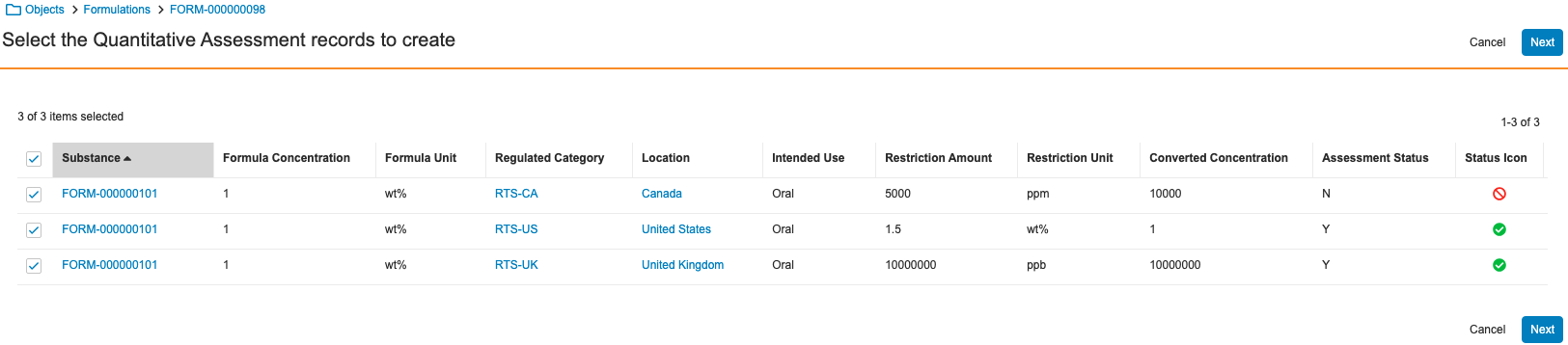
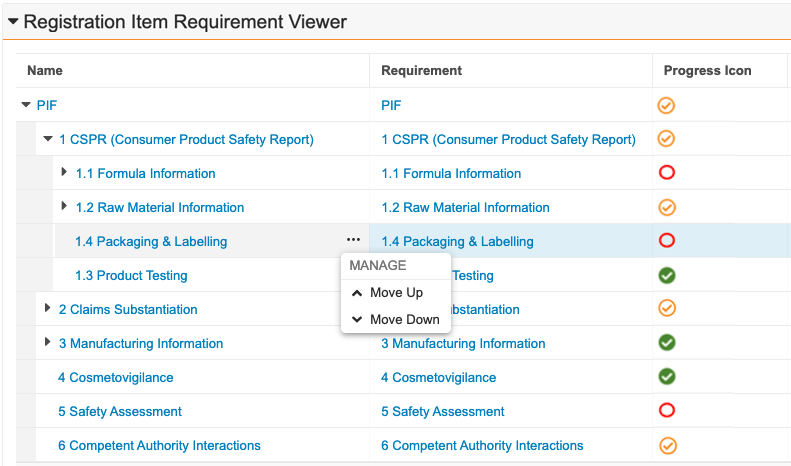
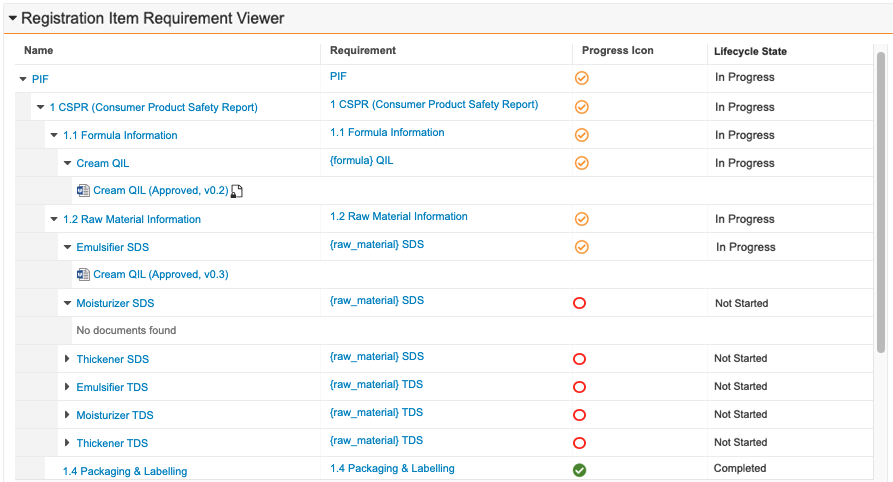
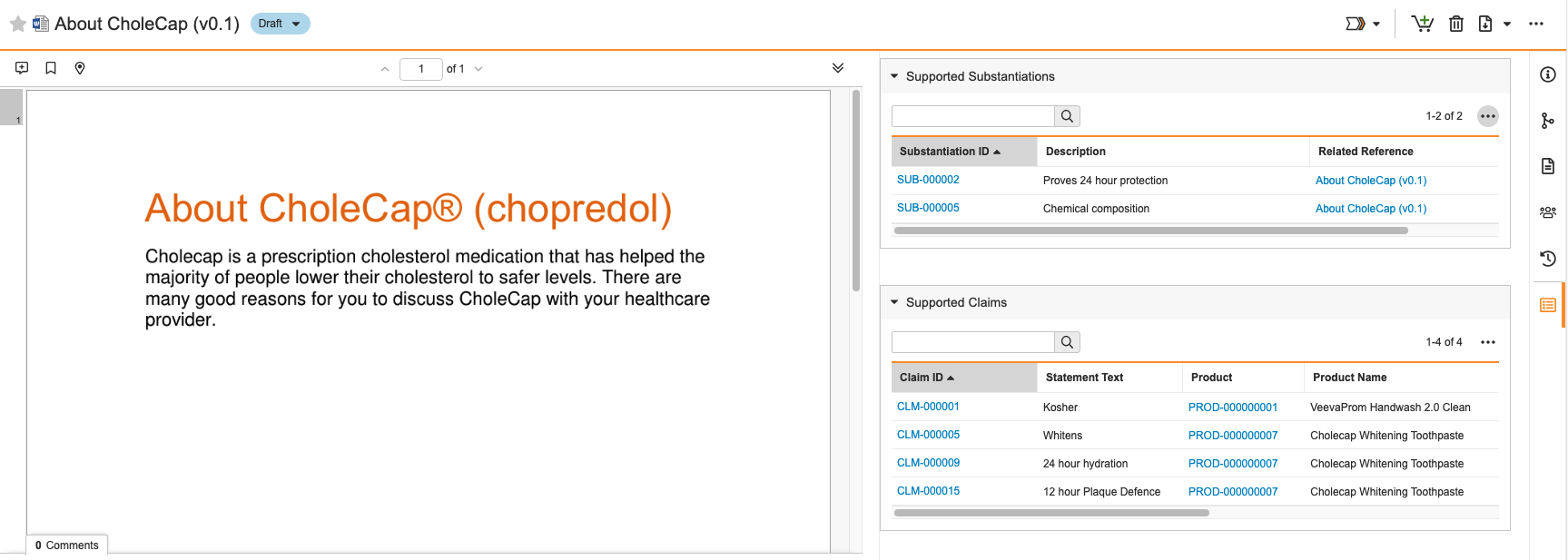
Prerelease Date: October 30, 2023 | Release Date: November 17 2023 & December 1, 2023
The following applications may have different release dates: Safety, QualityOne Client Applications, RegulatoryOne, and Veeva Claims.
We are pleased to bring you Vault 23R3. Read about the new features below. You can find information on enabling new features in 23R3 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Platform
In addition to the below release notes, the Vault Platform Veeva Connect community offers general release communications, release highlights, and key feature demos.
Working with Documents
Lifecycle & Workflow Actions for Collaborative Authoring
With this release, Admins now have the ability to configure both Workflow Action Steps and Entry Actions to automatically start or end a Collaborative Authoring session. The new actions available mirror manual user actions:
- Check out to Microsoft Office
- Check in from Microsoft Office
- Cancel editing in Microsoft Office
Collaborative Authoring is widely adopted and integrated with Authoring Workflows. However, Workflow Owners today need to manually check out/in the documents in their Authoring Workflows, which detracts from a seamless user experience between Collaborative Authoring and Workflows.
Admins can also configure notification steps in workflows that will send a summary notification to the Workflow Owner whenever one of these actions is initiated through a workflow or state change.
Learn more about Collaborative Authoring Workflow Actions.
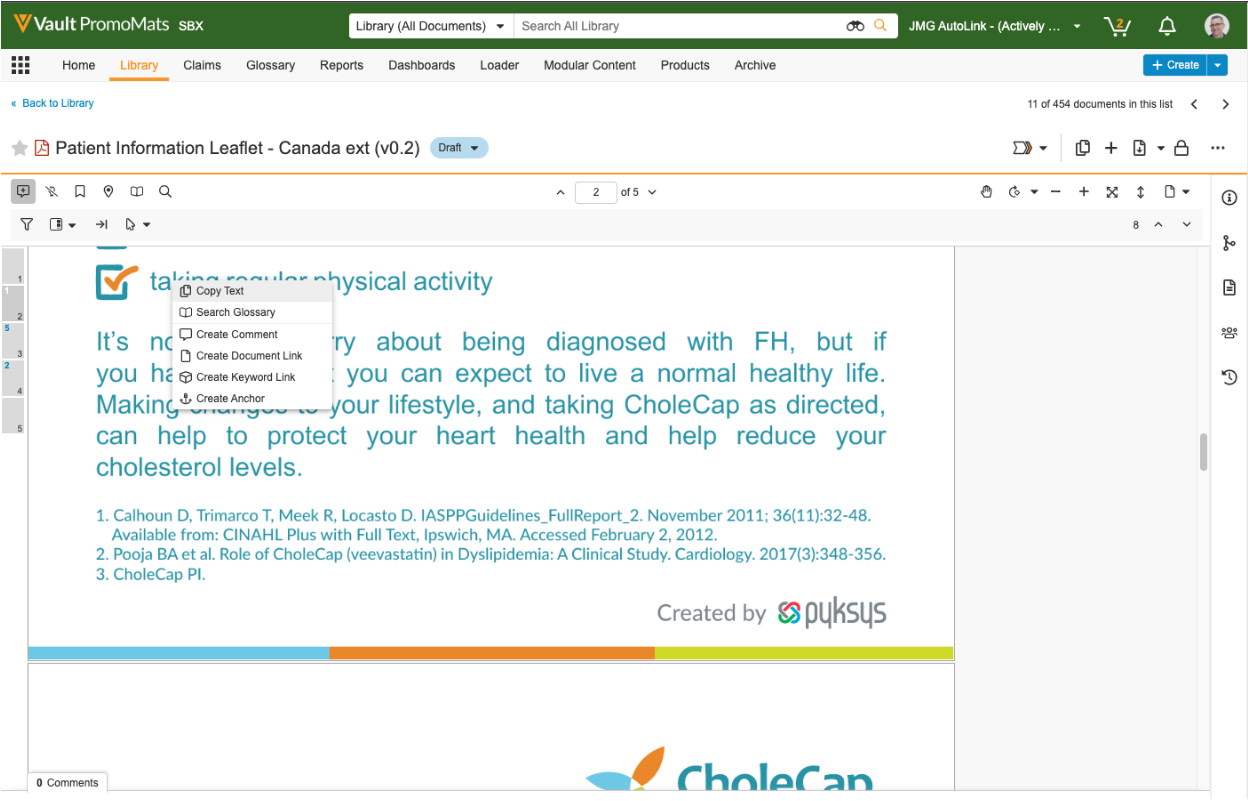
Document Viewer Context Menu
Users can now right-click within the Document Viewer to quickly and intuitively access common actions on document content. This enhancement provides a single point of access to some of the most frequently used features in the Document Viewer, such as copying text, searching the glossary (if enabled), and creating annotations.
Learn more about the Document Viewer context menu.
Improved Merge Fields & Bookmarks Performance
Merge Fields and Bookmarks (from Microsoft Word) will now render faster than ever with improved performance up to 70% for tokens and bookmarks added to Word documents.
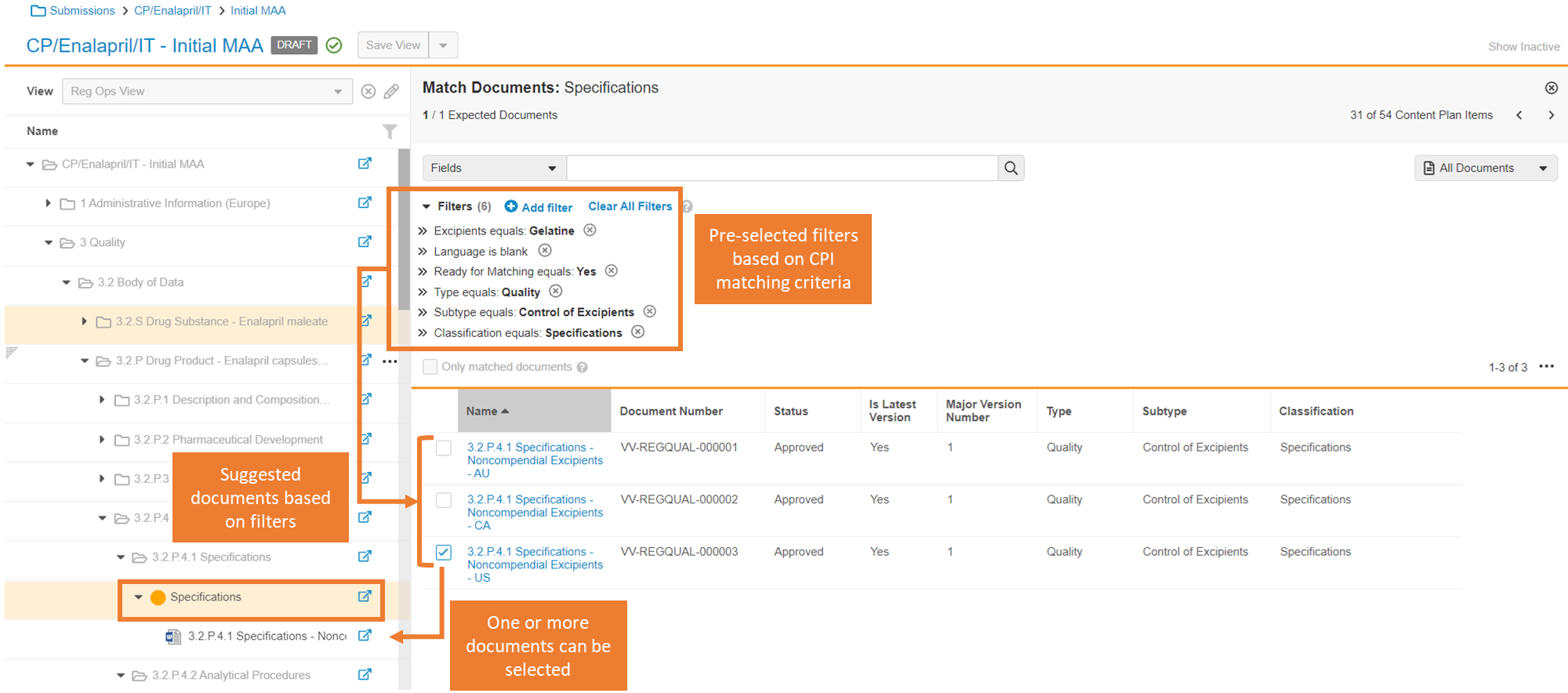
Atomic Security for EDL Item Actions
Admins will now have the ability to leverage Atomic Security on EDL Items (also known as Expected Documents in Clinical and Content Plan Items in RIM) to more granularly control the following actions through Atomic Security: Actions within EDL Item lifecycle states:
- Create Placeholder
- Create Document from Template
- Upload
- Match Documents
RIM and RegulatoryOne Vaults will also support more granular control on these additional actions for manual matching through Atomic Security: Actions within EDL Item lifecycle states:
- Add Document
- Remove Document
- Lock Version
- Unlock Version
- Exclude Document
- Include Document
Atomic Security: Controls will also allow controlling the use of the Add button based on an EDL Item lifecycle state. This will apply for RegulatoryOne Vaults in particular as those Vaults standardly have the Add button for manually matching documents enabled.
These enhancements overall will help RIM customers leveraging Submission Content Planning. When managing Content Plans, there are often scenarios where a subset of users needs to maintain the ability to edit specific fields on Content Plan Items (EDL Items) in specific lifecycle states without still having the ability to change the matching of documents/versions. This will allow the ability to restrict these actions while still allowing users to be able to edit fields in specific EDL Item lifecycle states.
Learn more about atomic security for objects.
Enhancements to Updating Last Matched Date on EDLs
For Vaults leveraging Expected Document List functionality, the Last Matched Time field on Expected Document Lists will no longer update when the matching job runs and no documents were matched or unmatched for Expected Documents in that EDL.
This ensures the Last Matched Time data is more meaningful as it now only updates when Vault actually matches or unmatches documents within a given EDL, and reduces the overall audit entries logged.
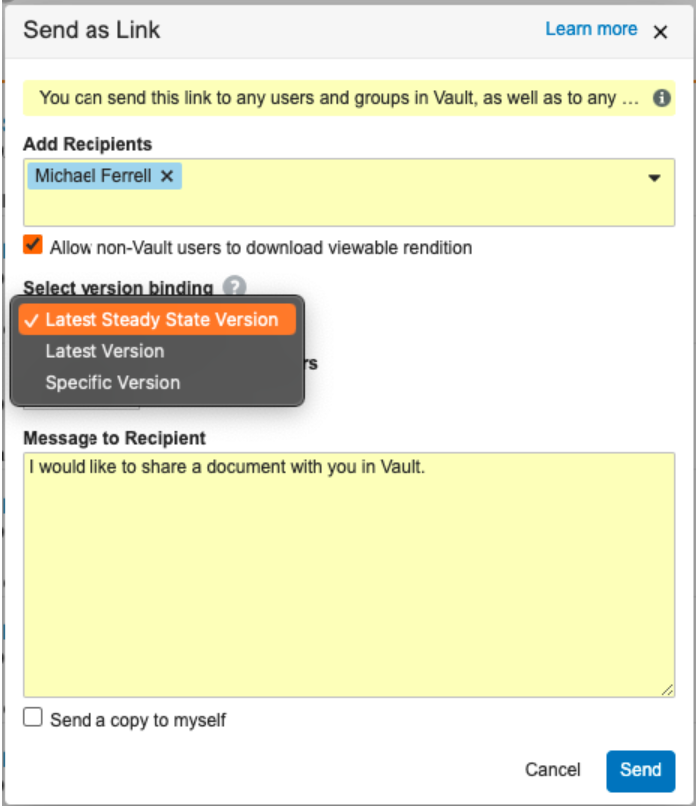
Send as Link Version Binding
When using Send as Link, users can now specify a version binding rule to control access for non-Vault users and to control which version is displayed by default for Vault users.
Options include:
- Latest Steady State Version: This is the default option, but is only available on documents with a Steady State version, which are generally major versions in final states (for example, Effective or Approved).
- Latest Version: This creates a link to the most recent version of the document, regardless of document status. If a new version is created, the link recipients will always see the newest version when accessing the link. This was previously the only option.
- Specific Version: This creates a link to the specific version the user is currently viewing, and the link recipients will always be directed to this specific version.
Full List of Attachments for All Records in the Formatted Output
Users can now include a list of attachments for related objects when generating a Formatted Output. Previously, users had to generate a Formatted Output from each related record to get a complete picture. With this new feature, admins can configure templates to include a list of all relevant attachments in a single output.
For instance, in Vault QMS, the Change Control (parent) formatted output could now include a list of attachments on the Change Actions (child) in addition to the list of attachments on the Change Control itself.
Intelligent Document Update
In 23R2, we introduced Intelligent Record Update, which ensured that a record’s audit trail and Last Modified Date were not updated when no change was made. With 23R3, we are extending this to documents to ensure consistent behavior and reduce unnecessary audit trail entries.
Vault now only updates documents if any changes have been made. Prior to this release, if a document was saved without any changes, Vault updated the Last Modified Date. For customers with integrations or custom SDK code, this often resulted in a large volume of unnecessary audit trail entries.
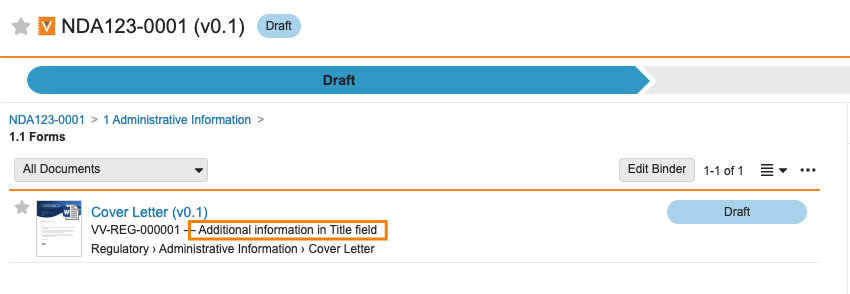
Document Title Displayed in Binder Compact View
Users will now be able to see a document’s Title in addition to the Document Name when viewing documents in a binder using the Compact View. The Title will be displayed next to the Document Number (in the same manner as when viewing documents in the Library Detail View and Compact View).
Title will only be displayed when the field is filled out. Many Vaults have renamed the Title field - two common examples are Additional Information or Description.
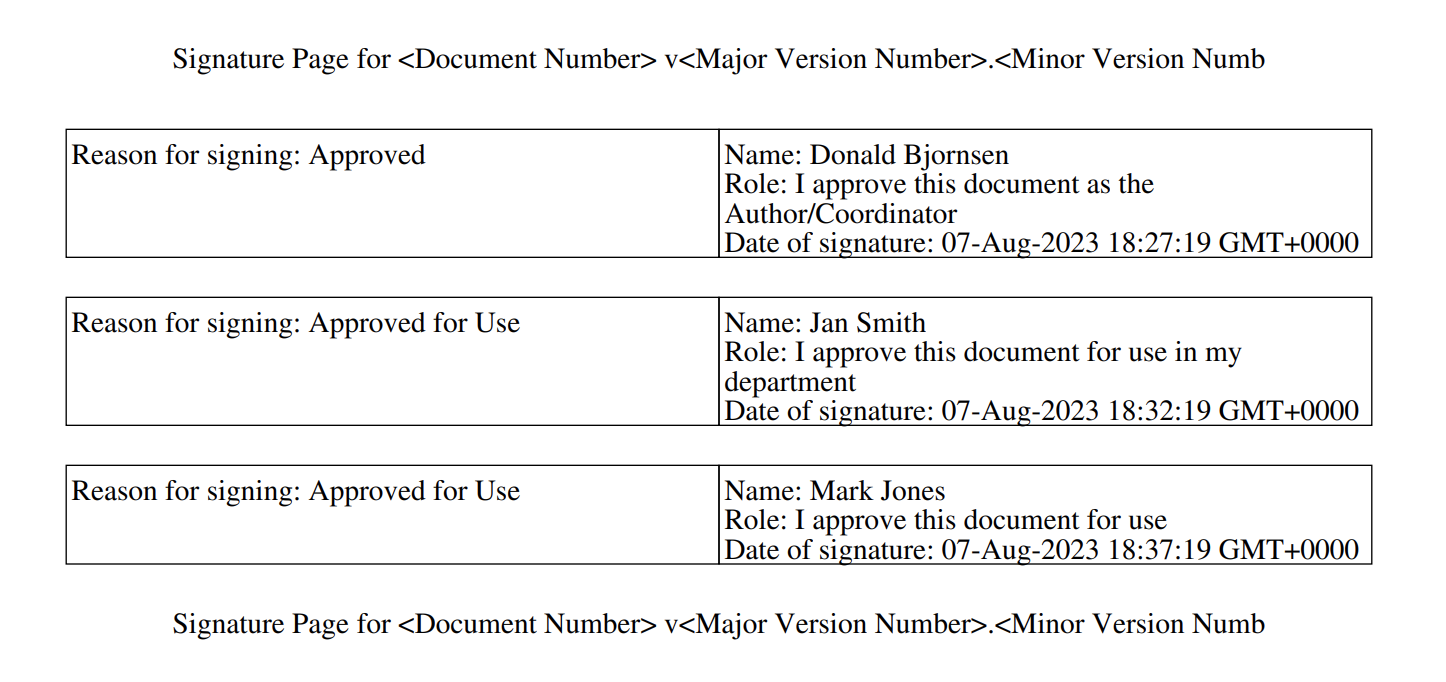
Download Preview for Basic Signature Page Template
Admins will now be able to download previews of basic signature page templates. This allows changes to basic signature page templates to be more easily assessed, avoiding the additional effort of applying a new eSignature to test documents to assess changes.
The preview will display any tokens as translated, user-friendly labels:
Audit Resync with Source CrossLink Action
This feature creates an audit event for the Resync with Source action. When Resync with Source is clicked on a CrossLink document, the message “‘Resync with Source’ initiated” will show up on the audit trail.
Vault Objects
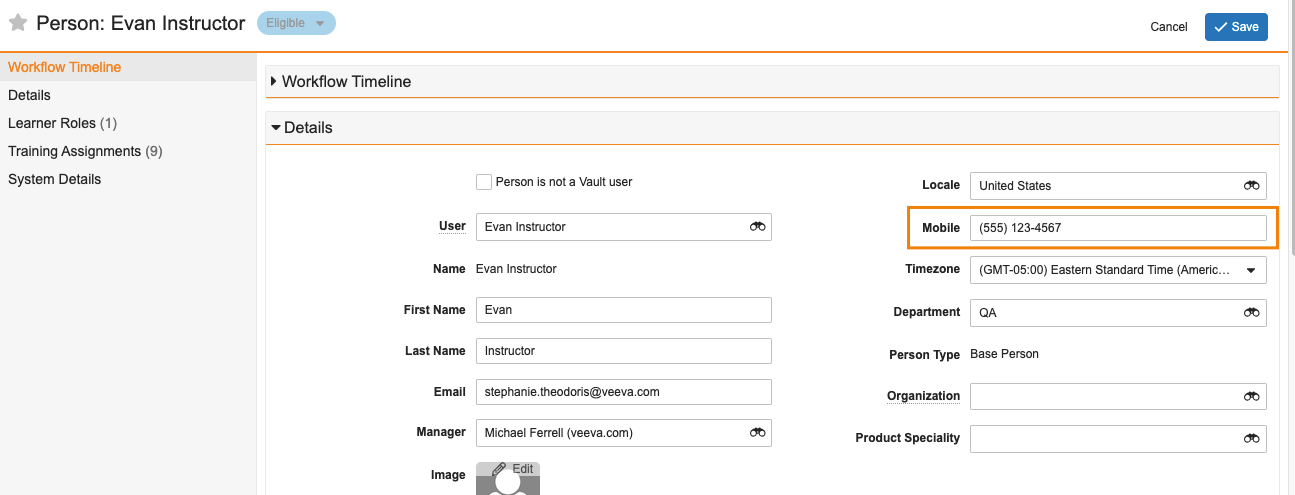
Configurable Format Mask for Standard & System Fields
In 23R2, we introduced Display Format, which gives Admins the ability to configure format masks on Text and Number fields, allowing user input to be displayed in a standardized format, such as displaying a set of numbers as a phone number. With that release, this functionality was only available to be configured on custom (__c) fields.
With 23R3, this is now extended to standard (__v) and system(__sys) fields as well, allowing customers to leverage Display Format on almost any object in Vault, such as applying phone number formatting to the Mobile field on Person.
Format mask configuration remains restricted for certain standard text and number fields:
- First Name, Last Name, and Veeva ID fields on Person objects
- EDL-related objects (EDL, EDL Item, EDL Template, EDL Item Template)
Note: You can configure format masks for standard fields on Checklist-related objects, but those fields will not display correctly on checklist custom views such as previewing a checklist design, using Visual Designer, or completing a checklist.
Learn more about using format masks.
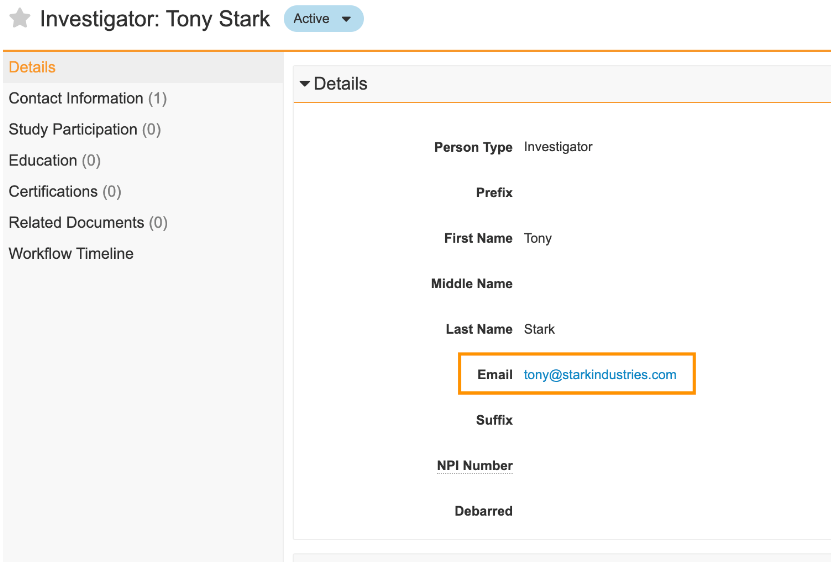
Display Format: Email Link
Admins can now use the Display Format functionality introduced in 23R2 to configure format masks on Text fields to wrap any entered text as “mailto” hyperlinks using a new EmailFormat() function. This function does not check that the user input matches a valid email address format, though this could be accomplished using the Regex() function in a validation rule.
This enhancement will allow users to click an email address hyperlink in Vault to immediately begin drafting an email in the user’s email client.
Display Format: Percent Input
When leveraging Display Format to display number fields as percentages, users will now be able to input numbers as percentages as well. For instance, a user can now enter “25” in a number field to mean “25%”. Prior to 23R3, a user would have needed to enter “0.25” to save “25%”.
This enhancement makes the data entry process more intuitive for users working with percentages.
Learn more about using format masks.
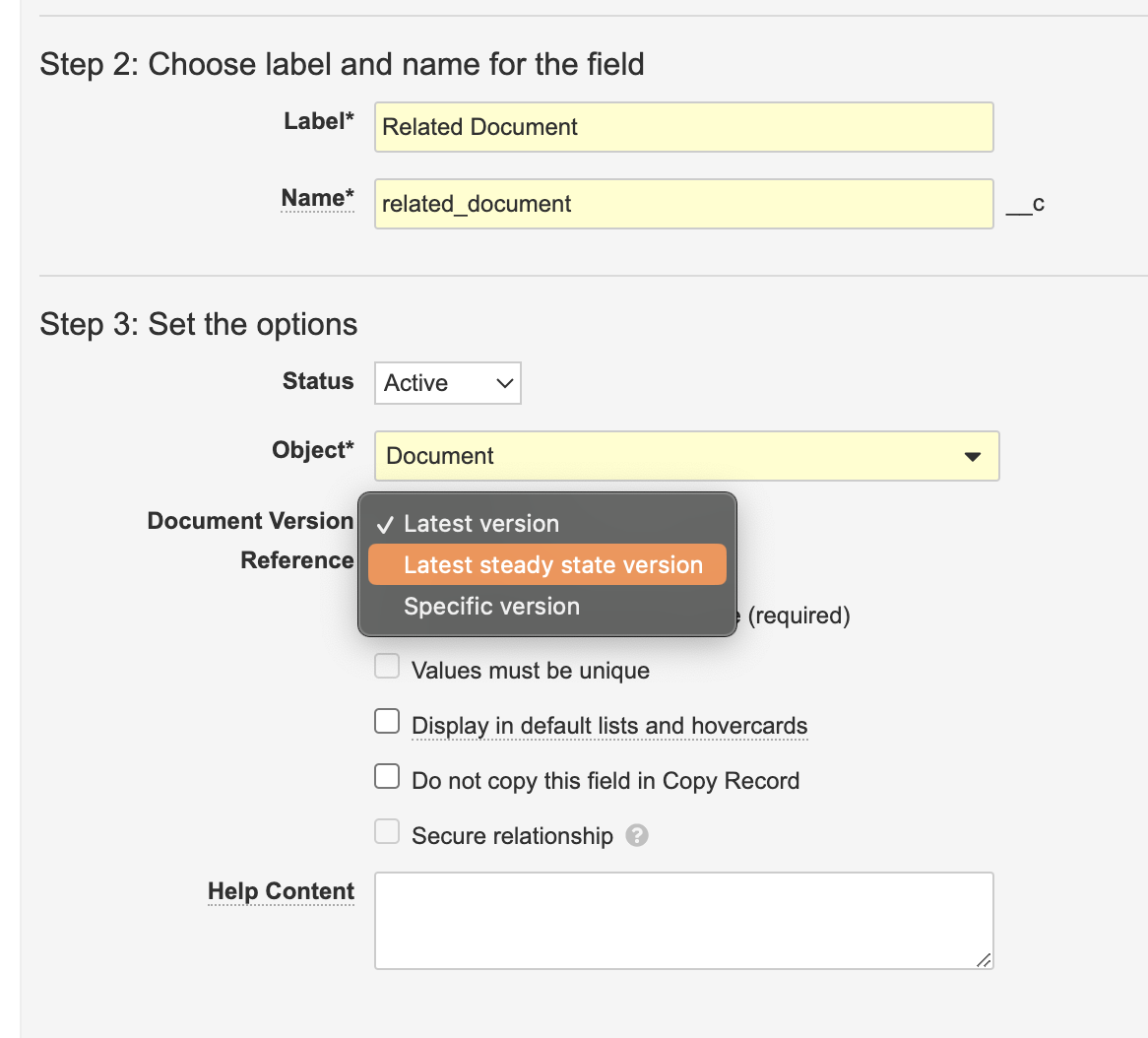
Document Reference: Latest Steady State Version
When configuring Document References fields on Objects, Admins will have a new version binding option called Latest Steady State Version. With this option checked, the Document Reference field will be binded to the latest steady state version of the referenced document.
Prior to 23R3, Document Reference fields had binding options for Latest Version and Specific Version. Adding Latest Steady State Version allows the field to dynamically update as the document is versioned, but only if it is a steady state version.
Learn more about Document Reference fields on Objects.
Vault Object Performance Improvement
We’ve improved our Object Framework to support good consistent performance when working with object records on larger vaults.
Lifecycle & Workflow
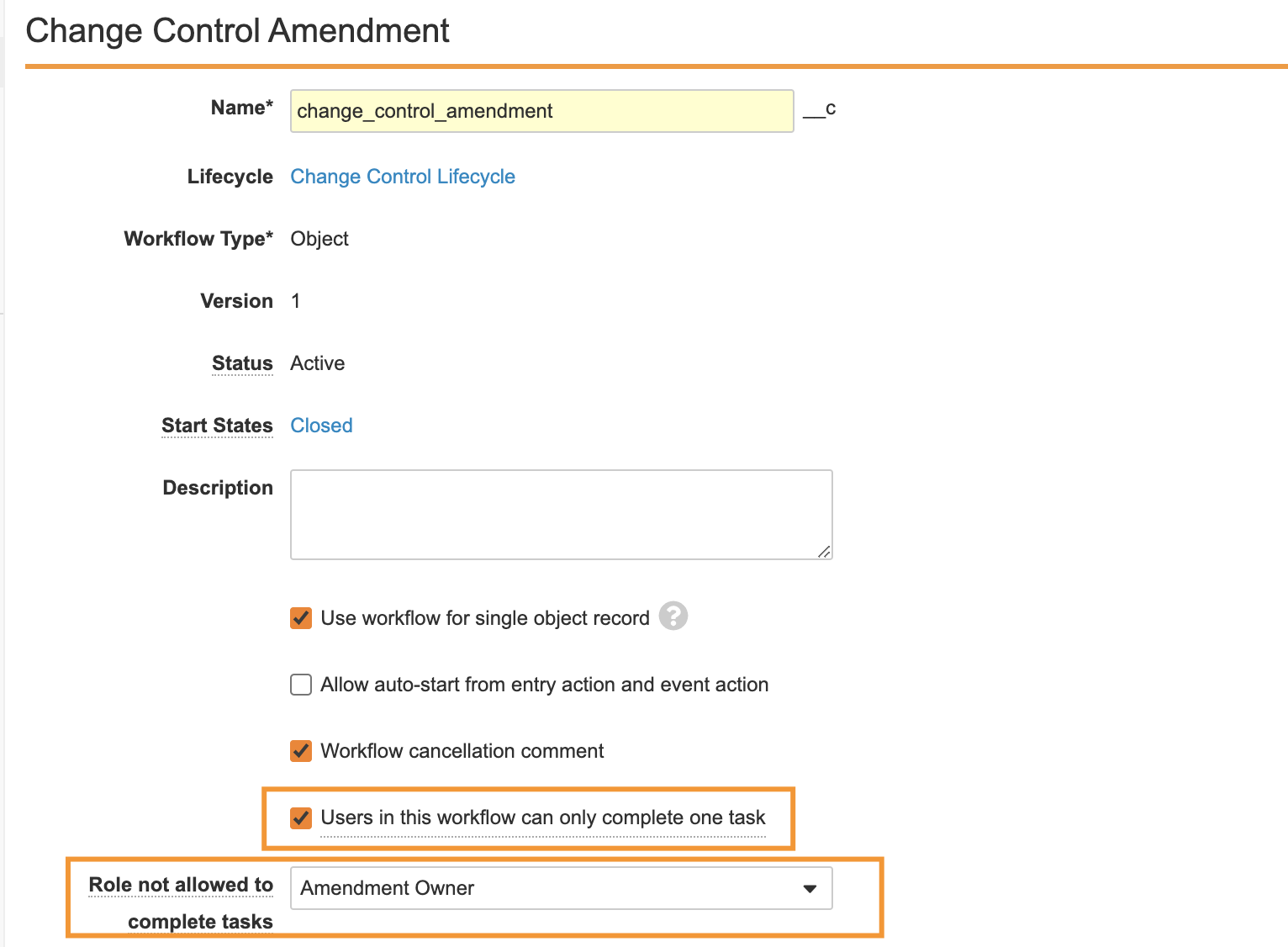
Workflow Segregation of Duties
Workflows now provide the option to enforce policies that preclude certain users from completing tasks in the workflow based on their Role membership or their task completion history in the same workflow.
With this enhancement, two new options are added to the workflow configuration:
- Checkbox: Users in this workflow can only complete one task
- Dropdown: [Role] cannot complete any task in this workflow
This enhancement allows for clearer segregation of duties to prevent non-compliance and errors during approval tasks. This is particularly critical for Quality customers in being able to enforce quality procedures on GxP content.
Learn more about Configuring Document Workflows and Configuring Object Workflows.
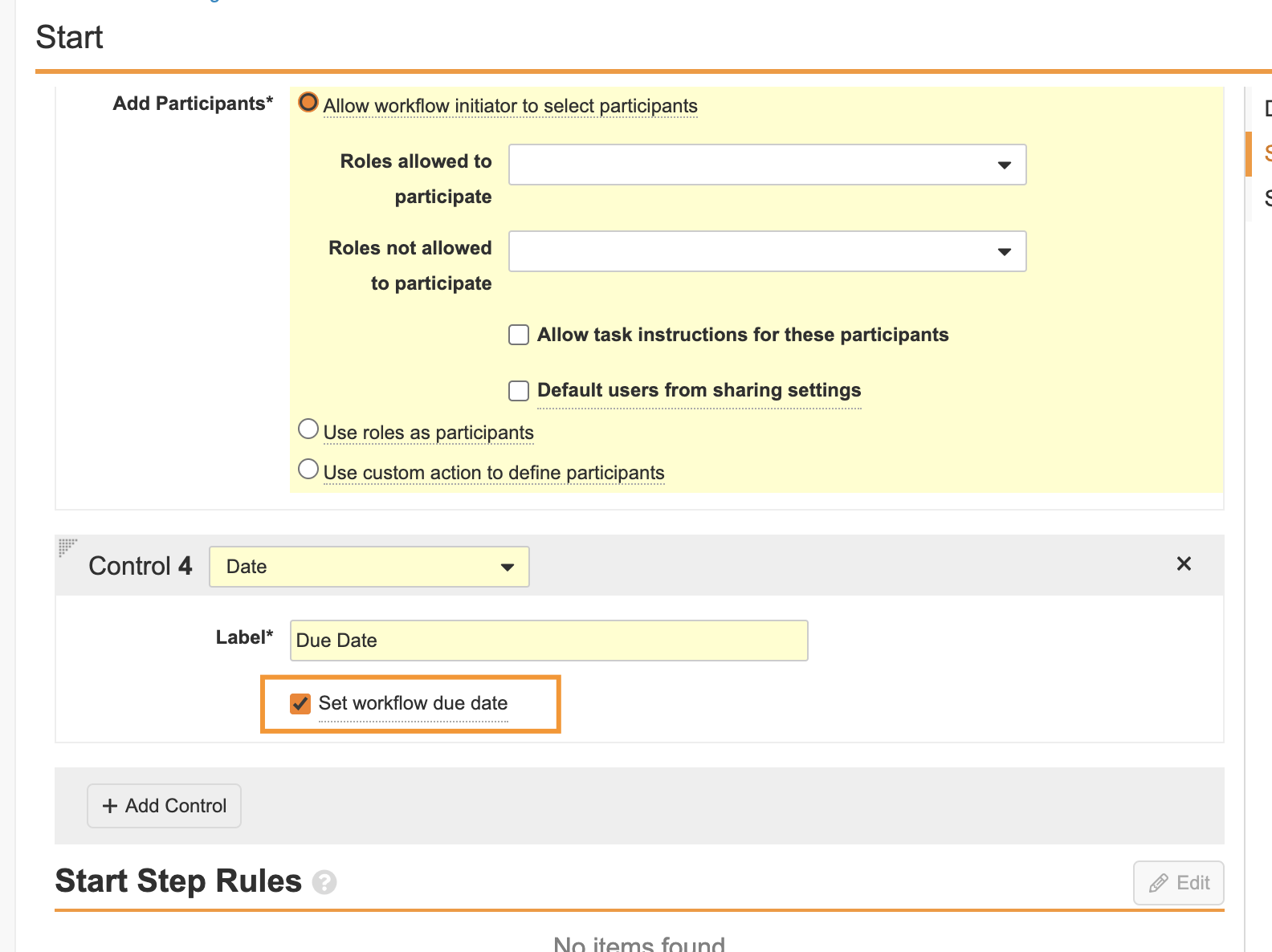
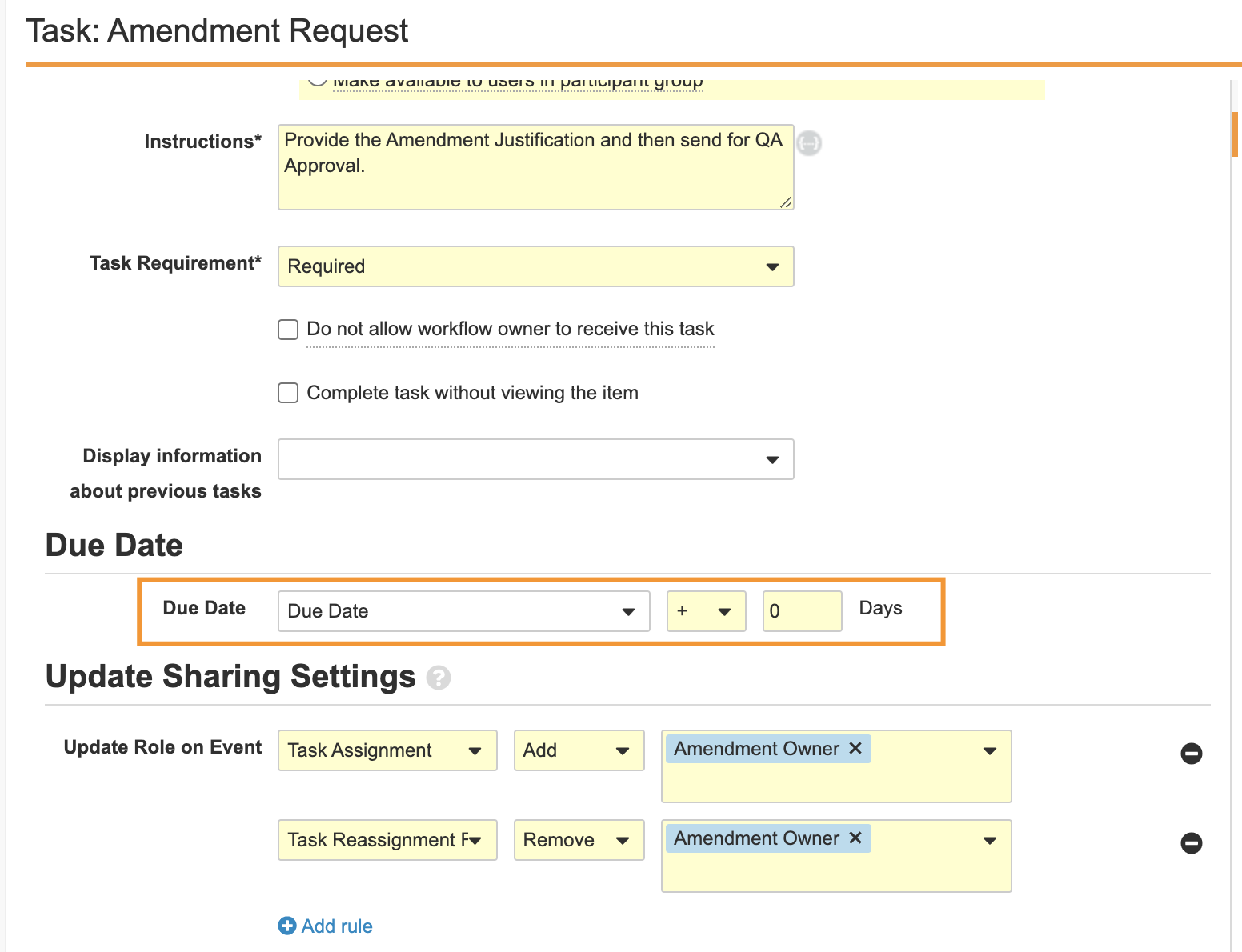
Workflow Due Date
Admins can now set date prompts in workflow Start steps as the workflow due date, enabling the workflow to track its own overall due date, in addition to task due dates.
Workflow due dates also give workflow owners better visibility into the timelines for active workflows.
This enhancement also enables the Active Workflows Home page to display due date indicators for active Workflows when the workflow is approaching its due date or is overdue. Additionally, task due dates that are configured with the workflow due date as their date can be updated in a single action from the Active Workflow actions.
This functionality was previously only supported in Legacy Workflows, but now applies to document and object workflows. This change allows workflow owners to more easily view workflow timelines and update task due dates with fewer clicks.
Learn more about Configuring Document Workflows and Configuring Object Workflows.
Workflow Task Options for Update Sharing Settings Actions on Tasks Made Available to Users
When a workflow task is configured to be Available to users (rather than Assigned to all users) and the task contains Update Sharing Settings actions, Vault now applies the Task Assignment action rule to the user that accepts a task as well as to users that were added to the participant group associated with the task.
Task Completion and Cancellation action rules now apply to the users and groups in the participant group associated with the task and the user currently assigned to the task.
Previously, when users accepted the task, the update sharing settings rules were not applied and when the task was canceled or completed, rules were not applied to groups included in the participant group associated with the task.
This enhancement ensures consistency in applying Update Sharing Settings actions so that the actions take place upon task creation, but also when tasks are accepted or users are added to the group associated with the task. This prevents scenarios where a user is added to an in-progress workflow but is not able to see the record.
Skip Object Lifecycle Event Actions in Record Migration Mode
Vault now skips Create Record object lifecycle event actions when objects are created via Vault Loader or the API with Record Migration Mode enabled.
When performing migrations of data, Event Actions are generally not applicable - allowing these to be skipped via Record Migration Mode helps provide a smoother data migration process and also ensures the functionality is consistent with Document Migration Mode (which already skips Event Actions for Document Lifecycles).
Learn more about event actions.
Document Numbers Available in Document Workflow Notification Template Tokens
A new notification template token is now available for document workflows that includes the document number along with the document name to help users receiving notifications to easily understand which documents are in the workflow.
The new token is labeled: Workflow Documents with Document Number and is available for all envelope object based notification templates.
Many customers work off of Document Number, so this enhancement helps improve the usability of workflow notifications. This enhancement does not apply to Legacy Workflows.
Learn more about configuring notification templates.
Email Participants Notification Template Supports Document Workflow Tokens
The email notification template for the Email Participants workflow action now supports the use of document workflow notification template tokens to provide more information about the workflow and its contents to users receiving the notification.
Learn more about configuring notification templates.
Reporting & Dashboards
Dashboard Resizing
With 23R3, users will be able to drag and resize charts on a canvas-like background, where they can choose the width of the dashboard component.
Prior to 23R3, the options for organizing dashboards were limited to two sizes, and two interfaces (2 columns or 3 columns), with most charts being fixed width based on the number of columns.
This enhancement will allow greater flexibility in how a dashboard is displayed. For instance, this would allow for multiple charts to be expanded in width, allowing for better highlighting more than one key metric, or for allowing wider charts to be able to view more data at a glance.
Learn more about resizing dashboards.
Report on Multi-Record Workflows
Vault now supports reporting on multi-record workflows. Multi-record workflows were introduced in 22R3 to allow users to send multiple object records in a single workflow, increasing productivity and streamlining the user experience. With this enhancement, customers can now report on the details of multi-record workflows.
Customers do not need to update existing reports. This change automatically applies to any existing Workflow with [Object] report types, where the workflow is configured to allow multiple records to be sent together. This enhancement is not applicable for matrix reports.
Learn more about reports and object workflows.
Display Format in Reports
In 23R2, we introduced the ability to configure Display Formats on Text and Number fields for objects using format masks. This allows for data such as phone numbers and percentages to be displayed in a standard format. With 23R3, we are extending this functionality to ensure that the configured format is how data is displayed in report filters, conditional fields, prompts and dashboard prompts.
This ensures consistency for users when interacting with this data both on the objects themselves as well as in reports on those objects.
Learn more about Creating Reports and Creating Dashboards.
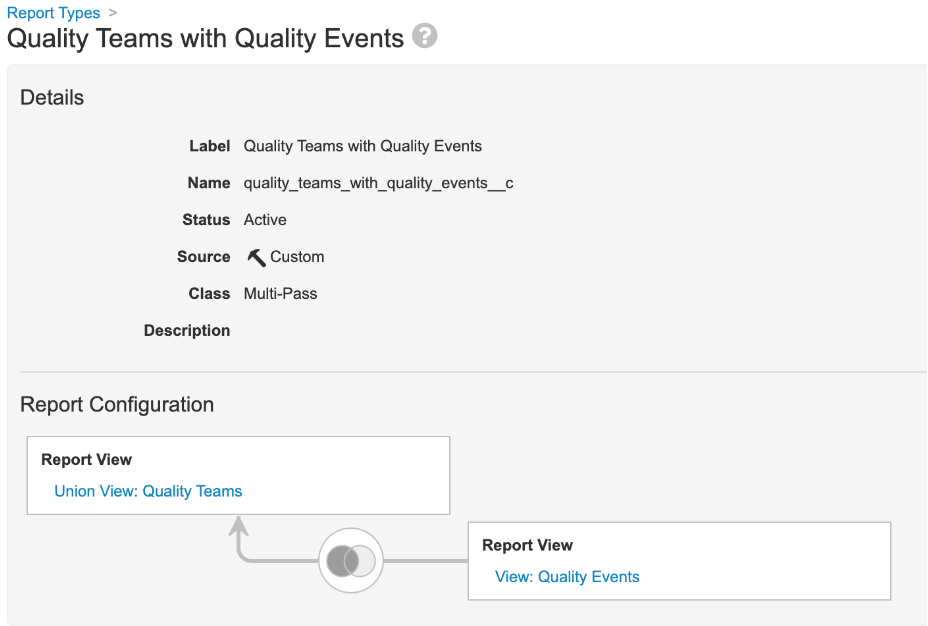
Support Union-All View Join with Other Views
In 23R2, we introduced the Union-All report type, which allows users to combine different objects into a single object, displaying the results as stacked rows in the same columns. With 23R3, this functionality is being extended. Customers can now join Union-All report views with other report views in multi-pass reporting.
For instance, in QMS Vaults leveraging Quality Teams, you could leverage a Union-All report to combine Quality Team Member objects together in a single object, and then join that report to a Quality Event report.
Last Ran in Export Cover Page Report
With this feature, customers can now see the report’s Last Ran date on the Excel export cover page. This would apply when exporting to a formatted excel and a user selects the Add a cover page option.
A new ${runDateTime} token has also been added, which can then be added to the PDF export and Excel template cover pages.
Combine Report Prompts Enhancement
Reports that prompt for the same data (name, data type) across different objects with the same operator will now be able to be combined into a single prompt using the Combine Report Prompts option added in 23R2. Prior to 23R3, Vault could only combine prompts when the data was being prompted on the same object multiple times in a report.
This enhancement expands that capability, further reducing the potential for users to need to enter the same data multiple times when running a report.
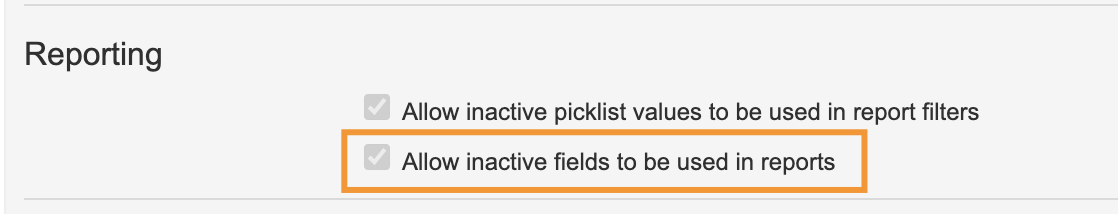
Hide Inactive Fields in Reports
Admins now have the option to hide inactive fields in reports. These inactive fields are available for reporting by default. A new setting has been added for Admins to hide the inactive fields in report views and reports.
Existing reports with inactive fields will continue to work and show the fields even if Admins decide to hide inactive fields.
Workflow with Object Report Enhancement
As of this release, Task Duration and Workflow Duration are populated in workflow reports regardless of the status of the task.
User groupings are based on user name and not by user ID, making it consistent with other report types.
Search & Filtering
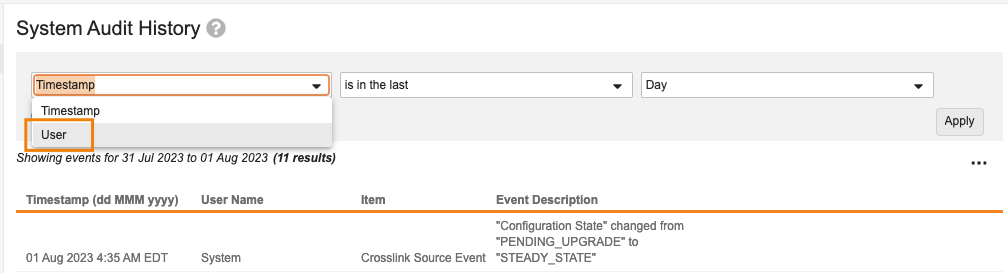
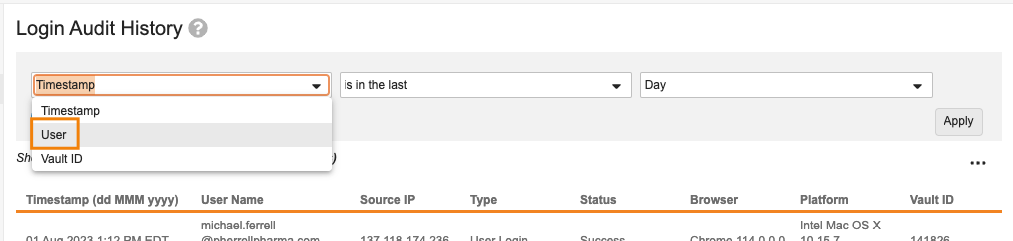
User Filter in Login & System Audit Logs
Admins with access to the Logs tab will now be able to leverage a User filter on the Login Audit History and System Audit History pages. This will enable Admins to more easily find the number of logins or configuration changes made by a specific user.
Adding a filter for User can be used in addition to or in place of the Timestamp filter. If User is applied as a filter, but not Timestamp, Vault will return results for all time.
No Results Redirect
When a user visits a searchable tab which defaults to any view other than All, text searches that produce no results will automatically redirect to the All view where the user may find better results. This occurs if the search is the first action a user performs after landing on the tab. If a user has intentionally clicked on a Recent view or added/removed filters, Vault will not redirect.
Many times, users may not even notice that they are not on the All view when performing a search. This can result in extra clicks as the user would receive no results, and then need to change views and re-perform the search. This feature will eliminate those extra clicks by automatically redirecting them to the corresponding All view.
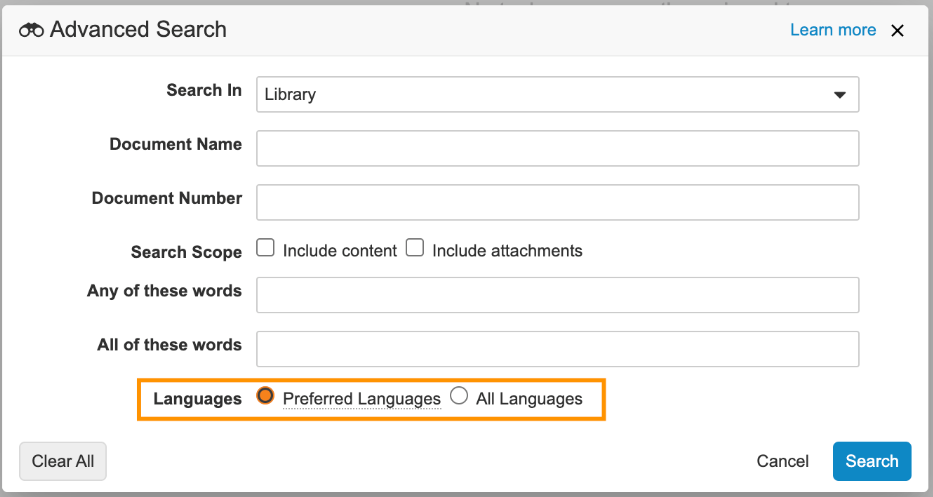
Language Preferences
For Vaults using multilingual capabilities, users performing searches will now only see results in the Language set on their User record. If needed, users can use Advanced Search to expand their search to records in any language.
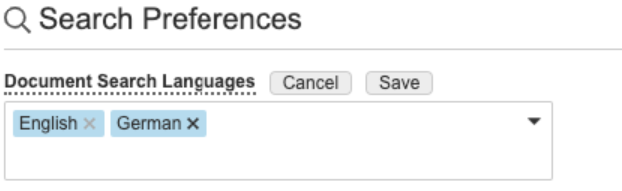
Additionally, a Search Preferences section is now available on the User Profile page to allow users to add additional preferred languages to be leveraged in search. For instance, if a user’s primary language on their User account is German, but they also need to standardly see English documents in their search results, they can add English as an additional preferred language in their User Profile.
Prior to 23R2, when a search was performed, Vault would attempt to search across all languages. This enhancement will improve search performance by reducing the amount of data that Vault needs to search through before returning results, and also benefits users by ensuring that Vault only returns the most relevant results by only returning results based on the user’s language.
With the initial release of this feature, each user’s Search Preferences will be set automatically based on documents they have viewed, created, or edited. On an ongoing basis, Vault will also automatically update a user’s Search Preferences when a user creates or updates documents in a new language.
Learn more about Language & Region Settings.
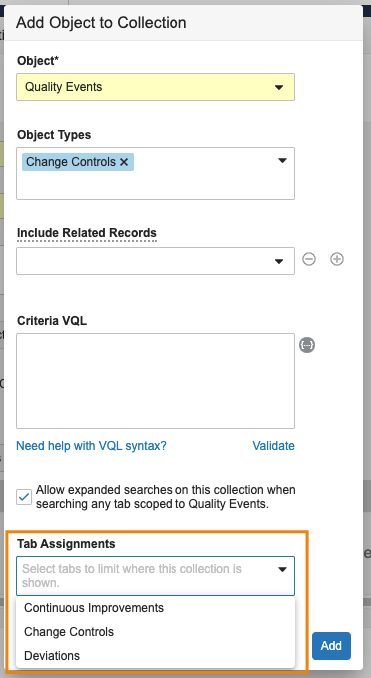
Assign Search Collection by Tab
Admins now have the ability to assign Search Collections to specific tabs. Prior to 23R3, search collections could be assigned to all tabs scoped to a particular object. For QMS customers in particular, Quality Events are broken down into separate tabs by object type (Change Control, Deviation, Complaint, or others), and it’s common that QMS customers need to set up separate search collections for each type of Quality Event. This resulted in all Quality Event search collections being available on every Quality Event tab.
With this enhancement, when there are multiple search collections pointing to the same object, Admins will be able to specify which tab each collection should be available on. This reduces the duplication of collections in the QMS use case, but also ensures that users are able to focus on searching the appropriate collection.
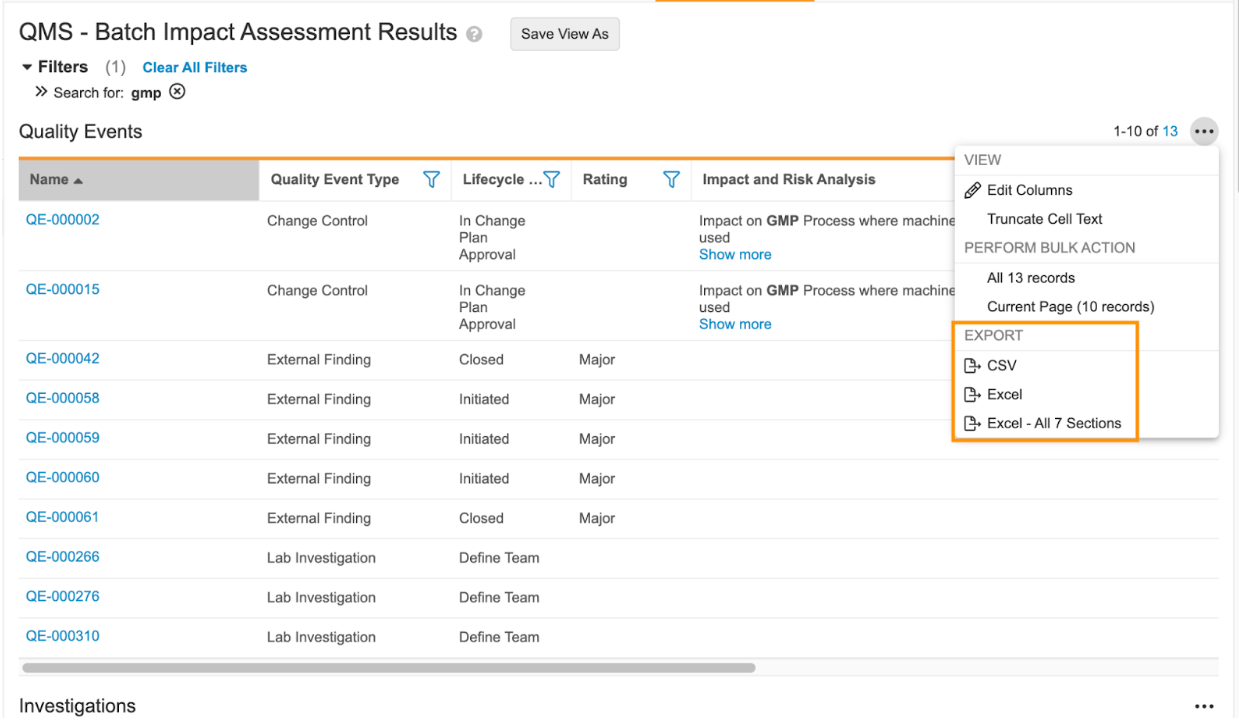
Export All Expanded Search Sections to Excel
With 23R3, for customers leveraging Expanded Search functionality, users now have an action on each section to export all search results across all sections to a single Excel file.
Many customers use exports to Excel to provide critical information to auditors. This enhancement reduces user effort in performing multiple exports across different objects (when using Expanded Search).
Learn more about configuring search collections to enable Expanded Search.
Formulas
Vault Formulas: Standard Deviation, Median, Mode & CountA
Vault formulas now support the following additional functions:
Median(): This function returns the median of the given numbers.Mode(): This function returns the mode of the given numbers.CountA(): This function returns the number of populated values.StDevP(): This function returns the standard deviation for an entire population.StDevS(): This function returns the standard deviation for a sample representing a population.
These functions are needed by new Vault applications, such as Vault LIMS, which needs to provide their customers with more statistical functions. Median() has also been a frequently requested function from customers across all Vault applications.
Learn more about Vault formulas.
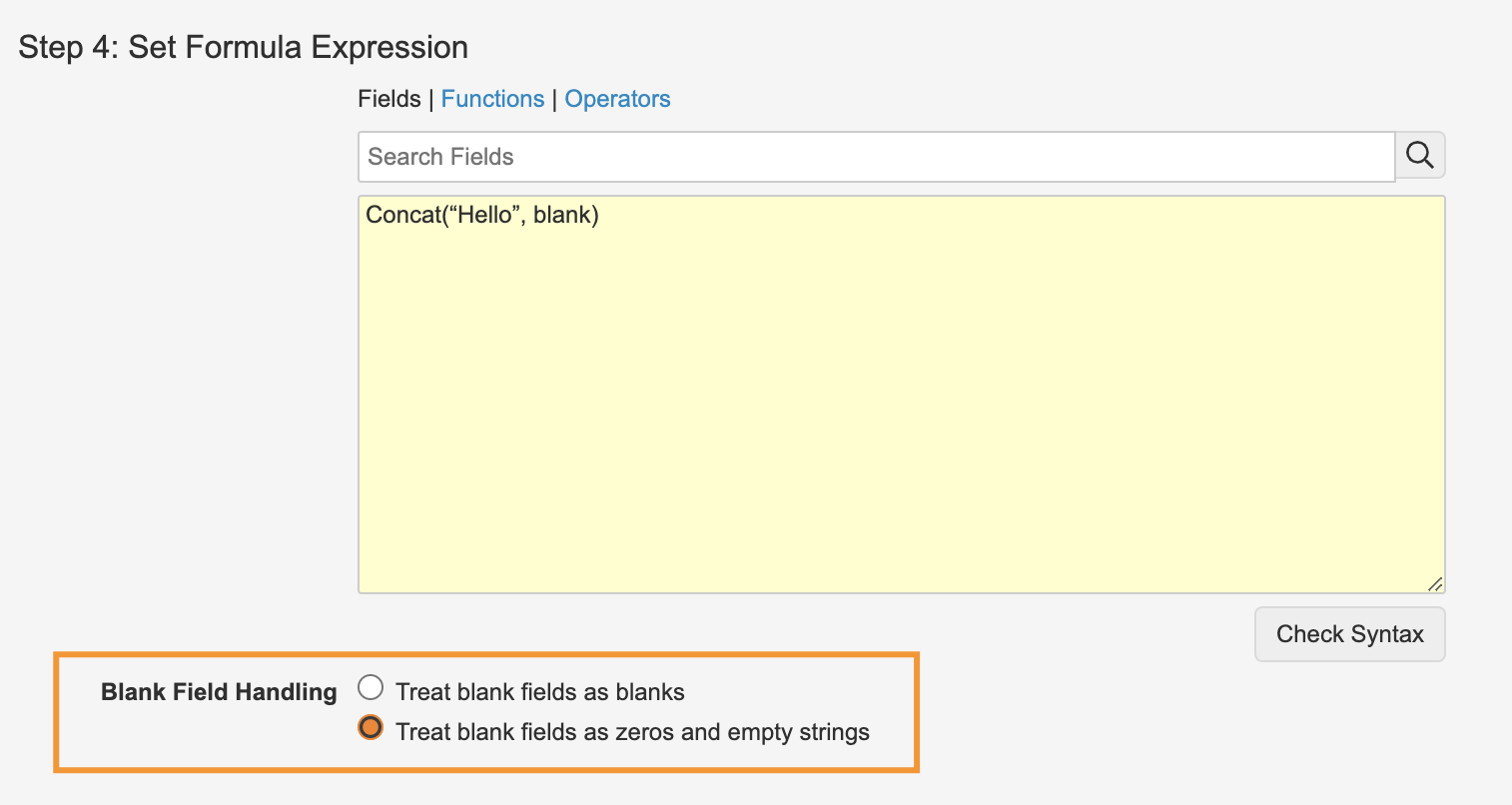
Enhanced Blank Handling in Object Formula Fields
When leveraging Vault formulas in object formula fields, users now have more flexibility in how blank values are handled. Users are now given the choice to continue treating blanks as blanks, the default behavior to date, or to handle them as zeros or empty strings.
If the latter option is chosen, functions like 5 + blank will return 5 instead of blank and Concat(“Hello”, blank) will return Hello instead of blank.
This enables customers to write simpler functions that would normally require multiple IsBlank and If functions and brings the behavior more in line with how Excel handles blank values.
Learn more about Vault formulas.
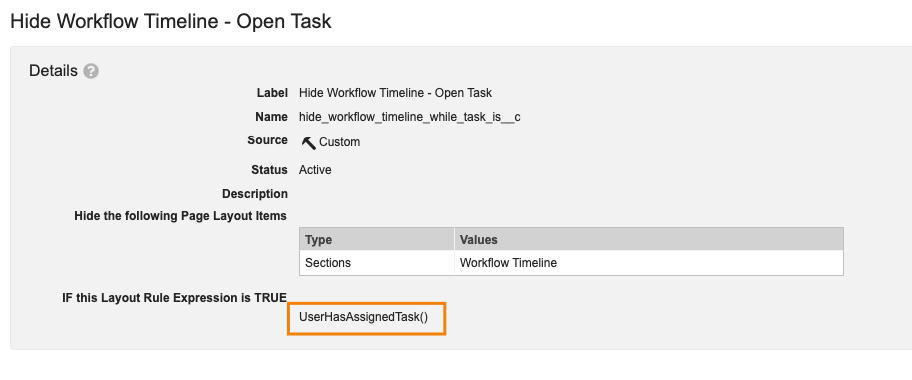
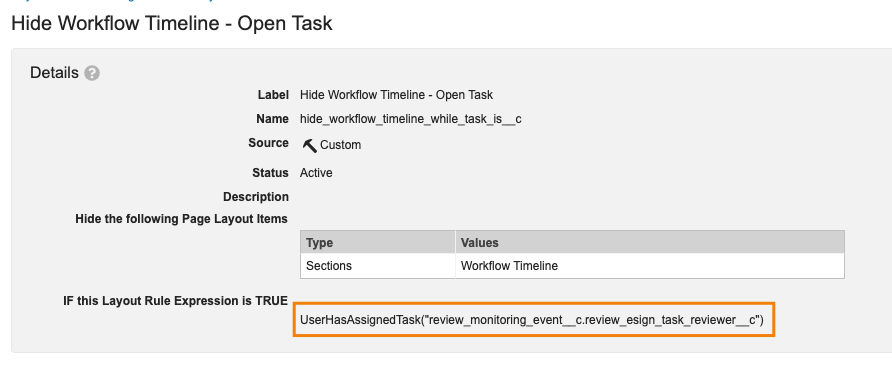
Vault Formula Function: UserHasAssignedTask()
For customers leveraging Layout Rules, Vault now supports a new function for hiding layout sections, fields, or controls for users who have a task assigned on that object record: UserHasAssignedTask().
Being able to hide information for users in this way can help streamline the layout and view for the user by removing information that is not necessary for them to see when they are focusing on task completion.
This function can be leveraged without specifying a particular task, which would apply the rule for a given user anytime they have any assigned task. For instance, in this example the Workflow Timeline would be hidden for any user who is viewing the record while having any open task:
Admins can also specify a particular task in the parentheses. For example, this would specifically hide the Workflow Timeline for a user when they are assigned the Review & eSign Task only:
When specifying a particular task, the format should be WorkflowName.TaskName. Note that this feature only applies to tasks that are Assigned - this would not apply to tasks that are Available, Completed or Cancelled.
Access Control
VeevaID: Registration, Login, & Portal Access
VeevaID is an Identity Provider (IdP) system for Veeva Applications (such as Study Portal, SiteVault Free, Vault Training). VeevaID allows end-users (Clinical Site Staff) to self-register, access applications they are subscribed to, and maintain their account.
Both Vault Applications as well as non-Vault applications can send registration notifications to users. Once registered with their VeevaID and application memberships, a user can use their VeevaID credentials to log on to different Vault as well as leverage those same VeevaID credentials to access non-Vault applications.
VeevaID and Study Portal will eliminate the need for clinical site staff in particular to manage and keep track of different credentials across the various applications and sponsors they work with.
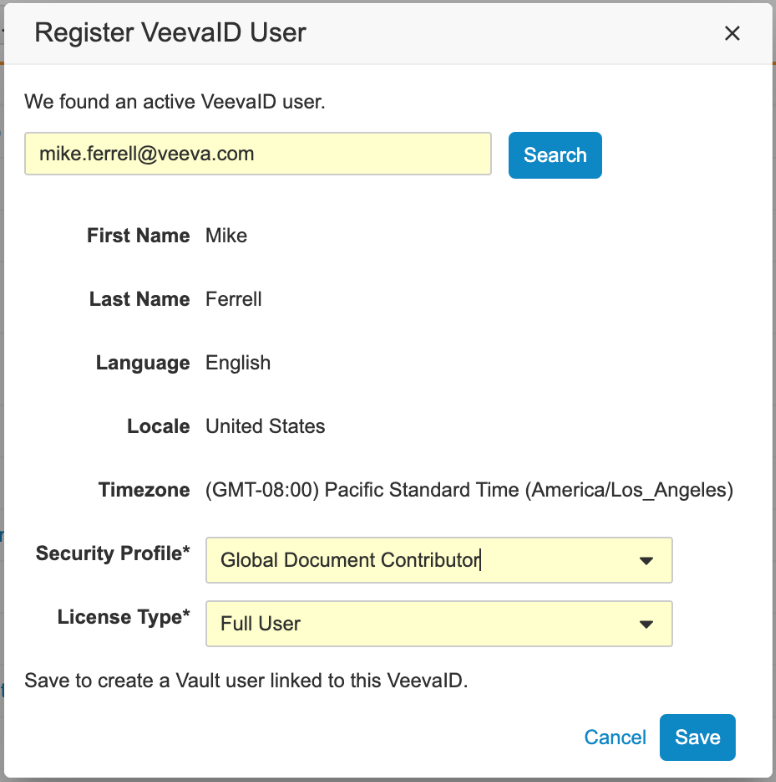
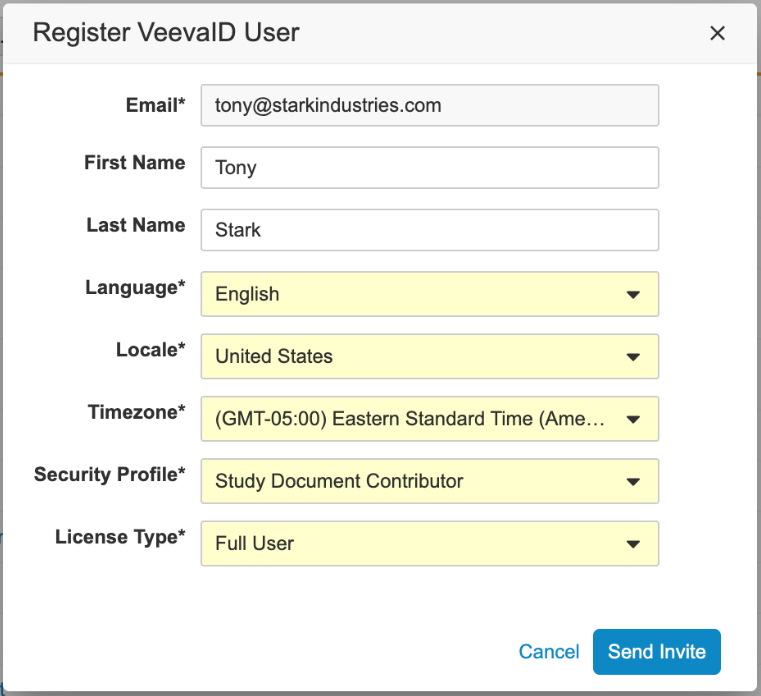
A VeevaID user can register for a VeevaID account by responding to an invitation email, or by going to id.veeva.com. To register, user’s will enter their first and last name, email, language, locale and timezone.
Once registered, users will be able to login to the VeevaID portal to access any systems that they have been granted access to with their VeevaID account.
VeevaID: Vault Platform Integration
The Vault platform allows the registration of an existing or new VeevaID user in a Vault. Once VeevaID users are added to a Vault, an administrator can manage their access in the same way as for any other Vault user. When a VeevaID user is added to a Vault, users are assigned to a system-managed VeevaID policy.
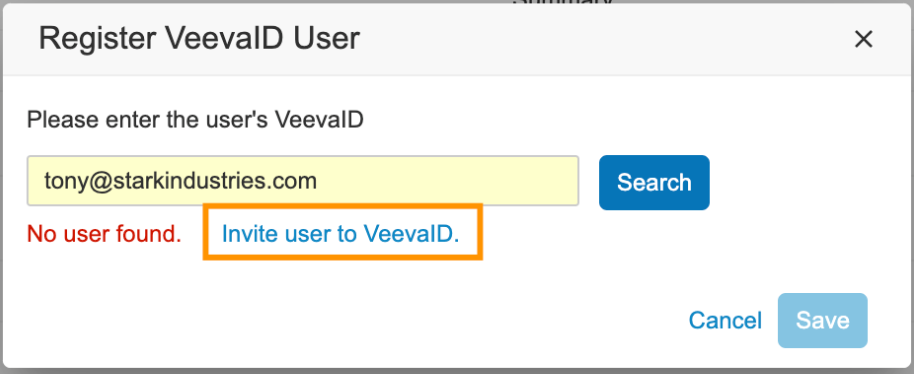
If a VeevaID account does not exist, admins will also have the option to directly send an invitation to VeevaID.
Once added to one or more Vaults, a VeevaID user is able to login to Vaults they have access to from the standard https:/login.veevavault.com URL, using their VeevaID credentials, as well as login to Vaults they have access to using Vault Mobile.
Administration
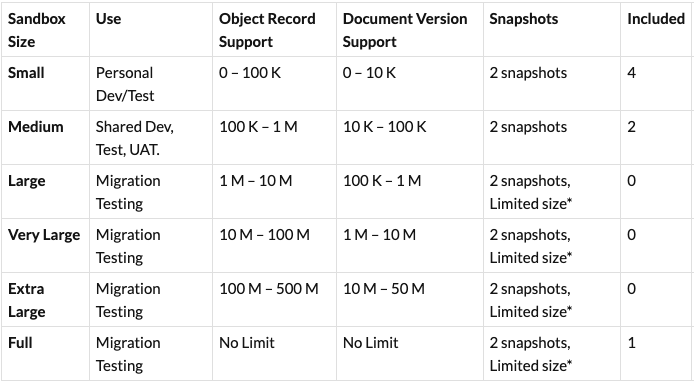
Additional Sandbox Sizes in Vault
Starting in 23R3, we will further adjust sandbox sizes as shown in the table below. These changes in 23R3 are in response to working with and listening to customers as they refine their environment management process.
*Snapshots on Large, Very Large, Extra Large, and Full sandboxes are limited to 10 million records, 100,000 document versions, and 50 GB of source content.
The specific changes with 23R3 include:
- Customers will now receive 4 Small, 2 Medium, and 1 Full sandbox with their production license. Existing Large sandboxes will be converted to Medium in 23R3.
- The document version limit on a Small sandbox is increased from 2K to 10K. The limit on the number of Vault Owners will be removed.
- The document version limit on a 23R3 Medium sandbox is increased from 10K to 100K when compared to an existing Large sandbox.
- This object record limit on a 23R3 Medium sandbox is decreased from 2M to 1M when compared to an existing Large sandbox.
- Three new sandbox sizes are introduced in 23R3: Large, Very Large, and Extra Large. These are not included with a production license but provide add-on purchase options for customers that are priced based on data volumes, not based on production license amount.
- Customers are no longer able to purchase add-on Full sandboxes.
Learn more about Sandboxes and Sandbox Snapshots.
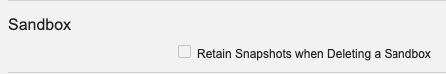
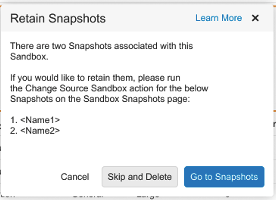
Retaining Snapshots for Deleted Sandboxes
Admins will now have the ability to retain Sandbox Snapshots for a Sandbox when it is deleted. Prior to 23R3, when a Sandbox was deleted, any associated Snapshots from that Sandbox were deleted as well.
This enhancement removes the potential need to maintain a Sandbox only for the purpose of maintaining its Snapshot, and provides customers with greater flexibility in environment management.
After 23R3, if the Retain Snapshots when Deleting a Sandbox feature is enabled by an Admin, Vault will warn the user when attempting to delete a Sandbox, and advise them to run a new Change Source Sandbox action on the Snapshot if it should be maintained.
Ignore Configuration Object Records Towards Sandbox Limits
When calculating the total number of object records for sandbox usage, Vault will automatically exclude system-managed objects as well as objects that are used in Vault configuration (and therefore standardly included when cloning and refreshing Vaults).
This will provide customers greater flexibility with sandbox size data limits by excluding objects that are always present. An example of this would be Controlled Vocabularies in RIM Vaults. This is a standard set of object records that is included whenever a RIM Vault is cloned or refreshed, so these objects will no longer count towards the sandbox data limit.
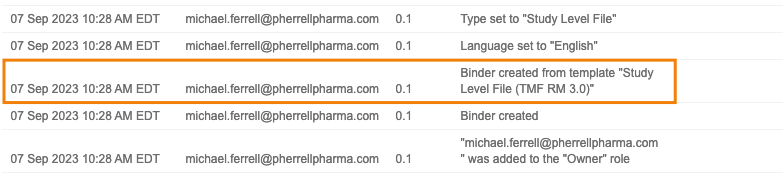
Audit Event for Binders Created from Template
When creating a binder from a binder template, Vault will now log this as an event in the binder’s audit trail to clearly indicate which template was used for creation. For customers leveraging binder templates, this will make it possible to identify the template used. Prior to 23R3, this was not tracked and was not readily determinable.
Learn more about binder templates.
Object References in Field Rules
Field Rules can now be configured with a Target Field Lookup (target_field_lookup) that allows admins to configure object reference lookups to be used when evaluating field rules. For example, Admins can configure a field rule for a child object that uses parent__cr.name__v for an object reference instead of using IDs. This eliminates the need to maintain reference lookup values, and instead developers can code their integrations to dynamically resolve object references.
Evaluate Field Rules: Reference Lookups & Default Values
When evaluating field rules using IntegrationRuleService#evaluateFieldRules, if the field rule configures a reference lookup and a default value, Vault now returns the default value instead of an error when there is no match in the reference lookup.
This applies when:
- The field rule is for an integration rule with Documents as the primary query object
- The query object is a relationship to a related object (
__vror__cr; meaning the query result data is a subquery) - The field rule has both a reference lookup and a field default
- The related object’s query field is not populated
This enhancement helps reduce unnecessary errors and user exception messages in Vault connections.
We encourage developers to review any custom code and configuration to ensure their field rules are evaluated properly.
Usability Updates
Sticky Create New Reference Record
When selecting a related object record in an object reference field, the option to create a new record is now frozen in the drop-down so that it is always easily accessible. This eliminates the need for a user to scroll through the entire list of available records to get to the Create option.
This enhancement applies to object reference fields that have the Allow create new reference record option enabled.
Large Video Rendition Support
Vault can now render video files up to a size of 100 GB and up to a video duration of 2 hours. Prior to 23R3, video files above 4 GB were not rendered. Videos that were uploaded prior to 23R3 within our supported limits can be re-rendered for a viewable rendition.
Expanding this limit enables users to upload and share larger, higher-resolution videos - in particular, Commercial customers who use PromoMats as a Digital Asset Management (DAM) solution.
Email to Vault: Improved Handling for Bounced Emails & Processing Failures
For Vaults leveraging email processors, Vault will now create Email records for bounced inbound emails if:
- The email exceeded the size limit (30 MB per email, including attachments), or
- The email was sent from an email address that is not considered an Allowed Sender
Prior to 23R3, Vault created Bounced Email records if the email was marked as spam or the sender failed SPF or DKIM authenticity checks. This enhancement expands the tracking of errors allowing Admins to report on additional types of bounced emails, inspect these emails, and take the next steps to try sending these emails again without needing to review the email log.
A Failure Reason field is also added to Email records to make it easier to troubleshoot ingested emails that were not successfully processed by the email processor.
Finally, an audit trail is also now available for Email records to make it easier to track the transition of Email records across different lifecycle states.
Checklists
Checklist: Store Score for Each Question
For customers leveraging Checklist functionality, Vault now stores score data on each checklist question. Prior to 23R3, this data was only available on each checklist section and on the overall checklist.
Adding this data to each question enables customers to report on average scores for each checklist question, which is a commonly used key performance indicator.
This feature does not apply to quizzes used in Quality Training and Study Training as the Points and Learner Points fields are used to calculate quiz scores.
Learn more about Checklist Scoring & Weighting.
Vault File Manager
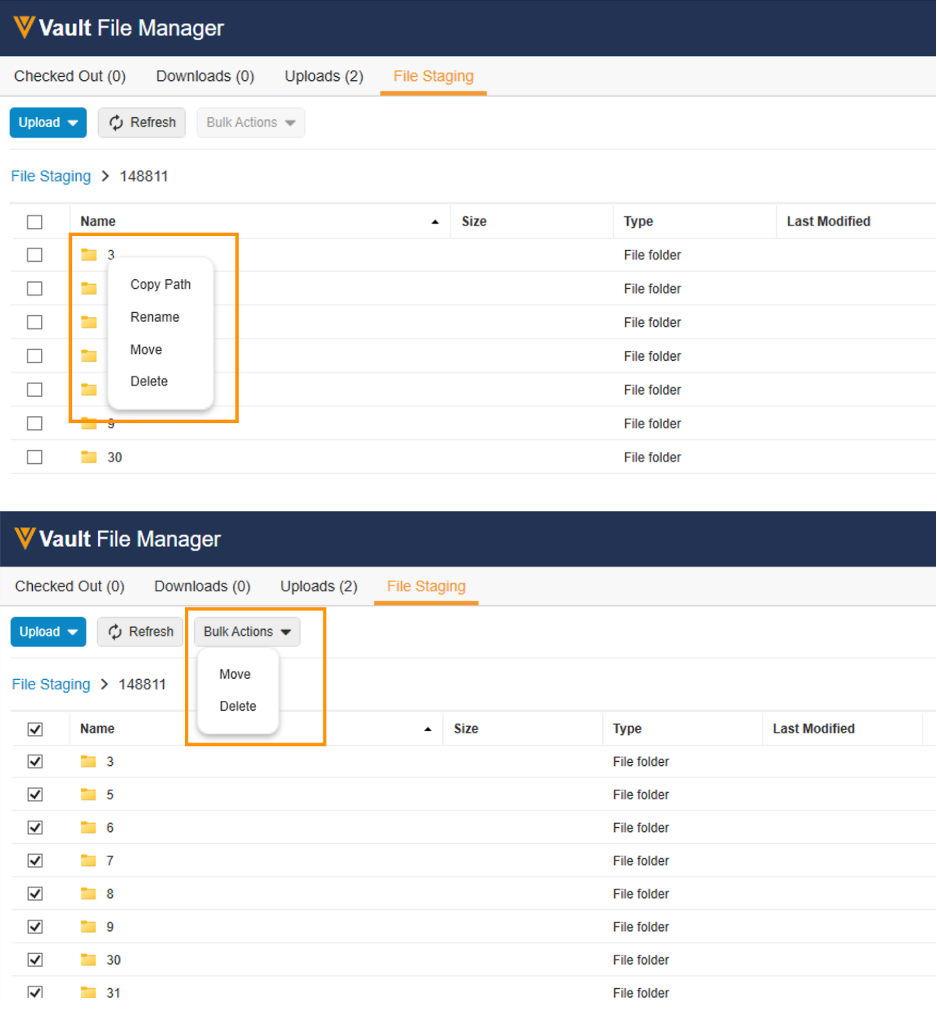
Move, Rename & Delete File Staging Contents with VFM
In 23R2, we enhanced Vault File Manager with a new File Staging tab, allowing Admins to stage content in Vault File Manager for importing into Vault, rather than using a separate file transfer protocol secure (FTPS) client.
With 23R3, this is being further enhanced to allow customers to perform the following additional actions within the File Staging tab in Vault File Manager:
- Rename and delete folders/files
- Move files between folders
Additionally, users can copy the file staging path for documents to more easily add that information into a CSV file for Vault Loader. These actions can be performed on individual files/folders (using right-click), or in bulk (by selecting multiple items and using the Bulk Actions dropdown).
Learn more about File Staging.
Default File Size Increased to 500GB
The default file size limit for Vault File Manager is now 500 GB, up from 400 GB by default previously. Prior to 23R3, customers could request the limit be increased from 400 GB to 500 GB if necessary, and with this release, we are removing the need for customers to request this increase.
Vault Mobile
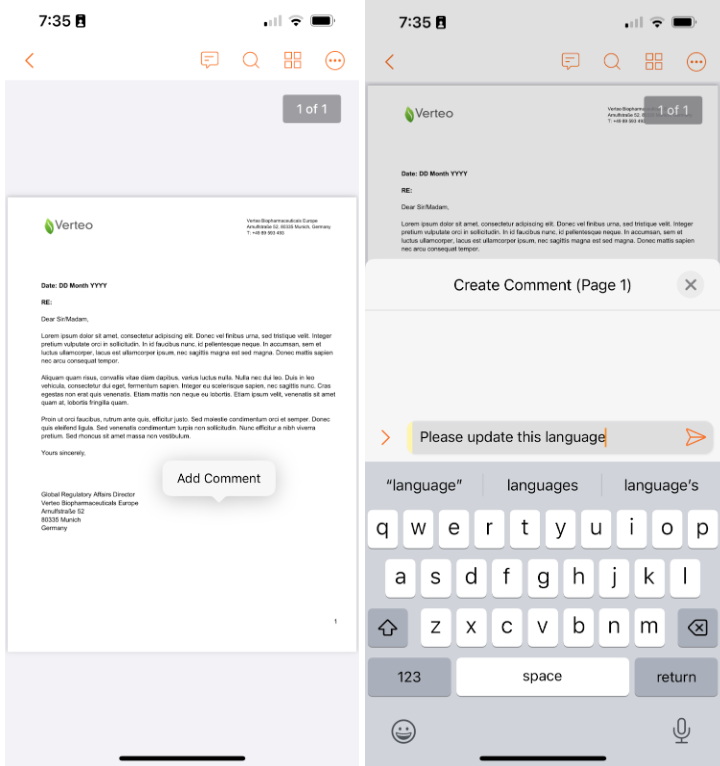
Create & Reply to Document Comments
Users can now view, reply to, and create document comment annotations via the Vault Mobile app. Most review workflows in Vault leverage annotations for reviewers to be able to provide feedback - allowing the ability for comment annotations to be managed in Vault Mobile allows these review workflows to be performed without needing to re-route to the browser.
VeevaID Login Support
VeevaID is an Identity Provider (IdP) system for Veeva Applications (such as CTV, SiteVault Free, Vault Training) being introduced in 23R3. VeevaID allows end-users (Clinical Site Staff) to self-register, access applications they are subscribed to, and maintain their account. With this enhancement, VeevaID users will also be able to log into the Vault Mobile app to access their Vaults.
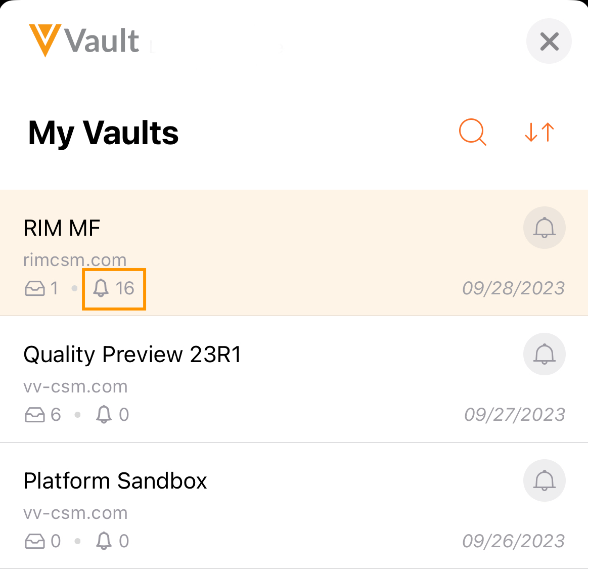
Display Notification Count in Vault Selector
Users can now see their Vault notification count from the Vault Selector list in Vault Mobile, making it easier for users to see how many unseen notifications they have across their Vaults so they can stay better informed.
Additionally, login audit events will only be triggered if the user actively accesses a given Vault in the mobile app. Prior to 23R3, the mobile app authenticated into each Vault a user had access, triggering a login audit event to every Vault, even if the user didn’t actually switch into that Vault.
Support for Android Tablets and Landscape Mode
Vault Mobile can now be downloaded and used on Android tablets. Additionally, the app can be used in landscape mode (horizontally) to accommodate tablet cases (for Android tablets and iPads).
Enhanced Document Viewer (Android Only)
Viewing documents in the Android Vault Mobile app has been enhanced to now include in-document text search and thumbnail view. This brings feature parity with the iOS Vault Mobile app, which already supports document text search and thumbnails.
Platform Data Model Changes
See 23R3 Platform Data Model Changes.
Vault Connections
RIM to Clinical Operations Vault Connection
RIM/Clinical: Default Study on Clinical CrossLinks based on Product field
RIM documents can be specific to a product family, and adding studies to them is consequently only a technical requirement needed for the document to be successfully generated in Clinical Operations Vaults via the connection.
With this enhancement, the behavior of the RIM to Clinical Operations Connection, will now work as such:
- If no Study field is populated on a RIM document to be CrossLinked, then the connection will auto-populate the Study field in Vault Clinical Operations based on the Active Lead Agent Product associated with the RIM document.
- If a Study field is populated on a RIM document to be CrossLinked, then the connection will respect the Study and populate the CrossLinks with the Study from RIM
This enhancement aims to reduce manual workload, and promote compliance.
When a CrossLink is created and a Study field is populated using this feature, if a new Study Product record is created, there will be no automatic reprocessing of CrossLinks to add additional Studies to the documents. In order for new Studies to be added the source documents will need to be modified in order for a metadata sync to occur.
RIM/Clinical: Improved User Exception Message Processing for Document Transfer
This feature improves how Vault handles User Exception Messages (UEMs) by:
- Preventing the creation of duplicate messages for existing issues for documents
- Retrying the processing of connection items related to documents that failed in previous job runs
- Revising the wording of UEMs for common failures of document creation to give clearer guidance
Administrators managing the connection between Vault RIM and Vault Clinical will no longer be required to review duplicate UEM records. Furthermore, they will receive more assistance in identifying and resolving common issues related to connection processing failures.
The behavior change of this feature only impacts RIM Vaults with an active RIM to Clinical Operations Vault Connection. Clinical Operations Vaults with an active connection will continue to see the same behavior as before this feature.
RIM/Clinical: Superseded Document Transfer Revamp
When the RIM to Clinops Connection: Transfer steady and superseded state document versions setting is enabled, Vault applies Query Object Rules (QORs) across all eligible versions for transfer, rather than just the most-recently-Approved version.
Additionally:
- Vault no longer supports QORs with IN clauses when this setting is enabled.
- The RIM to Clinops Connection: Transfer steady and superseded state document versions is now Support-enabled and no longer appears on the Application Settings page.
RIM/Clinical Operations: Remove Superseded Transfer Flag from Admin UI in RIM
The Transfer steady and superseded state document versions feature flag was removed from the RIM Vault Admin section. The flag is now only available to be turned on via a support request to Veeva.
Safety-RIM Vault Connection
RIM/Safety: Registered Product for Safety Cases
The Safety-RIM Product Connection automatically transfers Product Family, Product, Product Variant, Substance, Product Registration, and other records from RIM to Vault Safety. This automatic transfer from RIM to Vault Safety aligns product and registration data across these application families. This eliminates potential user errors of manually creating these records in Vault Safety and provides a common set of products in RIM against which to record Safety Cases.
Quality to RIM Vault Connection
QMS Feature Parity for QMS<>RIM
With this release, customers can connect Vault QMS directly to their RIM Vault in order to facilitate streamlined regulatory impact assessments using the standalone Change Control object. The functionality available in the Quality to RIM Vault Connection is now fully supported regardless of which QMS model (Quality Event versus standalone Change Control) your organization leverages.
This feature adds new fields to the standalone Change Control object in Vault QMS in support of the ability to connect to RIM vaults. It requires configuration to be enabled.
Quality to Clinical Operations Connection
Quality-Clinical Operations: Issue Management Connector
Clinical teams identify, triage, and resolve issues encountered in the course of their clinical operations. Some issues require the quality assurance (QA) organization to investigate, perform root cause analyses, and institute corrective and preventative actions (CAPAs).
The Quality-Clinical Operations Issue Management Connection allows Vault Clinical Operations users to send an Issue to the QMS application in a customer’s Quality Vault. Upon receipt, Vault QMS creates a GCP Deviation record that is used by QA to perform an investigation, root cause analysis, and any appropriate CAPAs. The connector creates a hyperlink in the GCP Deviation record that points to the originating Issue record in the Clinical Operations Vault. It also creates a hyperlink in the Clinical Operations Vault’s Issue record pointing to the corresponding GCP Deviation record in Vault Quality. Study information associated with the Clinical Operations Issue is automatically populated in the GCP Deviation record via the Quality-Clinical Operations: Study Data Connection.
The Quality-Clinical Operations Issue Management Connection standardizes how Vault Clinical customers manage quality issues. It eliminates the need to triage clinical issues outside Vault, and build investigation, root cause, and CAPA processes in their Clinical Operations Vaults. It supports the Vault QMS application’s standalone Deviation object and the Quality Event Deviation object.
Clinical Operations
In addition to the below release notes, the Vault CTMS, Vault eTMF, Veeva Site Connect, Vault Study Startup, and Vault Study Training Veeva Connect communities offer general release communications, release highlights, and key feature demos.
Several features listed in the Vault Connections section also affect the Clinical Operations application family.
CTMS
CTMS Content Field Defaulting & Enhanced Monitoring Event Seeding
For blinded studies, management of unblinded/restricted study material is important to ensure that access to secure, sensitive study information is only available to authorized individuals. In Vault eTMF, management of blinded/unblinded document content is achieved through use of a standard Content field that is defaulted based on User setup. This feature expands support for managing blinded/unblinded content to Vault CTMS objects.
When Monitoring Events are created, the Content field is defaulted based on the object type, and related records (for example, Issues, Follow Up Items) are defaulted to the same Content value as the related Monitoring Event. Only authorized Users are allowed to create Unblinded Monitoring Events. In determining the Previous Monitoring Event, Blinded and Unblinded Monitoring Events follow parallel paths. Vault evaluates the Content field and Monitoring Event dates according to current logic and automatically populates (where applicable) either the Previous Blinded or Previous Unblinded Monitoring Event field. The Content field can also be evaluated during Monitoring Event seeding actions, and workflows can be configured to branch based on Monitoring Event type.
For records created that are not related to a Monitoring Event, the Content field is defaulted based on User setup and follows the same logic currently used in Vault eTMF.
Enhanced Download & Upload Actions for Trip Report Templates
This feature enhances the download and upload actions on Trip Report Templates to support additional elements for:
- Instructions
- Answers with Conditional Requirements
- Question Branching
- Custom Fields
Currently, customers can use a CSV file to create Trip Report Templates including sections, the individual questions and responses, whether or not responses and comments are required, and the order of display (if desired). However, adding instructions, defining conditional requirements, and creating dependent questions previously required manual setup or Vault Loader. Now, these elements, as well as custom fields, can be included in the CSV file.
Additionally, the Download Trip Report Template action is updated to download a CSV file populated with the data from the Trip Report Template, which can then be leveraged to create new Trip Report Templates. Downloading a CSV file from a Trip Report with no data will continue to download a blank CSV template for authoring.
These enhancements improve and streamline the process for Trip Report Template creation.
Learn more about Uploading Questions to New Trip Report Templates.
Monitoring Schedule Extensibility
In this release, we are adding several capabilities to improve the extensibility of Monitoring Schedules for CTMS customers. Monitoring Schedules now include the ability to schedule custom Monitoring Event Types. When customers add their custom Monitoring Event Type to the Monitoring Event Type picklist and Vault will create custom Monitoring Events.
Monitoring Schedules now also include the ability to specify the Monitoring Visit Method for the corresponding Monitoring Event.
Monitoring Events will also now assign matching Study Team Personnel at the Study Country or Study level as the CRA for a Monitoring Event if there is a single Study Person with the Study Team Role. When multiple Study Persons exist with the same role at the matching level, Vault will not default the CRA.
Relate Trip Report Template to Monitoring Event
This feature introduces the ability to track which Trip Report Template is used to generate questions on a Monitoring Event. Once configured, when the action to Seed Trip Report Question Responses is taken, Vault will stamp the Trip Report Template used on the Monitoring Event, improving traceability and reporting.
Expansion of Resolved Issues Seeding into Monitoring Events
This feature expands the way resolved issues are seeded into Monitoring Events. Previously, if the seeding action was taken after the Monitoring Event Actual Visit Start Date was populated, Vault would not seed resolved issues where the resolved date was after the Actual Visit Start Date. Now, if the seeding action is taken and the Actual Visit Start Date is populated, Vault seeds resolved issues with a resolved date after the Actual Visit Start Date and on or before the current date
Last Subject Milestone for Only Discontinued Subjects
This feature updates the behavior of Automated Enrollment Milestones. Now, the Last Subject Out and Last Subject Treated milestone dates populate if at least one Subject has an Enrollment Date and all Subjects have Withdrawn Dates, accounting for situations in which all subjects at a site withdraw instead of complete the study.
This update applies to customers who have enabled the “Enable Automated Enrollment Milestones” feature under Application Settings.
eTMF
Document QC Step Support for Study Metadata Extraction
TMF Bot continues its extension and enhances the Document QC Step to check metadata. Thanks to this enhancement, TMF Bot will be able to identify, if enabled at any Document workflow step, classification and metadata issues. Both possible issues and suggested resolutions will be presented to the end users in the same document panel. This will help our customers improve TMF documents’ quality and accelerate inspection readiness.
TMF Bot Misclassified Document Tag
As part of the TMF Bot maintenance process, we encourage customers to regularly review the performance of the TMF Bot after deployment using Prediction Metrics. This enables the identification of patterns in cases where the TMF Bot struggles to classify, or misclassifies, certain document types. By doing so, customers can assess whether adjustment or re-training of their deployed model is necessary.
While Prediction Metrics offer an efficient way to assess the overall performance of deployed models, there is currently no straightforward method to identify and review individual documents that were misclassified by the Bot. To address this limitation, we are introducing a new standard document tag called TMF Bot Misclassified. Whenever an end user manually reclassifies a document before completing out their inbox, the system will automatically append the TMF Bot Misclassified tag to that document. Consequently, it will become easy to use this tag as a filter in the Library or in reports to identify and analyze misclassified documents.
This feature exclusively applies to documents loaded into the Inbox; the Document QC Step remains outside the scope of this feature.
Model Training Filters & Custom Model Automated Retraining
TMF Bot comes by default with a trained model which is automatically deployed to each customer’s environment. While the default model provides solid predictions, some customers have opted to enhance TMF Bot predictions confidence by creating custom models. However, using custom-trained models involves two challenges. Firstly, custom models require manual maintenance, as customers must remember to periodically retrain them. Secondly, some customers compile custom lists of documents for training because steady state documents are not guaranteed to be final. Compiling those lists via VQL queries and/or Loader exports is time-consuming.
To simplify the utilization of TMF Bot’s custom models, we are introducing a new feature: Model Training Filters and Custom Model Automated Retraining. With this feature, administrators can add a custom VQL Query into their trained model. Combined with Excluded Classifications (a 23R2 feature), this allows them to refine the training set without the need to manage lengthy lists of document IDs. Furthermore, this feature also enhances the Auto-Train Models job to give customers an option to refresh their custom-trained models on release night, ensuring that they stay up-to-date without manual intervention.
Study Startup
In-Flight Survey Updates
This feature allows users to regenerate a Site or Outreach Target survey from an updated survey template. If the previous survey has been partially or fully completed, Vault will iterate through the completed answer and pre-populate them on the newly regenerated survey.
Today, the only way to update the questions within a survey is to generate a new survey from a template. The questions that a site may have previously answered are lost and the site is required to repopulate the survey from scratch. This is viewed as wasted effort and a burden to the site which may even decrease sites response rate.
Regenerating and retaining any previously answered questions will provide a more streamlined experience for sites and will allow customers to gain efficiencies in a process where time and duration is vitally important for Study Startup activities.
Learn more about Feasibility Surveys.
Red Flag Survey Questions
This feature allows Business Admins to define flags on available answers in checklists, so that when those answers are selected in a Site or Outreach Target survey, the flag is copied to the parent survey record.
In previous releases, to progress Sites through the Qualification in an automated fashion:
- We relied on survey scoring
- Out of the many questions in a survey, a select few were true “dealbreakers” (for example, the Site must be rejected based on the wrong answer) or “red flags” for a closer review (for example, If the site is missing a key piece of equipment that the sponsor/CRO may be able to provide)
Reviewing these in previous releases required a separate report that keyed in on specific questions. This new feature bridges this gap by allowing Admins to trigger survey review workflows from the response of red flag questions.
Learn more about Checklist Question & Answer Setup.
Milestone Package Document Export
Clinical Trial Applications submitted to IRBs, Ethic committees, or Health Authorities often necessitate the manual upload of clinical documents into designated systems (for example, CTIS). As a result, it becomes imperative that all accurate documents and document versions required by these institutions are meticulously submitted. The introduction of the Clinical Application Management feature in 22R2 allowed for the tracking of specific document versions necessary for submission.
To streamline the export process, we are introducing Milestone Package Document Export. This feature offers a standard action within Milestones, enabling the export of all Complete State documents linked to Milestone Package Documents. Executing this action generates a compressed ZIP file containing the viewable rendition and attachments associated with the exported documents. To align with the naming conventions expected by these diverse institutions, the Viewable Rendition will be automatically renamed based on the value specified in the Export Name of the Milestone Package Document. This enhancement aims to increase efficiency, reduce manual workload, and foster compliance.
Survey Failure Notifications
This feature introduces detailed failure notifications that are sent to the user who initiates the sending of a Site or Outreach Target survey in Clinical Operations Vaults. Failure notifications will now be sent when the survey creation fails and is triggered via entry action into a particular lifecycle state.
Study Startup, eTMF
Milestone Workspace Usability Enhancements
The Matched Documents grid in Milestone Workspace will now feature a more intuitive layout of information, with links to the Document listed first.
Site Connect
Document Exchange Page
Site Connect customers will now have access to a Document Exchange page and tab. This page provides a history of documents that have been exchanged with each site as well as a view of any outstanding tasks.
This page provides Clinical Operations users with a single place to track all documents and document requests exchanged with their study sites.
Within the Document Exchange page, Clinical Operations users can also:
- Cancel open tasks that are no longer required or applicable.
- Send Site Packages to Connected Sites
Clinical Operations users can easily navigate to this new Document Exchange page directly from their Study Site’s Site Document Exchange section using the new Show in Tab button within the section.
Safety Distribution Resend Actions
Site Connect Admins can now resend safety distributions to distribution task recipients. This user action will show up on any Distribution Task Recipient with an Email Status of Sent, Failed, Opened, or Viewed. Existing Distribution Task Recipient records will be updated accordingly when new emails are generated.
In addition, safety distributions can now be sent to any newly defined Study Site personnel. This can be done from a new Resend to new Study Personnel user action on Distribution Tasks in the Sent or Failed lifecycle state.
Site Connect User Field
With this field, Site Connect customers will be able to flag which Study Site Personnel are to be added as VeevaID Site Connect Users. This field will only trigger the addition of VeevaID Site Connect Users once the site experience for Site Connect has been moved out of SiteVault and into Clinical Operations - targeted for 2024.
Additional Vault Clinical Docs Support
Site Connect customers can now exchange the following document types with Sites:
- IP Storage Condition Documentation
- Non-IP Storage Documentation
- Maintenance Logs
- IP Recall Documentation
- IP Return Documentation
- Acceptance of Marketed Product Material
- Non-IP Shipment Documentation
- Non-IP Return Documentation
- IP Transfer Documentation
The configuration for any relevant document types must be updated so that they map to the new Vault Clinical Docs artifacts.
Clinical Global & Remote ID Document Fields
In preparation for the migration of StudyConnect from SiteVault to Clinical Operations Vaults, we have added several new document fields to facilitate the migration of this data. These fields are hidden and the system automatically sets them via a job during migration. This job also updates the Last Modified By value for these documents post-update. For additional details about these new fields, please see 23R3 Clinical Operations Data Model Changes.
Clinical Operations Data Model Changes
See 23R3 Clinical Operations Data Model Changes.
Commercial
In addition to the below release notes, the Vault PromoMats Veeva Connect community offers general release communications, release highlights, and key feature demos.
PromoMats
Portals: Auto-on User Interface Update
In this release, the updated Portal User Interface (UI) will be enabled on all PromoMats and Medical Vaults. The UI includes a new homepage, which provides a modernized look and feel. The UI update also provides a more intuitive experience allowing users to find their content quicker. Admins benefit from an updated administration interface, allowing multi-document select, rich text support, further customization options, and other enhancements.
Learn more about the updated Portal UI.
Portals: Logic Change for Recently Added Document Widget
In this release, we have updated the logic for the Recently Added Document Widget. Previously, in order for a document to be displayed in the widget, the content had to have been added to the Portal in the last 45 days and also entered a steady state within the last 45 days. This created issues where new Portal content wasn’t displayed in the widget due to when it entered its steady state. With this enhancement, the Recently Added document widget will now populate based on Steady State documents most recently added to the Portal.
Learn more about Document Widgets.
Portals: Style Changes for Content Filters
With this release, the style of Content Filters has been changed to make them easier to read and interact with. The changes include:
- Increased size of icon, text, and button
- Default color of the filter name is black
- When a user hovers over a filter, the filter name color changes to the brand color
Learn more about Content Filters.
Portals: New Portal Selector Page
We have introduced the ability to view Portals in Grid View or Card View, and are providing filtering options for an end-user when they are navigating the Portals accessible to them. This addresses issues for customers with many Portals, and makes scaling easier for when new Portals are created in the future. End-users can now efficiently search and interact with Portals, and can favorite the Portals that they frequently visit.
Learn more about the Portal Selector page.
Portals: Additional Custom Document Widgets
Portal Administrators will have more options when working with document widgets in the new Portal UI. A maximum of 10 document widgets can be used on each Portal, but now Portal Administrators will be able to choose between system-generated widgets, custom document widgets, or a combination of both. This removes the previous limit of 5 custom document widgets.
Learn more about Document Widgets.
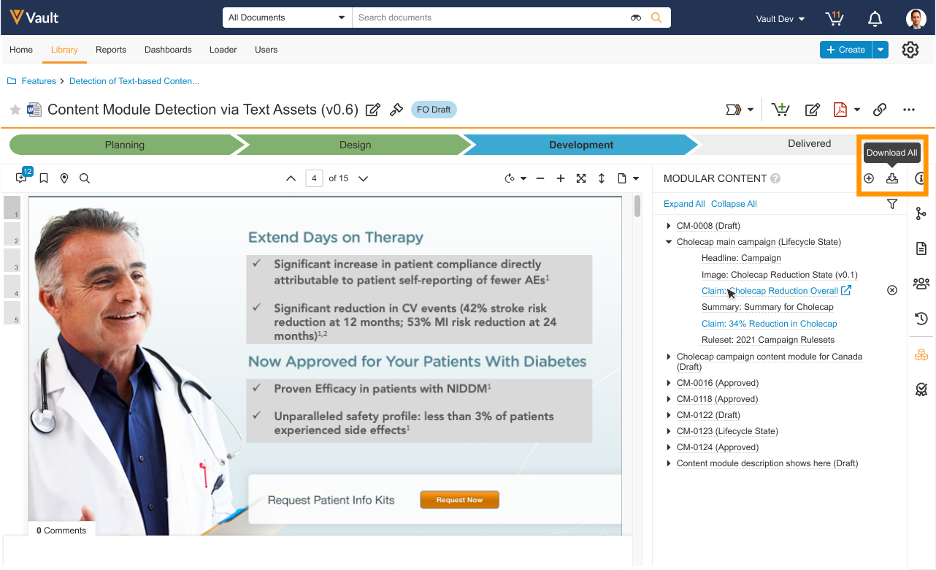
Modular Content: New User Interface
The new modular content user interface simplifies and enhances the process when creating, editing, and viewing a Content Module record. The user interface includes different sections, such as Claim, Reusable Text, and Images, to display each corresponding Content Module Asset. Users with the appropriate permissions can easily add, edit, and remove assets within each section. Each asset is shown as a card within the user interface. Users can click on the card to see a quick view of the Content Module Asset without navigating away from the page. This makes it easier for users to see which assets are in each section of the module and compare them against their corresponding assets. Users with the appropriate permissions can also edit Content Module Assets in the quick view panel and reorder cards by dragging and dropping them into place within their section. Editors can easily group assets within a section together to assist with asset placement within a template when authoring content.
This is automatically enabled for all PromoMats customers.
Learn more about modular content.
Modular Content: Download Content Modules
To better assist content authors in creating content using Content Modules in PromoMats, we have added the ability to download a Content Module and all its Content Module Assets from the Modular Content Doc Info Panel and from a Content Module record. Users can select the new download icon to initiate the export of claims, text assets, record fields, and data/image assets into a ZIP file to a maximum of 100 GB in size after compression.
Once a user clicks the Download icon, Vault generates an Excel file containing all metadata, text assets, and rules for Content Modules and Content Module Assets. Vault extracts source files and renditions for documents linked as Content Module Assets. The user receives a notification when the export package is complete and ready for download. Vault displays an error message if the maximum ZIP size is exceeded.
Learn more about modular content.
Claims Linking: Enablement Update for Enhanced Suggest Links
With this release, Enhanced Suggest Links is automatically enabled for all PromoMats customers without any Text Asset records. This feature also removes the Admin flag for all Platform Suggest Links functionality.
Standard Metrics Duration Reports & Dashboards
The Standard Metrics Track Duration data is now available through reports and dashboards. To enable this feature, customers must complete the configuration on the Standard Metrics State Mappings page and activate the Standard Metrics Duration job. The first time the job runs, it collects data from up to a year before the current date, so customers may see the job run more than once initially. Aggregated industry-level data is still available via Pulse Content Metrics delivered by the Veeva Business Consulting team.
Learn more about Standard Metrics Durations..
eCTD: Simplified Labeling Document Criteria
Labeling documents no longer require a Product value that matches the promotional material in order for Vault to pull the document into eCTD Compliance Packages. Previously, when adding labeling documents, the value in the product__v document field had to match a Product value on the promotional material for the system to recognize it as labeling. Now, to pull labeling documents into eCTD Compliance Packages, ensure the document type matches the type configured under Settings > Application Settings > Compliance Package Generation. Vault will recognize all documents with this document type as labeling.
eCTD: Naming Alignment for 2253 Form
When generating eCTD Compliance Packages, Form 2253 will now be named using the name provided on the template form. When applicable, this also applies to the Supplementary Form. Prior to this release, the name of the generated form consisted of the template form name and the Compliance Package name. This enhancement more closely aligns our eCTD functionality with FDA guidance.
eCTD: Bulk Fill Submission Date for Compliance Packages
Customers adopting the new eCTD bulk generate functionality released in 23R2 now have the ability to bulk fill the Submission Date when completing metadata for the Compliance Packages.
eCTD: Support for CrossLinked References
Users can now generate eCTD Compliance Packages for materials that are substantiated using references or labeling documents that are CrossLinks.
Claims Linking: Search Synonym Support
Enhanced Suggest Links now supports search synonyms set up in the Thesaurus when finding and matching text within documents to Text Assets.
Learn more about Configuring Search Synonyms.
Commercial Data Model Changes
See 23R3 Commercial Data Model Changes.
Medical
In addition to the below release notes, the Vault MedComms and Vault MedInquiry Veeva Connect communities offer general release communications, release highlights, and key feature demos.
MedComms
Enhanced Suggest Links for Scientific Statements
Enhanced Suggest Links is the new and improved matching functionality to link Scientific Statements automatically to Medical Content. It uses a new matching algorithm to allow for more flexibility with the Statement, reducing the need to use as many Scientific Statement Variations. Scientific Statements now support multiple countries and multiple products. The Enhanced Suggest Links feature finds matches on primary or secondary countries and products on Medical Content. Enhanced Suggest Links may be triggered automatically using a Lifecycle Entry Action or as a user action, thereby helping to speed up the process for getting Scientific Statement links to a document.
Learn more about Scientific Statements.
Portals: Auto-on User Interface Update
In this release, the updated Portal User Interface (UI) will be enabled on all PromoMats and Medical Vaults. The UI includes a new homepage, which provides a modernized look and feel. The UI update also provides a more intuitive experience allowing users to find their content quicker. Admins benefit from an updated administration interface, allowing multi-document select, rich text support, further customization options, and other enhancements.
Learn more about the updated Portal UI.
Portals: Additional Custom Document Widgets
Portal Administrators will have more options when working with document widgets in the new Portal UI. A maximum of 10 document widgets can be used on each Portal, but now Portal Administrators will be able to choose between system-generated widgets, custom document widgets, or a combination of both. This removes the previous limit of 5 custom document widgets.
Learn more about Document Widgets.
Entry Criteria & Entry Action Options for Binders
This feature enables configurable entry criteria and a configurable entry action to manage the binder content. The entry criteria checks if the documents in the binder are in a selected state. The entry action allows Admins to change the state of the binder contents when the binder reaches a selected state. Customers can leverage this feature to enhance the Multichannel Presentation and Slide approval process.
Portals: New Portal Selector Page
We have introduced the ability to view Portals in Grid View or Card View, and are providing filtering options for an end-user when they are navigating the Portals accessible to them. This addresses issues for customers with many Portals, and makes scaling easier for when new Portals are created in the future. End-users can now efficiently search and interact with Portals, and can favorite the Portals that they frequently visit.
Learn more about the Portal Selector page.
Portals: Logic Change for Recently Added Document Widget
In this release, we have updated the logic for the Recently Added Document Widget. Previously, in order for a document to be displayed in the widget, the content had to have been added to the Portal in the last 45 days and also entered a steady state within the last 45 days. This created issues where new Portal content wasn’t displayed in the widget due to when it entered its steady state. With this enhancement, the Recently Added document widget will now populate based on Steady State documents most recently added to the Portal.
Learn more about Document Widgets.
Portals: Style Changes for Content Filters
With this release, the style of Content Filters has been changed to make them easier to read and interact with. The changes include:
- Increased size of icon, text, and button
- Default color of the filter name is black
- When a user hovers over a filter, the filter name color changes to the brand color
Learn more about Content Filters.
Product Model Enhancements
We are standardizing the way customers manage products in Vault Medical with three goals in mind:
- Ensure that customers’ products are represented in a consistent manner across Vaults;
- Give customers the ability to manage product families and be able to use alternative names, such as brand names and trade names, when referring to products;
- Set up customers for success by offering a standard product model that makes it easier to adopt new features and logic using standard objects and fields.
MedInquiry
Automatically Suggest Responses
The Suggested Responses feature enabled Vault MedInquiry to automatically suggest the answer to medical information requests.
If the captured inquiry aligns to an approved frequently asked question, Vault will automatically match to it. Beyond text comparison, additional precision for matching will be provided by metadata on the case, such as Product, Country and Language.
If a successful match is made, Vault will automatically generate a response to this question. This suggested response will consist of response notes (the answer to the question), a package of fulfillment documents and an email template.
Advanced Search User Interface for FAQs & Standard Responses
Vault MedInquiry has standard objects for Frequently Asked Questions (FAQs) and Standard Responses. This feature enhances the Medical Inquiry User Interface (UI) to automatically find an FAQ that matches the inquiry being captured. If the user wishes to change the FAQ that has been suggested, they can select a different one from a new dialog. The new dialog provides visibility of the Standard Responses to the FAQ.
Learn more about FAQs & Standard Responses.
Automatically Identify Product from Incoming Case Emails
The Product field can now be automatically populated on Case Requests generated from system-ingested inquiries raised via email. When this feature is enabled in settings, the Product will be matched from the contents of the email at ingestion, and populated in the newly created Case Request record. This feature increases intake automation.
Learn more about email ingestion.
Revoke Case Response Links
Standard Responses are used to pre-package answers to requests for medical information. The Standard Response record includes response notes and individual standard fulfillment documents, which are accessible via a response link. When configured, this feature revokes access to response package links when one of two events happen:
- When a Medical Information user manually revokes access to a package link by way of a user action;
- When a Standard Response related to Case Responses moves out of steady state by way of an entry action or a user action.
Capture Product Quality Complaints
Medical Inquiries are a frequent source of Product Quality Complaints. With this release, a standard Product Quality Complaint object type is added to the existing Event object, alongside the existing Adverse Event object type. This additional data can then be shared to Quality.
Medical Data Model Changes
See 23R3 Medical Data Model Changes.
Training
Study Training receives data model updates in parallel with the Quality Suite: Vault Training application as well as functionality updates from the following features:
- “Add To Calendar” Link (Classroom Training)
- Session Location Field in Create Class Dialog
- Imported Training Assignment Handling Update
- Create Person Records Automatically
- Set Quiz Completion Date for External & Evaluation Training Assignments
- Curriculum Matching: Support Multi-Select Picklist Fields
- User Interface Updates for Admin Alerts
Study Training
In addition to the below release notes, the Vault Study Training Veeva Connect community offers general release communications, release highlights, and key feature demos.
Finalize Matrix Changes When Adding New Training Requirements
With this feature, users can finalize a training matrix via the Training Matrix Builder when Training Requirements are added to or removed from a Curriculum, even after the Training Matrix is finalized. When the matrix changes are finalized, Vault executes the Training Matrix Builder automation, setting Training Requirements to the Ready for Use state, and creating Training Assignments and Training Requirement Impact Assessment records as needed.
Create User Automatically
This feature automates adding an existing domain user to the Study Training Vault if the Study Person has a user account in the Clinical Vault. This feature reduces the administrative overhead for Vault Admin and IT users who must create domain users and associate them with the correct Person record.
Invite Sites to Register for VeevaID
This feature automatically initiates the VeevaID user invitation action. Sites can now use a single login account to access Study Training and other Vault applications. VeevaID is an Identity Provider (IdP) system for Veeva applications (such as CTV, SiteVault Free, Vault Training). The VeevaID portal allows end-users (Clinical Site Staff) to self-register, access applications they are subscribed to, and maintain their account.
Quality
In addition to the below release notes, the Vault QMS, Vault QualityDocs, Vault Training, Vault LIMS, and Validation Management Veeva Connect communities offer general release communications, release highlights, and key feature demos.
Several features listed in the Vault Connections section also affect the Quality application family.
QualityDocs
Process Navigator: Administrator Defined Document Columns
With this release, Admins are now able to configure default columns that display in the documents grid of the Process Navigator Detail Page and the Favorites Page. Admins can define default columns for each Visual Hierarchy Object Type. If enabled, the Allow User to Override the Default Fields setting in the configuration permits a user’s preferences to override the default columns set by the Admin. Additionally, the Visual Hierarchy Configurations link can now be found under Application Configurations > Quality Configurations on the Admin > Configuration page.
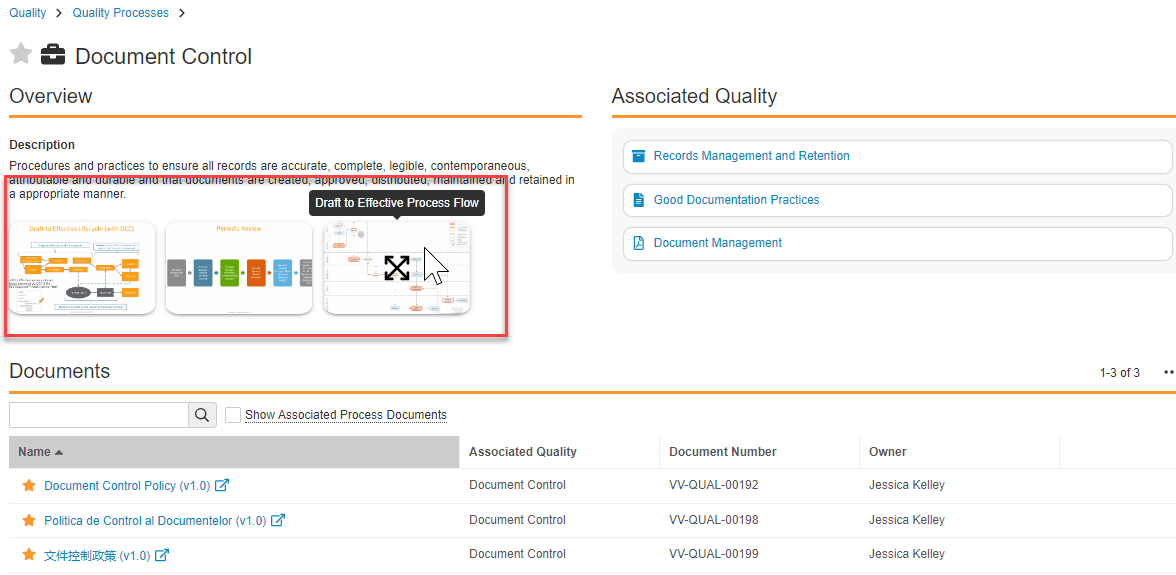
Process Navigator: Support for Images
Up to three (3) images (diagrams, tables, charts, or other images) can be added by a Process Owner or Admin to the overview section of the Process Navigator Detail Page. The images display as thumbnails. Hovering over a thumbnail image displays the name of the document in a tooltip and clicking on the thumbnail opens the enlarged image in a modal window. Buttons in the modal window enable navigation from one image to the next. Images are stored in new optional fields (visual_hierarchy_image1__v, visual_hierarchy_image2__v, visual_hierarchy_image3__v) with field type Object (Documents) on the Visual Hierarchy (visual_hierarchy__v) object.
Station Manager
Improved Document Viewer for Android Station Manager
A new improved document viewer has been implemented in the Android Station Manager application that improves performance and in-document searching. The feature does not change or impact end user functionality.
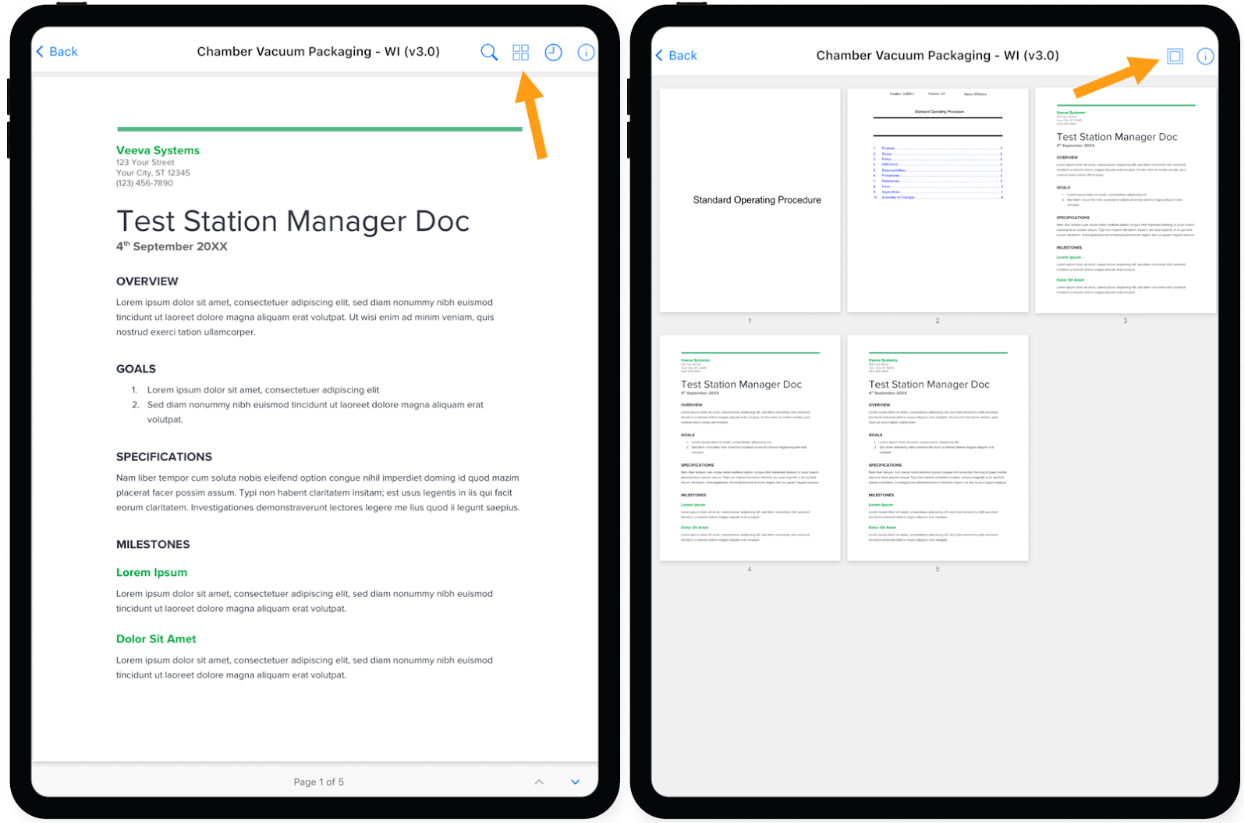
Document Page Thumbnail View for iOS Station Manager
The iOS Station Manager application now allows a user to display all pages of a document in a thumbnail view to more easily navigate to the desired page.
Training
Add to Calendar Link for Classroom Training
The Classroom Training feature in Vault Training provides customers the ability to schedule, publish, and complete classroom training. When a classroom training is scheduled using the updated Instructor-Led Training interface, the “Add To Calendar” link allows Learners and Instructors to add the event to their Google or Outlook calendars. The link goes out as an email notification and an in-app notification. If event details change, users are notified and Vault sends a new calendar link. Overall, this feature provides participants with awareness on when and where classroom training has been scheduled.
Session Location Field in Create Class Dialog
The new “Add to Calendar” Link feature populates a classroom training’s location into the calendar invitation. For added visibility, this Session Location is now visible to Instructors scheduling a training session in the Create Class dialog.
Imported Training Assignment Handling Update
When a Training Requirement is moved to the Retired state, Vault will now cancel any imported Training Assignments (where Creation Source is Import) which are in an open state, such as Assigned.
Create Person Records Automatically
In order to enhance the onboarding and offboarding experience, this feature automatically creates eligible Person records when a User account is active and also automatically sets Person records as ineligible for training when the User account is inactivated.
Set Quiz Completion Date for External & Evaluation Training Assignments
After a Learner passes a quiz for an External or Evaluation Training Assignment, Vault populates the Quiz Completion Date. Previously, this was only done for Vault Document Training Assignments.
Curriculum Matching: Support Multi-Select Picklist Fields
The Curriculum Matching feature adds flexibility to the training matrix and improves automation for the assignment of training to Learners. Building on the functionality released in 23R2, Curriculum Matching now allows the multi-select picklist field type on Person and Curriculum. Vault Admins can use multi-select picklist field types to determine a match as long as there is a match on one of the values and/or if one value on the Person picklist field matches one value on the corresponding Curriculum picklist field.
This feature was named SmartMatch in previous releases. It is updated to Curriculum Matching in 23R3, and the change will be reflected in Vault UI in 24R1.
User Interface Updates for Admin Alerts
Admin alerts have been updated for styling.
QMS
Auditor Profiles & Qualifications
Knowing who is authorized to perform a role on an audit can be difficult. An audit planned with an individual who is not qualified to do that work can be costly in both time and resources if that fact is discovered shortly before the audit is scheduled to occur.
To address this scenario efficiently, Vault QMS customers can now capture key demographic information about auditors, and manage the variety of qualifications that may be required for an auditor to perform any given role on various types of audits. Further, customers can now track and manage their own auditors’ progress towards those qualifications (including the history of audits performed by that auditor) to assess eligibility of an auditor to perform various types of audits all within Vault Quality. The resulting benefits are:
- When populating an audit’s Quality Team, only authorized and qualified individuals will be available for selection within each team role of the audit
- Progress of any given auditor towards requisite qualifications can be tracked and found within Vault’s Auditor Profiles.
With this feature, customers’ audit teams will have clarity on what qualifications are required for an individual to appropriately perform a specific role during an audit, and who within their organization meets those qualifications. Lastly, the Auditor Qualifications engine is tied directly in to Vault QMS’ security, such that as Auditors satisfy the requisite criteria for certain Qualifications, they can be automatically granted specialized access to functions within the Vault - and further will automatically appear as eligible within specific roles on Audits in the system. This feature requires configuration to be used.
Complaint Automated Email Ingestion
Organizations receive emailed complaints from consumers. Sometimes organizations capture consumer complaints through a website or call center, where they are logged in a Customer Relationship Management (CRM) system. When a complaint requires quality assurance attention, a Vault QMS user manually creates a complaint record. Alternatively, some organizations build integrations with systems that intake consumer complaints, and automatically create Vault QMS Complaint records.
This feature helps Vault QMS customers when it is not feasible to rely solely on manual creation of complaints, or an integration with another system to automate complaint creation. It enables customers to build an end-to-end automated complaint process by allowing organizations to configure a Vault Email Inbox to process Complaints received via email. When the Complaint Email Inbox receives an email, Vault automatically creates a Complaint. A system administrator can configure Vault to store the original email and any attachments on the new complaint record.
If the Complaint Email Inbox receives an email about a complaint that already exists in Vault (for example, sender replies to the original email, or replies to an automated notification sent via External Notification from Vault that acknowledges receipt of the complaint) that is sent to the Vault Email Inbox, Vault will record receipt of the additional email on the original Complaint record. Vault can also copy the subsequent emails, and their attachments, to the Complaint record.
Quality Incidents Data Model & Lifecycle
Maintaining a quality mindset during routine day-to-day tasks is an important component of a life science organization’s culture. Organizations want to enable their employees to notice and report incidents that have the potential to affect product quality.
While, in previous releases, organizations could create Quality Events, Deviations, and Nonconformances to capture and triage quality incidents, this could be inefficient because not all incidents require a full quality event process. Managing quality incidents as quality events skews quality metrics and creates a compliance burden for organizations during audits.
This feature adds a Quality Incident object and lifecycle to the QMS data model. As a result, anyone with a Vault QMS account can create a Quality Incident record. Quality organizations can triage an incident to determine if it is necessary to initiate a quality event. Information from a quality incident can be automatically copied to a quality event, including attachments on the incident that represent pictures and video documenting the incident.
Learn more about setting up Quality Incidents.
QRM: Risk Visualization Heatmap
A risk assessment can contain hundreds or even thousands of risks. Prioritizing risks, identifying patterns, and developing a comprehensive understanding of the overall risk profile is challenging when an assessment contains so much data.
This feature enhances the Risk Builder tool, enabling customers to present the risk assessment in a visual heatmap format. A heatmap organizes risks based on an assesment’s two-dimensional or three-dimensional Risk Matrix. Each cell in a heatmap represents a particular combination of Severity, Occurrence or Severity, Occurrence and Detectability. Cells are color coded to reflect the various Risk Levels defined in the assement’s Risk Matrix. The count inside a cell identifies the number of Risks within the assessment corresponding to the cell’s Risk Score. Users can click into a cell to display a list of all the associated Risks. In addition, the heatmap also provides easy to use Action Filters:
- Count of all risks
- Risks that do not have Risk Response assigned
- Risks that are flagged
- Count of Risks in each Risk Level.
Heatmap for a Risk Assessment Using a Two-Dimensional Risk Matrix:
Heatmap for a Risk Assessment Using a Three-Dimensional Risk Matrix:
Presenting risks in a visual heatmap helps organizations quickly understand the distribution of risks across Risk Levels, identify patterns, and prioritize mitigation activities.
The Risk Visualization heatmap feature is automatically enabled in Vaults that already use the Risk Builder tool. Learn more about how Admins can enable Risk Builder.
QRM: Risk Builder Filters
Vault QMS’s risk management feature set (QRM) enables organizations to manage risks associated with enterprise and operational processes, including support for risk assessments. The Risk Builder tool provides risk managers with an intuitive way to enter risk data in a risk assessment using a spreadsheet-like user interface.
A risk assessment can contain hundreds or even thousands of risks, so risk managers need a way to narrow the list based on certain risk criteria. This release introduces the ability to filter the list of risks within a risk assessment using the Risk Builder tool.
Risk filtering is automatically enabled in Vaults that already use the Risk Builder tool. Learn more about how Admins can enable Risk Builder.
QRM: Additional Risk Assessment Methodologies
Vault QMS’s risk management feature set (QRM) enables proactive management of risk for enterprise and operational processes using the Failure Mode and Effects Analysis methodology for processes (pFMEA). However, there are additional risk assessment methodologies commonly used by our customers. This feature enhances the Vault Quality data model to introduce support for the following methodologies:
- dFMEA (Design FMEA)
- uFMEA (Use FMEA)
- Generic
- What - If
- HAZOP (Hazard and Operability Analysis)
- HACCP (Hazard Analysis Critical Control Point)
- Hazard - Harm
The data model changes are automatically added to customer Vaults, enabling Admins to configure these risk assessment methodologies for their organization. If you are interested in learning about the Veeva best-practice configurations for each of these risk methodologies, please reach out to your Veeva account partner. Learn more about risk assessment methodologies supported in QRM.
Create Related Record Enhancement
The Create Related Record entry action allows Quality Admins to automate creation of a related record based on the lifecycle progress of another record. Create Related Record automatically transfers some data from the source record to the new record.
In this release, the entry action is being enhanced, allowing system administrators to optionally configure whether attachments on the source record are copied to the new record.
This capability is being introduced to support creation of a quality event from a Quality Incident. Pictures and video of the incident that are stored as attachments can be automatically copied to the quality event.
QMS Inbound Email Address Configuration
This feature introduces a new configuration screen for QMS system administrators to specify the type of object record created when Vault receives an email to a particular email address. Administrators can use this screen to configure automatic creation of Complaints and Supplier Change Notifications from information contained in the received emails.
Inherit Quality Team from Multiple Source Objects
With this release, the Quality Teams framework of Vault QMS has been enhanced to support a single team’s Team Role which can inherit role memberships from multiple parent objects’ teams. In prior releases, in shared-parentage scenarios where a subprocess may be related to multiple parent processes (like an issue escalation, for example) the subprocess may have needed one team definition per object type to account for each type of parent process.
Now, in shared-parentage scenarios like Extension Requests, which may be derived from CAPAs, or Change Controls, or Audits, a single Extension Request team definition can be used for all of the types of Extension Request, intelligently inheriting its role memberships from the appropriate parent process record at runtime, with no interaction from the user during day-to-day activities.
The introduction of this functionality should facilitate reduced complexity in existing Quality Event-based QMS configurations, and dramatically simplify the configuration needs for Standalone Quality Event-based configurations.
End-user function will not be affected by this feature until Admins configure this feature to take advantage of these new capabilities. Admins reviewing the definition of Quality Teams will observe some labeling changes in Admin to facilitate this new feature immediately upon release.
Quality Team Governor Increases
In support of the growing maturity and scale of our Vault QMS customers, this release introduces enhancements to the performance and scalability of the Quality Teams feature set throughout QMS. As a result, several of the configuration limits in this area previously enforced by Vault have been relaxed. Admins will no longer need to contact Veeva Support regarding configurations approaching these published values.
Quality Teams configurations can now globally support:
- Up to 100 teams per Vault
- Up to 10 roles per Quality Team
- Up to 20 team members per Quality Team Role
This feature requires configuration to change the end-user behavior in your vaults, and your configured teams’ functions will not change until action has been taken by an Admin. Learn more about managing Quality Teams.
Standard Data Model for Legacy Archive Data
With the need to preserve legacy archives from now-retired systems being a constant in Life Sciences systems, Vault QMS has introduced a standardized way for customers and partners to import, store and access legacy archive data.
This release introduces a new set of objects to support legacy archive data:
- Legacy System: Identifiers for each legacy system with content being brought to Vault
- Legacy Process Record: Houses the process data (CAPAs, findings, or others) from legacy systems
- Legacy Process Artifact: Houses artifacts associated with migrated process data (attachments, snapshots, audit trails, or others)
These purpose-built objects provide a standardized framework within which partners can import migrated legacy data in a uniform way. Support includes standard relationships between these types of content or data as well as places to store related audit trails, attachments and associated metadata. The model’s core is all standard, however support for extension via additional configurable metadata fields is supported. Lastly, the Legacy Process Record and Legacy Process Artifact objects have been optimized for higher volumes of data, mitigating risks involved when considering very large volumes of legacy data from prior systems.
These objects will be made available to Vault administrators for configuration with this release, however will require configuration to be exposed to, or to impact your users. Learn more about migrating legacy data to Vault QMS.
QMS Feature Parity for MedTech Objects
With this release, in a step similar to the recent change for Vault QMS’s pharma-focused objects and Quality Event, the Vault data model for MedTech provides standalone data models for Non-Conformances, CAPAs, and Change Controls. These objects were previously managed as object types on the Quality Event object. No impact or action is needed for customers using the current Quality Event data model. Customers that are planning to implement one of the above processes are encouraged to leverage the standalone data model.
Learn more about related record configurations in Vault QMS.
Surveillance
eMDR UI Enhancement: View & Edit Fields on AER
This new feature supporting electronic Medical Device Reporting (eMDR) expands on the pre-existing view-mode functions for Adverse Event Reports and MedTech Complaints, and now allows single-screen editing of fields directly on both the AER and MedTech Complaint records without the need of going into the underlying objects. This dramatically reduces needed clicks to fill out an AER’s sections A-H, and makes the overall AER more readable at a glance. User’s security across the objects is still respected.
While the feature supports setting up this streamlined interaction as the default behavior for users, this new experience does not forbid advanced users from directly navigating to those underlying objects to make their edits. Learn more about using eMDR in Vault Product Surveillance.
VPS: Display Validation Errors in UI
With this feature, Surveillance displays validation errors within form sections on Adverse Event Reports. An icon appears next to errors with additional information displayed when a user hovers over it.
eMDR: 23R3 Validation Updates
In accordance with our commitment to keeping our customers in line with latest governing regulations, this feature implements the newest FDA Validations updated by FDA in August 2022. These enhancements will be automatically enabled as part of the VPS Run Validation action for the eMDR, EUMIR and HealthCanada Reports, and do not require any action to take advantage of.
The new validation rules that are implemented are:
- No longer accept patient age without patient age unit
- No longer accept blank product code
- No longer accept implant date greater than explant date
- No longer accept reports that do not include Adverse Event Problem codes
- No longer accept reports that do not provide a contact email address
In addition, the maximum length of characters for the following fields has been increased.
- Form Code: Maximum length now 6 characters
- Exemption Number: Maximum length now 10 characters
Learn more about Vault Product Surveillance.
Reportability Assessment Enhancements
This feature adds several enhancements to reportability assessment functionality:
- Adds a standard lifecycle for the Adverse Event Report Validation object
- Allows users to create a new VPS: Reportability Assessment object record even if all other records are in Closed or Cancelled state type
- Improves processing for Reportability Assessment Effects
Learn more about Vault Product Surveillance.
Validation Management
Additional Prompts for Test Steps
With this feature, test script authors can define additional prompts to further customize instruction-based prompts or response-based prompts that executors will need to follow during test execution. Additional prompts enable organizations to build highly configurable test scripts to satisfy their discipline-specific requirements for Equipment, Cleaning, Utilities, Facilities, Process Validation, and others.
This feature extends how prompts are defined for setup steps and execution steps in the Test Authoring interface, Test Execution interface, and Review interfaces. The supported additional prompts in this release include:
- Text Instruction
- Text Response
- Number Response with Unit of Measure
- Date/Time Response
A maximum of 15 additional prompts can be defined for each test step. Existing behavior for Setup Steps and Execution Steps relating to comments, attachments, discrepancies, verdicts, requirements, and test step changes has been extended to support additional prompts. Learn more about adding prompts in test steps.
Workflow Participants Support for Validation Teams
Currently, Validation Teams can define roles and control access at the record level but do not work with workflows. With this feature, customers can configure their workflows to select participants from roles or leverage roles from Validation Teams as workflow participants.
The workflow will get users from roles through Validation Team Assignment as well as standard user reference fields such as Requirement Owner, Activity Owner, Deliverable Owner, Author, Executor, and Discrepancy Owner. Learn more about setting up Validation Teams.
Check Minimum Permissions to Access Test Authoring, Test Execution, & Review Protocol UIs
With 23R2.4, Vault Validation Management will now check that the user attempting to access the Test Authoring, Test Execution, and Review Executed Protocol pages is assigned the minimum-required Read permissions at the object and field levels. The permission check also considers new fields and objects introduced with the Additional Prompts for Test Steps feature introduced in 23R3. Learn more about Validation Management permissions.
Previously, a permission check was not performed prior to loading the Test Authoring, Test Execution, or Review Executed Protocol pages. Now, the user will receive an error message indicating that they have insufficient permissions assigned when a necessary permission is missing so that they can contact their Admin.
Prior to the release, existing customers will need to review their permission set configurations to ensure that the permission for the base__v object type on the Validation Requirement (val_requirement_svo__v) object is granted. Otherwise, users will receive an error message when attempting to open the Test Authoring, Test Execution, or Review Executed Protocol pages.
LIMS
LIMS Spec Data Redesign
The LIMS data model has been redesigned from the ground up to focus on a centralized testing structure that can be utilized not just for batch release testing, but also in the future for stability studies and environmental monitoring.
The core of these changes occurred on the design side, where the entire instructional set of what will be executed at runtime now resides inside a Spec Data record, which is a combination of the criteria that make up one or more Specifications, and the Tests that need to be executed.
Spec Data contains all the information about what Sample Plan will be followed, when the Samples should be pulled for testing, what Tests shall be executed for each Pulled Sample, and which Results from those Tests shall be evaluated against specification criteria.
The redesign incorporates all existing LIMS functionality including versioning, criteria evaluation, reopening and retesting Lab Tests, managing result changes, and presenting the exception summaries at the Test, Sample, and Spec Execution level.
Note: Spec Execution is the new object that describes which Spec Data is being executed, and a Batch (for example) may have one or more Spec Executions associated with it, depending on the use case.
This new way of working in LIMS must be enabled by Veeva Support with Product approval, and will require Vault Services to help with the migration of necessary data.
LIMS Change Analysis
As part of the Spec Data redesign, there is a new process for managing interrelated design data records. Change Analysis facilitates the accurate upversioning of your underlying LIMS data. When a new version is created, the Change Analysis process identifies all dependent design data records, allowing you to manage updates to these records all in one place, set a common new effective start date, and approve all the items together with the same Effective Start Date. This functionality is only available in the new LIMS data model.
LIMS Version Locking
Enhanced as part of the Spec Data redesign, a new Locked property on Sample Plan, Test Definition, and Spec Data helps ensure no unwarranted changes can occur to design data records that are undergoing review or have already been made effective. This property is automatically set to true by some processes such as Change Analysis, but can also be toggled by configured business processes where appropriate.
These changes only apply to the new LIMS data model.
LIMS Editor Role Assignment
With this release, there are no longer independent lifecycles for child objects of Sample Plan, Test Definition, or Spec Data. Instead, LIMS automatically grants Edit access to those child records depending on whether the user’s role on the parent record includes Edit access.
This same security is also applied to the Lab Test, automatically granting Edit access to Inputs and Results if the user’s role has Edit access to the Test.
These changes only apply to the new LIMS data model.
Create Design Data Copy Action
As part of the Spec Data redesign, there is a new way to copy your Test Definitions, Sample Plans, and Spec Data, in a way that ensures all the necessary child records are copied successfully. This action should be used instead of the default Copy Record action, as it incorporates all the automated functionality of Create New Version (including the automatic linking to latest versions for referenced design data). This action is only available in the new LIMS data model.
LIMS Criteria Evaluation
Enhanced as part of the Spec Data redesign, Criteria Evaluation records are now created upfront rather than upon result evaluation. These Criteria Evaluation records serve as the definitive source of truth for what must be conformed to for each purpose, indication, and market, in order to determine pass or failure status. Every time a Criteria Evaluation record is evaluated and re-evaluated, a Criteria Evaluation History record captures the changes.
These changes only apply to the new LIMS data model.
Lab Sample & Consumable Labeling
Uses LIMS integration framework to send label printing requests to third party label solutions for consumables, samples, tests and assets.
This feature is configurable in the new LIMS data model.
Support for Manual Completion of Lab Tests for Externally Tested Materials
Enhanced as part of the Spec Data redesign, this new user action better supports our Virtual Lab customers who do not enter the result data via the Test Execution interface. After uploading the result data, users can execute this Manual Test Completion action, and LIMS shall automatically perform data validation, calculation, and evaluation for all Results in all Steps of the Test.
This feature is configurable in the new LIMS data model.
Retest & Resample Parity
Included as part of the Spec Data redesign, these are explicit Resampling and Retesting actions that can be configured to be included in User Actions, Entry Actions and Workflows. This functionality was available previously through default Vault functionality.
Quality Data Model Changes
See 23R3 Quality Data Model Changes.
Regulatory
In addition to the below release notes, the Vault RIM Registrations, Vault RIM Submissions & Submissions Archive, and Vault RIM Publishing Veeva Connect communities offer general release communications, release highlights, and key feature demos.
Several features listed in the Vault Connections section also affect the Regulatory application family.
RIM
Health Authority Meeting Object
With this release, we are expanding Health Authority (HA) Interactions management in Vault RIM to enable organizations to prepare and plan for meetings with Health Authorities.
The Health Authority Meeting object allows organizations to record important details about each meeting, such as the type, format, and date, along with a summary of what was discussed. Furthermore, meeting materials can be easily linked to each meeting record.
Users can track the progress of HA meetings using object lifecycle states and connect meeting information to other key areas, such as HA Questions, Commitments, and Regulatory Objectives.
New Labeling Document Fields
With this release, new shared document fields and their associated picklists are added to support RIM best practice for Labeling: Labeling Components Included (field and picklist), Carton or Container Label Type (field and picklist), and Tracked Changes/Redlines.
RIM Registrations
Submission Wizard Support for Registrations Fields
This feature will ensure all fields needed for Registrations are system-managed when the Submission Wizard is enabled.
Use for Registrations Wizard Updates
Team members can now use the Use for Registrations field on the Application and Regulatory Objective-related records to specify related records that should be considered when Vault creates or updates Registered Details.
When initiating the Create Registrations or Manage Registered Details action, Vault will only create or update Registered Details for related records where Use for Registrations is set to “Yes.”
This new field provides increased flexibility for managing Registered Details and prevents Vault from creating unwanted registered details.
Support for Multiple Drug Trade Names with Translations
In this release, Vault Registrations adds fields to the Regulatory Text and Regulatory Text relationship objects to support multiple trade names for Drug Products. Vault Registrations wizards have also been updated to support Regulatory Text Translation relationships.
Drug-Led Combination Product Registration Enhancements for IDMP
In accordance with the device reporting for IDMP updates introduced in IG V2.1.1, organizations are now obligated to provide additional details for drug-led combination products. To comply with this requirement, the following fields are added to the related Product relationship records on the Event, Application, Submission, Regulatory Objective, and Registration:
- Co-Packaged or Integral Device checkbox
- Product
- Product Variant
The Create Registrations and Manage Registered Details functionalities will take these new fields into account and create a new related record when Co-Packaged or Integra Device is set to Yes.
Wizard Support for Full Indication Text & Orphan Designation
With this release, Vault Registrations has added the Full Indication Text and Regulatory Authorization (Orphan Designation) relationships to the related Indications on the Event, Application, Submission, and Regulatory Objective. When using bulk create and update actions such as Create Registrations, Create Related Records, and Managing Registered Details, the Full Indication Text and Regulatory Authorization (Orphan Designation) records are created or updated on the Registration. This enhances efficiency and accuracy of management of these data records, increasing compliance with IDMP requirements.
Submission Date on Submission Country for EU MRP/DCP
With this release, Submission Managers can use the new Submission Date field on the Submission Country object to track the submission date to the local health authority for non-centrally authorized procedures such as MRP and DCP in the European Union.
EU eAF Application Setting Update
This feature consolidates Application Settings for eAF Product Reports into a single location, allowing for a more flexible configuration of reports generated upon execution of the Generate eAF Reports action.
Customers with this reporting functionality enabled will be migrated to the new Application Setting to ensure continuity.
Support for EUDAMED XML Schema v2.0.8
With this feature, Vault generates EU UDI Submissions using the EUDAMED XML Schema v2.0.8, which now expects a country code of EL for Greece instead of GR.
UDI Enhanced Support for Device Changes
With this feature you can now select a Regulatory Objective when generating a UDI Submission to only include devices that were impacted by that Regulatory Objective. This is particularly helpful for generating UDI submissions to update EUDAMED after the initial set of devices on a Registration has already been registered.
UDI Data Model Updates for EU and US
With this release, we are updating the data model by moving the source locations for some of the UDI attributes. This data model update will bring the following benefits to our customers:
- Migration of the initial set of UDI data into Vault will become much easier.
- Faster identification and correction of the source values will be possible when there are UDI submission validation errors.
With this data model update:
- Most source data for a Basic UDI-DI will now be available on the Product record that has that device identifier.
- The Packaging record that has that device identifier will display most source data for UDI-DI.
EU UDI XML Generation Updates
We are introducing a new Application Setting to complement the UDI Source Data Model updates, which were added to streamline data collection for UDI Submissions. Once the data model updates are configured, Admins can enable the setting to indicate that XML generation will use the new source locations for Basic UDI and UDI attributes.
Support for Certification Bodies in Multiple Markets for Medical Devices Applications
In previous releases, the Notified Body field was restricted to supporting only the European Union (EU) on medical device applications.
In this release, business users can now use the same field to select approved Certification Bodies for other major markets.
Furthermore, the Notified Body field is relabeled to Certification Body and will only display relevant Certification Bodies for the selected Lead Market on the Application.
Review & Refine Related Records
In this release, we are improving the Create Related Records bulk action by introducing a new Preview and Refine step. This step enables users to preview the records that Vault will create or, in some cases, update based on the Event and a user’s selections.
During the Preview and Refine step, users can deselect records they do not want to create or update for a particular market. If a deselected record is referenced by another record in the preview, Vault visually flags those dependencies for review. Additionally, if a user has a large data set to review, they can save the preview to return to later or even have a colleague help complete the review.
The new Preview and Refine step adds transparency and greatly reduces the need for inactivating unwanted records, as users are now able to see and refine the exact records that will be created and updated for all impacted markets.
RIM Registrations, RIM Submissions, RIM Submissions Archive
Active Dossier Module 1 & Module 2 Support
To provide support for additional document types within a dossier, the Active Dossier scope has been expanded. Customers using Active Dossier will now see Product Information (Labeling) and Quality-related documents that reside outside Module 3 as well as all Common Technical Document summaries (Module 2) in the Active Dossier Viewer. New Active Dossier Template records and RIM Reference Model records are introduced to support this.
Administrative Information documents for Quality include:
- Application Form
- Certificates
- Certified Product Document (CPD/CPID)
- Declarations
- Environmental Risk Assessment
- Good Manufacturing Practice (GMP) Compliance
- Information About the Experts
- Manufacturing Authorization
- Normative Documents
- Other Supporting Quality Documents
Product Information now supported includes:
- Braille
- Carton or Container Labels
- Consultation with Target Patient Groups
- Core Datasheets
- Labeling and Package Leaflets
- Mock-ups
- Patient Information Leaflets (PIL)
- Prescribing Information
- Summary of Product Characteristics (SmPC)
- Supporting Documents
Summary documents now supported include:
- 2.2 Introduction
- 2.3 Quality Overall Summary
- 2.3 Introduction
- 2.3.S Drug Substance - ${active_substance__v}
- 2.3.P Drug Product - ${product__v}
- 2.3.A Appendices
- 2.3.R Regional Information
- 2.4 Nonclinical Overview
- 2.5 Clinical Overview
- 2.6 Nonclinical Written and Tabulated Summaries
- 2.6.1 Introduction
- 2.6.2 Pharmacology Written Summary
- 2.6.3 Pharmacology Tabulated Summary
- 2.6.4 Pharmacokinetics Written Summary
- 2.6.5 Pharmacokinetics Tabulated Summary
- 2.6.6 Toxicology Written Summary
- 2.6.7 Toxicology Tabulated Summary
- 2.7 Clinical Summary
- 2.7.1 Summary of Biopharmaceutic Studies and Associated Analytical Methods
- 2.7.2 Summary of Clinical Pharmacology Studies
- 2.7.3 Summary of Clinical Efficacy
- 2.7.4 Summary of Clinical Safety
- 2.7.5 References
- 2.7.6 Synopses of Individual Studies
Generate Active Dossier from Event (Manual Support)
This feature adds a new Dispatched value to the Active Dossier Status picklist to support tracking documents that are ready for use in a submission for a market but not yet submitted. This supports situations where a change can be implemented before submission or does not require approval at all. In these instances, users can now surface documents that would otherwise not have been included in the Active Dossier.
The Active Dossier Editor and Viewer are also made accessible from the Event to support viewing and manual generation of Active Dossier records. Active Dossier records can be created from the Active Dossier Editor opened from an Event record by dragging and dropping a Vault document link and selecting one or more related Activities for which to generate records.
RIM Submissions, RIM Submissions Archive
Map Multiple Document Types to Active Dossier Template
To support more granularity in the Active Dossier Template, multiple document types can now be mapped to the same template record. In some sections of the current Active Dossier Template, multiple document types apply for a single section. Previously, only a single document type could be mapped to an Active Dossier Template. Additional flexibility was developed so customers can track Active Dossier elements at their preferred level of document classification.
For example, this will allow Active Dossier Template mapping for Facilities and Equipment for documents written at the 3.2.A.1 level, or to multiple documents at the 3.2.A.1.X level.
Active Dossier Consolidated Sections
Active Dossier Viewer is being made easier to navigate and manage by simplifying the Active Dossier structure. The following tokens will be removed from the Active Dossier Template to reduce duplicate sections:
- 3.2.S: Product Variant and Manufacturer
- 3.2.P: Product Variant and Manufacturer
- 3.2.P.4: Manufacturer
- 3.2.A.1: Product, Product Variant, and Active Substance
- 3.2.A.2: Product Variant and Manufacturer
- 3.2.A.2: Manufacturer
Removing the tokens for these sections will condense the sections that appear in the AD Viewer, without reducing the number of documents or data being displayed. A new Application Setting will allow Admins to enable this functionality beginning in 23R3.
Tokens resulting in duplicate sections:
Tokens removed and Admin setting enabled:
RIM Submissions
Suggested Matching
Suggested Matching introduces a new Match Document Mode to improve the efficiency of document matching within Content Plans. When a user selects Match Document Mode and selects a Content Plan Item (CPI) to match, a new prefiltered document picker will suggest potential documents to be included from the Library. Suggestions are based on auto-matching criteria set on the CPI.
Benefits of using Match Document Mode compared to previous manual matching efforts include:
- Reduced effort for searching or filtering the Library for documents.
- Streamlined document matching within the Content Plan viewer, instead of in a separate tab or window.
To prevent confusion between Match Document Mode and other matching actions, the Add button has been removed from the Matched Documents section on the Content Plan Item page layouts. Also, the Match Document action in the Content Plan Item Actions menu is relabeled to Auto-Match Documents in RIM Vaults.
Respect Matched Document Atomic Security in Content Plans
With this release, new Atomic Security actions are introduced for matched documents on the Content Plan Item object and object lifecycle. These atomic security permissions additionally secure various Content Plan matched document operations in RIM and allow more control over matching operations within the Content Plan Item lifecycle. When users drag and drop documents from their desktop or the Library, Vault now respects the configured Add Document Atomic Security on the Content Plan Item. Additionally, when synchronizing Content Plans, Vault respects the configured Atomic Security for the following actions: Add Document, Remove Document, Lock Version, Exclude Document, and Include Document depending on the action being taken on the matched document by the synchronization.
Support Additional Fields in Sync Content Plan
With this release, additional fields will be compared when synchronizing Content Plans. During the comparison between the Global Content Plan and Submission Content Plans, Vault now includes the Content Plan field Section Owner as well as the following Content Plan Item object fields in the comparison: Expected Steady State Count, Source for Published Document, Publishing Element, Include eSignatures, and Deliverable Owner.
Respect Use for Content Planning Field in Dispatching Global Content Plans
Event record relationships are copied to the Global Submission record upon its creation, and further propagated to local Submissions. In some cases, the Event relationships are created at a more granular level for managing Registrations than are needed for Submissions. Beginning automatically in 23R2, if Use for Content Planning = No on a target Submission relationship record, the resulting local Submission Content Plan will respect that setting upon Global Content Plan Dispatch.
Submission Wizard: Respect Atomic Security for Relationships
The Submission Wizard will respect Relationship Atomic Security when creating or updating related records on Submissions and Regulatory Objectives. In lifecycle states such as Archived or HA Received, Submission or Regulatory Objective relationship records are often locked via Atomic Security. Now if the Submission Wizard encounters an action to create or update locked relationship records, the action will fail. For example, if Submission Product is set to Read access in the HA Received state, the wizard does not create a new Submission Product relationship record when run on a Submission in the HA Received state.
RIM Submissions Archive
eSTAR Submission Format Support
With this release, MedTech customers can import Submissions Archive 510(k) electronic submissions in the eSTAR format. In order for the Submission to be processed as an eSTAR, Submission managers must select eSTAR as the Dossier Format on the Submission record.
This eSTAR format is an XFA interactive form that guides applicants through a comprehensive medical device submission.
Viewer: Collapse All User Action
With this release, a Collapse All user action is available in the Submissions Archive Viewer section nodes where Expand All is shown. The rows collapsed as a result of this action are excluded from the total count, allowing users to further expand other areas.
Viewer: Display Country-Specific Sections
With this release, the Submissions Archive Viewer displays country-specific sections based on a combination of the eCTD County Code and Country Code fields.
RIM Publishing
Link Evaluator
Link Evaluator introduces a new Evaluate Links user action to efficiently review and correct hyperlinks within a Submission Content Plan (CP) or its Content Plan Items (CPI). When a user selects Evaluate Links from a CP or CPI All Actions menu, a new page containing the Link Evaluator will open.
A navigable Linking Pane lists all CPIs with filtering and visual indicators to indicate where valid or broken links are found. Selecting a CPI in the Linking Pane loads all hyperlinks for that CPI. The resulting Linking Table contains quick filters for All Links, Broken Links, External Links (inter-document links), and Automated Links (coming soon). Users can click on all hyperlinks from the Linking Table to test their validity, check that the correct target opens, and fix any incorrect or broken links.
Benefits of using Link Evaluator include:
- Quick and comprehensive identification of all hyperlink sources and targets in a single screen.
- Easy review of all internal and external hyperlinks, whether embedded in source, manually created in Vault, or created with automation.
- Efficiently identifying and retargeting broken hyperlinks for all documents in the submission.
Automated Hyperlinking
Vault Submissions Publishing can now intelligently resolve and publish external document hyperlinks. This allows authors to create hyperlinks earlier in the writing process, even before the target is uploaded. This reduces the time spent searching Vault for hyperlink targets and manually creating the links.
To set the links, authors highlight the desired text string in the source document and run a custom macro. The macro adds a Veeva Specific URL which contains key details for Vault to identify the target document and specific place within it to set the hyperlink. The Veeva Specific URL also acts as a placeholder for hyperlink targets that do not yet exist in Vault.
Veeva will provide a template macro to customers which can be modified to suit their individual business needs. The macro template relies on following a recognizable pattern of text. The pattern is a comma separated list which includes a Clarifier (such as Study 12345) followed by a Target Destination Type (Section, Table, Figure, etc.) and the specific Target Destination:
Customers can also choose to execute an alternate macro that requires manual entry of the Clarifier, Target Type, and Target Destination in a dialog window. The alternate macro may be useful for documents that do not follow a standard writing style that contains easily identifiable text strings.
Once the document has been uploaded to Vault and matched to a Content Plan Item, users can run the Create Automated Links action from the Content Plan Viewer that converts the Veeva Specific URLs to relative path links. Upon publishing, the relative paths are added to the published document.
In addition to the macro and configuration, Automated Hyperlinking requires the use of a related feature, the Link Evaluator. Both successful links and failed links can be viewed by users in the Link Evaluator. Link Evaluator will be the interface for identifying links that were created, and especially, those links that failed to create. Link Evaluator will be the only way to identify and resolve links that failed to identify a valid target.
Configurable Validation Criteria
With this release, Vault provides five new validation criteria rules that can be configured to meet the needs of multiple regions for non-eCTDs. The validation criteria rules validate the file path length, file size, folder name length, file name length, and valid characters.
South Korea eCTD 1.0
With this release, RIM Publishing Vaults support the KR FDA v1.0 (DTD 1.0) specification. Users can now create Content Plans and generate Submissions that are compliant with KR v1.0 specification. Vault validates these submissions based on the corresponding KR Validation Criteria Version.
Deprecate FDA Sync Gateway
With the introduction of the FDA Async Gateway, customers now have a more stable and faster solution to submitting submissions to the FDA and Health Canada via the FDA electronic submissions gateway. Starting with this release, Vault will prevent the activation of new Sync profiles, as the Set Active button on Sync Gateway profiles is disabled. All Publishing customers should be migrating profiles to the Async Gateway. Beginning in 24R1, the FDA Sync Gateway will no longer be supported in Vault.
RIM-PromoMats Content Plan Creation & Document Matching Performance Improvements
Content Plans created from the RIM to PromoMats Vault Connection will now automatically be created as Inactive. Using an express queue, the connection auto-matches and locks documents to the appropriate Content Plan Items (CPI), then Vault activates those matched items and the upstream parent items.
Users should see improved efficiency, as this eliminates extra steps of creating thousands of CPI records as active, then immediately inactivating them.
RIM Data Model Changes
See 23R3 RIM Data Model Changes.
Safety
The Safety 23R3 release, including all Platform features, is targeted for tentative availability on November 27, 2023 & December 8, 2023.
Safety
In addition to the below release notes, the Vault Safety and Vault SafetyDocs Veeva Connect communities offer general release communications, release highlights, and key feature demos.
Multi-E2B Import Limit Increase
Vault Safety now supports the import of up to 1,000 E2B files in zip or batch XML format to Inbox Items and Imported Cases. Previously, the default limit was 100. This feature improves the efficiency of importing multi-Case files.
Learn More
- User Help: Import an Inbox Item
Safety Inbox Loader: Multi-Case CSV Data Import
With this release, Admins can configure the creation of Inbox items from multi-Case CSV files. This feature streamlines intake from multi-Case CSV files by automatically bulk-importing up to 1,000 Cases to Inbox Items using a pre-defined mapping. Health authorities, such as Health Canada, often provide Case listings in tabular formats. Additionally, Marketing Authorization Holders (MAHs) use custom tabular files to collect Cases. Previously, customers manually identified and entered Cases in Vault Safety, which was time-consuming and prone to errors. An import log attached to the source document summarizes the imported data and includes warning and error messages.
This enhancement streamlines the triage and intake process and ensures faster and more accurate data entry, significantly improving efficiency and reducing the risk of human error.
Learn More
- Enablement: Enable Safety Inbox Loader Multi-Case CSV Data Import
- Admin Help: Manage Safety Inbox Loader Multi-Case CSV Data Import
- User Help: Import an Inbox Item: About Multi-Case Tabular Data Import
Merge and Follow-up of Localized Fields
Vault Safety can carry over localized fields from inbox items to domestic Follow-up Cases through the Case Compare page. When merging with a previous Case version to create a Follow-Up Case, new and edited localized fields are copied over to the Follow-up Case if the corresponding global fields have not been ignored or deleted.
In addition to carrying over localized field data, if an Inbox Item has new Case Narrative data in a non-English language, the system will also carry it over to the Domestic Follow-up Case. As part of these enhancements, the system prevents creating a Global Follow-up Case from a Domestic Case or merging a Domestic Case to a Global In-flight Case.
This feature is Auto-On in Vaults with Domestic Case Processing and/or Local Case Import to Inbox Item enabled.
Learn More
- User Help:
Case Access by Local PV Email
This release introduces a new feature for Case Access Groups. Admins can now assign Access Groups and manage access to Inbox Items, Cases and their associated documents based on the sender’s email address. Previously, access to emailed Inbox Items lacked control based on the sender’s region or email. This posed a compliance risk as it prevented the restriction of access to personally identifiable information (PII) in accordance with local data privacy regulations. Managing Inbox Item access based on the sender’s email ensures compliance with regulatory requirements and enhances Safety measures.
Learn More
- Enablement: Enable Case Access by Local PV Email
- Admin Help: Manage Case Access Group Security: Assign Case Access by Local PV Email
Copy Dosage During Intake and Case Processing
With this release, Vault Safety provides the ability to copy Product Dosage records during Intake and Case Processing. Currently, Intake users and Case Processors must often manually enter multiple Product Dosages that are very similar. Additionally, Vault Safety now optimizes keyboard navigation between fields in the Product Dosage section. This feature improves efficiency by reducing manual re-entry of information and enhancing field navigation.
Learn More
Case Volume Metrics
Vault Safety introduces a new capability to capture overall Case volume. With this functionality, you can monitor the Case volume in each step of the Case Lifecycle. You can visualize Case Volume Metrics in chart format, such as the number of completed and in-progress Cases as well as the comparison between optimal days and optimal hours. Admins can also export the Case Volume Metrics for further analyses. The metrics frequency is configurable to track Case volume on a daily or hourly basis. This feature enhances Case Processing efficiency metrics and reduces manual effort.
Learn More
- Enablement: Enable Case Volume Metrics
- User Help: View Case Volume Metrics
Case Assignment
Vault Safety now supports assigning Inbox Items and Case Processing activities to Case Assignment Teams, which are based on Case Access Groups. Case Assignment Teams can help distribute work based on metrics (such as team members and assigned Cases) and ensure activities are delegated to the appropriate teams and team members while balancing workload. Team leaders have access to these metrics and can assist in individual assignments.
In addition, with two (2) fields, Current User Caseload and Team Caseload, Admins can track the number of Inbox Items and Cases assigned to a user or to a team, respectively. In addition, the system updates these fields when a team member on the Case Assignment Team is assigned a new Inbox Item or Case.
Learn More
- Enablement: Enable Case Assignment
- Admin Help: Manage Case Access Group Security: Manage Case Assignment Teams
Auto-Population of Case Product Registration Holder
To support Organizations with products registered with unique Marketing Authorization Holders (MAHs) in different countries, Vault Safety now populates the value in the Registration Holder/Applicant field from the Product Registration record in the Registration Holder field on the Case Product record. If this field is blank, the Registration Holder is populated with the Organization value from the Product Registration.
Learn More
- Enablement: Enable Auto-Population of Case Product Registration Holder
- Admin Help: Manage Products: Add a Product Registration
- User Help: Enter Case Data: Registration Section
Harmonized Assessment Result Generation
For all Case generation methods, Vault Safety offers more consistent generation of Source Type on Case Assessment Results. The primary reporter Case Contact determines the Source Type for postmarket and non-Clinical Trial Study Cases. Once set on the Assessment Results, the Source Type does not change, even if the primary reporter’s qualification changes. In addition, during Case import, Company and Reporter data is populated based on the E2B file exactly, giving more control to Case Processors to identify and follow up on missing or incorrect information.
Learn More
Active Case Licensing
Vault Safety now displays active Case count statistics for the Safety Management application. Previously, customers had to request this information from Veeva. This feature enhances transparency for all parties involved and offers more accurate annual tracking of the utilization of Case licenses.
Learn More
- Admin Help: Application Licensing for Active Case Versions
Japan Drug Dictionary (JDD)
Vault Safety now includes a central Japan Drug Dictionary (JDD), available when processing Domestic and Localized Cases for Japan. This feature supports the lookup and coding of JDrug codes for External Products, which are exported to PMDA E2B(R3) reports when generating Transmissions. Previously, adding these codes required manual offline lookup and entry. New JDD versions are uploaded to the central dictionary when available. Admins can specify which version will be used for JDrug coding on the Vault.
Learn More
- Enablement: Enable the Japan Drug Dictionary
- Admin Help: Manage the Japan Drug Dictionary
- User Help: Code Japan Drug Dictionary Products
MedDRA Query Building Blocks
Vault Safety introduces MedDRA Query Building Blocks. A more efficient way to create and maintain Datasheets and Watchlists. Building Blocks are reusable queries that combine Standard MedDRA Queries (SMQs), Custom MedDRA Queries (CMQs), and MedDRA Terms at any level of the hierarchy. Once configured, you can apply a single building block to multiple Datasheets and Watchlists. If a Building Block is updated, the changes can be pushed to any Datasheet or Watchlist using it. This automation reduces the administrative burden of maintaining large groups of products with the same adverse event lists, whether for Expectedness evaluations or Watchlist monitoring.
This feature offers an alternative to manually maintaining individual MedDRA Terms on each Datasheet and Watchlist. Using Building Blocks also reduces the need to use Vault Loader for maintenance, which is complicated, time-consuming, and error-prone.
Learn More
- Enablement: Enable MedDRA Query Building Blocks
- Admin Help:
Product Family Datasheets
Vault Safety now supports creating Datasheets for Product Families. Upon creation, the Product Family Datasheet is inherited by all Products within the family that do not have a specified Datasheet. If the Product Family Datasheet changes, those updates are automatically applied to those Products, which simplifies Datasheet management.
Learn More
- Enablement: Enable Product Family Datasheets
- Admin Help: Manage Datasheets and Auto-Expectedness: Product Family Datasheet
Hierarchical MedDRA Terms for Datasheets and Watchlists
To save Admins time and effort, Vault Safety now supports configuring Adverse Event terms on Datasheets and Watchlists at any level of the MedDRA hierarchy. Previously, terms could be coded at the Preferred Term (PT) and Lower Level Term (LLT) levels only. When coding Adverse Events on Cases, any MedDRA LLT is considered a match if it is under the term on the Datasheet or Watchlist.
This feature is Auto-on, however Admins may apply optional additional configuration to some components.
Learn More
- Enablement: Enable Hierarchical MedDRA Terms for Datasheets and Watchlists
- Admin Help:
MedDRA Hierarchical Browser for Non-Case Coding
With this release, Vault Safety introduces an improved MedDRA Browser that supports coding Datasheets, Watchlists, and Queries at any level of the MedDRA hierarchy. When searching for a term in the browser, a new picklist in the Search bar enables Admins to specify the level of the hierarchy in which the system should search.
Learn More
- Admin Help:
Local Datasheet Unexpectedness Override
When the same MedDRA Criteria are Unexpected on the Local Datasheet and Expected on the Core Datasheet, Vault Safety now evaluates Adverse Event Expectedness based on the Local Datasheet. Previously, the system prioritized the Core Datasheet when the same MedDRA Criteria appeared on both Datasheets with this setup. In the opposite scenario, where the MedDRA Criteria is Expected on the Local Datasheet and Unexpected on the Core Datasheet, Vault Safety continues to evaluate Adverse Event Expectedness based on the Local Datasheet. The system continues to use the most conservative Expectedness across the Core and Local Datasheets to set the Case-level Expectedness.
Learn More
Extend Definition of Suspect to Drug Not Administered
Admins can now configure Vault Safety to evaluate Case Products with a Drug Role of Drug Not Administered as suspect products. When turned on, this functionality extends across Inbox Item intake, Case processing, ICSR Submissions and Distributions, and aggregate reports. Previously, the definition of suspect could include Case Products with a Drug Role of Suspect or Interacting only.
Learn More
- Enablement: Enable Extend Definition of Suspect to Drug Not Administered
- Admin Help: Reporting Rule Parameter Reference: Suspect
Support Cross Reporting for Non-Company Scenarios
With this release, Vault Safety supports generation of cross reports for Non-Company sponsored studies and Non-Company Products. Previously, Vault Safety could not generate cross reports for any Study or Product that did not have a registration.
Business Admins can now specify a Study as a Non-Company Sponsored Study. The system will then consider Investigational→Investigational and Investigational→Marketing cross reporting scenarios for the products within the Study.
Similarly, Business Admins can specify a Product as a Non-Company Product. The system will then consider Marketing→Investigational and Marketing→Marketing cross reporting scenarios for the Product.
Learn More
- Admin Help:
- User Help: Cross Reporting
Configurable Substance Matching for Cross Reporting
With this release, Vault Safety includes agency-specific methods for Substance matching during cross reporting. This feature supports agencies that require Case Substances to match exactly with agency-registered substances. With the added flexibility, for each agency Admins can set cross reporting evaluations of Case Substances as a subset, a partial match, or an exact match.
Learn More
- Enablement: Enable Configurable Substance Matching for Cross Reporting
- Admin Help: Manage Organizations: Add an Organization
- User Help: Cross Reporting: Cross Reporting Substance Matching
Cross Reporting without Expectedness Substitution
When evaluating cross reporting, expectedness can now be optionally calculated from General Reporting logic. If this option is selected, Cross reports to all destinations consider the expectedness values at the Case Assessment Expectedness or Case Assessment levels. Previously, expectedness could only be calculated based on substituted Products or Study Datasheets. This feature ensures compliance and accurate reporting.
Learn More
- Enablement: Enable Cross Reporting without Expectedness Substitution
- User Help: Cross Reporting
Case-Level Nullification and Amendment Reasons for Transmissions
Vault Safety now supports Case-level Reasons for Amendments and Nullifications. When available, this data is exported to E2B files. This feature allows Case Processors to enter the value once at the Case-level, improving efficiency and compliance.
Learn More
- Enablement: Enable Case-Level Nullification and Amendment Reasons for Transmissions
- User Help:
Export Product (Reported) to CIOMS I and FDA 3500A
With this release, when the Case Product (Reported) field is populated, it is exported by default on CIOMS I reports in the 14 Suspect Product(s) field and on FDA 3500A reports in the C1.1 Name and Strength and D1 Brand Name fields. Previously, the Product (Coded) field was populated when the Product (Reported) field was expected. Admins can manage whether to export the reported or coded Product by default.
Learn More
- Enablement: Enable Export Product (Reported) to CIOMS I and FDA 3500A
- User Help:
Show Unmasked Open-Label Products on Blinded Forms
Vault Safety now includes the option to export unmasked open-label Products within blinded Studies on blinded CIOMS I and FDA 3500A forms.
If Support previously enabled this feature in your Vault, you do not need to take any action to continue unmasking open-label Products within blinded Studies on blinded forms.
Learn More
- Enablement: Enable Show Unmasked Open-Label Products on Blinded Forms
- User Help:
Mask Dosage in Blinded Distributions
When Study Content Protection is set to “Mask Unblinded Content”, Vault Safety can now mask dosage information when generating CIOMS I, FDA 3500A, and E2B reports. Enabling masked dosage prevents exposing sites or personnel to unblinded dosage information they should not see.
If Support previously enabled this feature in your Vault, you do not need to take any action to continue masking dosage information.
Learn More
- Enablement: Enable Mask Dosage in Blinded Distributions
- Admin Help: Generate Masked Safety Reports: Dosage Masking for Study Products
Study Content Protection for Generated Documents
Blinded users can now be configured to view masked documents when a Study is unblinded. Previously, Vault Safety activated blind protection on masked documents, which prevented blinded users from accessing them during the unblinding of Study Cases.
EMA Clinical Trial Submissions: Non-Study Suspect Products
Vault Safety now supports submitting Clinical Trial Cases with the Spontaneous Report Type. This feature aligns with the European Union (EU) Good Vigilance Practice (GVP) Module VI guidelines, particularly for Clinical Trial Cases involving adverse events related solely to non-investigational medicinal products available in the EU. In order to accomplish this, a new Report Type Override field on Transmissions enables overriding the Report Type at the Transmission level. This feature enhances compliance with regulatory requirements.
Learn More
- Enablement: Enable EMA Clinical Trial Submissions: Non-Study Suspect Products
- User Help:
PMDA Clinical Trial Reporting Enhancements
With this release, Vault Safety provides clinical trial-related PMDA E2B(R3) generation enhancements to address the revised pharmaceutical affairs law requirements for clinical trial submissions and TIKEN scenarios.
- When reporting Study Cases for Studies registered in Japan, the system populates the J2.12 Clinical Compound Number (CCN) data element based on the Study’s CCN when available. When the CCN is blank on the Study, the system continues to map the CCN of the Primary Case Product Registration from the Local Reporting Details. In both scenarios, the J2.13.r section repeats for each Study with a Japan Study Registration that has a Clinical Compound Number field with the same CCN transmitted in J2.12.
- When a foreign Case has multiple Japan-reportable investigational Product registrations with the same substance, the system provides a way to configure multiple CCNs for a single Case primary Product.
- In a scenario where a foreign Case includes both postmarking and investigational registrations and meets the Under Partial Change Trial criteria so that the PMDA requires only a postmarketing Transmission, selecting the new TIKEN field in the Product Reporting Details section prepends “TIKEN” on the G.k.11 Additional Information on Drug (free text) data element. In addition, Admins can now set up product registrations with the new “Under Partial Change Trial (TIKEN)” registration type.
- In generated PMDA E2B(R3) reports, on the J2.4.k License Category of New Drug and J2.14.i Expectedness data elements, the J-OID Code System version is updated to 1.2.
This feature is Auto-on, however some components require additional configuration.
Learn More
- Enablement: Enable PMDA Clinical Trial Reporting Enhancements
- Admin Help: Set Up the Localized Business Admin Library: Japan Localized Study Fields
- User Help: E2B Generation Data Mapping: PMDA E2B(R3)
PMDA Due Dates Based on Localized Case Assessments
In 23R2, Vault Safety introduced PMDA Local Assessments and Expectedness as part of the PMDA Local Case Processing Automations feature, with the plan to update the associated Reporting Rules in a later release. In 23R3, the PMDA Reporting Rules run on and calculate due dates for each Localized Case Assessment on a Localized Case for Japan. Admins control whether Localized Due Dates are calculated based on the Global Case New Info Date or the Localized Case Local Awareness Date.
Learn More
- Enablement: Enable PMDA Due Dates Based on Localized Case Assessments
- Admin Help: Set Up the Localized Business Admin Library: Set Up Localizations
- User Help: Complete Intake and Process Cases for the PMDA: Evaluate Reporting Obligations for Localized Case Assessments
PMDA Local Reporting Details Generation and Submission Linking
To support PMDA multi-Submission requirements, Vault Safety now gives Case Processors the ability to generate Local Reporting Details that drive Submission generation. Completed in a single action, this feature generates Local Reporting Details, Product Joins, and Localized Case Comments based on Case Product Registrations that are reportable to Japan. Previously, these records required manual creation and linking, which was time-consuming and error-prone.
Learn More
- Enablement: Enable PMDA Local Reporting Details Generation and Submission Linking
- User Help: Complete Intake and Process Cases for the PMDA: Local Reporting Details Section
PMDA Submission Validation Enhancements
Vault Safety has updated the PMDA Submission Validation Criteria to reflect the PMDA Data Model changes made in the 23R1 release. This enhancement also addresses previously reported PMDA validation issues to ensure compliance with PMDA regulatory reporting requirements.
Learn More
- User Help: Case and Submission Validation
CIOMS II Line Listing Enhancements
Vault Safety introduces additional enhancements to the CIOMS II Line listings. You can now incorporate narrative previews, reporter comments, and company comments on the CIOMS II Report. Additionally, the system now populates the reporter’s country when the Event Country field is empty. By supporting additional fields, the CIOMS II has gained flexibility. As a result, the CIOMS II Line Listings has increased adaptability and encompasses a wider array of reporting requirements.
Learn More
- Enablement: Turn Off Narrative Preview for CIOMS II Reports
- User Help: Create CIOMS II Reports
Criteria Page on Aggregate Reports
Vault Safety now provides an option to display a criteria page at the beginning of each of the Aggregate Reports using an “Include Criteria Page on Documents” checkbox. The following summary information is displayed on the criteria page:
- Report Parameters (for example, the Document Name and Organization)
- Filter Parameters (for example, the Product Family, report period start and end date, and States to Include)
- Output Parameters (for example, Indicate Unexpected Terms and any additional fields displayed)
- Legend (for example, * indicates an Unexpected Event)
Previously, while some of the filter criteria could be displayed in the header and footer of an Aggregate Report, it was not user-friendly. Using a summary criteria page, this feature highlights and clarifies valuable information on Aggregate Reports.
Learn More
- Enablement: Enable Aggregate Reports Criteria Page
- User Help:
DSUR: Generate Masked Appendices
Vault Safety now generates masked versions of the following documents for the DSUR when masked generation is specified:
- Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions from Clinical Trials.
- Appendix: List of Subjects Who Died During the Reporting Period
Previously, only the DSUR Interval Line Listings of Serious Adverse Reactions and Cumulative Tabulation of Serious Adverse Events from Clinical Trials were generated as masked.
Learn More
- User Help:
DSUR and PBRER: Summary Totals and Separate Log Files
This release introduces several changes to the DSUR and PBRER aggregate reports.
The DSUR Cumulative Tabulation of Serious Adverse Events from Clinical Trials and the Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials now display totals for each MedDRA System Organ Class (SOC) and MedDRA Preferred Term (PT) as well as a grand total.
The PBRER Cumulative Tabulation of Serious Adverse Events from Clinical Trials and the Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources display totals for each SOC and PT as well as a grand total.
Additionally, DSUR and PBRER Log files are now generated as separate files using the new “Log” Document Classification type. The Summary Tabulation of Adverse Drug Reactions from Postmarketing Sources Log also includes additional information to validate the report and correlate the tabulation results with the log of Cases.
Admins must upload new templates before enabling this feature.
Learn More
- Enablement: Enable DSUR and PBRER Summary Totals and Separate Log Files
- Admin Help: Configure Aggregate Report Templates
- User Help:
PSUR: Summary Totals and Separate Log Files
The PSUR Summary Tabulation of Serious Unlisted Adverse Drug Reactions and Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions now display totals for each MedDRA System Organ Class (SOC) and MedDRA Preferred Term (PT) as well as a grand total.
The styling of the tabulation report has also been updated for better usability.
Additionally, Summary Tabulation of Serious Listed and Non Serious Adverse Drug Reactions Logs are generated separately in a single Log file, using the new “Log” Document Classification type. The Log includes a “Report Tab” column to distinguish Cases as “Serious Unlisted” or “Serious Listed and Non Serious”.
Admins must upload a new template before enabling this feature.
Learn More
- Enablement: Enable PSUR Summary Totals and Separate Log Files
- User Help: Create PSUR and CIOMS II Line Listing Reports
DSUR and PBRER: Enhanced SAR Line Listing Review
This feature improves clarity when reviewing the DSUR and PBRER Interval Line Listings of Serious Adverse Reactions (SAR) by displaying additional relevant information and styling enhancements:
- The report now displays Case Assessment Results in the “Comments” column for each listed product and event.
- A Trial Number row groups the System Organ Class (SOC) terms and Cases below it.
- The report displays subtotals of Cases for each Trial and SOC and a grand total of Cases.
- The report applies shading to the “Trial #” row and includes a line to separate multiple products within the same Case.
Learn More
- Enablement: Enhanced SAR Line Listing Review for DSUR and PBRER
- User Help:
AS2 Connection: Health Authority Submissions
With this release, AS2 Connections now supports a number of Health Authorities. Previously, AS2 Gateway Connections only supported partner distributions. See the following sections for details of the supported Health Authorities.
AS2 Connection: EMA
AS2 Connections now supports submissions to the EMA.
Learn More
- Admin Help: Configure EMA AS2 Connection
AS2 Connection: MHRA
AS2 Connections now supports submissions to the MHRA.
Learn More
- Admin Help: Configure MHRA AS2 Connection
AS2 Connection: FDA
AS2 Connections now supports submissions to the FDA.
Learn More
- Admin Help: Configure FDA AS2 Connection
AS2 Connection: PMDA
AS2 Connections now supports submission to the PMDA.
Learn More
- Admin Help: Configure PMDA AS2 Connection
AS2 Connection: HC
AS2 Connections now supports submission to Health Canada.
Learn More
- Admin Help: Configure Health Canada AS2 Connection
AS2 Connection: Russia (RZN)
AS2 Connections now supports submissions to the Russian Health Authority (RZN).
Learn More
- Admin Help: Configure RZN AS2 Connection
AS2 Connection: China
AS2 Connections now supports submissions to the Chinese Health Authority (NMPA CDE).
Note: This feature is currently available only to Early Adopters.
Learn More
- Admin Help: Configure NMPA AS2 Connection
Validation Query Builder: Simple Expressions
With this release, Vault Safety introduces the capability to configure validation criteria for Case data through the Vault Expression Engine. Previously, Vault Safety customers had to use either custom JSON or an SDK to validate Case data, a complex and cumbersome process often necessitating technical support for implementation. The Vault Expression Engine, which employs a more user-friendly language, now empowers Vault Safety customers to set up these validators, thereby enhancing process efficiencies and ease of administration.
Learn More
- Admin Help: Configure Custom Validation Criteria
- User Help: Case and Submission Validation
Safety-RIM Product Connection (Safety)
The Safety-RIM Product Connection automatically transfers Product Family, Product, Product Variant, Substance, Product Registration, and other records from RIM to Vault Safety. This automatic transfer from RIM to Vault Safety aligns product and registration data across these application families. This eliminates potential user errors of manually creating these records in Vault Safety and provides a common set of products in RIM against which to record Safety Cases.
Learn More
- Enablement: Enable the Safety-RIM Vault Connection
- Admin Help: Configuring the Safety-RIM Vault Connection
- User Help: About the Safety-RIM Vault Connection
Safety Feature Enablement Updates
Enablement updates apply to the following features in this release:
See the following articles to learn more about and configure these features:
- Upgrade/Downgrade Transmission State Evaluation:
- Use Adverse Event PT on CIOMS and MedWatch 3500A:
Safety & SafetyDocs
MedDRA UI Enhancements for Non-Case Coding
With this release, Vault Safety and Vault SafetyDocs have enhanced the MedDRA control interface for Datasheets, Watchlists, MedDRA Queries, Product-Event Combinations, and Product-Event Dispositions to display the full hierarchy to which a term has been coded. This updated interface also supports auto-coding to any level in the MedDRA hierarchy. These enhancements provide greater granularity, making it easier to understand where a term is situated within the MedDRA hierarchy, which is important for tracking purposes.
There is no change to the MedDRA interface for Cases and Inbox Items that include reported terms.
Learn More
- Enablement: Enable MedDRA UI Enhancements for Non-Case Coding
- Admin Help:
SafetyDocs
Import Literature References from Database Files
With this release, Vault SafetyDocs can intake literature references in bulk from third-party databases using the Research Information Systems (RIS) format. All relevant information can be automatically mapped into the system, which saves time and reduces the need for manual data entry, allowing for easier literature processing.
Learn More
- Enablement: Enable Importing Literature References from Database Files
- User Help: Import Literature References from Database Files
PSMF: Table of Contents Page Linking Change
This feature changes how hyperlinks generate in the PSMF Table of Contents (ToC). In PSMF PDFs, ToC links will now resolve within the same document. This enhancement makes it easier for non-Vault users to navigate the PSMF.
Learn More
PSMF: Table of Contents Page Numbering Change
With this release, Vault SafetyDocs applies continuous, sequential page numbering to the PSMF PDF, including the generated Table of Contents. Page numbers throughout the PDF are now displayed as a single series from 1 to N in Arabic numerals.
Learn More
PSMF: Suppress Logbook & eSignature Page on Generation
With this release, Vault SafetyDocs can suppress the auto-generation of the standard PSMF logbook and/or eSignature page when generating a PSMF PDF where a custom logbook or eSignature page has been defined. This enhancement gives users more control over customizing their logbooks and eSignature pages.
Learn More
- Enablement: Enable PSMF Automatic Logbook Generation: 23R3 Update
- User Help:
Aggregate Report Template Management
With this release, Vault SafetyDocs provides templates for all major Aggregate Reports (PBRER, PSUR, DSUR, PADER, and CIOMS II) as well as custom reports. These templates auto-generate tasks that break down the report authoring process into manageable steps for authors based on guidance from regulatory agencies. Having pre-defined report templates with auto-generated tasks reduces the need for manual data entry and duplication, enhancing the efficiency and accuracy of Aggregate Report authoring.
These templates are a useful starting point but customers are encouraged to customize them to align with their business processes.
Note: This feature is a building block for expanding future Aggregate Reporting capabilities in Vault SafetyDocs and will be available for full configuration in an upcoming release. For more information, contact your Veeva representative.
Learn More
- Admin Help: Configure Templates for Aggregate Report Authoring
- User Help: Author Aggregate Reports: Generate Aggregate Report Sections
Aggregate Reporting: Extract Report to Document
Vault SafetyDocs now provides the ability to run a Vault Report and automatically generate a document from it to make relevant data easily available for Aggregate Report authoring. Previously, users needed to locate the report, run it, export it to an Excel or PDF format, and then re-upload it as a document. With this feature, this process has been automated to reduce manual work and allow authors to easily access data from line listings, tabulations, and other custom reports for their Aggregate Reports.
Note: This feature is a building block for expanding future Aggregate Reporting capabilities in Vault SafetyDocs and will be available for full configuration in an upcoming release. For more information, contact your Veeva representative.
Learn More
- Admin Help: Configure Templates for Aggregate Report Authoring
- User Help: Generate Aggregate Report Documents from Vault Reports
Literature Abstract Auto-Translation
Vault SafetyDocs now offers the ability to translate non-English literature abstracts into English using the AWS Translate service. The system also identifies the language of literature abstracts so that users can easily determine if they need to be translated. Once the translation is complete, pharmacovigilance teams can review the literature abstracts to determine if there are any ICSRs to be processed or signal investigations to be started.
To control translation costs, Admins can configure limits to the number of articles that can be translated and maximum abstract length that can be sent for translation. Using this feature will save costs and time that went into evaluating whether an article needs to be fully translated, in turn increasing the efficiency and accuracy of literature processing.
Note: Customers must use their own AWS account when setting up this feature.
Learn More
- Enablement: Enable Literature Abstract Translation
- Admin Help: Configure Literature Abstract Translation Settings
- User Help: Translate Literature Abstracts
Data Model Changes
See 23R3 Data Model Changes: Safety.
QualityOne
In addition to the below release notes, the QualityOne Veeva Connect community offers general release communications, release highlights, and key feature demos.
QMS
Periodic Review
The Periodic Review feature allows for more streamlined and collaborative periodic reviews, with all information collated from multiple data sources. Periodic Review enables customers to set up reusable templates for different types of reviews, capture data related to quality events that inform the review process through automated generation, and assign tasks associated with the review.
Annual Product Quality Review
The Annual Product Quality Review (APQR) feature allows customers to capture data related to an annual evaluation of a product assessment. As part of a periodic review process, customers can assign data compilation tasks, auto-generate reports into documents, and generate a Final APQR Report based on the data stored in the record. The Final APQR Report consists of Quality data from multiple sources, the summarized results of the review, and the recommendations from distinct subject matter experts.
Learn more about APQR.
Generate Document from Report
This feature enables customers to generate a document from an exported Report directly from a Periodic Review Item as part of a periodic review process, such as APQR. Running this action generates an associated document based on an export of a specified report.
Learn more about Document Generation.
Enhanced Configurability for Product Group
In this release, we have added the ability for Admins to configure the attribute System manages field value for the Name (name__v) field on the Product Group (product_group__v) object. Customers can now add configurability for naming conventions on the Product Group object, allowing more flexibility for customers to provide custom naming conventions that suit their needs.
HACCP Flow Diagram: Easily Identifiable Process Steps
This feature introduces additional process step types for HACCP Plans, represented as easily identifiable shapes on the HACCP Flow Diagram. The following new process step types are available from the Diagram Symbols panel:
- Material: Represents a step in which a material is processed.
- Logistics: Represents a step in which a material is stored or distributed.
- Connector: Represents a connection to another HACCP Flow Diagram.
Learn more about the HACCP Flow Diagram Process Steps.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Managing Hazard Analysis
This feature offers the ability to view and manage Process Hazard Analysis directly from the HACCP Flow Diagram. Customers can select any type of HACCP Plan Process Step except Connector to create, review, and update hazard analysis information for that process step in the Information panel.
Learn more about managing Hazard Analysis with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
COA UX Enhancements
This feature adds additional UX improvements to previously delivered features, such as the Analyze COA, Analyze Inspection, and Delete Inspection actions, job logging and notifications, and additional minor backend functionality to enhance COA post-processing capabilities.
Learn more about the UX improvements for COA analysis.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
QMS & HSE
Skip Checklist Assignment for Previously Assigned Checklists
This feature enhances the behavior of the existing Manual Checklist Assignment user action available in the Audit lifecycle. Triggering this action now will skip assigning checklists to previously assigned users in an Audit Checklist, avoiding duplicating checklist tasks for the same users and assigning checklists to any newly-added users. This creates more clarity for users working on a checklist and removes any confusion pertaining to duplicate tasks. If any custom object type exists for the Audit Checklist Assignment object, customers must manually add the Checklist field to the custom object type.
Learn more about audit checklists.
QualityOne Audit Checklist on Android Enhancements
This feature delivers some essential enhancements for seamless enterprise deployment, along with valuable updates driven by customer feedback.
The QualityOne Audit Checklist Mobile 23R3 release is scheduled for tentative availability on December 12, 2023.
Ad Hoc Section Support
This feature allows users to create Audit Checklist sections directly from the QualityOne Audit Checklist Mobile (Android) application. Auditors have more flexibility in how they want to structure the content for their Audits by creating sections in the moment without leaving the application or accessing Vault.
SSO SAML Support
This feature allows customers with an SSO SAML provider to access QualityOne Audit Checklist Mobile (Android) with a single set of credentials, enabling a more secured authentication method.
Risk Management: Support for 2D and 3D Matrices in the Same Vault
This feature provides the flexibility to create 2D and 3D Risk Matrices in the same Vault, eliminating the need to choose between 2D or 3D. Both types of Risk Matrices can be used in the same QualityOne Vault, depending on Risk Study requirements (for example, using 2D matrices for HAZOP studies and 3D matrices for p-FMEA studies).
Learn more about Risk Management.
Data Model Changes
See 23R3 Data Model Changes: QualityOne.
RegulatoryOne
The RegulatoryOne 23R3 release, including all Platform features, is scheduled for tentative availability on December 12, 2023.
In addition to the below release notes, the RegulatoryOne Veeva Connect community offers general release communications, release highlights, and key feature demos.
Compliance Management
Quantitative Assessments
This feature introduces the quantitative assessments data model and functionality, which allows users to compare the total concentration of substances in a formula to local market restrictions and to determine whether a formulation is compliant in that region early in the product development lifecycle. Vault automatically populates certain fields on new Quantitative Assessment records based on field values, ensuring compliance information matches data captured in existing records.
Learn more about Quantitative Assessments.
Automate Quantitative Assessments
This feature automates the creation of Quantitative Assessment records for substances of concern. Vault suggests which records to create after iterating through a list of ingredients for regulated substances and determining whether their concentrations are within acceptable limits in specified local markets, saving users time and reducing errors.
Learn more about automatically creating Quantitative Assessments.
Registration & Dossier Management
Registration Item Requirement Hierarchy Viewer Enhancement: Ordering
As of this release, Registration Item Requirements created by the Generate Requirements action maintain their default order when viewed in the Registration Item Requirement Hierarchy Viewer and users can reorder requirements directly in the viewer, minimizing the time spent modifying the order before generating a Dossier Binder.
Learn more about reordering requirements in the Registration Item Requirement Hierarchy Viewer.
Defining Registration Item Requirement Order for Dossier Binders
As of this release, customers have the ability to define a default order for Requirements, which carries over to the Registration Item Requirements created by the Generate Requirements action. Binders generated from these Registration Item Requirements maintain the default order, so users do not have to reorder them each time they run the Create Dossier action.
Learn more about defining default order for Requirements.
Registration Item Requirement Hierarchy Viewer Enhancement: View Matched Documents
As of this release, the Registration Item Requirement Hierarchy Viewer displays the matched documents associated with each requirement and provides users with the ability to navigate to each document directly from the viewer, reducing the time users spend reviewing all documents in a dossier.
Learn more about the Registration Item Requirement Hierarchy Viewer.
Remove Create Registrations in Bulk Feature
As of this release, we have removed the Create Registrations in Bulk feature, which we no longer support. We have replaced this functionality with the Local Impact Assessment feature (released in 22R1), which allows users to generate the appropriate Registration records within the registration process and makes the Create Registrations in Bulk feature unnecessary. All customers previously using the Create Registrations in Bulk feature have already moved to other features prior to this release, and no customer is directly impacted by the removal of this feature.
23R3 Registration & Dossier Management Enhancements
This feature introduces several data validations and error handling which ensure customer data is correct and clean, including:
- Each Registration Item Requirement can only have one (1) EDL and one (1) EDL Item related children.
- The Registration Item Requirement Hierarchy Viewer displays an error if any of the Registration Item Requirements link to multiple EDLs or EDL Items.
Additionally, when users run the Local Impact Assessment action, Vault updates the Regulatory Process Type field when applicable, ensuring the field always reflects the correct value.
This feature also removes the Relational Token and Object Mapping tabs. These are replaced by the equivalent pages on the Application Configuration section of the Admin > Configuration page introduced with the Admin UI to Manage Relational Tokens & Object Mappings feature in 23R1.
Data Model Changes
See 23R3 Data Model Changes: RegulatoryOne.
Veeva Claims
The Veeva Claims 23R3 release, including all Platform features, is targeted for tentative availability on December 12, 2023.
In addition to the below release notes, the Claims Veeva Connect community offers general release communications, release highlights, and key feature demos.
Veeva Claims
Direct Access to Claims from Substantiation Documents
Most claim Substantiations reference an underlying substantiation document linked by a related reference field on the record. This feature introduces the new Substantiations Doc Info panel, where users can view all supported Substantiation and Claims for a document, allowing them to easily see the context of where a substantiation document is used.
Learn more about accessing claims from substantiation documents.
Simplified Substantiation Creation
Most claim Substantiations reference an underlying substantiation document linked by a related reference field on the record. This feature simplifies the process of uploading a new reference document while creating a substantiation record by allowing users to upload a new document or reference an existing document when creating a Substantiation record. In previous releases, users had to upload the document before they could reference it in a new or existing Substantiation.
Learn more about substantiating claims.
Copied from Local Adaptation Link
Claims users commonly copy Local Adaptation records to create new Local Adaptation records. When copying Local Adaptations, it is important to establish a link between the copied from record and copied to record to maintain traceability so that users can refer back to the key details from the original record. This feature introduces the read-only Source Local Adaptation and Source Local Adaptation Claim reference fields on the Local Adaptation object, which auto-populate when a user copies a Local Adaptation record.
Learn more about copying Local Adaptations.
Comment Thread Viewer Enhancements
This feature introduces improvements to the comment thread viewer, making for a more seamless and visually enhanced user experience. Anytime users add a new comment or reply in the thread viewer, the corresponding row in the thread viewer is highlighted, with the applicable thread expanded, to help them visually confirm that their comment has been added.
Learn more about comments.
Comment Thread Notification Enhancements
With this feature, users can now navigate directly to comments and replies in the comment thread viewer from their email and Vault notifications. Vault also highlights the corresponding row in the viewer when users navigate to an existing comment or reply from a notification and when applicable, expands the appropriate thread.
Learn more about configuring comment notifications.
Admin Settings Configuration Enhancements
This feature enables Admins to configure settings for existing Veeva Claims features, such as Comments and Localize Pack Copy (including translator groups), from the Admin > Configuration page, simplifying the configuration steps for these features. In previous releases, Admins had to define these feature settings in Claim Admin Setting records.
Learn more about configuring Comments and Localize Pack Copy.
Specify Column Order Display for Action Settings
With this release, Admin users can specify the column display order users see in the dialogs when they run the Create Local Adaptations Run As System and Localize Pack Copy actions.
Learn more about configuring the Create Local Adaptations Run As System and Localize Pack Copy actions.
23R3 Veeva Claims Enhancements
This feature introduces several UI enhancements to improve the overall user experience. Vault now shows users a banner after they run certain actions, confirming that the action executed so users can avoid unnecessarily rerunning the actions. This feature also removes irrelevant instructional text from action dialogs that do not list any records.