have more strict definitions of patient (i.e. EMA).
Learn More
- Enablement: To leverage the “Patient Known to Exist” field, an admin can add the field to the Case and Imported Case page layouts.
- Admin Help: Reporting Rule Parameter Reference
- User Help: Enter Case Data (Patient Known to Exist field)
Bulk Unblind Configuration
Vault Safety can now bulk unblind multiple cases in a given study. This includes both the removal of blind protection for previously unblinded cases and snapshotting study arm product information for blinded cases. Cases currently in workflow will not be modified by this action.
Learn More
- Enablement: Enable Bulk Unblind
- Admin Help: Bulk Unblind a Study
Organized Data Collection of Reports from Patient Support Programs and Market Research Programs Configuration
Vault Safety now supports organized data collection of reports from PSPs (Patient Support Programs) and MRPs (Market Research Programs). Admins can create Study placeholders for Studies with unspecified products and intake users can select Company Products as Suspect Products. Upon case promotion, reporting obligations are evaluated using Product Registrations for the suspect Company Products.
Learn More
- Enablement: Enable Organized Data Collection
- Admin Help: Create a Study with Unspecified Products
SiteVault
User Administration with Multiple Security Policies
For SiteVaults that have more than one active security policy (for example, basic Vault authentication and a single sign-on provider), this feature allows the user administrator to select a security policy when creating or editing a user. This feature only pertains to vaults with multiple security policies and the Extensible SiteVault Permissions feature enabled.
Support eConsent Simple Signatures
This feature adds support to enable study participants and other signatories to complete eConsent forms without creating a MyVeeva for Patients account. In SiteVault, site staff can send eConsent forms without required contact information. This feature is tentatively scheduled to be generally available with the Vault 22R1 release.
Veeva eConsent: Additional Signatory Enhancements
This feature includes general enhancements to the Veeva eConsent Additional Signatories feature, including making it even easier to create and manage Signatory records and providing users more flexibility in sending eConsent forms to signatories.
Veeva eConsent: Contact Information Update Request
With this feature, when a patient or signatory on a study updates their contact information in MyVeeva for Patients, their contact information is also updated in SiteVault. Site Administrator users receive a notification that alerts them to the changes. This feature is tentatively scheduled to be generally available with the Vault 22R1 release.
Connected Study Details User Action
SiteVault users working on Connected Studies can now use the Connected Study Details user action to access more details regarding a document’s exchange with a Sponsor/CRO. This action opens a dialog box that displays tracking information as well as the document comment history with the Sponsor/CRO.
Sponsor/CRO Information on Documents
With this feature, SiteVault users working on Connected Studies can now see details regarding whether a document version has been sent to Sponsor/CRO, the date it was initially sent, and the last sent date directly in the document’s metadata.
New Exchangeable Document Type on Connected Studies
This feature adds support to transfer documents of type IP Excursions to a Sponsor/CRO’s vault for a Connected Study.
SiteVault Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the SiteVault data model to support new features:
QualityOne
External Collaboration Management on CAR
This feature allows internal users to manage the creation, activation, and inactivation of user accounts for third-party organizations. External Collaborators (third-party users) can respond to CARs (SCARs) in Vault using the External User license assigned. Internal users manage a “contact list” of persons associated with the third-party organizations, tagging CARs (SCARs) with a relevant External Collaborator for Vault to automatically create, activate, or inactivate. When the user account for the External Collaborator is created, activated, or inactivated, the relevant email is sent to those persons as part of the CAR (SCAR) process. This feature enables Admins to utilize and configure a pool of shared External User licenses to facilitate this process using the External Collaborator User Template. Learn more about External Collaboration Management.
QualityOne Team Enhancements
This feature extends the QualityOne Team capability to allow Admins the flexibility of configuring Team roles and members. Learn more about QualityOne Teams.
Manage Invalid Users with Tasks
Admins can now control invalid users when managing QualityOne Teams. For a given Team, if any user in that Team is invalid, users can choose to remove the invalid user from the team or to replace them with an alternative, valid user. When invalid users are replaced, Vault automatically handles task reassignment by moving open tasks assigned to the replaced user.
Role Restriction & Child Object Team Enablement
Admins can now restrict a team member to a particular role, similar to a workflow’s participant group role restriction. . Team Roles are restricted by declaring the role as Exclusive: if a user is a team member of an exclusive Team Role, that user cannot also be a member of any other role for that record. Additionally, Admins can now create a Team on child objects.
Display Key Team Roles for Filtering & Reporting
Users can now surface key Team Role assignments as User fields, displaying and using the key in related lists, searches, filters on library views, and reports, allowing team membership changes to synchronize with the record’s field.
COA Ingestion Enhancements
This feature extends our processing capability to allow for a wider range of COA formats that can be accurately ingested. Learn more about COA Inspections.
Additional Matching Options for Component Matching Variants
This feature adds additional matching options for Admins to choose from when defining a COA Component Matching Rule on a Component Matching Variant. The new COA Component Matching Rules are:
- Contains
- Starts With
- Ends With
If a Component Matching Variant does not have a COA Component Matching Rule defined, Vault uses an Exact Match criteria when evaluating a Component Matching Rule’s validity.
Additional Matching Options for Matching Rule Variants
This feature adds additional matching options for Admins to choose from when defining a Variant Type on a Matching Rule Variant. The new Variant Types are:
- Contains
- Starts With
- Ends With
Improved COA Analysis Job Logging
This feature allows Admins to access debugging information related to COA ingestion via a Job Log. The Job Logs can be accessed by navigating to Admin > Operations > Job Status. A summary of the ingestion analysis is provided in the Job Log to give Admins the necessary information needed to quickly triage and resolve COA ingestion-related issues.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Risk Management: Detectability Support for Qualitative & Quantitative Risk Matrices
This feature adds support for modeling Detectability as another axis of the Risk Matrix. Adding the Detectability axis supports assessment methodologies such as the FMEA (Failure Mode and Effects Analysis) Risk Assessment, which requires the Detectability attribute of an identified risk. QualityOne customers can create 3D risk matrices (Severity x Occurrence x Detectability) within their Vaults with this feature. Learn more about Risk Management.
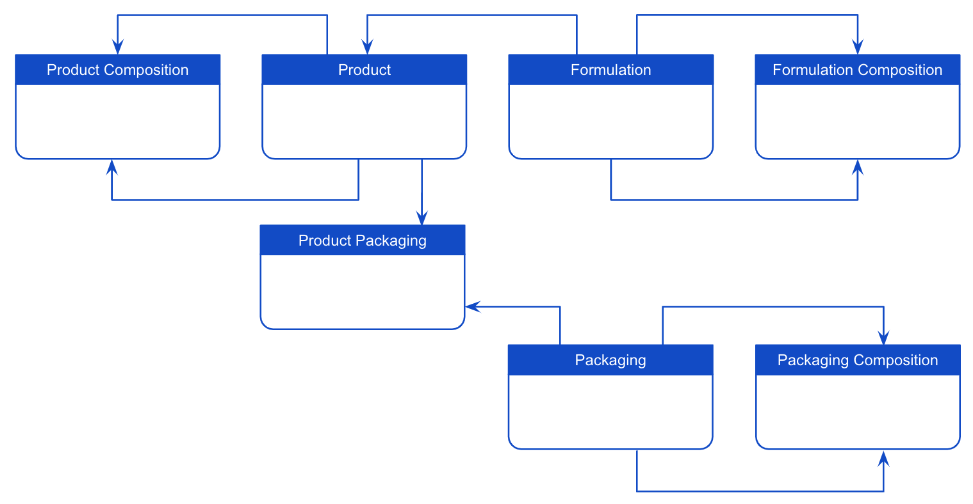
Product Hierarchy Data Model Extension for Packaging & Materials
This feature provisions new objects (Packaging, Product-Packaging, Material, Material-Organization) in QualityOne to extend the standard product hierarchy data model. Customers can leverage Material-related ERP (Enterprise Resource Planning) data to use with QualityOne.
Inspections Compatibility with Extended Product Hierarchy Data Model
This feature updates QualityOne Inspection Management functionality to align with the newly-provisioned Material (material__v) object that supports the Extended Product Hierarchy Data Model.
Update View 5 Whys Analysis Action
This feature offers a new record action “View 5 Whys Analysis” to launch the 5 Whys diagram, replacing the original “View 5 Whys Analysis” user action. The “View 5 Whys Analysis” user action is renamed to “View 5 Whys Analysis (Legacy)” and is targeted for removal in a future release. Learn more about the new 5 Whys Analysis record action.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on February 22.
Formulation Questionnaires
Using checklists, this feature allows regulatory users to send targeted questionnaires, such as raw material questionnaires (RMQs), via email to contacts at external organizations, such as suppliers, and enables those contacts to respond securely via public access links (PALs) without logging into Vault. Learn more about Formulation Questionnaires.
Local Impact Assessment
This feature helps users assess whether a new Registration Item’s objective can be accomplished by amending an existing registration or if a new registration is needed. Vault uses Admin-defined Regulated Category Attribute Impacts to search active registrations and identify the records that match the Registration Item. Vault also indicates to users if the matched registrations need to be amended. Lastly, Vault starts the appropriate registration process based on the user’s selection of a registration. Learn more about Local Impact Assessments.
Create Dossier Binder
This feature gives users the ability to create a binder with a list of locked steady state expected documents for a requirement while maintaining the requirement hierarchy as a hierarchy of sections. Admins can configure the new Create Dossier Binder action on the Registration Item Requirement object to allow users to create Dossier Binders from a parent record. Learn more about Dossier Binders.
Generate Requirements for Registration Objective
This feature allows Admins to configure the Generate Requirements action on the Registration Objective, allowing users to generate requirements for a collection of Registration Items. This may be helpful when multiple products, such as shaded products, can be registered together through the submission of a single dossier. Learn more about generating requirements.
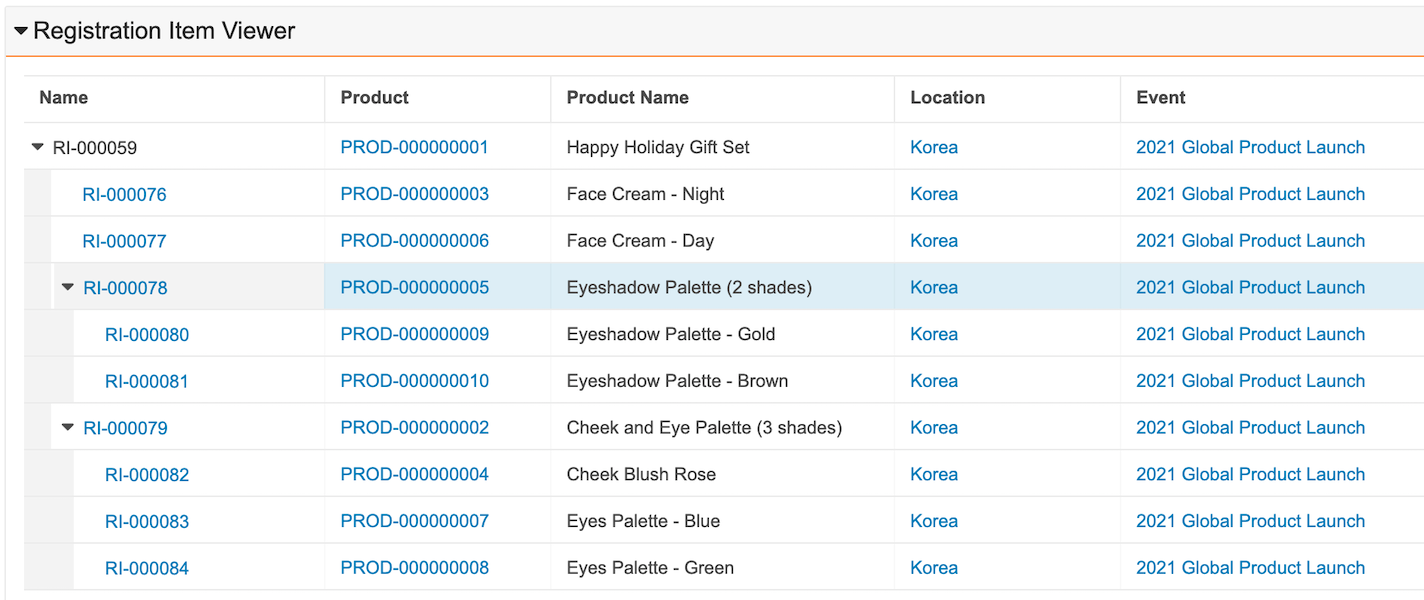
Registration Item Hierarchy Viewer
When configured, this feature allows users to view multiple related Registration Items in a hierarchical view. Users can identify related Registration Items and any dependencies between them all in one viewer, improving the user experience by limiting the need to navigate to multiple other pages. Learn more about the Registration Item Hierarchy Viewer.
Split Registration Items Enhancement: Recursion
This feature enhances the Split Registration Items feature and gives users the flexibility to view generated Registration Items in an output structure that preserves the hierarchical relationship between them in addition to viewing the records in a flat list. Customers can utilize the Registration Item Hierarchy Viewer to easily visualize the output when several multi-level records are generated from the Split Registration Items action on a Registration Item with a Split Rule linked to a recursive relational token. Learn more about configuring Split Registration Items.
Track Authority Questions
This feature unlocks the standard Regulatory Request object in Registration & Dossier Management Vaults, giving users the ability to track authority questions.
Enable Checklists in RegulatoryOne
This feature enables Checklists in all RegulatoryOne Vaults, including Veeva Claims.
Product Hierarchy Data Model Extension for Packaging
Consumer and cosmetic products often come inside product packaging that can form multilevel hierarchical relationships. With this release, we are expanding the shared standard product hierarchy data model to include product packaging data (Packaging, Product Packaging, and Packaging Composition objects) to reduce reliance on customization.
These data model changes are automatically included in RegulatoryOne, but Admins must make configuration changes to make them available.
Note this feature is also available for Veeva Claims.
Veeva Claims
Veeva Claims features are targeted for tentative availability on February 22.
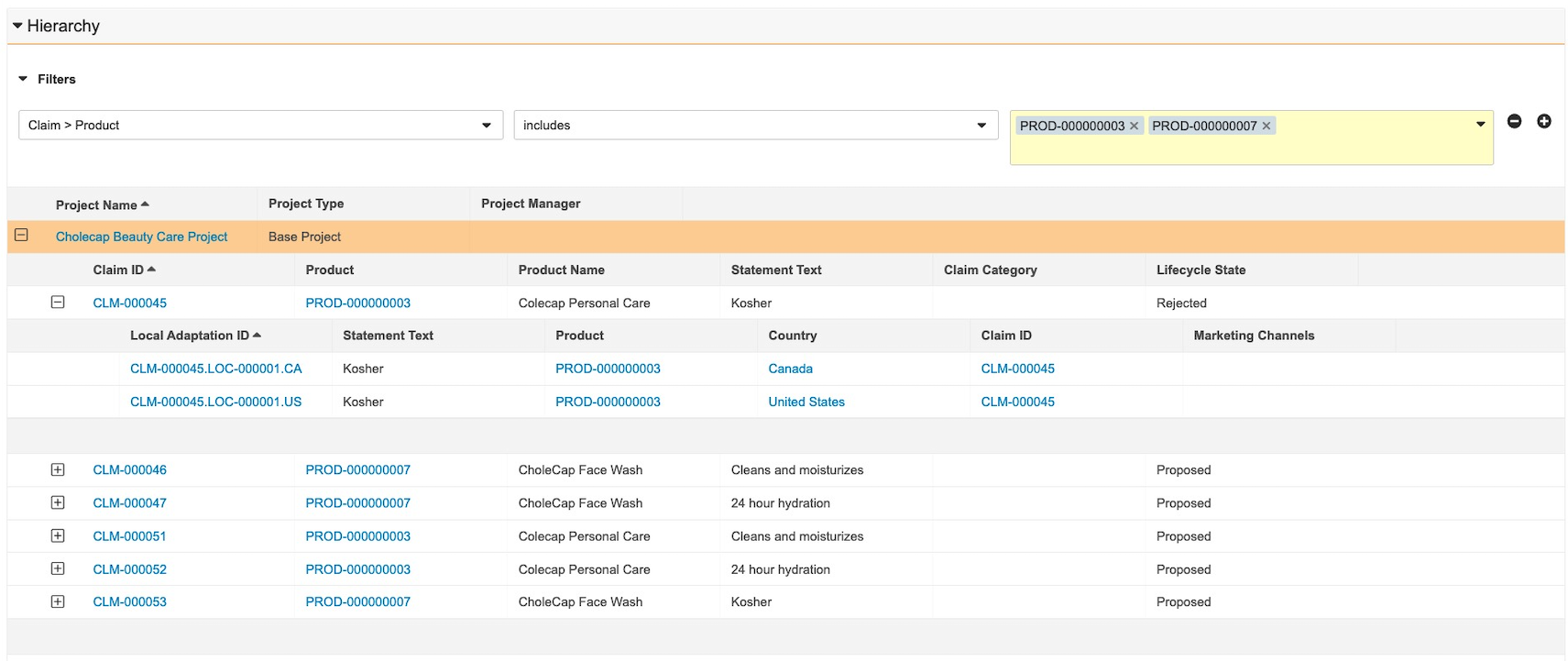
Project Hierarchy Viewer
When configured, this feature enables users to navigate through a large data set of Claims and Local Adaptations within a project with ease. Users can filter the list of Claims and Local Adaptations in the project to narrow down their scope. Through a hierarchy navigator, users can jump easily between different levels of a Project. Users can view and add related sections for Claims and Local Adaptation objects while maintaining the context of the Project. Learn more about configuring the project hierarchy viewer.
Dynamic Deep Copy Project
This feature improves the speed of starting new Claims projects. When configured, this feature gives users control over the copy Project record behavior by allowing users to specify which related sections of Claim and Local Adaptation records they want to copy over to the new Project record. Learn more about configuring dynamic deep copy project.
Claims UX Enhancement
This feature enhances the UX for Claims users. The feature primarily enhances the search and list experience in the Selectively Create Claims dialog and the Generate Local Adaptations dialog.
Product Hierarchy Data Model Extension for Packaging
See: feature description.
Enable Checklists in RegulatoryOne
See: feature description.
Enablement Details
| Feature | Enablement | Application |
| Working with Documents | ||
| Caption Bookmark Exclusions | Configuration | Platform |
| Annotate Usability Improvements | Auto-on | Platform |
| Text Indexing and Combination Enhancements | N/A | Platform |
| Collaborative Authoring Troubleshooting Page | Auto-on | Platform |
| Google Drive Integration: Create New Document | Auto-on | Platform |
| Enhanced Bulk Document Update: Validation Behavior Changes | Auto-on | Platform |
| Legacy Feature Enablement Updates | Auto-on | Platform |
| EDLs: Configurable States for Document Version Locking | Configuration | Platform |
| Vault Objects | ||
| High Volume Object Multi-Value Picklist Field | Auto-on | Platform |
| Lifecycle & Workflow | ||
| Consistent Steps Across Workflows | Auto-on | Platform |
| Populate Object Reference Fields with Workflows & Lifecycles | Configuration | Platform |
| Enhanced Stages Chevron Card for Workflows | Auto-on | Platform |
| Show More on Tasks in Home Pages | Auto-on | Platform |
| Remove Default Task Link in Label | Configuration | Platform |
| Bulk Actions on Record Actions | N/A | Platform |
| Reporting & Dashboards | ||
| In Operator for Name Report Filters | Auto-on | Platform |
| Optional Prompts | Auto-on | Platform |
| Additional Document Field Support in Report | Auto-on | Platform |
| Usability & UI Updates | ||
| Breadcrumb Enhancements for Object Tabs | Auto-on | Platform |
| Notifications: View Full Content in Panel | Auto-on | Platform |
| Notifications: Email Burst Threshold | Configuration | Platform |
| Email to Vault: Create Records for Bounced Emails | Auto-on | Platform |
| Updated UI Style on Users & Groups Pages | Auto-on | Platform |
| Workflow Action Menu for Objects: Drop-Down Behavior Update | Auto-on | Platform |
| Rich Text/Long Text Pop-up Window Size Update | Auto-on | Platform |
| Auditing | ||
| Audit Trail Enhancements to Track Lookup Field Changes in Object Records | Auto-on | Platform |
| Administration | ||
| Scheduled Data Exports: Initial Full Data Export | Configuration | Platform |
| Scheduled Data Exports: Escaping Characters in CSV Output | Auto-on | Platform |
| Scheduled Data Exports: Including Inactive Document Fields by Default | Auto-on | Platform |
| Scheduled Data Exports: Increased Precision on Field Values | Auto-on | Platform |
| Scheduled Data Exports: Uploading CSV Files to S3 with Bucket Owner Full Control ACL | Auto-on | Platform |
| Object and Document Field Types for Integration Field Rules | Auto-on | Platform |
| Configuration Management | ||
| Configuration Management: Allow Outbound & Inbound Packages enabled by default on Align Vaults | Auto-on | Platform |
| Data VPK: Validate VQL in Dataset Filter | Auto-on | Platform |
| Access Control | ||
| Object Control Security | Configuration | Platform |
| Search | ||
| Expanded Search with Related Records | Configuration | Platform |
| Field Centric Strict Matching | Auto-on | Platform |
| Checklists | ||
| Checklists: Add Reference Documents to Questions | Configuration | Platform |
| Checklists: Dependent Sections | Configuration | Platform |
| Checklists: Hide Unseen Questions on Review Page | Auto-on | Platform |
| Auto Claims Linking | ||
| Auto Claims Linking: View Claim Details from Select Claims Dialog | Configuration | Platform |
| Auto Claims Linking: Ignore Superscript and Subscript Characters When Matching to Claims | N/A | Platform |
| Vault Formulas | ||
| Workdays & Holidays | Auto-on | Platform |
| RecordByLabel() Function in Expressions | Configuration | Platform |
| Significant Figures in Expressions | Auto-on | Platform |
| Vault File Manager | ||
| VFM Download Rendition Usability Enhancements | Auto-on | Platform |
| Pause & Resume Uploads & Downloads with Vault File Manager | Auto-on | Platform |
| Vault Loader | ||
| Vault Loader: Return Updated Roles in Success Log | Auto-on | Platform |
| Vault Loader: Log Skipped Lines in Failure Log | Auto-on | Platform |
| Vault Loader: Disabling Workflow System Objects from Load or Extract Requests | Auto-on | Platform |
| Vault Java SDK | ||
| Enhancements to Reference Lookups | Auto-on | Platform |
| Object and Document Field Types for Integration Field Rules | Auto-on | Platform |
| SDK Runtime Logs | Auto-on | Platform |
| Vault Tokens | Auto-on | Platform |
| Platform Data Model Changes | Auto-on | Platform |
| Vault Connections | ||
| PromoMats/RIM: Conditional Transfer Support Using Query Object Rules (QORs) | Configuration | Promomats<>RIM |
| Clinical Operations | ||
| Milestone Roll Up Enhancements | Admin Checkbox | CTMS, Site Connect, Study Startup, Vault Payments, eTMF |
| Support to Identify Non-enrolling Sites | Auto-on | CTMS |
| Non-User Viewing of Documents in Surveys | Configuration | Study Startup |
| Restrict Doc Study to Single Value | Admin Checkbox | CTMS, Clinical Operations - All, Site Connect, Study Startup, eTMF |
| Automate Milestone State Changes: Additional Date Field Triggers | Auto-on | CTMS, Clinical Operations - All, Study Startup, eTMF |
| Site Document Exchange Section | Admin Checkbox | Site Connect |
| Monitoring Schedule Planning | Configuration | CTMS |
| Site Payment Holdbacks | Configuration | Vault Payments |
| Subject Payment Tracking Rules | Configuration | Vault Payments |
| Site Overhead Calculations | Configuration | Vault Payments |
| Transfer CSV Reports for TMF Transfer | Auto-on | eTMF |
| TMF Bot - Auto-classification Customer Feedback (22R1) | Auto-on | eTMF |
| Site Connect: Additional Vault Clinical Docs Support | Configuration | Site Connect |
| Site Information on Documents | Configuration | Site Connect |
| Cancel Site Connect Agreement Invitation | Configuration | Site Connect |
| Mark Site Connect document as Rescinded | Configuration | Site Connect |
| Site Connect Details User Action | Configuration | Site Connect |
| Clinical Operations Data Model Changes | Auto-on | CTMS, Site Connect, Study Startup, Vault Payments, eTMF |
| Commercial | ||
| Document Notifications: Support for "Based On" Relationship | Configuration | PromoMats |
| Modular Content Approval Document Enhancements | Auto-on | PromoMats |
| External Viewer: Number Of Views | Configuration | MedComms, PromoMats |
| Medical Inquiry UI: Admin Page | Auto-on | MedComms |
| Medical Inquiry UI: Case Contact Section | Configuration | MedComms |
| Allow Case Response Emails to be Deleted | Configuration | MedComms |
| CRM Data Sharing: Support Merged CRM Accounts | Admin Checkbox | MedComms |
| Automated Image Rendition: Include Source Document Name | Configuration | PromoMats |
| eCTD Binder Generation Failure Notifications | Auto-on | PromoMats |
| Commercial Data Model Changes | Auto-on | MedComms, PromoMats |
| Quality | ||
| Send Email to Non-Vault Users | Configuration | QMS |
| APQR/QMR | Configuration | QMS |
| Generate Document from Report: Support for APQR Item & QMR Item Lifecycles | Configuration | QMS |
| Recurrence Check: UI Enhancements | Auto-on | QMS |
| Generate Merged PDF Document Action | Configuration | QMS |
| Generate Document for Standard QMS Processes | Configuration | QMS |
| Standard Cycle Time Metrics: Audit Object & Quality Event Custom Object Type Support | Configuration | QMS |
| Curriculum View on Learner Homepage | Auto-on | Training |
| Admin Alerts: Training Materials, Prerequisite Rules, & Substitute Rules | Auto-on | Training |
| Admin Alerts: Quiz & Recurrence | Configuration | Training |
| Initial Training Due Date | Configuration | Training |
| Training Jobs: New Jobs to Process Training Assignments | Auto-on | Training |
| Imported Training Assignments: Sharing Settings Not Updated | Auto-on | Training |
| Updates to Cancel Training Assignments Job | Auto-on | Training |
| Training Requirement Impact Assessment: Optional Task Assignee | Auto-on | Training |
| Training Requirement Impact Assessment: Action to Re-Evaluate Records | Configuration | Training |
| Assignment Details Creation Allowed for Imported Records | Auto-on | Training |
| Training Materials: Prevent Duplicate Documents | Auto-on | Training |
| Learner Application Role Updates in Sharing Settings | Auto-on | Training |
| Prevent Duplicate Assignment Detail Records | Auto-on | Training |
| HVO Migration Preparation: Field Configuration Limitations | Auto-on | Training |
| eMDR: Usability Enhancements | Configuration | Surveillance |
| Quality Data Model Changes | Auto-on | QMS, QualityDocs, Station Manager, Surveillance, Training |
| Regulatory | ||
| Matched Document Ordering | Auto-on | RIM Submissions |
| Submission Wizard | Configuration | RIM Submissions |
| Global Content Plan | Configuration | RIM Submissions |
| Select Section in Add to Active Dossier Dialog | Auto-on | RIM Submissions, RIM Submissions Archive |
| Show Only Pending Toggle in Active Dossier Editor | Auto-on | RIM Submissions, RIM Submissions Archive |
| Pull Objective Data Enhancements | Auto-on | RIM Registrations |
| Create Related Records Enhancements | Auto-on | RIM Registrations |
| China XSD 1.0 eCTD Publishing & Validation | Configuration | RIM Publishing |
| Asynchronous FDA Gateway Support | Configuration | RIM Publishing |
| Resubmission of Previously Transmitted Submissions | Configuration | RIM Publishing |
| Providing Additional Data in the Publishing Progress Indicator | Auto-on | RIM Publishing |
| Content Plan Creation & Copy Notification Token | Configuration | RIM Submissions, RIM Submissions Archive |
| Generic Token Support for Submission Pharmaceutical Form | Auto-on | RIM Submissions |
| 22R1 RIM Data Model Changes | Auto-on | RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive |
| SiteVault | ||
| User Administration with Multiple Security Policies | Auto-on | SiteVault Enterprise, SiteVault Free |
| Support eConsent Simple Signatures | Auto-on | SiteVault Enterprise, SiteVault Free |
| Veeva eConsent: Additional Signatory Enhancements | Auto-on | SiteVault Enterprise, SiteVault Free |
| Veeva eConsent: Contact Information Update Request | Auto-on | SiteVault Enterprise, SiteVault Free |
| Connected Study Details User Action | Configuration | Site Connect, SiteVault Enterprise, SiteVault Free |
| Sponsor/CRO Information on Documents | Configuration | Site Connect, SiteVault Enterprise, SiteVault Free |
| New Exchangeable Document Type on Connected Studies | Configuration | Site Connect, SiteVault Enterprise, SiteVault Free |
| SiteVault Data Model Changes | Auto-on | Site Connect, SiteVault Enterprise, SiteVault Free |
| QualityOne | ||
| External Collaboration Management on CAR | Configuration | QualityOne |
| QualityOne Team Enhancements | Configuration | QualityOne |
| Manage Invalid Users with Tasks | Configuration | QualityOne |
| Role Restriction & Child Object Team Enablement | Configuration | QualityOne |
| Display Key Team Roles for Filtering & Reporting | Configuration | QualityOne |
| COA Ingestion Enhancements | Auto-on | QualityOne |
| Additional Matching Options for Component Matching Variants | Auto-on | QualityOne |
| Additional Matching Options for Matching Rule Variants | Auto-on | QualityOne |
| Improved COA Analysis Job Logging | Auto-on | QualityOne |
| Risk Management: Detectability Support for Qualitative & Quantitative Risk Matrices | Configuration | QualityOne |
| Product Hierarchy Data Model Extension for Packaging & Materials | Configuration | QualityOne |
| Inspections Compatibility with Extended Product Hierarchy Data Model | Configuration | QualityOne |
| Update View 5 Whys Analysis Action | Configuration | QualityOne |
| RegulatoryOne | ||
| Formulation Questionnaires | Configuration | RegulatoryOne Compliance Management |
| Local Impact Assessment | Configuration | RegulatoryOne Registration & Dossier Management |
| Create Dossier Binder | Configuration | RegulatoryOne Registration & Dossier Management |
| Generate Requirements for Registration Objective | Configuration | RegulatoryOne Registration & Dossier Management |
| Registration Item Hierarchy Viewer | Configuration | RegulatoryOne Registration & Dossier Management |
| Split Registration Items Enhancement: Recursion | Configuration | RegulatoryOne Registration & Dossier Management |
| Track Authority Questions | Configuration | RegulatoryOne Registration & Dossier Management |
| Enable Checklists in RegulatoryOne | Auto-on | RegulatoryOne Compliance Management, RegulatoryOne Registration & Dossier Management, RegulatoryOne Regulatory Documents |
| Product Hierarchy Data Model Extension for Packaging | Configuration | RegulatoryOne Compliance Management, RegulatoryOne Registration & Dossier Management, RegulatoryOne Regulatory Documents |
| Veeva Claims | ||
| Project Hierarchy Viewer | Configuration | Veeva Claims |
| Dynamic Deep Copy Project | Configuration | Veeva Claims |
| Claims UX Enhancement | Auto-on | Veeva Claims |
| Product Hierarchy Data Model Extension for Packaging | Configuration | Veeva Claims |
| Enable Checklists in RegulatoryOne | Auto-on | Veeva Claims |
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.” | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|