Release Date: December 10, 2021
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Working with Documents
Auto-Populate Rendition PDF Metadata
With this release, Vault populates the basic document metadata in a viewable rendition’s PDF Document Properties based on the original source document’s properties. This feature works with all supported Microsoft Office source document types.
Required Overlays
With this release, Vault prevents the download of viewable PDF renditions when overlays fail to apply. Admins can also mark overlay templates as optional, allowing downloads when overlays fail to apply.
Notifications on Replies to Notes
This enhancement notifies users whenever another user replies to one of their note annotations. Vault also limits the number of notifications from a single annotation event, preventing redundant notifications. For example, if someone adds a new note, replies to an existing note on a Favorite document, or mentions the user when composing a note or reply, Vault only sends one notification.
Copy Text from Viewer
With this enhancement, Vault users can copy text from Vault documents by holding down the C key while selecting text, saving the copied text to the user’s clipboard. This option is available in both View and Annotate modes.
Editable Bookmarks
When enabled, this feature allows users to edit certain Bookmark attributes on the Doc Info page. Once saved, the edited bookmarks persist in the viewable rendition. Supported actions include Rename, Move Up, Move Down, Promote, Demote, and Delete.
Google Drive Integration: Create Draft & Upload New Version
This enhancement allows users to browse for and select files from Google Drive when creating a draft or uploading a new document version in Vault.
Google Drive Integration: Upload to Placeholder
This enhancement allows users to browse for and select files from Google Drive when uploading a file to a placeholder in Vault.
Today Link for Date Fields on Doc Info Page
This enhancement adds a Today link next to any Date field that will automatically stamp today’s date when clicked. This link appears while editing on the Doc Info page, or on the bulk documents edit page.
Vault Objects
Today Link for Date Fields on Object Record Detail Page
This enhancement adds a Today link next to any Date field that will automatically stamp today’s date while editing information on an object record details page.
Lifecycle & Workflow
Verdict Information & Reporting on Removed Documents
With this release, Vault shows verdict information for documents removed from workflows manually or by a content action on the Workflow Timeline view. Additionally, reporting and notification tokens on Verdicts can now capture removed documents. Once a document is removed, it’s state is set to Removed in the envelope.
Bound Version Create Draft
With this feature, Admins can now enable documents in workflows to remain in their steady state version, even if the Create Draft action is performed on the document. By default, all workflows on documents continue with the latest version of the document.
Remove eSignature from documents
Workflow administrators can now remove signatures on documents when a workflow is cancelled or during the workflow using a Workflow Cancellation Action or a Content Action. Signatures can only be removed on the current active workflow, and can be removed for a specific task and verdict.
Skip Document State Change in Object Lifecycle Entry Action
Workflow administrators can skip the state change of related documents in an object lifecycle entry action. This allows administrators to skip state change for related documents already belonging to a steady state.
Reporting & Dashboards
Export Report to Formatted Excel
This feature allows users to export reports as they are displayed in the report viewer. Users can download the full data or grouped summary of the report in an Excel format. This reduces the effort required to format the exported Excel sheet and improves usability.
Vault now displays a single Export to Excel option in the Actions menu for all Excel related export actions, which provides several export options: the default Data only option; the Formatted option, which exports the formatted Excel; and the Template option, if users upload a pre-formatted Excel template.
Multi-Pass Formula Fields Enhancements
With this release, Vault supports formula fields on Multi-Pass reports created using Binder, Relationship, and Workflow report views, along with improved performance on formula fields on Multi-Pass reports overall. Formula field performance was also improved in multi-pass reports.
Configuration Management
Configuration Migration: Migrating Document Tags between Vaults
Admins can now migrate Document Tags between Vaults using Vault Packages (VPK) and can view Tag components in Vault Configuration and Comparison reports. *Auto-on in Vaults with Outbound and Inbound Packages enabled.
Configuration Migration: Comparison of XMLString Component Attributes when Deploying Packages
Admins can view and compare values of a component’s XMLString attributes between the inbound package and the target Vault before deploying the package. *Auto-on in Vaults with Inbound Packages enabled.
Vault Compare Report: Header Rows Frozen by Default
Column headers in each sheet in the Vault Compare workbook are now frozen by default so that users can easily understand which fields they are viewing while scrolling through the report.
Vault Configuration and Compare Reports: Reporting Full MDL of Object Lifecycle State Entry Action Rules
Vault Compare and Vault Configuration Reports now report on the formula used in an object lifecycle state entry action by providing the full value of the rule attribute in the Objectlifecyclestateentryaction sub-component.
Vault Configuration Report: Cover Page Title for Object Data Workbook
The Object Data workbook generated with the Vault Configuration Report now adds “Object Data” to the title in the cover page so that users can quickly understand which workbook they are viewing.
Vault Configuration and Compare Report: Details for Auto Managed Group Field Order Security Settings
Security settings for Auto Managed Group Field Order in Vault Compare and Vault Configuration Reports now display each object and its fields in individual rows, making it easier to understand the configuration and aligning it with how Vault displays the settings in the UI.
Migration Mode with Relaxed Validation Rules
With this release, Vault bypasses validation rules and reference constraints when creating records in migration mode via Vault Loader or configuration migration packages. Migration mode continues to allow record creation in any lifecycle state.
With this change, the audit trail will now append “in migration mode” in the event_description. For example, if the previous description was “Vehicle : VEH-000007 created”, the new description is “Vehicle : VEH-000007 created in migration mode”.
Access Control
User Role Object Moving to HVO
The User Role system object (user_role__sys), which is used to store user role assignments, is moving to high volume to deliver increased performance and scalability.
Administration
Jobs: Display Start Time for Completed Jobs
The job start time is now displayed in the job history table on the Job Status page allowing Admins to quickly see when a job began and ended.
Search
Multidirectional & Phrase Synonyms
The synonyms feature has been enhanced in several ways. First, there is a new option to set any row in the thesaurus to multidirectional. This will make it so a user can search for any entry or synonym on that row and the search will expand out to all of the other entries and synonyms. This is useful for terms like “child” and “adolescent” that should both always match no matter which term is searched.
Vault also now supports phrases as entries. With this, users can search for more complex concepts. For example, searching “myocardial infarction” can match “heart attack” and vice versa. Phrases wrapped in double quotes also now match to any synonyms as well.
The limits have also been updated to increase flexibility. Each row in the thesaurus can have 250 characters in up to 5 entries and 15 synonyms. This supports use cases like name search where there may be many variations on a first name (e.g. Liz, Lizzy, Elizabeth, Liza, etc), and long acronyms where the expanded form exceeded the 50 character limit per synonym that was in place previously.
HVO Tabs: Search by Name from the Top Search Box
When navigating to an HVO tab, users can now use the main search box to quickly find records by name. This is a case-sensitive, starts-with search, so it has limited capability when compared to Vault’s standard object search.
Usability
Action UI: Documents: Expose Drop-Down from Chevron for One Action
With this release, Vault displays the drop-down when users click the state change and workflow action chevrons. Previously, when only one state change or workflow action was available on the chevron, clicking the chevron immediately started the state change or workflow action.
Vault File Manager
Download Rendition Files to Vault File Manager
With this release, users with the appropriate permissions can perform the Download Rendition File to Vault File Manager action when downloading Vault document renditions. If the file is over 4 GB in size, Vault suggests downloading the file to Vault File Manager instead of attempting to download in the web browser using the standard Download Rendition action.
Vault Loader
Vault Loader: Updating User & Group Role Assignments on Object Records
Vault Loader now provides the ability to assign and remove users and groups from object record roles for objects that have custom and matching sharing rules enabled. *Auto-on in Vaults with Vault Loader enabled. Learn more about updating object record roles with Vault Loader.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Platform data model to support new features:
Added the Bookmarks Edited (bookmarks_edited__sys) system-managed field to support the Editable Bookmarks feature.
Vault Connections
PromoMats/RIM: RIM Reference Model Support
This feature adds support for the RIM Reference Model to the PromoMats to RIM Connector. When document types are not defined in the Connector, the RIM Reference Model is used to determine the document types to be used with the Connector.
Clinical Operations
Milestone Gating for EDL Item Requiredness
This feature extends Milestone Gating by adding a check, either as configurable entry criteria or a workflow decision step, that ensures all related EDL items have a Requiredness value of “Required” or “Not Required.” It also introduces one-way settings that allow Vault admins, in the EDL Requiredness picklist, to enable the standard “Pending Decision’’ and disable the standard “Optional” values.
Site Connect: Distribution Task Return Field Field Now Dedicated to Site
This feature has made an update to dedicate the Distribution Task object’s Return Document Version and Return Comments fields to always contain the Site Document Version and Site Comments. Previously, Vault could contain site Document Version and Site Comments in the Document Version and Document Comments fields under certain conditions.
Customers currently tracking the documents received from a Site within a related list section of the Study Site record should hide the Document Version / Document Comments field and instead expose the Return Document / Return Document Comments fields.
Commercial
Document Notifications: Support for “Based On” Relationship
This feature expands the functionality of the “Based On” relationship and allows Admins to configure a notification Vault sends if the Parent Document in the “Based On” relationship experiences a document entry action, user action, or a document event action. Learn more about parent document notifications.
Modular Content Approval Document Additional Lifecycle Support
With this release, Admins can associate the Module Content Approval Document with any lifecycle while still successfully generating the Approval Document. If the Approval Document subtype is associated with multiple lifecycles, Vault associates the first lifecycle listed alphabetically as that document’s lifecycle when performing the Generate Approval Document action.
Transparency Support in Automated Image Renditions
With this release, Admins configuring Automated Image Renditions can select the option to Preserve Transparency for .PNG, .GIF, .BMP, and .TIFF file formats on each Rendition Type where transparency is required.
Medical Inquiry: CRM Inquiry Pull Job Timing Enhancement
This enhancement adjusts the date & time range used to retrieve medical inquiries from CRM and now uses the date & time of the last successful Account pull to ensure that the Accounts referenced are present in Vault.
Quality
Recurrence Check: Support for MedTech Complaints
With this release, Vault QMS expands its Recurrence Check capabilities to Medtech Complaints and introduces an intelligent and streamlined process for end-users to check whether a Medtech Complaint is a recurrence of another. The previously complex manual process is replaced with a simple user action that quickly finds potential recurrences and displays them in an easy to scan list view. Once the recurrence check is complete, Vault QMS will automatically store the results which can then later be utilized for reporting and trending.
Generate Document from Report: Support for Record Check Lifecycle
The Generate Document from Report user action allows a report to be executed from a Record Check Result record. Once the report is run, a document is automatically created in either PDF, Excel, Excel Template, or CSV format and accessible from the record check result record from which the action was executed. This allows a user to utilize the power of recurrence checks and Vault reporting to provide robust trending on recurring Deviations, Complaints, and Medtech Complaints.
Recurrence Check: Display Match Criteria on User Dialog
The QMS Record Check user action has been enhanced to easily view the match criteria parameters for a selected record check. Users can view the exact match fields, lifecycle states, and time thresholds that will be evaluated when executing a recurrence check.
Quizzes: Question & Answer Randomization
This feature allows Training Admins to randomize quiz questions within a Quiz Section Design and randomize quiz answers within a Quiz Question Design. Every time a Learner attempts the resulting Vault Training Quiz, Vault randomizes the display order of questions, answers, or both.
China Link E-Learning Support
Vaults on China Link can now use E-Learning courses: from uploading e-learning courses to completing e-learning courses as a Learner.
Direct Assignment: Hide Inactive or Ineligible Persons in the Learners Field
Persons with Training Eligibility equal to Ineligible or Status equal to Inactive will no longer appear in the Learners field during a Direct Assignment action.
Email Participant Action Available for Training Assignment
From the workflow timeline for a Training Assignment, an authorized user can email task participants, such as the Learner, by selecting the Email Participants action. This allows Training Admins or Managers to remind the Learner to complete a training task.
Issue Training Assignment Entry Action Update
With this update, if the Issue Training Assignment entry action cannot find an open Training Requirement Impact Assessment (TRIA), the action will now update the document version on the Training Requirement. Previously, an update did not occur.
Training Requirement Impact Assessment (TRIA) for All Training Requirements
The Training Requirement Impact Assessment (TRIA) process notifies Training Admins when assets in a Training Requirement are updated so the Admin can decide what should happen with the Training Requirement. In previous releases, TRIA was only supported for multi-document, classroom, or other types of Training Requirements. With this release, a new setting in Admin > Settings > Application Settings will allow TRIA to be used for all Training Requirements, including single-document Training Requirements.
QRM: Template Based Risk Evaluation
With this release, users can create new Risks for an assessment from existing Template Risks, saving time and effort spent creating assessments. The system will automatically copy all the available information from the Template Risk to Assessment Risk by matching the name and the type of the fields. To take full advantage of this feature, Assessment Risk should not have Risk Matrix or Process Step as a mandatory field.
QRM: Promote the Assessment Risk Event to Risk Event in the Register
With this feature, users can promote one or more Assessment Risk records to one or more (up to 5) Risk Registers. The system automatically copies all the available information from the Assessment Risk to Risk (Event) by matching the field name and type. This feature is intended to consolidate all Risks identified as part of multiple assessments, conducted via different methodologies, for a particular context (such as Product, Supplier, Process, Site, or other). The newly created Risk records in the Register are linked to the originating Assessment Risks.
Generate Document from Object Record Action: QRM Objects
With this feature, Admins can set up a user action or entry action on a lifecycle state to generate a document from Formatted Output or from Document Template for a risk assessment or a Risk Register. This will enable Admins to have a snapshot of the record created and attached directly to the record itself in the form of a Vault document.
QRM Data Model Updates
This release brings several data model updates to support the Quality Risk Management suite of features:
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Quality data model to support new features:
Made the following changes to support the Quizzes: Question & Answer Randomization feature:
- Added the Randomize Questions? (
randomize_questions__v) field to the Section Design object. - Added the Randomize Answers? (
randomize_answers__v) field to the Question Design object.
The following changes were made to support the Recurrence Check: Support for MedTech Complaints feature:
- Added the Medtech Complaint (
mt_complaint__v) object type to the Record Check Result and Record Check Result Match Records objects. - Added the Record Check Result (
record_check_result__v) shared document field.
Regulatory
Capture Active Dossier Submitted Status
With this release, Submitted  is available as a new country status in the Active Dossier, providing visibility into upcoming changes that have been submitted but are not yet approved or made current. When generating Active Dossier records from a submission, Vault automatically sets the Submitted status on newly-created Active Dossier Item and Active Dossier Item Detail records, and updates such records where the status is blank.
is available as a new country status in the Active Dossier, providing visibility into upcoming changes that have been submitted but are not yet approved or made current. When generating Active Dossier records from a submission, Vault automatically sets the Submitted status on newly-created Active Dossier Item and Active Dossier Item Detail records, and updates such records where the status is blank.
Restrict Submissions Archive Access to Full User Licensed Vault Users
With this release, Vault will enforce Submissions Archive application licensing. In Vaults created in 21R3 and earlier, users without a Full User application license are warned about unlicensed usage of the application. In Vaults created in 22R1 and later, users without a Full User application license are blocked from accessing any Submissions Archive functionality.
Content Plan Creation: Novel Excipient Update
When creating content plans with novel excipients, Vault creates corresponding repeating content plan sections (for example, 3.2.P.4.6) within repeating excipient sections (for example, 3.2.P.4) only when the novel excipient’s Submission Inactive Ingredient record is a Novel Inactive Ingredient.
Display Inactive Applications & Submissions in Viewer
When enabled by an Admin, users are able to select and view the content of inactive applications and submissions within the Submissions Archive Viewer.
Extend Matched Document Tokens for Content Plans
This feature enables generic support for tokenizing object and text fields on matched documents in the Content Plan. For example, the Clinical Site document field can be used within the Published Output Location to organize Case Report Forms into site folders in the published output.
Translation Document Relationship Flag
With this release, the status of the standard Translation document relationship introduced in 21R2 is controllable via two-way Vault Admin flag. Customers experiencing conflicts with existing custom Translation document relationships can inactivate the standard relationship, which hides the relationship from all document pages.
22R1 RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model:
Added the Submitted (submitted__v) picklist value to the Active Dossier Status (active_dossier_status__v) field to support the Capture Active Dossier Submitted Status feature.
Safety
Safety features are targeted for tentative availability on December 16, 2021.
Duplicate Detection by Case Identifiers Auto-on
Vault Safety will now search for duplicates using Case Identifiers. Inbox Item UIDs and Case Identifiers will be cross compared with Case UIDs and Identifiers during duplicate detection.
Learn More
Identifiable Reporter and Patient Per ICH E2D on Inbox Item Auto-on
Vault Safety will now evaluate the Identifiable Patient and Identifiable Reporter fields on the Inbox Item based on the ICH Guideline Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting E2D, which looks at certain fields from the Patient and Case Contacts section. In addition, if you mark one or more of these fields as Masked, the patient or reporter will qualify as identifiable.
Learn More
Always Serious and Product or Study-Independent Watchlists Configuration
You can now create watchlists without specifying a Study or Product. Additionally, you can set Seriousness criteria for adverse events on a watchlist. If a Case contains a matching adverse event(s), the Seriousness will automatically be assigned to the Case Adverse Event, if Seriousness is not already specified.
Learn More
- Enablement: Enable Adverse Event Watchlists: Enable Always Serious and Product or Study-Independent Watchlists
- Admin Help: Configure Adverse Event Watchlists
- User Help: How Case Watchlist Tags and DMEs are Assigned: Default Case Seriousness
Auto-Listedness Using Core Datasheets Configuration
Vault Safety will now generate expectedness records for the product’s Core Datasheet (CCDS) to support listedness calculations during case processing. A new field will also be available for Core Datasheet roll up at the Assessment and Case level.
Learn More
- Enablement: Enable Listedness and Expectedness From Core Datasheets
- Admin Help: Manage Datasheets and Auto-Expectedness
Expectedness from Core Datasheets Configuration
When generating a submission, Vault Safety will evaluate expectedness for a Study Case using the Development Core Safety Information (DCSI) datasheet within the investigator brochure (IB).
Vault Safety can also calculate expectedness from a product’s Core Datasheet without additional configuration to Local Datasheets, if your product or organization does not require them.
Learn More
- Enablement: Enable Listedness and Expectedness From Core Datasheets
- Admin Help: Manage Datasheets and Auto-Expectedness
FDA Submission Automation: Relatedness Assessment Source Parameter Auto-on
The FDA does not require a study submission if a sponsor assesses a case as Not Related while the investigator assesses the case as Related for a SUSAR in a clinical trial. With this release, users will no longer have to inactivate the submission to the FDA, as it will not be generated due to now evaluating the relatedness for FDA SUSAR cases based on the Sponsor’s Assessment. Additionally, the Assessment Source parameter will be introduced for use in Custom Rulesets.
Learn More
- Admin Help: Reporting Rule Parameter Reference
Submission Automation: Upgrade and Downgrade Parameters Auto-on
Vault Safety will introduce new parameters to calculate whether a Case is an upgrade or a downgrade in order to support additional submissions scenarios for the FDA, EMA, ROW, and custom rule sets. This feature will also support the one-last-time and one-more-time reporting rules, while considering the appropriate due date and local expedited criteria.
Learn More
- Admin Help: Reporting Rule Parameter Reference
Submission Rules: Support for MedDRA Queries (SMQs and CMQs) Configuration
With this release, Vault Safety will extend the Safety Rule Engine to allow greater configurability for Custom Rule Sets. New rule parameters will be introduced to support the selection of specific Products and/or Studies when deciding if a rule should be executed. Additionally, MedDRA Queries (SMQs and CMQs) can be used to determine if a case should be reportable in order to support situations such as “Lack of Efficacy” or customer-managed lists of non-reportable terms.
Learn More
- Enablement: Enable Support for MedDRA Queries in Submission Rules
- Admin Help: Use MedDRA Queries
- User Help: Reporting Rule Parameter Reference
Custom Safety Rules SDK
Vault Safety now has an SDK entry point to allow customized logic for submission rules.
Learn More
For assistance with the custom safety rules SDK, contact Veeva Services.
Custom Safety Validations SDK
Vault Safety now has an SDK entry point to extend Case validation with custom criteria and evaluation logic.
Learn More
For assistance with the custom validations SDK, contact Veeva Services.
Bulk Narrative Import and Status Check API Endpoints
Two new Vault Safety API endpoints are available to import multiple case narrative documents and translations in one operation. This is to improve case migration performance in Vault Safety. The Vault Developer Release Notes provide more information.
Learn More
SiteVault
Study Export
This feature allows users to export all major versions of all documents for a study. Study document renditions are exported as a ZIP file and, when unzipped, are in the eBinder folder structure.
QualityOne
Linking Quality & HSE Events to Risk Register
This feature will allow users to link Quality and HSE Events to Risk Register. Quality and HSE Managers can leverage this link to be proactive in identifying, managing, and mitigating potential risks across their organizations. Learn more about the link.
Note: For HSE Events, this feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
COA Ingestion Enhancements
This feature extends our processing capability to allow for a wider range of COA formats that can be accurately ingested. Learn more about the COA ingestion enhancements.
Note: This feature is currently available only to early adopters. Contact your Customer Success Manager for more information.
Multi-Page Header Matching Strategy Support
This feature expands the support of the header matching strategies to account for COA details that do not reside on the first page of a COA document.
Processing COA Files with Table Breaks
This feature allows Vault to process tables within COA documents that contain a break, such as when a table spreads across multiple pages.
Automatic Detection of COA Date Formats
This feature allows Vault to extract commonly used date formats directly from a COA document to remove dependency on the set value of the Expected Value Format field of a Header Matching Rule Variant.
Lenient Expected Value Formats for Dates
This feature improves the user experience of date format configuration in a COA document by relaxing the case-sensitivity and delimiter matching requirements.
Improved COA Analysis Job Logging
This feature provides debugging information related to COA ingestion for users to quickly resolve potential configuration issues for problematic COA formats.
Improved COA Table Analysis
This feature improves the number of COA formats that can be accurately ingested with a specific focus on improving table processing accuracy.
Related Record Setup Enhancements
This feature extends the Related Record Setup capability to allow Admins the flexibility of configuring the “Create Related Record” action on the “Create Record” Event Action. Learn more about the Related Record Setup enhancements.
UI Enhancement for Related Record Setup
This feature allows Admins to view a clickable list of related record components to easily access the details page of each component.
Support Related Record Creation during Creation of Source Record
This feature improves and supports Related Record Setup to be configured on the “Create Record” event action on the object lifecycle, allowing related record creations to trigger during the creation of the source record. Depending on configuration, users can also nest multiple levels of related record creation by triggering the initial “Create Related Record” event action. Vault then triggers the same action on the created related record to create another related record, with the initial related record as the source record now. Admins can nest the levels of related record creation up to a default depth limit of 5 levels.
Admin Menu for Related Record Setup
This feature allows Admins to access the Related Record Setup page from Admin by navigating to Configuration > Application Configuration > Component Setup section and selecting Related Record Setup from the list.
Admin Menu for Comment Setup
This feature allows Admins to access the Comment Setup page from Admin by navigating to Configuration > Application Configuration > Component Setup section and selecting Comment Setup from the list. Learn more about the Admin navigation.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on December 21st.
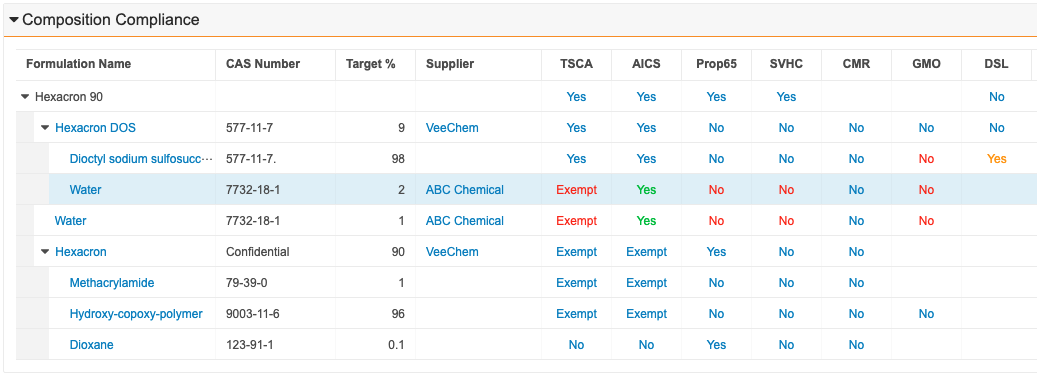
Formulation Composition Viewer Enhancements
A complex formulation can contain numerous constituent chemicals. It is important to quickly distinguish a single formulation’s attributes and identify any red flags when viewing that formulation in the Formulation Composition Viewer. This feature allows Admins to configure the viewer to display shading between the hierarchical levels as well as highlighting a row when users hover over specific records. Users can also identify constituent chemicals that don’t meet regulatory requirements by assigning a color to the compliance assessment status displayed in the viewer. Learn more about the Formulation Composition Viewer.
Veeva Claims
Veeva Claims features are targeted for tentative availability on December 21st.
Copied from Project Link
Claims users commonly copy Project records to initiate new projects. When copying projects, it is important to establish a link between the copied from record and copied to record to maintain traceability and maintain a reference so that users can refer back to the key details from the original project. This feature introduces a new read-only Project reference field as a part of the Project object which is auto-populated when a user copies a project. Learn more about the new field.
Auto-Populate Statement Translations for Local Adaptations
Local Adaptations for each country can have multiple associated Statement translations in country-specific languages. Veeva Claims stores Statement translations and semantic variations in a self-referencing Statement library. When configured, this feature eliminates the extra effort by users to search and assign Statement translations from the Statement library to each Local Adaptation record by automatically assigning Statement translations to a Local Adaptation record based on the Admin-configured country-language mapping. Learn more about configuring the Populate Statement Translations action.
Enablement Details
| Feature | Enablement | Application |
| Working with Documents | ||
| Auto-Populate Rendition PDF Metadata | Admin Checkbox | Platform |
| Required Overlays | Auto-on | Platform |
| Notifications on Replies to Notes | Auto-on | Platform |
| Copy Text from Viewer | Auto-on | Platform |
| Editable Bookmarks | Admin Checkbox | Platform |
| Google Drive Integration: Create Draft & Upload New Version | Admin Checkbox | Platform |
| Google Drive Integration: Upload to Placeholder | Admin Checkbox | Platform |
| Today Link for Date Fields on Doc Info Page | Auto-on | Platform |
| Vault Objects | ||
| Today Link for Date Fields on Object Record Detail Page | Auto-on | Platform |
| Lifecycle & Workflow | ||
| Verdict Information & Reporting on Removed Documents | Auto-on | Platform |
| Bound Version Create Draft | Auto-on | Platform |
| Remove eSignature from Documents | Configuration | Platform |
| Skip Document State Change in Object Lifecycle Entry Action | Configuration | Platform |
| Reporting & Dashboards | ||
| Export Report to Formatted Excel | Auto-on | Platform |
| Multi-Pass Formula Fields Enhancements | Auto-on | Platform |
| Configuration Management | ||
| Configuration Management: Migrating Document Tags between Vaults | Auto-on | Platform |
| Configuration Management: Comparison of XMLString Component Attributes when Deploying Packages | Auto-on | Platform |
| Vault Compare Report: Header Rows Frozen by Default | Auto-on | Platform |
| Vault Configuration and Compare Reports: Reporting Full MDL of Object Lifecycle State Entry Action Rules | Auto-on | Platform |
| Vault Configuration Report: Cover Page Title for Object Data Workbook | Auto-on | Platform |
| Vault Configuration and Compare Report: Details for Auto Managed Group Field Order Security Settings | Auto-on | Platform |
| Access Control | ||
| User Role Object Moving to HVO | Auto-on | Platform |
| Administration | ||
| Jobs: Display Start Time for Completed Jobs | Auto-on | Platform |
| Search | ||
| Multidirectional & Phrase Synonyms | Configuration | Platform |
| HVO Tabs: Search by Name from the Top Search Box | Auto-on | Platform |
| Usability & UI Updates | ||
| Action UI: Documents: Expose Drop-Down from Chevron for One Action | Auto-on | Platform |
| Vault File Manager | ||
| Download Rendition Files to Vault File Manager | Auto-on | Platform |
| Vault Loader | ||
| Vault Loader: Updating User & Group Role Assignments on Object Records | Auto-on | Platform |
| Platform Data Model Changes | Auto-on | Platform |
| Vault Connections | ||
| PromoMats/RIM: RIM Reference Model Support | Configuration | Promomats<>RIM |
| Clinical Operations | ||
| Milestone Gating for EDL Item Requiredness | Configuration |
CTMS, Clinical Operations - All, Study Startup, Vault Payments, eTMF
|
| Site Connect: Distribution Task Return Field Field Now Dedicated to Site | Auto-on |
Site Connect, SiteVault Enterprise, SiteVault Free
|
| Commercial | ||
| Document Notifications: Support for "Based On" Relationship | Configuration | PromoMats |
| Modular Content Approval Document Additional Lifecycle Support | Auto-on | PromoMats |
| Transparency Support in Automated Image Renditions | Configuration |
MedComms, PromoMats
|
| Medical Inquiry: CRM Inquiry Pull Job Timing Enhancement | Auto-on | MedComms |
| Quality | ||
| Recurrence Check: Support for MedTech Complaints | Configuration | QMS |
| Generate Document from Report: Support for Record Check Lifecycle | Configuration | QMS |
| Recurrence Check: Display Match Criteria on User Dialog | Auto-on | QMS |
| Quizzes: Question & Answer Randomization | Configuration | Training |
| China Link E-Learning Support | Auto-on | Training |
| Direct Assignment: Hide Inactive or Ineligible Persons in the Learners Field | Auto-on | Training |
| Email Participant Action Available for Training Assignment | Auto-on | Training |
| Issue Training Assignment Entry Action Update | Auto-on | Training |
| Training Requirement Impact Assessment (TRIA) for All Training Requirements | Configuration | Training |
| QRM: Template Based Risk Evaluation | Configuration | QMS |
| QRM: Promote the Assessment Risk Event to Risk Event in the Register | Configuration | Quality |
| Generate Document from Object Record Action: QRM Objects | Configuration | QMS |
| QRM Data Model Updates | Auto-on | Quality |
| Quality Data Model Changes | Auto-on |
QualityDocs, Training, QMS
|
| Regulatory | ||
| Capture Active Dossier Submitted Status | Auto-on | RIM |
| Restrict Submissions Archive Access to Full User Licensed Vault Users | Auto-on |
RIM Submissions Archive
|
| Content Plan Creation: Novel Excipient Update | Auto-on | RIM Submissions |
| Display Inactive Applications & Submissions in Viewer | Admin Checkbox | RIM Submissions Archive |
| Extend Matched Document Tokens for Content Plans | Auto-on | RIM Submissions |
| Translation Document Relationship Flag | Admin Checkbox | RIM |
| 22R1 RIM Data Model Changes | Auto-on |
RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive
|
| SiteVault | ||
| Study Export | Auto-on |
SiteVault Enterprise, SiteVault Free
|
| QualityOne | ||
| Linking Quality & HSE Events to Risk Register | Configuration | QualityOne |
| COA Ingestion Enhancements | Auto-on | QualityOne |
| Multi-Page Header Matching Strategy Support | Auto-on | QualityOne |
| Processing COA Files with Table Breaks | Auto-on | QualityOne |
| Automatic Detection of COA Date Formats | Auto-on | QualityOne |
| Lenient Expected Value Formats for Dates | Auto-on | QualityOne |
| Improved COA Analysis Job Logging | Auto-on | QualityOne |
| Improved COA Table Analysis | Auto-on | QualityOne |
| Related Record Setup Enhancements | Auto-on | QualityOne |
| UI Enhancement for Related Record Setup | Auto-on | QualityOne |
| Support Related Record Creation during Creation of Source Record | Auto-on | QualityOne |
| Admin Menu for Related Record Setup | Auto-on | QualityOne |
| Admin Menu for Comment Setup | Auto-on | QualityOne |
| RegulatoryOne | ||
| Formulation Composition Viewer Enhancements | Configuration |
RegulatoryOne Compliance Management
|
| Veeva Claims | ||
| Copied from Project Link | Auto-on | Veeva Claims |
| Auto-Populate Statement Translations for Local Adaptations | Configuration | Veeva Claims |
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some "Auto-On" features have a checkbox setting that hides the feature; these will show "Auto-On." | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|