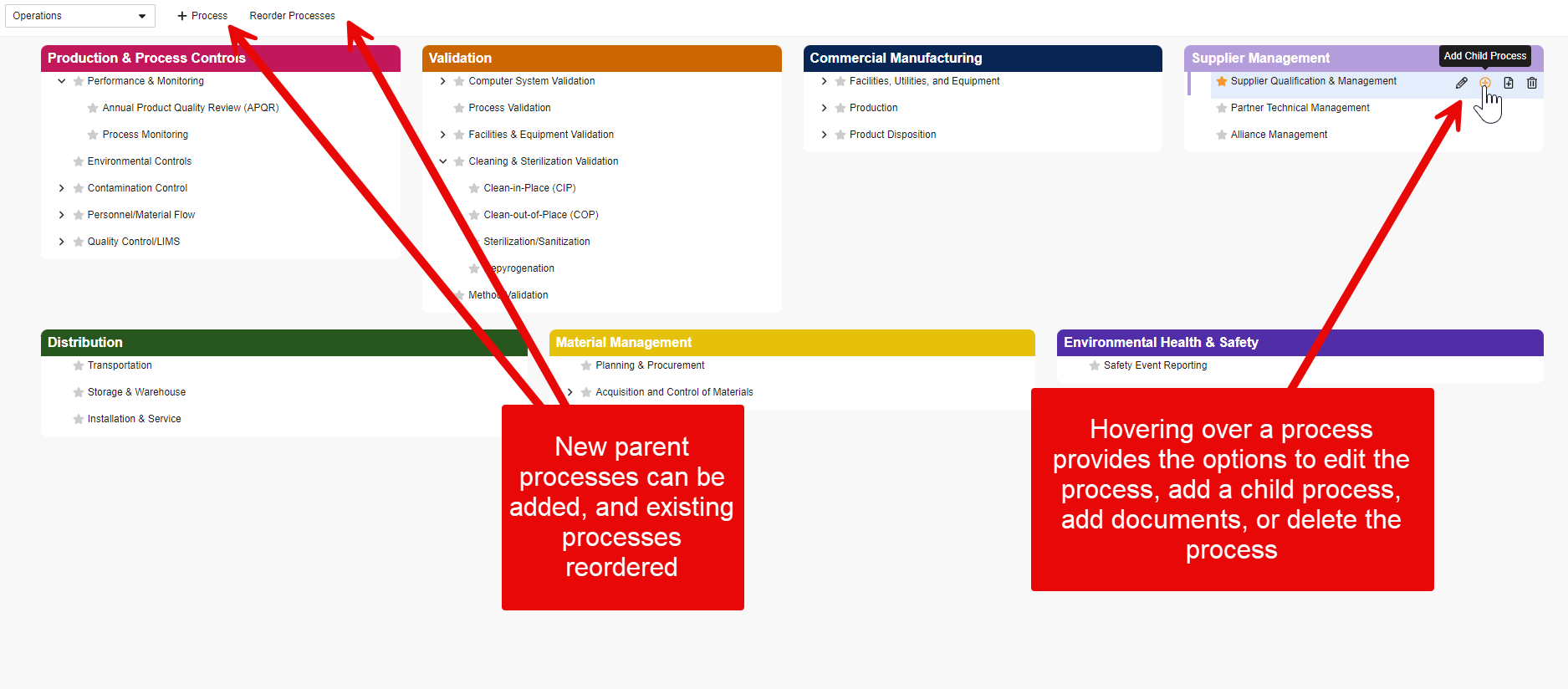
Limited Release Dates: May 3, 2024 Postponed to May 9, 2024 (24R1.2); June 7, 2024 (24R1.3); June 28, 2024 (24R1.4) | General Release Date: August 9, 2024
The following applications may have different release dates: Safety, RegulatoryOne, and Veeva Claims.
We are pleased to bring you new functionality with each limited release. These release notes are updated with upcoming new features one week before the limited release date. See the following explanations for enablement options:
- Auto-on: Automatically activated and no configuration is required before using the feature; in some cases, a new feature is dependent on another feature that must be enabled or configured.
- Admin Checkbox: Admins must turn on the feature with an Admin checkbox. Some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.”
- Configuration: Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates.
- Support: On/off option controlled by Support.
- Available for Use: Used only by the eConsent, eCOA, and SiteVault applications. Sponsors must make a study-specific configuration change to implement new capabilities.
Platform
Documents
VeevaID Login Support for Vault File ManagerAuto-on24R1.2
VeevaID users will now be able to log in to Vault File Manager to perform typical functions such as check-out, download, and check-in to support document editing.
VeevaID is an Identity Provider (IdP) system for Veeva Applications (such as Study Portal, SiteVault Free, Vault Training). VeevaID allows end-users (Clinical Site Staff) to self-register, access applications they are subscribed to, and maintain their account.
Learn more about Veeva ID.
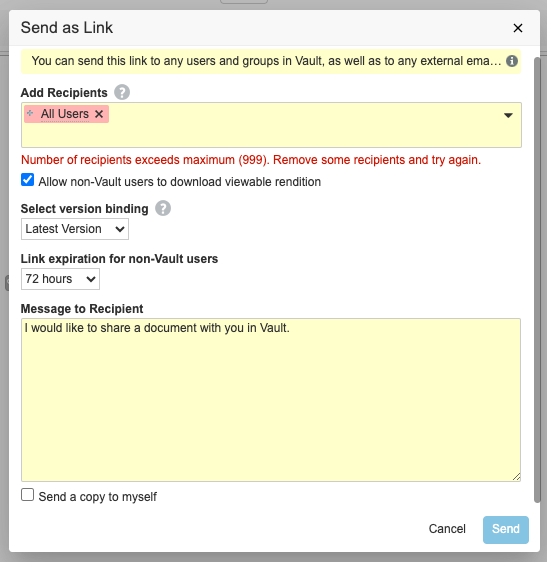
Send as Link: Limit Number of RecipientsAuto-on24R1.2
When using the Send as Link function, Vault will now limit the number of recipients in a single action to fewer than 1,000. This count is based on the total number of recipients across users, groups and external email addresses that are added. When calculating the number of targeted recipients, any individual user only counts once. For instance, if a user is targeted directly by email address, and again by membership in a targeted group, that user only counts once against the maximum threshold.
This enhancement prevents users from accidentally sending a document to a large group of recipients, which can happen if a user selects a group such as All Internal Users. This also prevents potential performance impact to email notifications being sent from Send as Link actions (for instance, if Vault needs to send over 1,000 email notifications, that can create a delay due to the volume).
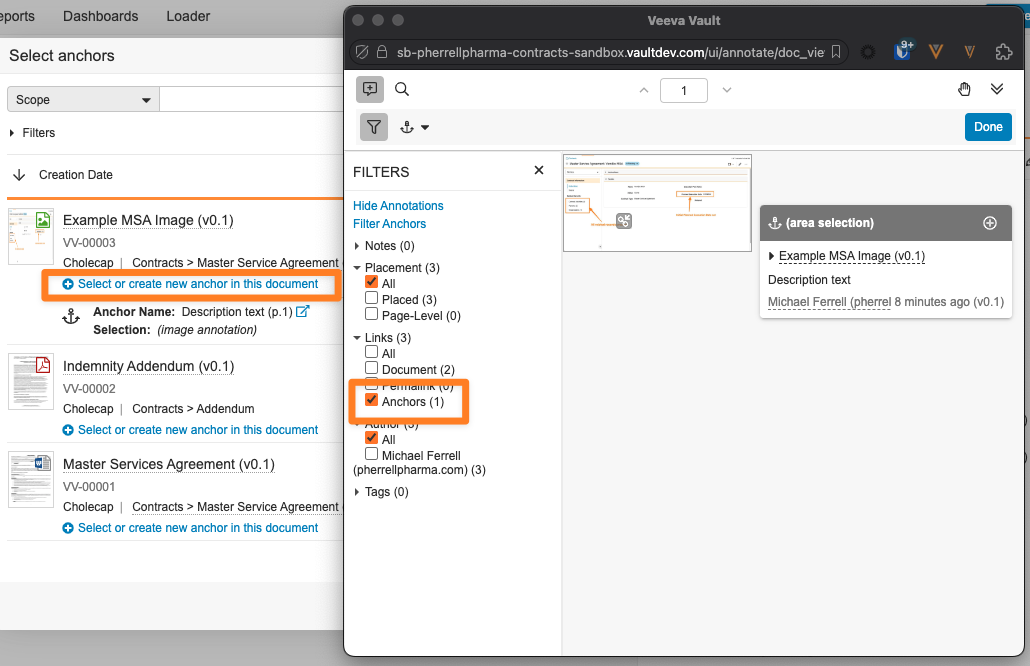
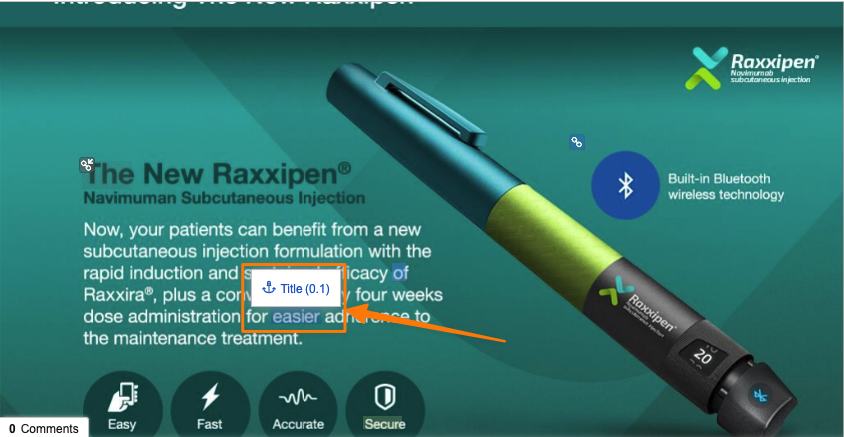
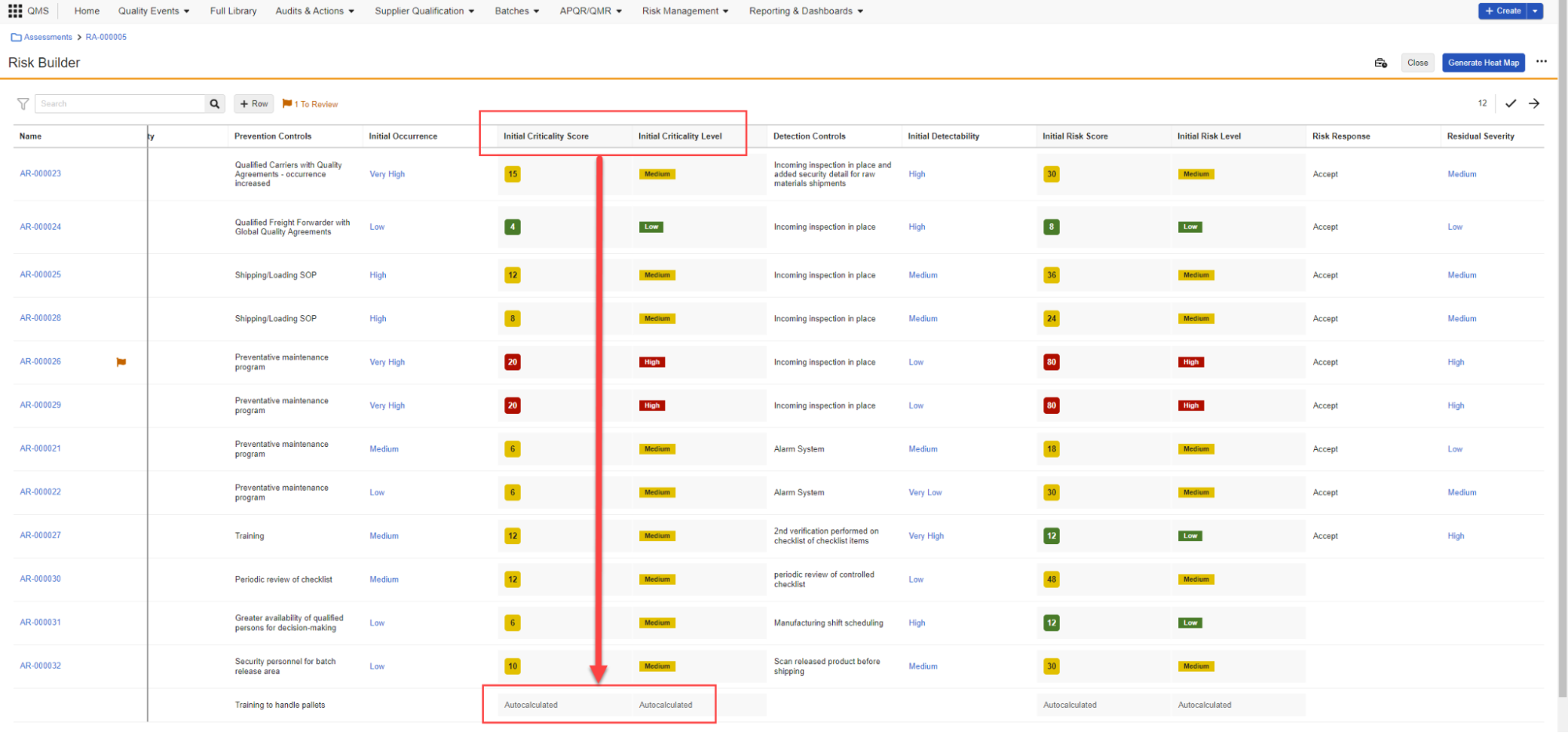
Select Anchor & Permalink ImprovementsAuto-on24R1.2
When leveraging the Select or create new anchor option in the Select anchors dialog, the mini-browser that opens will now automatically filter only on anchors to allow users to more easily find and select the correct anchor for their document link.
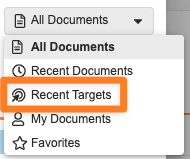
Additionally, when creating a permalink link annotation, the Select target dialog will now include a Recent Targets view, with an option to sort by Selection Date, allowing users to more easily reuse permalink targets:
Vault will now also remember the last view that a user used in the Select target dialog and apply that the next time they perform an action to create a permalink link annotation.
Learn more about Using Link Annotations & Document Links.
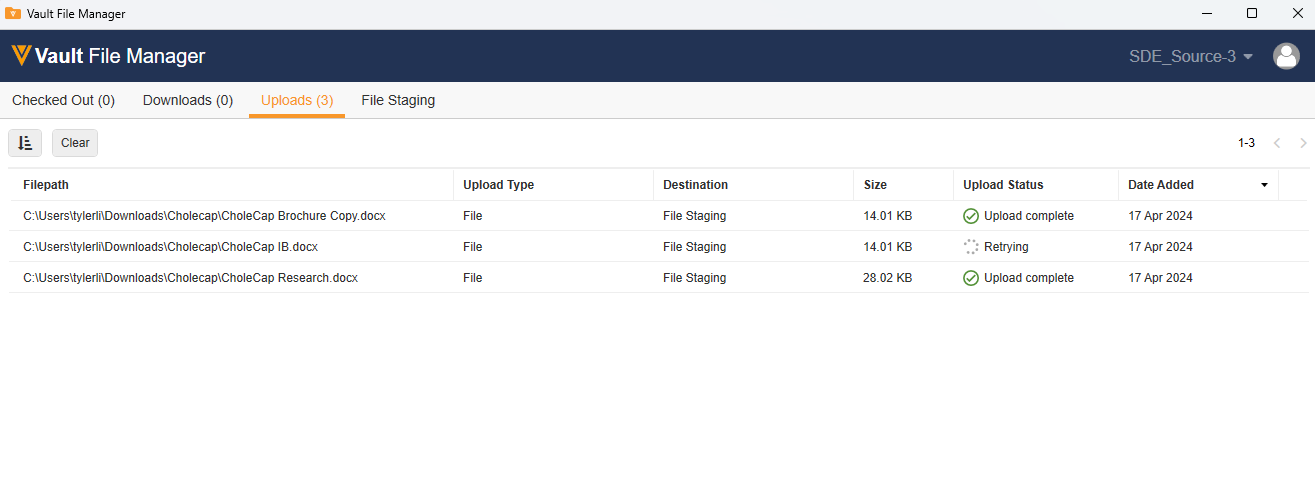
Improved Directory Upload to File Staging in Vault File ManagerAuto-on24R1.2
When uploading a single directory or multiple directories to the File Staging tab within Vault File Manager, Vault File Manager now shows the upload progress of each file within the directories. If there is an error uploading a file in the directory, users will now be able to easily identify which file had an error and what the error was.
With this change, all files within the directory will be shown as a separate line. Each line notes the full file path as well as the Upload Status, Dated Added, Upload Type, Size, and Destination.
Learn more about Vault File Manager.
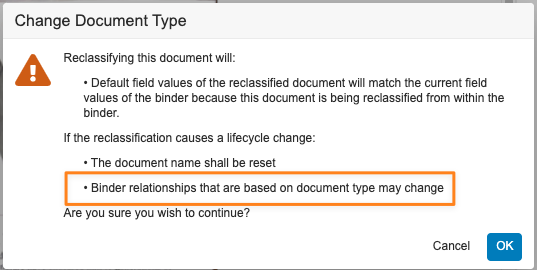
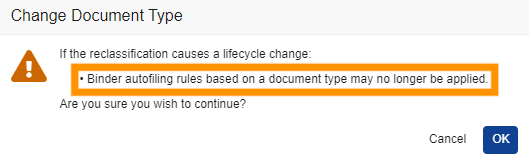
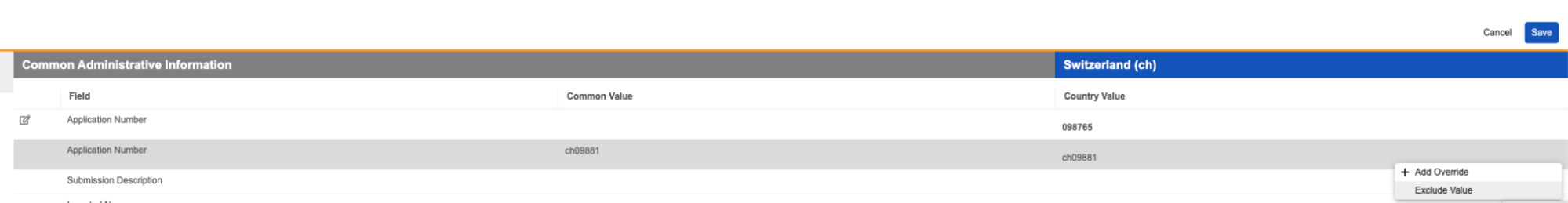
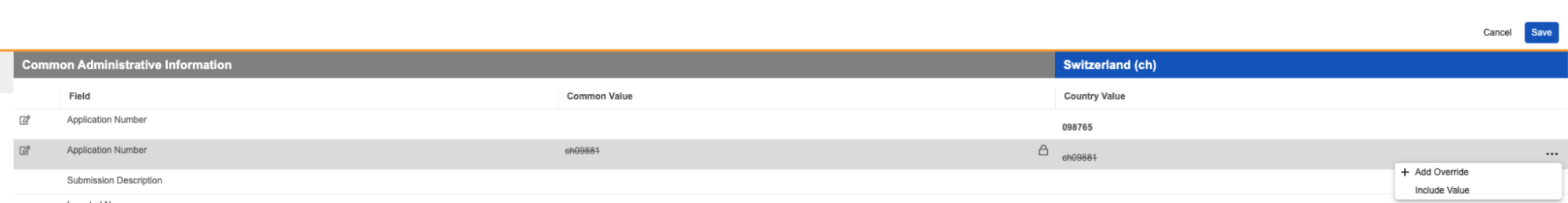
Clearer Reclassification WarningAuto-on24R1.2
When reclassifying documents that are matched to binders via autofiling, Vault will now display a clearer message to users indicating that the binder relationship may change: “Binder autofiling rules based on a document type may no longer be applied.” Prior to 24R2, the message Vault displayed did not clearly indicate the warning is based on autofiling rules that are based on document types.
Before 24R1, the warning would show as:
After 24R1, the warning will show as:
Learn more about Reclassifying Documents.
Clearer Error Handling when Reclassifying Documents Under Legal HoldAuto-on24R1.2
If a user is viewing a document while another user places that document under legal hold, Vault continues to display the Reclassify link until the page is refreshed. If the user attempts to click the Reclassify link, Vault will prevent the reclassification per legal hold restrictions.
With 24R2, this behavior will not change, but Vault will now also provide a message to the user making clear to them why the reclassification is not allowed: “Cannot reclassify document : Document is associated with one or more approved Legal Holds.”
Learn more about Applying & Removing Legal Holds.
Configuration Migration: Remove Document Type Filter WarningAuto-on24R1.2
Prior to 24R2, when deploying document configuration using configuration migration packages, Admins received a warning if the package contained a document type with a filter and the filter wasn’t applied to its subtypes or classifications. This warning was not actionable, so with 24R2, this warning is no longer displayed.
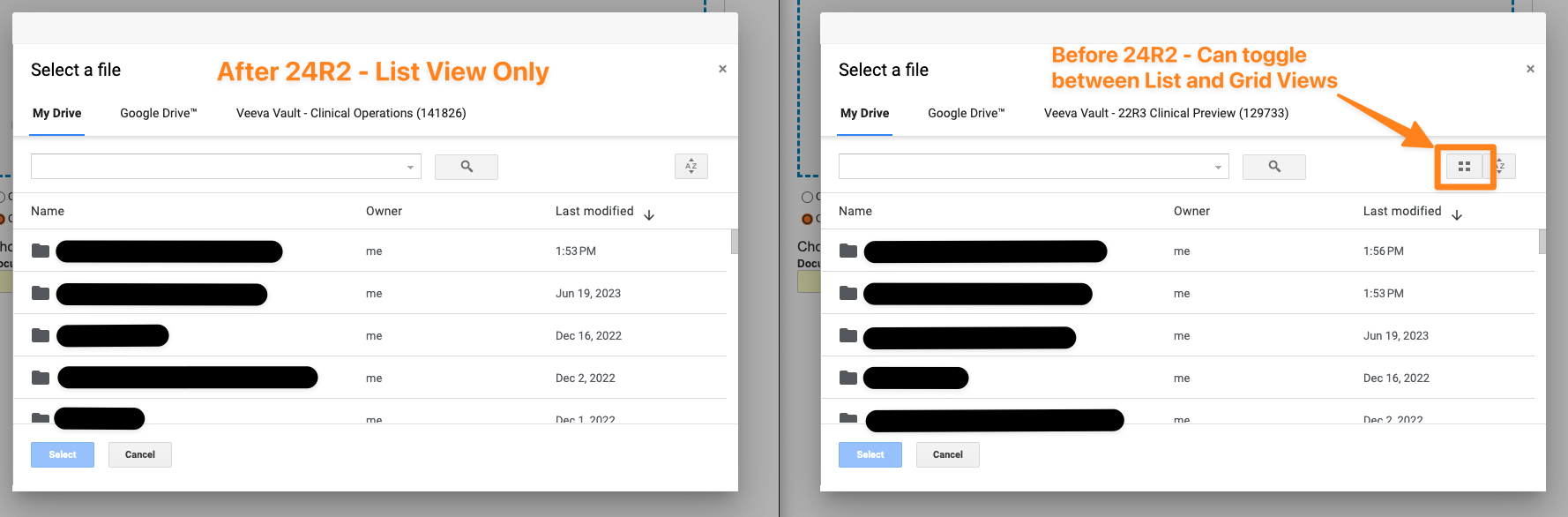
Google Drive Integration: List View Only in File DialogAuto-on24R1.2
For customers leveraging the standard Google Drive Integration, when adding files to Vault from Google Drive, users will no longer be able to switch between List and Grid views within the Google Drive dialog:
This change is being made based on changes to the API between Vault and Google, but users will still be able to access, search, and sort within the Google Drive dialog.
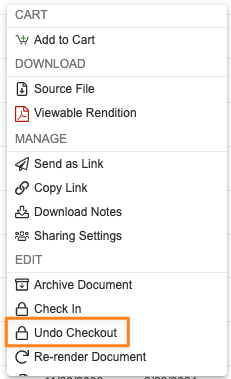
Standardized Undo Checkout PermissionsAuto-on24R1.2
Vault supports several different checkout methods for editing documents - standard checkout, checkout to Vault File Manager, Collaborative Authoring, and using Google Drive. Each method provides a mechanism to cancel/undo an existing checkout. Going forward, the permissions governing when cancellation can be performed of another’s user’s checked out document will be consistent across all checkout types.
In order for a user to cancel/undo a checkout that another user owns, users will need to have Edit Document permission in the document lifecycle state security settings, as well as one of the following:
-
Have the Document Owner role for the document
-
Have the All Document Actions permission in their Permission Set
-
Have the Cancel Checkout permission in their Permission Set
Ensuring that the same permissions apply across all checkout types will reduce confusion and make it easier to manage permissions and permission changes.
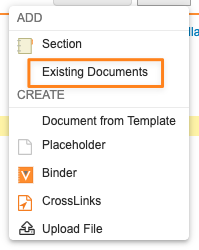
Error Message When Adding a Duplicate Document to a Binder SectionAuto-on24R1.2
When a user attempts to add a document to a binder section and an existing version of that document is already bound to the binder section, using the Add Existing Documents function, Vault now displays a clear message to users letting them know that the document cannot be linked twice in a single binder section.
Prior to 24R2, Vault already prevented users from adding documents to a binder section more than once, but no message was provided to users. Including this message will reduce confusion for users so they know why the document was not linked again.
Learn more about Editing Binders.
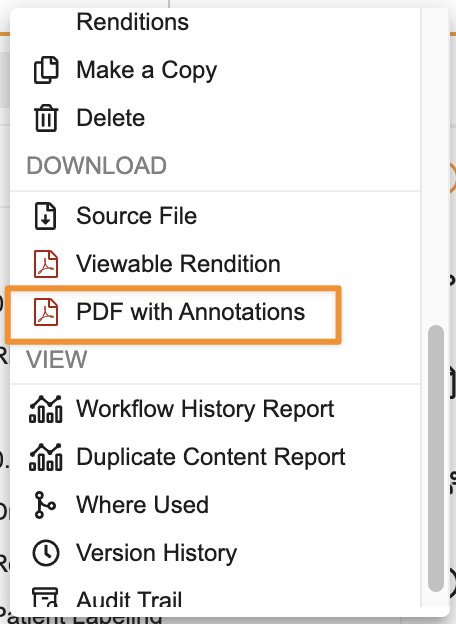
Viewer & Annotations Usability ImprovementsAuto-on24R1.3
A number of usability improvements have been added to the document viewer, including:
-
All file types will fit-to-width by default in the doc viewer
-
More responsive video player resizing
-
Download PDF with Annotations is now available in the Actions menu (in addition to the file download dropdown)
-
Keyboard Shortcut to save annotation (CTRL+Enter / CMD+Enter)
-
Tooltip to navigate Link annotations when in View mode with Grab unselected
- Tooltip to navigate Link annotations also appears when text is selected and the link is on that text
-
Better visual differentiation of Approved versus Auto Link annotation types:
- Auto Link
- Approved Link
-
When expanding the document information panel, the document viewer toolbar options will more dynamically respond to the reduced viewer size by moving options into the dropdown:
-
Mini-Browser default size increased to 1000px
-
Mini-Browser now has a way to traverse through annotations
Learn more about the Doc Info Page.
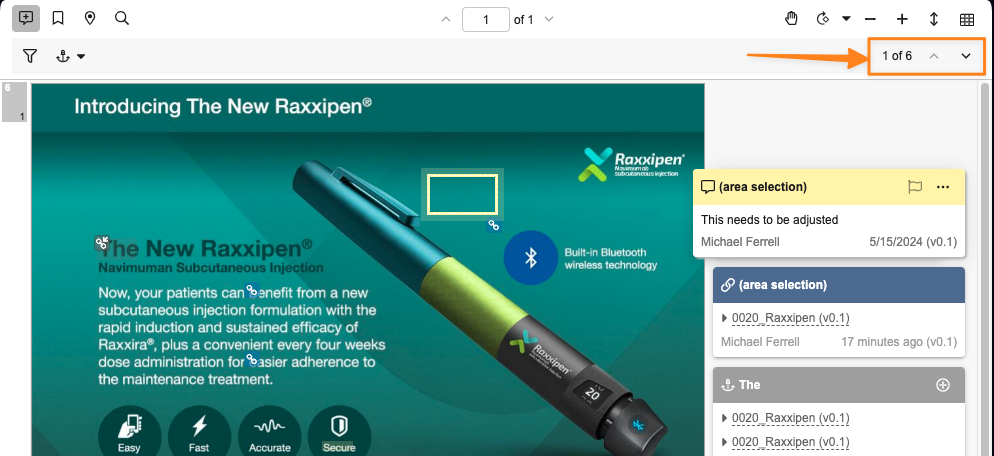
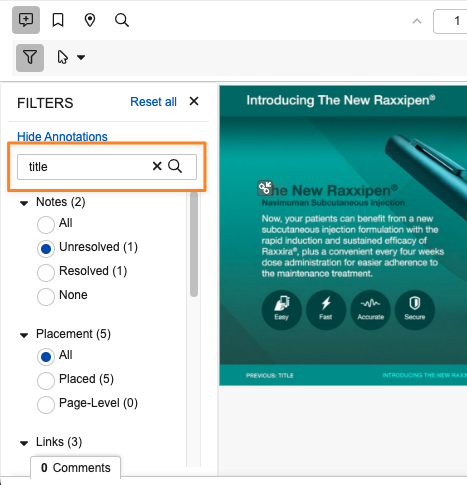
Filter Annotations By Keyword and New vs Brought-ForwardAuto-on24R1.3
Along with the new filtered annotation export abilities, you can now also filter annotations by keywords, or by whether they are new to this document version or brought forward from a previous version. In addition, there is a new Reset all option to clear all selected filters.
New filtering options, by keyword search, or by ‘new to this version’ vs ‘brought forward’:
Filter by Text searches for text (up to 100 characters) in the annotation title, comment and replies, and hides annotations that do not contain that text.
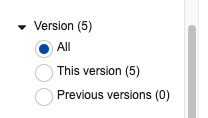
Filter by Version differentiates annotations based on the version in which they were created. This is helpful for users who need to focus on brought forward annotations (for instance, to make sure their placement is correct), or on new annotations (to focus just on annotations that have not already been seen on prior versions).
Reset all clears all filters to show all annotations. This also updates the current user’s saved preferences in the filter panel.
Allow Fields & Relationships to Be Copied for Large DocumentsAuto-on24R1.3
If a document contains source files over 4 GB, the Make a Copy action was not available prior to 24R2. Now users can make copies of these documents, but Vault will only copy the fields and relationships, not the large source file.
This allows customers to retain the Based On relationship when, for example, localizing a global material. In this scenario, the process should be to download the large size asset from the global document, create the localized version of it, then upload that file to the copied document in Vault. This avoids wasting a very large first version of the document that isn’t needed for the localized document.
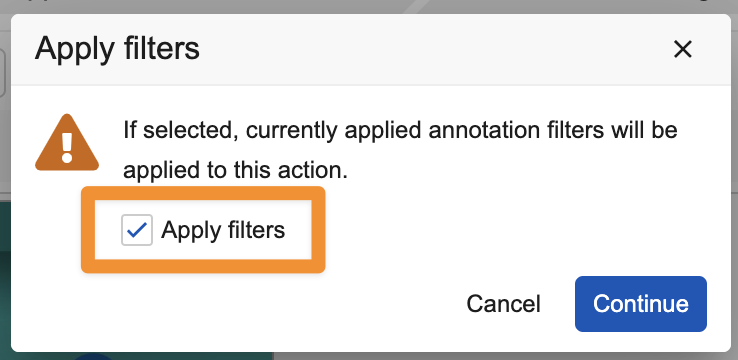
Apply Filters to Annotation ExportsAuto-on24R1.3
This feature introduces a new Apply filters option when exporting annotations from a document using Export Annotations, Download Notes, or downloading the PDF With Annotations file, allowing users to apply the same annotation filters that they have applied on the Doc Info page. This option does not appear if no filters have been applied. This is helpful when you want to send a file back to the creative agency, for example, with only unresolved comments for them to address. You could also use the filters to only download comments related to particular users, exclude links, or only include those that have been tagged in a particular way.
Apply filters is deselected by default when the dialog opens, and if all annotations are hidden by filters, this option is not shown at all, and all annotations are included in the export. When downloading the PDF with Annotations file, if filters are applied, the export will use those filters. If no filters are applied, the existing option for users to choose whether to exclude resolved annotations will be displayed.
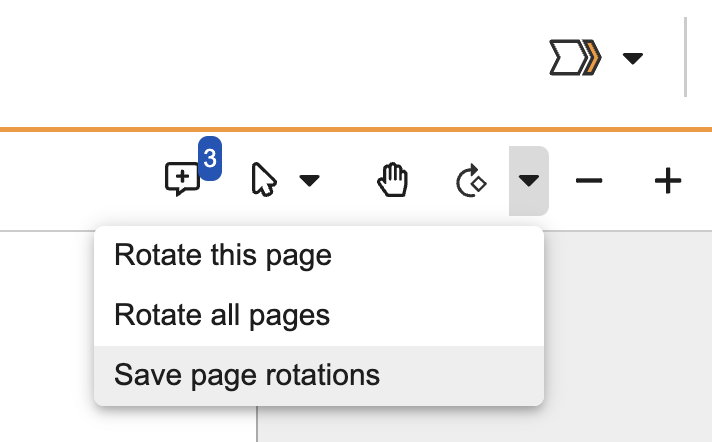
Saved Page RotationAuto-on24R1.3
After rotating pages in the document viewer, users with the appropriate permission can save that rotation. This applies to any user when viewing the document or downloading the Viewable Rendition, but it does not modify the source file.
Prior to 24R2, to permanently fix a page that has the incorrect orientation, users would need to correct the issue in the source file and upload a new version. Rotating in the document viewer only applied rotations based on the specific user who performed the rotate action and the rotation would reset once the user navigated away from the document.
This enhancement allows for simple changes, such as correcting orientation issues, to be performed right in Vault and persisted for other users and the viewable rendition going forward.
To save any rotations applied to a document, there will be a new Save Page Rotations option in the dropdown menu on the Rotate this page button. Vault will also warn users if they have applied rotations and are attempting to navigate away without saving.
Users must have the Manage Viewable Rendition permission in their document lifecycle role security settings or have a security profile that grants the Vault Owner Actions: Re-render permission in order to save page rotations.
Learn more about Rotate in the document viewer.
EDL Item Batch Matching Job Limit
The Batch Matching functionality for EDL Items will now limit the number of documents that can match to a single EDL Item to 3,000 documents. This enhancement helps improve Vault performance.
With EDL Item matching, there are several options for matching documents: Batch Matching (using the Match EDL Items to Documents job), Continuous Matching, and Manual Matching. Prior to 24R2, there has already been a limit of 1,000 documents per EDL Item for both Continuous Matching.
The limit for Batch Matching is higher to account for the Include Previously Matched Documents Field option in RIM. If that option is disabled, Vault will prevent multiple Submissions under the same Application from including duplicate documents - but the Batch Matching job performs the deduplication after initial matching so having the limit be set to 3,000 provides flexibility for that use case.
This feature was deferred to a future release.
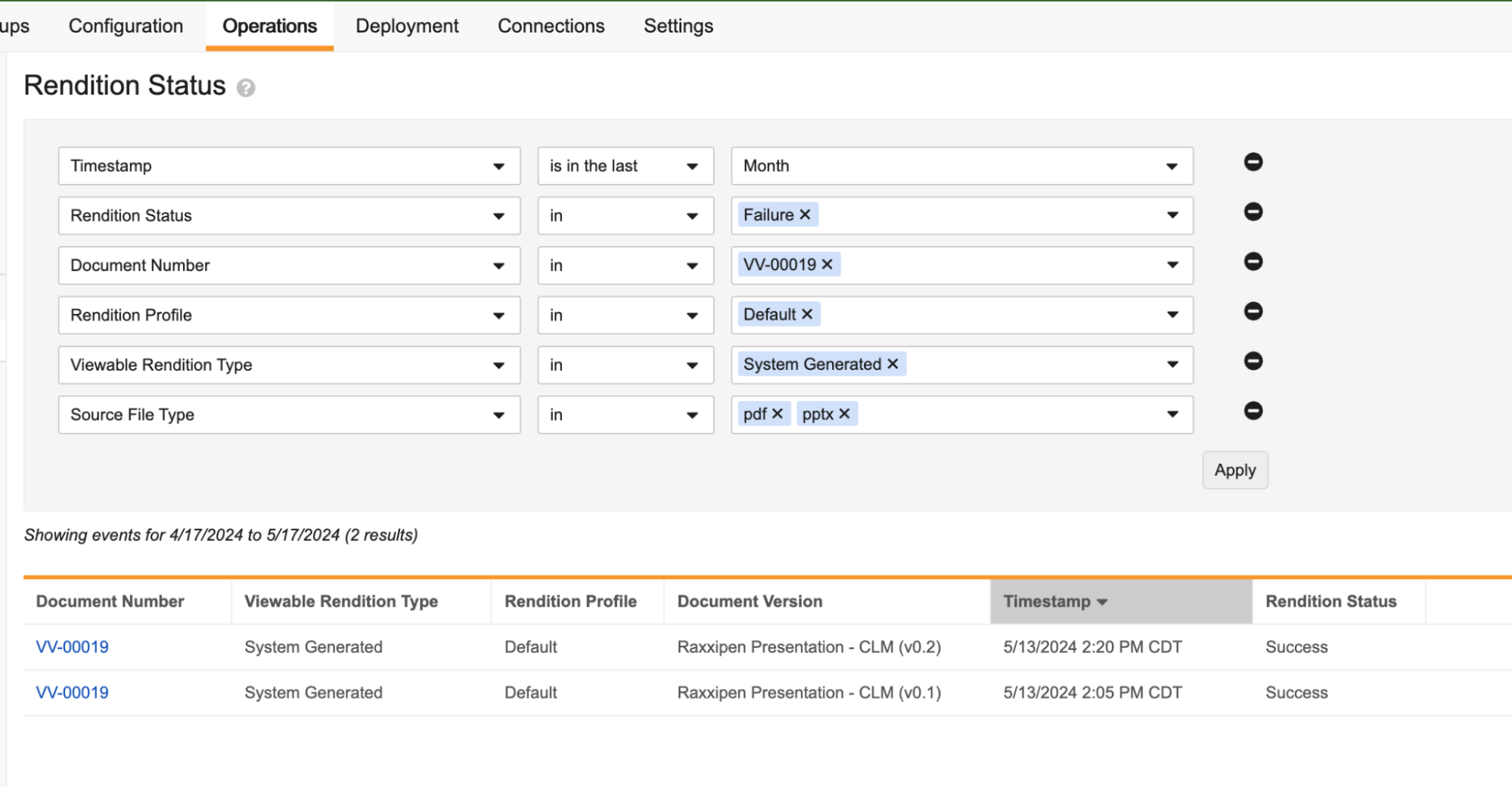
Improved Usability of Rendition Status for AdminsAuto-on24R1.3
With 24R2, additional fields, including Rendition Profile and Viewable Rendition Type (System Generated or Manual), are now available to add as columns on the Admin > Operations > Rendition Status page. In addition, all fields are now available as filters at the top of the page.
Document Logos in Branding SettingsAuto-on24R1.3
From the Vault Branding Settings page, Admins can upload a Primary and Secondary document logo.
With 24R2, these options will be used in conjunction with the Print Record feature, and in a future release, these will be able to be leveraged in Overlay and Signature Page templates. This allows customers to more easily customize their outputs with the organization logo, without needing to manually add the logo into documents/records.
Improved OCR for ImagesAuto-on24R1.3
Vault’s Optical Character Recognition (OCR) functionality has been improved to better detect text from images, resulting in more recognized text that can be used with other Vault features. This includes search, annotations, Auto-Linking, and more.
In 24R1, this enhancement was introduced for Commercial Vaults, and with 24R2, this enhancement now applies to all Vaults.
Improving OCR for these image files will ensure a more consistent user experience when leveraging features that rely upon recognized text.
Learn more about Optical Character Recognition.
Enforce Edit Sharing Settings Permission in Latest Version for Send as LinkAuto-on24R1.3
Vault will now require the Edit Sharing Settings permission on the latest document version in order to add users to the Viewer role via Send as Link. This will apply regardless of the Version Binding selected by the user in the Send as Link dialog.
Prior to 24R2, when sending a link and adding users to the Viewer role, the Edit Sharing Settings permission was only required on the specific version being sent, though the Viewer role is added to all versions.
This enhancement ensures consistency in a user’s ability to update the Sharing Settings using Send as Link, and matches existing behavior in Vault. For instance, a user can only modify Sharing Settings on a document manually when they have Edit Sharing Setting permissions on the latest version.
Optimized Renditions for PDF Merge Use CasesAuto-on24R1.4
Vault optimizes downloadable renditions in Content Plan Section and Content Plan Item Merge, and other features where multiple PDFs are merged into one. It will now eliminate duplicate fonts, images, and other elements, producing a smaller PDF file.
Lifecycle & Workflow
New Workflows Deployed as ActiveAuto-on24R1.2
To reduce the number of steps required when migrating workflow configurations from Sandbox to Production, Vault will now deploy new workflows as Active if all the configuration is valid. Prior to this change, only updates to existing workflows would result in the workflow becoming active, and all new workflows would deploy in the Inactive status.
Jobs Can Be Migrated as ActiveAuto-on24R1.2
To eliminate the need to manual activate jobs that have been migrated from Sandbox to Production, Vault will now deploy jobs as Active if they are Active in the Sandbox and all the configuration is valid. Prior to this change, all jobs regardless of whether they are Active or not would deploy in the Inactive status.
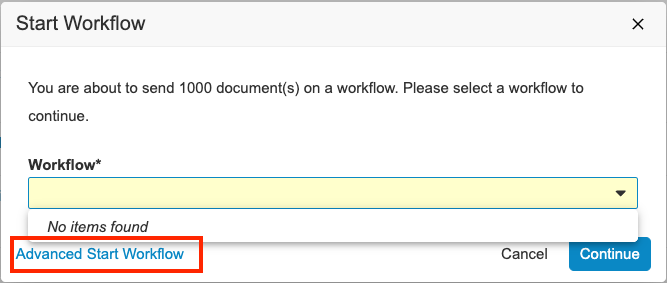
Advanced Start Workflow Available from Document ReportAuto-on24R1.2
When using the Start Workflow action from the results of a document report, we have now included the new Advanced Start Workflow link. From there, you can refine your selection (if you don’t want to send all the documents in the results on the workflow), select your workflow, and even loop back through those documents multiple times using the Finish + Start Next Workflow button. This is particularly useful if you’re executing ‘taskless’ workflows on the documents; using workflows as a way to perform a series of configured actions.
Start Multi-Record Workflows from Object ReportsAuto-on24R1.2
Object reports are often designed to return a very specific set of object records that require action, often via one or more workflows. Directly from the results of an object report you can now execute a multi-record workflow, including the Advanced Start Workflow option that allows you to refine your selection before initiating one of the available workflows.
Limit Workflow Task Participants Setting Extended to Add ParticipantsAuto-on24R1.3
The Limit Workflow Task Participants setting (Admin > General Settings > Workflow) that is enforced when starting a workflow is now also enforced when using the Add Participants action. This feature is particularly helpful when Admins need to prevent users from accidentally selecting large user groups, which could result in needing to cancel a lot of individual tasks.
Learn more about limiting workflow task participants.
Gray Status Indicator for Workflows & Tasks Without a Due DateAuto-on24R1.3
Prior to this release, if a workflow task, active workflow, or user task did not have a due date set, Vault displayed the same green status indication shown when the due date is over five (5) days away:
This could be misleading when prioritizing tasks. With this release, tasks with no due date show a gray status indicator instead:
Learn more about status indicators.
Default to Advanced Start Workflow When There Are No Workflows Matching All ItemsAuto-on24R1.3
When using the Start Workflow action from a list of documents or records to initiate a multi-item workflow, the available workflows for some items may not match those available for others (according to lifecycle state or other configured user action conditions). This results in the Start Workflow menu containing no items and requires using the Advanced Start Workflow link instead:
In 24R2, if Vault detects that there are no matching workflows for all selected items, it automatically redirects to the Advanced Start Workflow page instead. This flow provides much more flexibility and options when trying to start workflows, including a first step to refine your selection.
Read more about Advanced Start Workflow for object workflows and for document workflows.
Objects
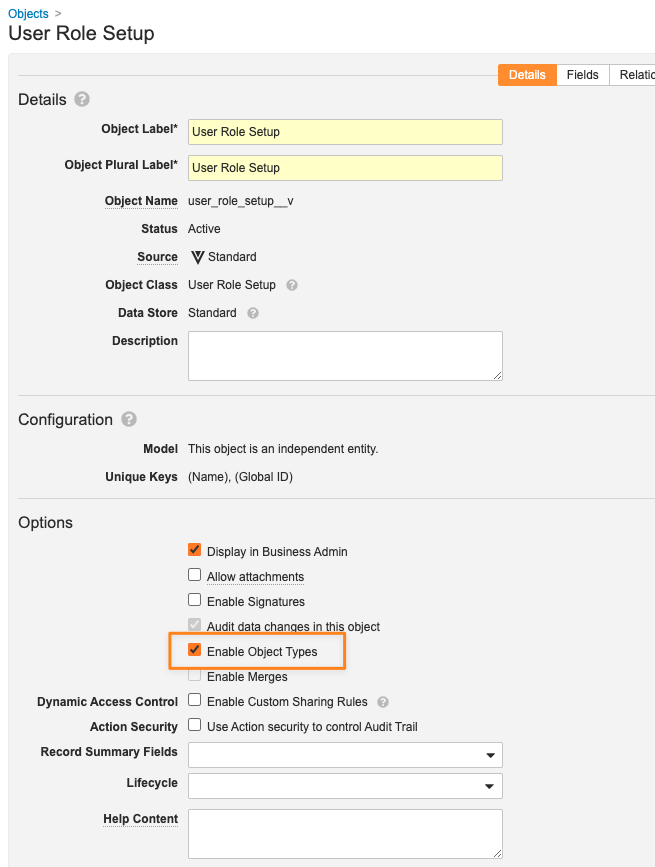
Matching Field Limit Increase Applied to User Role Setup Object TypesAuto-on24R1.2
In 24R2, the maximum number of custom matching fields on User Role Setup objects was increased from five (5) to six (6). For RIM Vaults specifically, where Vault allows for multiple Object Types on the standard User Role Setup object, the limit remains as five (5) per object type.
With 24R2, customers will now be able to add up to six (6) custom matching fields per User Role Setup Object Type.
Learn more about User Role Setup Object Types.
Object Type TranslationConfiguration24R1.2
To better support users in Vaults with multilingual labels enabled, Object Type labels can now be translated. Users who view Vault in a language other than the base language will now see translated object type labels where available in the Vault UI, including record detail pages, reports, and formula fields. If no translation is available in the user’s language, Vault displays the label in the base language.
Admins who can edit localized labels for a single language will now see additional fields for localized Label and Plural Label when viewing object types.
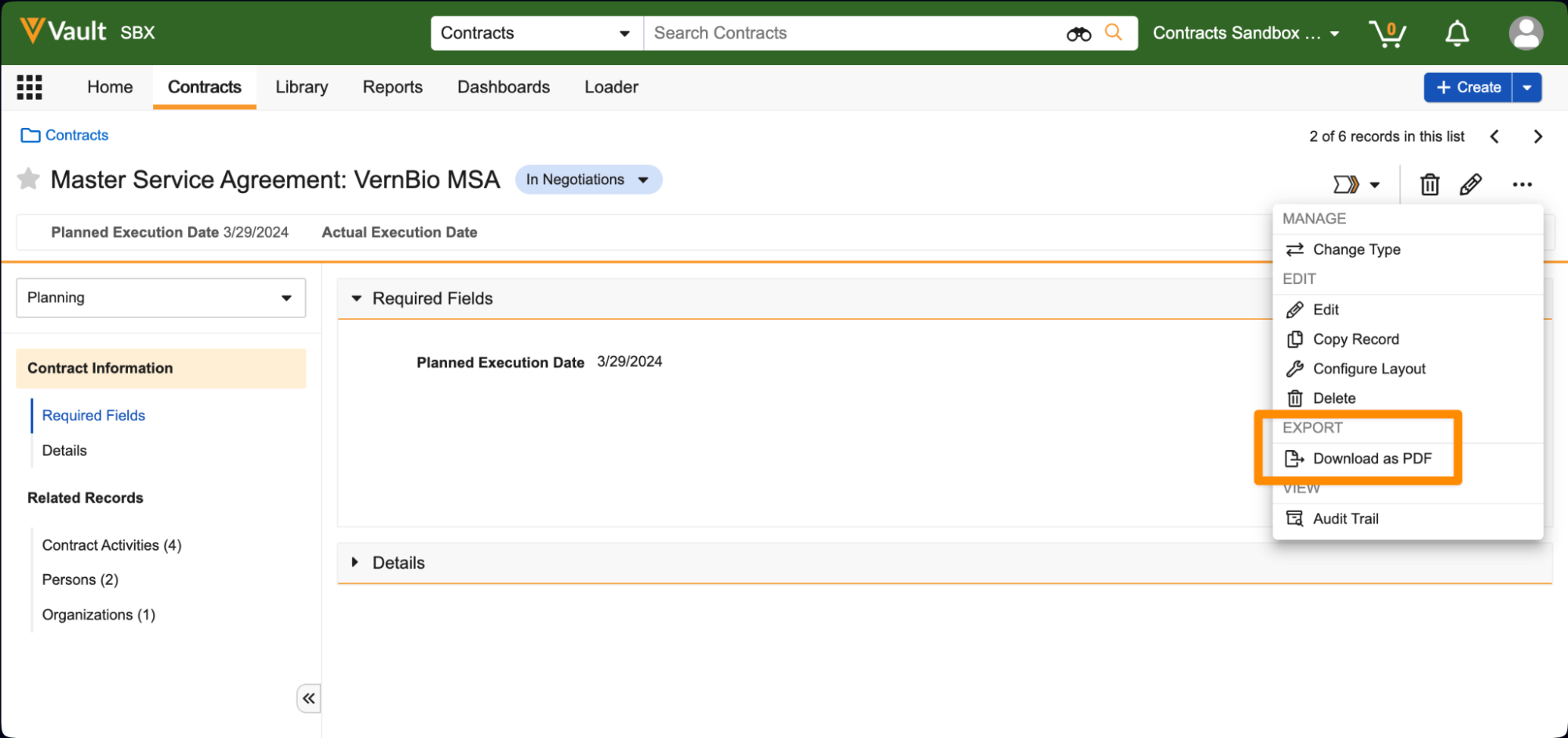
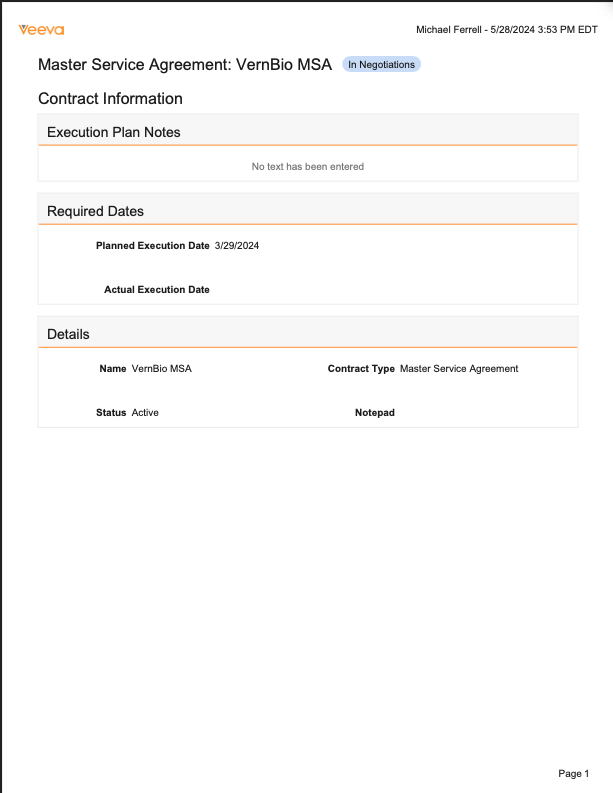
Print RecordAuto-on24R1.3
Users can now leverage a standard Download as PDF action on object records to generate a downloadable PDF based on the current layout that the user sees for that record.
Prior to 24R2, the only way to have downloadable PDF versions of object records was to configure Formatted Outputs. This enhancement provides a simple, configuration-free option for users to generate user-friendly PDFs that will be formatted based on the currently selected layout for that record. If multiple layouts are available for that record, users can switch layouts to change how the downloaded PDF will appear.
Some layouts may have application-specific UI overrides for a field or section that may not print to PDF. Those are represented by a Ø in the PDF.This feature is auto-on for all Vaults except Quality and Safety Vaults, and is available on all objects, though Admins can disable this via a checkbox called Enable Download PDF in Admin > Settings > General Settings > User Interface Options.Of note, we are also introducing another feature called Document Logos in Branding Settings, which will allow customers to define a custom logo to use in the Print Record outputs.
Yes/No Checkbox Field Enhancement
Yes/No fields displayed as checkboxes will now allow null values. Null values display separately from false values so that users can tell them apart. Prior to this release, checkboxes were not allowed to have null values, resulting in Vault updating some or all records in the object when adding a checkbox. The original behavior is discontinued as of this release.
This feature was deferred to a future release.
Allow Currency ISO Picklist Status To Be EditableConfiguration24R1.3
This feature adds six (6) new values to the standard Currency ISO picklist, which is used in the Abbreviation field of the Currency object. In addition, Admins can now change the status of values in this picklist. Prior to this release, Admins could not change the status, so it was not possible to hide these options from end users.
Object Field DescriptionsConfiguration24R1.3
In addition to adding help text to Object Fields for users to see when they hover over the field, Admins can now also add descriptions that are visible when configuring the object in Admin UI only. Object Field Descriptions are not exposed to users, but are used as a way for Admin users to understand the purpose of an object and how it should be used.
Visual Checklist Designer EnhancementsAuto-on24R1.3
The Visual Checklist Designer is a great user experience that accurately represents how a checklist will appear to the respondent user. With all the recent enhancements to this intuitive interface, Vault now directs users to the Visual Checklist Designer by default when designing checklists. You can still return to the object detail page view from the Actions menu if you need to update a field on the Checklist Design record, such as the name of the record, enabling or disabling ‘weightings’, or configuring it as an aggregate checklist, or when you need to proceed the Checklist Design record through a workflow for review and approval.
The only scenario where Vault will not default to the Visual Checklist Designer page is if the Checklist Design record is configured as an aggregate checklist since the Visual Checklist Designer does not yet support managing Sub-Checklist Design records.
For users with only Read access to the underlying Checklist Design objects, when accessing a Checklist Design, it will now appear as a View Only page. Learn more about using the Visual Checklist Designer.
Checklist Design Translation EnhancementsAuto-on24R1.3
This enhancement allows you to now manage Checklist Design translations from Visual Checklist Designer. In addition, you can upload a different Question Design Reference Document for each language you want to support.
Unscored Checklists Use Null ValuesAuto-on24R1.3
To help customers differentiate between unscored checklists and those scored as 0 (zero), Vault no longer represents unscored checklists as having a score of 0 (zero). Instead, Vault leaves the score as null. If a scored question is skipped in the checklist, Vault sets its Sum Score field to a null value. This feature also provides more accurate results when reporting on the average score of a checklist, section, or question across multiple checklists.
This enhancement does not affect Vault Training’s Quizzes, as they use a Points field instead to calculate the quiz score. In addition, Vault will not re-score any checklists that were already completed.
Checklist Section Dependency LimitAuto-on24R1.3
This enhancement introduces a new limit that prevents dependent sections from going beyond three (3) levels. In addition, Vault will also not allow users to create dependent sections after a level-3 dependent question. Any existing Checklist Designs that have nested dependencies of more than three (3) levels are still functional, and any runtime checklists can still be generated from them. This matches the limit already in place for three (3) level dependent questions.
Default Attribute for Object TypesConfiguration24R1.3
On objects with object types, you can specify a default object type. However, when using VPK to import one of these new objects, this could fail because the default object type is set prior to the additional object types being created for the newly imported object. This has now been corrected, reducing the manual MDL steps required to ensure this step of the import completes the first time through.
This is achieved by storing the Default Object Type on the Object Type components themselves, instead of the Object. There is a new read-only Default Object Type checkbox, that will indicate this on the Admin UI.
Domain & VeevaID Indicators Removed from Person ObjectAuto-on24R1.3
Prior to this release, user names were suffixed with “({domain})” for cross-domain users or “(VeevaID)” for users authenticated via VeevaID, but this means these suffixes appear in document tokens. These indicators have now been removed from the Person object’s Name field on all Person records.
Common UI & Search
What's New General Release Notification: Mark as ReadAuto-on24R1.2
With each General Release, there is a What’s New notification that is automatically available to all users in the Notifications menu. This notification is pinned at the top of the notification list.
Starting with 24R2, users will be able to mark this notification as read, and once marked as read, the notification will begin to move down the list as other notifications are received.
This enhancement ensures that users are still made aware of new features, while also providing the flexibility to have the notification not remain at the top of the list.
This update does not apply to the following applications: CDMS, Align, CRM.
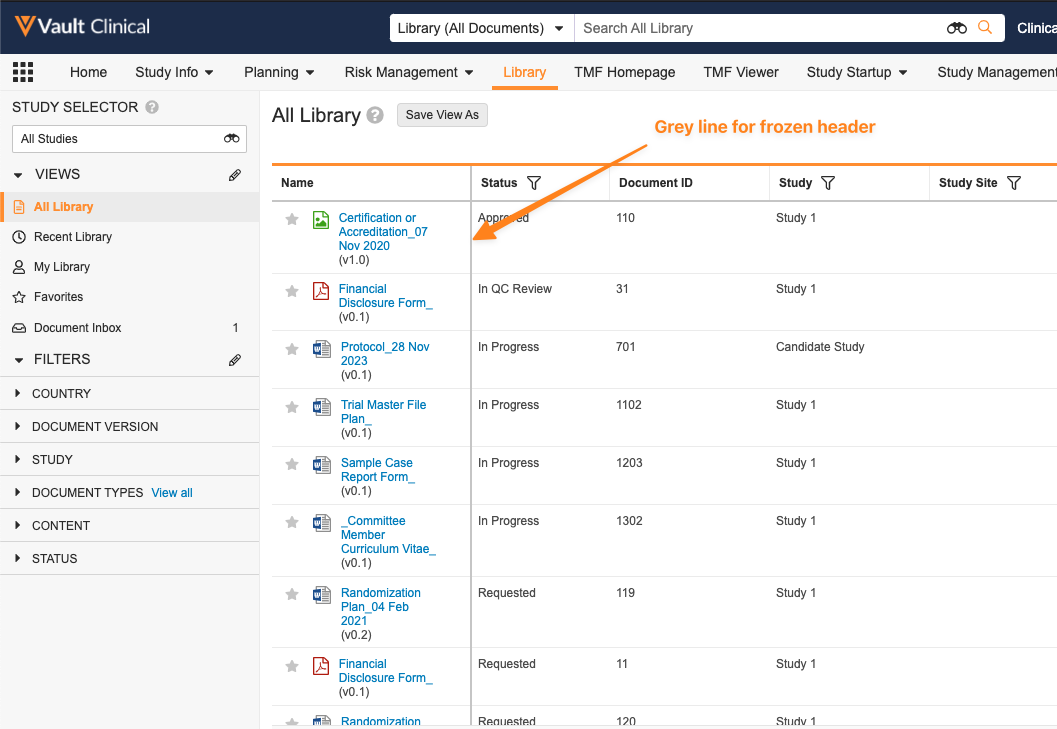
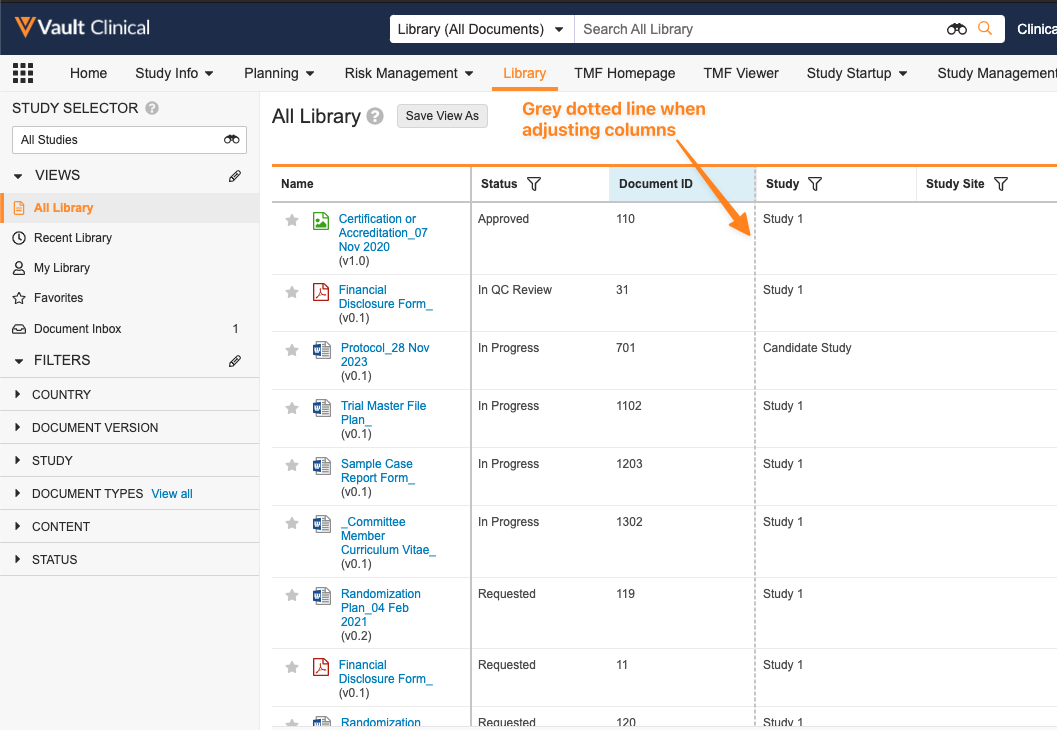
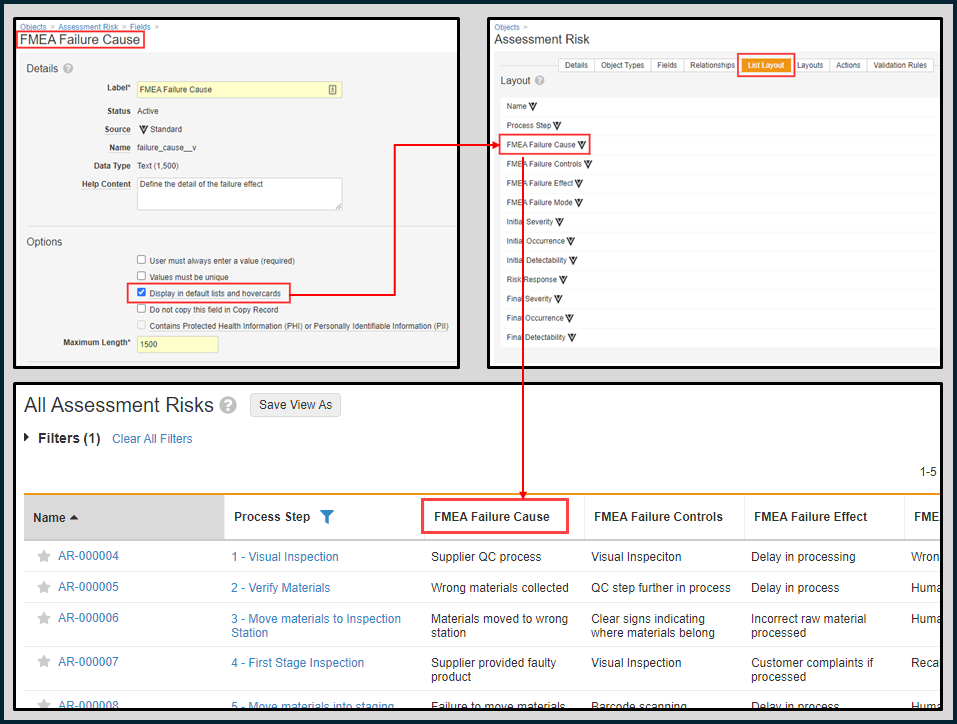
Object Data Grid Freeze Line Design Changesimages24R1.2
When using a grid view (either on an object tab or in the Library), we have enhanced the user experience in a couple of ways:
- When freezing columns, the line indicating which column is frozen will be gray rather than blue, making it look less like something that is being actively moved.
- When moving columns via drag-and-drop or moving the freeze line, Vault now shows a dotted gray line rather than a dotted blue line, in accordance with the change above.
Keyboard ShortcutsAuto-on24R1.3
Navigating Vault and accessing quick actions has been made easier. On any page, you can use a new hotkey combination of Ctrl + / which opens a list of available keyboard shortcuts for the current page. Global keyboard shortcuts include things like jumping to the search bar (Shift + Ctrl + F), or selecting an item from the Create menu (Shift + Ctrl + C).
On record detail pages, you can begin editing the record with Shift + Ctrl + E, save with Shift + Ctrl + S, or open the Actions menu using Shift + Ctrl + M and arrow down through the available actions.
On documents, you can use those same keyboard shortcuts to edit and save fields, and open the action menu. For a full list, see the help page for Keyboard Shortcuts. As we enhance Vault, more keyboard shortcuts will be added over time to support other common functions, with the ultimate goal of allowing power users to never have to leave their keyboard.
Note: Replace CTRL with CMD on Mac OS.
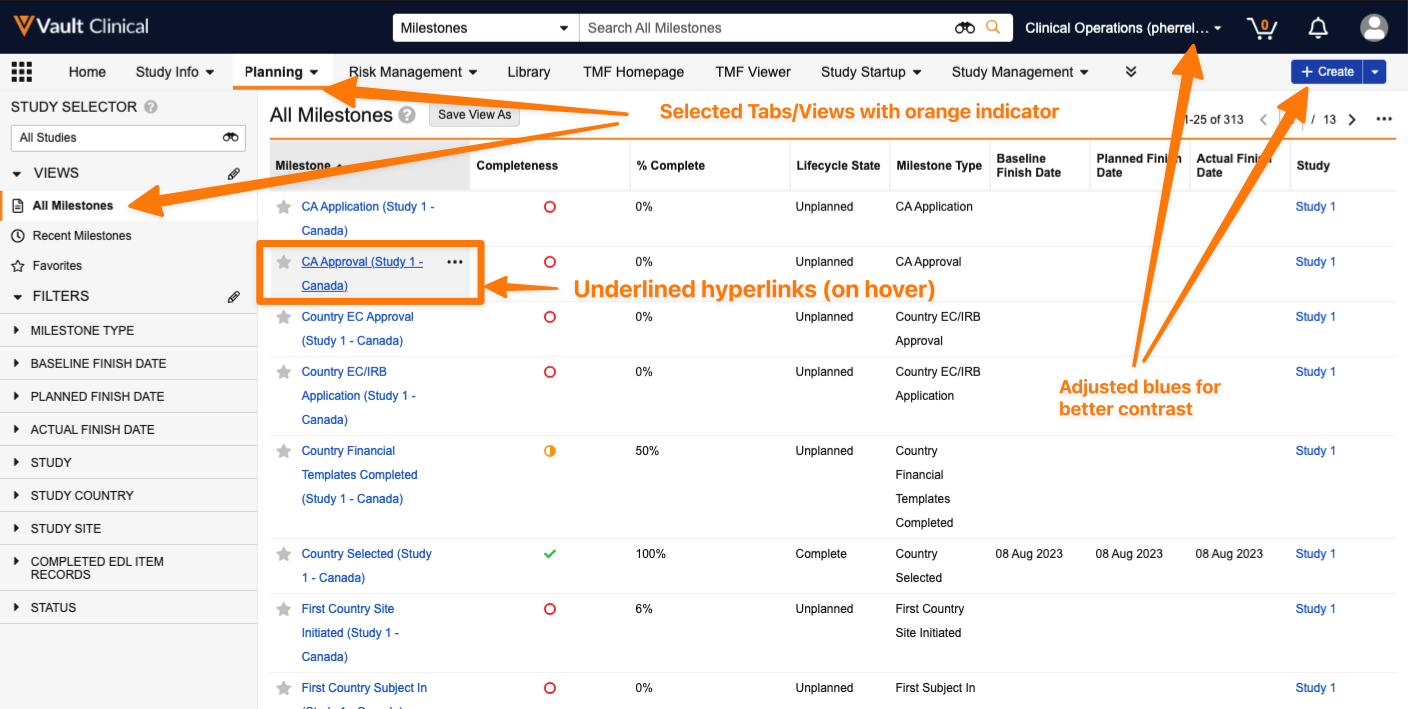
Improved Color ContrastAuto-on24R1.3
Several updates have been made to the Vault user interface to enhance color contrast and improve clarity and visibility. These changes aim to provide a more visually distinct and user-friendly experience.These changes include:
-
A slightly darker blue header bar for Production Vaults
-
A slightly brighter Create button
-
Text for Selected Tabs and Views will be in black text with an orange indicator
-
Hyperlinks are a more discreet blue and when hovering over a hyperlink, the link will stay blue and add an underline indicator
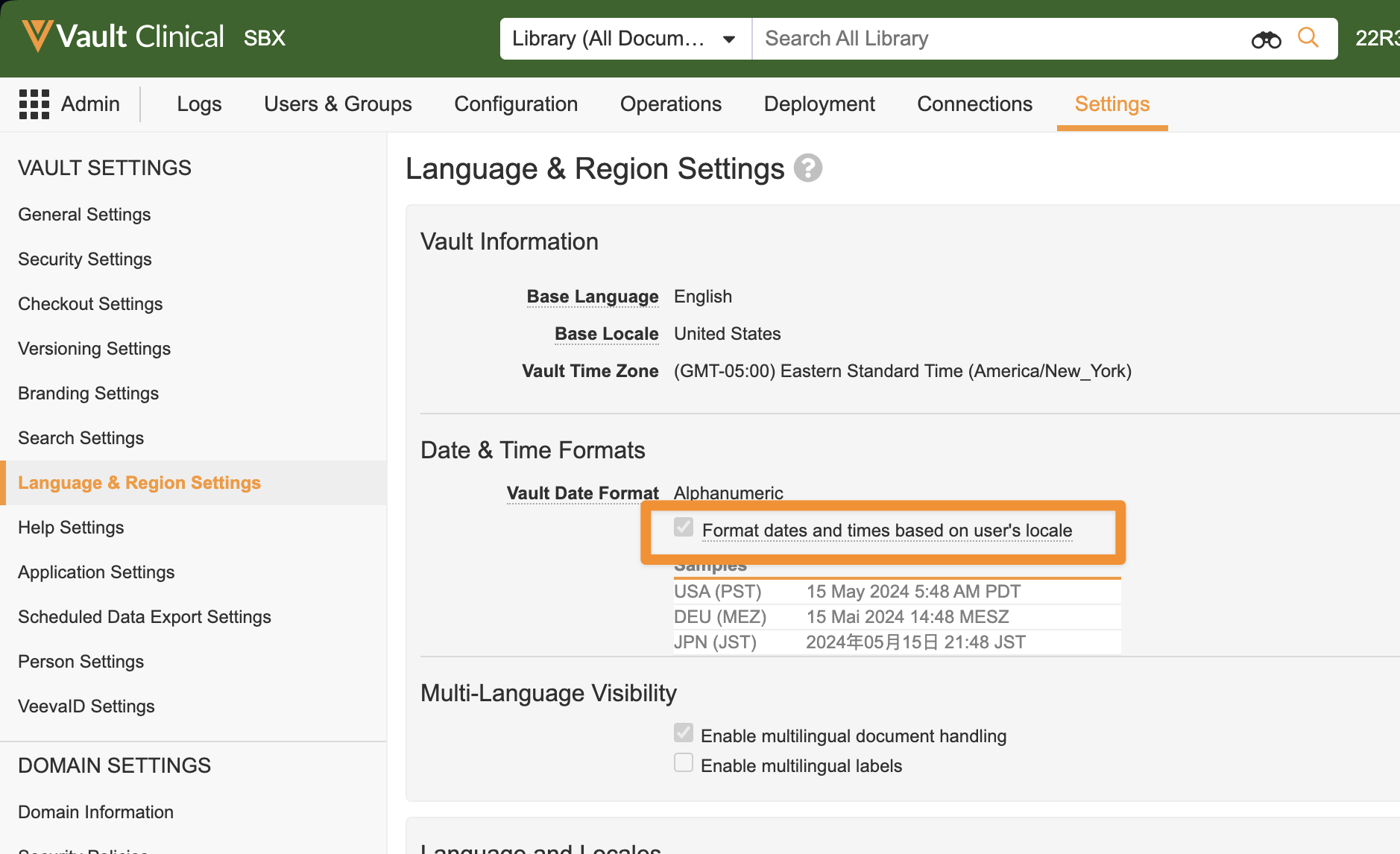
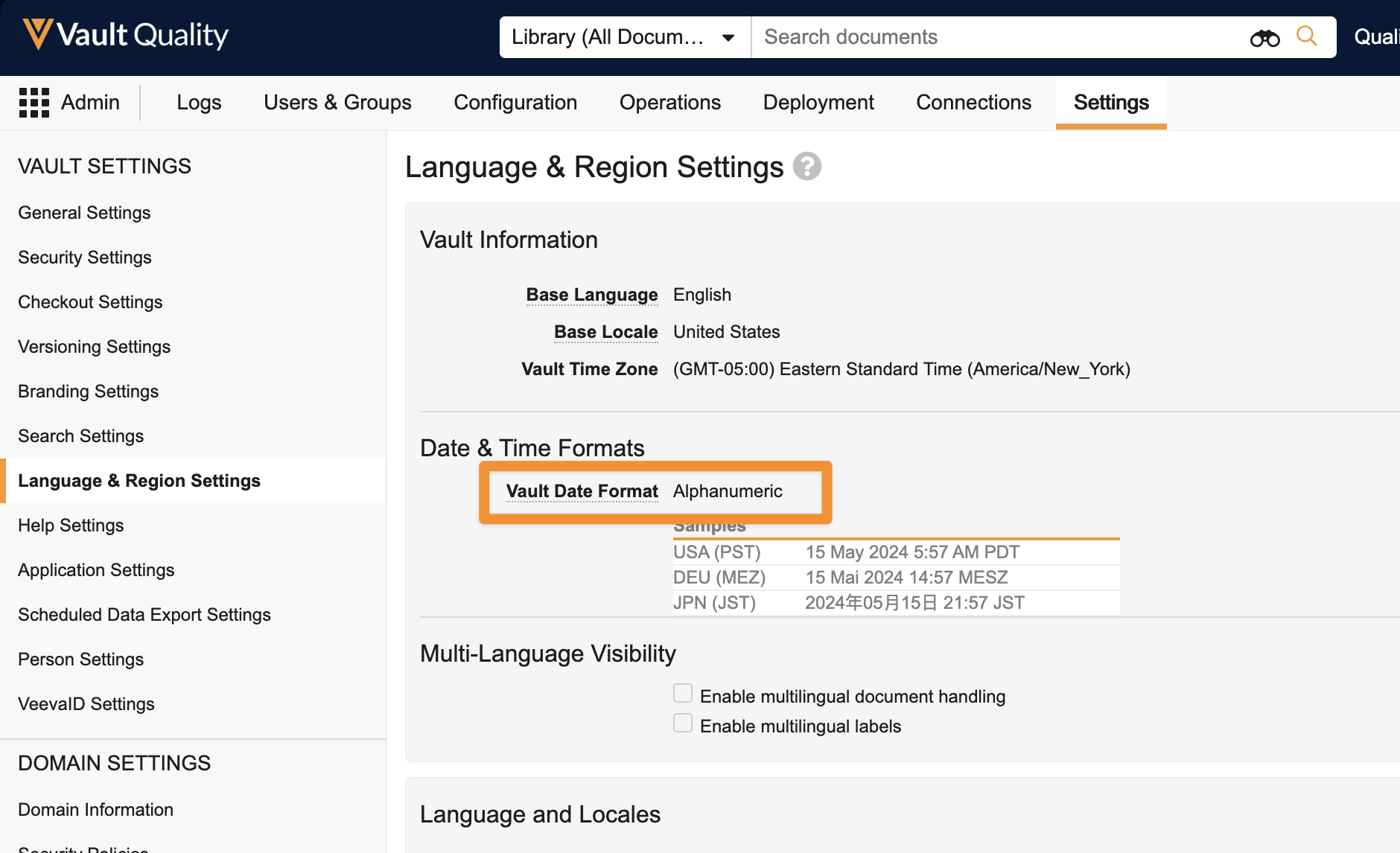
Flexible Date Formatsimages24R1.3
Date and Date/Time fields now accept various date formats. Empty fields display a placeholder in the selected Vault date format. Users can enter dates in numeric format with slash or dot separators or without separators using zeros for single-digit months and days according to their locales. Dates can also be entered in alphanumeric format. The date will then be formatted to the configured Vault date format setting.
All Date and Date/Time fields will display based on the locale for each user and all Vaults. The existing checkbox Format dates and times based on the user’s locale setting will be removed from all Vaults, cutting the amount of date format variations that can be available in half.
This feature simplifies the date formats and provides flexible date input across Vault, making it easier for users to read and enter dates.Existing Vaults will retain the existing Date Format that is currently configured. New Vaults created after 24R2 will have the Alphanumeric option set as the standard default for Date Format. Alphanumeric is more readable and less ambiguous across locales, and is the setting that the majority of Vaults use currently.
For Vaults that currently have the Format dates and times based on user’s locale option unchecked, users who’s Locale differs from the Vault Locale will need to be informed that dates will now appear to them based on their Locale.
Learn more about Vault Date & Time Formats.
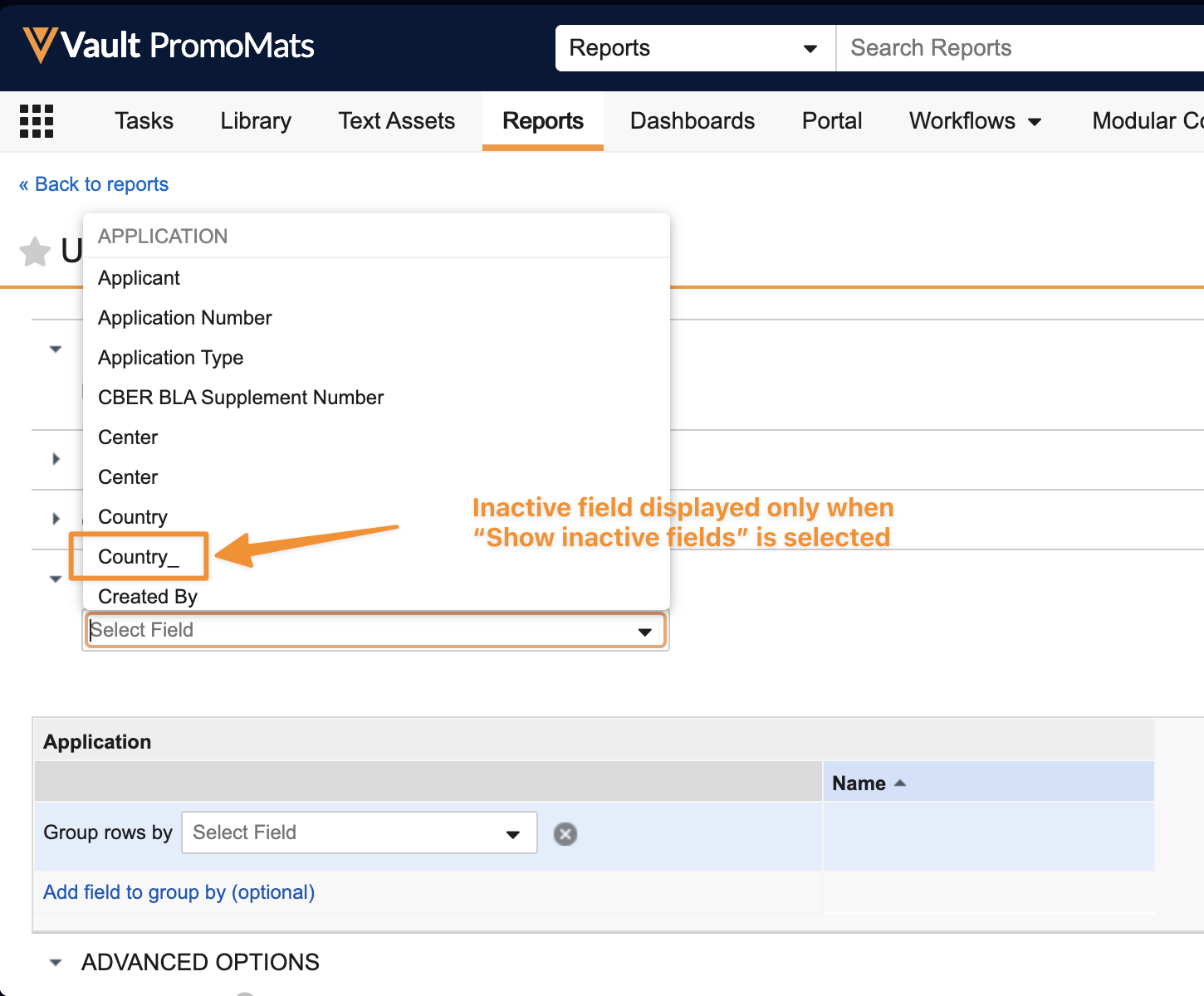
Hide Inactive Fields from Object SearchAuto-on24R1.3
As more standard fields are added to Vault applications over time, there may be duplicates of custom fields that customers have already implemented in the meantime, causing confusion in searches and filters where both similarly named fields are now available. To help with this, when searching or filtering object records in an object tab, inactive fields are no longer searched or displayed. If a search needs to be conducted on an inactive field, users should create a report to do this instead.
Wildcard Limits in SearchAuto-on24R1.3
To ensure fast and reliable performance of Vault search, we have introduced some limits for the number of wildcards you can include in your search terms. There are two new rules:
- Each search term can contain up to two (2) wildcards *
- Your search can include up to ten (10) search terms that contain wildcards *
This limit is enforced anywhere a user can enter a text search. Trailing wildcards can be used to find results that start with a search term (veev* vaul*), or wildcards can be used to substitute a letter or number inside of a term (v*eva v*u*t).
Learn more about wildcard searching
English Language Default for Help LinksAuto-on24R1.3
Learn More links from Vault now route users with Japanese or Chinese-language Vaults to English-language Vault Help documentation, where options for translated documentation are visible if available.
Update Select All and Clear Selected Behavior in Column Header FiltersAuto-on24R1.3
In the column header filters you see in grid views across Vault, the Clear option has been relabeled as Clear Selected, and is only enabled if at least one value has been selected. In addition, the Select All Matches option (which only shows if you type-ahead for a value) has been relabeled as Select All, and is disabled if all the values matching your type-ahead term have already been selected.
Improved Search Behavior for Email Suppression ListAuto-on24R1.4
The search feature on the Email Suppression List page (Admin > Operations > Email Suppression List) now looks across the Name and Suppression Reason columns, in addition to the Email Address, which was already searchable.
Support for Hyphens in Picklist Entry ValuesAuto-on24R1.4
We have expanded the supported character set for picklist value names to support the use of hyphens. After this change, the supported character set will be as follows:
-
Lowercase letters a-z
-
Digits zero (0) through nine (9)
-
Hyphens and underscores, limited to a single sequential instance of either
-
Cannot begin or end with a hyphen (“-“) or underscore (“_”)
Example valid values are as follows:
-
test_value_1__c -
test-value-1__c -
test-value_1__c
Example invalid values are as follows:
-
_test_value_1__cis invalid due to the leading underscore -
-test-value-1__cis invalid due to the leading hyphen -
test_value-__cis invalid due to the trailing hyphen -
test-_value_1__cis invalid due to sequential use of more than one instance of a hyphen or underscore
This change will apply to picklists entries across objects and documents. Picklist value names with hyphens are not supported within expressions. Customers that require evaluation of picklist entries in expressions should not leverage hyphens in picklist value names.
Reporting & Expressions
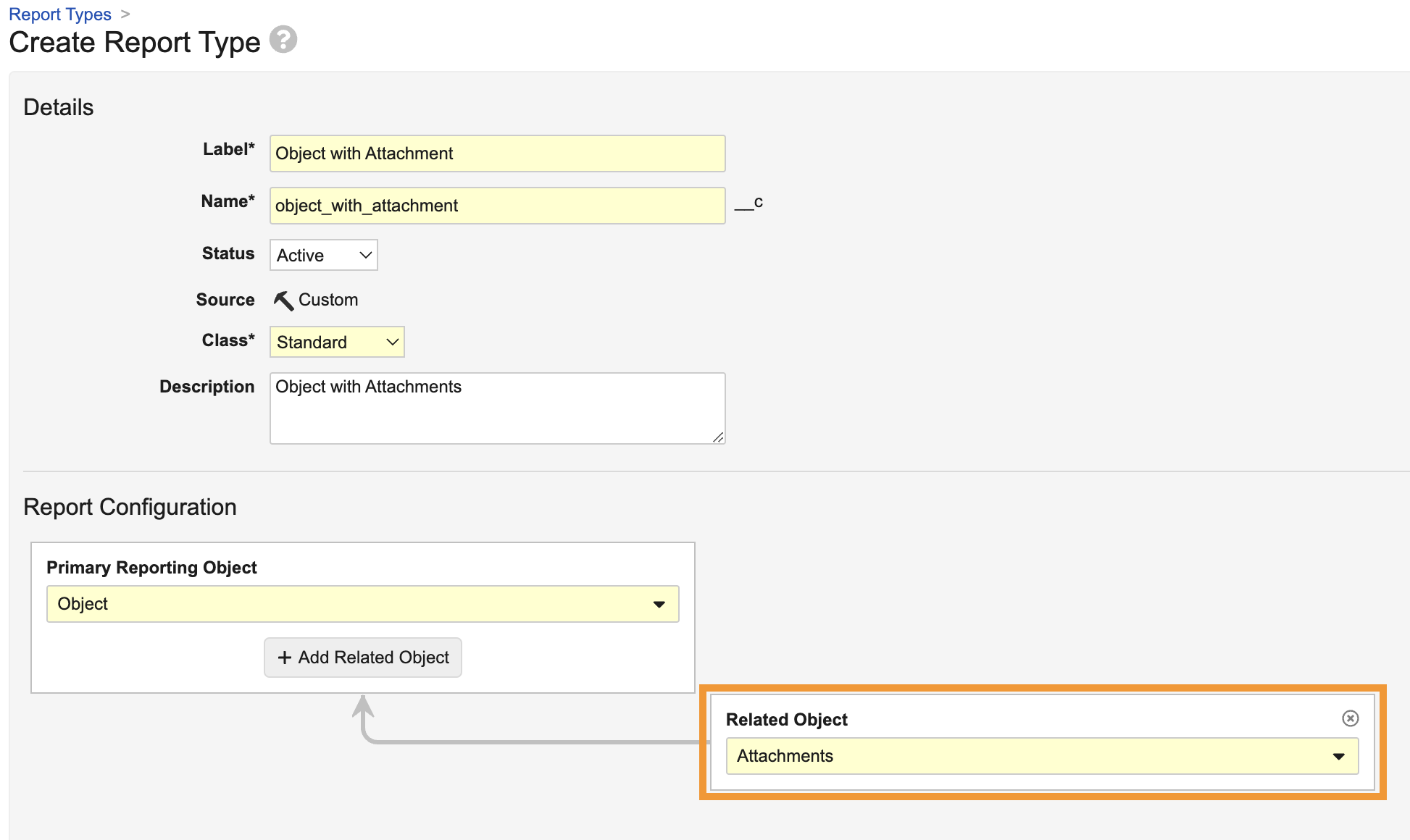
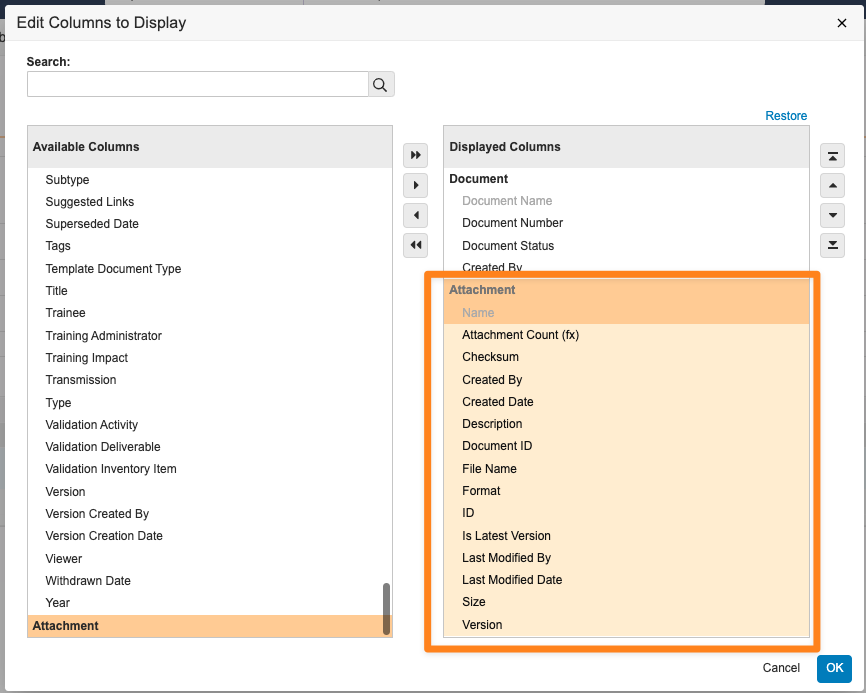
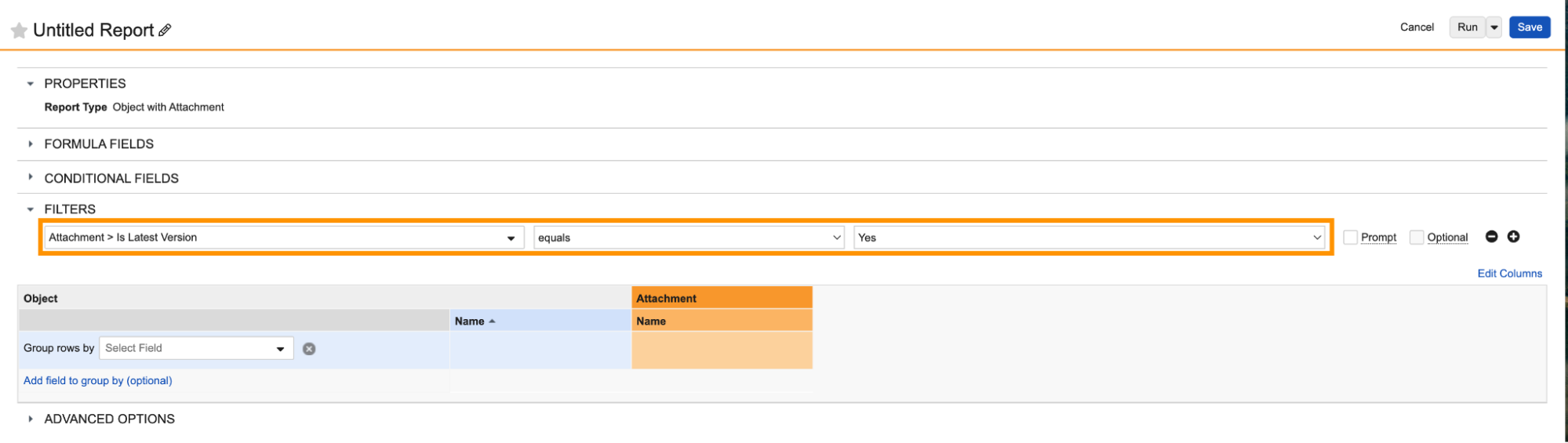
Report on Object AttachmentsAuto-on24R1.4
Admins can now create report types that include attachments on any object with the Allow Attachments setting enabled.
When the Attachments object is included in a report type, users can create reports that include the following standard attachment information, which users can include as report columns or use in report filters:
Reporting on attachments has been a common need from customers across Vault applications; for instance, in QMS, a customer may need to identify which Quality Event records have attachments and the details included in those attachments. Prior to 24R2, users could only obtain this information through the API.
Attachment reports are also supported as Report Views in multi-pass reports, and support pulling information on all attachment versions (though by default, attachment reports will filter for the latest version).
Learn more about Attachments and Configuring Report Types.
Expression EnhancementsConfiguration24R1.2
This release introduces some new capabilities to Vault’s expressions functionality (used in formula fields on objects, validation rules, formula fields on reports, and more).
-
New system variables: expressions are now able to return the Domain of the Vault, the Vault ID, and the timezone of the Vault. These can be accessed in any expressions with the following tokens:
@Vault.domain,@Vault.id,@Vault.timezone -
DateTimeValue: this is a new function that accepts a date, datetime, or text in the format of a date or a datetime, and returns a datetime value. Datetimes are assumed to be in GMT, but users can specify a timezone through a second parameter.
-
IsAppEnabled: this new function accepts the name of a Vault Application as a string and returns true or false depending on whether the app is enabled in the Vault
-
See the Vault Formula Reference Guide for a list of application strings
-
Blank Handling: all functions in object formula fields now honor the Treat blank fields as zeros and empty strings attribute.
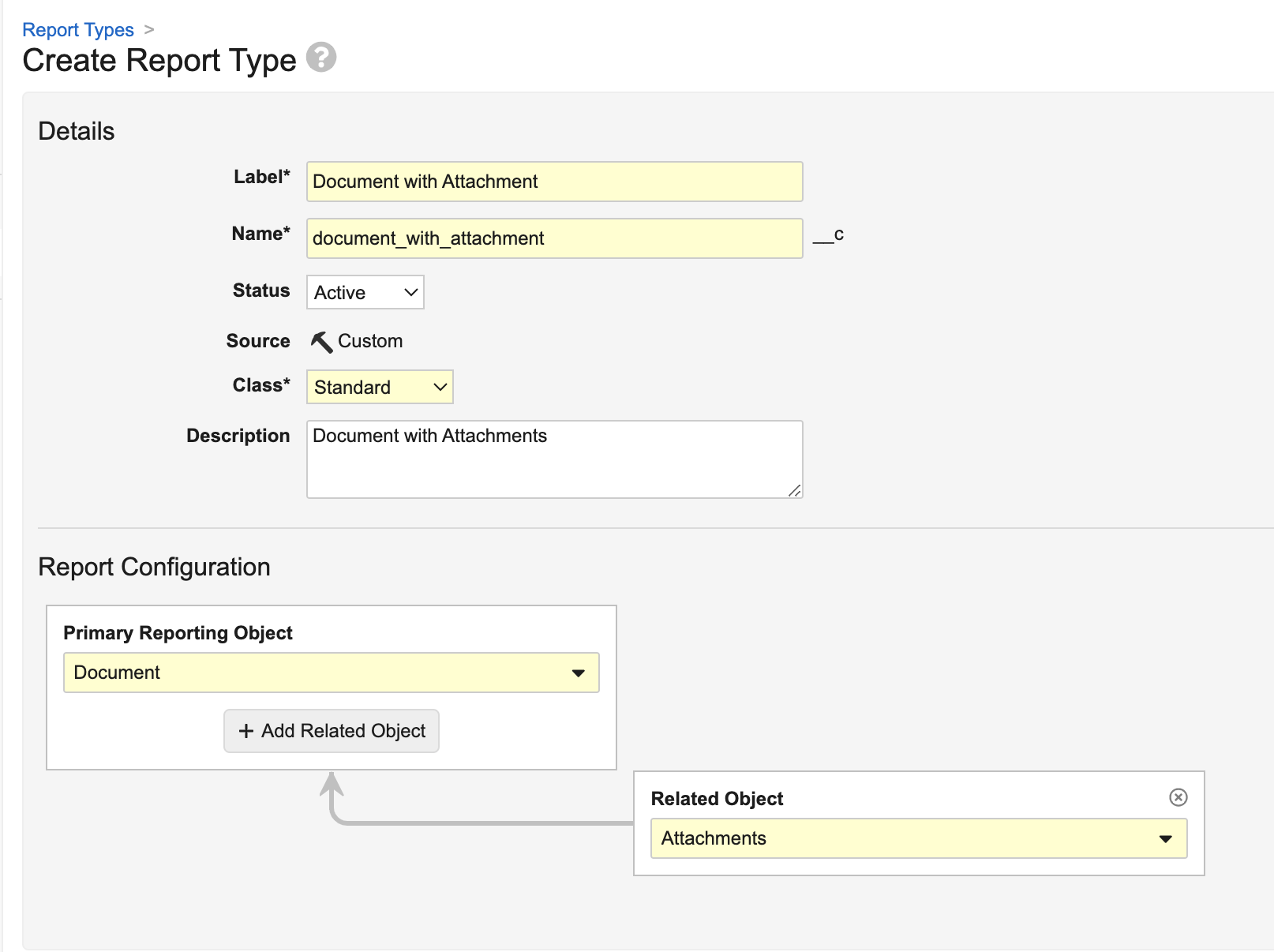
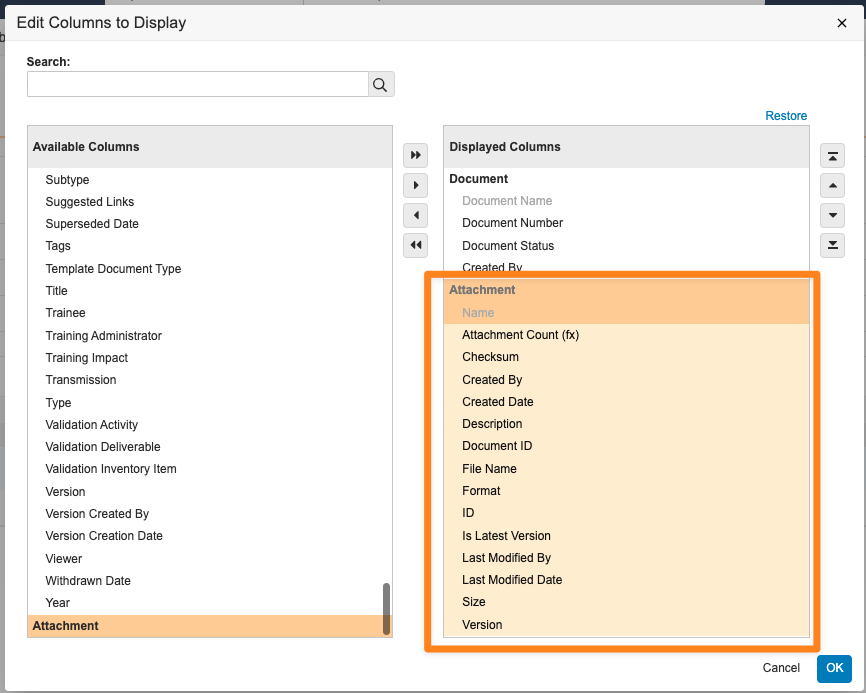
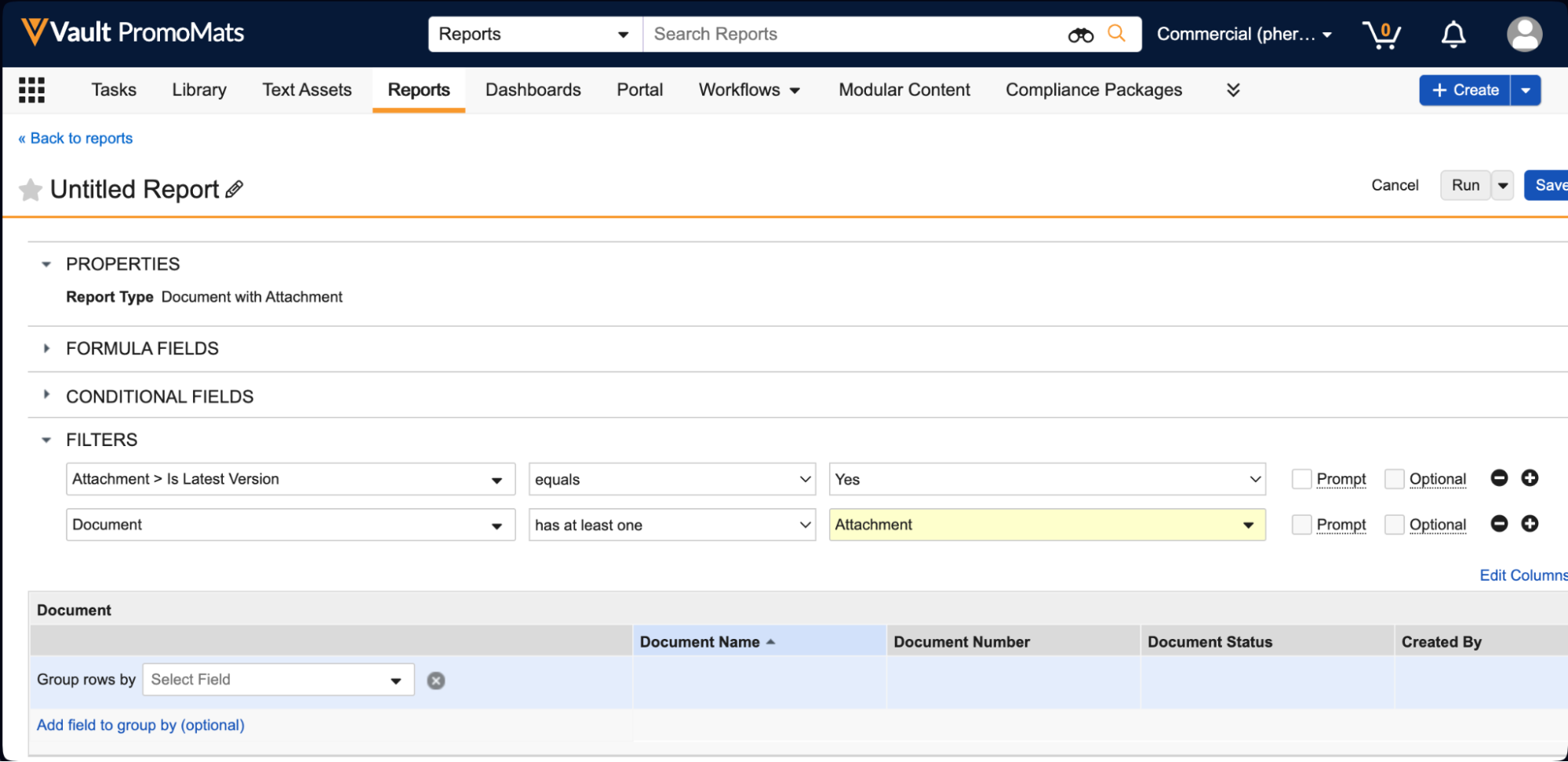
Report on Document AttachmentsAuto-on24R1.3
Admins can now create Report Types for Documents that include Attachments.
When attachments are included in a report type, users can create reports that include the following standard attachment information, which users can include as report columns or use in report filters:
Reporting on attachments has been a common need from customers across Vault applications; for instance, in QMS, a customer may need to identify which Quality Event records have attachments and what those attachments are. Prior to 24R2, users could only obtain this information through the API.
Attachment reports are also available as Report Views in multi-pass reports, and allow users to pull information on all attachment versions (though by default, attachment reports will filter for the latest version):
Learn more about Attachment and Configuring Report Types.
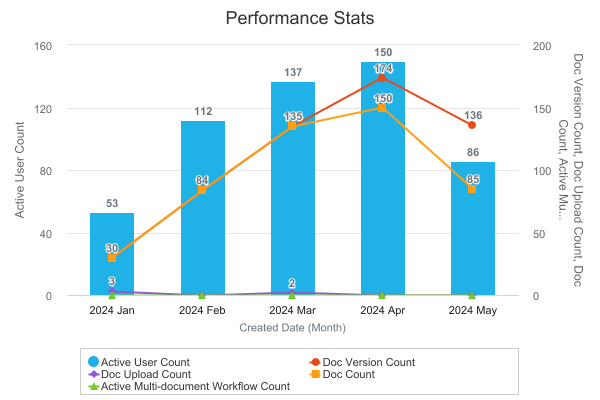
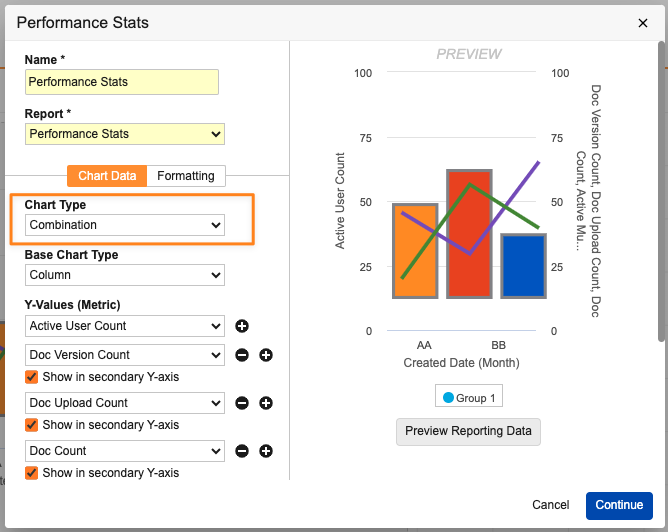
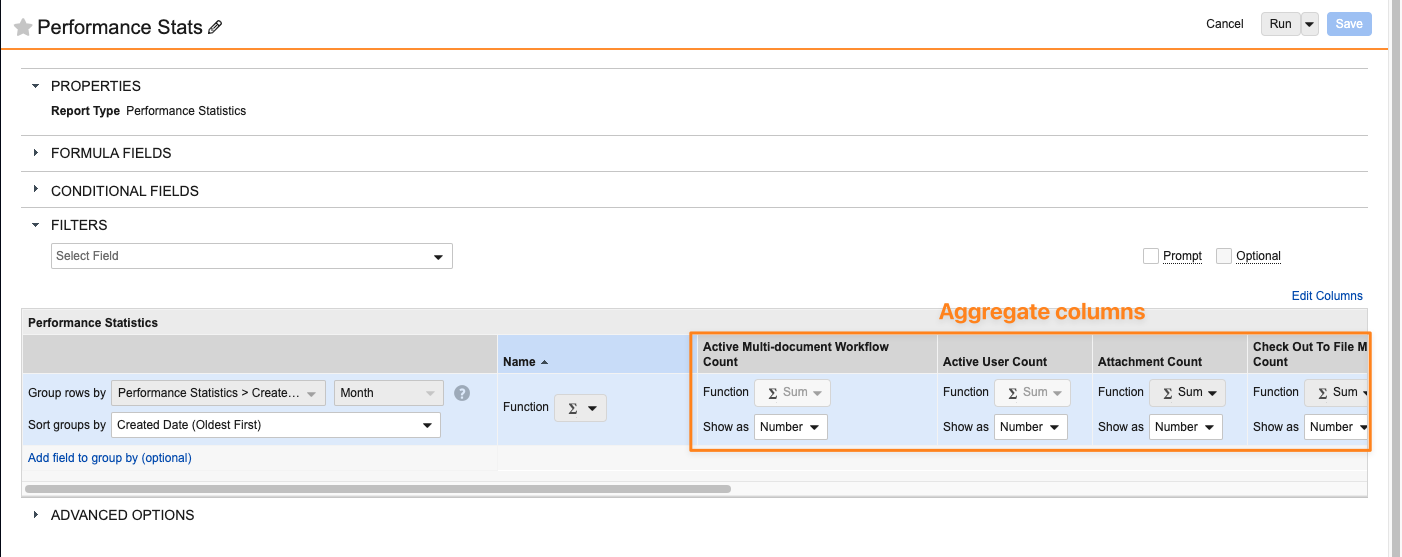
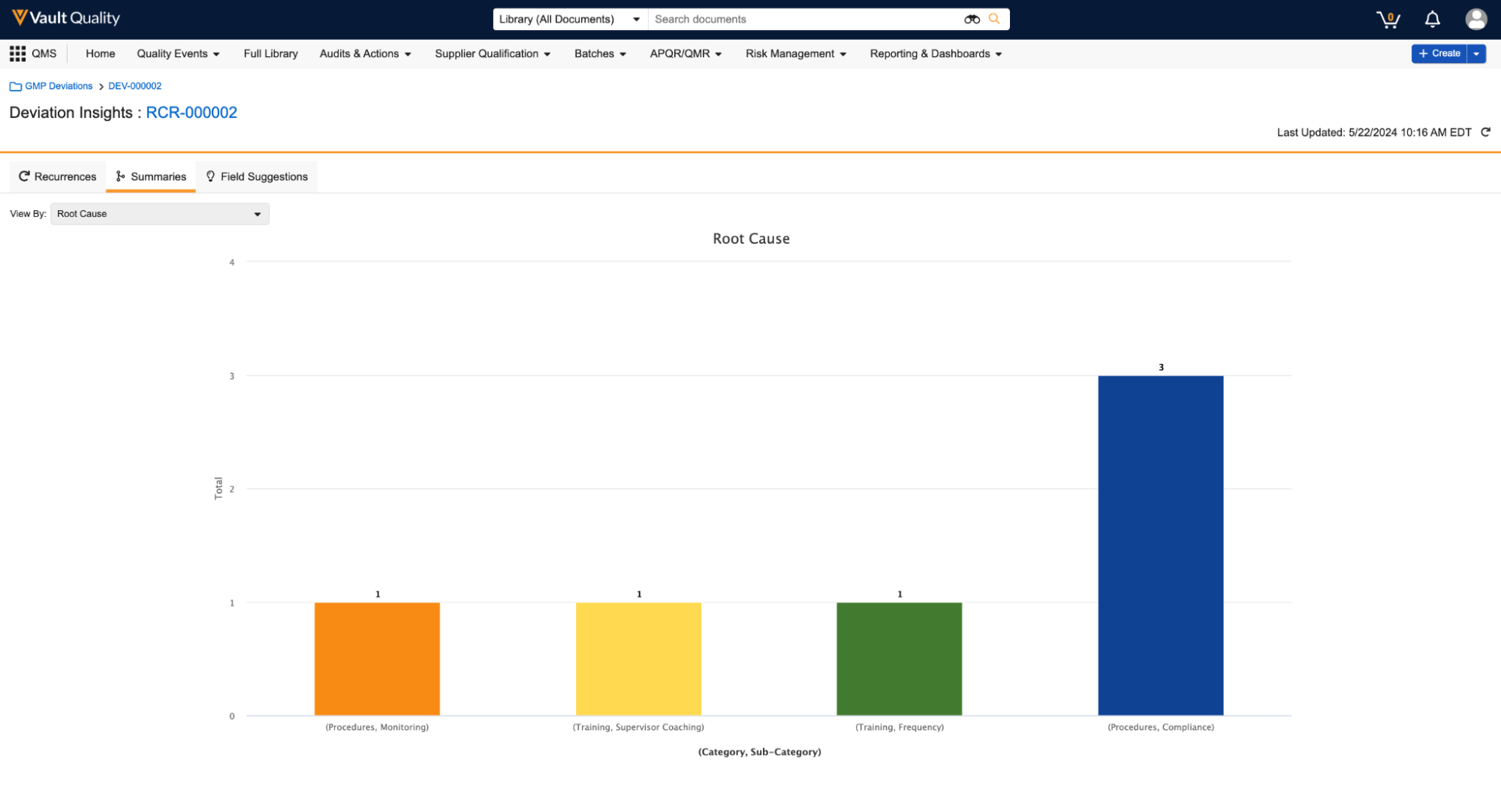
Combination Chart in DashboardsAuto-on24R1.3
Users can now add multiple metrics into a single Combination Chart in a dashboard, which allows for an easier visualization of metrics compared against each other. Prior to this release, users could not add multiple metrics to a single chart and were required to create a separate dashboard component for each metric.
The new Combination Chart functionality allows users to include multiple metrics when creating a bar, column, or line chart. In addition, users can add up to nine (9) secondary metrics and include a secondary Y-axis that displays the secondary metrics as lines on the chart.
When setting up a Combination Chart, the available values for the Y-axes are based on aggregate columns in the underlying reports (for example, Record Count columns).
Learn more about Creating & Editing Dashboards.
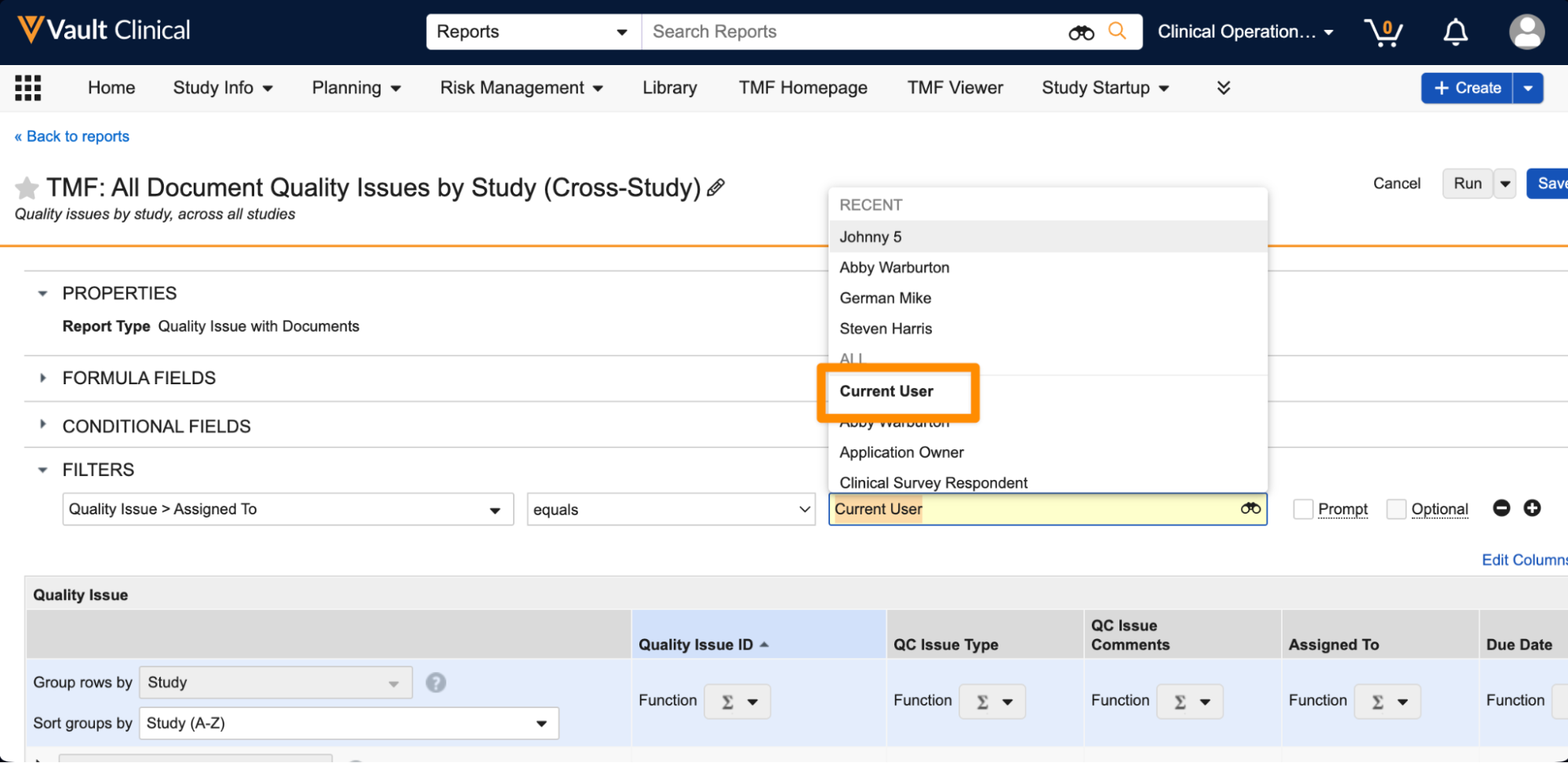
Current User Filter for All User Fields in ReportsAuto-on24R1.3
Report filters now support Current User as a selection for all fields that reference the User object. This filter ensures that the report results are dynamically filtered based on the user who is running the report.
Prior to 24R2, Current User was available in some user fields, including System fields (such as Created By and Last Modified By), workflow user fields (such as Workflow Owner and Task Owner), and standard document role fields. With this enhancement, Current User is now available on all fields that reference users.
Learn more about Using Report Filters.
define in FormulasConfiguration24R1.3
Vault Formulas now support the use of #define statements, which allow users to simplify long expressions by replacing long blocks of text with a shorter set of characters. This can make formulas easier to read in certain cases, especially formula fields in multi-pass reports. To leverage this, #define must be declared at the beginning of a formula.
As an example, here #define is used to significantly shorten the Due Date name, allowing for it to be used in the expression itself in a more concise and readable way:
#define wf_dd join_ref_1_v.workflow.workflow_dueDate_v
if(today() > wf_dd, "Late", "On-Time")
With this syntax, #define is declared first, then the shortened text is added, and then the existing field name that is being defined is added.
Learn more about formulas in our Vault Formula Reference Guide.
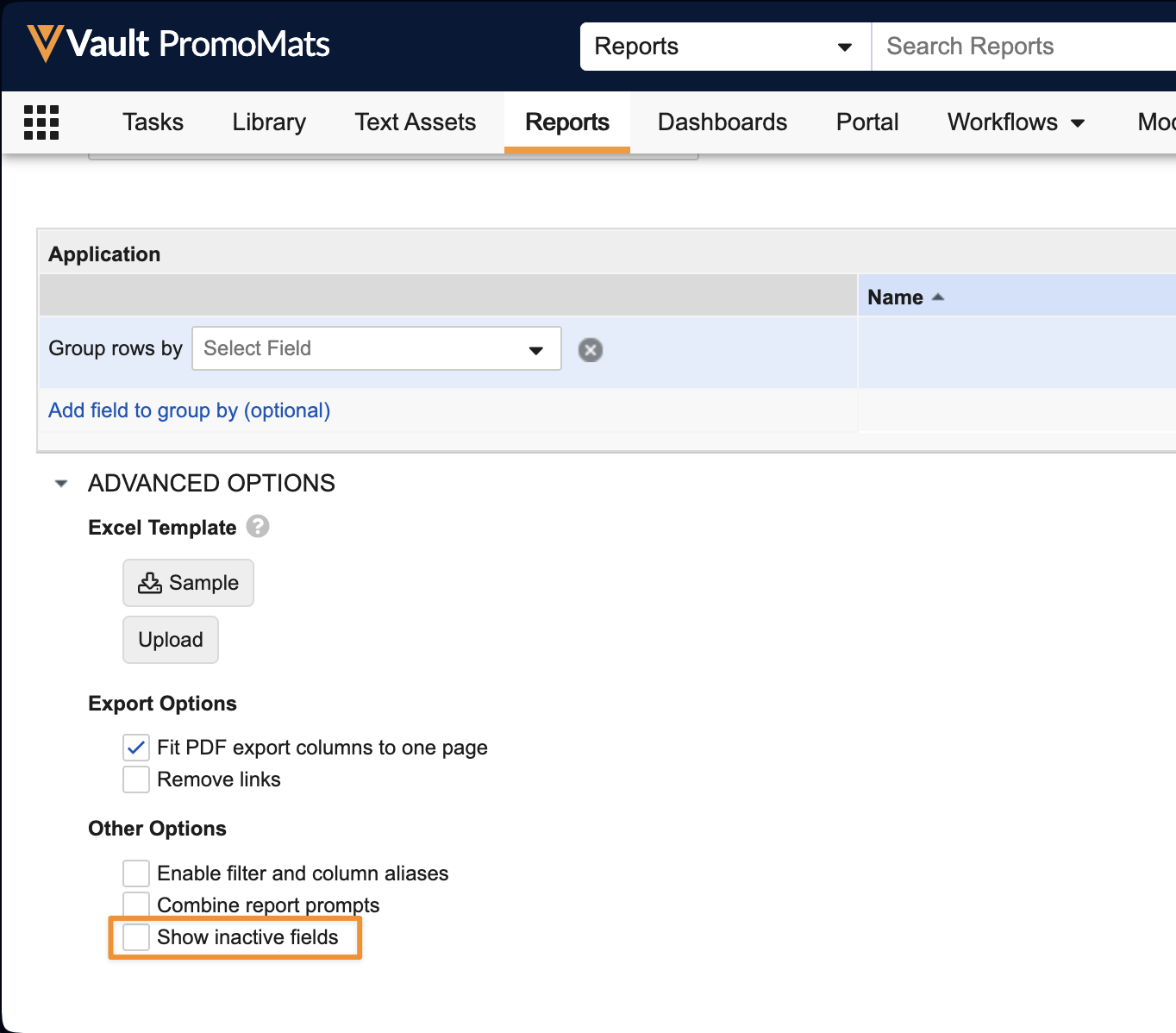
Show Inactive Fields Option in ReportsAuto-on24R1.4
Reports will now exclude inactive fields by default from formula fields, conditional fields, filters, grouping, and columns. If a Vault has the Allow inactive fields to be used in reports setting checked in Admin > Settings > General Settings, a new option will be available under Advanced Options to allow inactive fields to be shown, if needed.
This enhancement reduces confusion for users in building reports by focusing information on the active fields only, while still maintaining the ability to use the inactive fields where needed for historical purposes.
Limit Flash Report from Being Sent to More than 10K UsersAuto-on24R1.4
With 24R2, Vault will not send flash report emails for reports with more than 10,000 unique users. Users will receive Vault notifications and have access to the flash report. The user who scheduled the flash report will receive an email notifying them that the email was not sent if the limit is exceeded.
Audit
Static Values for On Behalf Of UserAuto-on24R1.3
In audit trails, if an action is taken on behalf of another user via delegated access, the user’s name and username are now stored as the On Behalf Of User value in plain text rather than dynamically looking up the current information according to the User ID. This serves two purposes: to more accurately represent the data at the time of the entry, and to improve audit trail performance.
Security
Security Profile Limits Exclude Standard Security ProfilesAuto-on24R1.2
The number of Security Profiles that a given Vault can have is limited to 100 or less. Prior to 24R2, Standard (__v) Security Profiles were included in this count, but going forward, this limit will only be enforced on the number of custom (__c) Security Profiles.
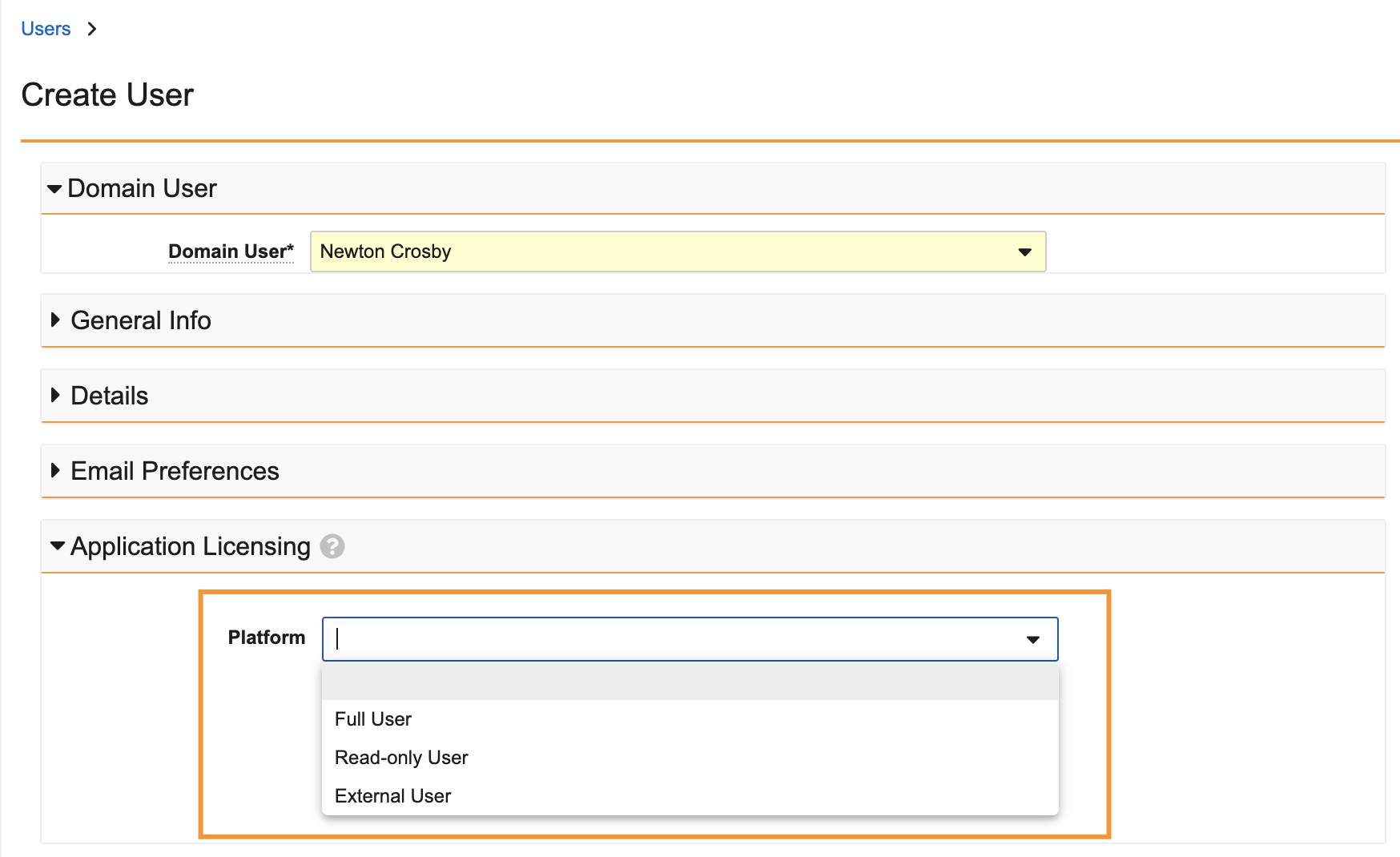
Streamlined User Licensing ManagementAuto-on24R1.3
For applications that license based on users, Vault will now provide a more streamlined experience for managing licensing by focusing all license management on the Application Licensing fields and ensuring that a user’s permissions are in line with their assigned licenses. This change applies to Quality, RIM, Commercial, Consumer Products, and Safety (for SafetyDocs).
With 24R2 specifically:
- The License Type field will be hidden from the User layout as all licensing will be managed via Application Licensing fields.
-
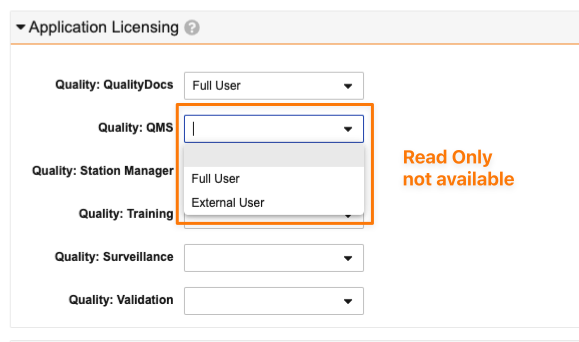
Application Licensing fields will no longer accept invalid values for a given application when creating new users or updating existing users.
- For instance, QMS is not contracted with Read Only licenses, so Read Only will no longer be available in the picklist:
-
Existing users will not have any changes made by default, until they are next changed.
-
New users will follow the validation rules.
-
Updates to an existing user that has an invalid value will flag that for correction before you can save.
Important: If you have an integration that creates or updates users in Vault, you will need to ensure that the integration is not setting invalid values. If it is, this will need to be updated prior to 24R2 to ensure the integration continues to work as expected.
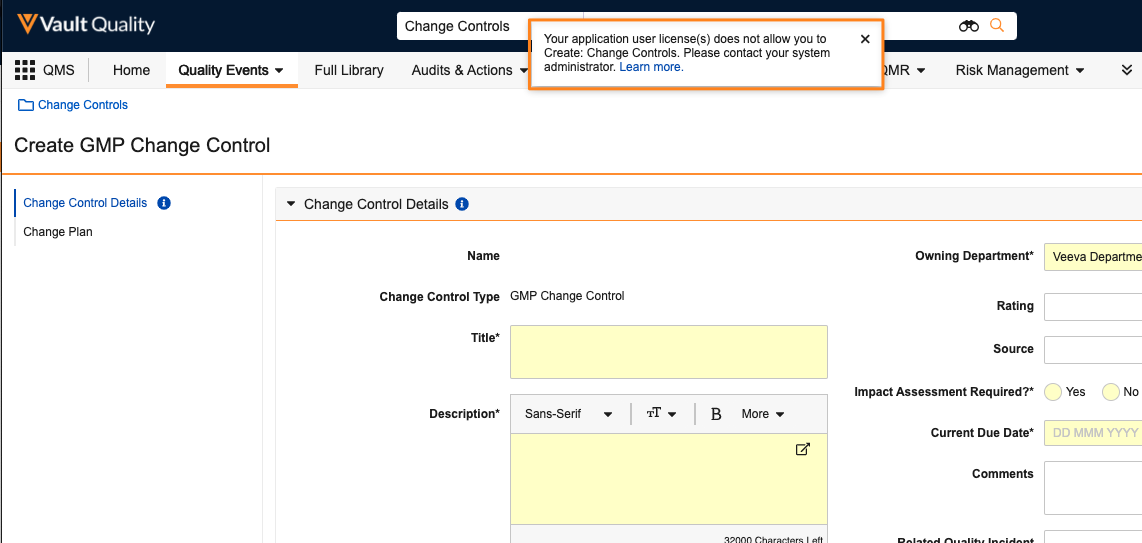
-
For customers with multiple applications in a given Vault, if an existing user accesses an object or tab that is not supported by the Application Licensing that they have assigned, Vault will show a warning message. Users will still be able to access and create those records until 25R1, though with 25R1, users will no longer be able to access objects and tabs that are not within their license.
- For example, Change Control is a QMS-specific object, so if a user’s Security Profile grants them access to that object, but they don’t have a value in the QMS Application Licensing field, Vault will warn them.
-
In 25R1, they would no longer be able to access the objects or tabs that they don’t have a license for, even if the Security Profile allows
-
If you only have one application in a given Vault (i.e. just QualityDocs), this change should not have any impact
-
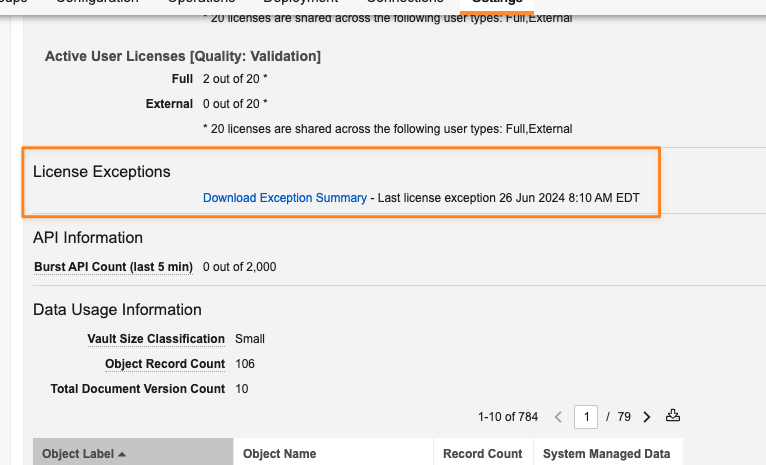
Admins will be able to access and download an Exception Summary to identify any issues to address. Vault refreshes license type exceptions within the summary every 12 hours at 01:10 GMT and 12:10 GMT. The summary captures object and tab exceptions every four (4) hours.
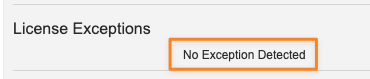
- If there are no exceptions to address, the section will note that:
Learn more about application licensing for users.
VeevaID: 30-Day Multi-Factor AuthenticationAuto-on24R1.3
VeevaID users are now required to use multi-factor authentication (MFA) once every 30 days through an email verification code, regardless of the browser or device they are using. Prior to 24R2, MFA was leveraged the first time a user logged in using VeevaID from a new browser or device, but ensuring that this is used once every 30 days improves the overall user security of VeevaID.
Learn more About VeevaID.
Vault Selector Respects VeevaID NameAuto-on24R1.2
With VeevaID, organizations have the ability to change how their Vault is named to ensure it’s displayed in a meaningful way to VeevaID users.
With 24R2, VeevaID users will see the updated name on both the VeevaID home page as well as in the Vault selector. Prior to 24R2, the updated name would only display on the home page.
This enhancement creates a consistent and less confusing experience for VeevaID users who have access to multiple environments across organizations.
Learn more about Administering VeevaID.
Application Licensing in Platform VaultsAuto-on24R1.3
In line with the enhancements to streamline the experience of managing users in user-based licensed applications, Platform Vaults now display an Application Licensing section:
This enhancement ensures that Admins have a consistent experience managing users across all Vaults that are licensed by users.
For existing users, the Application Licensing value will be set to the same value as the License Type field as part of the 24R2 upgrade.
Remove Username Conflicts with VeevaIDAuto-on24R1.3
Usernames in Vault no longer need to be unique if they are associated to different domains. With the introduction of VeevaID, there are scenarios where a VeevaID username may match an existing Vault username or vice versa.
Prior to 24R2, the existing requirement for uniqueness could cause conflicts - for instance, if an Admin attempted to add a new domain user, they would receive an error if that user already had an existing VeevaID account. Conversely, if a user already existed in the Vault and an Admin created a new VeevaID user record for them, VeevaID would append the username to ensure uniqueness.
With this enhancement, Admins will no longer receive an error if a user already has an existing VeevaID account, and the VeevaID username will not be appended if there is an existing user account.
Learn more About VeevaID.
Tools
Users No Longer Require the All Document Create Permission for Vault LoaderAuto-on24R1.2
When creating or updating documents, versions, relationships, attachments, roles, or renditions using Vault Loader, users no longer require the All Document Create permission in their permission sets. You don’t need this additional permission to do these actions in Vault, so it is no longer required for Vault Loader either.
Suppress Emails for Sandbox from SnapshotsAuto-on24R1.2
When a Sandbox vault is created from a Snapshot, users no longer receive the welcome email, unless they are the Vault Owner.
API Burst Throttling LoggingAuto-on24R1.3
With this release, Vault no longer adds entries to the System Audit History when the API burst threshold has been reached. API burst information can be found in the API Usage Logs and API response headers.
Vault Mobile
Note: Vault Mobile does not have a Limited Release, and only releases with Vault General Releases. Features listed here will be available for testing and assessment during the pre-release period for the next General Release. Learn more about the mobile pre-release here.
Show Document Name & Latest Version Link in Document ViewerAuto-on24R1.2
When viewing a document in Vault Mobile, users can now see the name of the document at the top, and if they are not viewing the latest version, they will also see a link to the current version. Similarly, if a user is viewing a minor version, and a steady state version exists, users will see a link to the latest steady state version.This enhancement helps ensure that it’s clear to users that they are viewing the correct document, and reduces the risk that a user might inadvertently reference an unapproved version of the document.
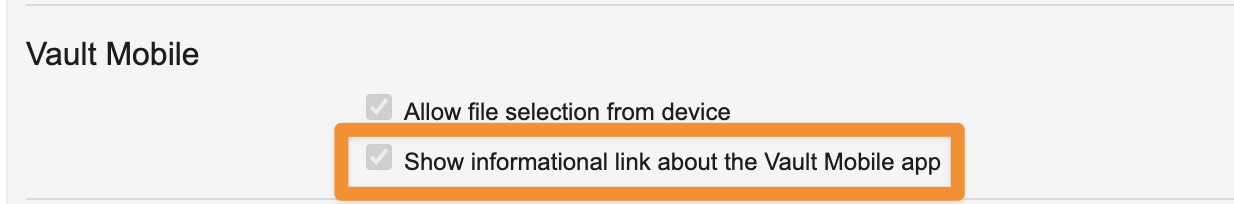
Vault Mobile Learn More Links in WebAuto-on24R1.3
To better ensure users are aware of Vault Mobile and able to take advantage of its functionality, the Vault web user interface now shows a message on applicable tabs to inform users about Vault Mobile and make it easy for them to download it.
Any tabs that are supported on Vault Mobile (Library, Tasks, Home, and Dashboards) now show this message. The message includes a hyperlink to a landing page where users can learn about Vault Mobile and use QR codes to download it.
The links display only if the user does not already have a Vault Mobile device registration (meaning, they have not used Vault Mobile to access the Vault); if a user has already used Vault Mobile to access the Vault, they will not see these messages/links.
This enhancement is auto-on by default, though Admins do have the ability to turn this feature off in Admin > Settings > General Settings > Vault Mobile.
Full Re-authentication Fallback for Biometrics
A feature called Device Enforced App Access was introduced in 23R2, which allows users to reauthenticate into Vault Mobile using their device’s biometrics. Prior to 24R2, if a user’s biometrics failed, they would be able to complete authentication using their device’s backup method (typically a PIN).
After 24R2, if a user’s biometrics fails for authentication, the user is now required to fully re-authenticate using their username and password. While biometrics are a secure method for authentication, using a full re-authentication as a fallback method provides a more secure approach when biometrics don’t work. This feature has no impact on eSignatures in Vault Mobile, which already required a full re-authentication fallback.
Platform Data Model Changes
See 24R2 Platform Data Model Changes.
Vault Connections
Quality-LIMS Connection
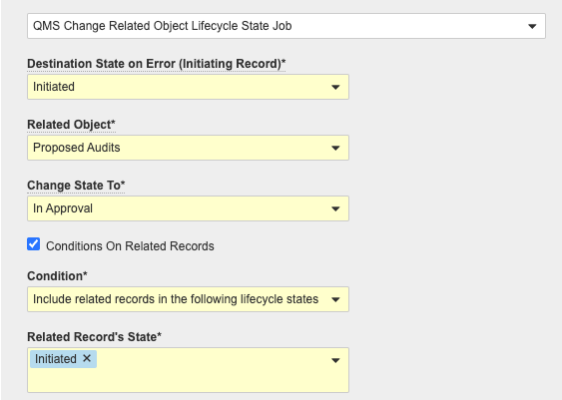
Quality-LIMS Connection: Reference ObjectConfiguration24R1.2
Vault LIMS continues to add functionality to support the connection between Quality and LIMS. This feature will provide new integration and integration points to support the automated syncing of reference object records from Quality Vaults to LIMS. The shared objects that are supported in the reference object syncing connection are the following: Batch, Related Batch, Material, Related Material, Product, Product Family, Product Variant, Product Marketed, Organization, Asset Family, and Asset.
RIM-PromoMats Connection
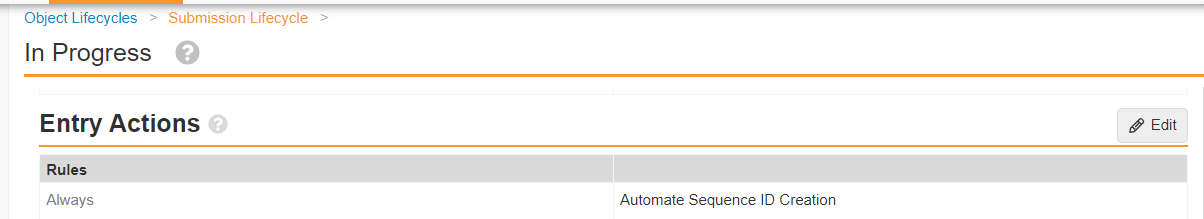
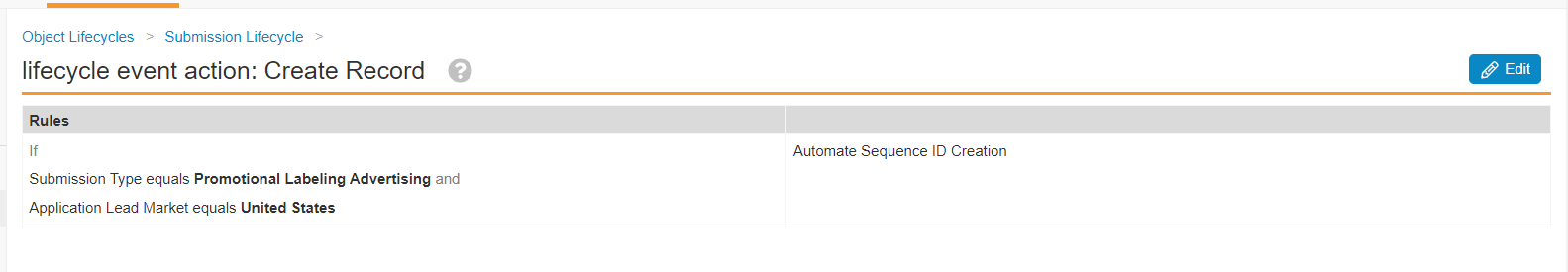
RIM-PromoMats: Configurable Action to Automate Sequence IDConfiguration24R1.2
To provide more flexibility for customers who may plan submissions far in advance of being ready to assign a Sequence ID, a new Action has been added: “Automate the Sequence ID.”
In 21R1, Veeva introduced the Admin setting to automatically populate the Sequence ID (xml_submission_id__v) field when a new Submission record is created. The Admin setting applies to all new Submission records Vault-wide, and will use the next available 4-digit sequence for that Application. The existing setting is used by the RIM-PromoMats Connection when creating 2253 Submission records; however, assigning a Sequence ID at the time of record creation is not always desirable.
With 24R1, “Automate the Sequence ID” can be configured as an Event Action or Lifecycle State Entry Action. Customers can specify when and on what Application Types/Submission Types the Sequence ID field is auto-populated. For example:
- Entry Action on Submission Lifecycle In Progress state: Sequence ID will only be populated when Submission record advances to In Progress
For RIM-PromoMats Connection customers, the “Automate the Sequence ID” Action will need to be executed upon record creation, therefore:
- Event Action: Update record upon Create
Submissions Publishing customers should ensure “Automate the Sequence ID” is configured to occur before any action that automatically enables Continuous Publishing to prevent failure of the Publishing job to initiate.
RIM-PromoMats: Enhanced Label Management in RIMAuto-on24R1.3
Beginning with 24R1, a label that originates in RIM can be transferred to PromoMats, where a CrossLink is created and included in the Compliance Package. With 24R2, when the Connection creates the new RIM Submission record and Content Plan for AdPromo submissions, system logic will be used to determine if the label in the Compliance Package is a document that originated in RIM, and if that label has been previously submitted to the Application.
The label’s origin will determine the system-managed steps taken, as follows:
| Scenario | Connection Automated Steps |
| 1. Label in the Compliance Package is not a CrossLink that originated in RIM | Create a CrossLink document in RIM, match CrossLink to Content Plan |
| 2. Label in the Compliance Package is a CrossLink that originated in RIM, AND the label was previously submitted | Identify source label in RIM, AND Create a Reference Leaf in Content Plan |
| 3. Label in the Compliance Package is a CrossLink that originated in RIM, AND the label was not previously submitted | Identify source label in RIM, AND match source label to Content Plan |
| 4. Label in the Compliance Package is a CrossLink that originated in RIM, BUT the source was deleted, or is an Obsolete/Superseded state | Error and User Exception Message sent |
The additional logic and system-managed steps provide support for customers who manually manage label transfer. It also eliminates creation of a circular CrossLink to a CrossLink for labels that originated in RIM and were returned to RIM via the Compliance Package transfer.
RIM-Clinical Operations Connection
RIM-Clinical Operations: Improved User Exception Message ProcessingAuto-on24R1.3
Admins and Users of the RIM-Clinical Operations Connection will encounter more efficient processing of User Exception Messages (UEMs). Previously, unresolved item processing errors resulted in new Active UEMs for the same error following the next job run, potentially multiplying into an unmanageable number of records. Now, backend refactoring will inactivate old UEMs in the next job run, and create a single Active UEM that contains all items run in the job, making error resolution simpler. Jobs will look at the last Active UEM and process only failed and new items.
RIM-Clinical Operations: Update to Performance Statistics
Upon further review, this internal feature is not visible to non-Veeva developers.
Safety-RIM Connection
Safety-RIM Connection: PSMF Annex H SupportAuto-on24R1.2
The Safety-RIM connection now transfers all data from RIM Vaults needed to generate Annex H of the PSMF in SafetyDocs Vaults. Additionally, with this release, SafetyDocs includes a standard report that organizations can use to generate the annex. These enhancements allow the PV team to own the Annex H document fully, streamlining the PSMF management process and compliance.
Learn more about the generating the PSMF Annex H document in your Safety Vault using product registration data received from your RIM Vault.
Safety-EDC Connection
Safety-EDC Connection: SAE Transfer EnhancementsAuto-on24R1.2
This release introduces enhancements to the Safety-EDC connection. If a customer has connections to both CDMS and Safety from their CTMS Clinical Operations Vault, Studies match in all Vaults based on the common Link (link_sys) values, regardless of existing matching criteria such as Study Number.
Learn more about the Safety-EDC Vault Connection.
Safety-EDC Connection: Custom Field SupportConfiguration24R1.3
With this release, the Safety-EDC connection supports custom fields. Admins can create and map custom fields across safety-related form types in EDC to Safety. When transferring SAEs, any custom field values that have been effectively incorporated into the CDMS Subject Information data model seamlessly transition through the Inbox Item to the relevant Case record, optimizing operational efficiency and data integrity.
Learn more about mapping fields for the Safety-EDC Vault Connection.
Safety-EDC: EDC Local Labs SupportAuto-on24R1.3
With this release, the Safety-EDC connection now facilitates the transfer of local lab data across safety-related forms in EDC to Safety. When transferring SAEs, any local lab test values integrated into the EDC subject casebook seamlessly transition through the Inbox Item to the corresponding lab section in the Case record, enhancing operational efficiency and data integrity.
Safety-EDC Connection: Support Case & Subject Deletion Scenarios from EDCAuto-on24R1.3
The Safety-EDC connection now effectively manages Case deletion scenarios from EDC. When a user clears an SAE form in EDC, Vault Safety generates an Inbox Item containing all Case details. The Inbox Item now includes an EDC Reason indicating that any corresponding Case must be voided or nullified.
In the event of a subject deletion in EDC, Vault Safety generates an Inbox Item for each Case associated with that subject with an EDC Reason field value indicating the subject was deleted in EDC.
When a Case is downgraded in EDC, Vault Safety generates a new follow-up Inbox Item, allowing users to compare and observe the removal of seriousness criteria.
Medical-Safety Connection
Medical-Safety Connection: Follow-ups, Multiple Reactions & ProductsConfiguration24R1.3
This feature introduces two improvements when sharing of Adverse Events between Vault MedInquiry and Vault Safety:
-
Additional Adverse Event information can be easily related to an initial Adverse Event in Vault MedInquiry and shared as a new Inbox item directly from Vault MedInquiry to Vault Safety.
-
Multiple products and reactions (Event Products and Event Reactions) can be captured under a single Adverse Event record in Vault MedInquiry and shared to Vault Safety as multiple Case Products and Case Adverse Events under a single Inbox Item.
Quality-RIM Connection
Quality-RIM: Resolve Reconnecting IssuesAuto-on24R1.2
Prior to this release, customers using the Quality-RIM Connection encountered issues after changing the connection’s remote Vault, and the resolution often required assistance from Veeva’s Customer Support team. This feature enables a customer’s Vault Owner to resolve the issue by inactivating Transactions and Transaction Logs without filing a Customer Support ticket. For more information, please refer to the Quality-RIM Connection.
Quality-RIM: Quality Inbound Job Processing ImprovementsAuto-on24R1.3
This feature introduces two improvements that to the Quality Vault for the Variant Management feature:
-
Prior to this release, it was possible for the Quality-RIM Connection job in the Quality Vault to return a “failed” status because there were no Transaction records to process. This occurred when the Quality Vault received multiple simultaneous messages from the RIM Vault. In this case, the first queued job processed all of the pending Transactions. The subsequent jobs “failed” because there were no more Transactions to process. This feature updates the job scheduling logic to mark a job as “failed to run” if another instance of the QMS-RIM Change Control job is already queued to run.
-
Additionally, jobs that run and find no transactions will now be marked as complete.
This feature does not have any impact on the RIM side of this Connection.
Clinical Operations
Several features listed in the Vault Connections section also affect the Clinical Operations application family.
CTMS
Complex Trials: Additional MetricsConfiguration24R1.3
With this feature, we continue to enhance support for complex trials by expanding subject metrics in two key areas:
-
Customers can now manage complex trial subject metrics without tracking the underlying subject data in Vault. Using the ‘Metrics Only’ calculation, customers can enter ‘Actual’ values for Subject Group Metrics, and Vault will perform the appropriate study rollups.
-
Actual Finish Dates on Last Subject Group Milestones (e.g., Last Subject Screened) can now be automatically populated prior to the completion of all enrollment activities at the study site. This allows customers to track completion of individual subject group enrollment activities prior to completion of the entire study at a study site.
Clinical Operations-Medical CRM Connection: Specify CRM Account Identifier per ConnectionConfiguration24R1.3
This feature provides additional support for customers using the Clinical Operations-Medical CRM Connection who connect to multiple CRM organizations. Previously, customers were required to select a single unique identifier field for Persons (Clinical Operations) and Accounts (CRM) in Vault Settings. In situations where there were multiple connections to separate CRM instances, this meant that the CRM Account Identifier had to match in each instance. With this feature, customers can now select a unique CRM Account Identifier per connection, providing greater flexibility.
Clinical Operations-Medical CRM Connection: Vault Setting RelabelAuto-on24R1.3
This feature updates the Vault Setting label from ‘Enable CRM Connection’ to ‘Enable Global CRM Connection Identifier Mapping.’ Prior to this release, the CRM Account unique identifier was set in Vault Settings. Now, customers are able to specify CRM Account unique identifiers per connection or choose a global identifier under Vault Settings. Thus, the label has been updated to reflect this change.
CTMS, Study Training
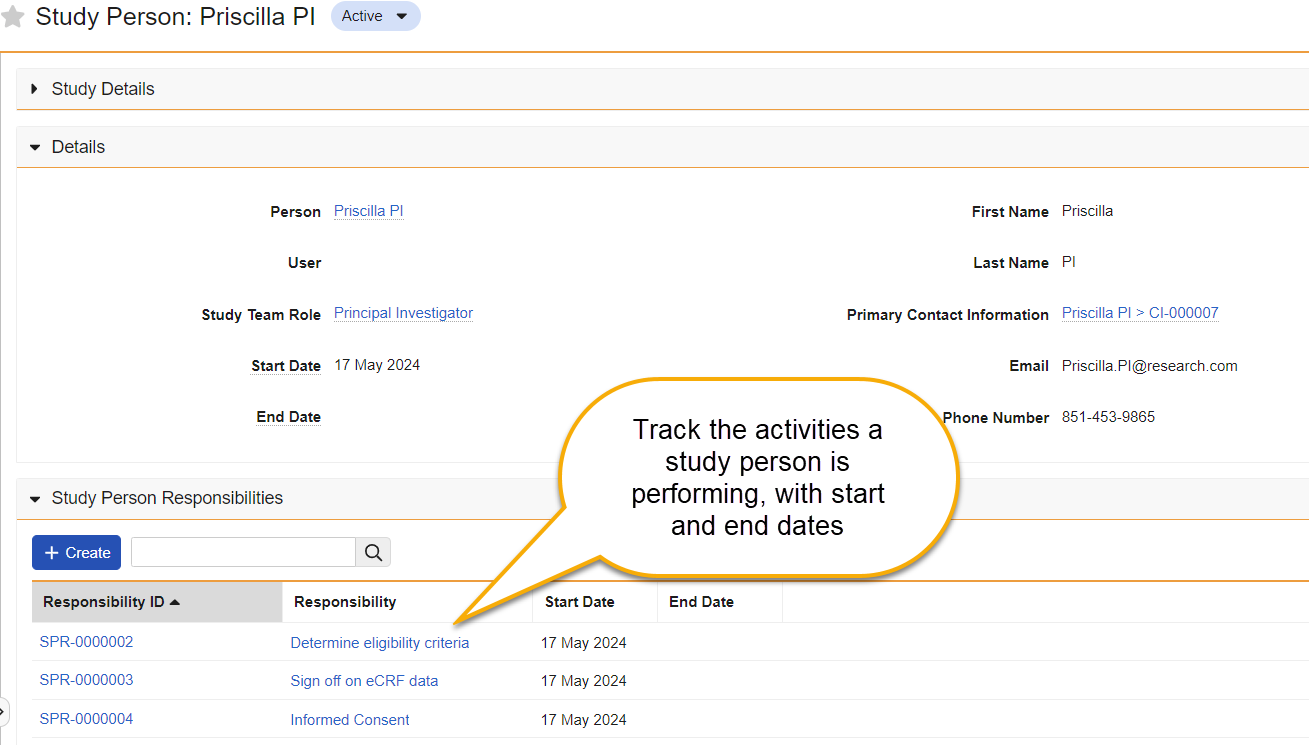
Study Person ResponsibilitiesAuto-on24R1.3
Study teams are composed of sponsor representatives, site staff, and other study personnel who are responsible for ensuring the ethical conduct of the clinical study, including protecting human subjects’ rights, safety, and welfare, verifying protocol compliance and data integrity, and ensuring adherence to all applicable regulations. They accomplish this through the performance of various study tasks and activities. Tracking who is responsible for different study tasks is important to ensure that only trained, authorized individuals are performing them.
To support this, in this release we are introducing the concept of Study Responsibilities. With this feature, customers can now track the specific Study Responsibilities (such as performing informed consent, assessing eligibility criteria, managing IRB/EC submissions, etc.) that each person is assigned. Study Training customers can transfer this information from Vault Clinical to Study Training to develop ‘responsibility-based’ training requirements.
Vault Payments
Repeating Visit ManagementConfiguration24R1.3
In this release, we’re adding support for Payments customers who use subject visit cycles (i.e., a grouping of subject visits that is repeated for a number of cycles). Using visit cycles provides greater flexibility in customizing visit schedules based on study design (for example, collecting additional data for a specific set of subjects). While the same set of visits may repeat in each cycle, customers may want to assign different fees for each visit based on the visit cycle, such as when there are slight variations in the procedures performed between cycles. Now, with this feature, Vault Payments customers can define different fees for the same repeating visit based on the visit cycle.
eTMF
Metadata Extraction Generally AvailableConfiguration24R1.2
The capabilities of the TMF Bot have expanded significantly over time. Beyond its initial function of automatically classifying documents, it now also possesses the ability to set metadata. Initially we focused on Study metadata (23R1) and more recently we extended to Country and Site metadata (24R1).
TMF Bot Metadata Extraction is now generally available, eliminating the need for support enablement. Customers can now independently create, test, and deploy Metadata Extraction models in their Vaults.
Country & Site Check in Document QC StepAuto-on24R1.2
TMF Bot continues its extension and enhances the Document QC Step to check Country & Site metadata. Thanks to this enhancement, TMF Bot will be able to identify, at any Document workflow step, classification and metadata issues (incorrect Study, Country or Site). Both possible issues and suggested resolutions will be presented to the end users in the same document panel. This will help our customers improve TMF documents’ quality and accelerate inspection readiness.
TMF Bot Enhanced Prediction MetricsAuto-on24R1.3
Considering the dynamic nature of eTMF, it’s crucial to regularly evaluate the performance of a trained model to maintain a consistent and successful execution of TMF Bot over time. Trained model Performance Metrics and Prediction Metrics enable the respective measurement of performances before & after deployment.
In our 24R2 release, we have implemented enhancements to these Performance & Prediction metrics for metadata extraction, aiming to improve accuracy and precision. These enhancements include:
-
Adding metrics to calculate extraction for Study Country & Study Site.
-
Introducing a “Documents Extracted” metric for an accurate count of documents the TMF Bot was able to evaluate.
-
Updating metadata extraction metrics to account for scenarios where users set metadata upon upload and adjusting the success rate calculation accordingly.
-
Ensuring consistency in metric calculation between Prediction Metrics and Trained Model Performance Metrics.
Simplified Model TrainingAuto-on24R1.3
Several customers have implemented custom training models for TMF Bot. However, our investigations revealed instances where the settings or training sets on these models resulted in hindered performance after deployment.
To optimize model performance and streamline management, we are simplifying TMF Bot Model training.
-
The following fields will become system managed and set with default value: Prediction Confidence Threshold (PCT): 0.85; Minimum Documents per Type: 10; Auto-Deploy: TRUE
-
The Advanced Model Parameters field will no longer be visible.
-
The option to select a training source based on a CSV of Document IDs is deprecated. Instead, date-based training, relying on the Training Window Start Date, will be the only training source option available.
Customers will still be able to narrow the scope of their training set via Excluded Classifications or a VQL string in the Document Criteria - VQL field. This VQL criteria can be used to exclude, for example, documents created via a migration account, or documents that sit under a Non-TMF document type in your system.
Study Startup, eTMF
Recurring Milestone Schedule Automated Date UpdateAuto-on24R1.3
This auto-on feature automates updates to the Next Create Date field on the following cases:
- Auto-set Next Create Date = Initial + Offset fields on activation of Recurring Milestone Schedule object record
- Null Next Create Date field on inactivation of Recurring Milestone Schedule object record
When this feature was released in 24R1 populating these field values required configuration, now this automation streamlines the process and bridges this gap.
All Clinical Operations Applications
CTMS Transfer: Deletion in SourceAuto-on24R1.3
CTMS Transfer automates the daily transfer of study data from the CRO to the sponsor when both organizations utilize Vault CTMS. Over time, some of these previously transferred data may be deleted by the CRO in the Source Vault. However, until now, the Sponsor Vault lacked a mechanism to track these deletions.
Recognizing the critical importance of ensuring that changes made in the Source Vault by the CRO are accurately mirrored in the target Vault, CTMS Transfer has been enhanced to now highlight records that have been deleted in the source system thanks to a new Deleted in Source System field. This enhancement ensures that sponsors can confidently conduct thorough analyses and make informed decisions based on the most current data available.
Clinical Workflow ContextAuto-on24R1.3
This feature extends the Milestone Workspace by supporting the view and initiation of workflows on EDL Items and Documents. Workflow Variables are introduced on object and document workflows started from the workspace. This will subsequently support new tabs, Current Workflows and Workflow History, within the workspace where related workflows will be aggregated in a singular table and users will be able to view both active and completed workflows initiated from the workspace. Additionally, users will be able to navigate directly into a workflow envelope from the Milestone Workspace workflow tab to complete assigned tasks. This feature is auto-on and will be automatically available in Vaults where the Milestone Workspace is configured.
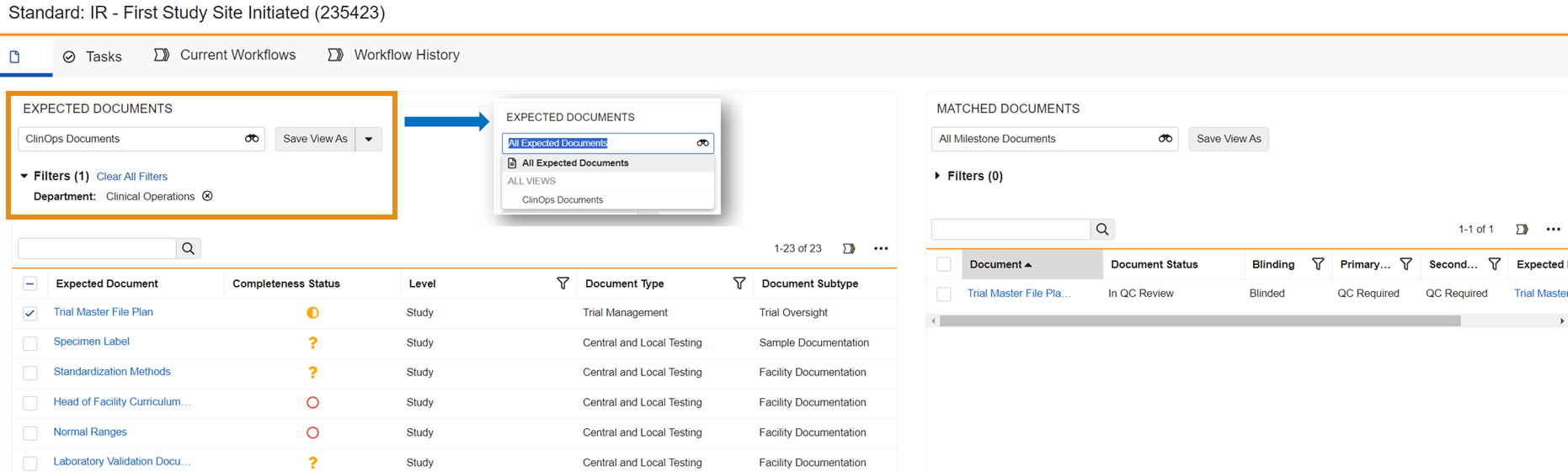
Milestone Workspace View EnhancementsAuto-on24R1.3
Usability continues to be enhanced in the Milestone Workspace. Workspace users will now have the ability to filter on columns in the Expected Documents Object Data Grid (ODG) as well as ‘select all’ within any ODG. The Milestone Workspace will also allow for Saved Views in any data grid’s filter criteria. This feature is auto-on and will be automatically available in Vaults where the Milestone Workspace is configured.
Recalculate Completeness Metrics Action on MilestoneConfiguration24R1.3
This configurable feature introduces a manual action that will trigger the recalculation of completeness metrics on milestones, this will ensure a true reflection of the metrics on actual time, so users don’t have to wait for the system to update it or provide inaccurate data.
eConsent: Send to Site ActionConfiguration24R1.4
In this release, Veeva is introducing a dedicated solution for sharing eConsent forms between sponsors and sites. With this new product architecture, sponsors can more independently configure eConsent and other connected study products in their Vaults as their business needs differ. This new approach continues to support the distribution of eConsent forms to single or multiple sites on a consent form by consent form basis.
To accommodate this you must configure a new eConsent Send to Sites action in your Clinical Operations Vault to replace the previous Distribute via Clinical Network action.
Site Connect
Site Connect Site Users in Clinical OperationsAuto-on24R1.3
The Site experience of Site Connect is now in Clinical Operations Vaults. Sponsors and CROs can manage Site User access to Study Sites in Clinical Operations Vaults. This change is auto-on, but existing SiteVault users will not automatically have users created in Clinical Vaults. Sponsors and CROs need to ensure that the appropriate Study Site Personnel have had their Site Connect User field enabled in order to provide uninterrupted access to Site Connect.
Existing connectivity to SiteVault will continue to be supported and any Study Sites that were Connected prior to this feature, will automatically remain Connected after its release. SiteVault sites can now optionally connect and disconnect themselves using the Site-driven Connect to SiteVault feature.
Site Connect Site Users will have a similar experience in Clinical Vaults, including access to existing SiteVault functionality and gaining access to new Site Connect features. Sponsor/CRO users will also be able to view Site Connect tabs, including the Site user’s Site Home view, within their Clinical Vault. For more information on individual Site Connect features available in Clinical Vaults, continue though the Site Connect Release Notes below.
Add Site Personnel into Site ConnectAuto-on24R1.3
This new feature enables Site User access to Studies through the Study Directory. Similarly to how Study-specific access is managed for Clinical Vaults, customers can now grant Study Site access to Site Users through a Site Connect User field on the Study Person record. This supported functionality is auto-on for Vaults with Site Connect, but the field is not automatically added to the Study Person object layout. Tracking of email invitations and access start and end dates are also supported.
Study Contacts Section for SitesAuto-on24R1.3
Customers can now share Sponsor/CRO Study Contacts with their Study Sites using Site Connect users. The Study Contact feature includes a new object called Study Contacts, two object types, and an associated lifecycle. A Study Contacts tab is accessible to Sponsor/CRO users within the existing Site Connect menu tab. Study Contacts will be managed by Sponsor/CRO users and able to be viewed by Site Connect Site users. An additional action to create a Study Contact from an existing Study Person record will also be available for ClinOps users.Study Contacts can pull in existing Study and Global Directory data, from Persons, or have independent contact information.
The Site Home menu will include a new Study Contacts section that will display the shared Study Contact records.
Study Announcements Section for SitesAuto-on24R1.3
This auto-on feature allows Sponsors/CROs to publish announcements such as trial deadlines and news in Vault Clinical so they can broadcast relevant information to the Sites they work with. Once Published, these posts will be visible to ‘Site users’ in Site Connect so they can reference it later and will also be sent to ‘Sites’ as email notifications.
It will include the following components:
-
New ‘Study Announcements’ tab
-
New ‘Study Announcements’ object & lifecycle
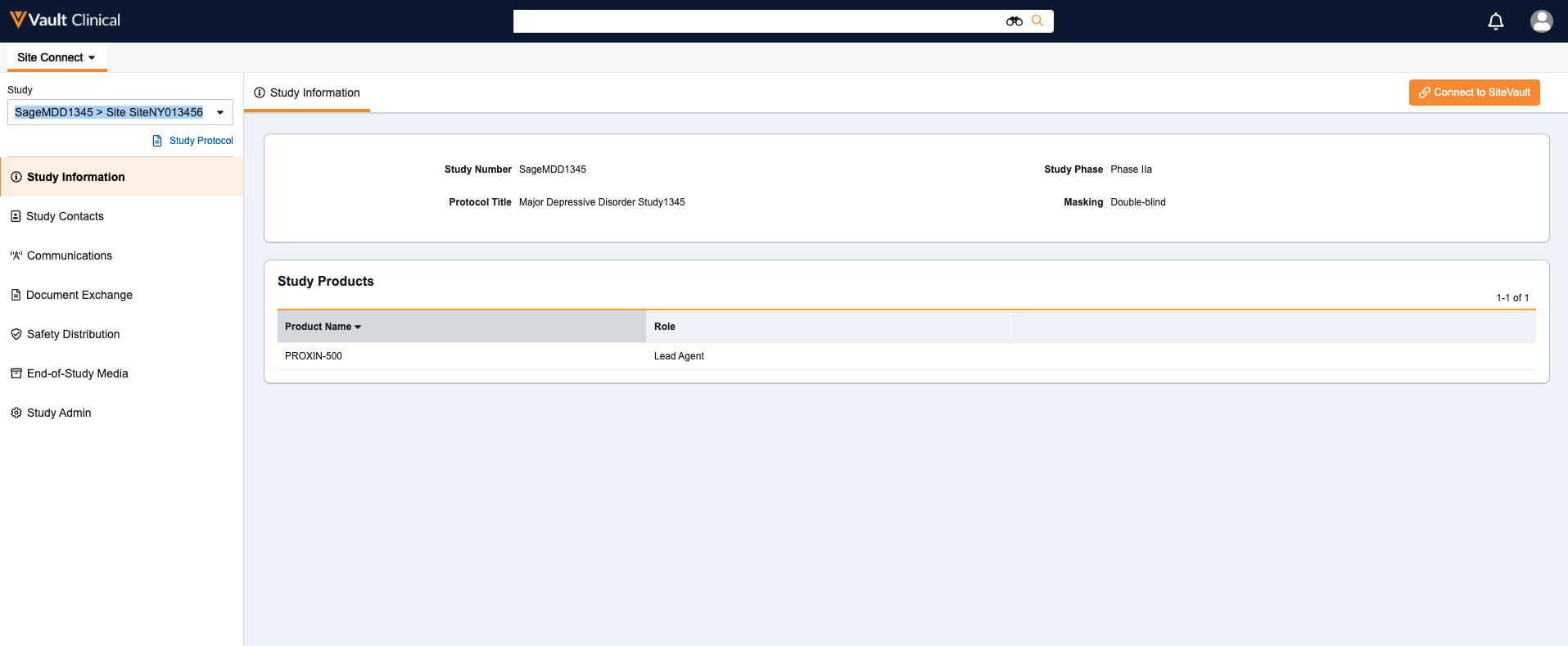
Study Information Section for SitesAuto-on24R1.3
This auto-on feature provides a new section within Site Connect ‘Site Home’ that displays an overview of the Study with a set of standard fields describing the trial in a way relevant to study sites. This information includes Study Number, Protocol Title, Study Phase, Masking and Study Products.
Study Protocol LinkAuto-on24R1.3
Site Home now includes a Study Protocol link below the Study Selector that allows Site Connect users to quickly open the Study’s Protocol without navigating to another section or page. This new feature is auto-on and displays the most recent version of the Protocol that has been sent to the Site in a pop-up window.
End-of-Study Media Section for SitesAuto-on24R1.3
This auto-on feature provides a new section in Site Connect to display ‘End-of-Study-Media’ documents (e.g. CRFs) that have been sent to the ‘Site’. Site Users can view and download these documents in bulk, just like they can in the Document Exchange section.
It will include the following components:
-
New section within Site Connect Site Home
-
Filtered list of documents to Completed CRFs
-
Bulk Download/File to Site Vault user actions
Study Selector in Site HomeAuto-on24R1.3
Site Connect users can easily navigate between studies for the Sponsor using the Study Selector on the Site Home page. The Study Selector dropdown only displays studies that the user has access to using the format [Study Number] >Site [Study Site Number]. Type ahead search allows users to quickly locate and select the desired study.
eSignature in Site ConnectAuto-on24R1.3
This feature introduces a standard workflow, Site Connect Site eSignature, in Clinical Vaults that allows a Site User in Site Connect to eSign a document shared through Document Exchange. This auto-on workflow is available as a user action, eSign to Complete, for Site users on Update & Return Document Actions.
eSignatures from SiteVault Viewable in Document ViewerAuto-on24R1.3
Documents originating from SiteVault that are eSigned will now have their signature pages visible in the Clinical Vault document viewer. Additionally, the Site Rendition in Clinical Vaults is now the rendition being sent to the site when a document is eSigned rather than the rendition received from the site. The Site Rendition combines the Viewable Rendition and eSignature page. This adjustment to Site Rendition behavior is auto-on for Clinical Vaults with Site Connect enabled.
Study Admin Section for SitesAuto-on24R1.3
With this feature, we’re introducing a new Study Admin section within the Site Home page. In this section, Site Admin users have access to two new tabs, Site Staff Access and Settings, where they can manage site personnel and settings on a study-specific basis. Read more about Site Staff Access under the Self-Service Site Access feature.
In Settings, Site Admins can now manage which studies are connected to SiteVault (if used). When connected, Site Admins have additional options to allow the study sponsor/CRO to exchange documents with SiteVault, enable auto-filing of safety documents and end of study media, and enable upload of files, providing greater flexibility and control for sites using SiteVault.
Self-service Site AccessAuto-on24R1.3
This feature streamlines site staff management by allowing Site Admins to add and remove site staff under the Site Staff Access tab in the Study Admin section. Site Admins can add up to five (5) site staff. When permitted, adding site staff provides access to study records without prior approval. If approval is required, Site Admins follow the same process to add site staff and request access. These records display as Access Requested until processed by the study sponsor. This permission is controlled by the study sponsor on a study-specific basis.
Allow Sponsor/CRO to Exchange Documents with SiteVaultAuto-on24R1.3
With this feature, Site Admins can choose to allow sponsor users to take actions on the site’s behalf, simplifying document exchange and interaction. When enabled, sponsor users can file documents to SiteVault and complete tasks using SiteVault documents. When disabled, sponsor users will not be able to do so. This feature is disabled by default.
File to SiteVault & Upload from SiteVault in Site HomeAuto-on24R1.3
This auto-on feature allows Sites that have connected their SiteVault in Site Connect Site Home to file documents to their SiteVault and to upload documents from SiteVault into Clinical Operations Vaults. These actions can also be taken in bulk.
Document Exchange for All SitesAuto-on24R1.3
This auto-on feature allows Site Connect customers to send documents to all sites. The Send to sites document/expected document actions and the Send Site Packages action will now work with all study sites on Studies with Document Exchange in the Connected Study Type field.
All Site Connect Site Users will receive email notifications containing document request details and links to the documents that have been sent to them by the Sponsor/CRO.
Bulk Document Download in Site Connect Site HomeAuto-on24R1.3
This feature allows Site users to bulk download documents from the Site Home document grid. When at least one document is selected, the Download option is available from the action menu. This action creates a zip file containing either the site rendition or source file of each selected document. Once documents are processed, users receive email and Vault notifications with a link that downloads the zip file to the user’s computer.
Site-driven Connect to SiteVaultAuto-on24R1.3
This feature introduces a streamlined and simplified way for Site users to connect themselves to SiteVault for their Studies. Site users will now have the ability to initiate this connection directly within Site Connect, bypassing the need for Sponsor’s involvement and also removing the need for Connected Study Invitations. Site Connect Site users can connect by clicking on the Connect to SiteVault button from within Site Home, entering the Universal Site Number (USN) associated with their SiteVault Site, and then selecting the relevant Study to complete connection establishment.
Once connected, Site users will then be able to perform additional actions such as File to SiteVault and Upload from SiteVault to complete Sponsor tasks.
Connected Study Invitations DeprecatedAuto-on24R1.3
With the introduction of the Add Site Personnel into Site Connect and the Site-driven Connect to SiteVault features which streamline getting sites up and running with Site Connect, the previous connection method based on sponsor-driven invitations and site agreements becomes obsolete and is deprecated. Note that any site that was using SiteVault on a Connected Study prior to the 24R2 release will automatically be Connected after the 24R2 release, ensuring uninterrupted access to their eISF.
Standardized Safety Distribution Email TemplateAuto-on24R1.3
With this auto-on feature a new standardized email format will be used for Safety Distributions:
-
The Safety Distribution Email Notification (
safety_distribution_email__v) will no longer be used -
The new standard email format will contain the same details and document links for site recipients to access safety documents
With this update, Site personnel will have a consistent experience across all Sponsors/CROs using Site Connect Safety Distribution.
Clinical Operations-EDC Connection
Clinical Operations-EDC Connection: Repeating Visit ManagementConfiguration24R1.3
In this release, we’re enhancing the Clinical Operations-EDC Connection to add support for visit cycles (i.e., a grouping of subject visits that is repeated for a number of cycles). Now, using this feature, customers can send the following additional information from EDC to CTMS:
-
Visit Group Definitions (called Event Groups in EDC) and Repeating Visit Labels (to sync labels between CTMS and EDC).
-
Visit Group and Visit Sequence on Subject Visits
In Clinical Operations Vaults, Payments customers can now use this information when defining different fees based on the visit cycle.
Clinical Operations Data Model Changes
See 24R2 Clinical Operations Data Model Changes.
Commercial
Several features listed in the Vault Connections section also affect the Commercial application family.
PromoMats
Portals: Enhancements to Document CardAuto-on24R1.2
Enhancements have been made to document cards on the Portals homepage that allow users to quickly find more information on documents. Users can now see the file format for a document represented by an icon similar to other areas of the Vault UI. Additionally, the document type/classification is now visible without having to hover over a document card. The format is the same as it is today on hover (Document Type > Subtype > Classification).
Portals: Update to Portal Tab VisibilityAuto-on24R1.2
With this release, Portals Tab visibility is based solely on user permissions, meaning users with permissions can still see the tab even when no portals exist. Prior to this release, if users had access to the Portals Tab but no portals existed, the tab was hidden.
Modular Content: UI ImprovementsAuto-on24R1.2
With this enhancement, we have made usability improvements to the Modular Content UI, including surfacing the Content Module Details on the user interface, a navigation panel to the left of the modules, and a refreshed Quick Look panel.
eCTD: Change Material State on SubmissionConfiguration24R1.2
Administrators can now configure an entry action on the Submission Ready Lifecycle to change the state of the eCTD Source document when the state of the submission ready copy is updated. We recommend configuring this on the Submitted state of the Submission Ready Lifecycle to set the eCTD Source document to the Steady State state type. The action will only run on documents where the Main Promotional Material? field is equal to Yes. This is a system-managed field on the clean material submission ready copy and must be activated if not configured already.
Multichannel: New Email Preview FormatsAuto-on24R1.2
With this release, Vault includes more current email formats when previewing Approved Email content prior to publishing to field teams. This release also removes older email formats and clients.
Harvesting ClaimsConfiguration24R1.3
This feature allows you to extract link annotations from documents to populate the Claims library directly from the user interface. Users can harvest the annotated text as Match Text, bring forward linked references, and copy the document into the individual Claim records. You can immediately approve Claims upon creation if the document you are harvesting from is Approved. This helps streamline the Claims management process by providing teams with automated Claim creation.
Modular Content: Create a Local CopyConfiguration24R1.3
Content Modules contain various Assets that may require localisation. This feature significantly improves the process of localizing Content Modules. Vault can now identify if an Asset was previously localized, and recommends using the existing Asset where appropriate. If no local Asset exists, Vault streamlines the localization process by allowing you to create local copies of Assets directly from the Content Module record.
Modular Content HTML Previews: CRM Email ModulesConfiguration24R1.3
Using CRM Email Modules, you can create variations of Content Module Assets and assemble them in Veeva CRM to create an Approved Email, providing a more personalized customer experience.
This feature allows you to visualize variations of Content Module Assets with predefined Templates, providing a more streamlined and practical way of reviewing CRM Email Module content.
Modular Content: Commenting for MLRAuto-on24R1.3
With this release, we streamlined the review and approval process for Content Modules in Vault PromoMats. This new functionality enables users to comment on Content Modules, Content Module Assets, or Content Module Combinations directly from the Modular Content tab.
Commercial Content Taxonomy for Life SciencesConfiguration24R1.3
This feature introduces standard document types that reflect the Commercial Content Taxonomy for Life Sciences. This enhancement improves interoperability of Vault-to-Vault Connections, reduces the burden on Vault administrators, and simplifies the user experience.
The new document types are provisioned as inactive and require configuration to enable.
Portals: Documents added to Portal on Behalf of SystemConfiguration24R1.3
This enhancement simplifies the security requirements for documents added to Portal Widgets via the Portal user interface. Now, when users add content to a Widget, they do not require the Edit Fields permission on the document to complete the action, and the action is completed by Vault on the user’s behalf. This removes the need to grant a Portal Manager Edit Fields permissions on documents.
Text Asset Reason for CopyConfiguration24R1.4
When copying a Text Asset, Vault now allows you to track the Reason for Copy. The following reasons are available:
- Local Adaptation/ Re-Use
- Metadata Copy
- Modification
This feature improves the ability to track Text Asset reuse and adaptation.
Modular Content Commenting Usability EnhancementsAuto-on24R1.4
With this release, we have improved the Modular Content commenting user experience. You can now navigate directly to specific Asset comments within the comment panel by clicking on the relevant Asset. Additionally, if you select a specific comment, Vault highlights the relevant Asset.
Make a Local Copy: User Action Permission RefinementConfiguration24R1.4
With this release, when a user performs the Make a local copy user action to make a copy of a Content Module, Vault checks their permissions against the types of Content Assets within the Content Module to ensure that they have the permissions necessary to create copies of these Content Assets. Users only require permissions for the types of Content Assets that exist within the Content Module that they are copying.
Commercial Data Model Changes
See 24R2 Commercial Data Model Changes.
Medical
Several features listed in the Vault Connections section also affect the Medical application family.
MedInquiry
Capture Multiple Reactions & Products Under EventsConfiguration24R1.2
The new Event Reaction and Event Product objects allow structured capture of multiple reactions/events and multiple products in an Adverse Event or Product Quality Complaint. Multiple Event Reactions and Event Products generate the corresponding repeating sections in the E2B(R3) report.
Telephony Support: Embedded Call ControlsConfiguration24R1.3
This feature enables MedInquiry users to answer and make phone calls and answer live chat requests within Vault. This feature speeds up the inquiry intake process with a pop-up that redirects users to the Case Intake UI. Users are no longer required to use two systems to log cases while talking to HCPs on the phone or live chat since the communications can be handled within Vault.With this feature we are also releasing a standard telephony integration with Amazon Connect and a set of common telephony methods that customers may use to integrate other telephony providers.
CC & BCC: Usability Enhancements & Automatically Add RecipientsConfiguration24R1.3
This feature simplifies the addition of CC and BCC recipients on Case Response emails. Responding to a query often requires having additional recipients as either CC or BCC. This feature adds a new UI component that allows Medical Inquiry users to add CC and BCC recipients without manually creating join objects.
Additionally, Admins can configure two new actions on the Case Response object lifecycle:
- Add CC or BCC to Email can be configured as user action or entry action. Common use cases include automatically copying MSLs on responses concerning respective products.
- Match or Create Person can be configured as user action, entry action, or create record action. An example use case is where an email address of an MSL is synced into a text field in Vault from an external source such as Veeva CRM or a web form, and Vault needs to match the text field value to the Person record of that MSL.
MedInquiry Keyword TrackingConfiguration24R1.3
This feature introduces keyword tracking, which allows Vault to gather insights on the usage of specific terms across requests for medical information.Medical Affairs teams often prepare reports detailing key topics mentioned across inquiries. This functionality enables the creation of Medical Inquiry Keywords and variations that can be tracked across Case Requests and compiled into easily-produced reports to highlight trends across inquiries to further drive new content creation strategies.
CRM Data Sharing: Error Handling EnhancementsAuto-on24R1.3
This set of enhancements improves error handling for CRM Data Sharing of Accounts and Inquiries between Veeva CRM and Vault MedInquiry to ensure consistency across jobs. The success/failure processing for the Pull Account and Pull Inquiry jobs are updated to match the Push Inquiry job. Failed records are now automatically picked up in the next job run.
- Separate date fields are added for the Pull Inquiry and Push Inquiry jobs to record when each job was last successfully run for the Case record.
- Multiple CRM Org processing is also updated for the Pull Account, Pull Inquiry, and Push Inquiry jobs.
Respond to Multiple Requests in One ResponseConfiguration24R1.3
This feature allows users to bundle multiple answers in a single response when addressing multiple questions from the same requestor. Medical Inquiry users frequently respond to multiple inquiries within the same Case. With this release, each request does not require a corresponding response. Instead, Vault allows users to prepare individual responses before selecting which responses should be included in the Case Response sent to the requestor. In cases where an HCP’s preferred response channel is email, this functionality prevents users from needing to issue multiple separate response emails to address multiple HCP requests.
MedInquiry, Safety
Follow Up Events Received in MedicalConfiguration24R1.3
This functionality enables Vault to relate additional Adverse Event information to an initial Adverse Event and to share this information as a new Inbox item directly from MedInquiry to Vault Safety. It is common for Adverse Events to include additional information after the initial capture, where further information is shared with the Medical Affairs team as a follow up. Admins can configure the new Create Event Follow Up action on the Case object lifecycle to allow the creation of new records that are directly linked to previous Cases and Adverse Events. In addition, if the Copy Record action is run from an Adverse Event marked as Follow Up, the new record is automatically linked to the source Case and Adverse Event.
MedComms
Scientific Communication PlatformsConfiguration24R1.3
Organizing your Scientific Communications can help you generate insights for effective content utilization and engagement. The new Scientific Communications Platform provides a standardized object-based architecture that allows you to organize your Scientific Communications, including Objectives, Positioning, Pillars, Statements, References, and Resources. This new functionality is a foundational hierarchy for Scientific Communication management.
Portals: Documents added to Portal on Behalf of SystemConfiguration24R1.3
This enhancement simplifies the security requirements for documents added to Portal Widgets via the Portal user interface. Now, when users add content to a Widget, they do not require the Edit Fields permission on the document to complete the action, and the action is completed by Vault on the user’s behalf. This removes the need to grant a Portal Manager Edit Fields permissions on documents.
Portals: Enhancements to Document CardAuto-on24R1.2
Enhancements have been made to document cards on the Portals homepage that allow users to quickly find more information on documents. Users can now see the file format for a document represented by an icon similar to other areas of the Vault UI. Additionally, the document type/classification is now visible without having to hover over a document card. The format is the same as it is today on hover (Document Type > Subtype > Classification).
Portals: Update to Portal Tab VisibilityAuto-on24R1.2
With this release, Portals Tab visibility is based solely on user permissions, meaning users with permissions can still see the tab even when no portals exist. Prior to this release, if users had access to the Portals Tab but no portals existed, the tab was hidden.
Multichannel: New Email Preview FormatsAuto-on24R1.2
With this release, Vault includes more current email formats when previewing Approved Email content prior to publishing to field teams. This release also removes older email formats and clients.
Medical Data Model Changes
See 24R2 Medical Data Model Changes.
Training
Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
Study Training
VeevaID Invitation Based on Study Person TypeAuto-on24R1.2
The Clinical Operations Person record’s Person Type field now determines whether a Learner receives a VeevaID invitation when they are brought over to Study Training with an assigned Study Team Role.
Quality
A feature listed in the Vault Connections section also affects the Quality application family.
Quality: Allowed Application LicensesAuto-on24R1.3
Beginning in 24R2, system administrators can only select valid application license types for each application. The valid application license types for applications in the Quality suite are shown below.
| Quality Suite Application | Valid Application License Types |
| QMS | Full, External |
| QualityDocs | Full, External, Read Only |
| Station Manager | Full |
| Training | Full, External, Learner |
| Study Training | Full, External, Learner |
| Validation Management | Full, External |
| Surveillance | Full, External |
| Batch Release | Full |
| LIMS | Full |
For additional information, please refer to Streamlined User Licensing Management.
Vault Batch Release
Batch Release Execution App Page (Edit / Actions)Support24R1.2
Vault Batch Release is a new Vault Quality Suite application that offers an automated, end-to-end solution for dispositioning batches. Vault Batch Release features a dedicated page aimed at simplifying batch disposition for quality personnel post-completion of Checks containing Items. This user-friendly interface consolidates all pertinent records including Batches, Dispositions, Checks, and Items. Checks and their respective Items are displayed in a structured “tree-grid” format with visual status indicators, enhancing the visibility of completion statuses. Links to related items such as deviations and documents expedite information review and status assessment. Standard pages will cover all other application needs including list views, reports, and the administration of Disposition Plans. Material-specific Disposition Plans are used to determine what Checks need to be completed to disposition a batch.
Note: Vault Batch Release is only available to customers enrolled in the Early Adopter Program. To learn more, please contact your Account Partner or Customer Success Manager.
Batch Release Workflow IntegrationSupport24R1.2
With the launch of Vault Batch Release, a new workflow is available to help coordinate the review and approval of Dispositions after all their Checks have been completed.
QualityDocs
Process Navigator: Hierarchy BuilderAuto-on24R1.4
This feature provides a streamlined experience of Process Navigator for both Users and Administrators. Users are able to view the entire process tree for a given Visual Hierarchy object type, as well as any associated processes and process documents, on the Process Navigator tab. Content can be located much more quickly and easily without the need to navigate or drill down through subprocess pages.
Process Owners and others with the appropriate permissions are able to create, reorder, edit, and delete parent and child hierarchies, as well as assign documents to processes, directly and easily from the Process Navigator tab. Visual Hierarchy administration is no longer required to be performed from the Business Admin module.
Governance limits require that no more than 5000 records are created per Visual Hierarchy object type and no more than 200 Hierarchy Document records are associated to a single Visual Hierarchy record.
User View:
Administrator View:
Enable Deletion of Documents with Controlled CopiesAuto-on24R1.3
The system no longer prevents Administrators from deleting documents that have Controlled Copy User Input and Controlled Copy Trace object record references. The related object records are not deleted.
Support up to 3 External Collaborators for Document External CollaborationAuto-on24R1.2
Users can send a document for review or approval to up to three External Collaborators at a time. Prior to this release, only one External Collaborator was able to be assigned to a task.
Training
Note: All features apply to Study Training in addition to Vault Training, unless otherwise indicated.
Instructor-Led Training: Learner Self-Attendance & Learner E-SignatureAuto-on24R1.4
This feature allows Learners to sign off or acknowledge that they have attended an Instructor-Led session, either with or without an eSignature.
Some regulations require that Learners acknowledge their own attendance to Instructor-Led sessions. Without this feature, the Instructor is the single point of confirmation that the training was completed, and is seen as a risk by customers.
- This feature can be enabled or disabled per class.
- Instructors can generate a QR code that can be displayed on a screen or printed. Learners can scan the code to acknowledge their attendance and receive a prompt to record their eSignature (if enabled).
- This feature can be used with or without an eSignature requirement.
- The class can be completed but the Learner’s Training Assignment will remain open until the eSignature is completed.
- Limitation: These functionalities are applicable to the new ILT page only.
For more details and configuration, see Instructor-Led Training.
Learner Homepage: Update Filter Permission BehaviorAuto-on24R1.4
Learners can now use filters on the Learner Homepage, regardless of permissions for a Curriculum.
Enforce Valid License Types for Vault Training & Study TrainingAuto-on24R1.3
Refer to Quality: Allowed Application Licenses
External & On-the-Job Training (OJT): Support Document-Based TrainingConfiguration24R1.3
Learners can now view documents related to the Evaluation and External Training Requirements. This allows Learners to see one holistic view and enables the triggering of retraining on External or Evaluation Training Requirements in the event that the related document is up-versioned.
Administrators can associate documents with any Training Requirement, creating a consistent experience across all types.
Learners do not need to mark documents complete. Completion occurs against the whole Training Requirement and not the individual pieces.
Vault creates a Training Requirement Impact Assessment when an associated document is up-versioned. This is now consistent for all requirement types.
Learner View of Evaluation with Documents:
Limitations:
- Training Content Sets will not be created for existing Evaluation and External Training Requirements.
For more details and configuration, see Configuring On-the-Job Training and Configuring External Training Requirements.
On-the-Job Training (OJT): Learner Selects EvaluatorConfiguration24R1.3
Learners can now select one or more Evaluators from a list for an Evaluation Training Requirement. This speeds up the process of determining the correct Evaluator for the Learner in cases where the Learner knows who their Evaluator should be. For example, the Learner knows that they work on the same shift as Jim Smith, so they select Jim as their Evaluator.
A Training Admin must configure the Evaluation Training Requirement to allow the Learner to select their Evaluator. Training Admins can also change the assigned Evaluator.
For more details and configuration, see Configuring On-the-Job Training.
Training Matrix VisualizationConfiguration24R1.3
Users can now see a visual representation of how an individual training record fits within the training matrix overall.
In the past, Training Admins have used various methods to visualize the configuration of a Learner’s training matrix, from Excel files to navigating through Vault objects. These manual methods lead to errors, out-of-date information, and are generally difficult to consume.
Users can access a visual representation of a Learner’s training matrix from a Person, Training Requirement, or Training Requirement Impact Assessment object record page. Once expanded, Vault displays up to 1,000 Curriculum and 50 Learner Role records for a given visualization. Inactive and retired Learner Roles, Curricula, and Training Requirements are excluded from view.
Manager Homepage: Individual Learner PageAuto-on24R1.3
Note: The Manager Homepage is also known as the My Team page, accessed from a tab of the same name.
Managers can now view details about a direct report’s learning progress.
Managers have a need to understand their direct reports’ training status. Prior to this feature, Managers could only view high-level information, such as due dates. This view will allow more details as a live view, without the need for flash reports.
This feature allows managers to see due and completed training for an employee. They can also view whether a direct report has completed training on-time or late and nudge direct reports, reminding them to complete their assignments.
Recommendation: Enabling the Training Matrix Visualization feature is recommended to utilize the full capabilities of the Manager Homepage.
Manager view of Open Assignments on Individual Learner Page:
Manager view of Completed Assignments on Individual Learner Page:
For more details and configuration, see Manager Homepage.
Learner Homepage History Tab: Export Completed AssignmentsAuto-on24R1.3
Learners can now export completed training from the History tab to PDF format. This provides a compliant report for Learners to export and provide as proof of training.
An Admin can add History tab filters, allowing Learners to filter by the configured fields.
A new Due Date column is available in the History tab.
For more details and configuration, see History Tab.
Learner Homepage History Tab: Ability to Search by Specific FieldsConfiguration24R1.3
Users can now search by certain fields within the History tab. This creates more ways for a Learner to search and narrow down completion records.
For more details and configuration, see History Tab.
Learner Homepage: Update Filter Permission Behavior
Learners can now use filters on the Learner Homepage, regardless of permissions for a Curriculum.
This feature is deferred to a future release.
Hierarchical Copy Allowed for Training ObjectsConfiguration24R1.3
When performing the Copy Record action for a Learner Role, Curriculum, or Training Requirement, the user can now additionally copy its related records. This saves time for a Training Admin when, for example, they need to duplicate a Learner Role for another Learner.
Training Limits UpdatedAuto-on24R1.3
With this update, the number of Learners who can receive assignments via a single Direct Assignment is increased to 500. Additionally, the number of Study Learner Roles allowed to reference a single Study is increased to 150. This increases assignment efficiency and allows the viewing of additional Studies in the Training Matrix.
Learner Homepage History Tab: Performance Improvements & Updates to SortAuto-on24R1.2
Performance improvements were made to the History tab on the Learner Homepage to quickly display completed Training Assignments:
- It is no longer possible to sort by Curriculum or Learner Role columns.
- The list of field values in the filter shows up to 50 values. To locate a field out of view, users can type ahead to search more.
- To filter on a Training Assignment object field, Learners require Read permission to that field.
Quiz UI Improvements for Mobile AppAuto-on24R1.2
While taking a quiz on the mobile app, users can navigate back to the previous page. Previously, this was not possible.
Learner Homepage History Tab: Assignment Due DateAuto-on24R1.2
An additional Due Date column is automatically added to the Learner Homepage, displaying the due date of completed training assignments. The column is available to sort assignments, and Learners cannot remove it.
QMS
Standalone Quality Events EnablementAuto-on24R1.3
For organizations deployed with all of their core QMS processes modeled as object types of the Quality Event object, this release introduces a new self-service administration page for changing a Vault from a Merged Quality Event data model, into a standalone object data model for core QMS processes. This feature provides flexibility for organizations looking to move to the new standalone data model.
A ticket to Veeva Support is no longer required to convert a Vault to use the new standalone object model for a given process. Administrators can find the new option under Admin > Configuration > Application Configurations > Quality Data Model Enablement. The processes eligible for migration from Merged Quality Event to standalone are:
- Change Control
- Complaint
- Continuous Improvement
- Deviation
- Finding
- Lab Investigation
- Nonconformance
These one-way flags are permanent, and, if enabled for a process, will prevent new records of that process from being created as the corresponding Quality Event object type, and instead require that all new records are created as records of the corresponding standalone object.
Learn more in our Veeva Connect community post about the decision to move to the standalone QMS data model. You can also learn more on Vault Help about how to manage the Quality Event data model.
Quality Recurrence Check InsightsConfiguration24R1.3
When working with Deviations and Complaints, the importance of knowing insights related to recurring records is key for organizations in order to anticipate the next actions they should take for the current record.
To reduce hours, costs and improve tracking, the following needs have been met to give users the best experience when working with the Recurrence Check functionality:
- Knowing preventively if an additional Investigation is needed
- Verifying If a CAPA is in place and how effective it is
- Viewing the most common root causes and field values associated with the recurrences
With this new release, we are introducing the Recurrence Check Insights page available for Deviation, Complaints and Nonconformance objects (and their relative standard or custom object types), as well as for Deviation, Complaints, Medtech Complaint and Nonconformance Quality Event object types.
After completing a recurrence check, if configured by an Admin, Vault notifies the user that the insights are ready to be viewed.
The user must click on the blue hyperlink to navigate to the Recurrence Check Insights page which has three (3) different tabs available.
Recurrences Tab: In this tab, the recurrences are shown and the user will be able to filter the view to show associated Investigations, CAPAs, or Effectiveness Checks and see if these apply to the current record. A button to create new Investigations, CAPAs, or Effectiveness Checks records is also available to speed up the creation of the related process.
Administrators can configure which fields to display in the view for Investigations, CAPAs and Effectiveness Checks.
Summaries Tab: In this tab, related record data is displayed in a bar chart to enable the user to compare the numerical values of the configured information.
Administrators can configure up to three (3) related records to be displayed and up to two (2) fields can be added to group the chart.
Field Suggestions Tab: In this tab, users will be prompted with suggestions to Accept or Reject updates to fields based on the statistics pulled from the recurrences, which can be viewed by hovering over the View Statistics help text.
Administrators can configure a maximum of three (3) fields to be selected from the primary object, the only supported data types are object reference and single-value picklist. Once these have been entered, the comparison settings should be added. Two (2) matching tiers are available, (Probable and Likely) where a % minimum similarity score can be provided.
Learn more about configuring Quality Recurrence Check Insights.
Quality Teams ManagementConfiguration24R1.3
As Quality Teams become more prevalent within the QMS suite, administrators have voiced their interest in managing Quality Team memberships for multiple records directly through the user interface. This becomes particularly crucial when a user departs from the company, takes a prolonged absence, or transitions to a different role. In such instances, an administrator may find themselves needing to update numerous records associated with that user one by one which can lead to a significant amount of working hours.
Another option is to leverage the Quality Team public API introduced in 23R2. This facilitates the use of a loader-like CSV format for adding and removing users from existing Quality Teams. It conducts the same validations as updates performed through the user interface. Even though it could be a quicker solution, it requires having knowledge of the API beyond a normal business administrator scope.
In 24R2, Vault will allow administrators to add, replace, or remove users on multiple Quality Teams of the same type through the UI. The operation in the background will utilize the Quality Teams public API and respects the same validation as updates through the user interface. Once the operation is chosen, you can select up to 250 records, and at completion the user will be notified with a CSV file that lists the results of the attempted operation.
When landing on the Quality Team Management page, administrators can select which action they want to execute.
Add User: Choose the Add User action and select the desired Quality Team in the list to update, then the list of records related to that Quality Team will be available. After selecting the records that you want to update, a page with the available roles will show up. Type into the role to search for the users who need to be added and if any of the roles allow more than one participant, you can add multiple users.
Clicking on Confirm starts the operation and the user receives a notification with a CSV results file when it is completed.
Remove User: Choose the Remove User action, type in the user who needs to be removed, and select the Quality Teams to be updated. The list of available records will appear, where it is possible to add filters to narrow down the search. Select the desired records and after clicking on Continue a summary text will appear with the changes to be made.
Clicking on Confirm starts the operation and the user receives a notification with a CSV results file when it is completed.
Replace User: Choose the Replace User action, type in the user who needs to be replaced, and select the Quality Team to be updated. The list of available records will appear, where it is possible to add filters to narrow down the search. Type in a replacement user for each record or, type in the replacement user in the first row and click on the down arrow to add the same user for all the records. After clicking on Continue, a summary text will appear with the changes to be made.
Clicking on Confirm starts the operation and the user receives a notification with a CSV results file when it is completed.
The Quality Teams Management tab will be available and provided as inactive on the Tabs administration page. Learn more on how to manage Quality Teams in bulk.
Supplier Change Notifications: Extract Entities from AttachmentConfiguration24R1.3
When a supplier change notification (SCN) is received, it is often accompanied by email attachments from the supplier describing the changes. The user processing the SCN typically opens the attachments looking for impacted materials. When impacted materials are found, the user manually associates the materials’ qualification records to the SCN record. This is time consuming and prone to error.
This feature automates the manual process of locating and linking impacted material qualification records to the SCN. It makes it easier for Vault QMS customers to receive and appropriately interpret material qualification impacts.
System administrators can configure a new user action, Extract and Relate Entities from Attachment, to the Supplier Change Notification lifecycle. When the action is invoked by a user, Vault automatically scans all email attachments associated with the Supplier Change Notification record, looking for material qualifications specified in the user action. When matches are found, the action automatically links the material qualifications to the Supplier Change Notification.
The action is configurable, allowing system administrators the ability to define the role(s) permitted to run the action on Supplier Change Notification records. It is also flexible, enabling Admins to identify the object in their Vault that represents material qualifications, and which field within that object contains the name you wish to match upon.
Because this feature requires configuration to adopt, it will not impact your existing SCN processes until the user action is added to the Supplier Change Notification lifecycle. Learn more about the Extract and Relate Entities from Attachment action.
Action Paths & Steps for Change ControlConfiguration24R1.3
Organizations often sequence execution of Change Actions, rather than executing them all simultaneously. However, users must coordinate which Change Actions to execute in the appropriate order.
Beginning with this release, a System Administrator can configure a Change Control or Change Plan to utilize a Change Action Path to organize Change Actions in sequential Steps. Vault QMS will automatically start Change Actions in the proper order, based on their sequencing in the Change Action Path’s Steps.
The following example shows how the Change Action Path feature alters the way Change Actions are executed. Assume your organization creates a GMP Change Control to reset the steady state temperature of a freezer from -80oC to -70oC in a Microbiology lab. The Change Actions defined for the GMP Change Control include:
- Update SOPs that mention -80oC
- Update logbook used to QC freezers temps each day
- Change temperature of freezer to -70oC
- Perform stability tests on samples after 1 week at -70oC
Prior to this feature, each Change Action is simultaneously placed into an In Implementation state when the GMP Change Control reaches the In Change Execution state (see diagram below). However, some Change Actions need to be executed before others, so the Change Action owners must communicate with each other to coordinate the sequencing. Eventually, when all of the Change Actions are Completed, the GMP Change Control moves to the In Final Approval state.
Now assume your organization’s System Administrator configured a Change Action Path that is assigned to GMP Change Controls when they are created. The Change Action Path is defined as follows:
- Applicability: GMP Change Controls
- Step 1: Pre-Implementation
- Step 2: Implementation
- Step 3: Post-Implementation
When Change Actions are defined for this GMP Change Control, they are assigned to the Change Action Path Steps as follows:
- Step 1: Pre-Implementation
- Change Action: Update SOPs that mention -80oC
- Change Action: Update logbook used to QC freezers temps each day
- Step 2: Implementation
- Change temperature of freezer to -70oC
- Step 3: Post-Implementation
- Perform stability tests on samples after 1 week at -70oC
Now when the GMP Change Control enters the In Pre-Implementation state, Vault QMS starts the Change Actions in the Pre-Implementation Step by automatically moving them to the Start Implementation state as shown in the diagram below.
When the Change Actions in the Pre-Implementation Step are finished and move to the Closed state, Vault QMS moves the parent Change Control to an In Implementation state, and this starts the Change Action in the Implementation Step by automatically moving it to the Start Implementation state as shown in the diagram below.
Next, when the Change Action in the Implementation Step is finished and moves to the Closed state, Vault QMS moves the parent Change Control to a Post-Implementation state, and this starts the Change Action in the Post-Implementation Step by automatically moving it to the Start Implementation state as shown in the diagram below.
Finally, when the Change Action in the Post-Implementation Step is finished and moves to the Closed state, Vault QMS moves the parent Change Control to the In Final Approval state as shown in the diagram below.
The benefit of this feature lies in the ability to organize Change Actions into sequential Steps, and to automatically start Change Actions based on the Step sequence when the previous Step is finished. Since a Step can include multiple Change Actions, it’s also possible to execute Change Actions in parallel within a Step.
This feature is available for Quality Event and standalone Change Controls, as well as standalone Change Plans. Learn more about how to configure Change Action Paths & Steps.
Complaint Intake & Promote to ComplaintConfiguration24R1.2
Organizations intake information about potential product complaints, and this information must be triaged to determine if an actual product complaint process is necessary. In order to support the capture and triage of potential product complaints, this release introduces the following new objects included in the standard QMS data model:
- Complaint Intake: represents a potential complaint to be triaged. If necessary, one or more actual QMS complaint records can be created and related to the source Complaint Intake record.
- Reported Product: represents the product associated with a Complaint Intake record. Each Complaint Intake record can have one or more Reported Product records.
- Reported Product Batch: represents the product batch associated with a Reported Product record. Each Reported Product record can have one or more Reported Product Batch records.
The data model updates support the ability to create related standalone Complaint or Quality Event Complaint records. This release also introduces two standard join objects:
- Complaint Intake Complaint: joins Complaint Intake records to their related standalone Complaint records.
- Complaint Intake Quality Event Complaint: joins Complaint Intake records to their related Quality Event Complaint records.
A new Promote to Complaint action for the Complaint Intake object makes it possible to create one or more complaints that are related to the source Complaint Intake record. System administrators can configure the action to be used on a Complaint Intake record’s lifecycle state via user action or entry action. The action can also be configured to execute in a workflow step within a workflow that routes a Complaint Intake record. When this action executes, information from a Complaint Intake is copied to the resulting QMS complaint record(s) created from this action, including any attachments on the Complaint Intake record. The Complaint Intake record’s related Reported Products are also copied to the QMS complaint.
The following diagram shows an example of a Complaint Intake record that includes two Reported Products and no Reported Product Batches. The Promote to Complaint action creates two Complaint records, each with the appropriate Product records.
The next diagram shows an example of a Complaint Intake record with one Reported Products with two Reported Product Batches. If the Promote to Complaint action is configured to “Split by Batch”, two Complaint records are created, each with the associated Product and Batch records. If the Promote to Complaint action is not configured to “Split by Batch”, one Complaint record is created with the associated Product record and Batch records.
This feature sets the stage for:
- Future Release: New Vault Connections that will enable automatic creation of quality complaints in Vault QMS based on input from Medical and Safety Vaults.
- Vault QMS integrations with customer applications that receive complaint information from patients and healthcare providers to create Complaint Intake records.
- Enabling Vault QMS users to manually create Complaint Intake records, triage them, and create complaints as described above. Of course, QMS users will continue to have the ability to create complaint records directly, bypassing the Complaint Intake process.
Recurrence Check for Complaints: Enable Tiers & Creation of Related EventsConfiguration24R1.3
Prior to 24R2, recurrence check for Complaints and MedTech Complaints was configured differently than other Quality Events. Due to the anticipated high volume and structured way of identifying recurrences, recurrence check for Complaints was originally designed to assume that returned records were recurrences and users had the ability to specify which records were not recurrences. In addition, upon completion of the recurrence check for Complaints, related events and Quality Events were not created.
In 24R2, we are enhancing the recurrence check for Complaints and providing an option for administrators to configure it to behave like other Quality Events. Administrators can enable comparison settings for Complaints and set the comparison settings so that Complaints can be grouped into the three match tiers (Likely, Possible, Unlikely). Verdicts can be provided and upon completion of the recurrence check, if any of the records has a verdict not equal to “is unrelated,” the corresponding related events or related Quality Events will be created.
Filling out the fields in this section will only be mandatory if the Enable Comparison Settings checkbox, which only appears on Complaints Record Check configuration, is selected. If Enable Comparison Settings is not selected, there is no change in behavior, meaning that no tiers will be available, automatic related events creation will not occur, and “is unrelated” will be the only verdict selectable.
Learn more on how to configure recurrence check.
Recurrence Check: Support Execution as an Entry ActionConfiguration24R1.2
Today, you can only manually execute recurrence checks with a user action configured on one or more lifecycle states. For instance, when initially reviewing a quality event before sending it for an investigation to find potential recurrences, you can configure a recurrence check to be mandatory.
In this release, the Run Quality Record Check action has been enhanced and is now available to be executed as an entry action. This allows for the recurrence check to be automatically run when entering into a specific lifecycle state, eliminating the need for users to manually run the recurrence check.
Learn more about Configuring Recurrence Check.
Duplicate Check: Enable Verdicts without Transitioning Complaint Lifecycle StateConfiguration24R1.2
The Duplicate Check feature was introduced with the 22R3 release to replace complex manual processes to identify duplicate and follow-up complaints with an intelligent search functionality.
Previously, when a Complaint has been found and a verdict to mark the record as a duplicate or follow-up needs to be provided, the lifecycle state which the duplicate or follow-up complaint will transition into is a required input. With this feature, the transition to a specific lifecycle state will no longer be required. This will solve situations when the complaint still needs to be processed to closure while ensuring that a verdict has been selected and a related event record is still created.
When configuring Duplicate Check, Admins have to select a picklist value as a verdict, but the Destination Lifecycle State is now an optional field. Additionally, the Do not transition complaints in the following lifecycle states field added in 24R1 only displays when at least one Destination Lifecycle State on at least a single verdict has a value.
Recurrence Check: Refresh Results from Comparison PageAuto-on24R1.2
When a user wants to resume a recurrence check, the system uses the data based on the last time the check was run. That could have been several days ago and additional data, such as new related records or field value changes, could now be in the system. In order to get the latest results, the user would have to use the option to discard the previous record check and then execute a new record check.
In 24R2, a Refresh button with information regarding the date and time of the latest update will be displayed in the header of the record check interface. This button allows the user to refresh the results based on the latest data added to Vault.
For any new record check executed following the release, if the Allow user to override discovered match terms option is enabled, selecting the Refresh button will also give the user the opportunity to add, edit, or remove search terms.
If a record check is executed prior to this release and is In Progress, a user will not be able to override match terms even if the Allow user to override discovered match terms option was enabled when the recurrence check was originally executed.
Learn more about Configuring Recurrence Check.
Standard Layout & Data Model UpdatesAuto-on24R1.3
The 24R1 release introduced Action Layouts, allowing a System Administrator to tailor the user experience viewing an object record’s fields. Layouts define how an object record’s fields are organized for display, as well as rules that determine such things as when fields are required or hidden.
The Vault QMS application builds upon this capability by providing Standard Layouts across many core QMS processes. These are read-only example Layouts for standard objects included in the QMS application. Standard Layouts are not used to display object records to end users. Rather, they are provided for system administrators to copy and adapt when creating custom Layouts for their organization’s business requirements. Standard Layouts make it easier for system administrators to adopt the new Action Layout capabilities in their own implementations. System administrators cannot edit, delete, or activate Standard Layouts, or set them as a Default Layout. Note that Standard Layouts are not counted towards an organization’s custom Layout limit.
In order to deploy Standard Layouts, this release also introduces standard object types and fields referenced by the Standard Layouts. The standard fields and object types are Inactive by default, but can be made Active by system administrators. Standard Layouts are not available for Quality Event object types. However, Standard Layouts, standard fields, and standard object types are included for the following QMS objects:
Standalone Quality Events
- Change Control
- Change Plan
- Complaint
- Continuous Improvement
- Deviation
- Finding
- Lab Investigation
- Nonconformance
Processes that Support Quality Events / Standalone Quality Events
- CAPA Actions
- Change Action
- Effectiveness Checks
- Extension Requests
- Impact Assessment
- Investigation
- Issue Escalation
- MedTech CAPA
- Quality Incident
- Root Cause
- Supplier Corrective Action Request (SCAR)
- Supplier Change Notice (SCN)
APQR / QMR
- APQR
- APQR Item
- QMR
- QMR Item
- QMR Task
Quality Risk Management
- Assessment
Supplier Qualification
- Audit
- Audit Program
- Proposed Audit
- Organization
- Qualification
Quality Team Related Object Security MDL UpdatesAuto-on24R1.3
This release introduces structural improvements to the underlying workings of the Quality Team Related Object Security features within Vault QMS. These automatically enabled changes will streamline the ability of admins to migrate configurations of Quality Teams involving Related Object Security between environments. The impact of this feature is invisible to end users, but administrators interested in migrating configurations between environments can notice that they are represented as MDL changes to the structure of the Quality Team configuration itself. No action is required to take advantage of this new structure, and saving a Quality Team with Related Object Security configuration will update it to the new structure automatically.
QMS Change Related Object Lifecycle State ActionConfiguration24R1.3
When planning an audit, organizations can have Audit Programs that span many thousands of Proposed Audits related to suppliers and facilities. The existing Change Related Object Lifecycle State action, used in order to move the Proposed Audits into a specific state when the parent Audit Program transitions to the In Approval state, has two types that can introduce two problematic scenarios:
- The synchronous Change Related Object Lifecycle State action has a limit of 1000 records, meaning organizations would have to split up the Audit Program into multiple programs, which is not ideal from a tracking and tracing point of view and may result in additional work for the end user.
- The asynchronous Change Related Object Lifecycle State allows the Audit Program to go into a destination state even though Proposed Audits may not have successfully transitioned to the appropriate destination state. This potentially leaves the parent Audit Program in a lifecycle state that is not consistent with the lifecycle states of associated Proposed Audits.
In 24R2, we are introducing a new action called QMS Change Related Object Lifecycle State Job. This will execute a no-limit asynchronous job. If any errors are found transitioning the related object to the lifecycle state, the parent process record will be transitioned back to a destination state previously chosen by the Admin.
The entry action will be available in the lifecycles of the Audit Program and FMEA Risk Assessment Objects.
If there is any error while transitioning the related object into the following state, it will be written in the job log, and the action will continue with the next record. Once all records have been processed, the job log will return the number of records successfully and not successfully processed. In the meantime, the parent record will transition into the Destination State on Error selected and no additional entry actions (if configured) will be executed.
Risk Builder: Mitigation Action Filter & Workflow ExecutionAuto-on24R1.3
This release includes a series of enhancements to Risk Builder.
Allow Users to Edit Fields Not in Assessment Risk List Layout: System Admins can configure which Assessment Risk fields are displayed by default in a list of Assessment Risk records.
As of the 24R1 release, fields displayed in Risk Builder that were not in the Assessment Risk object’s List Layout were not editable.
This feature allows users to edit any Assessment Risk field displayed in Risk Builder, regardless of whether the field is configured to “Display in Lists and Hovercards,” as long as the user has permission to edit the field.
Add Mitigation Action Filter on Heatmap: An Assessment’s Heat Map now shows the number of Assessment Risks that do not have Mitigation Actions.
Clicking the To Mitigate filter takes the user to the Risk Builder page and displays Assessment Risk records that have no Mitigation Actions. This feature is automatically enabled if the Mitigation Action object is active. The Mitigation Action filter is not available if the Heat Map is generated for a historical Risk Assessment snapshot.
Display Hovercard Information for Object Reference Fields in Risk Builder: Prior to this release, when a user hovered over fields that represented object records in Risk Builder, Vault did not display the record’s hovercard to reveal information about the record. Beginning in this release, Vault displays hovercards when a user has Risk Builder open in View mode. System Admins can configure the fields displayed in an object’s hovercard.
Change Lifecycle State and Initiate Workflow on Assessment Risks in Risk Builder: This feature allows a user to initiate any available user action on an Assessment Risk record, including changing its lifecycle state or initiating a workflow, directly from Risk Builder. The user must be in View Mode.
View Audit Trail: Beginning in this release, users can view the audit trail of an Assessment Risk record directly from within Risk Builder.
Risk Builder: Auto-Calculation of Criticality ScoresAuto-on24R1.2
This release includes a series of enhancements to Risk Builder:
Displaying the Assessment Risk Mitigation Column: The previous release introduced the Assessment Risk Mitigation object, which allows users to view, assign, and remove records that mitigate Assessment Risks directly within Risk Builder. This object is inactive by default, so system Admins must first make the Assessment Risk Mitigation object active.
Previously, an additional step was necessary to make the Assessment Risk Mitigation column display in Risk Builder: Users must also display the Risk Response column. When the Assessment Risk Mitigation object is active and the Risk Response column is displayed, the Assessment Risk Mitigation column appears to the right of the Risk Response column in Risk Builder.
Beginning in this release, you can add and remove the Assessment Risk Mitigation column from display in Risk Builder just like any other field, regardless of whether the Risk Response column is displayed. System Admins can add or remove the Assessment Risk Mitigation column in an Assessment object’s layout configuration that defines the default Risk Builder columns.
Additionally, if a system Admin has configured an Assessment to allow users to change the columns in Risk Builder, then users can also add or remove the Assessment Risk Mitigation column.
System Admins must still make the Assessment Risk Mitigation object active before the Assessment Risk Mitigation column can be selected for display.
Realtime Auto Calculation of Criticality Score and Criticality Level: Vault calculates the Assessment Risk record’s Criticality Score and Criticality Level fields.
- Criticality Score is the product of the Assessment Risk’s Severity and Occurrence ratings.
- Criticality Level is based on where the Criticality Score fits within the ranges defined for each Criticality Level.
Previously, these fields were calculated when an Assessment Risk record was saved in Risk Builder. Beginning in this release, the Criticality Score and Criticality Level fields are automatically calculated immediately after an Assessment Risk record’s Severity and Occurrence fields are populated.
Make Criticality Label Dynamic in the Heat Map: Previously, the Heat Map label for the Criticality axis was not configurable.
Beginning in this release, system Admins can change the Criticality Level object’s label, which will change the label of the corresponding axis in the Heat Map.
Surveillance
VPS: Validation & UI EnhancementsAuto-on24R1.2
This release includes a series of enhancements & optimizations across the Vault Product Surveillance (VPS) application. Internal validations no longer perform unnecessary data checks while permission checks have been modified in some areas of the AER workflows to handle cases where the user lacks rights to the object storing data that should be shown on that page.
The following enhancements have been made to support the VPS application:
- VPS-rendered PDFs now support showing the MDR Contact Office site and person in eMDR G1.
- EUMIR: 2.4 now supports picklist dependencies to guide user value selections.
- EUMIR: 4.3.1 now provides more precise validation messages.
- EUMIR: Data truncation is now honored identically across the VPS UI and generated PDF & XML artifacts, with indications inline where data has been appropriately truncated in rendered PDFs.
- EUMIR: 1.3.2, 1.3.3 subsections c,d, as well as 1.3.4 subsections b, c have had their validation granularity adjusted, now supporting more helpful error messages if a person, or part of a person’s definition (first name, last name) are missing from the data set.
- eMDR: additional translation support has been added for Validation messages in sections A, D, and H6.
VPS: Health Canada EnhancementsAuto-on24R1.3
Continuing with the clarity and usability enhancements to VPS we started in 24R1.2, this release adds new validation messages and user feedback for VPS users working on eMDR, Health Canada, and EUMIR forms.
A few of the enhancements introduce visible functional changes, such as:
- EU MIR will now attempt to intelligently convert any weight captured on a Complaint in pounds (lbs) to kilograms (kg), using a ratio of 1 lb = 0.45359237 kg to the nearest 2 decimal places.
- EU MIR will now detect Age values with weeks as unsupported, and will prompt the user to convert the data appropriately before allowing PDF generation.
- Reporter, Complainant, and Healthcare Facility sections of Health Canada forms have received a UI update, making it easier to capture data for and review these sections of the form.
QMS, Surveillance
MedTech Standard Layouts & Data ModelAuto-on24R1.3
This release introduces a standard action layout for the following objects and object types:
- VPS: Reportability Assessment
- eMDR
- EU MIR
- Health Canada
- MedTech CAPA
To work with these standard layouts, make a copy of the standard layout itself and activate it for use. You cannot directly activate standard layouts. These standard layouts serve as a great starting point for organizations looking to leverage Vault’s Action Layouts feature with Vault QMS & VPS processes. You can use criteria VQL and layout rules to keep your teams focused on what’s important and relevant to them.
Validation Management
Ignore Required Fields if N/A Verdict SelectedAuto-on24R1.2
In 24R2, Validation Management no longer requires Executors to provide record data or attachments if the actual results, attachments, or additional prompt response prompts are required for an In Progress test step and the Executor selects the N/A verdict. When an Executor selects the N/A verdict and attempts to complete the test step, a warning dialog now appears to verify that the N/A verdict is correct.
If an Executor executes a test step change for a completed test step with a N/A verdict, the verdict is changed to Pass or Fail, and there is at least one (1) required field that is missing a value or attachment, Vault moves the test step back to the In Progress lifecycle state, clears the completed date/time, and marks the test step as no longer complete. An audit trail entry exists for the test step detailing the changes being made. The Complete button is disabled until all required fields are populated. If a test step was completed with the N/A verdict when the test step change is initiated for the verdict, Pass or Fail is selected as the new verdict, and there are no missing required fields, the test step change executes successfully and the verdict is modified to the value specified.
Requirement, Test Script, & Test Protocol Naming SchemesConfiguration24R1.3
In 24R2, the Validation Management application has enabled Vault to generate the Name (name__v) for Validation Requirement, Test Script, and Test Protocol records. Upon creating requirements, test scripts, and test protocols, Vault automatically applies the relevant naming scheme and determines the correct sequence number, eliminating the need for manual entry and preventing duplicate IDs.
The names of Validation Requirements will follow the format {Validation Entity Acronym} + {Requirement Type Acronym} + {Requirement Suffix} + {Sequence Number (6 digits)}. For example, VAL-UR-MECH-000001.
The names of Test Protocols and Test Scripts will follow the format {Validation Entity Acronym} + {TP or TS} + {Sequence Number (6 digits)}. For example, VAL-TP-000005 or VAL-TS-000010.
With this feature, we enhance the deep copy test script action to increment the run number if it has a numeric value. Vault only increments the run number if you configure this feature for Test Scripts.
Reassign Test Step Executor EnhancementsAdmin Checkbox24R1.3
In 24R2, Vault Validation Management allows any user with permission to perform the Reassign Test Step Executor action to reassign the Executor on a test step to another user. A user can execute this action from both the Test Script Execution Interface as well as the object record details page. This feature also does not update the completion date/time field value when the Executor is reassigned for a completed test step. Today, reassignment of an Executor on a test step is too restrictive and only the assigned Executor can reassign the test step when the step is in progress or completed.
Multiple Test Script AuthorsConfiguration24R1.3
In 24R1, Vault Validation Management introduces a new Co-Author role and allows multiple authors to work on the same test script simultaneously. This will empower both the Author (“lead author”) and Co-Authors with appropriate permissions to seamlessly add, edit, or delete test steps directly from the Test Authoring Interface, fostering real-time collaboration and streamlining the script authoring process.
Enforce Valid License Types for Validation Management UsersAuto-on24R1.2
Beginning in 24R2, Vault will introduce a streamlined user management experience. The goal of this feature is to create transparency between the way licenses are displayed and managed in Vault, and the way licenses are defined contractually, reducing the effort required to reconcile Vault licenses.
System Administrators will only be able to select valid application license types for each application. The valid application license types for the Vault Validation Management application are:
- Full User
- External User
The impact of this change is as follows:
- Application Licensing fields will no longer accept invalid values when a System Administrator creates a new user or updates an existing user.
- The upgrade will not change the Application Licensing field values for existing users. System Administrators can access a summary of invalid application license assignments.
- If your organization uses multiple Quality applications, and an existing user attempts to access an object or tab that is not supported by their assigned application license, Vault will show a warning message, but allow the user to access the object or tab.
LIMS
Sample Movement & Lab Location TrackingAuto-on24R1.4
This feature introduces two new objects: Lab Location History and Lab Location Hierarchy.
Lab Location History creates records every time a Lab Sample, Asset, or Consumable is moved. If an app section is configured on the requisite page, users can view the full history of movement including datetime stamps and the user moving the object in question.
Lab Location Hierarchy traverses Lab Location records and creates a string of full hierarchical location, up to 50 in a string.
Admins can configure this feature by adding app sections to Lab Sample pages as well as Asset and Consumable pages to view hierarchy. The Samples Found In app section can be configured on Lab Location pages to show all Lab Samples found in a Location, or descendants of a hierarchical location.
Aliquoting & PoolingConfiguration24R1.4
This feature allows for the creation of aliquot lab samples from an existing lab sample, for both batch release and stability samples. Aliquoting can be done in either a planned manner based on the Spec Data, or at runtime in an ad hoc manner via a user action. When aliquots are created based on the Spec Data, lab tests and lab criteria evaluations can be associated with the resulting aliquot samples (if defined in the Spec Data).
This feature introduces the following new objects:
- Lab Sample Lineage: Shows the aliquoting relationship between source and resultant samples
- Lab Aliquoting User Input: Used for ad hoc aliquoting to define the number of aliquot samples to be created
This feature also introduces the Enable Full Sample Action Automation Vault application setting. This setting in combination with fields on the Spec Data and Spec Data Sample Actions allows for automated planned Aliquot sample creation.
Editable Calculation & Instrument fieldsAuto-on24R1.4
This feature allows users with elevated permissions to use record actions to input/override results into calculated or instrument fields. When calculation errors occur or users run into instrument integration issues, outages, etc, this will allow for labs and their management personnel to continue to input results in a timely manner.
Users with this elevated permission are able to edit the result through a record action. This allows them to input an initial value for these result entry types or update an existing value. When the user does such an update, they will be prompted with an eSignature workflow to sign off on the result update. These types of updates will also be flagged as exceptions in Test Execution and other objects that are capturing exceptions.
Evaluate Additional CriteriaConfiguration24R1.3
In 24R2, Vault LIMS now supports configuring multiple criteria sets per Spec Data. After configuring your primary set of criteria for results in your Spec Data, and adding multiple markets to your Spec Data, you can now specify which criteria are relevant for each market. If the criteria for a given market is different from the primary, you can specify that as an override to the default criteria formula. This setup allows the same criteria to be shared across many markets, which helps reduce the amount of repeated work in your Spec Data setup. Additionally, you can configure whether the markets on your Spec Data will always be evaluated when executing this Spec Data, or only evaluated on demand.
Test Set EnhancementsAuto-on24R1.3
In 24R2 LIMS will now support a streamlined way to add/remove tests to a test set as well as adjust the order in which they’re listed. This is important to help support the lab analyst and the order they enter data or the order which the instruments will inject samples.
Label Barcode Scanning & Sample ManagementConfiguration24R1.3
Lab Analysts and Sample Managers can now utilize barcode scanners to initiate receiving samples, moving samples throughout the lab and sample disposal. Today in Vault LIMS, finding a sample requires the user to type in the sample ID or navigate Vault platform functionality to select the samples individually as required. In 24R2, Vault LIMS supports using a third party barcode scanner to quickly search and find a sample record in LIMS. Once one or more sample records have been identified, the user can begin sample movement workflows such as receiving samples, moving the sample to a new location, and updating the sample as disposed.
Enable Associated RecordConfiguration24R1.3
LIMS now includes Enable Related Record capabilities which include the same functionality as the Create Associated Record capabilities in Quality.
Product Usability EnhancementsConfiguration24R1.4
This feature includes various usability improvements to existing features and capabilities.
Quality Data Model Changes
See 24R2 Quality Data Model Changes.
Regulatory
Several features listed in the Vault Connections section also affect the Regulatory application family.
RIM
Health Authority Question ExtractionConfiguration24R1.4
With this release, Vault RIM provides the ability to extract questions from correspondence documents to create Health Authority Question (HAQ) records. In Question Selection mode, users can highlight the question text within the document viewer. HAQ records are automatically created and their fields are populated based on matching fields from the correspondence document. Once the selection of questions is complete, the system provides the ability to review the questions in a dedicated tab. Users can view and edit the records individually or in bulk. This feature streamlines the HAQ creation process.
RIM Registrations
Enhanced Support for Parallel XEVMPD & IDMPAuto-on24R1.2
In this release, the Vault Registrations data model and aggregation algorithms are updated for XEVMPD and IDMP. This auto-on enhancement supports the continued concurrency of these transactions.
Dependent Labeling Deviation GranularityAdmin Checkbox24R1.2
Vault Registrations has added a new Skip Duplicate Check for Dependency Automation Trigger Application Setting. Prior to this release, Labeling Deviation records were required to have a unique combination of Activity and Labeling Concept. However, this uniqueness check is inconsistent with the ability to cascade Labeling Deviations to dependent countries. Additionally, more than one deviation can be captured per labeling concept. To address this, the CCDS Section picklist can be configured as multi-value. The ability to skip the duplicate check removes the requirement to have a unique combination of Activity and Labeling Concept. These enhancements support more granular Labeling Deviations as well as deviations that are cascaded to dependent markets.
Enhancement to Product-Based Activity Creation from Impact Assessment ReportAuto-on24R1.2
With this release, the Create Related Records wizard in Vault Registrations is updated to more accurately create records when running the wizard from an Impact Assessment Report (IAR) and the Activity Scope is Product-based (“Application and Product” or “Country, Application and Product”). In some cases, the Product field may be blank on the Registration records included in the IAR, for example if the Registration Scope is “Marketed Product Group’’. In this case, Vault calculates the product based on the Product field on the Registered Product records associated with the Registrations in the IAR.
Registrations Data Model UpdatesAuto-on24R1.3
With every release, we update the data model to better support evolving needs and new feature functionality. These data model updates are automatically included in all RIM Vaults, but Admins must make configuration changes to make them available.
- The Registrations data model updates for this release include:
- Allowing Admins to inactivate picklist values.
- Adding a National Code field for tracking codes such as NDC in the United States or PZN in Germany.
- Enabling audit on several objects to enhance compliance.
- Adding a Medicinal Product Container object type on the Shelf Life or Condition object to more clearly distinguish shelf life information tracked at this level, versus shelf life information tracked at the Packaged Medicinal Product level.
- Updates to Rimobjectmapping records to make them visible in the Vault UI and allow the exclude_from_matching property to be editable.
- Updating the behavior of manufacturing details fields in various relationship objects so that:
- The Submission Wizard does not consider them when determining unique records.
- Fields in Application relationship records are editable, even after those records have been used as the source for completed Submissions.
- Fields are editable in the Manage Registered Details wizard.
See 24R2 RIM Data Model Changes for specific object and field details.
IDMP Data Visualization Enhancements: High Volume ViewAuto-on24R1.3
With this release, the IDMP Viewer is enhanced to better visualize large volumes of data. Data is now arranged in columns (the number of columns is limited to ten (10)). Field headers are frozen and users have the ability to scroll horizontally. Differing values (between submission data and additional data sets) in like fields are highlighted to easily identify data changes. Data filtering is enhanced so users can filter records, view only fields in which there are differing values in like records, or view only text override fields (used to generate submission FHIR messages).
The grid view is enhanced to better support high-volume records, such as the PDS Packaged Medicinal Product Element. Vault displays data in a grid with limited columns and horizontal/vertical scrollbars.
These enhancements support the review and comparison of large datasets.
IDMP Viewer CompareAuto-on24R1.3
IDMP Viewer Compare improves the ability to visualize and compare IDMP submission data. This feature enhances the review process of IDMP data submissions.
RIM Registrations, RIM Submissions
Global Content Plan Creation: Support for Custom Matching FieldsAuto-on24R1.2
When generating Global Submission records and Global Content Plans, Vault will now consider custom fields on Event, Submission, Application and Regulatory object relationships when calculating and removing duplicate records. Previously, the system did not include custom fields in its calculation and would remove what it considered duplicate relationships that were identical, except for their custom field values.
This enhancement prevents the system from removing relationships that were intended by the customer to be included in the GCP. Vault can also now use the custom fields to support Automatching and Suggested Matching of documents to the GCP.
Admin Configuration Page for RimobjectconfigurationsAuto-on24R1.3
Admins will now be able to configure Rimobjectconfigurations using a new Admin configuration page. Rimobjectconfigurations define properties for various RIM objects in the data model that are leveraged across multiple Submissions and Registrations features such as the Registrations and Submission wizards, Global Content Plan, and Copy Content Plan. These properties are centered around the display and editability of fields in the Registrations wizards, and the uniqueness check in Submissions-related functionality. Previously, these properties could only be updated through the Vault API.
RIM Submissions
Suggested Matching Enhancements: Maintain User Context, Update to Clear All (Filters) ButtonAuto-on24R1.2
Two enhancements have been added to improve the Match Document Mode user experience. When a user enters Match Document Mode with a specific Content Plan Item selected, the Viewer will open to that specific CPI, rather than opening to the first item in the Content Plan. This prevents users from losing their place in the Viewer and reduces time spent navigating back to the CPI they want to match. Upon launching Match Document Mode, a new Clear All Filters option is available by default, reducing the number of clicks to remove the default matching filters.
Respect Uppercase Lettering in Published Output Location
Vault Submissions now respects upper case letters in the Published Output Location field when the submission record’s dossier format is set to Other. This will be respected in the following actions: Split CPI, Bulk Update Tokens from CP and Update Tokens from CPI, Create/Update/Copy CP.
Note that document naming based on the Published Output Location field requires Vault Submissions Publishing.
This feature is deferred to a future release.
Exclude Use for Registrations from GCP Matching RulesAuto-on24R1.2
The Use for Registrations field has been removed from being used as a matching field for Event relationships when dispatching or generating comparisons of Global Content Plans.
Submission Wizard Enhancements 24R1.3
New enhancements to Submission Wizard increase efficiency and improve the user experience, particularly when managing Application data over time where changes may be necessary.
Through a new Application Setting, users will have additional flexibility in managing Application relationships and relationship fields. Application relationship fields were previously locked when a Submission record using that metadata reached an excluded lifecycle state, such as when a submission was sent to a Health Authority. This prevented users from updating Application relationship fields that may change throughout the life of an Application. Now, not only can these fields be updated, but the new field values will automatically cascade onto existing relationships fields on Submissions and Regulatory Objectives in active states. Admins can determine the fields that will become editable and the Lifecycle States to be respected by the new settings.
When a Regulatory Objective record is created due to a local-market change (rather than a global Event), the Regulatory Objective needs to be linked to any existing Registration records. With this new feature, relationships to existing Registrations can be created via the Submission Wizard. Users will now be prompted by the UI to create the relationships, and will do so more efficiently than manually relating records one at a time.
New filtering options allow users to more easily identify the Application relationships to add to their Submissions and Regulatory Objectives. Over the life of an Application, the list of potential related records can become long. This filtering replaces the page-by-page scrolling and improves efficiency and user experience of adding relationships using Submission Wizard.
Support Auto-matching of RLCP Documents into SCPConfiguration24R1.3
With this release users will be able to select a related Report Level Content Plan when creating Submission Clinical Studies and Submission Nonclinical Studies. This field value will then be used as a matching criteria to further constrain the list of documents matched to the corresponding Module 4 and Module 5 Submission Content Plan Items.
Dispatch Global Content Plan Across TemplatesAuto-on24R1.4
This feature supports the dispatch of a Global Content Plan (GCP) to Submissions with a different Content Plan Template (CPT) than the GCP. Previously, GCPs could only be dispatched to Submissions using the same template as the GCP.
Customers can set up mappings between Content Plan Item Templates in a new Content Plan Template Mapping object. The GCP dispatch is updated so if the target Submission has a different CPT than the Global Content Plan, the dispatch distributes the matched documents only based on the mappings.
This expands the use cases for GCPs to support Investigational Applications (CTAs, INDs) and MedTech Applications, where there is no single common or harmonized structure across all markets. In addition, this feature supports the dispatch across the different Module 1 structures for CTDs.
RIM Submissions Archive
Support for Additional Document User Actions in the ViewerAuto-on24R1.4
Submissions Archive users are now able to execute the following User Actions directly from the Submissions Archive Viewer:
- Open Content Plan
- Open Content Plan Item
- Open Source Document
When configured, these actions support using the Viewer to review in-process submissions, and are now available in the new Archive Viewer interface.
Submissions Archive: Singapore eCTD 1.0
With this release, import, display and export of Singapore 1.0 DTD for eCTD submissions are now supported.
This feature is deferred to a future release.
EAEU latest updatesAuto-on24R1.2
With this release, the latest updates to the EAEU submission specification are now supported in the Submissions Archive. The following updates are now available:
- Updated document codes to display 3.2.R.1 documents properly
- Section 1.8.2 has been updated
- Sections 1.8.2.x have been added within the structure
Submissions Archive: South Africa eCTD v3.0Auto-on24R1.2
With this release, import, display, and export of eCTD v3.0 for South Africa are now supported.
China Non-eCTD Import HandlingAuto-on24R1.3
Submissions Archive now supports import of China NeeS submissions that include index.xml and index-sm3.txt files, as made effective by National Medical Products Administration in March 2024. Vault recognizes these dossiers as Non-eCTD.
Limit on Duplicate Content Detection During ImportAuto-on24R1.3
During import, RIM Submissions Archive will detect up to 10 duplicate documents per document included. The limitation applies to documents created by RIM Submissions Archive during import processing and is in effect when Vault-level Duplicate Content Detection is enabled.
RIM Submissions, RIM Submissions Archive
Active Dossier Auto-Calculation ImprovementsAuto-on24R1.3
With this release the Active Dossier auto-calculation logic has been improved to handle additional scenarios:
- Documents split or merged into separate documents
- Documents in eCTD submissions where Vault is unable to locate the XML in Submissions Archive
- Later document versions approved before earlier versions
Support for these additional scenarios will allow the Active Dossier auto-calculation to be more robust and reduce the need for extraneous manual status updates. A new document relationship, ‘Originates From’, is added to support the first scenario.
Active Dossier Viewer ImprovementsAuto-on24R1.3
The Active Dossier Viewer has been updated with this release to allow users to filter and navigate to the data they are looking for more quickly. These improvements include:
- Support for additional and more flexible filtering options. This includes filtering on non-transactional objects (Product, Product Variant, Active Substance) or transactional objects (Application, Regulatory Objective, Submission, Event).
- More intuitive display of relevant records and country columns based on selected filters.
- Support for collapsing the Active Dossier header and global filters
- Displaying the global filters header (pre-filtered) when viewing or editing Active Dossier from a transaction record
- When viewing or editing Active Dossier from a transaction record, the records will be filtered directly based on the transaction record instead of the transaction’s relationships
Support Editability of Fields on Active Dossier Item DetailAuto-on24R1.3
With this release, users can edit the Application, Regulatory Objective, Submission, Event, and Activity fields on the Active Dossier Item Detail object. Previously, these fields were system-managed and could not be edited after the Active Dossier Item Detail record was initially created. This enhancement allows users to enrich Active Dossier Item Details records manually created from dragging and dropping documents into the Active Dossier Editor before it was possible to set the Application, Regulatory Objective, Submission, Event, and Activity fields in the dialog. This also allows users to modify these fields if they need to be corrected on existing records.
Update to Active Dossier Template for 3.2.A.3 Novel ExcipientsAuto-on24R1.4
Prior to this release, the 3.2.A.3 records in the Active Dossier were not created as expected according to the ICH eCTD specification. This is because the Active Dossier Template section was set up to be tracked by and repeat on inactive ingredients; however, according to the ICH eCTD specification, 3.2.A.3 is not meant to be tracked against the inactive ingredient.
With this release, the Active Dossier Template record for 3.2.A.3 is therefore being updated to remove the incorrectly placed ${inactive_ingredient__v} token.
RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive
RIM Bot: Simplified Model TrainingAuto-on24R1.3
To help customers with improved training and deployment of RIM Classification Bot (RIM Bot), a new simplified training model is being introduced. After analyzing the performance of trained models over time (TMF Bot and RIM Bot), Veeva has observed significantly lower performance of custom-trained models compared to system-trained models. Over time, more classifications are added to Vaults than were originally trained, which in turn results in a downward trend in the Bot performance.
To help customers improve initial performance and accuracy of their trained Bot, and to maintain performance over time, more training parameters will be system-managed. The option to select a training source CSV will be removed, and all Bots will be trained via the Training Window Start Date. The Advanced Model Parameters field will be removed from view, and the following training parameters are being updated and set to read-only:
- Prediction Confidence Threshold: 0.85
- Minimum Documents per Document Type: 10
- Auto-Deploy: True
RIM Bot: Auto-on in All RIM VaultsAuto-on24R1.3
This feature will automatically create, train, and deploy a Document Classification Trained Model in all Submissions Vaults that do not already have one deployed, as long as there are more than 1,500 steady state documents. Training and deployment of this model may take 48 to 72 hours in some cases. Once Vault deploys the model, documents within the customer’s Document Inbox may be auto-classified by the RIM Bot. Documents that have been auto-classified still require a user to confirm or correct the suggested classification before the document is available in the Library.
Learn more about RIM Bot
RIM Publishing
Canada Validation Criteria v5.2 & REP XML RulesConfiguration24R1.2
With this release, Vault Submissions Publishing supports validation of Health Canada eCTD submissions against the Health Canada validation criteria version 5.2, which now includes Regulatory Enrollment Process (REP) rules I01 - I11. Version 5.2 also includes the following updates to rules that were made available in 24R1:
- Updated text (but not logic) in B32, B44, B47, B48 rules regarding PDF Analysis
- B49: Added searchable documents
Define RLCP Output Document Type for Section Level MergeAuto-on24R1.2
Report Level Content Plan users can now define the published output document type, subtype, and classification for documents produced using Section Level Merge. With 24R1, merged output was classified as a Submission Ready document. Now, users have the option to define a more useful classification to support filtering, searching, and matching into Submission Content Plans.
A new Content Plan field, RLCP Section Output Document Type, can be added to the RLCP Viewer and populated as needed. For example, when using Section Level Merge to produce a single PDF containing a Table of Contents and tables & figures, users can choose Clinical > Study Reports > Tables and Figures.
View Submission Administrative Information Based on Region & Regional XMLAuto-on24R1.2
When viewing Submission Administrative Information on a Submission or Submission Country record, viewing is only available when the region on the Published Output Location matches the Submission Country.
In the event that a regional.xml Published Output Location is accidentally or purposefully updated to include a different country code than the Submission Country, users will receive an error (The Regional XML is not available) when attempting to view Submission Administrative Information. For example, for a US Submission, if the regional.xml was updated to m1/za/us-regional.xml, the Submission Administrative Information would show an error.
Support Empty Published Output Location Field for Delete Lifecycle OperationsAuto-on24R1.2
When publishing a Content Plan Item with its XML Leaf Operation field set to Delete, Vault automatically clears the item’s Published Output Location (POL) field and the xlink:href within the regional or index XML. Previously, manually clearing the Published Output Location field for a Delete operation could cause Publishing issues. Now, regardless if the Published Output Location field is cleared or remains populated, Publishing will execute and Vault will clear any values in the Published Output Location field.
Section Level Merge for SCPConfiguration24R1.3
Submission Content Plan users can now choose to merge documents matched to multiple Content Plan Items within a Content Plan section. A new Yes/No field on the Content Plan allows users to determine the section for merging the Published Output Location of the merged document.
Previously, Vault Submissions Publishing could merge multiple documents matched to the same Content Plan Item (CPI), or merge an entire Submission Content Plan. Users will now have a third option to publish a Submission and only merge the sections indicated as ‘‘Yes” in the Merge Section column. Admins will configure a new User Action on the Content Plan Lifecycle with the Merge Sections checkbox enabled. This added flexibility allows users to define the level of merging to suit their authoring style and Publishing needs. Section Level Merge will support non-eCTD submission requirements, and can combine multiple CPIs (such as a Table of Contents and report body) into a single published PDF. All internal bookmarks from individual merged documents will be included in the merged PDF. This functionality was previously only available for Report Level Content Plans.
NeeS: Bosnia ValidationConfiguration24R1.3
With this release, Bosnia Validation Criteria for non-eCTD Submissions are supported within Vault Submission Publishing.
The Validation Rules can be uploaded to Vault through Veeva-provided VPK. The VPK contains the Validation Rules, the updates to the Content Plan Template and updated Controlled Vocabularies.
US eCTD Validation Rule 1255 UpdatesAuto-on24R1.3
US Validation Rule 1255 now reflects the latest FDA Specifications for File Format Types Using eCTD Specifications. Per the specification, PDF archive format copies are no longer required for Microsoft Word (DOC, DOCX) or Excel (XLS, XLSX) files. Rule 1255 also validates the following for CDER submissions:
- File formats SMLX, PUMASCP, JMD, and QMD
- Update name for file format JL
South Africa (ZA) eCTD 3.0 Publishing & Validation UpdatesAuto-on24R1.3
With this release, RIM Publishing supports the ZA SAHPRA v3.0 specification. Users can now create Content Plans and generate submissions that are compliant with ZA SAHPRA v3.0 specification updates. Vault validates these submissions based on the corresponding ZA SAHPRA v3.0 Validation Criteria Version. A VPK package is available to update Vault with the latest Validation Criteria and Content Plan Template package.
Switzerland (CH) Application Number UpdatesAuto-on24R1.4
Users who are publishing CH eCTD submissions often need to include an Application Number that is different from the overall XML Application Number on the Application record. Users can manually override the default value with the Add Override action. With 24R2, an additional option to Exclude Value is available in addition to the Add Override action. The Exclude Value option provides users with an interface to make these one-time updates for a given submission, without the new Application Number override carrying forward to future submissions.
Copy Product, Product Variant, & Manufacturer Source Document Fields to Archived DocumentsAuto-on24R1.4
The copy of Source Document Fields to the Archived Documents during RIM Publishing has been enhanced to now include the following Fields:
- Products (
drug_product__v) - Product Variants (
product_detail__v) - Manufacturers (
manufacturer__v)
When both fields are on the Content Plan Items matched document type and configured on the Submission Archive document type, Vault will copy those fields at publishing time.
RIM-Clinical Operations Connection
RIM-Clinical Operations: Update to Standard Field RulesConfiguration24R1.3
With this release, the RIM-Clinical Operations connection has added the Site Lead Investigator standard field to the Site object along with a standard field rule to enable the transfer of the Principal Investigator Name when creating the site in Vault RIM. Additionally, standard field rules have been added to enable the transfer of the Ready for Publishing and Redacted document fields. Prior to this, users were required to enter this data manually. This streamlines the clinical site and document creation process, enhancing productivity and data quality.
RIM Data Model Changes
See 24R2 RIM Data Model Changes.
Safety
The Safety 24R1.4 release, including all Platform features, is scheduled for tentative availability on July 8 11, 2024. For the latest central dictionary updates for your Safety Vault, see Safety Central Dictionary Updates.
Several features listed in the Vault Connections section also affect the Safety application family.
Safety
Sender-Based Email Attachment HandlingConfiguration24R1.2
With this release, Admins can now efficiently manage the handling of emails and attachments received from their PV email inboxes. This feature introduces new settings that allow Admins to associate inbound Vault emails with Transmission Profiles, providing granular control over email management. Depending on configuration, Vault generates an Inbox Item per email or attachment, streamlining organization and user accessibility. Moreover, both multi- and single-E2B ZIP and XML format attachments are automatically imported into Inbox Items, eliminating manual user intervention and enabling seamless integration of structured attachment formats. This advancement automates the handling of email attachments and facilitates touchless intake. This feature eliminates the need for manual triage and classification of email attachments.
Learn More
- Enablement: Enable Sender-Based Email Attachment Handling
- User Help: Email to Inbox Item
Follow-up Parent Information Case UpdatesAuto-on24R1.2
With this release, Vault Safety creates a new version of Parent Information Cases for Global Follow-up Cases. Now, for Follow-up Cases with an associated Parent Information Case, Vault applies new information from import to the Follow-up Parent Information Case. When creating a Follow-up through the Case Compare page, Vault applies new information from the Inbox Item and copies missing information from the previous Parent Information Case. This feature improves data quality and compliance.
Learn More
MedDRA Query Reporting Rule Parameter Eligibility UpdatesAuto-on24R1.2
With this release, Vault Safety enhances the logic governing the evaluation of the Include MedDRA Query and Exclude MedDRA Query reporting rule parameters to determine more precisely the reportability of cases containing Adverse Events configured within a MedDRA Query. Previously, the rule engine used an approach known as Most Conservative Product/Assessment (MCP/A) to identify the single “most reportable” Product/Assessment. However, the enhanced logic now evaluates all Case Adverse Events as a Case-level snapshot rather than at the individual Assessment level. This refined methodology provides a more efficient evaluation of reporting rules and ensures a consistent evaluation method across multiple destinations. This enhancement does not require you to change your currently configured rules, allowing you to maintain the continuity of your current workflow and processes.
Learn More
- User Help:
Japan Domestic Case: Calculate Due Dates for Localized Case AssessmentsConfiguration24R1.2
With this release, users have the flexibility to calculate Due Dates on Localized Case Assessments without leaving the related Japan Domestic Case. The new Calculate Due Dates on Localized Case Assessments action is available on Domestic Cases when the Japan Localization is set to generate Localized Assessments for Case Product Registrations. Previously, the option to calculate Due Dates on Localized Case Assessments was available on Localized Cases only. This feature improves the Domestic Case processing experience.
Learn More
- Enablement: Enable Japan Domestic Case: Calculate Due Dates for Localized Case Assessments
- Admin Help: Set Up the Localized Business Admin Library for Japan: Assessment Generation
- User Help: Complete Intake and Process Cases for the PMDA: Assessments Section
PMDA E2B(R3): Export Substance Codes from JDrug CodesAuto-on24R1.2
With this release, Vault Safety improves the export of Substance codes to PMDA E2B(R3) reports. When Company Products or External Products have a Japan Product Code Type of J-Drug Code, Vault exports the first seven (7) digits of the Local Product Code or Local MPID Code, respectively, to the G.k.2.3.r.1 Substance / Specified Substance Name data element.
This feature is Auto-on in Vaults with the Japan Drug Dictionary enabled or J-Drug Code specified as the Japan Product Code Type in the Product Registration. Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable PMDA E2B(R3): Export Substance Codes from JDrug Codes
- User Help:
PMDA E2B(R3): Prevent Export of Unsupported UCUM CodesAdmin Checkbox24R1.2
With this release, Vault Safety prevents the export of unsupported Unified Code for Units of Measure (UCUM) codes to PMDA E2B(R3) reports. In addition, Vault can automatically replace unsupported UCUM codes in PMDA Submissions. This reduces instances of Submissions being rejected for unsupported codes and eliminates the need to send Cases back to Global for correction.
Learn More
- Enablement: Enable PMDA E2B(R3): Prevent Export of Unsupported UCUM Codes
- User Help:
Adverse Event in Jurisdiction Parameter Country DifferentiationConfiguration24R1.2
With this release, Vault Safety Admins can configure safety reporting rules to use either Primary Reporter Country or Event Country to determine if the Adverse Event occurred within the jurisdiction of a specific Agency. This allows Vault to comply more closely with regulations from global health agencies including the EMA (Europe) and the TGA (Australia).
Learn More
Reporting Rule Level Control of Auto-Submit for TransmissionsConfiguration24R1.2
With this release, Vault Safety supports the configuration of automatic submissions for Transmissions generated using a specific reporting rule. This feature introduces a new Output Reporting Rule Parameter for the Auto-Submit feature which can be set on a rule-by-rule basis. This could allow, for example, only non-serious cases to be auto-submitted to a specific destination, providing enhanced control for touchless processing. Previously Auto-Submit was controlled at the Transmission Profile level. As a result, when Vault generated a Submission or Distribution to a specific destination, Vault marked that Transmission as Auto-Submit.
Learn More
Reporting Family Configuration ScenariosConfiguration24R1.2
With this update, Vault Safety Admins can configure Partner Distribution Lists (Reporting Families) to include the following:
- All Products stored in the Vault.
- All Studies stored in the Vault.
- All Products within a designated Product Family.
- All Products coded to a specific Product Registration.
Previously, this functionality was limited to specific Products and Studies. This enhancement provides Admins with greater Reporting Family configurability, especially in scenarios where additional Products, Studies, or Product Family members are configured in Production.
Learn More
- Enablement: Enable Reporting Family Configuration Scenarios
- User Help: Manage Partner Distribution Lists
Strict Transmission Version EnforcementAdmin Checkbox24R1.2
With this release, Vault Safety can enforce the order in which you are able to send Transmissions via Gateway and Email to all destinations except the PMDA. If set, you cannot submit a Follow-up report if the initial report for the same Case Number is not first completed. This helps ensure that Submissions are not sent out of order which can lead to downstream compliance issues.
Learn More
- Enablement: Enable Strict Transmission Version Enforcement
- User Help:
Note: PMDA support for this feature will be provided in a future release.
Include Source Document in Email TransmissionConfiguration24R1.2
With this release, Vault Safety provides a configurable option to automatically include source documents as email attachments for distribution. The feature introduces a new Transmission Profile picklist field called Attach Source Document(s). If you have an obligation to share source documents with a certain partner, based on PVA requirements, this feature eliminates the step of manually attaching source documents during the Transmission workflow. Additionally, if you use the Auto-Submit feature, this new configuration option completely automates the process.
Learn More
- Admin Help:
PBRER: Include Spontaneous Events Without AssessmentsAuto-on24R1.2
With this release, users can create PBRER reports that include spontaneous events without Case Assessments. Previously, adverse events were gathered for inclusion based on the presence of a case assessment, PBRER reports omitted any adverse event without a Case Assessment record. This enhancement aligns with ICH guidance, recognizing that spontaneous Cases imply causality and thus don’t mandate assessments or assessment results. Additionally, this feature ensures accurate aggregate reports for Cases migrated without Case Assessment records.
Learn More
DSUR & PBRER: Exclude Cases with Study Products Unrelated to Products in the Reporting FamilyAuto-on24R1.2
With this release, DSUR and PBRER reports only include Cases with Products directly defined in the Reporting Family or linked to a Study defined in the Reporting Family. Previously, when a Reporting Family was set up with a Product (not a Study), DSUR and PBRER reports included Cases from the Studies of the Products in the Reporting Family. This feature streamlines reporting processes and reduces errors, improving safety monitoring and regulatory compliance.
Learn More
Default Frequency for Case Volume MetricsAuto-on24R1.2
With this release, if the Case Volume Metrics Frequency setting is left blank, Vault will automatically set it to Hourly. This feature was released in 24R1.2 without documentation.
Learn More
- Enablement: Enable Case Volume Metrics
Product Coding: Additional Matching Fields for Product AliasesConfiguration24R1.3
With this release, Vault Safety introduces a more advanced matching algorithm for Product coding with Product Aliases during E2B or JSON import. To make Product Aliases more unique, Admins can define additional criteria for Product Aliases, such as the Country, Route of Administration, Dosage, Dose Form, and Dose Strength. Vault ensures that imported Products align with library Products based on these factors. Once the Product coding criteria are satisfied, Vault proceeds with Product matching and auto-coding. By enhancing the precision of Product Alias matching, imported Products sharing the same Alias name can still be auto-coded, reducing the need for manual Product coding and enhancing Case Intake efficiency.
Learn More
- Enablement: Enable Product Coding: Additional Matching Fields for Product Aliases
- Admin Help: Manage Products: Create Product and Substance Aliases
- User Help: Inbox Item Study and Product Matching: Product and Substance Alias Matching
Automated Classification of Inbox Item SignificanceConfiguration24R1.3
For Inbox Items imported from the Safety-EDC Vault Connection, Vault Safety can classify Inbox Items as Initial Cases and whether a Follow-up Inbox Item is significant or non-significant. To classify follow-up significance, Vault determines whether there is an update to the reportable fields that could impact the seriousness, expectedness, and relatedness of the matching Case. When Vault marks an Inbox Item for follow-up, other Inbox Items related to the same Case are set to the Marked as Follow-up (Not Current) state. Additionally, when you promote an Inbox Item, Vault sets the non-current Inbox Items that are related to the same Case to the Superseded state. These improvements significantly assist intake users with prioritizing Cases for processing and follow-up actions, ultimately streamlining Case Intake efforts.
Learn More
- Enablement: Enable Automated Classification of Inbox Item Significance
- Admin Help: Configure the Inbox Item Follow-Up Case Compare Layout: Customize Fields
- User Help: Import an Inbox Item: Inbox Item Significance
Follow-up Compare with Summary SettingAuto-on24R1.3
With this release, Vault Safety introduces a new setting, Enable Follow-up Compare with Summary.
Note: This setting will control the functionality of the Follow-up Compare with Summary feature, which will be introduced in a future release.
Case Compare Study Product Matching Logic UpdateAuto-on24R1.3
This feature ensures that open label Products are matched only to open label Products. Open label Products will not be matched to unblinded Products.
Learn More
- User Help: Inbox Item Follow-Up: Merging Child Records
Prevent Merge to In-flight Locked CasesAuto-on24R1.3
With this release, Vault Safety prevents merging follow-up information to an In-flight Case during Case Compare if that Case is locked to another user. Now, merging to an In-flight locked Case is only permitted if the active Case is locked to the same user performing the merge. Currently, you can still create a new Follow-up Case version if the initial Case is locked.
Learn More
- User Help:
Online Questionnaires for Post-Marketing Follow-upsConfiguration24R1.3
With this release, Vault Safety streamlines the end-to-end follow-up questionnaire process for post-marketing Cases. Vault automatically selects online questionnaires using follow-up rules and shares them with the right Case Contacts through email. If applicable, Case Processors can review the questionnaires before they are sent and add questions. The answers will be available directly on the Case for review by the Case Processor.
Learn More
- Enablement: Enable Online Questionnaires for Post-Marketing Follow-Ups
- Admin Help: Set Up Follow-Up Online Questionnaires
- User Help: Send a Follow-Up Online Questionnaire to Case Contacts
Generate Domestic and Localized Parent InformationAuto-on24R1.3
With this release, Vault Safety generates Domestic Parent Information during promotion and follow-up. During promotion, if a Global or Domestic Case has a Parent Information Case, Vault generates a Localized Parent Information Case and synchronizes all Parent Information Case child records to the Localized Parent Information Case.
Learn More
- User Help:
Clinical Trials: Isolate Blinded Product Information UpdatesConfiguration24R1.3
Vault Safety now fully isolates unblinded Product information so that unblinded Case Products can be independently assessed. Prior to this enhancement, Vault Safety segregated a subset of blinded and unblinded data. This feature has now been extended to all Product data to support reporting to the PMDA, aggregate reporting, and Distribution requirements. This advanced approach to protecting blinded information helps Medical Reviewers better differentiate between blinded and unblinded data.
Learn More
- Enablement: Enable Clinical Trials: Isolate Blinded Product Information
- User Help: Isolate Blinded Clinical Trial Information
Japan: Calculate Non-Study Product Expectedness for Study CasesAuto-on24R1.3
When processing Study Cases that are reportable to Japan, Vault Safety now calculates Expectedness on Localized Case Assessments for non-Study Products registered in Japan.
Learn More
PMDA: Clinical Trial Development Phase 1-3 and J OID Code List UpdateAuto-on24R1.3
To support the latest PMDA updates, Vault Safety’s Development Phase controlled vocabulary type picklist now includes “Phase 1/3”. When this Development Phase option is selected on a Japan Study, Vault exports E2B Code “6” to the J2.13.r.3 Development phase data element of generated PMDA E2B(R3) reports. In addition, the J OID Code List for that data element is updated to version 1.2.
Learn More
PMDA: Global Case Investigational to Investigational Cross ReportingAuto-on24R1.3
With this release, Vault Safety updates cross-agency investigational to investigational cross reporting for the PMDA. When calculating reporting obligations to the PMDA on a global Case, Vault determines whether any Studies registered with a non-PMDA agency have Products registered with the PMDA. If so, Vault generates Submissions to the PMDA.
Learn More
FDA E2B(R3)Auto-on24R1.3
With this release, Vault Safety supports generating and submitting Investigational New Drug (IND) and Postmarket reports in the FDA E2B(R3) format. This feature includes the core International Council for Harmonisation (ICH) and regional data elements required for compliant Transmissions.
This feature is Auto-on in Vaults with Transmission Profiles for FDA E2B(R3) Submissions and Distributions enabled.
Learn More
- Enablement: Enable FDA E2B(R3) Submissions and Distributions
- Admin Help: Configure FDA AS2 Connection
- User Help:
Japan Periodic Reporting: Post-Market Serious (J-PSR)Configuration24R1.3
With this release, Vault Safety supports Japan Periodic Safety Reports (J-PSRs) for drugs approved for marketing in Japan. When generated, the aggregate report includes postmarket Serious Domestic Cases submitted to the PMDA during a defined period. Vault Safety summarizes the required information in the J-PSR Cumulative Tabulation of Adverse Events Form 3 and J-PSR Line Listing of Adverse Events Form 4 tabulations. This feature helps marketing authorization holders (MAHs) collect and submit safety information within drug re-examination periods, meeting PMDA periodic reporting requirements.
Learn More
- Enablement: Enable Japan Periodic Reporting: Post-Market Serious (J-PSR)
- User Help: Create PMDA Post-Market Aggregate Reports
PMDA: Blinded Study Product ReportingAuto-on24R1.3
With this release, Vault Safety supports PMDA reporting requirements for blinded Study Products, including evaluating reporting rules. You can now specify the primary blinded investigational Product for blinded Products. When reporting to the PMDA, the reporting rule engine assesses reportability based on the blinded Product’s primary blinded investigational Product. In addition, when Case Product Registrations (CPRs) are generated on Localized Cases, Vault considers the primary blinded investigational Product and generates one (1) CPR for each blinded Study Product. Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable PMDA: Blinded Study Product Reporting
- Admin Help: Set Up the Localized Business Admin Library for Japan: Specify the Primary Investigational Product for Blinded Study Arm Products
- User Help: Report to the PMDA: Blinded Study Product Reporting
PMDA Submission Validation EnhancementsAuto-on24R1.3
Vault Safety updates the PMDA Submission Validation Criteria to enhance PMDA reporting compliance. This introduces the following improvements:
- Some validations previously run only at the Submission level now run at the Case level
- Unnecessary Validation Criteria are downgraded to Warnings
- New Validation Criteria are included
- Known issues are addressed
Learn More
- User Help: Case and Submission Validation
Rule Engine Product Selection StandardizationAuto-on24R1.3
The Vault Safety Rule Engine now standardizes the way in which Products within a Case are assessed for reportability. Prior to this release, you could configure your Vault to assess reportability against either the Primary Product or all Products associated with the Case. As of this release, Vault evaluates all Products associated with the Case only. This enhancement ensures that Vault evaluates Reporting Rules in the manner most compliant with global reporting requirements.
Learn More
- User Help: Reporting Rules Product Selection
Product Family Rule ParameterConfiguration24R1.3
Vault Safety now supports defining Individual Case Safety Reports (ICSR) Reporting Rules for Cases containing Products within a certain Product Family. This allows for simpler configuration and maintenance as Reporting Rules do not need to be updated because of a change in the Product Library.
Learn More
- Enablement: Enable Product Family Rule Parameter
- Admin Help: Manage Products in Vault Safety
- User Help: Reporting Rule Parameter Reference: Product Family
Reporting Family Configuration Scenarios for SubstancesConfiguration24R1.3
In 24R1.2, we introduced Reporting Family Configuration Scenarios that allowed Vault Admins to configure Partner Distribution Lists (Reporting Families) to include the following:
- All Products stored in the Vault.
- All Studies stored in the Vault.
- All Products within a designated Product Family.
- All Products coded to a specific Product Registration.
This release extends this functionality so that Admins can configure Partner Distribution Lists that include Products which contain a specific Substance.
Learn More
- Enablement: Enable Reporting Family Configuration Scenarios
- User Help: Manage Partner Distribution Lists
Downgrade/Upgrade Scenario EvaluationConfiguration24R1.3
With this release, Vault Safety supports the evaluation of specific downgrade/upgrade scenarios with respect to Seriousness, Expectedness, and Relatedness. The feature introduces two (2) new reporting rule parameters called Downgrade Scenario and Upgrade Scenario. With these parameters, you can define specific scenarios that should be considered downgrades/upgrades when evaluating reporting rules for more control when reporting ICSRs. For example, the definition of a downgrade for a specific regulatory authority could be limited from “Serious, Unexpected, Related” to “Non-Serious, Unexpected, Related” if specified by that authority.
Learn More
- User Help:
Configurable "From" Email Address for TransmissionsConfiguration24R1.3
Vault Safety can now send emails to other organizations using a custom domain. This feature allows customers to configure their own From Email Address, for example, safety@verteo.com. Previously, Vault Safety emails only used an @veevavault.com domain. Now, with the ability to create custom domains, customers can send emails from Vault Safety using an email address associated with their company.
Learn More
- Enablement: Enable Configurable From Email Addresses for Transmissions
- Admin Help: Configure Email Transmissions: Use Custom Outbound Domains and Emails
- User Help: Send an Email Transmission
Strict Transmission Version Enforcement for PMDAAdmin Checkbox24R1.3
With this release, Vault Safety can enforce the order in which you are able to send Transmissions via Gateway and Email to the Pharmaceuticals and Medical Devices Agency (PMDA). If set, you cannot submit a Follow-up report if the initial report for the same Case Number is not first completed. This helps ensure that Submissions are not sent out of order which can lead to downstream compliance issues.
Learn More
- Enablement: Enable Strict Transmission Version Enforcement
- User Help:
Transmission Output TemplatesConfiguration24R1.3
Vault Safety now supports configurable generation of multiple Transmissions when specific Reporting Rules are met. This addresses the Submission requirement where, when a foreign Clinical Trial Case is reported in China, the case must be submitted to the Center for Drug Evaluation of the NMPA as well as to China’s National Health Commission. This feature can support additional use cases including, for example, multi-partner Submissions.
Learn More
- Enablement: Enable Transmission Output Templates
- Admin Help: Manage Transmission Output Templates
- User Help: Reporting Rule Parameter Reference: Transmission Output Template
Aggregate Reports - Support Conditional Expectedness from DatasheetsAuto-on24R1.3
With this release, Vault Safety’s aggregate reports consider the same Datasheet Expectedness fields that Vault uses during case processing. These reports now factor in conditional expectedness and include medical conditions from the datasheet in their calculations. Moreover, the expectedness calculation logic accounts for high-level MedDRA terms on the datasheet, as well as expectedness based on age and sex. This feature applies to the following aggregate reports: CIOMS II, DSUR, PBRER, and PSUR.
Learn More
- Admin Help: Manage Datasheets and Auto-Expectedness
AS2 Connections: Korea (MFDS)Configuration24R1.3
Vault Safety now supports AS2 Connection submissions to the Korean Ministry of Food and Drug Safety. This extends already-supported AS2 communication between Vault and external partners and health authorities, removing the need to rely on external systems for this functionality.
Learn More
- Enablement: Enable AS2 Connections
- Admin Help: Configure MFDS AS2 Connection
AS2 Connection: Troubleshooting ToolsAuto-on24R1.3
Vault Safety now provides the following tools to troubleshoot AS2 Connection issues:
- Ability to send an existing test file to a destination to check if the connection is successful. This eliminates the need to generate a compliant case to test the AS2 Connection with a partner or health authority.
- To troubleshoot the receipt of messages from a destination, Admins can view the latest blocked IP addresses that are not on the Connection Allowed list that the destination used to send the AS2 request to Vault Safety.
These tools empower users to troubleshoot their AS2 Connections independently.
Learn More
- Enablement: Enable AS2 Connections
- Admin Help: Troubleshoot AS2 Connections
Fast Inbox Item LoadAuto-on24R1.4
Vault Safety now loads Inbox Item sections asynchronously after the initial page displays. This improves the performance of the Inbox Item page and enhances the user experience.
Learn More
- User Help: Inbox Item Field Reference
Case Version Compare with SummaryConfiguration24R1.4
With this release, Vault Safety introduces a new Case Version Compare page, which can compare two (2) Case versions at once. Additionally, Vault Safety displays a summary of new and modified information on the Case Version Compare page to help users identify changes more quickly. This feature allows customers to visually focus on the key differences between cases to make more informed decisions during Case comparison.
Learn More
- Enablement: Enable Case Version Compare with Summary
- Admin Help: Configure the Inbox Item Follow-Up Case Compare Layout
- User Help: Case Version Compare
Product Browser for Faster Case Product SelectionConfiguration24R1.4
This feature streamlines Product coding by introducing a Product Browser for quick and efficient selection of Company and External Products during Case processing. By replacing the single product drop-down list, which required the extra step of manually choosing the Product Type, Vault Safety improves support for more extensive product libraries.
Learn More
- Enablement: Enable Product Browser for Faster Case Product Selection
- User Help: Auto-Code and Browse Products: About Product Browsing
Product Auto-Code Search ImprovementsAuto-on24R1.4
When auto-coding Case Products using the Product Browser, Vault Safety now searches for matches against Products, Aliases, Registrations, and Trade Names while also considering the Country Obtained. Additionally, after auto-coding a Product, Vault always displays both the Registration and Trade Name fields. When users hover over the Company Product value, the hovercard includes the Product Family, Product, Trade Name, Registration, and Country when available. Finally, on Domestic Cases, Vault maps the auto-coded Product (Reported) value to the English field and includes the Company Product, Trade Name, and Registration if matched.
Learn More
Product Browser ImprovementsAuto-on24R1.4
This feature introduces several improvements to the Product Browser available on Inbox Items and Cases. The browser now includes the Trade Name column, which displays Product Trade Names that Admins have configured in the library, and the Localized Product Name column, which appears when browsing Products on Domestic Cases only. In addition, Vault now displays the Product (Reported) value below the Search bar, and users can now sort any column in ascending or descending order.
Learn More
Product Reported on External Product BrowsersAuto-on24R1.4
Vault now displays the Product (Reported) value below the Search bar in the WHODrug Browser and JDrug Browser. Showing the Product (Reported) value adds clarity to users since Vault uses that term, when available, for the initial search.
Learn More
- User Help:
PDMA E2B(R3) Import to Inbox ItemAuto-on24R1.4
With this release, Vault Safety supports PMDA E2B(R3) import to Inbox Item, including merging to an In-flight Case and promoting to a Follow-up Case through the Inbox to Case Compare page. PMDA E2B(R3) import is supported for Japan Domestic Cases. After Inbox Item promotion, Vault creates a corresponding Japan Localized Case and sets the Classification to “Domestic”.
Learn More
- User Help: PMDA E2B(R3) Case Import
Support for Custom Translation Connectors for Intake 24R1.4
With this release, the Vault Safety Auto-Translation Framework now supports Intake translation for custom connectors. Previously, Vault Safety only supported Amazon Translate. Using custom connectors gives customers the ability to tailor translation processes to meet unique organizational needs.
Case Access Groups: All Access Constrain by LocalizationAuto-on24R1.4
Case Access Group security has been enhanced to ensure that users assigned to the system-provided All Access Group can access Inbox Items, Cases, and Case child records based on the Localization value specified on their User Access Group Assignment.
Learn More
User Help: Manage Case Access Group Security: Create User Access Group Assignments
Agency-Based Auto-Expectedness for Clinical Trial Study CasesConfiguration24R1.4
With this release, Admins can customize Expectedness evaluations on Clinical Trial Study Cases to consider how the Agency requires Expectedness to be calculated. This enhancement introduces the Base Clinical Trial Expectedness On field to the Organization object. When the field is set to “New Info Date”, Vault calculates Expectedness using new information on the Case. For Follow-up Cases, Vault will recalculate Expectedness, disregarding active dates. Adjusting Expectedness according to agency specifications supports more accurate reporting.
Learn More
- Enablement: Enable Agency-Based Auto-Expectedness for Clinical Trial Study Cases
- Admin Help: Manage Organizations: Base Clinical Trial Expectedness On
- User Help: How Case Assessment Expectedness is Generated: Expectedness Evaluations for Clinical Trial Study Cases
Show Calculation Basis for Clinical Trial Study ExpectednessConfiguration24R1.4
For Clinical Trial Study Cases, Vault Safety now displays whether Expectedness is calculated based on Case New Info date or Adverse Event Onset Date. The new Based On field is available on the Case Assessment Expectedness object and provides additional details on Vault Expectedness auto-calculation.
Learn More
- Enablement: Enable Show Calculation Basis for Clinical Trial Study Expectedness
- User Help: Enter Case Data: Expectedness Section
PMDA E2B(R3) Export for Blinded Study Product ReportingAuto-on24R1.4
To support PMDA reporting requirements, Vault Safety extends Blinded Study Product Reporting to include exporting Substances to PMDA E2B(R3) reports. When Case Study Products are blinded and have an investigational Product with a registration that is reportable to the PMDA, users can define a Case Product Registration (CPR) that links the blinded Study Product to an actual Company Product in the Library. Then, when generating reports, Vault exports Substances based on the Product associated with CPR to the G.k.2.3.r.1 Substance / Specified Substance Name data element.
Learn More
Safety Feature Enablement Updates
Enablement updates apply to the following feature in this release:
Enablement Update: Automated Cross ReportingAuto-on24R1.2
With this release, the Cross Reporting feature is Auto-on. This feature was originally introduced in 22R1 with Configuration-enablement (see Automated Cross Reporting in What’s New in 22R1). Although this feature is Auto-on, some components require additional configuration. This enablement change primarily impacts customers using version 3 of the FDA ICSR Reporting Rule Set.
See the following articles to learn more about this feature:
- Enablement: Enable Automated Cross Reporting
- User Help: Cross Reporting
SafetyDocs
Order PSMF Logbook by Section/AnnexAdmin Checkbox24R1.2
With this release, Admins can choose to order logbook entries by date of entry or by section/annex. Previously, the logbook was ordered exclusively by date. This enhancement contributes to the usability of the logbook by providing additional flexibility.
Learn More
- Enablement: Enable Order PSMF Logbook by Section/Annex
- User Help: Manage PSMF Binders and Logbooks: PSMF Logbook
PSMF Version Binding ValidationConfiguration24R1.2
Vault SafetyDocs can now specifically verify PSMF Content documents for steady-state binding when approving a PSMF binder prior to PDF generation. Previously, Vault would run steady-state verification for all documents within a binder, including obsolete documents, which should never be included in the generated PDF, and auto-generated documents such as reports, table of contents, and the PSMF Logbook. These would block approval as they may not have a steady-state version available. This enhancement ensures that no draft documents are included in the generated PDF, without considering obsolete or auto-generated documents during approval.
Learn More
- Enablement: Enable PSMF Version Binding Validation
- User Help: Manage PSMF Binders and Logbooks: Review and Approve PSMF Binders
Update PSMF Logbook TemplateAuto-on24R1.2
The PSMF Logbook template has been updated with a wider column for Summary of Changes. This provides a clearer view of the data and a better user experience.
Note: All custom sharing rules on the PSMF Logbook Report will be reset.
Code Product-Event Combinations to MedDRA QueriesConfiguration24R1.2
In this release, Vault SafetyDocs provides the ability to code Product-Event Combinations to a MedDRA query (SMQ or CMQ) rather than to a single MedDRA term. This enhancement adds more flexibility to Signal Management.
Learn More
- Enablement: Enable Code Product-Event Combinations to MedDRA Queries
- User Help: Signal Management: Create a Product-Event Combination
Create Safety Investigations from Product-Event DispositionsConfiguration24R1.2
In this release, Vault SafetyDocs provides a new action to create Safety Investigations directly from Product-Event Dispositions. This helps streamline the process of managing your safety investigations.
Learn More
- Enablement: Enable Create Safety Investigations from Product-Event Dispositions
- User Help: Signal Management: Create a Safety Investigation
Signal Management Disposition and Reporting Period ChangesAuto-on24R1.2
This release introduces several data model changes to better support the Signal Management process:
- The reporting period is now available at the Product-Event Disposition level for point-in-time reference whereas Safety Investigations are no longer tied to a reporting period.
- You can now prioritize Validated Signals using the standard Safety Investigation Priority picklist.
- A new lifecycle state, Monitoring, has been added to the Product-Event Disposition lifecycle.
- SafetyDocs now provides several standard Checklist types to reduce the need for custom Checklist types and improve alignment.
- For the Validation Outcome picklist, the “Refuted” picklist value label has been updated to “Non-Validated” to align with industry terminology.
Learn More
- Enablement: Enable Signal Management
- User Help: Signal Management: Product-Event Dispositions Section
SafetyDocs Standardization 24R2 24R1.3
With this release, Vault SafetyDocs adds a number of standard components to ensure customers can easily adopt best practices for major business processes. Additional Standardization capabilities will be available in upcoming releases.
Merge Binder to PDF Document Inclusion UpdateAuto-on24R1.3
This enhancement updates the document inclusion rules of the Merge Binder to PDF action to include all documents in the binder upon merging. Previously, this action included only documents that could be viewed by the user running the action. This ensures that outputs include all the documents from their respective binders.
Learn More
- User Help: Merge a Binder to PDF
JAPIC Literature IntakeConfiguration24R1.3
In order to support Literature processing for Japan, Vault SafetyDocs has expanded its literature management capabilities by allowing the import of literature article list files from JAPIC. Additionally, users can review articles to assess whether reporting to the PMDA is required, create Inbox Items from the articles, and promote them to PMDA Safety Measure Report or Research Report Cases.
These updates allow users to efficiently process literature articles in line with the PMDA’s requirements.
Note: Your Vault must be licensed for both Vault Safety and Vault SafetyDocs to create Inbox Items from JAPIC articles and promote them to Cases as part of this feature.
Learn More
- Enablement: Enable JAPIC Literature Intake
- Admin Help: Create Literature Standard Search Terms
- User Help:
Risk ManagementConfiguration24R1.3
With this release, Vault SafetyDocs supports tracking the authoring, approval, and distribution of global and local Risk Management Plans (RMPs) and Additional Risk Minimization Measures (aRMMs). Identified risks can also be categorized and tracked with measures to be taken globally and in each market. Supporting this key element of the pharmacovigilance process enhances compliance with overall drug safety.
Note: This feature will be available for full configuration in an upcoming release. For more information, contact your Veeva Representative.
Generate Final Aggregate Report ZIPConfiguration24R1.3
Vault SafetyDocs now offers an action to generate the final Aggregate Report as a ZIP file organized in the order of the created Aggregate Report Sections. Prior to this, the Sections needed to be downloaded and ordered individually, which was time-consuming. The ZIP file can be included in the published output that is sent to health authorities or distributed to partners. This feature enhances the end-to-end user experience and usability of Aggregate Report creation and distribution.
Learn More
- Enablement: Enable Partner Distribution Tracking and Generate Final Aggregate Report ZIP
- User Help: Author Aggregate Reports: Generate Final Aggregate Report ZIP
Partner Distribution Tracking for Aggregate ReportsConfiguration24R1.3
With this release, Vault SafetyDocs supports specifying whether Aggregate Reports are being distributed to partners or submitted to health authorities. This ensures compliance with the distribution clauses defined in PVAs without the need for duplicate data entry and setup.
Learn More
- Enablement: Enable Partner Distribution Tracking and Generate Final Aggregate Report ZIP
- User Help: Author Aggregate Reports: Create an Aggregate Report Destination
Update Aggregate Reports Setting LabelAuto-on24R1.3
The Generate Tabulations Using Tasks setting has been relabeled to “Generate Tabulations Using Sections” since Aggregate Report Tasks are now called Aggregate Report Sections.
Safety Data Model Changes
See 24R2 Safety Data Model Changes.
QualityOne
QMS (QualityOne)
External Collaboration Management on Audit Findings and Action ItemsConfiguration24R1.2
This feature extends External Collaboration Management functionality in QualityOne QMS to the Audit Finding and Action Item objects, enabling users to work with External Collaborators to complete and follow up on Audit Findings and Action Items in Vault. Internal users manage a “contact list” of persons associated with the third-party organizations, tagging Audit Finding and Action Item records with a relevant External Collaborator for Vault to automatically create, activate, or inactivate. When the user account for the External Collaborator is created, activated, or inactivated, the relevant email is sent to those persons as part of the process.
This feature automates the creation, activation, and deactivation of accounts for external collaborators such as suppliers. Users can easily bring in External Collaborators using a license from a “revolving door” pool of external licenses without needing to purchase licenses for each individual external user, and Vault automatically manages user accounts for External Collaborators with little to no need for Admins to manage user account creation, activation, and deactivation.
Previously introduced External Collaboration Management functionality includes the CAR, NCR, Change Control, Audit, and Inspection objects, as well as Document Review & Approval for Document Control users.
Learn more about working with External Collaborators.
HACCP Flow Diagram: Create Hazard Analysis, OPRP, and CCP for a Process StepAuto-on24R1.3
This feature adds the following additional functionality to the HACCP Flow Diagram’s Information panel:
- Users can now click a plus (+) icon in the Information panel to create Hazard Analysis records for a HACCP Plan Process Step, reducing the number of keystrokes required to add new Hazard Analysis records.
- Users can now add Operational Prerequisite Program (OPRP) and Critical Control Point (CCP) records to a HACCP Plan Process Step directly from the Information panel instead of having to launch a new browser window for data entry.
- Updates to records now automatically display in the Information panel without users having to manually refresh.
These enhancements improve the user experience when interacting with the HACCP Flow Diagram by reducing clicks, and provide a broader view of the manufacturing process at-a-glance.
Learn more about performing Hazard Analysis and working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Support for Custom Auto-Numbering ActionAuto-on24R1.3
This feature provides a way for users to execute a custom action that automatically updates the sequencing of HACCP Plan Process Steps on a HACCP Flow Diagram. Once the custom action has been implemented by Veeva Services, users can execute the action to apply business rules tailored for their organization’s business process to re-sequence HACCP Plan Process Steps. This reduces the margin of error and tedious administrative effort of manually re-ordering process steps due to additions and removals.
Learn more about using the auto-numbering action.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Manage Hazard Analysis for Groups within DiagramAuto-on24R1.3
This feature enables users to create and view Hazard Analyses for HACCP Plan Process Step Groups in a HACCP Plan on the HACCP Flow Diagram. Users can add Hazard Analysis, OPRP, and CCP records directly from the Information panel using the plus (+) icon without having to manually refresh the panel to see updates.
Learn more about performing Hazard Analysis and working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Hazard Analysis ImprovementsConfiguration24R1.3
With this feature, users can conduct a hazard analysis for HACCP Plan Process Steps and HACCP Plan Process Step Groups within the Information panel of the HACCP Flow Diagram. These improvements assist users in systematically identifying and assessing the risk of potential hazards at each step of a process.
Learn more about performing Hazard Analysis and working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Support for Completeness Check
This feature allows users to store the completeness status of hazard analysis for each HACCP Plan Process Step and HACCP Plan Process Step Group in a HACCP Plan. When the Hazard Analysis Completeness Check (![]() ) icon is enabled, an icon (
) icon is enabled, an icon (![]() ) displays on HACCP Plan Process Steps and HACCP Plan Process Step Groups where the status of the hazard analysis is incomplete. Inactivating the Hazard Analysis Completeness Check hides the icons on HACCP Plan Process Steps and HACCP Plan Process Step Groups.
) displays on HACCP Plan Process Steps and HACCP Plan Process Step Groups where the status of the hazard analysis is incomplete. Inactivating the Hazard Analysis Completeness Check hides the icons on HACCP Plan Process Steps and HACCP Plan Process Step Groups.
HACCP Flow Diagram: Complete Risk Assessment and Decision Tree within Diagram
This feature allows users to complete the following hazard analysis activities from the Information panel for a selected HACCP Plan Process Step or HACCP Plan Process Step Group:
- Identifying possible hazards (for example, biological, chemical, physical) associated with each HACCP Plan Process Step.
- Completing risk assessment by selecting the applicable risk matrix and evaluating the likelihood and severity of the hazards.
- Completing a decision tree to determine the type of control measures required (for example, CCP or OPRP) and listing measures to control or eliminate each identified hazard.
Initial Support for HACCP Localization
This feature lays the foundation for the localization of HACCP plans, providing HACCP team members working in factories with the ability to view and edit HACCP Plans in their local language. Localization is limited to languages supported by Vault.
HACCP Plan: Localization Support (Data Model)Configuration24R1.3
This feature provides the foundational data model to support the localization of HACCP plans, enabling users to store translated master data and transactional data to create translated copies of HACCP Plans.
Learn more about working with HACCP Management.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
QMS (QualityOne) & HSE
Audit Checklist Assignment AutomationConfiguration24R1.3
This feature makes the functionality of the Manual Checklist Assignment user action available as the Audit Checklist Assignment entry action and the Audit Checklist Assignment system action. This allows users to automatically assign checklists to specific Auditors by changing the lifecycle state on an Audit record or moving through a workflow, without the need to manually run a user action.
Learn more about assigning Audit Checklists and configuring Audit Checklist Assignment actions.
Auto-Create Proposed AuditsConfiguration24R1.3
With this feature, users can now automatically create the associated Proposed Audit records in an Audit Program by running the Create Proposed Audits user action. When users run the action, Vault creates Proposed Audits from Organization or Facility records based on the Planned Start Date and Planned End Date of the Audit Program, the configured date field on the applicable Organization or Facility records, and specific optional metadata that is configured in the user action.
Learn more about auto-creating Proposed Audits and configuring Proposed Audit auto-creation.
QualityOne Data Model Changes
See 24R2 QualityOne Data Model Changes.
RegulatoryOne
The RegulatoryOne 24R1.4 release, including all Platform features, is scheduled for tentative availability on July 9, 2024.
Compliance Management
Packaging Composition ViewerConfiguration24R1.2
This feature allows users to review Packaging Compositions in a hierarchical view, inclusive of related Formulations, enabling regulatory teams to easily determine the weight and percentage of recycled content in packaging for reporting to authorities.
Learn more about Packaging Composition Viewer.
Packaging Composition Viewer: Qualitative AssessmentsConfiguration24R1.3
This feature allows users to determine whether packaging compositions contain any dangerous materials in order to track compliance against various regulations, such as REACH, Heavy Metals, Prop65, and SVHC for reporting to authorities. This allows organizations to assess packaging needs as part of product launch and maintenance.
Learn more about Packaging Composition Viewer.
Registration & Dossier Management
Object Mapping Enhancement: Support for Formula & Lookup FieldsAuto-on24R1.2
This feature allows Admins to create Object Mappings for non-editable source fields such as lookups and formula types of fields, supporting more flexibility when mapping fields.
Learn more about configuring Object Mappings and Field Mappings.
24R2 Registration & Dossier Management EnhancementsAuto-on24R1.2
These enhancements ensure data correctness by validating the following conditions when users create or update records and run actions:
- Dossier Binders cannot include binders.
- Root dossier Requirements must reference a root Requirement Library.
- Requirements must reference either a Registration Item or a Registration Objective.
- Each EDL and its child EDL Items must reference the same dossier Requirement.
- Users cannot select the Use Source checkbox on dossier Requirements that directly reference an active EDL.
Learn more about Dossier Binders and generating Requirements.
Generate Requirement Enhancement: Living DossiersConfiguration24R1.3
This feature allows users to re-generate a dossier’s requirements, which creates new dossier Requirement records. This allows users to update global and core dossiers over time to reflect changes.
Learn more about living dossiers.
Copy & Amend Enhancement: Multiple TemplatesConfiguration24R1.3
This feature allows users to build a dossier from a source that uses a different dossier template. This gives different groups the flexibility to define their own template to be compliant while allowing them to build their dossiers quickly and efficiently.
Learn more about configuring multiple templates.
RegulatoryOne Data Model Changes
See 24R2 RegulatoryOne & Veeva Claims Data Model Changes.
Veeva Claims
The Veeva Claims 24R1.4 release, including all Platform features, is scheduled for tentative availability on July 9, 2024.
Veeva Claims
Pack Copy Enhancement: All Elements ViewConfiguration24R1.2
With this release, the All Elements View is available to configure on the Pack Copy object, which gives users a consolidated view of all elements assigned to different panels of a Pack Copy record.
This feature also allows Translators to provide translations for a local Pack Copy by editing the element text field and gives them the ability to add columns of their choice, freeze columns, and click on hyperlinks to view more details of all records displayed in the viewer.
Learn more about translating Pack Copy element text.
Owner Role for Clone Pack Copy & Localize Pack Copy ActionsAuto-on24R1.2
This Pack Copy enhancement sets the current user as the owner of records generated by the Clone Pack Copy and Localize Pack Copy actions when Dynamic Access Control (DAC) is configured for the Pack Copy object so that the current user can view and edit records created by them through above actions.
Learn more about configuring the Clone Pack Copy and Localize Pack Copy actions.
24R2 Veeva Claims EnhancementAuto-on24R1.3
This feature adds security enhancements to improve the overall administration of the application. Upon manually creating a Panel record, Vault now sets the value of the Pack Copy Ref field. This feature also enhances the Copy Claims to Another Product action to prevent copying Claim object fields that are configured not to be copied through the Copy Record action.
Learn more about the process of copying Claims.
View & Assign Available Product ClaimsConfiguration24R1.3
Organizations can manage Claims at different levels of the Product hierarchy, and often these Claims are reused across different levels of the hierarchy. This feature provides users with a consolidated view of all the Claims available to a particular Product through its product composition, such as Claims related to the Product’s parent and child Products. This allows users to review the applicable Claims in a single place rather than manually collecting that list from multiple Product detail pages.
Additionally, the feature allows users to selectively assign the available Claims to a Product through a new user action so that they can identify and manage the usage of the assigned Claims.
Learn more about viewing and assigning product claims.