Prerelease Date: March 21, 2022 | Release Date: April 8, 2022 & April 22, 2022
The following applications may have different release dates: Safety, QualityOne Client Applications, RegulatoryOne, and Veeva Claims.
We are pleased to bring you Vault 22R1. Read about the new features below. You can find information on enabling new features in 22R1 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Working with Documents
Editable Bookmarks
When enabled, this feature allows users to edit certain Bookmark attributes on the Doc Info page. Once saved, the edited bookmarks persist in the viewable rendition. Supported actions include Rename, Move Up, Move Down, Promote, Demote, and Delete.
Copy Text from Viewer
With this enhancement, Vault users can copy text from Vault documents by holding down the C key while selecting text, saving the copied text to the user’s clipboard. This option is available in both View and Annotate modes.
Google Drive Integration: Upload Files
This enhancement allows users to browse for and select files from their Google Drive when creating a new document, uploading a new document version, or uploading a file to a placeholder in Vault. This enhancement also updates the UI when uploading documents.
Caption Bookmark Exclusions
This feature builds on Vault’s ability to generate bookmarks in a PDF Viewable Rendition based on captions in Microsoft Word documents. With this release, Admins can exclude specific captions when generating bookmarks and can control generation of “Lists of” bookmark sections based on captions, which previously required enablement from Veeva Support.
Annotate Usability Improvements
With this release, we have made the following enhancements to improve the user’s ability to interact with documents and annotations:
- Annotation filters: Filter by Author includes reply authors, and the Links section differentiates document links from permalinks.
- Permalinks have the distinct document field “Annotations (Permalinks),” which is available in Library view, Reporting, and other locations.
- Rotate Page is replaced by Rotate Page and Rotate All Pages, allowing users to temporarily rotate all pages during a viewing session.
- Zoom, Fit, and Full Screen are applied with buttons instead of menus.
- Toolbar buttons that previously dismissed unsaved annotations are visibly disabled while composing annotations.
- Users can now scroll, zoom, apply fullscreen, fit width, and height while composing annotations.
- Admins can opt into a new “strict” permission requirement for Copy Text functionality.
Text Indexing & Combination Enhancements
This enhancement improves Vault’s ability to correctly index, find, and highlight text in documents that contain specific kinds of punctuation that serve as word separating characters, including periods, commas, semicolons, and dashes. This enhancement also improves its ability to handle formatting variations of single and double quotation marks. Users see better results when performing certain actions on documents, including Find in Document, Create annotation, and Suggest Links (PromoMats).
Notifications on Replies to Notes
This enhancement notifies users whenever another user replies to one of their note annotations. Vault also limits the number of notifications from a single annotation event, preventing redundant notifications. For example, if someone adds a new note, replies to an existing note on a Favorite document, or mentions the user when composing a note or reply, Vault only sends one notification.
Required Overlays
With this release, Vault prevents the download of viewable PDF renditions when overlays fail to apply. Admins can also mark overlay templates as optional, allowing downloads when overlays fail to apply.
Today Link for Date Fields on Doc Info Page
This enhancement adds a Today link next to any Date field that will automatically stamp today’s date when clicked. This link appears while editing on the Doc Info page, or on the bulk documents edit page.
EDLs: Configurable States for Document Version Locking
Customers can configure which document lifecycle states support document version locking for EDL matched items, allowing customers to lock document versions in states other than Steady or Superseded. The EDL Match Job will take a little longer to complete the first time it runs after this update due to the data model updates. The completion time for subsequent runs of the EDL Match Job will return to their normal run time.
Collaborative Authoring Troubleshooting Page
This feature introduces a logs page that allows users to view all Microsoft Graph API errors that occur when using Collaborative Authoring with Microsoft Office. This provides users with a self-service method for troubleshooting issues with Collaborative Authoring. Learn more about the Collaborative Authoring Error Log.
Auto-Populate Rendition PDF Metadata
With this release, Vault populates the basic document metadata in a viewable rendition’s PDF Document Properties based on the original source document’s properties. This feature works with all supported Microsoft Office source document types.
Enhanced Bulk Document Update: Validation Behavior Changes
This feature introduces several changes to bulk document update validation:
- For any UI errors which did not previously have a defined error message, Vault now displays a generic error message.
- Vault now displays an error if a user attempts to update a read-only field on a document.
Deprecate Office Online Feature
After the 22R1 release, any documents that are checked out to Office Online will no longer be accessible, and the system will automatically cancel the checkouts. Documents that are checked out to Office Online can be checked back into Vault only until 22R1. The ability for users to check out using the legacy Office Online feature was removed with the 21R2 release.
Legacy Feature Enablement Updates
With this feature, the Enable checkout to Office Online feature is now disabled on all Vaults.
Document Upload UI Enhancements
This enhancement updates the icons and UI when creating a new document or draft and uploading a new version or to a placeholder.
Vault Objects
High Volume Object Multi-Value Picklist Field
This feature provides support for the multi-value picklist field in high volume objects. You can create up to two (2) multi-value picklist fields per high volume object.
Today Link for Date Fields on Object Record Detail Page
This enhancement adds a Today link next to any Date field that will automatically stamp today’s date while editing information on an object record details page.
Lifecycle & Workflow
Remove eSignature in Workflows
Workflow administrators can now remove signatures on documents when a workflow is cancelled or during the workflow using a Workflow Cancellation Action or a Content Action. Signatures can only be removed on the current active workflow, and can be removed for a specific task and verdict.
Show More on Tasks in Home Pages
This feature makes it easier for users to manage their tasks by providing additional task and workflow information on the homepage. It also enables Admins to configure whether or not users may complete a task directly from the homepage.
Consistent Steps Across Workflows
This feature makes workflow steps consistent across Workflows on Documents and Objects. The Action step now combines the former object workflow State Change, Update Record Field, and Update Related Record Field steps, and allows users to perform them conditionally. Additionally, state changes performed within an Action step no longer check entry criteria or fire entry actions when the target state is the same as the existing state. Learn more about configuring workflows on documents and objects.
Populate Object Reference Fields with Workflows & Lifecycles
This feature provides the ability to update object reference fields in lifecycle or workflow actions using expressions. Users may return specific records using the new RecordByLabel() function.
Enhanced Stages Chevron Card for Workflows
In previous releases, users had to navigate to the timeline view to see actions on open tasks. With this release, lifecycle stage chevron cards now display actions on open tasks as well as additional workflow information, saving users clicks and providing easier access to this info.
Bulk Actions on Record Actions
Applications and customers may now expose SDK Record Actions within Vault’s bulk actions. Admins will now see a Record Actions option within the bulk action interface for objects. Learn more about bulk object record actions.
Verdict Information & Reporting on Removed Documents
With this release, Vault shows verdict information for documents removed from workflows manually or by a content action on the Workflow Timeline view. Additionally, reporting and notification tokens on Verdicts can now capture removed documents. Once a document is removed, it’s state is set to Removed in the envelope.
Create Draft Maintains Current Version in Workflows
With this feature, Admins can now enable documents in workflows to remain in their steady state version, even if the Create Draft action is performed on the document. By default, all workflows on documents continue with the latest version of the document.
Skip Document State Change in Object Lifecycle Entry Action
Workflow administrators can skip the state change of related documents in an object lifecycle entry action. This allows administrators to skip state change for related documents already belonging to a steady state.
Remove Default Task Link in Labels
This feature allows Admins to turn off the default link provided with the task label.
Reporting & Dashboards
Export Report to Formatted Excel
This feature allows users to export reports as they are displayed in the report viewer. Users can download the full data or grouped summary of the report in an Excel format. This reduces the effort required to format the exported Excel sheet and improves usability.
Vault now displays a single Export to Excel option in the Actions menu for all Excel related export actions, which provides several export options: the default Data only option; the Formatted option, which exports the formatted Excel; and the Template option, if users upload a pre-formatted Excel template.
Optional Prompts in Reports
With this release, report creators can make prompts optional, allowing users to run a report without adding a value to the report prompt. Users may view all the records or a subset of the records from the same report. Reports are more flexible, allowing one report to solve the use cases of two reports previously. Users can also filter on a null or blank value.
Name Fields Support the In Operator for Report Filters
With this release, users can view and select the names of the object or document records and add them as filter values in a report. This feature also adds the conditional operator “in” to the report filters for the Name fields. Users can then view record values before adding them to the filter, and can select up to 100 values in a single filter. This user experience is similar to the object reference field selector and is supported for both standard and HVO objects.
Multi-Pass Formula Fields Enhancements
With this release, Vault supports formula fields on Multi-Pass reports created using Binder, Relationship, and Workflow report views, along with improved performance on formula fields on Multi-Pass reports overall. Formula field performance was also improved in multi-pass reports.
Additional Document Field Support in Reports
With this release, Vault supports the Checked Out By, Checked Out Date, Checked Out, and Description fields on Document reports.
Auditing
Audit Trail Enhancements to Track Lookup Field Changes in Object Records
This enhancement allows the Audit Trail to log an entry on object records with lookup fields whenever the values change due to source field changes, reference object changes, and changes caused by lookup field initializations.
Vault Formulas
Workdays & Holidays
This feature allows users to perform calculations in expressions that factor out weekends and holidays, making timeliness metrics and due dates more accurate. The new function NetWorkdays() returns the difference between two dates excluding weekends and optionally holidays. Workday() returns a date that is N days in the future excluding weekends and optionally holidays. Admins can define Holiday Schedules and their children Holidays, and can select a Holiday Schedule record for each user.
RecordByLabel() Function in Expressions
This feature allows users to return object references based on object record labels in expressions. The RecordByLabel() function accepts object labels and returns object references which can then be used to populate fields. This feature is only available in workflow and lifecycle actions.
Significant Figures in Expressions
This feature modifies the Round function to accept an optional parameter that can either be “significant” or “significant-astm”. This enables users to round their numbers down to the appropriate number of significant figures based on two common methodologies.
Search & Filter
Multidirectional & Phrase Synonyms
The synonyms feature has been enhanced in several ways. First, there is a new option to set any row in the thesaurus to multidirectional. This will make it so a user can search for any entry or synonym on that row and the search will expand out to all of the other entries and synonyms. This is useful for terms like “child” and “adolescent” that should both always match no matter which term is searched.
Vault also now supports phrases as entries. With this, users can search for more complex concepts. For example, searching “myocardial infarction” can match “heart attack” and vice versa. Phrases wrapped in double quotes also now match to any synonyms as well.
The limits have also been updated to increase flexibility. Each row in the thesaurus can have 250 characters in up to 5 entries and 15 synonyms. This supports use cases like name search where there may be many variations on a first name (e.g. Liz, Lizzy, Elizabeth, Liza, etc), and long acronyms where the expanded form exceeded the 50 character limit per synonym that was in place previously.
Field Centric Strict Matching
To facilitate multi-term synonym searches, we’ve changed Vault searches with more than one term to allow a search like “myocardial infarction” to appear as a phrase in the thesaurus and match to other phrases like “heart attack.” This update also makes the Strict matching option more field-centric, meaning that the minimum required matching terms must be in the same field rather than split between multiple fields. Strict matching is optional, so Admins can enable or disable it at any time under Admin > Settings > Search Settings.
Expanded Search with Related Records
You can now configure Search Collections to include relationships between objects. This allows expanded searches to return results in one object section because of their relationship to records matching the search criteria in another object section. For example, if there is a relationship between Product and Country to manage the list of countries in which each product is marketed, you could set up a Collection to return all of the country records related to the search results from Products. As you filter down Product results, the Country results update as well.
Search by Name on High Volume Object Tabs
When navigating to an HVO tab, users can now use the main search box to quickly find records by name. This is a case-sensitive, starts-with search, so it has limited capability when compared to Vault’s standard object search.
Filter Envelope Tasks by Document Fields
Users viewing OneWorkflow tasks on the Home tab can now apply document filters on those tasks. Once the user filters Task Type to show only Envelopes, filters become available for the documents contained within each Envelope.
Checklists
Checklists: Dependent Sections
Multiple-choice question responses can now control the appearance of one or more sections in a checklist.
Checklists: Add Reference Documents to Questions
Checklist designers can link one or more Vault documents to their questions as reference material for checklist respondents to review when providing answers in the checklist.
Checklists: Hide Unseen Questions on Review Page
The checklist review page no longer displays dependent questions that the respondent did not see when completing the checklist.
Auto Claims Linking
Auto Claims Linking: View Claim Details from Select Claims Dialog
With this release, when manually linking to Claims, users can click to view the Claim record in detail from the Select Claim dialog. This enables a more informed selection, and replicates the existing experience when linking to documents and anchors.
Auto Claims Linking: Ignore Superscript & Subscript Characters When Matching to Claims
This feature allows Vault to ignore superscript and subscript characters at the end of words in documents when determining matches to approved Claim records. This eliminates the need for a majority of wildcards in match text values, which were previously necessary to account for variable footnote and endnote references in content.
Usability Updates
Breadcrumb Enhancements for Object Tabs
As users view data in an Object tab, each click to view details of a record or drilldown to view a related record or document is tracked by a breadcrumb. Users can mouseover the breadcrumb to display a hovercard that identifies the previously viewed record before navigating back to that page. Previously bookmarked Object Record Detail pages no longer display the breadcrumb generated when the page was originally bookmarked. Such breadcrumbs are stale and prone to errors as data changes over time.
Learn More Links: Vault Help Update
With this release, “Learn More” links within Vault now target a new Vault Help site.
Norwegian Language Support
Vault now supports exporting and importing Norwegian dynamic messages.
Notifications: Email Burst Threshold
With this feature, Admins can set a burst threshold for the number of emails Vault sends for the same notification template in a 30-minute period. Once the burst threshold is reached, Vault throttles the sending of additional email notifications for that template. The burst threshold is a guardrail for customers whose email provider or infrastructure cannot handle a large influx of emails. Learn more about email administration.
Notifications: View Full Content in Panel
Users can now expand the content of longer notifications directly within the notification panel to view up to 25 lines of text without opening the notification page. This allows users to view longer notifications without having to navigate away from their current task. For notifications longer than 25 lines of text, the notification includes a hyperlink to navigate directly to the full text on the notification page.
Email to Vault: Create Records for Bounced Emails
With this feature, when Vault bounces an inbound email because it is considered spam or because the sender fails SPF or DKIM authenticity checks, it now creates an Email object record in the Bounced lifecycle state. This provides Admins with the ability to report on bounced emails, inspect these emails, and have Vault process them if deemed legitimate.
Workflow Action Menu for Objects: Drop-Down Behavior Update
With this release, Vault displays the drop-down when users click the state change and workflow action chevrons. Previously, when only one state change or workflow action was available on the chevron, clicking the chevron immediately started the state change or workflow action.
Workflow Action Menu for Documents: Drop-Down Behavior Update
With this release, clicking on a chevron button with only one workflow action available displays a drop-down instead of initiating that workflow action. Users can then click on the action label in the drop-down to initiate it, or click elsewhere to cancel. This enhancement helps prevent users from accidentally triggering workflow actions.
Rich Text/Long Text Pop-up Window Size Update
This change makes the Rich Text and Long Text field pop-up window size bigger when viewing a record so that it is the same size as when editing the record. This change provides a consistent experience between viewing and editing Rich Text and Long Text fields.
Updated UI Style on Users & Groups Pages
In this release, we have updated the Security Profiles, Permission Sets, and Groups list views (Admin > Users & Groups) with our new UI style (Create button, filter, and search UI element placement). There is no functionality change. This feature was added in 21R3.2.
End of Support for Microsoft Internet Explorer Browser
With the 22R1 release, Vault will no longer support the Microsoft Internet Explorer 11 browser application. Microsoft has announced IE 11 will be retired on June 15, 2022. Vault customers should explore transitioning to other browser applications in advance of this date. Refer to Vault’s list of supported browsers to identify an appropriate browser to use.
Administration
Changes to FTPS Cipher Suite
In this release, Vault will no longer support TLS1.0 and TLS1.1. These changes may affect custom integrations, but Vault UI users will not be affected.
Learn more about cipher suite changes.
Scheduled Data Exports: Initial Full Data Export
This feature provides users with the ability to export all of their Vault object data and document metadata records in an initial load of Vault data into their data lakes and data warehouses.
Scheduled Data Exports: Uploading CSV Files to S3 with Bucket Owner Full Control ACL
With this release, customers using a self-managed AWS S3 bucket as a storage option for Scheduled Data Exports can be the S3 object owner for every export file uploaded to the bucket. Additional AWS S3 configuration may be required. This feature is Auto-on in Vaults configured for Scheduled Data Exports.
Scheduled Data Exports: Increased Precision on Field Values
With this release, currency fields exported in Vault Objects now have an extra decimal place. For example, 1.0 now exports as 1.00. Additionally, DateTime fields on Document and Document Version exports now include seconds in the timestamp.
Scheduled Data Exports: Including Inactive Document Fields by Default
With this release, Vault automatically includes inactive document fields in exports when users select the Document entity in a scheduled data export. Prior to this release, Vault only exported active document fields in Document exports.
Scheduled Data Exports: Escaping Characters in CSV Output
Currently, when Vault exports picklist fields with Scheduled Data Exports, commas contained within picklist items are escaped by an extra comma. For example, ["value1a,,value1b"]. With this release, commas contained within picklist values are escaped by two pairs of double quotes. For example, ["""value1a, value1b"", value2"] for multi-value and ["value1a, value1b"] for single value.
Additionally, empty values for multi-value object reference fields no longer contain commas in the output, and the exported field value is empty.
Finally, field values with backslashes no longer include an additional backslash.
Jobs: Disable Session ID Token in External URL Call Configuration
Session ID tokens in the external URL call job configuration are no longer supported and will not work in this release. Please update your job definitions to use the Post Session Credentials via Form Data with Key “Session.id” option.
Jobs: Display Start Time for Completed Jobs
The job start time is now displayed in the job history table on the Job Status page allowing Admins to quickly see when a job began and ended.
Access Control
Object Control Security
Object Controls are interface controls delivered by Vault Applications, allowing Admins to put them in the Object Record Detail Page layout to provide a tailored experience. For instance, in Vault Safety, users see object controls when viewing or editing a case object record.
This feature allows Admins to configure which object controls Vault displays at runtime. Admins can configure security profiles and permission sets to control the visibility of these controls at the object level (View permission). They can also use Atomic Security for fine-grained control of visibility on the object record details page, based on the object record state and role assigned to users.
User Role Object Moving to HVO
The User Role system object (user_role__sys), which is used to store user role assignments, is moving to high volume to deliver increased performance and scalability.
Authentication & Security
New Certificate for SAML SSO & Spark Messaging Connection
Release Dates:
- New Certificate Testing Period: March 28, 2022 6pm PDT - April 29, 2022 6pm PDT
- New Certificate Rollover Event: April 29, 2022 6pm PDT
- Support for New and Old Certificate: April 29, 2022 - May 20, 2022 6pm PDT
- Final Certificate Rollover: May 20, 2022 6pm PDT
Vault is scheduled to rollover the signing certificate used to sign SAML Single Sign-on requests and Spark messaging connections. There is no expected downtime.
The new certificate can be downloaded from Vault Help.
Your IT organization must ensure that the new certificate is configured on your Enterprise Identity Provider system prior to the New Certificate Rollover Event on April 29, 2022. Please ensure that your Spark messaging integrations do not cache the old certificate. Failure to utilize the new certificate by this date may cause login issues for SAML users, and Spark messaging integrations may fail.
Learn more about the SAML/Spark certificate rollover on Veeva Trust.
Security Policy Inactivation
Starting from 22R1, security policies of authentication type password or SSO can be inactivated by a domain administrator. Once a security policy is inactivated, users can no longer select it when creating or updating a Vault User. This change does not affect existing users already assigned to the policy. Additionally, administrators can delete security policies without any users assigned to them.
OAuth 2.0/OpenID Connect profiles: Preferred Microsoft Authentication Library (ADFS)
When creating or updating an OAuth 2.0/OpenID Connect profile and selecting ADFS as an authorization server provider, Vault provides an additional option to select the preferred Microsoft Authentication Library (MSAL or ADAL). MSAL is recommended when using ADFS 2019 or later. Vault File Manager uses this setting to load the right Microsoft library according to the profile setting.
Password Security Policies: Password Reset Rate Limit
Security policy of authentication type password includes a new setting limiting the number of password resets that an unauthenticated user can request in a 24 hour window.
Admins can set the limit between 1 and 10 requests, or set it to unlimited (existing behavior). This rate limit setting mitigates unwanted password reset requests. Once the limit is reached, additional password requests are no longer possible for 24 hours and only a system administrator or an authenticated user can reset the password.
Vault Loader
Vault Loader: Updating User & Group Role Assignments on Object Records
Vault Loader now provides the ability to assign and remove users and groups from object record roles for objects that have custom and matching sharing rules enabled. Auto-on in Vaults with Vault Loader enabled. Learn more about updating object record roles with Vault Loader.
Vault Loader: Return Updated Roles in Success Log
With this release, when updating document roles using Vault Loader, the success log now contains the updated role IDs. Auto-on with Vault Loader enabled.
Vault Loader: Log Skipped Lines in Failure Log
With this release, Vault Loader failure logs now include any input CSV lines that were skipped due to errors. Auto-on with Vault Loader enabled.
Vault Loader: Disabling Workflow System Objects from Load or Extract Requests
With this release, the envelope__sys and envelope_content__sys system objects are no longer available for load or extract using Vault Loader. These objects drive the behavior of Vault workflows, and excluding them from Vault Loader ensures data integrity within these objects. Auto-on with Vault Loader enabled.
Record Migration Mode: Suppress Email During User Task Creation
With this release, when User Tasks are created during Record Migration Mode through Vault Loader or API, email notifications are not sent for assigned or completed tasks.
Vault Java SDK
SDK Runtime Logs
This feature provides Vault Admins and developers runtime logging for SDK requests. Daily logs are available via the Vault UI and Vault REST API, and log entries include SDK exceptions and custom code usages of LogService. Vault Admins can configure the current log level as DISABLED, EXCEPTIONS (default), ERROR, WARN, and INFO. SDK Runtime Log entries are available 15 minutes after the request has completed and can be accessed for 30 days. Logging is limited to 10KB for Exceptions and 40KB for LogService entries.
Vault Tokens
This feature introduces a new component type, Vault token, and adds the new Vault Tokens permission type. Admins with the Vault Tokens: Create permission can configure up to ten (10) Vault-wide tokens using MDL. Learn more in the Vault Developer Portal.
Vault also includes the following system provided Vault tokens that developers can use to reference Vault information:
${Vault.vault_dns__sys}${Vault.vault_id__sys}${Vault.vault_name__sys}
Enhancements to Reference Lookups
With this feature, we’ve introduced a new Reference Lookup Type, Generic, which enables Administrators to configure lookups for mismatched data types. Additionally, the new Generic option provides support for any-to-any mapping for all single-value fields which were previously unsupported, such as Boolean. Multi-value fields such as multi-value picklists are not supported.
Object and Document Field Types for Integration Field Rules
This feature allows Vault Admins to configure the field type for an Object or Document Query Field in Field Rules. Additionally, developers can access this value in Vault Java SDK and use the value to create call-back VQL queries with the necessary VQL functions such as LONGTEXT(), RICHTEXT, and TONAME().
Vault File Manager
Download Rendition Files to Vault File Manager
With this release, users with the appropriate permissions can perform the Download Rendition File to Vault File Manager action when downloading Vault document renditions. If the file is over 4 GB in size, Vault suggests downloading the file to Vault File Manager instead of attempting to download in the web browser using the standard Download Rendition action.
Pause & Resume Uploads & Downloads with Vault File Manager
This feature allows users to pause and resume in progress uploads and downloads in the Vault File Manager application. Users can also sort files on the Uploading and Downloading tabs by the order in which they will be uploaded and downloaded.
Configuration Management
Migration Mode with Relaxed Validation Rules
With this release, Vault bypasses validation rules and reference constraints when creating records in migration mode via Vault Loader or configuration migration packages. Migration mode continues to allow record creation in any lifecycle state.
With this change, the audit trail will now append “in migration mode” in the event_description. For example, if the previous description was “Vehicle : VEH-000007 created”, the new description is “Vehicle : VEH-000007 created in migration mode”.
Data VPK: Validate VQL in Dataset Filter
This feature allows users to validate Criteria VQL prior to saving a dataset filter.
Vault Configuration & Compare Reports: Reporting Full MDL of Object Lifecycle State Entry Action Rules
Vault Compare and Vault Configuration Reports now report on the formula used in an object lifecycle state entry action by providing the full value of the rule attribute in the Objectlifecyclestateentryaction sub-component.
Vault Configuration & Compare Report: Details for Auto Managed Group Field Order Security Settings
Security settings for Auto Managed Group Field Order in Vault Compare and Vault Configuration Reports now display each object and its fields in individual rows, making it easier to understand the configuration and aligning it with how Vault displays the settings in the UI.
Vault Compare Report: Header Rows Frozen by Default
Column headers in each sheet in the Vault Compare workbook are now frozen by default so that users can easily understand which fields they are viewing while scrolling through the report.
Vault Configuration Report: Cover Page Title for Object Data Workbook
The Object Data workbook generated with the Vault Configuration Report now adds “Object Data” to the title in the cover page so that users can quickly understand which workbook they are viewing.
Configuration Management: Migrating Document Tags Between Vaults
Admins can now migrate Document Tags between Vaults using Vault Packages (VPK) and can view Tag components in Vault Configuration and Comparison reports. Auto-on in Vaults with Outbound and Inbound Packages enabled.
Configuration Management: Comparison of XMLString Component Attributes when Deploying Packages
Admins can view and compare values of a component’s XMLString attributes between the inbound package and the target Vault before deploying the package. Auto-on in Vaults with Inbound Packages enabled.
Configuration Management: Allow Outbound & Inbound Packages Enabled by Default on Align Vaults
Align Vaults will now have Allow Outbound Packages and Allow Inbound Packages enabled by default. These settings can be found under Admin > General Settings > Configuration Management.
Platform Data Model Changes
See 22R1 Data Model Changes: Platform for changes relating to the above features.
Vault Connections
PromoMats/RIM: Conditional Transfer Support Using Query Object Rules (QORs)
This feature supports Query Object Rules for RIM Vaults using the PromoMats/RIM Connection, adding the ability to filter out the documents and records transferred in an Integration Rule. Query Object Rules are already supported in PromoMats Vaults.
PromoMats/RIM: RIM Reference Model Support
This feature adds support for the RIM Reference Model to the PromoMats to RIM Connector. When document types are not defined in the Connector, the RIM Reference Model is used to determine the document types to be used with the Connector.
Clinical Operations
Monitoring Schedule Planning
This feature allows for the triggered and ongoing creation of Monitoring Events in Vault CTMS based on updates to milestones in a CTMS Vault. This functionality automates the creation of future Monitoring Events and allows users to assess the timeliness of created monitoring visits. Vault can create monitoring Schedule Templates at a Study or Study Country level to define the expected schedule.
RBSM: Critical Data & Processes
This feature adds support for tracking Critical Data and Processes for a Study. As part of a Study Risk Assessment, users can define which data points and processes are critical to study execution and link the records to Study Risks.
Milestone Roll Up Enhancements
This feature delivers improvements to Milestone Rollup behavior. When the Milestone Rollup Enhancements setting is enabled and Rollup (max) and/or Rollup (min) Milestone Dependencies are configured, Vault will reassess rolled-up dates when a previous Milestone’s date is set to null, updated, or a related Milestone Dependency is inactivated. This feature supports “First” and “Last” Site and Subject-related Milestones tracked in Vault Clinical.
Support to Identify Non-enrolling Sites
This feature identifies Sites that did not enroll any Subjects when the Enable Automated Enrollment Milestones setting is enabled, and the “No New Subjects” field is checked for a Site, allowing users to quickly inactivate Subject-related Milestones for the non-enrolling Sites with additional configuration.
Site Payment Holdbacks
This feature extends support for Payments to allow for holdback and overhead tracking in the delivered application. Fees defined as holdback fees generate two payable items to track accrued costs and make the initial payment to the Site. A new system action allows updating holdback items in bulk at the Site, Study Country, or Study level if the matching payment criteria are still valid.
Site Overhead Calculations
Vault Payments can now define whether Fee and Fee Templates should expect the addition of Overhead. If added, Vault Payments will calculate the Total Amount field value with the matching Overhead Percentage.
Subject Payment Tracking Rules
This feature allows Vault Payments customers to add additional Payment Rules to their Fees. You can add Subject Status as a required criterion for a Visit or Procedure fee, and use Source Data Verification status as a criterion for a Visit fee.
CDMS/ClinOps: Support for Multiple CDMS Vaults
Single Study Refresh enables refreshing ClinOps/CDMS connected data only for a single study. Single Study Refresh replaces the current full refresh mode which refreshes the connected data across all the connected studies.
CDMS/ClinOps: Single Study Refresh
This feature adds support for re-syncing CTMS data under a single study. This feature replaces the “full refresh” functionality that was available via the “refresh inbound” action.
Clinical Email Ingestion: Relevant Communications
This feature leverages platform email ingestion functionality to support inbound email addresses that create a TMF document from an inbound message and relate it to a Study Communication Log record.
TMF Bot: Auto-Classification Enhancements
This feature incorporates several changes derived from feedback received from customers, including changing auto-on models to .95 Prediction Confidence, and excluding Documents with null pages when training the auto-classification model.
TMF Bot: Bulk Upload Pipeline
This feature optimizes the TMF Bot to prioritize documents uploaded via the UI over those uploaded in bulk via the API, allowing end user uploaded documents to be auto-classified first.
Transfer CSV Reports for TMF Transfer
This feature provides greater visibility into TMF Transfer activity on both the sending and receiving Vaults. Vault provides two CSV reports to each Agreement Transfer record to specify the Names and Global IDs of each transferred item:
- Compare CSV compares the items that have been transferred from Source to Target over the life of the Agreement.
- Transferred Items CSV shows the specific items transmitted by TMF Transfer as part of a specific transfer action.
Non-User Viewing of Documents in Surveys
This feature extends the Clinical capabilities on Checklists to allow non-users to view a Vault document in the context of completing a Checklist in Vault.
Automate Milestone State Changes: Additional Date Field Triggers
This feature introduces additional triggers that fire following the update of a milestone’s planned start, planned finish, and actual start dates; and which updates other milestone date fields, metrics, or state without relying on lifecycle actions.
Milestone Gating for EDL Item Requiredness
This feature extends Milestone Gating by adding a check, either as configurable entry criteria or a workflow decision step, that ensures all related EDL items have a Requiredness value of “Required” or “Not Required.” It also introduces one-way settings that allow Vault admins, in the EDL Requiredness picklist, to enable the standard “Pending Decision” and disable the standard “Optional” values.
Site Document Exchange Section
This feature introduces a new section to the Study Site page layout that increases visibility into documents sent and received from Sites, condensing the five existing sections currently used to view this information into a single section.
Site Connect Details User Action
Site Connect customers can now access more details regarding a document’s distribution to Sites via a Site Connect Details user action. This action launches a dialog to display tracking information and the document’s comment history exchanged with a site.
Summary Notification of Returned Documents
With this feature, Vault now notifies the specific Site Connect users who have sent document requests to sites over Site Connect when documents return in a summary email notification specific to a given Study Site.
Site Information on Documents
Site Connect customers can now see additional details regarding documents sent and received over Site Connect. A new Site Information section is available on documents to display the below items directly within the document metadata.
- Sent to Site(s)
- Initial Sent to Site(s) Date
- Last Sent to Site(s) Date
- Received from Site Date
Site Connect: Distribution Task Return Field Field Now Dedicated to Site
This feature has made an update to dedicate the Distribution Task object’s Return Document Version and Return Comments fields to always contain the Site Document Version and Site Comments. Previously, Vault could contain site Document Version and Site Comments in the Document Version and Document Comments fields under certain conditions.
Customers currently tracking the documents received from a Site within a related list section of the Study Site record should hide the Document Version / Document Comments field and instead expose the Return Document / Return Document Comments fields.
Cancel Site Connect Agreement Invitation
This feature allows Site Connect customers to cancel Pending Site Connect Study Invitations, deactivating the invitation and canceling the workflow task in SiteVault.
Mark Site Connect Document as Rescinded
With this feature, Site Connect customers can now take a Mark Rescinded user action on documents sent over Site Connect. Taking this action will update the Marked Rescinded by Sponsor/CRO field to Yes on the corresponding document in SiteVault.
Site Connect: Additional Vault Clinical Docs Support
This feature adds support to transfer the following new document artifacts via Veeva Site Connect.
- IP Storage Condition Excursion Documentation
- Additional Monitoring Activity
- Trial Initiation Monitoring Report
Restrict Document Study to Single Value
This feature introduces a Vault-wide setting that prevents non-system-managed users from populating more than one Study on a document. Documents cannot be limited to a single Study if you have configured Vault for streamlined document reuse.
Clinical Operations Data Model Changes
See 22R1 Data Model Changes: Clinical Operations.
Commercial
Medical Inquiry UI: Admin Page
The dedicated Medical Inquiry user interface was added to MedComms in 21R3. This feature updates the user interface configuration screen to provide a better user experience for Admins. The screen is used for both the initial setup of the user interface and ongoing maintenance. Admins can define the objects, fields, and user interface layout that medical inquiry users see.
Medical Inquiry UI: Case Contact Section
This feature adds a section to the Medical Inquiry user interface containing information on the Case Contact. If the contact already exists, Medical Inquiry users can modify the Case Contact record during the interaction. If the contact is new, users can capture and save their details as a new Case Contact record. This streamlines the process of capturing Case Contact details and collects all Case and Case Contact information in one place, rather than across multiple screens.
External Viewer: Number Of Views
This feature allows users to report on the number of views through an external viewer per document. Users can also report on which external viewer was used, such as which CRM Org or which Public Content Distribution channel, including SharePoint or Website. This assists marketing managers in understanding the effectiveness of content viewed through the external viewer. Learn more about the external viewer.
Allow Case Response Emails to be Deleted
Medical Inquiry allows users to respond to requests for medical information via email directly from MedComms. Vault stores this email content in the system as a referenceable, auditable record of what was sent to the contact. This feature allows users with the required permissions to permanently delete these records from the system.
Customisable “From” Email Address for Case Response Emails
This feature allows customers to configure the “From” email address on Medical Inquiry case response emails. When the contact receives the email, they see the sender as the address configured in the customer Vault rather than a generic Vault email address.
Document Notifications: Support for “Based On” Relationship
This feature provides the ability to notify the Document Owner of a document that is related to a document via the “Based On” Relationship. Admins can configure the “Send Notification to Based On Content” action on a state entry action or user action. The action sends a notification to the owners of documents created from a source document via the Make A Copy action. Learn more about parent document notifications.
CRM Data Sharing: Support Merged CRM Accounts
Today, duplicate accounts in Veeva CRM can be merged together. This feature adds support to the standard integration between MedComms and Veeva CRM for sharing Medical Inquiry data to reflect this in MedComms, maintaining consistency across the two products.
Modular Content Approval Document Additional Lifecycle Support
With this release, Admins can associate the Module Content Approval Document with any lifecycle while still successfully generating the Approval Document. If the Approval Document subtype is associated with multiple lifecycles, Vault associates the first lifecycle listed alphabetically as that document’s lifecycle when performing the Generate Approval Document action.
Modular Content Approval Document Enhancements
This feature expands the functionality and generation of the Content Module Approval Documents to support CJK languages, increase rich text formatting options, and support text background colors.
Automated Image Rendition: Include Source Document Name
For Automated Image Rendition types, Admins now have the option to concatenate the document name with the rendition type. Vault applies the prepended name when creating the rendition, and the name is visible on downloads.
Transparency Support in Automated Image Renditions
With this release, Admins configuring Automated Image Renditions can select the option to Preserve Transparency for .PNG, .GIF, .BMP, and .TIFF file formats on each Rendition Type where transparency is required.
Medical Inquiry: CRM Inquiry Pull Job Timing Enhancement
This enhancement adjusts the date & time range used to retrieve medical inquiries from CRM and now uses the date & time of the last successful Account pull to ensure that the Accounts referenced are present in Vault.
eCTD Binder Generation Failure Notifications
With this release, Vault provides more detailed notifications when eCTD binder generation failures occur due to insufficient permissions or other configuration related causes.
CRM Document Field Label Updates
This feature updates the labels for some CRM related document fields to align with Veeva’s best practices and documentation. The updated labels provide clarity on the intended use of the fields and their impact on the CRM integration.
Commercial Data Model Changes
See 22R1 Data Model Changes: Commercial.
Vault Mobile
Vault Mobile Share to Vault
With this release, Veeva Vault Mobile app users can ingest content into Vault by “sharing” files from their mobile device into the app using the standard sharing mechanism supported by the mobile OS. The app ships with one standard Sharing Action which allows users to create unclassified documents. Your developers can also build custom Sharing Actions to perform other actions on the ingested files based on your specific business needs.
eSignature Fallback for SSO Users
With this release, Veeva Vault Mobile app users who use single sign-on credentials to log into the app can use their SSO credentials while completing eSignatures whenever the biometric controls are not available on their devices.
Station Manager
Background Sync on iOS
With this release, iOS Station Manager will now attempt to conduct its sync while the mobile app is in the background. Previously, the iOS app could only sync while the user was actively using the application.
QR Code Reader
With this release, Station Manager will now provide a QR code reader within the app. A new icon will be made available on the document list page that will take a user to a QR code reader, which they can use to scan valid Station Manager QR codes.
This feature is available on both platforms:
QR Code Reader for Android Station Manager App
QR Code Reader for iOS Station Manager App
Quality
Send Email to Non-Vault Users
Organizations often need to send emails to internal and external recipients for various processes in QMS. With this release, customers can define distribution groups, notification templates, send emails and share documents for the following processes: Audits, Complaints, and Issue Escalation. This new functionality addresses several scenarios that organizations may encounter:
- When an Audit report is completed, that report often needs to be sent to an external recipient, such as the supplier contact, as well as persons associated with the Audit or supplier.
- In Complaint processes, customers often need to send correspondence letters and forms to the complainant or the reporter associated with the Complaint at various times during the Complaint lifecycle.
- If a serious issue is encountered, such as a critical deviation, upper management external to Vault may need to be notified of the serious event.
In addition, when using this feature with Complaint records, the sender of the email will be notified if the email was not successfully delivered to the recipient’s email address. Learn more about configuring external notifications.
APQR/QMR
With this release, Vault QMS now supports management of APQR and QMR processes.
The APQR process is an annual evaluation of the quality standard of a drug product to determine the need for adjustments in drug product specifications, manufacturing, and control procedures. It is a collaborative effort to generate the final APQR report, which is a compilation of data from multiple data sources, the summarized results, and the recommendations from distinct SMEs.
The QMR process is very similar to the APQR process but does not require the generation of a final summary report, but does require the capturing of meeting notes, meeting attendees, and post-meeting action items.
Both of these processes share common challenges which include but are not limited to:
- Data extraction and identification
- Scheduling, reminder, and progress updates
- Data and records which come from disparate systems
- Involvement of many different inputs from various stakeholders
With the APQR/QMR feature, customers will now be able to:
- Manage both the APQR and QMR process within QMS
- Create APQR and QMR templates for known documents that need to be collected
- Once those templates have been defined, APQR or QMR records can be created from the templates and provide a consistent starting point based on various scopes, such as Country, Product, or Facility
- This feature is designed to work in conjunction with the Generate Document from Report feature, which allows reports to be defined to extract key data that already resides in QMS
- Once all documents have been collected, an APQR Binder can be created from the APQR record and the Generate Merged PDF document action can then be leveraged to generate the final APQR report.
Generate Merged PDF Document Action
As part of the APQR process, customers are often required to produce a final report which combines summary, recommendation, and detailed report documents into a single document. In previous releases, the creation of this single document was often done manually, outside of Vault.
With this release, users can upload the individual documents into an APQR Binder and, using the Generate Merged PDF Document action, all of the documents in the APQR Binder are merged together into a single document in their binder order. The resulting document is attached to the APQR Binder through the APQR Reports document relationship and can be reviewed and approved like any other Vault document.
If any document in the APQR Binder is revised, the action can be run again. The existing Final Report document and the relationship linking it to the binder will be up-versioned and the report will include the latest content.
Generate Document for Standard QMS Processes
This feature enhances the Generate Document actions to allow execution on additional processes within QMS, and can now be configured to allow a user to generate a document from a list of available document templates, formatted outputs, or reports. This is useful when a default document is typically utilized but a different template may need to be generated based on the Country or Product of the record. Learn more about using and configuring Quality Document Generation.
QRM: Template Based Risk Evaluation
With this release, users can create new Risks for an assessment from existing Template Risks, saving time and effort spent creating assessments. The system will automatically copy all the available information from the Template Risk to Assessment Risk by matching the name and the type of the fields. To take full advantage of this feature, Assessment Risk should not have Risk Matrix or Process Step as a mandatory field.
QRM: Promote the Assessment Risk Event to Risk Event in the Register
With this feature, users can promote one or more Assessment Risk records to one or more (up to 5) Risk Registers. The system automatically copies all the available information from the Assessment Risk to Risk (Event) by matching the field name and type. This feature is intended to consolidate all Risks identified as part of multiple assessments, conducted via different methodologies, for a particular context (such as Product, Supplier, Process, Site, or other). The newly created Risk records in the Register are linked to the originating Assessment Risks.
QRM Data Model Updates
This release brings several data model updates to support the Quality Risk Management suite of features:
Click here to view the data model changes.
Recurrence Check: UI Enhancements
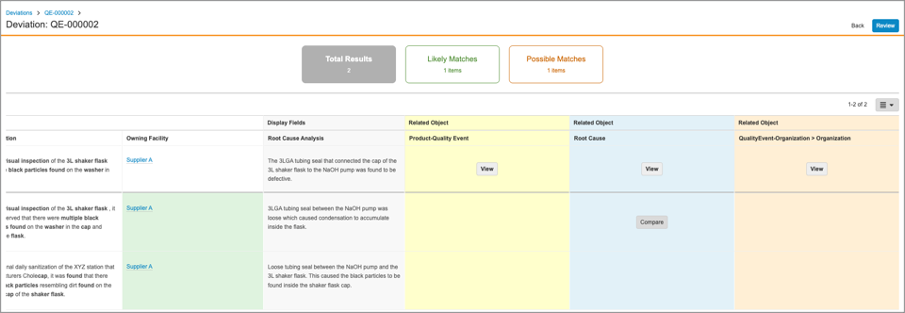
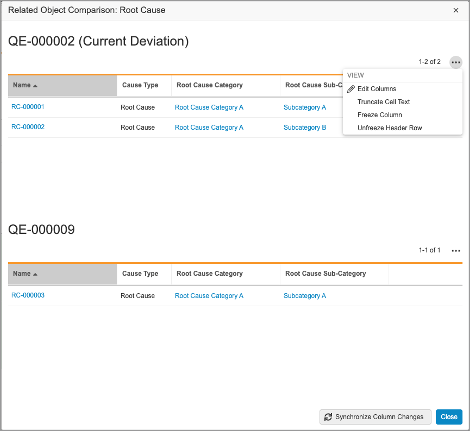
With this release, the Recurrence Check interface has been improved. Once potential recurrences are returned, the Comparison and Grid views have been enhanced to allow users to view related object data referenced by the record check for both the current record and the potential duplicate. Users can click on the Compare button to compare related object data in an easily-accessible user interface.
Recurrence Check: Display Match Criteria on User Dialog
The QMS Record Check user action has been enhanced to easily view the match criteria parameters for a selected record check. Users can view the exact match fields, lifecycle states, and time thresholds that will be evaluated when executing a recurrence check.
Recurrence Check: Support for MedTech Complaints
With this release, Vault QMS expands its Recurrence Check capabilities to Medtech Complaints and introduces an intelligent and streamlined process for end-users to check whether a Medtech Complaint is a recurrence of another. The previously complex manual process is replaced with a simple user action that quickly finds potential recurrences and displays them in an easy to scan list view. Once the recurrence check is complete, Vault QMS will automatically store the results which can then later be utilized for reporting and trending.
Standard Cycle Time Metrics: Audit Object & Quality Event Custom Object Type Support
With this release, Vault QMS extends the capability of Standard Cycle Time Metrics to include support for all custom Quality Event object types and to automatically capture the following cycle time metrics for all standard and custom Audit object types:
- Cycle Time: Duration in number of days from initiation to completion of the Audit
- Audit Preparation Cycle Time: Numbers of days an Audit record is in preparation prior to the Actual Start Date.
- Audit Reporting/Response Cycle Time: Number of days from the Actual End Date until completion of all post-audit or inspection activities, such as reporting, finding issuance, response, or others
- Planned vs. Actual Audit Start Delta: Number of days the Actual Start Date for the Audit is before (early) or after (late) the Scheduled Start Date
- Planned vs. Actual Audit Duration Delta: Actual duration of the Audit (Actual Start Date to Actual End Date) compared to planned duration (Scheduled Start Date to Scheduled End Date)
Learn more about standard cycle time metrics.
eMDR: Usability Enhancements
This Vault Product Surveillance feature allows Admins to add custom sections to the Adverse Event Report object page layout for the eMDR object type. It allows the users to view the information in the exact same format as it would be submitted to FDA by aggregating information from various objects and transforming it into the required format.
Learner Homepage: Curriculum View
Training Assignments on the Learner Homepage can now be grouped by Curriculum allowing Learners to prioritize training by Curriculum. When grouped, each Curriculum is represented as a card. The Curriculum card shows the number of Training Assignments assigned, Curriculum description, and other helpful information for Learners. From a Curriculum card, a user can navigate to a curriculum details page, which shows all Training Assignments assigned in the Curriculum, allowing the Learner to focus on a targeted list of Training Assignments. Learn more about the Learner Homepage.
Admin Alerts: Training Materials, Prerequisite Rules, & Substitute Rules
Admin Alerts inform the Training Administrator when they are about to make a change to a Training Requirement that could impact Learners’ Training Assignments. This feature explains what the impact of a change will be and how many users could be impacted. This set of auto-on alerts appear when changing Training Materials or modifying Prerequisite or Substitute rule configurations. For Training Materials, the feature also allows the Admin to adjust the settings that control impact directly from the warning message.
Admin Alerts: Quiz & Recurrence
Admin Alerts inform the Training Administrator when they are about to make a change to a Training Requirement that could impact Learners’ Training Assignments. This feature explains what the impact of a change will be and how many users could be impacted. This set of configuration-enabled alerts can appear for Quiz and Recurrence configuration changes.
Training Jobs: New Jobs to Process Training Assignments
This feature optimizes the performance of the Update Training Assignment job by introducing child jobs that perform the following functions alongside the parent Vault Training job:
- Transition the state of Training Assignments to the target state
- Cancel all Training Assignments for a Person in the Ineligible state
The child jobs allow Vault Training to assign Training Assignment records quickly and efficiently. Status and logs for the child jobs are available in a new Training Job Status page available at Admin > Configuration > Training Job Status. In addition, Training Assignments created or updated after this release have the originating Vault Training job ID written to a new field for quick troubleshooting. Learn more about Vault Training automation.
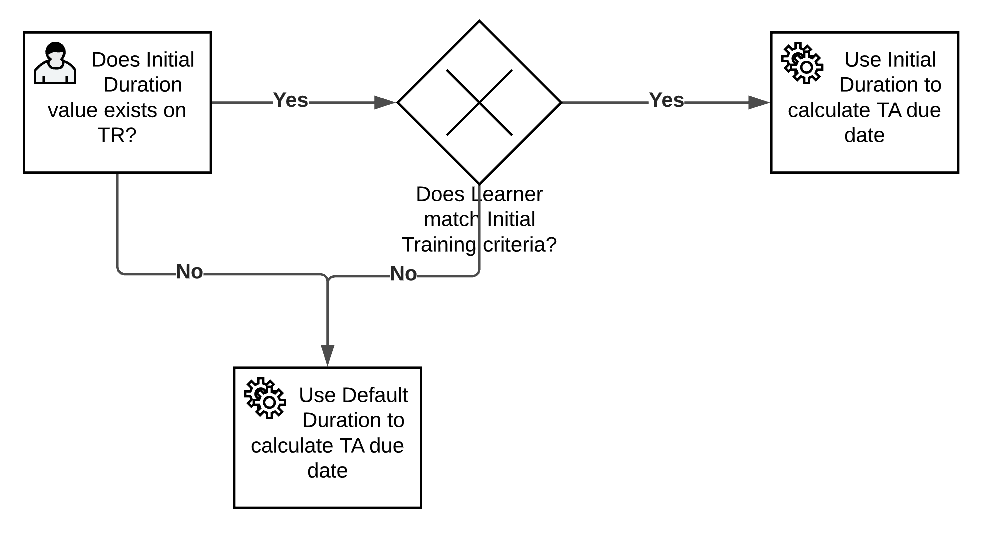
Initial Training Due Date
This feature allows an Admin to set a duration for initial training that is different from the general duration. This means that new hires or new transfers receiving this training for the first time can have due dates that are different from a Learner who has received this training before. Learners who have received this training before (for example, those receiving the assignment via recurrence) will be given assignments with the default due date. Learn more about configuring initial training due dates.
Quizzes: Question & Answer Randomization
This feature allows Training Admins to randomize quiz questions within a Quiz Section Design and randomize quiz answers within a Quiz Question Design. Every time a Learner attempts the resulting Vault Training Quiz, Vault randomizes the display order of questions, answers, or both.
Training Requirement Impact Assessment (TRIA) for All Training Requirements
The Training Requirement Impact Assessment (TRIA) process notifies Training Admins when assets in a Training Requirement are updated so the Admin can decide what should happen with the Training Requirement. In previous releases, TRIA was only supported for multi-document, classroom, or other types of Training Requirements. With this release, a new setting in Admin > Settings > Application Settings will allow TRIA to be used for all Training Requirements, including single-document Training Requirements.
China Link E-Learning Support
Vaults on China Link can now use E-Learning courses: from uploading e-learning courses to completing e-learning courses as a Learner.
Direct Assignment: Hide Inactive or Ineligible Persons in the Learners Field
Persons with Training Eligibility equal to Ineligible or Status equal to Inactive will no longer appear in the Learners field during a Direct Assignment action.
Training Requirement Impact Assessment: Action to Re-Evaluate Record
This feature provides the ability for a user to re-evaluate a Training Requirement Impact Assessment record in the event that the record cannot enter the Completed state. This allows a quick way to update the record, without requiring a configuration change.
Email Participant Action Available for Training Assignment Workflows
From the workflow timeline for a Training Assignment, an authorized user can email task participants, such as the Learner, by selecting the Email Participants action. This allows Training Admins or Managers to remind the Learner to complete a training task.
Issue Training Assignment Entry Action Update: Update Training Materials If TRIA Not Found
With this update, if the Issue Training Assignment entry action cannot find an open Training Requirement Impact Assessment (TRIA), the action will now update the document version on the Training Requirement. Previously, an update did not occur.
Assignment Details Creation Allowed for Imported Records
Assignment Details, a child record of Training Assignment, can now be created for imported Training Assignments. This allows historical Training Assignments to have Assignment Details, enabling reporting on Curriculum and Learner Role for historical Training Assignments.
Learner Application Role Updates in Sharing Settings
This feature allows updates to be made to the Learner Application Role in Sharing Settings for Training Assignment, Assignment Details, and other Training object records. Learners can then be added to the Learner Application Role in Sharing Settings using the Vault API.
Training Materials: Prevent Duplicate Documents
With this release, Training Admins are prevented from adding the same document multiple times to the Training Materials for a Training Requirement, regardless of whether they are attempting to add different versions of the document.
Prevent Duplicate Assignment Detail Records
This update prevents a user or Vault from creating duplicate Assignment Details records for a given Training Assignment. A duplicate Assignment Details record is considered the same Curriculum and Learner Role for a given Training Assignment.
Training Requirement Impact Assessment: Optional Task Assignee
The Assign Task To input for the Create Training Requirement Impact Assessment and Retire or Assess Impact on Training Requirements document entry actions is now optional. This allows configuration of workflow task participants at the Training Requirement Impact Assessment object level.
Imported Training Assignments: Sharing Settings Not Updated
The Update Training Assignments job will no longer add the Learner and Direct Manager, if none exist, to Sharing Settings for the most recent imported Training Assignment for a Learner or Training Requirement. This change improves the overall Update Training Assignment job performance.
Updates to Cancel Training Assignments Job
The Cancel Training Assignments job, which is executed from a document lifecycle’s Cancel Training Assignments entry action, now logs the following information:
- Whether Training Assignments were cancelled
- Training Assignments that were cancelled
- Error information, if there was an error during the job execution
HVO Migration Preparation: Field Configuration Limitations
In an upcoming release, Vault Training will migrate some objects’ Data Store setting to High Volume. To prepare for this, the following fields can no longer be created on the Training Assignment or Assignment Details objects:
- Field types that support field-level encryption
- More than two (2) multi-value picklist fields
Quality Data Model Changes
See 22R1 Data Model Changes: Quality.
Regulatory
Submission Wizard
A new wizard is available to simplify creation of Submissions, Regulatory Objectives, and their relationships as well as improve data consistency and integrity.
Users can select from a controlled list of relationships within the wizard, which are defined using new Application relationship objects to manage metadata at the Application level. New options are available in the RIM Maintenance tab to extract and load a zip of loader sheets for suggested Application relationships based on the current set of Submission relationships in the Vault.
Global Content Plan
With this release, users can create and update a Content Plan directly from an Event record, where Vault generates a Global Submission and builds the Global Content Plan structure based on the Event’s Activities and other relationships. Users can also view the Global Content Plan directly from the Event or Activity, where viewing from the Activity filters the Global Content Plan based on the content relevant to the Activity. Once finalized, the Global Content Plan can be copied in bulk to multiple submissions using a new dispatch action.
IDMP Viewer
This feature provides a data visualization UI that consolidates IDMP submission data across-medicinal products for a unified view. The viewer also highlights differences among like records within the submission set to facilitate data review.
Manage & Edit Table of Contents (TOC)
With this release, Submissions Publishing and Report Level Content Plan users can edit a generated Table of Contents (TOC) from within Vault RIM.
Pull Objective Data Enhancements
With this release, the Pull Objective Data action can create Submission details with a mix of object types when the internal names of the object types on the source and target objects match. Pull Objective Data also adds the following details to Submissions: Authorizations such as certificates, Regulatory Text such as device trade names and descriptions, and Product Organizations such as authorised representatives. Support is also added for copying additional standard fields that were added to the data model in previous releases.
Create Related Records Enhancements
With this release, Create Related Records can create Regulatory Objective and Submission details with a mix of object types when the internal names of the object types on the source and target objects match. Create Related Records also adds the following details to Regulatory Objectives and Submissions: Authorizations such as certificates, Regulatory Text such as device trade names and descriptions, and Product Organizations such as authorised representatives. Support is also added for copying additional standard fields that were added to the data model in previous releases. Finally, Create Related Records will now always create relationships between the source Event and any resulting Submissions and Regulatory Objectives.
Matched Document Ordering
This feature allows users to set a specific order for matched documents in the Content Plan hierarchy viewer when multiple documents are matched to a single Content Plan Item. By default, matched documents are sorted in ascending alphanumeric name order on a Content Plan Item when no specific order is set.
Users can also drag and drop documents to match to a specific matched document position under the Content Plan Item. Existing content plan actions including splitting, binder creation, copy content plan, and starting workflow from hierarchy viewer are also updated to utilize the matched document order.
Labeling Process Improvements
This feature extends the existing Vault RIM data model to better support tracking of labeling concepts and local label deviations by adding the ability to support multi-document changes, bolstering the relationship between deviations and health authority communications, and supporting the deviation approval process.
China XSD 1.0 eCTD Publishing & Validation
With this release, RIM Submissions Publishing supports the CN NMPA v1.0 (XSD 1.0) specification. Users can now create Content Plans and generate submissions that are compliant with CN NMPA v1.0 specification. Vault validates these submissions based on the corresponding CN NMPA v1.0 Validation Criteria Version.
Asynchronous FDA Gateway Support
With this release, Admins can configure a Gateway profile for US FDA submissions to send the Mail Delivery Notification (MDN) asynchronously.
Extend Matched Document Tokens for Content Plans
This feature enables generic support for tokenizing object and text fields on matched documents in the Content Plan. For example, the Clinical Site document field can be used within the Published Output Location to organize Case Report Forms into site folders in the published output.
Show Only Pending Toggle in Active Dossier Editor
With this release, a new toggle between ‘Show Only Pending’ and ‘Show All’ in the Active Dossier Editor allows users to quickly switch between viewing all Active Dossier Item Detail records and those with a Pending type status, for example Pending Current.
Content Plan Enablement Update
With this release, the ‘Enable Submission Content Planning’ and ‘Enable tree grid’ settings will be enabled for all RIM Vaults where they are currently disabled. This change aligns such Vaults with the standard RIM data model and allows for deprecation of the legacy Content Plan viewer. This will not impact Vaults where these settings are already enabled.
Content Plan Creation & Copy Notification Token
A new token, ${templateConstraints}, is now supported for the Content Plan Creation and Copy notifications, providing end-users visibility into whether records were created as Inactive or Excluded due to template constraints.
Content Plan Creation: Novel Excipient Update
When creating content plans with novel excipients, Vault creates corresponding repeating content plan sections (for example, 3.2.P.4.6) within repeating excipient sections (for example, 3.2.P.4) only when the novel excipient’s Submission Inactive Ingredient record is a Novel Inactive Ingredient.
Extend Content Plan Token Support for Submission Pharmaceutical Form
With this release, content plans now support tokens for text and object reference fields within the Submission Pharmaceutical Form object.
Capture Active Dossier Submitted Status
With this release, Submitted  is available as a new country status in the Active Dossier, providing visibility into upcoming changes that have been submitted but are not yet approved or made current. When generating Active Dossier records from a submission, Vault automatically sets the Submitted status on newly-created Active Dossier Item and Active Dossier Item Detail records, and updates such records where the status is blank.
is available as a new country status in the Active Dossier, providing visibility into upcoming changes that have been submitted but are not yet approved or made current. When generating Active Dossier records from a submission, Vault automatically sets the Submitted status on newly-created Active Dossier Item and Active Dossier Item Detail records, and updates such records where the status is blank.
Select Section in Add to Active Dossier Dialog
When adding a single document to Active Dossier in the Active Dossier Editor, users are now able to select and change the section in which to add the document via the Add to Active Dossier dialog.
Translation Document Relationship Flag
With this release, the status of the standard Translation document relationship introduced in 21R2 is controllable via two-way Vault Admin flag. Customers experiencing conflicts with existing custom Translation document relationships can inactivate the standard relationship, which hides the relationship from all document pages.
Display Inactive Applications & Submissions in Viewer
When enabled by an Admin, users are able to select and view the content of inactive Applications and Submissions within the Submissions Archive Viewer.
Restrict Submissions Archive Access to Full User Licensed Vault Users
With this release, Vault will enforce Submissions Archive application licensing. In Vaults created in 21R3 and earlier, users without a Full User application license are warned about unlicensed usage of the application. In Vaults created in 22R1 and later, users without a Full User application license are blocked from accessing any Submissions Archive functionality.
Gateway Enhancements
With this release, Vault now includes the extensions on all acknowledgements received through Vault, allowing for the acknowledgements to be viewed directly from within Vault. Additionally, the user listed in the Published Content Owner field will receive notifications when an acknowledgement is received for that submission.
Resubmission of Previously Transmitted Submissions
With this release, users can resubmit submissions that were already sent to the Health Authority and a technical rejection was received.
Providing Additional Data in the Publishing Progress Indicator
With this release, additional information is added to the Publishing Progress Indictor’s download CSV to enable better understanding and troubleshooting of publishing-related issues.
22R1 RIM Data Model Changes
See 22R1 Data Model Changes: Regulatory.
Safety
Safety features are targeted for tentative availability on April 14, 2022 & April 29, 2022.
Case UID Manual Intake on Inbox Item Auto-on
Users can now manually enter the Worldwide UID and an External System UID on the Inbox Item page to facilitate case intake and duplicate search.
Duplicate Detection by Case Identifiers Auto-on
Vault Safety will now search for duplicates using Case Identifiers. Inbox Item UIDs and Case Identifiers will be cross compared with Case UIDs and Identifiers during duplicate detection.
Learn More
Identifiable Reporter and Patient Per ICH E2D on Inbox Item Auto-on
Vault Safety will now evaluate the Identifiable Patient and Identifiable Reporter fields on the Inbox Item based on the ICH Guideline Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D, which looks at certain fields from the Patient and Case Contacts section. In addition, if you mark one or more of these fields as Masked, the patient or reporter will qualify as identifiable.
Learn More
Automated Case Promotion to Initial and Follow-Up Case Configuration
Vault Safety now supports automated Inbox Item promotion to an Initial or Follow-Up Case for external systems (E2B/JSON using Intake API or AS2 Gateway) that have their own case processing workflows. The system verifies whether Case promotion is valid, leverages Case Identifier matching, and has selectable merging methods based on seriousness.
Learn More
- Enablement: Enable Automated Case Promotion
- User Help: Automated Case Promotion
Merge Inbox Item to In-Flight Case Admin Checkbox
Vault Safety now supports merging information received in Inbox Item into the latest Case version. When merged, source documents will also be added to the latest Case version. To facilitate parallel processing, Intake users can now alert the case processor that there is new case information by marking an Inbox Item as a follow-up to the latest case. Inbox Items marked as follow-up can either be merged into the latest Case version or can be used to create a Follow-Up Case.
Learn More
- Enablement: Enable Merge to In-Flight Case
- User Help: Merge to In-Flight Case
Promote to Multiple Cases Configuration
Vault Safety can now promote a single Inbox Item to up to 100 cases. The cases will be related and contain all the information from the Inbox Item, including source documents. This functionality is primarily intended for the intake of literature and legal cases. This feature is not supported for E2B imported Inbox Items.
Learn More
- Enablement: Promote an Inbox Item to Multiple Cases
- User Help: Promote to Case: Promote an Inbox Item to Multiple Cases
Follow-Up with Any Matching UID Configuration
Admins can now configure Vault Safety to allow Inbox Items to be promoted to a Follow-Up Case when the Worldwide UID does not match but matches another UID, such as an External System UID or a Case Identifier.
Learn More
Import Product Matching by Company Product Substance, Trade Name, and Alias Configuration
This release enhances product matching when importing Inbox Items or AERs from E2B and JSON files by supporting product generic names, trade names, product aliases, and substance aliases.
Learn More
- Admin Help: Manage Products: Create Product and Substance Aliases
- User Help: Inbox Item Study and Product Matching: E2B Import Study and Product Matching
Inbox Item Global Actions for Manual Intake Auto-on
Vault Safety now supports global actions to create, edit, and save all Inbox Item records in a single click during manual Inbox Item intake. The system warns users when navigating away from an Inbox Item with unsaved data.
Learn More
- User Help: Manual Inbox Item Intake
Deep Duplicate Search Support
Vault Safety will now search for potential matches using all Case Adverse Events and Company or Study Products, including non-primary records. Additionally, users will be able to confirm new case creation when no potential match is found.
Learn More
- User Help: Promote to Case: Deep Duplicate Detection
Attach Custom Child Objects to Case on Follow-Up From Inbox Item Auto-on
When promoting an Inbox Item to a Follow-Up Case, custom child object records will now be added to the new Case version.
Learn More
- User Help: Inbox Item Follow-Up: Merging Child Records
Rank Suggestion and Validation for E2B-Imported Blinded Products Auto-on
Vault Safety will now suggest Rank 1 for Investigational Blinded Products (G.k.2.5 = Yes) on E2B R3 import to Inbox Item. These Case Products won’t need a Study Product or Company Product to be a primary suspect (rank 1).
Field Limit Updates for E2B Support
Vault Safety will now support the maximum E2B length for Dose Text, Reason Text, Result Value and Age value. First, Dose Text will now accept up to 2,000 characters (E2B limit for G.K.4.r.8 / B.4.k.6) on Inbox Item, Case Product Dosage and Localized Case Product Dosage. Second, Reason Text will now accept up to 2,000 characters (E2B limit for C.1.11.2 / A.1.13.1) on Transmission. Third, Result Value on Case Test Result will now accept up to 50 digits (E2B limit for F.r.3.2 / B.3.1d). Lastly, Age Value will accept up to 5 digits (E2B limit for D.2.2a).
Learn More
- Enablement: Enable Field Limit Updates for E2B
Foreign Localized Case Synching Support
On a follow-up Localized case, Vault Safety now performs a one-time data sync from the global case to the localized case when the associated transmission record is created. The system also prevents user edits to the follow-up Localized Case until it is ready for transmission evaluation.
Learn More
Always Serious and Product or Study-Independent Watchlists Configuration
You can now create watchlists without specifying a Study or Product. Additionally, you can set Seriousness criteria for adverse events on a watchlist. If a Case contains a matching adverse event(s), the Seriousness will automatically be assigned to the Case Adverse Event, if Seriousness is not already specified.
Learn More
- Enablement: Enable Adverse Event Watchlists: Enable Always Serious and Product or Study-Independent Watchlists
- Admin Help: Configure Adverse Event Watchlists
- User Help: How Case Watchlist Tags and DMEs are Assigned: Default Case Seriousness
Auto-Listedness Using Core Datasheets Configuration
Vault Safety will now generate expectedness records for the product’s Core Datasheet (CCDS) to support listedness calculations during case processing. A new field will also be available for Core Datasheet roll up at the Assessment and Case level.
Learn More
- Enablement: Enable Listedness and Expectedness From Core Datasheets
- Admin Help: Manage Datasheets and Auto-Expectedness
Expectedness from Core Datasheets Configuration
When generating a submission, Vault Safety will evaluate expectedness for a Study Case using the Development Core Safety Information (DCSI) datasheet within the investigator brochure (IB).
Vault Safety can also calculate expectedness from a product’s Core Datasheet without additional configuration to Local Datasheets, if your product or organization does not require them.
Learn More
- Enablement: Enable Listedness and Expectedness From Core Datasheets
- Admin Help: Manage Datasheets and Auto-Expectedness
Conditional Expectedness Configuration
This feature brings multiple enhancements to the configuration and evaluation of Datasheets for auto-expectedness. When configuring Datasheets, administrators can now specify seriousness criteria conditions, which define when a listed term is unexpected. Seriousness criteria can be configured at both the Datasheet and MedDRA Criteria (listed term) level. Admins can also now configure precise expectedness on Datasheets, which prevents the system from marking terms as unexpected if they are not listed on the Datasheet. Additionally, a new Expectedness MedDRA Criteria setting allows you to specify unexpected terms on a Datasheet.
Learn More
- Enablement: Enable Conditional Expectedness
- Admin Help: Manage Datasheets and Auto-Expectedness
Case Nullification Configuration
Vault Safety now supports the ability to void a case, including the option to nullify previous Transmissions using a configurable user action at the Case-level. You must specify a reason for nullification when you run the action, which will auto-populate in new system-generated transmission records related to the case. Additionally, upon initiating the voiding process, the system automatically cancels any in-progress workflows.
Learn More
- Enablement: Enable Case Nullification
- User Help:
Case Validations: Control Workflow Transitions Configuration
Vault Safety can now calculate the worst validation result for a case and transmission to be used in entry criteria and prevent lifecycle state changes. The system will also prevent gateway submissions for any transmissions with Hard Failures.
Learn More
- Enablement: Enable Control Workflow Transitions for Case Validations
- User Help: Enter Case Data (Validation Status field)
Strict Case Locking Admin Checkbox
Administrators can now enable a system setting to enforce strict case locking, which prevents users from making changes to a Case unless that user had locked the Case themselves.
Learn More
- Enablement: Enable Strict Case Locking
- Admin Help: Lock and Unlock a Case: Strict Case Locking
Provision Standard Verbatim Fields for Case Drug History and Case Diagnosis Configuration
In this release, Vault will allow for standard fields to support migration of verbatim values for Case Drug History and Case Diagnosis records.
Learn More
- Enablement: (Optional) To expose the new verbatim fields to users, an administrator can add the new fields to the following page layouts, if required. Note: These fields are primarily added for data migration and are not currently supported for E2B import or export or any type of report generation. These fields do not need to be added to page layouts for migration:
- Case Drug History:
- “Indication (Reported)” (
indication_reported__v) - “Reaction (Reported)” (
reaction_reported__v)
- “Indication (Reported)” (
- Case Diagnosis:
- “Name (Reported)” (
name_reported__v)
- “Name (Reported)” (
- Case Drug History:
- User Help: Enter Case Data
MedDRA Hierarchy for CMQ and Vault Reporting Configuration
Admins can now download the full active MedDRA version from the central dictionary to your vault instead of uploading dictionary .zip files. Admins can also perform a deep copy of MedDRA queries and associated records, as well as update the MedDRA hierarchy with higher-level terms associated with all LLT terms in your vault.
Additionally, Vault Safety now supports creating Custom MedDRA Queries (CMQs) using any level of the MedDRA hierarchy (SOCs, HLGTs, HLTs, PTs, and LLTs). Previously, MedDRA Queries could only be constructed using LLTs. The MedDRA Queries with non-LLTs will be supported through standard Vault Reporting and Dashboarding.
Learn More
- Admin Help:
Blind Protection Relatedness Override - CIOMS I and E2B Support
Vault Safety now provides the ability to blind protect unblinded double-blinded study cases in generated CIOMS I and E2B documents. Users can select an override for Case Assessment Results, if different from the blinded Assessment, to be displayed in the unblinded CIOMS I and unmasked E2B documents.
Learn More:
- Admin Help: Configure Blind Protection Relatedness Override for CIOMS I and E2B Generation
- User Help: Enter Case Data: Case Assessment Result Fields
Precise Inclusion of Age-Related and Medically Confirmed E2B Elements Auto-on
This release includes enhancements to E2B generation for precise inclusion of age-related elements (B.1 / D.2) and medical confirmation by a health professional (A.1.14 / E.i.8). This change impacts all standard E2B(R2) and (R3) formats that Vault Safety generates. The following table describes the impacted data elements:
| Element Name | E2B(R2) | E2B(R3) | Changes |
|---|---|---|---|
| Medically Confirmed by Healthcare Professional | A.1.14 | E.i.8 | Now only transmitted when the primary Reporter is not a health professional. |
| Patient Date of Birth | B.1.2.1 | D.2.1 | Now only transmitted when a full date is entered in the corresponding Vault Safety field (CCYYMMDD), except for PMDA where the Date of Birth is always masked if provided. |
| Date of Birth of Parent | B.1.10.2.1 | D.10.2.1 | Now only transmitted when a full date is entered in the corresponding Vault Safety field (CCYYMMDD). |
| Age at Time of Onset | B.1.2.2 | D.2.2 | Now only transmitted if the Date of Birth (D.2.1 / B.1.2.1) is not transmitted. For example, Date of Birth is partial or Masked. |
| Age Group | B.1.2.3 | D.2.3 | Now only transmitted when both the Patient Date of Birth (B.1.2.1 / D.2.1) and Age at Onset (B.1.2.2 / D.2.2) are not transmitted. For example, Date of Birth is partial or Masked. |
Learn More
- User Help: E2B Generation Data Mapping
E2B(R2) Enhancements Auto-on
This release includes the following E2B(R2) enhancements for all E2B(R2) report formats (ICH, FDA, HC):
- When “Drug Not Administered” is selected for a Product Drug Role, include drug characterization with “Suspect” (B.4.k.1)
- Importing and exporting the
< safetyreportversion >tag - Collating seriousness from all Case Adverse Events when transmitting Case Seriousness criteria (A.1.5.2)
- Masking E2B(R2) elements with “PRIVACY” for masked distributions
Learn More
- Enablement: Enable Safety Report Version Customization
- User Help:
E2B(R2) Partial Dates for Combination Product Expiration Date Support
Vault Safety now accepts partial dates in the Case Product “Expiration Date” field. For FDA E2B(R2) combination product reports, partial dates are exported to Expiration Date elements B.4.k.2.4.FDA.1a-b using the appropriate date format code.
Learn More
- Enablement: Contact Veeva Support to enable this feature. Once enabled, an admin must replace the existing “Expiration Date” field with the new Expiration Date control on the Product and Case Product page layouts. Note: If you enable this feature, you must manually migrate any existing Expiration Date data after adding the new control.
- User Help:
Reporter Masking for Post-Market Non-Literature Cases Admin Checkbox
Vault Safety provides a system-level setting to support masking reporter information in all outbound Submissions and Distribution for post-market non-literature Cases (E2B(R2), E2B(R3), CIOMS I, MedWatch3500A). If an admin enables this setting, reporter masking applies to all post-market non-literature Cases.
Learn More
- Enablement: Enable Reporter Masking System Setting
- User Help: Reporter Masking for Postmarket Non-Literature Cases
Generate Assessment Results With Clinical and Post Market Source Defaults Auto-on
This release enhances EMA E2B(R3) file generation by ensuring that the Source Type on system-generated Case Assessment Results is accurately set for postmarket submissions to the EMA EVHUMAN module. Table constraints for the DSUR Interval Line Listings of Serious Adverse Reactions have also been updated with further protections to ensure only related adverse events are listed. Additionally, administrators can now edit the name of system-provided Controlled Vocabulary records, if required.
Learn More
- Admin Help: Configure Controlled Vocabularies
- User Help:
Transmission Product Types Configuration
This feature enables products to be registered as different product types in different markets. For example, a product may be registered as a combination product with the FDA, and a drug with the EMA. This feature is controlled by a new Transmission Product Type setting, which can be set on a Transmission or at the Product or Study Registration level by an admin.
One scenario this feature supports is omitting device constituents from combination product E2B Reports. Because certain jurisdictions do not accept combination product submissions, you can exclude Device-type product constituents from E2B files generated for Combination Product reports. Unless this feature is enabled, E2B files generated for a Combination Product Case continue to include both Device-type and Drug-type Product Constituents.
Learn More
- Enablement: Exclude Device Constituents from E2B Exports: Prerequisites
- Admin Help: Manage Combination Products: Exclude Device Constituents from E2B Exports
- User Help: Create a Submission
Health Canada Transmission Profiles and Message Types Auto-on
Vault Safety now supports the Health Canada gateway for electronic submissions of clinical and post-market cases. A valid Health Canada E2B(R2) file with correct header information is also generated.
Learn More
- Enablement: Configure Health Canada Gateway: Prerequisites
- Admin Help: Configure Health Canada Gateway
- User Help:
PHI Masking on Foreign Submissions Configuration
Vault Safety can now generate safety reports with PHI masking for Submissions. Masking can be configured at the reporting rule level to apply to all cases or only foreign cases.
Learn More
- Enablement:
- To allow users to manually select masking for a Submission, an admin can add the “Patient Content Protection” and “Exceptions to Patient Content Protection” fields to the Submission Page Layout.
- To automatically apply masking with reporting rules, an admin can configure the Mask PII rule parameter.
- Admin Help: Reporting Rule Parameter Reference
- User Help:
Configurable Validation Criteria Configuration
Vault Safety now supports additional custom validation criteria using the standard validation criteria syntax, which will run as part of the Case validations. In addition, the Vault Safety validation engine now supports E2B(R2) formats.
Learn More
For assistance in configuring custom validation criteria, contact Veeva Services.
Ignore Validation Rule Auto-on
Validation Results on a Case or Transmission can now be changed to the Ignored state. This allows users to proceed with case processing and submissions while ignoring certain validation failures. Subsequent validation evaluations will not execute on ignored results.
Learn More
- Enablement: Enable Ignore Validation Rule
- User Help: Case and Submission Validation: Ignore Validation Results
FDA Submission Automation: Relatedness Assessment Source Parameter Auto-on
The FDA does not require a study submission if a sponsor assesses a case as Not Related while the investigator assesses the case as Related for a SUSAR in a clinical trial. With this release, users will no longer have to inactivate the submission to the FDA, as it will not be generated due to now evaluating the relatedness for FDA SUSAR cases based on the Sponsor’s Assessment. Additionally, the Assessment Source parameter will be introduced for use in Custom Rulesets.
Learn More
- Admin Help: Reporting Rule Parameter Reference
Submission Automation: Upgrade and Downgrade Parameters Auto-on
Vault Safety will introduce new parameters to calculate whether a Case is an upgrade or a downgrade in order to support additional submissions scenarios for the FDA, EMA, ROW, and custom rule sets. This feature will also support the one-last-time and one-more-time reporting rules, while considering the appropriate due date and local expedited criteria.
Learn More
- Admin Help: Reporting Rule Parameter Reference
Submission Rules: Support for MedDRA Queries (SMQs and CMQs) Configuration
With this release, Vault Safety will extend the Safety Rule Engine to allow greater configurability for Custom Rule Sets. New rule parameters will be introduced to support the selection of specific Products and/or Studies when deciding if a rule should be executed. Additionally, MedDRA Queries (SMQs and CMQs) can be used to determine if a case should be reportable in order to support situations such as “Lack of Efficacy” or customer-managed lists of non-reportable terms.
Learn More
- Enablement: Enable Support for MedDRA Queries in Submission Rules
- Admin Help: Use MedDRA Queries
- User Help: Reporting Rule Parameter Reference
Safety Rule Version Management Admin Checkbox
Admins can now configure an Active Version of the system-provided standard rulesets. When new rules are introduced into a standard ruleset (for example, FDA, EMA, and PMDA) as part of a release, admins can set the rule version to allow for adoption of the most recent version of a standard ruleset on a configurable basis.
Learn More
- Enablement: Manage Reporting Rule Versions: Prerequisites
- Admin Help: Manage Reporting Rule Versions
Automated Cross Reporting Configuration
In this release, Vault Safety extends the evaluation of reporting obligations to include cross-reporting (investigational to marketing registration) scenarios. Vault Safety evaluates the investigational and marketing registrations of study products, then identifies any additional reporting destinations for products and studies. Administrators can also specify destination overrides on product licenses.
Learn More
- Enablement: Enable Automated Cross Reporting
- Admin Help:
Identifiable Patient Definition Ruleset Parameter Configuration
In this release, Vault Safety allows submission rules to evaluate the Identifiable Patient Definition ruleset parameter to determine whether or not a case contains an Identifiable Patient as defined by E2D (ICH standard), or less strict “Patient Known to Exists” - a newly introduced case field. This allows greater specificity in submission to health authority and partners with less strict definitions of patient (i.e. FDA) while minimizing the potential for over reporting to destinations that have more strict definitions of patient (i.e. EMA).
Learn More
- Enablement: To leverage the “Patient Known to Exist” field, an admin can add the field to the Case and Imported Case page layouts.
- Admin Help: Reporting Rule Parameter Reference
- User Help: Enter Case Data (Patient Known to Exist field)
Bulk Unblind Configuration
Vault Safety can now bulk unblind multiple cases in a given study. This includes both the removal of blind protection for previously unblinded cases and snapshotting study arm product information for blinded cases. Cases currently in workflow will not be modified by this action.
Learn More
- Enablement: Enable Bulk Unblind
- Admin Help: Bulk Unblind a Study
Organized Data Collection of Reports from Patient Support Programs and Market Research Programs Configuration
Vault Safety now supports organized data collection of reports from PSPs (Patient Support Programs) and MRPs (Market Research Programs). Admins can create Study placeholders for Studies with unspecified products and intake users can select Company Products as Suspect Products. Upon case promotion, reporting obligations are evaluated using Product Registrations for the suspect Company Products.
Learn More
- Enablement: Enable Organized Data Collection
- Admin Help: Create a Study with Unspecified Products
Bulk Narrative Import and Status Check API Endpoints
Two new Vault Safety API endpoints are available to import multiple case narrative documents and translations in one operation. This is to improve case migration performance in Vault Safety. The Vault Developer Release Notes provide more information.
Learn More
Custom Safety Rules SDK
Vault Safety now has an SDK entry point to allow customized logic for submission rules.
Learn More
For assistance with the custom safety rules SDK, contact Veeva Services.
Custom Safety Validations SDK
Vault Safety now has an SDK entry point to extend Case validation with custom criteria and evaluation logic.
Learn More
For assistance with the custom validations SDK, contact Veeva Services.
SiteVault
Study Export
This feature allows users to export all major versions of all documents for a study. Study document renditions are exported as a ZIP file and, when unzipped, are in the eBinder folder structure.
Digital Delegation: Email Notifications
This feature provides the following general usability enhancements to the Digital Delegation feature:
- Users receive email notifications for pending and completed digital delegation tasks, for example, when delegations are ready for principal investigator (PI) approval and when the PI approves.
- Users can reinitiate study staff acceptance of delegations before PI approval. This enables users to update a study team member’s delegations after they’ve accepted but before the PI has approved, if needed.
The Digital Delegation feature can only be configured in vaults with the Extensible SiteVault Permissions feature enabled.
User Administration with Multiple Security Policies
For SiteVaults that have more than one active security policy (for example, basic Vault authentication and a single sign-on provider), this feature allows the user administrator to select a security policy when creating or editing a user. This feature only pertains to vaults with multiple security policies and the Extensible SiteVault Permissions feature enabled.
Sponsor/CRO Information on Documents
With this feature, SiteVault users working on Connected Studies can now see details regarding whether a document version has been sent to Sponsor/CRO, the date it was initially sent, and the last sent date directly in the document’s metadata.
Connected Study Details User Action
SiteVault users working on Connected Studies can now use the Connected Study Details user action to access more details regarding a document’s exchange with a Sponsor/CRO. This action opens a dialog box that displays tracking information as well as the document comment history with the Sponsor/CRO.
New Exchangeable Document Type on Connected Studies
This feature adds support to transfer documents of type IP Excursions to a Sponsor/CRO’s vault for a Connected Study.
Support eConsent Simple Signatures
This feature adds support to enable study participants and other signatories to complete eConsent forms without creating a MyVeeva for Patients account. In SiteVault, site staff can send eConsent forms without required contact information. This feature is tentatively scheduled to be generally available with the Vault 22R1 release.
Veeva eConsent: In-person eConsent
This feature enables SiteVault users to quickly connect a mobile device to MyVeeva for Patients so that patients can read and complete an eConsent form in person.
Users can select the View In-person Code action on the study participant’s record to generate a unique QR code to access the eConsent form in MyVeeva for Patients. Patients can use their own mobile devices or a site’s device when available.
Veeva eConsent: Contact Information Update Request
With this feature, when a patient or signatory on a study updates their contact information in MyVeeva for Patients, their contact information is also updated in SiteVault. Site Administrator users receive a notification that alerts them to the changes. This feature is tentatively scheduled to be generally available with the Vault 22R1 release.
Veeva eConsent: Additional Signatory Enhancements
This feature includes general enhancements to the Veeva eConsent Additional Signatories feature, including making it even easier to create and manage Signatory records and providing users more flexibility in sending eConsent forms to signatories.
Study PI Role for Workflows
With this feature, a system-managed Principal Investigator role is available for use in workflows for study-related documents and objects.
Standard Participant Workflows
This feature adds new standard workflows for the Participant object. Configuration is required to begin using the new workflows.
SiteVault Data Model Changes
See 22R1 Data Model Changes: SiteVault.
QualityOne
Risk Management: Detectability Support for Qualitative & Quantitative Risk Matrices
This feature adds support for modeling Detectability as another axis of the Risk Matrix. Adding the Detectability axis supports assessment methodologies such as the FMEA (Failure Mode and Effects Analysis) Risk Assessment, which requires the Detectability attribute of an identified risk. QualityOne customers can create 3D risk matrices (Severity x Occurrence x Detectability) within their Vaults with this feature. Learn more about Risk Management.
Linking Quality & HSE Events to Risk Register
This feature will allow users to link Quality and HSE Events to Risk Register. Quality and HSE Managers can leverage this link to be proactive in identifying, managing, and mitigating potential risks across their organizations. Learn more about the link.
Note: For HSE Events, this feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
External Collaboration Management on CAR
This feature allows internal users to manage the creation, activation, and inactivation of user accounts for third-party organizations. External Collaborators (third-party users) can respond to CARs (SCARs) in Vault using the External User license assigned. Internal users manage a “contact list” of persons associated with the third-party organizations, tagging CARs (SCARs) with a relevant External Collaborator for Vault to automatically create, activate, or inactivate. When the user account for the External Collaborator is created, activated, or inactivated, the relevant email is sent to those persons as part of the CAR (SCAR) process. This feature enables Admins to utilize and configure a pool of shared External User licenses to facilitate this process using the External Collaborator User Template. Learn more about External Collaboration Management.
Product Hierarchy Data Model Extension for Packaging & Materials
This feature provisions new objects (Packaging, Product-Packaging, Material, Material-Organization) in QualityOne to extend the standard product hierarchy data model. Customers can leverage Material-related ERP (Enterprise Resource Planning) data to use with QualityOne.
QualityOne Mobile for iOS Compatibility with Extended Product Hierarchy Model
This feature updates QualityOne Mobile for iOS to use the extended standard Product Hierarchy Data Model. Users can now log NCRs against Materials (including Products, Formulations, and Packaging) and submit them to Vault via the mobile application.
QualityOne Mobile features are targeted for tentative availability on May 3rd.
Inspections Compatibility with Extended Product Hierarchy Data Model
This feature updates QualityOne Inspection Management functionality to align with the newly-provisioned Material (material__v) object that supports the Extended Product Hierarchy Data Model.
FSMA Supplier Verification (FSVP/SCAC)
This feature offers the ability to verify the supplier’s capability of controlling the known or foreseeable hazards associated with the supplied material, in accordance with the FSMA (Food Safety Modernization Act) requirements, using a Material Verification Checklist. This checklist allows users to configure the FSMA requirements for both Supply Chain Applied Controls (SCAC) as well as Foreign Supplier Verification Programs (FSVP). Learn more about Material Verification Checklists.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
QualityOne Station Manager Enhancements
This feature enhances QualityOne Station Manager by allowing customers to generate custom QualityOne Station Manager URLs for documents, improving sync intervals and sync warning messages, updating document categorization to be record-based, enhancing Recent Document visibility, and adding application update enforcement.
QualityOne Station Manager features are targeted for tentative availability on May 3rd.
Custom URL & QR Code Support for Categories
This feature allows customers to generate custom URLs to view specific categories of documents in QualityOne Station Manager. Manufacturing users can open custom URLs on their tablet device by manually entering the custom URL or by scanning a generated QR code. The QualityOne Station Manager mobile application can open lists of documents pre-filtered to the categories specified in the custom URL. This feature enables customers to create shortcuts for manufacturing users to quickly navigate to the appropriate set of documents for their current task.
Sync Interval Update
The interval at which Station Manager periodically syncs with Vault has been updated from every 15 minutes to every 15-20 minutes. This variability improves the scalability and performance of the mobile application when distributed across a large number of devices.
Warning Mode
This feature allows customers to configure a time of day after which the QualityOne Station Manager mobile application must successfully sync with QualityOne Vault. If the application does not sync by the configured time, the warning messages will display that the documents may be “out-of-date”. Customers can also configure the default warning message that will display for users to see.
Record Based Categorization
This feature changes the way customers will manage categories for QualityOne Station Manager. Customers will now be using object records for categorization and use Vault’s access controls to restrict which users can manage categories and the assignment of categories to Station Documents. Record-based categories are flexible by allowing diverse scoping so that each customer can define and manage their own list of categories. Once the categories have been defined and assigned in Vault, they are displayed in the mobile application for selection by manufacturing users.
Recent Documents Enhancements
This feature allows operators to easily access the most recently viewed documents and videos from within the document or video viewer. Manufacturing users often need to repeatedly switch between multiple procedures and instructions when performing a task on the shop floor. Allowing users to switch between documents and videos without losing their place increases efficiency while performing the task at hand. In addition, the Recents tab is now displayed as the second tab after the All tab.
App Update Enforcement
This feature allows customers to enforce Veeva’s mobile application support policy for QualityOne Station Manager. When the application detects that it is one version behind QualityOne Vault, the mobile application begins a reportable countdown and continually notifies users to update the mobile application. Once the countdown ends, the mobile application logs the current user out. If the application is two versions behind QualityOne Vault, users may not log into the mobile application.
COA Ingestion Enhancements
This feature extends our processing capability to allow for a wider range of COA formats that can be accurately ingested. Learn more about the COA ingestion enhancements.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Multi-Page Header Matching Strategy Support
This feature expands the support of the header matching strategies to account for COA details that do not reside on the first page of a COA document.
Processing COA Files with Table Breaks
This feature allows Vault to process tables within COA documents that contain a break, such as when a table spreads across multiple pages.
Automatic Detection of COA Date Formats
This feature allows Vault to extract commonly used date formats directly from a COA document to remove dependency on the set value of the Expected Value Format field of a Header Matching Rule Variant.
Lenient Expected Value Formats for Dates
This feature improves the user experience of date format configuration in a COA document by relaxing the case-sensitivity and delimiter matching requirements.
Improved COA Analysis Job Logging
This feature provides debugging information related to COA ingestion for users to quickly resolve potential configuration issues for problematic COA formats. This feature allows Admins to access debugging information related to COA ingestion via a Job Log. The Job Logs can be accessed by navigating to Admin > Operations > Job Status. A summary of the ingestion analysis is provided in the Job Log to give Admins the necessary information needed to quickly triage and resolve COA ingestion-related issues.
Improved COA Table Analysis
This feature improves the number of COA formats that can be accurately ingested with a specific focus on improving table processing accuracy.
Additional Matching Options for Component Matching Variants
This feature adds additional matching options for Admins to choose from when defining a COA Component Matching Rule on a Component Matching Variant. The new COA Component Matching Rules are:
- Contains
- Starts With
- Ends With
If a Component Matching Variant does not have a COA Component Matching Rule defined, Vault uses an Exact Match criteria when evaluating a Component Matching Rule’s validity.
Additional Matching Options for Matching Rule Variants
This feature adds additional matching options for Admins to choose from when defining a Variant Type on a Matching Rule Variant. The new Variant Types are:
- Contains
- Starts With
- Ends With
Update View 5 Whys Analysis Action
This feature offers a new record action “View 5 Whys Analysis” to launch the 5 Whys diagram, replacing the original “View 5 Whys Analysis” user action. The “View 5 Whys Analysis” user action is renamed to “View 5 Whys Analysis (Legacy)” and is targeted for removal in a future release. Learn more about the new 5 Whys Analysis record action.
QualityOne Team Enhancements
This feature extends the QualityOne Team capability to allow Admins the flexibility of configuring Team roles and members. Learn more about QualityOne Teams.
Manage Invalid Users with Tasks
Admins can now control invalid users when managing QualityOne Teams. For a given Team, if any user in that Team is invalid, users can choose to remove the invalid user from the team or to replace them with an alternative, valid user. When invalid users are replaced, Vault automatically handles task reassignment by moving open tasks assigned to the replaced user.
Role Restriction & Child Object Team Enablement
Admins can now restrict a team member to a particular role, similar to a workflow’s participant group role restriction. . Team Roles are restricted by declaring the role as Exclusive: if a user is a team member of an exclusive Team Role, that user cannot also be a member of any other role for that record. Additionally, Admins can now create a Team on child objects.
Display Key Team Roles for Filtering & Reporting
Users can now surface key Team Role assignments as User fields, displaying and using the key in related lists, searches, filters on library views, and reports, allowing team membership changes to synchronize with the record’s field.
Related Record Setup Enhancements
This feature extends the Related Record Setup capability to allow Admins the flexibility of configuring the “Create Related Record” action on the “Create Record” Event Action. Learn more about the Related Record Setup enhancements.
UI Enhancement for Related Record Setup
This feature allows Admins to view a clickable list of related record components to easily access the details page of each component.
Support Related Record Creation during Creation of Source Record
This feature improves and supports Related Record Setup to be configured on the “Create Record” event action on the object lifecycle, allowing related record creations to trigger during the creation of the source record. Depending on configuration, users can also nest multiple levels of related record creation by triggering the initial “Create Related Record” event action. Vault then triggers the same action on the created related record to create another related record, with the initial related record as the source record now. Admins can nest the levels of related record creation up to a default depth limit of 5 levels.
Admin Menu for Related Record Setup
This feature allows Admins to access the Related Record Setup page from Admin by navigating to Configuration > Application Configuration > Component Setup section and selecting Related Record Setup from the list.
Admin Menu for Comment Setup
This feature allows Admins to access the Comment Setup page from Admin by navigating to Configuration > Application Configuration > Component Setup section and selecting Comment Setup from the list. Learn more about the Admin navigation.
Data Model Changes
See 22R1 Data Model Changes: QualityOne.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on May 3rd.
Formulation Questionnaires
Using checklists, this feature allows regulatory users to send targeted questionnaires, such as raw material questionnaires (RMQs), via email to contacts at external organizations, such as suppliers, and enables those contacts to respond securely via public access links (PALs) without logging into Vault. Learn more about Formulation Questionnaires.
Local Impact Assessment
This feature helps users assess whether a new Registration Item’s objective can be accomplished by amending an existing registration or if a new registration is needed. Vault uses Admin-defined Regulated Category Attribute Impacts to search active registrations and identify the records that match the Registration Item. Vault also indicates to users if the matched registrations need to be amended. Lastly, Vault starts the appropriate registration process based on the user’s selection of a registration. Learn more about Local Impact Assessments.
Create Dossier Binder
This feature gives users the ability to create a binder with a list of locked steady state expected documents for a requirement while maintaining the requirement hierarchy as a hierarchy of sections. Admins can configure the new Create Dossier Binder action on the Registration Item Requirement object to allow users to create Dossier Binders from a parent record. Learn more about Dossier Binders.
Generate Requirements for Registration Objective
This feature allows Admins to configure the Generate Requirements action on the Registration Objective, allowing users to generate requirements for a collection of Registration Items. This may be helpful when multiple products, such as shaded products, can be registered together through the submission of a single dossier. Learn more about generating requirements.
Split Registration Items Enhancement: Recursion
This feature enhances the Split Registration Items feature and gives users the flexibility to view generated Registration Items in an output structure that preserves the hierarchical relationship between them in addition to viewing the records in a flat list. Customers can utilize the Registration Item Hierarchy Viewer to easily visualize the output when several multi-level records are generated from the Split Registration Items action on a Registration Item with a Split Rule linked to a recursive relational token. Learn more about configuring Split Registration Items.
Registration Item Hierarchy Viewer
When configured, this feature allows users to view multiple related Registration Items in a hierarchical view. Users can identify related Registration Items and any dependencies between them all in one viewer, improving the user experience by limiting the need to navigate to multiple other pages. Learn more about the Registration Item Hierarchy Viewer.
Formulation Composition Viewer Enhancements
A complex formulation can contain numerous constituent chemicals. It is important to quickly distinguish a single formulation’s attributes and identify any red flags when viewing that formulation in the Formulation Composition Viewer. This feature allows Admins to configure the viewer to display shading between the hierarchical levels as well as highlighting a row when users hover over specific records. Users can also identify constituent chemicals that don’t meet regulatory requirements by assigning a color to the compliance assessment status displayed in the viewer. Learn more about the Formulation Composition Viewer.
Track Authority Questions
This feature unlocks the standard Regulatory Request object in Registration & Dossier Management Vaults, giving users the ability to track authority questions.
Enable Checklists in RegulatoryOne
This feature enables Checklists in all RegulatoryOne Vaults, including Veeva Claims.
Product Hierarchy Data Model Extension for Packaging
Consumer and cosmetic products often come inside product packaging that can form multilevel hierarchical relationships. With this release, we are expanding the shared standard product hierarchy data model to include product packaging data (Packaging, Product Packaging, and Packaging Composition objects) to reduce reliance on customization.
These data model changes are automatically included in RegulatoryOne, but Admins must make configuration changes to make them available.
Note this feature is also available for Veeva Claims.
Data Model Changes
See 22R1 Data Model Changes: RegulatoryOne.
Veeva Claims
Veeva Claims features are targeted for tentative availability on May 3rd.
Project Hierarchy Viewer
When configured, this feature enables users to navigate through a large data set of Claims and Local Adaptations within a project with ease. Users can filter the list of Claims and Local Adaptations in the project to narrow down their scope. Filters allow users to filter the project hierarchy only on the Product and Country values which are joined with the Project. Through a hierarchy navigator, users can jump easily between different levels of a Project. Users can view and add related sections for Claims and Local Adaptation objects while maintaining the context of the Project. Learn more about configuring the project hierarchy viewer.
Dynamic Deep Copy Project
This feature improves the speed of starting new Claims projects. When configured, this feature gives users control over the copy Project record behavior by allowing users to specify which related sections of Claim and Local Adaptation records they want to copy over to the new Project record. Learn more about configuring dynamic deep copy project.
Copied from Project Link
Claims users commonly copy Project records to initiate new projects. When copying projects, it is important to establish a link between the copied from record and copied to record to maintain traceability and maintain a reference so that users can refer back to the key details from the original project. This feature introduces a new read-only Project reference field as a part of the Project object which is auto-populated when a user copies a project. Learn more about the new field.
Auto-Populate Statement Translations for Local Adaptations
Local Adaptations for each country can have multiple associated Statement translations in country-specific languages. Veeva Claims stores Statement translations and semantic variations in a self-referencing Statement library. When configured, this feature eliminates the extra effort by users to search and assign Statement translations from the Statement library to each Local Adaptation record by automatically assigning Statement translations to a Local Adaptation record based on the Admin-configured country-language mapping. Learn more about configuring the Populate Statement Translations action.
Claims UX Enhancement
This feature enhances the UX for Claims users. The feature primarily enhances the search and list experience in the Selectively Create Claims dialog and the Generate Local Adaptations dialog.
Enable Checklists in RegulatoryOne
See: feature description.
Product Hierarchy Data Model Extension for Packaging
See: feature description.
Data Model Changes
See 22R1 Data Model Changes: Veeva Claims.
Enablement Details
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some "Auto-On" features have a checkbox setting that hides the feature; these will show "Auto-On." | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|