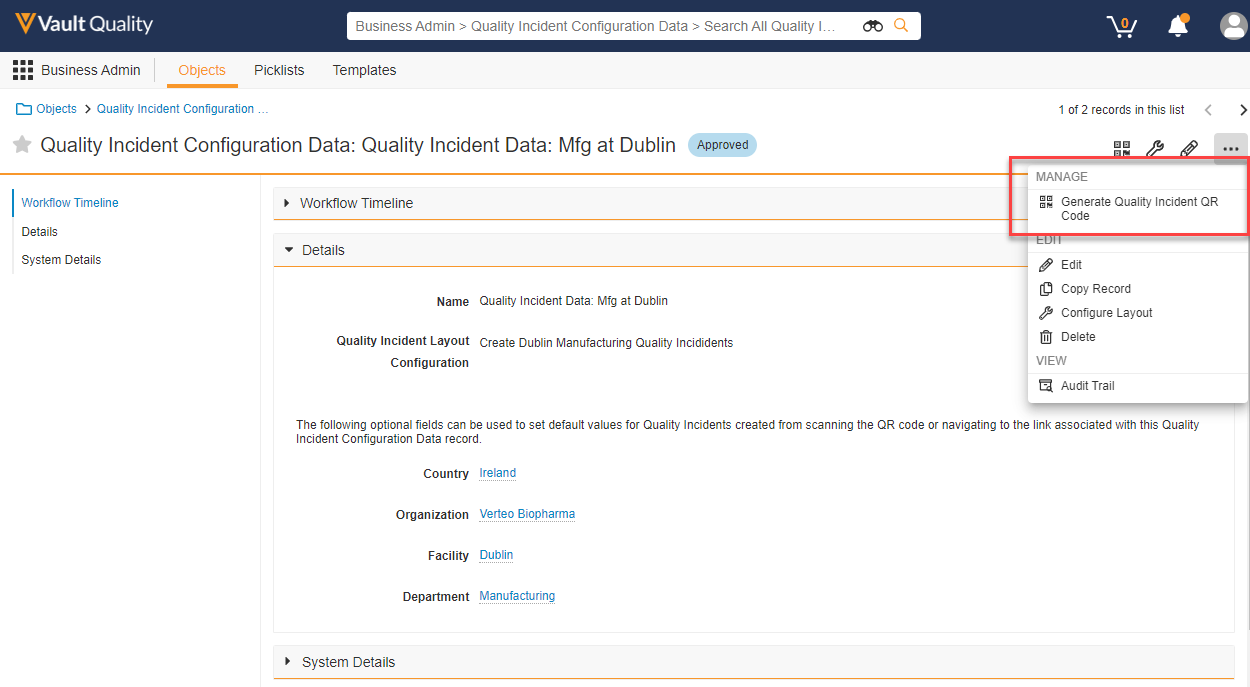
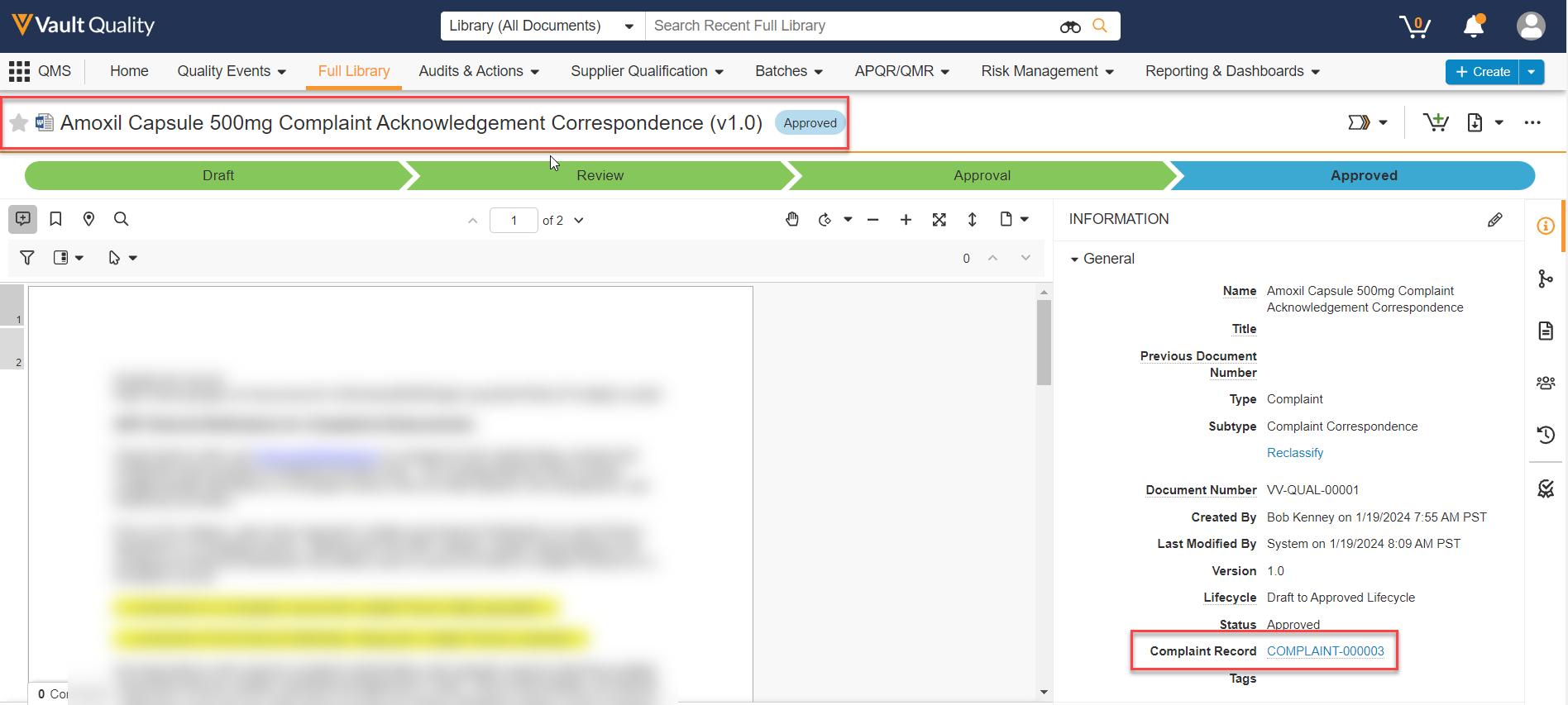
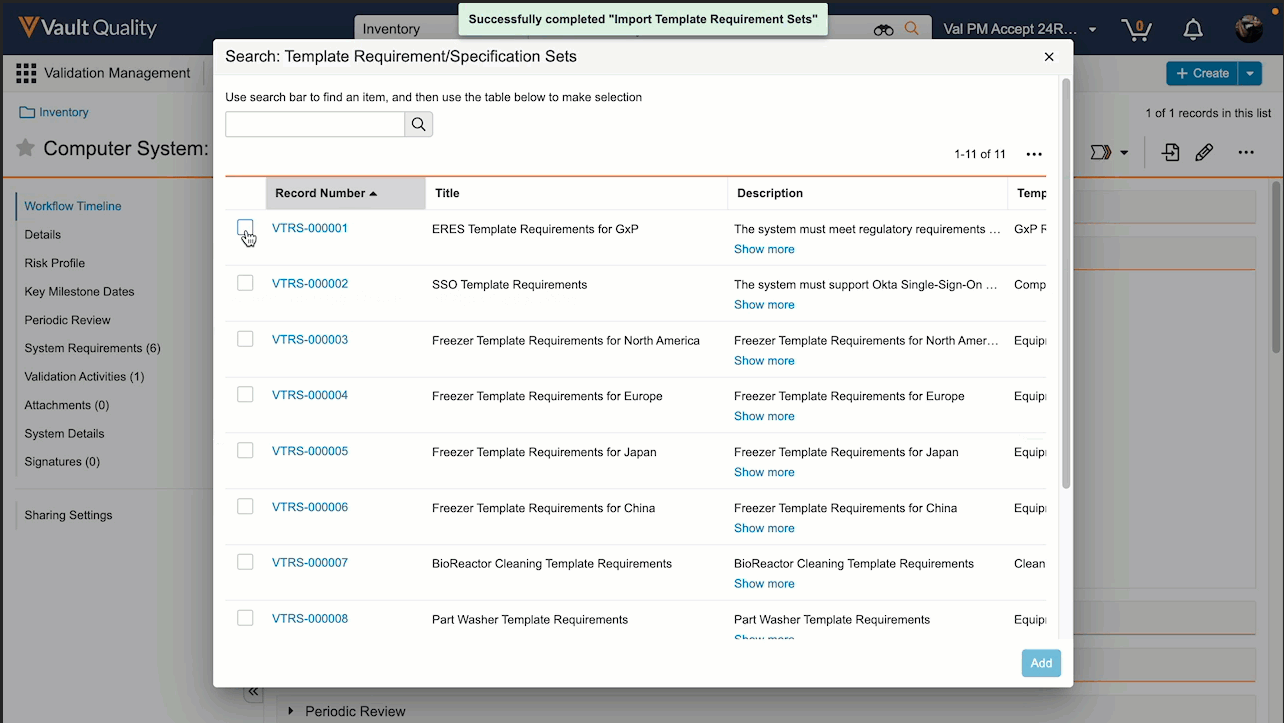
Limited Release Dates: December 8, 2023 (23R3.2); February 9, 2024 (23R3.4); March 8, 2024 (23R3.5) | General Release Date: April 19, 2024
The following applications may have different release dates: Safety, RegulatoryOne, and Veeva Claims.
We are pleased to bring you new functionality with each limited release. These release notes are updated with upcoming new features one week before the limited release date. See the following explanations for enablement options:
- Auto-on: Automatically activated and no configuration is required before using the feature; in some cases, a new feature is dependent on another feature that must be enabled or configured.
- Admin Checkbox: Admins must turn on the feature with an Admin checkbox. Some “Auto-On” features have a checkbox setting that hides the feature; these will show “Auto-On.”
- Configuration: Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates.
- Support: On/off option controlled by Support.
- Available for Use: Used only by the eConsent, eCOA, and SiteVault applications. Sponsors must make a study-specific configuration change to implement new capabilities.
Platform
Documents
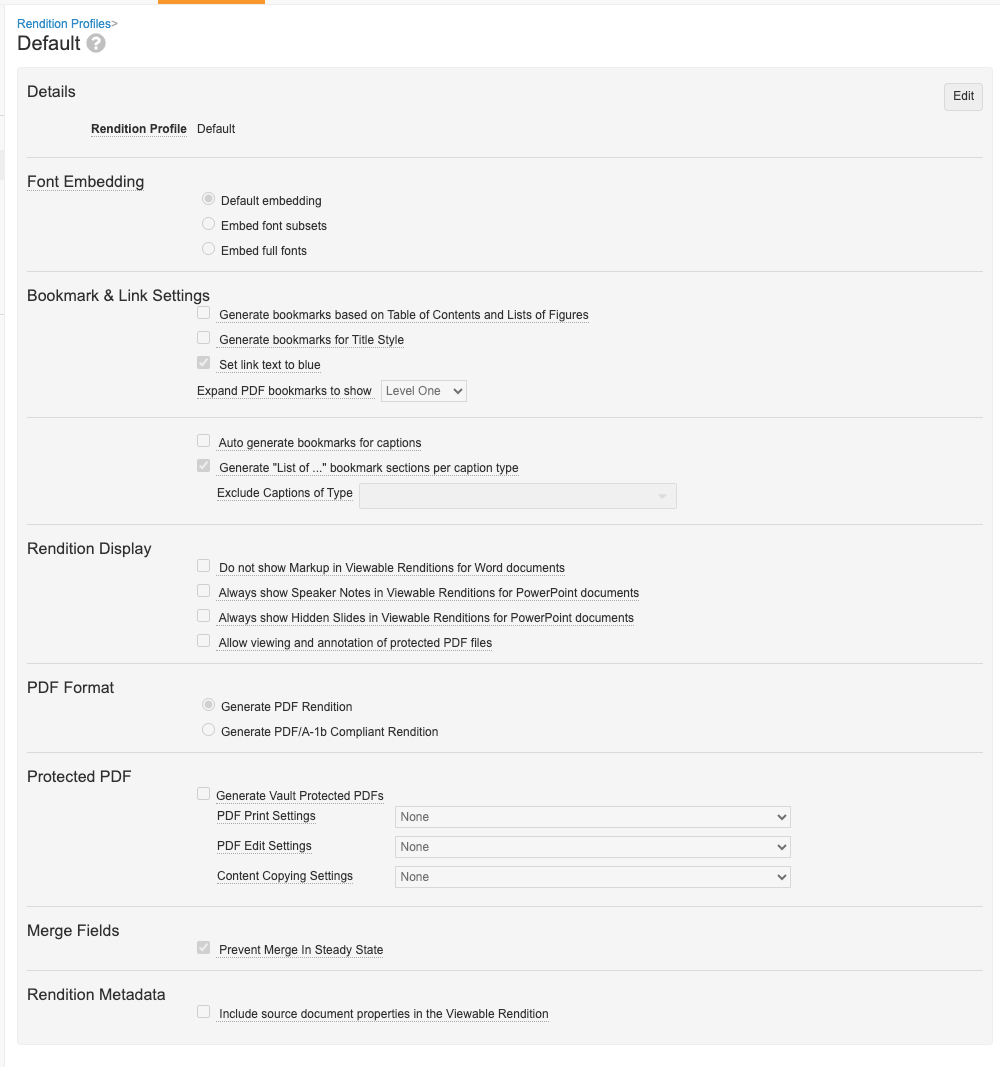
Consolidated Rendition Settings & ProfilesAuto-on23R3.4
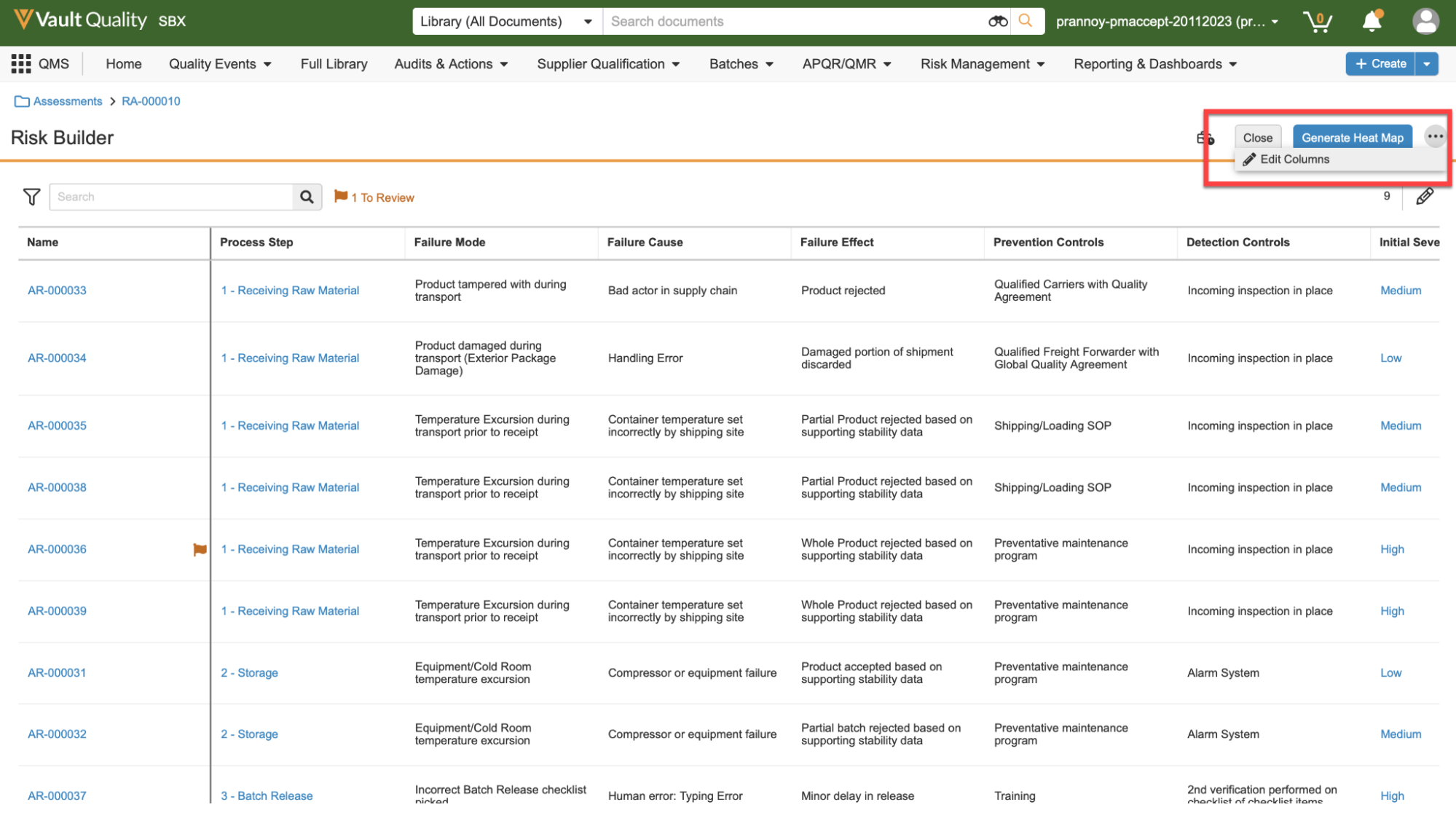
Admins will now manage all rendition settings via the Rendition Profiles page in Configuration. Prior to 24R1, there were “Vault-wide” rendition settings managed in Admin > Settings > Rendition Settings, as well as more specific settings managed within Rendition Profiles.
This enhancement streamlines the process and makes it easier for Admins to troubleshoot rendition questions by ensuring all settings are managed in one location: Rendition Profiles.
Any settings currently applied in Admin > Settings > Rendition Settings will be migrated to the default Rendition Profile to avoid any change in behavior for end users.
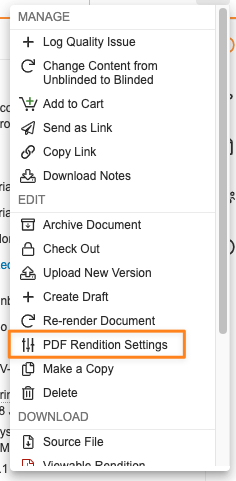
With this enhancement, the ability for end users to individually adjust the appearance of markup in Word, layouts in Powerpoint, and watermarks in PDFs will be deprecated. Users will no longer see the PDF Rendition Settings option in a document’s Actions menu:
Going forward, the Rendition Profile will determine how Vault handles displaying markup and layouts. With the removal of PDF Rendition Settings, the Display Hidden in PDF but Printable (“Print Only”) Watermark option is no longer available, as most customers use overlays to manage watermarks. This change will help provide additional control for customers and avoid scenarios where documents are approved with markup displayed.
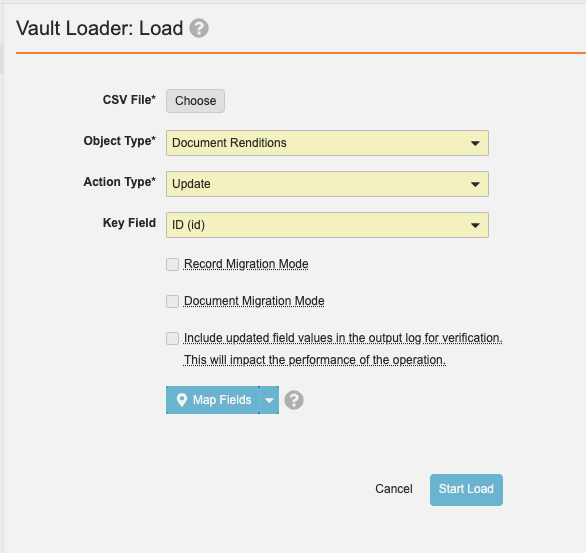
Bulk Rendition OperationsAuto-on23R3.4
Admins will now be able to bulk re-render documents using Vault Loader in the UI or via the API. Prior to 24R1, customers would need to contact Product Support to perform bulk re-rendering of documents.
The CSV file should include ID, major_version_number__v, and minor_version_number__v. The bulk re-rendering can be executed by Vault Loader through an Update action on Document Renditions with the CSV file as input:
This enhancement enables Admins to be able to perform these actions independently.
If any files fail to re-render, the process will not be stopped, but any failures will be noted in a summary file once the process is completed.
The process also runs asynchronously, meaning users will still be able to access documents while the process runs. Admins can also monitor the process from the Job Status page. A new Bulk Rendition Request job tracks the status of in-progress re-renders.
Learn more about re-rendering.
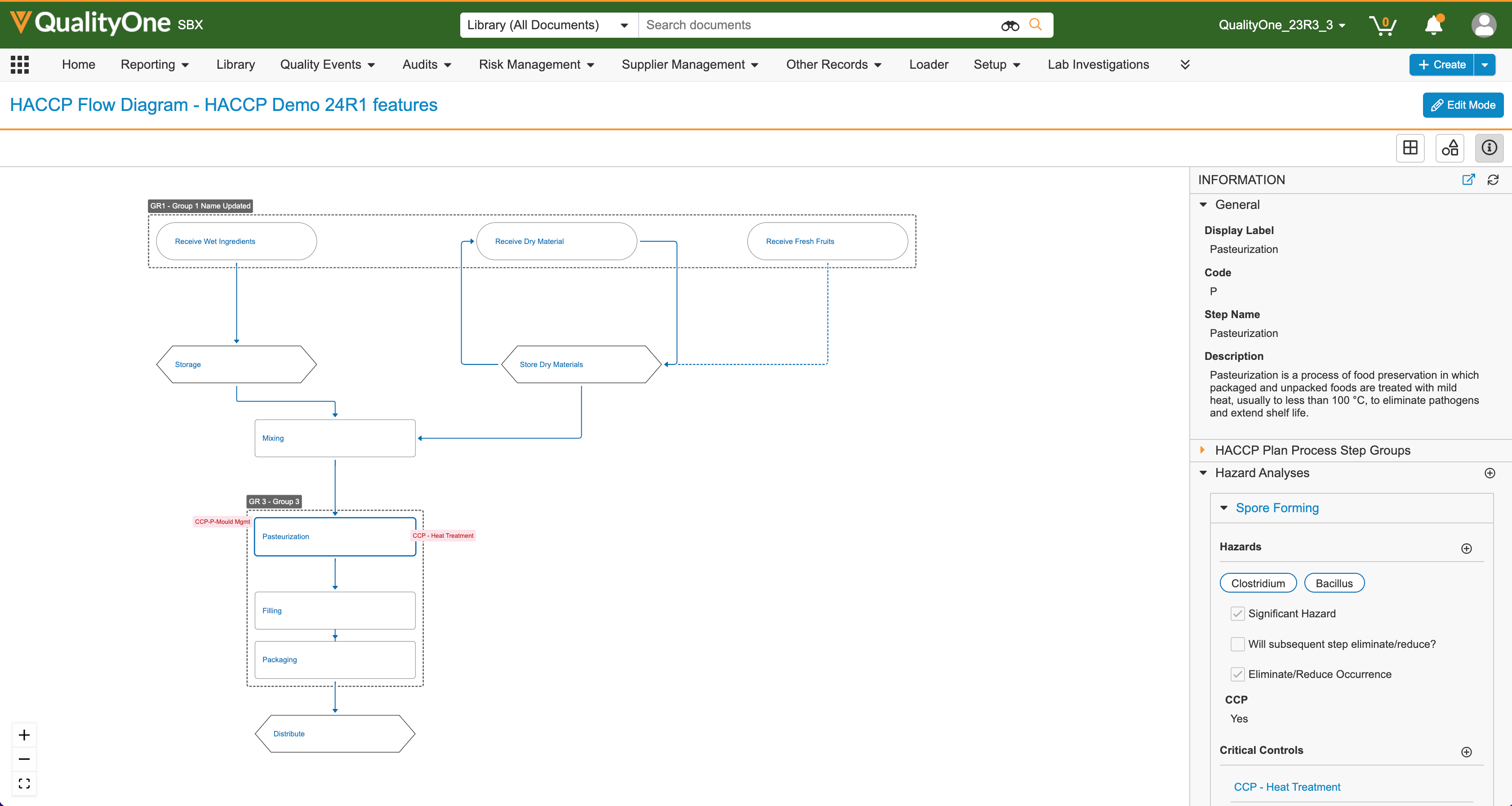
Doc Viewer: Context Menu UsabilityAuto-on23R3.4
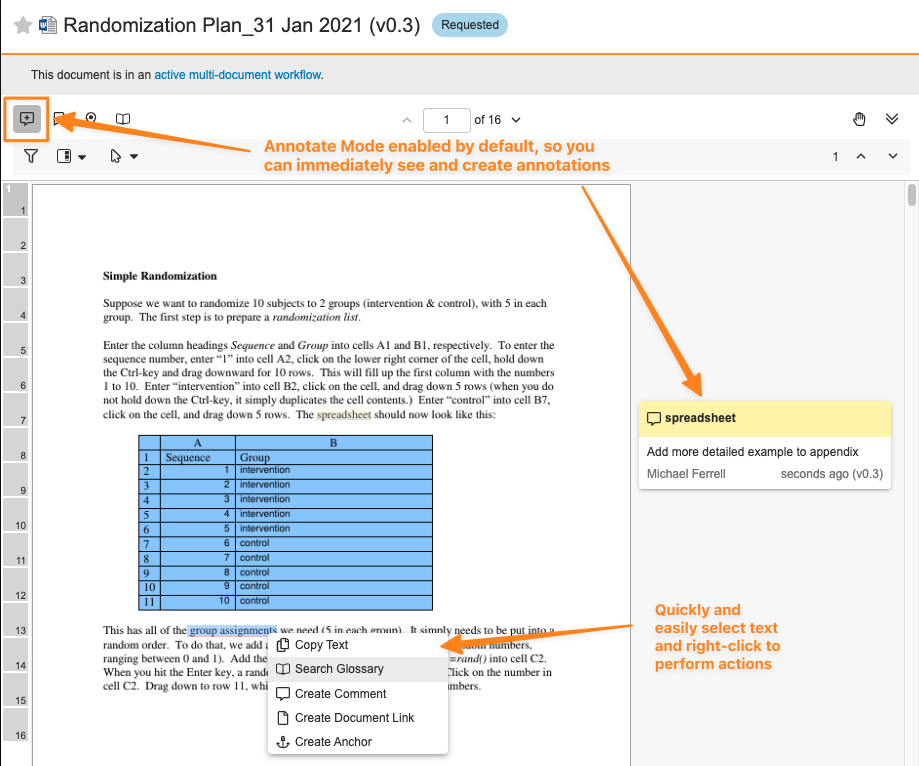
With 23R3, we introduced the Document Viewer Context Menu, which gave users the ability to right-click within the Document Viewer to quickly and intuitively access common actions on document content.
With 24R1, users can now select text or areas more easily to perform Context Menu actions and work with annotations as the Grab button will be disabled by default and Annotate Mode will be enabled. If Annotations exist or the user has Annotate permissions, Annotate Mode will be enabled by default. Prior to 24R1, if a user needed to perform a Context Menu action that required selecting text (such as performing a Glossary search), they would need to disable the Grab button first, which was selected by default.
Users can still enable Grab if needed by selecting the Grab button or by using CTRL+Shift (or CMD+Shift) on their keyboard.
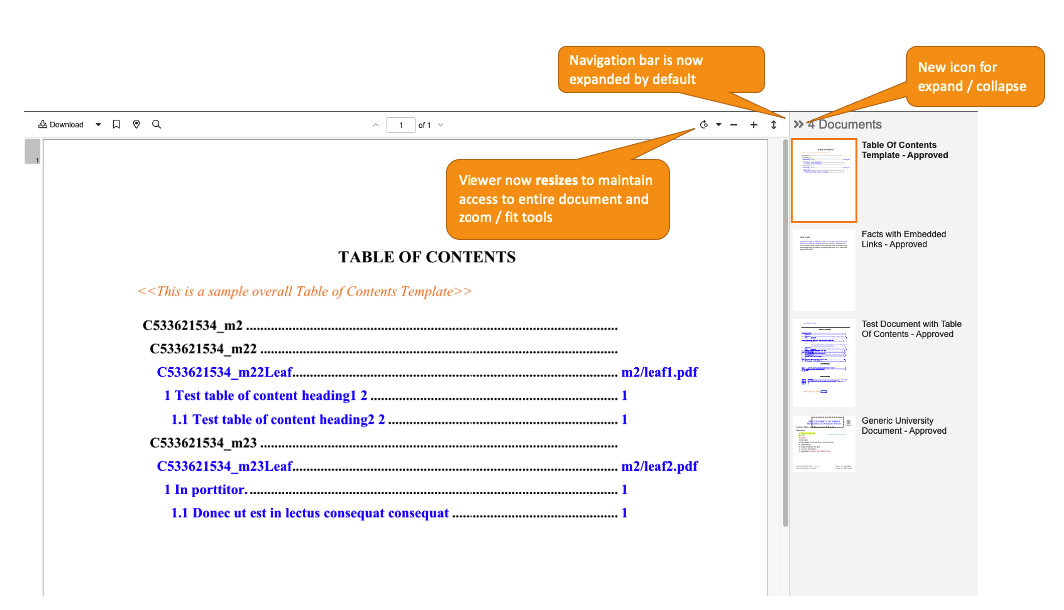
External Viewer: Improved Multi-Document NavigationAuto-on23R3.4
When using the External Viewer for multiple documents at once, users will now see several enhancements that improve the user experience and make the External Viewer more intuitive:
- The document sidebar automatically expands by default, making it clearer to users that multiple documents require review. Prior to 24R1, a user receiving multiple documents could miss that additional documents were included if they did not manually expand the document sidebar.
- The document sidebar will now remain expanded unless a user specifically chooses to collapse it. Prior to 24R1, the document sidebar would automatically collapse when a user selected a particular document.
- The icon to collapse/expand the document sidebar will be updated to left/right arrows.
- When the document sidebar is expanded, the viewer will automatically resize the document to ensure that the sidebar is not obscuring the document or any zoom controls (rotate, fit, zoom)
The External Viewer is accessed via direct URL and is often used to send documents to non-Vault users. Common uses for sending multiple documents via a direct URL to the External Viewer include:
- Sending Response Packages in MedInquiry
- Approved Email in Medical and PromoMats
- Safety Distribution via SiteConnect (for sites that do not have an active Agreement)
Select Anchor ImprovementsAuto-on23R3.4
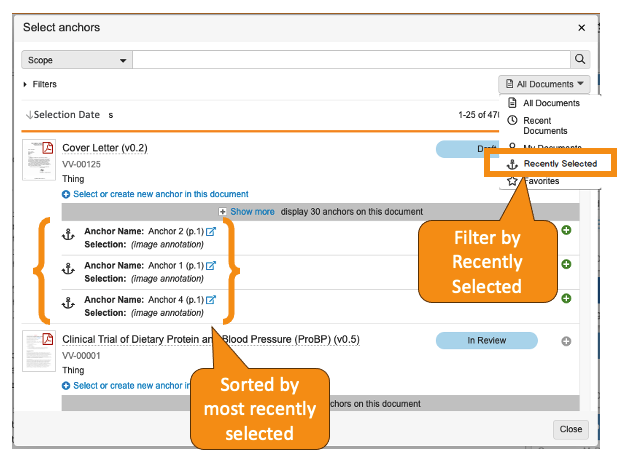
For users that leverage Anchors to link to specific locations within documents, the user experience has been enhanced to make it easier to find, reference, and select anchors.
Within the Select Anchor dialog box:
- Users now have a Recently Selected filter that displays the 25 documents most recently selected by that user.
- When a user selects any item in the filters menu, Vault now re-applies that filter the next time the user opens the Select Anchor dialog.
- Visible anchors under each document are now sorted based on that user’s most recent selections.
These enhancements allow heavy users of Anchors to more efficiently apply and re-use anchors across documents.
Learn more about Anchors.
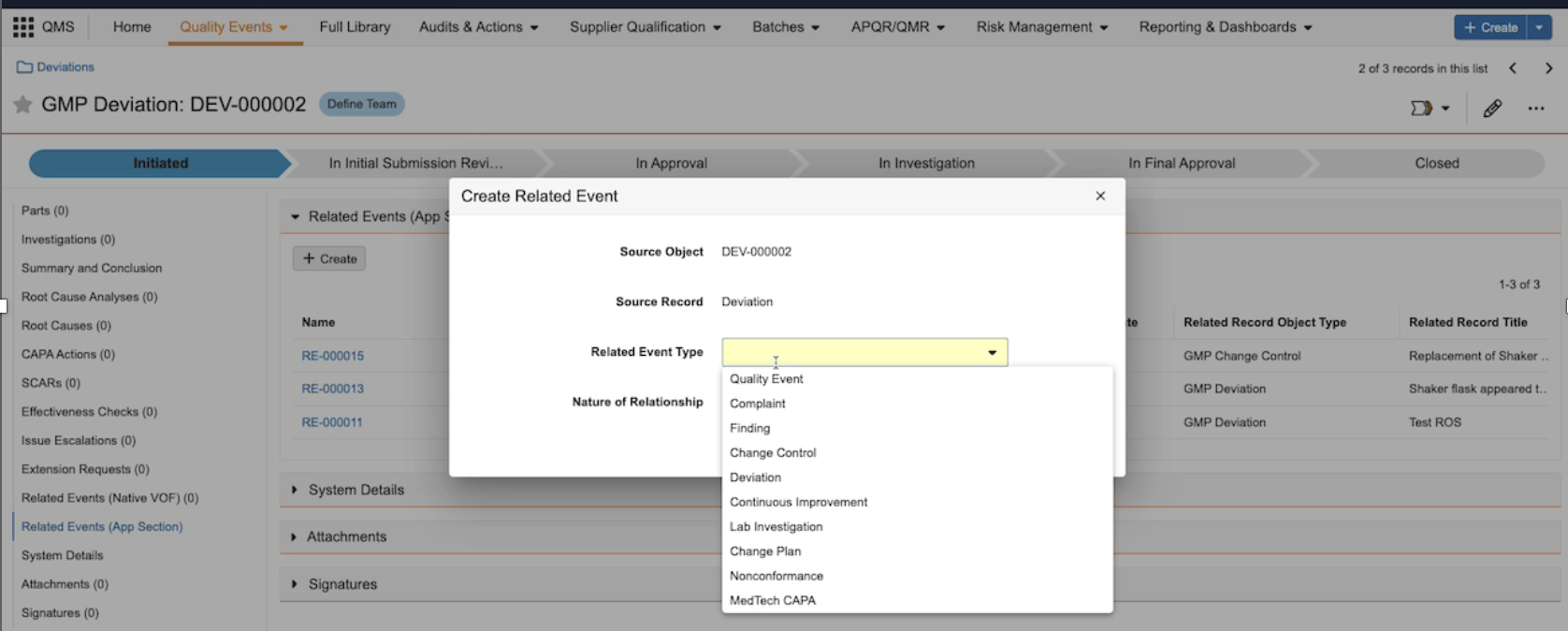
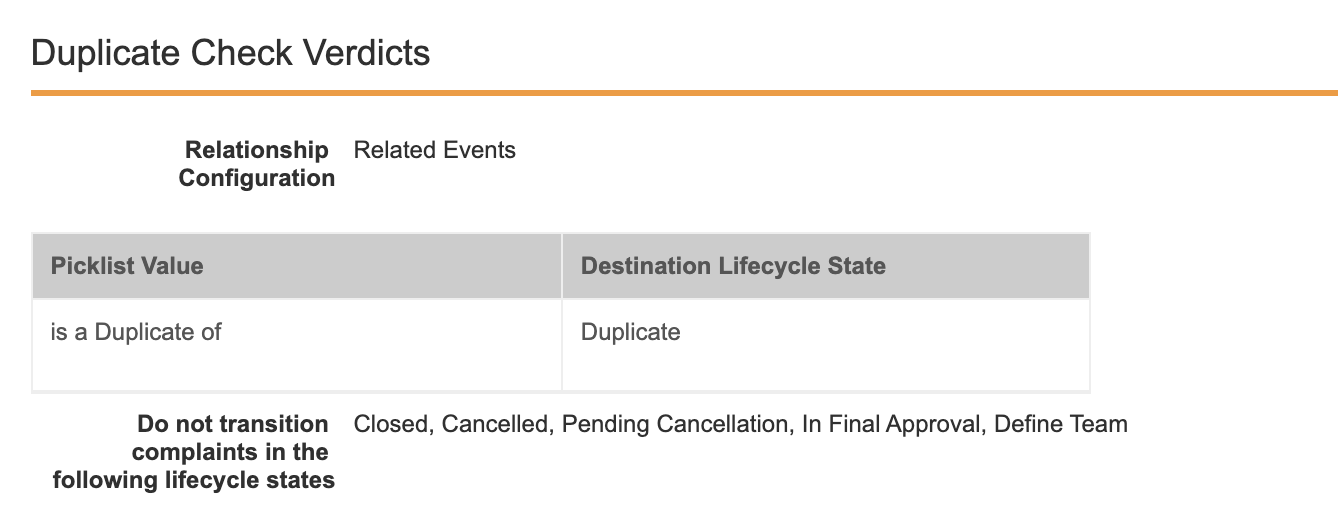
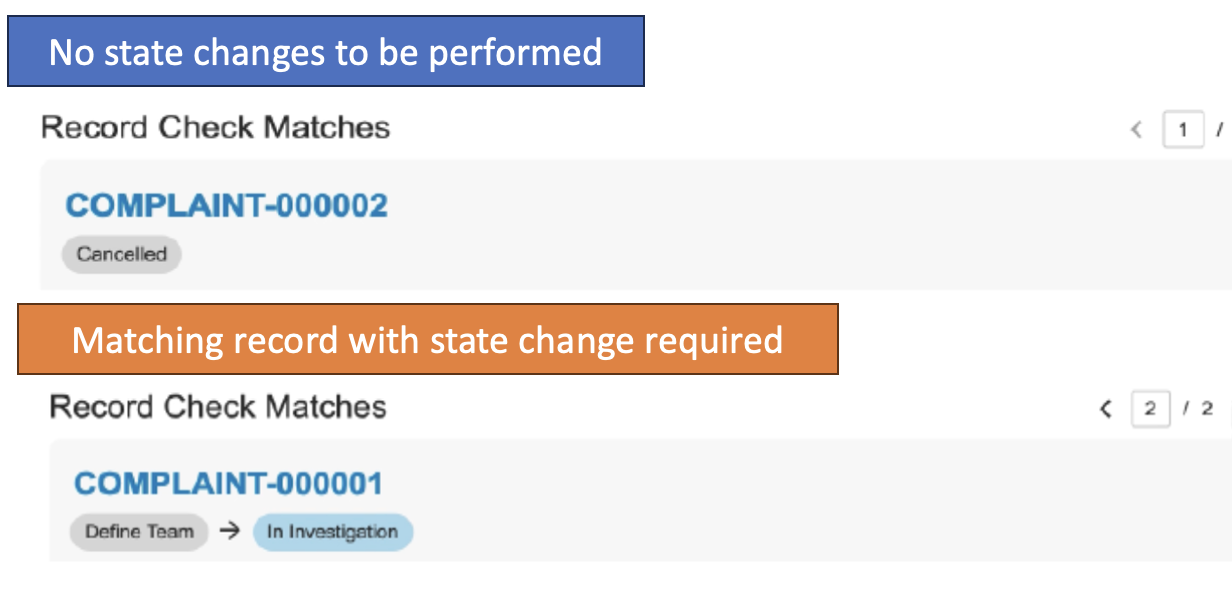
Crosslink a CrosslinkAuto-on23R3.4
Vault now supports the ability to CrossLink an existing CrossLink from one Vault to another, within a single domain. The primary use case for this enhancement is around Vault Connections - specifically, a document could be originally sourced in RIM, such as a protocol, then is crosslinked to eTMF (via the RIM to Clinical Operations Connection) and then crosslinked again to Study Training (via the Study Training to Clinical Operations Connection).
Prior to 24R1, to accomplish this, both the Study Training and eTMF Vaults would need to reference the source in RIM separately, which would require that Study Training would need to be pulling the document from the RIM document directly.
This enhancement provides a more comprehensive way of referencing documents across multiple Vaults and strengthens the productized Vault Connections. A Crosslinked Crosslink uses the viewable rendition as its source, and is not bound to the original source document.
Learn more about CrossLinking and Vault Connections.
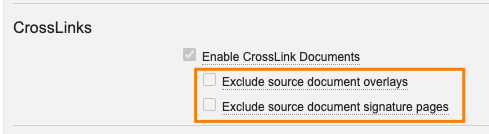
Crosslink Settings for AdminsAuto-on23R3.2
By default, Crosslinks will include the source document’s signature page and overlay - however, customers have had the ability to submit a Product Support ticket to request that the following options be made visible in Admin > Settings > General Settings:
With 24R1, we are making these options available by default for Admins - this reduces the need for customers to need to go through Veeva to be able to enable these options. The checkboxes will now be visible and editable to Admins by default, though they will be unchecked by default for any Vaults that had not previously exposed and edited them.
Non-Consecutive Document IDsAuto-on23R3.2
When documents are created, Vault automatically assigns a document ID (note, this is different from Document Number). With 24R1, we are making changes to how Document IDs are generated to support future functionality - these changes will result in document IDs consistently not being sequential numbers.
Prior to 24R1, there were already scenarios where Document IDs may not be sequential (i.e. due to document deletions), but these changes in 24R1 will result in Document IDs never being sequential.
We recommend that the Document ID field not be used to rely on the document creation order. Please use the Document Number field instead to track document sequence.
Document Templates LimitAuto-on23R3.2
With 24R1, Vault now has clearly documented and enforced limits for Vault Document Templates (both Basic and Controlled Document Templates) to ensure reliable document template performance.
Each Vault now has a limit of:
- 50 document templates at the Base Document type
- Once the limit is reached, no new templates will be able to be added to Base Document
- 500 document templates per document hierarchy
- Once the limit is reached, no new templates will be able to be added in the document hierarchy
- 5,000 document templates per Vault
- Once the limit is reached, no new templates will be able to be added anywhere in the Vault
The document templates limit includes both active and inactive templates.
The document hierarchy limit includes all of the local and inherited document templates in a given classification. For example, consider the following document type hierarchy with document templates which has a total of 500 templates and is at the limit:
VPS Adverse Event Report Document (300) > eDMR (150) > Attachment (50)
If a customer has document templates currently that are over any of these limits, the Veeva Account Team will be reaching out directly.
Learn more about document templates here.
Binder LimitsAuto-on23R3.2
Vault now has clearly documented and enforced limits for Vault Binders to ensure good binder performance. Customers and Veeva services can use binders in a predictable and easy to understand way.
With 24R1, the following limits will apply to binders:
- Binders are limited to 50,000 nodes. If a binder reaches this limit, binder nodes cannot be added, but they can be deleted
- This 50,000 node limit includes the nodes from a binder’s component binders. If a binder has reached its limit, binder nodes cannot be added to any of its component binders, even if the component binders have not reached the 50,000 node limit.
- A binder cannot be versioned via Create Draft or copied if it has a total size of 10,000 nodes or more. This node count includes the nodes within component binders. The entry action Set New Major Version is not blocked, even if the binder has 10,000 nodes or more.
There is no limit on the number of binders in a Vault. The Veeva Account Team will directly reach out to customers if a Vault contains binders that exceed these limits.
Learn more about binders in Vault here.
Preventing Unintended Document Template CreationAuto-on23R3.2
When leveraging Controlled Document Templates, templates are associated to document types using the Template Document Type field.
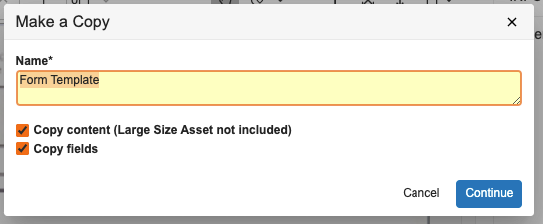
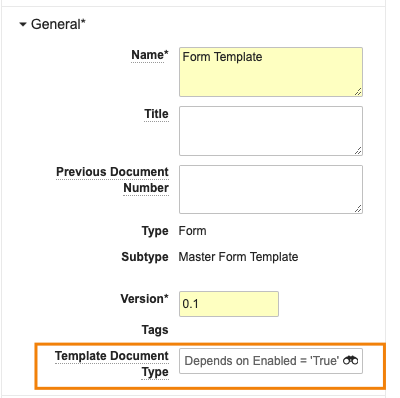
With 24R1, if a user performs a Make a Copy action on a template, Vault will no longer copy over the Template Document Type field value. This helps prevent the unnecessary and unintentional creation of new document templates. Users can still manually update the Template Document Type field after making a copy if appropriate. If needed, an Admin can also configure the field to copy by default, but the default behavior with this feature will be to not copy the value.
Here is an example Form Template in a Quality Vault, with the Template Document Type field filled out:
When a user performs a Make a Copy action:
The new document will be blank in the Template Document Type field:
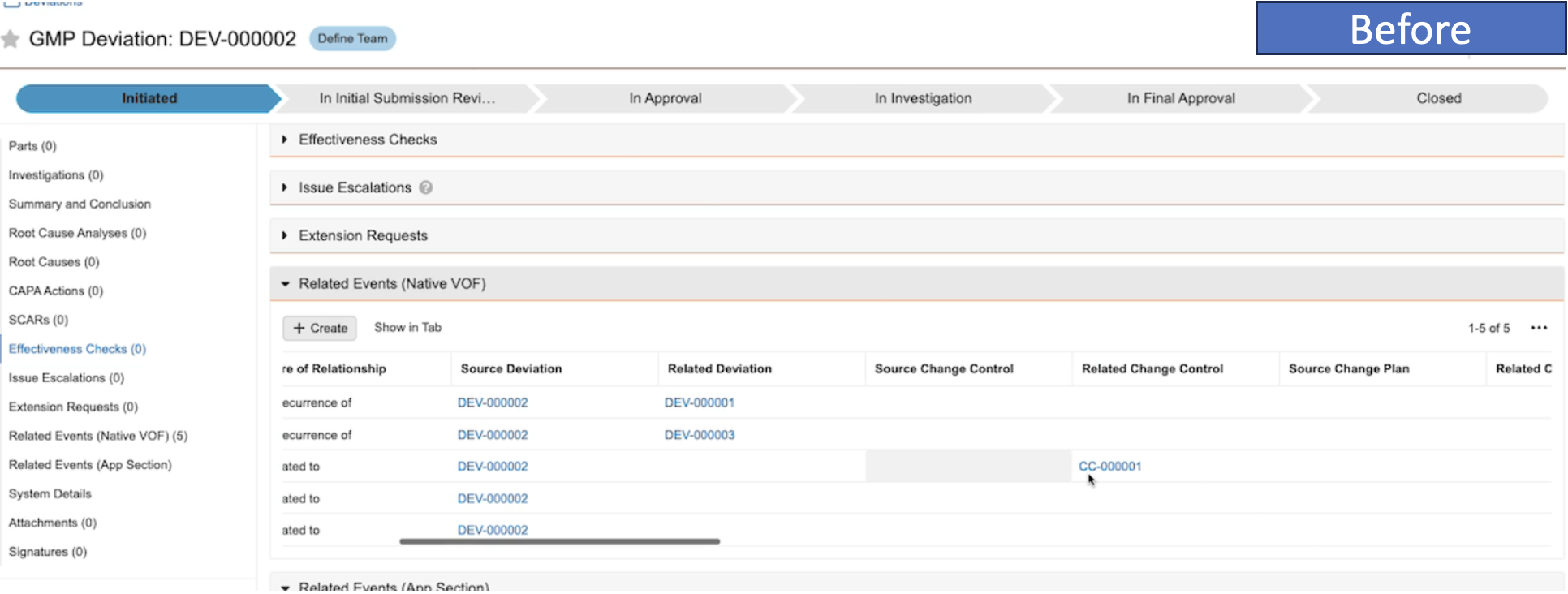
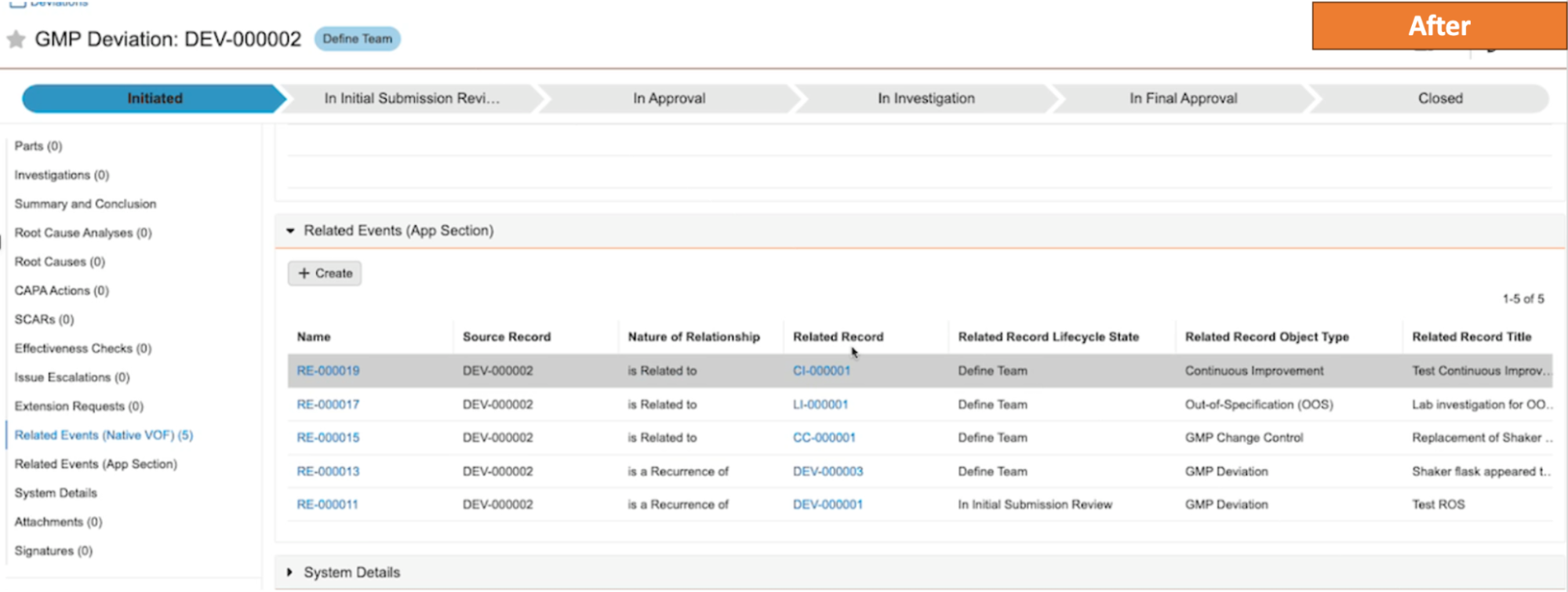
Improved Reporting on Nested BindersAuto-on23R3.4
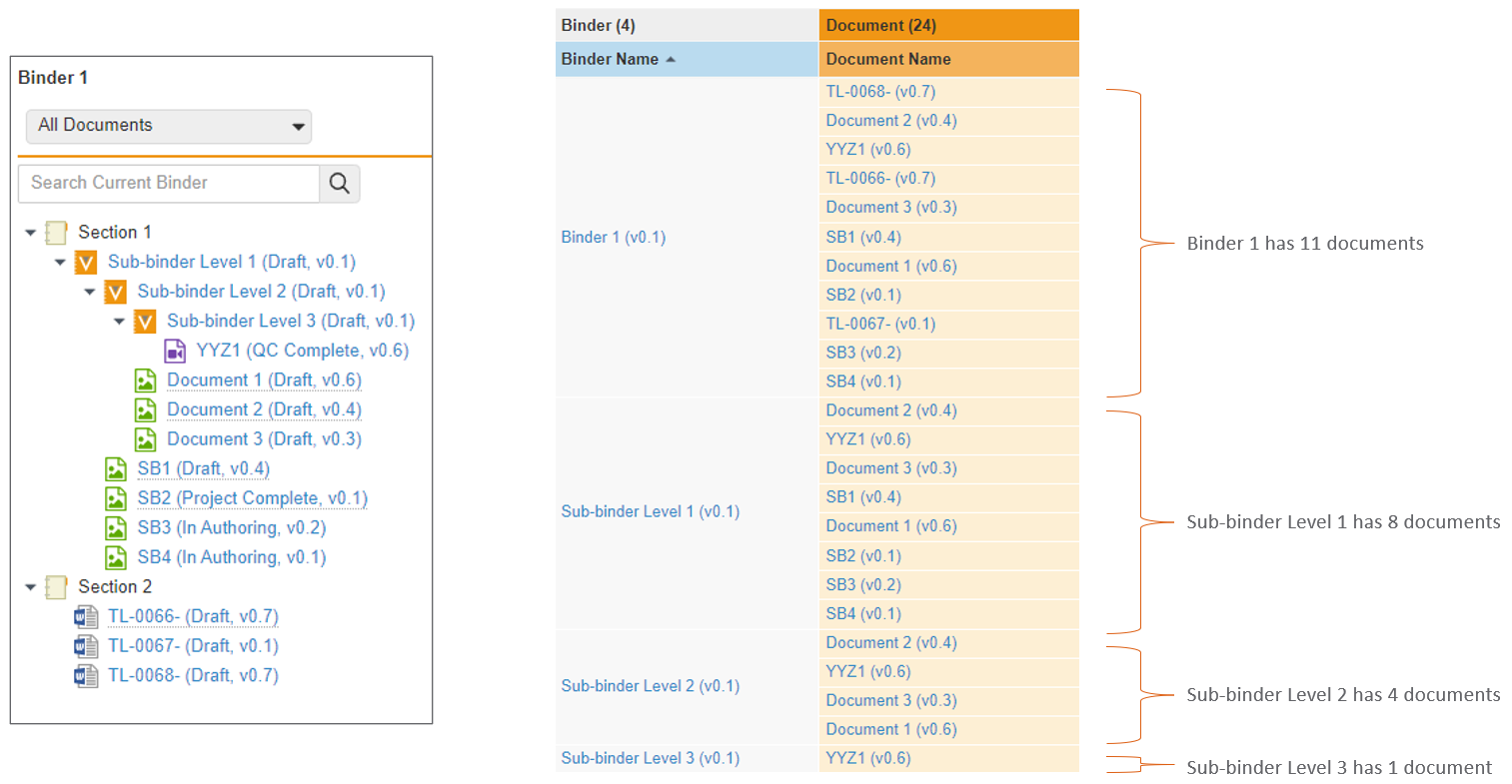
When reporting on binders that contain component binders, Vault will now display the component binder as a document within the parent binder. Prior to 24R1, Vault would display all documents within the component binder without any indication that those documents were not directly in the parent binder.
As an example, in the below scenario, there are several component binders that are visible in the structure on the left. Prior to 24R1, reporting on this would show that document YYZ1 belongs to both the component binders as well as the parent binder.
After 24R1, that same structure will be simplified - the parent binder will show the component binder within it, but document YYZ1 will only display in the component binder it directly belongs to.
This enhancement simplifies the report results and provides a more accurate picture of relationships when using binders within other binders.
Learn more about Binders.
Support for High-Efficiency Image RenderingAuto-on23R3.4
Vault now supports rendering of HEIF and HEIC files, including support for OCR, overlays, and signature pages. If the files are animated, Vault will render them as Video Renditions. Vault also supports Optical Character Recognition (OCR), Overlays, and Signature Pages for these renditions.
HEIC and HEIF files are common formats used by a camera application on mobile devices, across Android and Apple devices - adding rendition support for these will enable customers to better work with these file types.
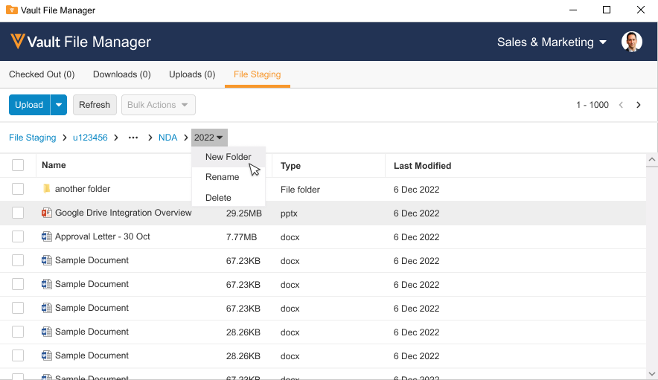
File Staging Enhancements for Vault File ManagerAuto-on23R3.4
Vault File Manager now supports the creation of folders directly within the File Staging tab. In 23R2, we enhanced Vault File Manager with a new File Staging tab, allowing Admins to stage content in Vault File Manager for importing into Vault, rather than using a separate file transfer protocol secure (FTPS) client.
With 23R3, Vault File Manager’s File Staging capability was enhanced to allow users to rename and delete folders/files, and move files between folders. Actually creating new folders though still required that a user create the folder on their desktop and then move the folder into the File Staging tab in Vault File Manager.
Now with 24R1, this process no longer requires that users perform the creation outside of Vault File Manager.
Many organizations may have firewalls or security policies in place that are incompatible with the use of FTPS - adding the ability to create folders within Vault File Manager enables customers to better leverage Vault File Manager to manage File Staging, without the need of a third party FTP client.
Learn more about File Staging.
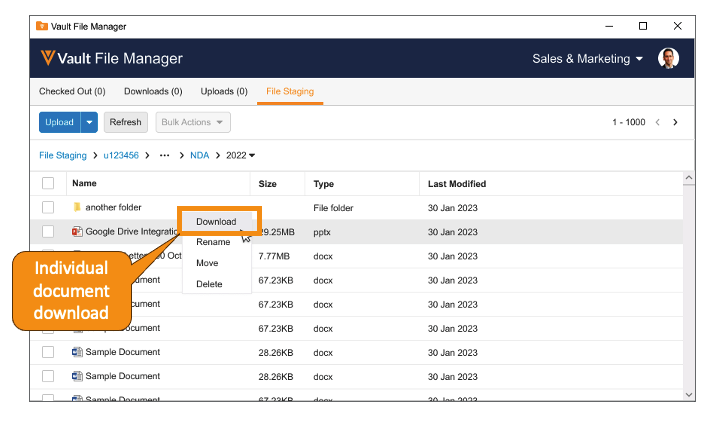
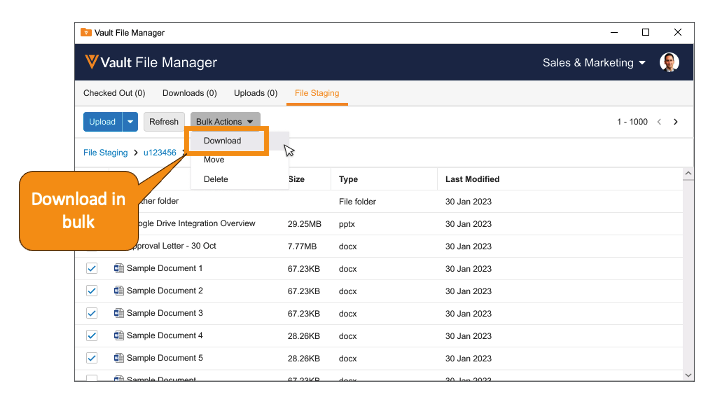
Download from File Staging with Vault File ManagerAuto-on23R3.4
Vault File Manager now supports the ability to download documents and folders, both individually and in bulk, from the File Staging tab. In 23R2, we enhanced Vault File Manager with a new File Staging tab, allowing Admins to stage content in Vault File Manager for importing into Vault, rather than using a separate file transfer protocol secure (FTPS) client.
Many organizations may have firewalls or security policies in place that are incompatible with the use of FTPS - adding the ability to download files ensures that Vault File Manager can adequately serve as a replacement for third-party FTP clients.
Learn more about File Staging.
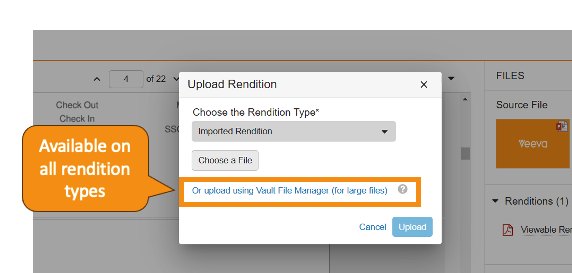
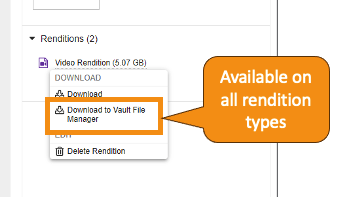
Expanded Rendition Type Support in Vault File ManagerAuto-on23R3.4
Vault File Manager now supports all standard rendition types for both download and upload actions. Prior to 24R1, Vault File Manager only supported upload for Large Size Asset renditions, and did not support download for all rendition types. In 24R1, you can download the following additional rendition typesvia Vault File Manager:
- Video Rendition
- Audio Rendition
- Commercial Vaults
- Distribution Package
- Veeva HTML
- Veeva Preview
- Assets
- eCTD Submission Package
- Clinical Operations Vaults
- Imported Audit Trail
- Historical Audit Trail
- Veeva eForm
- Site Rendition
- SiteVault
- Sponsor/CRO Rendition
Custom rendition types were previously supported for download only but will be supported for upload going forward.
Learn more about Vault File Manager and Rendition Types.
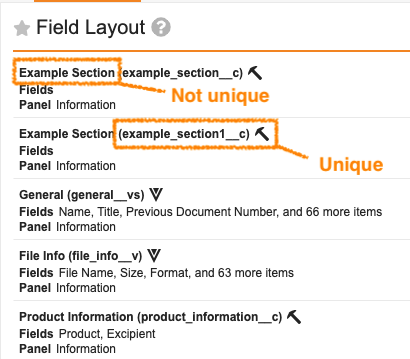
Reusable Labels for Document Field LayoutsAuto-on23R3.4
Vault will no longer require Document Field Layout labels to be unique. Uniqueness will still be enforced on the name.
This enhancement ensures that uniqueness with Document Field Layouts is handled in a manner consistent with other Vault components.
Learn more about Document Field Layouts.
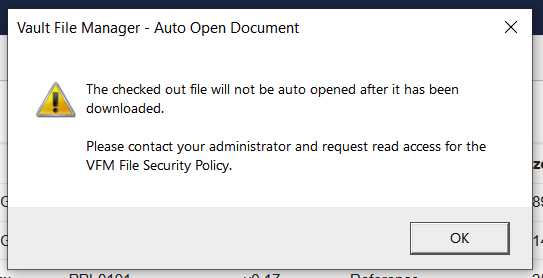
Improved Error Message for Missing VFM File Security PolicyAuto-on23R3.4
When checking out a document to Vault File Manager (VFM), a clearer error message is shown when VFM attempts to auto-open the file if a user does not have Read access to the VFM File Security Policy object in their permission set:
Prior to 24R1, a generic error message existed that did not provide the user with appropriate context to follow-up with an Admin. The error message now clearly states the issue:
Learn more about Vault File Manager.
Lifecycle & Workflow
Auto-Start Document WorkflowsConfiguration23R3.2
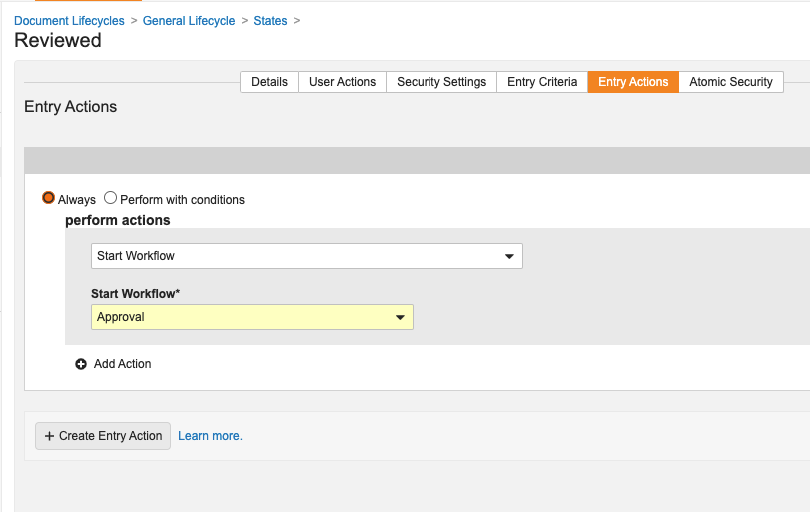
Users can now auto-start document workflows through document lifecycle state entry actions, allowing for the same kind of business processes automation as is available today in object workflows.
This allows for an uninterrupted flow for your documents from initial state to steady state, reducing the number of clicks and points of manual intervention.
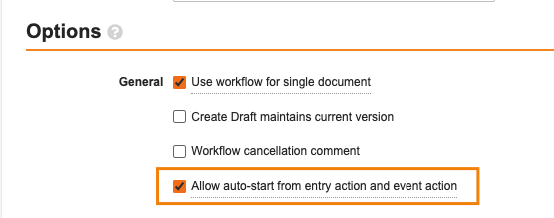
This enhancement applies to document workflows that are specific to a particular document lifecycle and are set to only be run for one document at a time. When the Use workflow for single document box is checked, the Allow auto-start from entry action option becomes available:
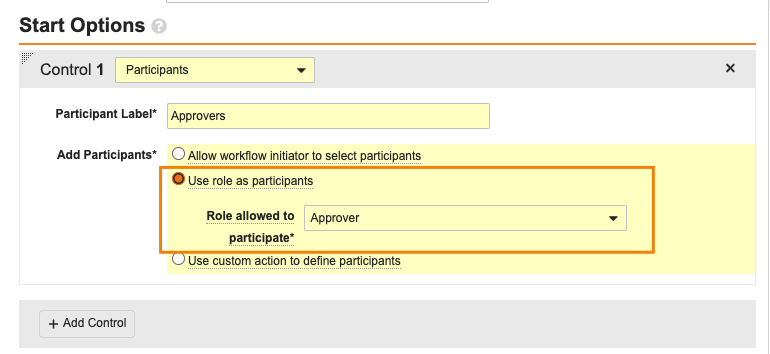
In order for Vault to auto-start workflows, the Participant Controls must use either the Use role as participant or Use custom action to define participants options:
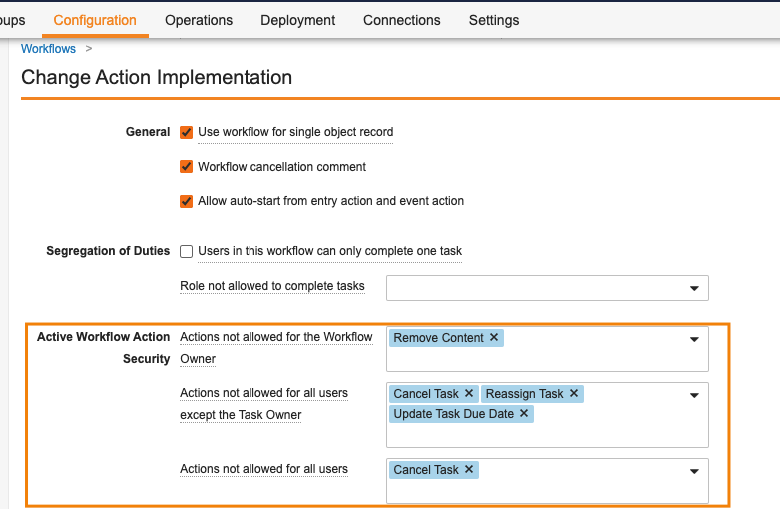
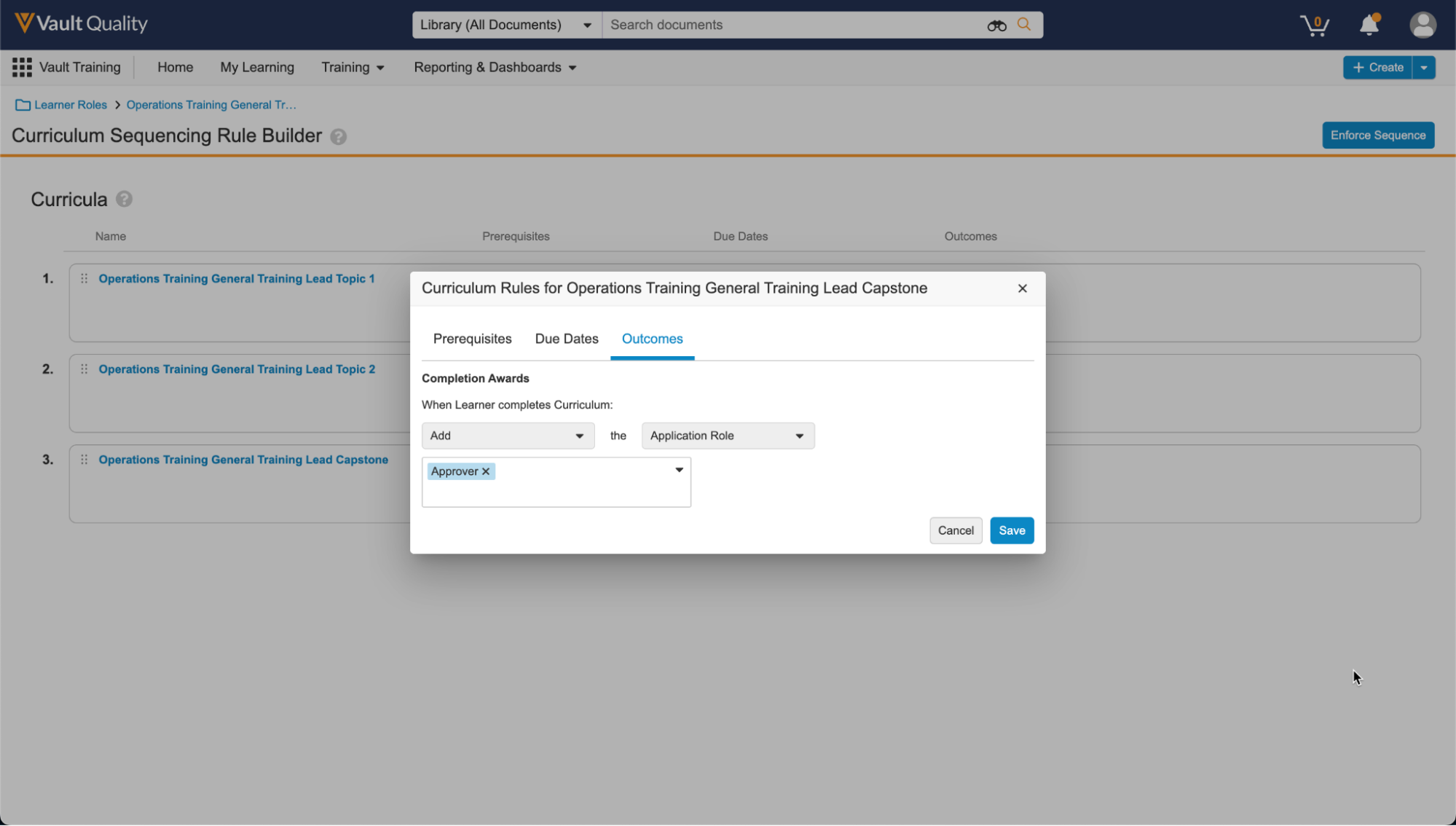
Workflow Action Security for Workflow & Task OwnersConfiguration23R3.2
This feature introduces a new option to configure workflows so that the Workflow Owner is not automatically allowed to perform certain actions, such as canceling the workflow, reassigning tasks, and updating due dates. An Active Workflow Action Security section is now available for Admins when configuring a workflow. With this section, Admins can define which actions are revoked from the Workflow Owner, All Participants, or All Participants other than the Task Owner. These actions include Cancel Workflow, Cancel Task, Reassign Task, Update Workflow Due Date, and Update Task Due Date.
For example, you may want to prevent Workflow Owners from canceling the workflow, or ‘All Participants other than Task Owner’ from canceling tasks (i.e. they can only cancel their own, even if their permissions say they can cancel workflow tasks in general).
Reset Saved Task InformationAuto-on23R3.4
In the event that a user enters some field information in a task completion dialog, and an error prevents them from successfully completing the task (for example, the value they entered does not conform to a Lifecycle State Entry Criteria), Vault holds on to that information in the dialog so they don’t have to reenter it again. However, if in between your attempts you edit the document fields or object record fields directly on the document info or record detail page, and save your changes, Vault will no longer hold on to those prior, stale values, to ensure you don’t accidentally overwrite any purposeful changes you’ve made in the meantime. This only applies for field prompts on tasks and verdicts that apply to all items in the workflow (for example, it does not apply where you’re allowed different verdicts per item).
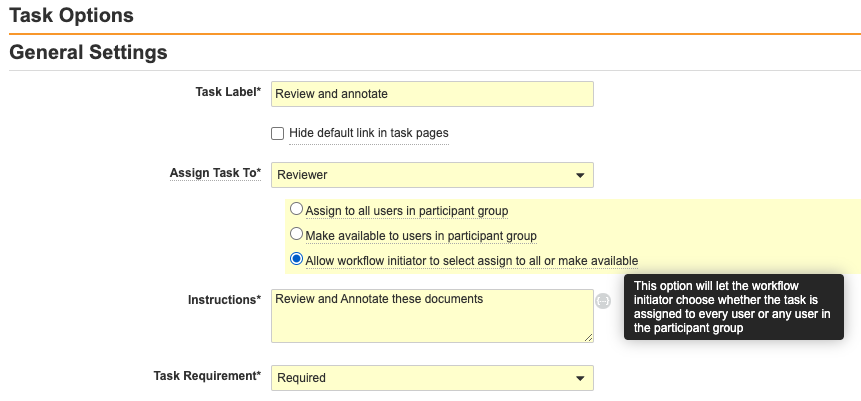
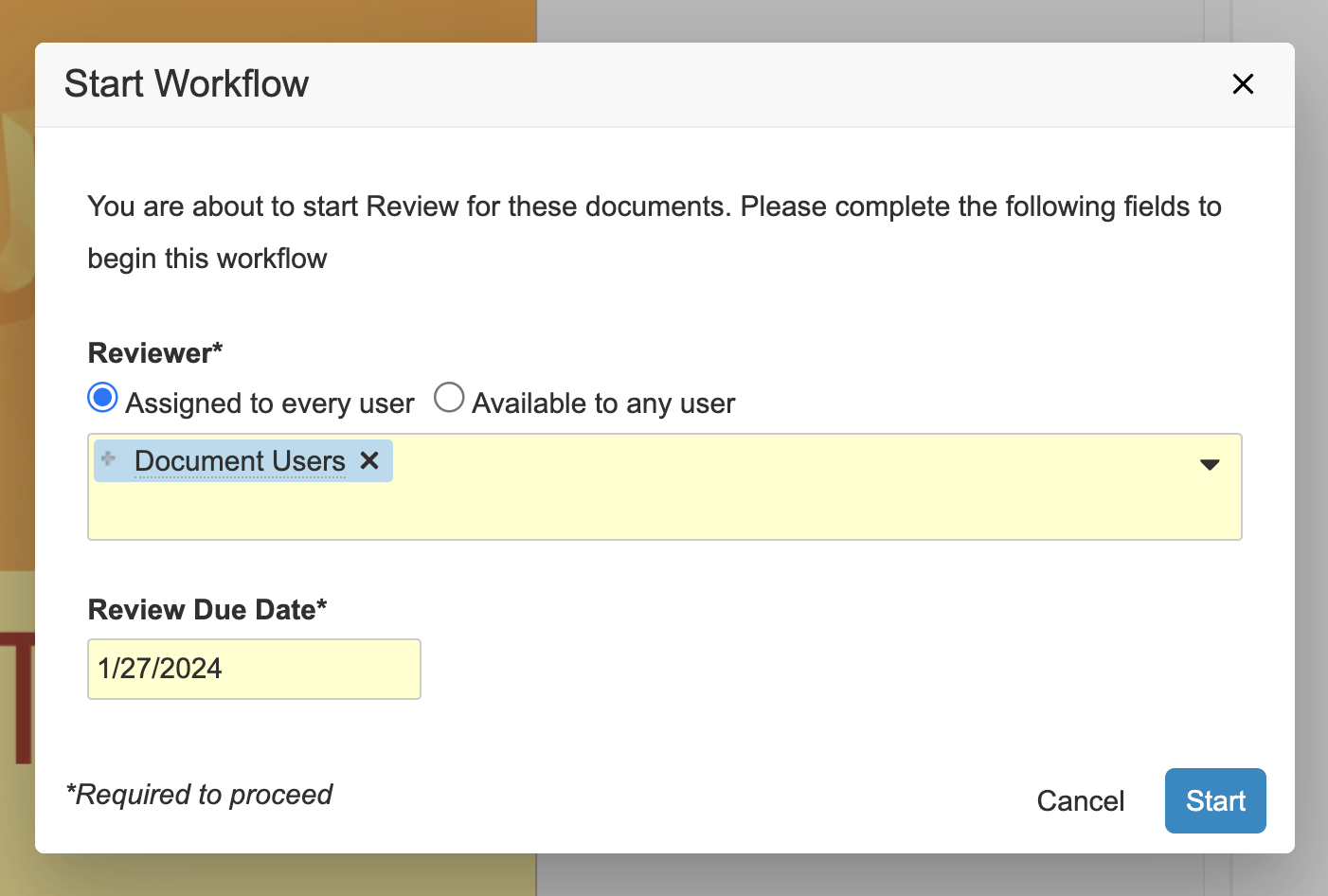
Workflow Initiators Select Task Assignment TypeConfiguration23R3.4
Traditionally, when Admins configured a task, they would determine whether that task should be assigned to all users in the participant group or made available to users in the participant group, where any user in the workflow participant group could accept and assume control of the task. However, in some scenarios it’s helpful to leave this decision up to the Workflow Initiator. In this release, we’re providing a third configuration option, which essentially allows the Workflow Initiator to determine the assignment type at the Start step of the workflow for the corresponding participant group.
With that configuration setup above, the Workflow Initiator will see two (2) options above the workflow participant selection at runtime. These options will display if at least one (1) task assigned to this workflow participant group has the new Allow workflow initiator to select option configured. The Workflow Initiator’s selection will only apply to tasks configured to allow the Workflow Initiator to make the selection and does not override any task not configured with this option.
Document Workflow Task Due Dates Support Document Date FieldsConfiguration23R3.4
Admins can now set Task Due Dates using document fields. As part of the due date configuration on the workflow task, you can choose whether the due date will automatically update when the document field is updated. If you update the document field to blank, Vault won’t update the due date. This feature is only available for single-document workflows.
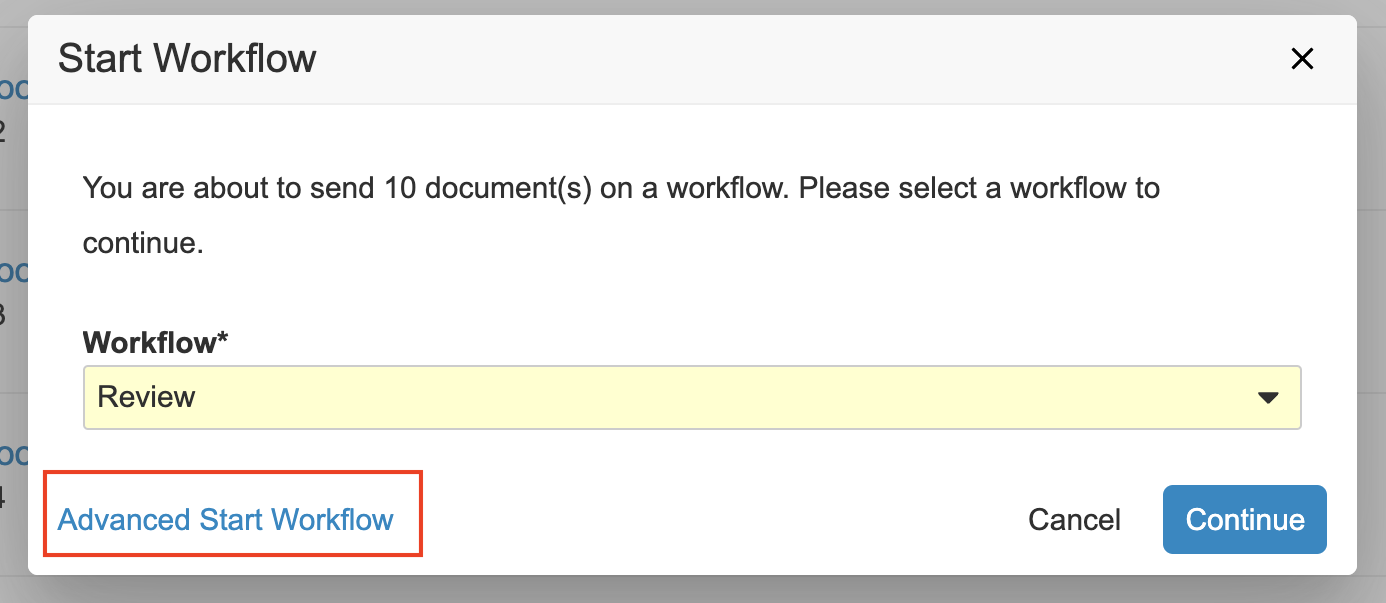
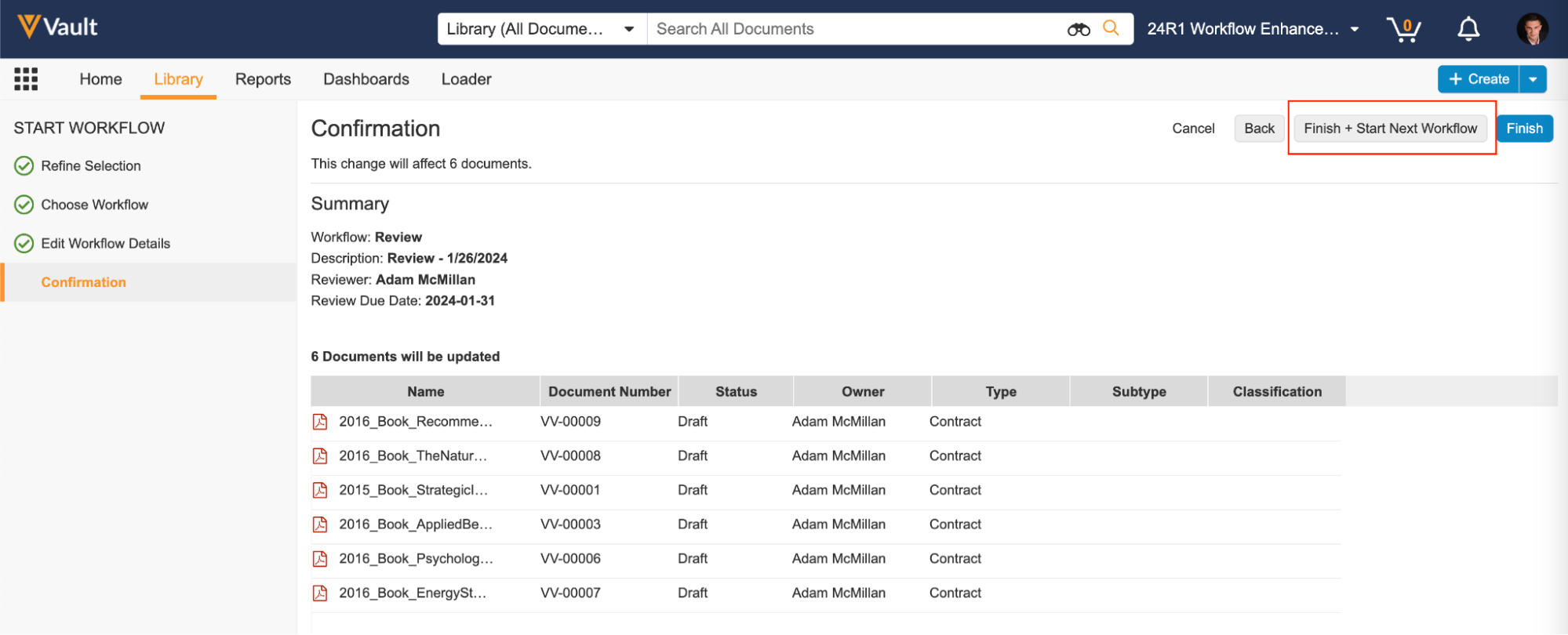
Advanced Start WorkflowAuto-on23R3.4
When starting a multi-document or multi-record workflow from a filtered list of items, sometimes you need a way of grouping those items into subsets, rather than sending them all on the same multi-item workflow together. To help with that, there is now a new link when starting a multi-item workflow to access advanced options, where you first refine your selection, select the workflow you want to begin, and enter any workflow Start step prompts, such as workflow participants, fields, and dates.
On the final step you can click the Finish + Start New Workflow button to both start that workflow and then return to the remaining items you deselected earlier to send those items on a workflow.
Alternatively, you can click the Finish button on the final step if your goal was to just deselect a few items before starting the workflow.
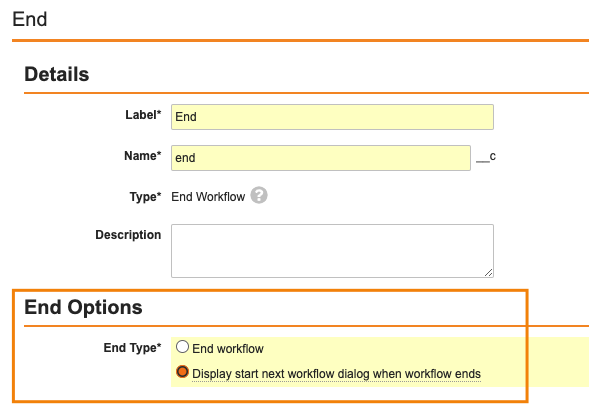
Start Next Workflow Prompt when a Multi-Record Workflow EndsConfiguration23R3.2
While it’s possible to auto-start a new object workflow as another workflow ends, sometimes you need the user to be presented with multiple options, allowing them to manually choose the next appropriate workflow.
To streamline that process, we’ve extended the Multi Record Workflow functionality to include the Start New Workflow prompt that can be configured to show to the user who completes the final task in the workflow.
This was already available for other workflow types, but is now also available for multi-record workflow.
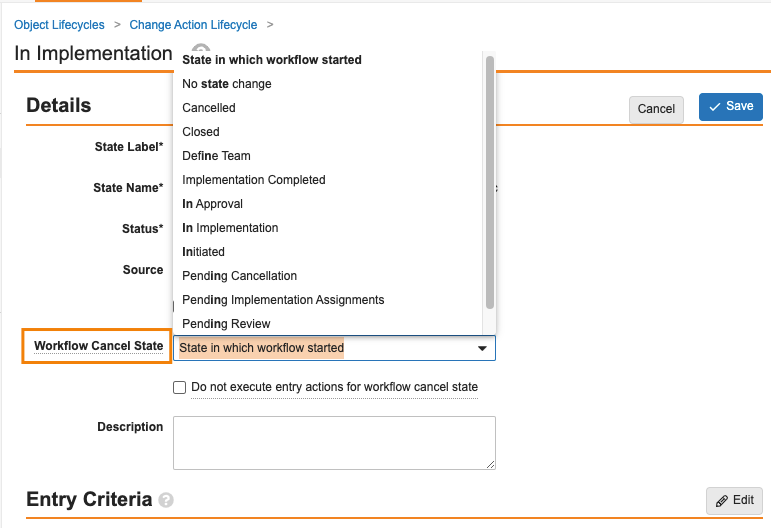
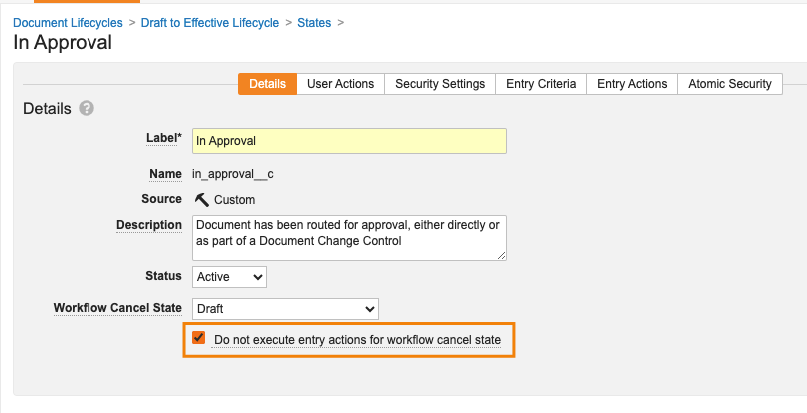
Object Lifecycle Workflow Cancel StatesConfiguration23R3.2
In 23R2, we introduced the ability to cancel object workflows based on lifecycle entry actions. While a very powerful feature in Vaults like QMS, where you may need the cancellation of a parent record (i.e. Change Control) to cancel workflows of child records (i.e. Change Actions), there were limitations in that an Admin could not define which state the Change Actions would go to upon cancellation. The default behavior was to revert to the state it was in before the workflow started.
With 24R1, we’re providing Admins the ability to control which state a record should go to when the workflow is canceled. This would apply in all workflow cancellation scenarios, but particularly ensures consistency when using entry actions to cancel multiple workflows for related records. This feature is possible with documents, and now is extended to objects.
This is controlled via a new field in each object lifecycle state:
By default, this option is set to State in which workflow started for all existing workflows to maintain consistency in behavior
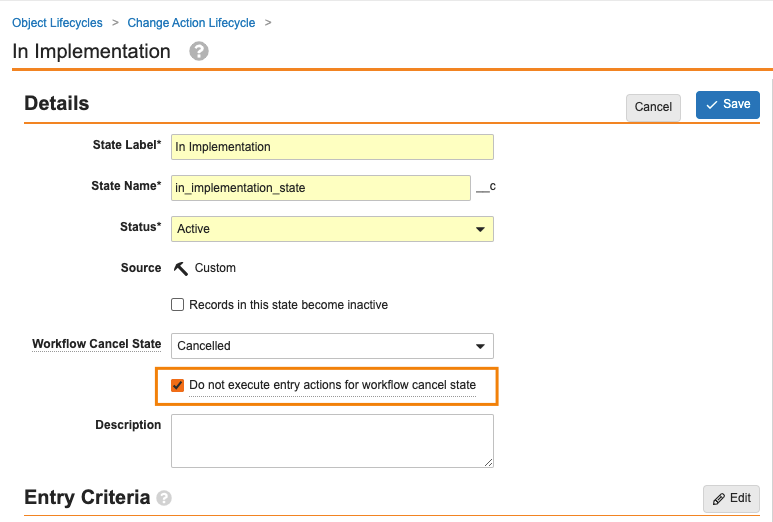
Workflow Cancel States Can Ignore Entry ActionsConfiguration23R3.2
In addition to the new ability to configure the Cancel state (which is now available for both Document and Object workflows), Admins also have the ability to control whether or not Entry Actions should execute based on cancellation:
If this option is enabled, when Documents or Object Records enter the lifecycle state designated as the cancel state, Vault will not execute any Entry Actions configured for that lifecycle state. However, Vault will still evaluate Entry Criteria.
This enhancement ensures that unnecessary actions do not take place when a document or object enters a state based on a workflow cancellation.
This change applies when canceling Object Workflows and Document Workflows, but does not apply to Legacy Workflows.
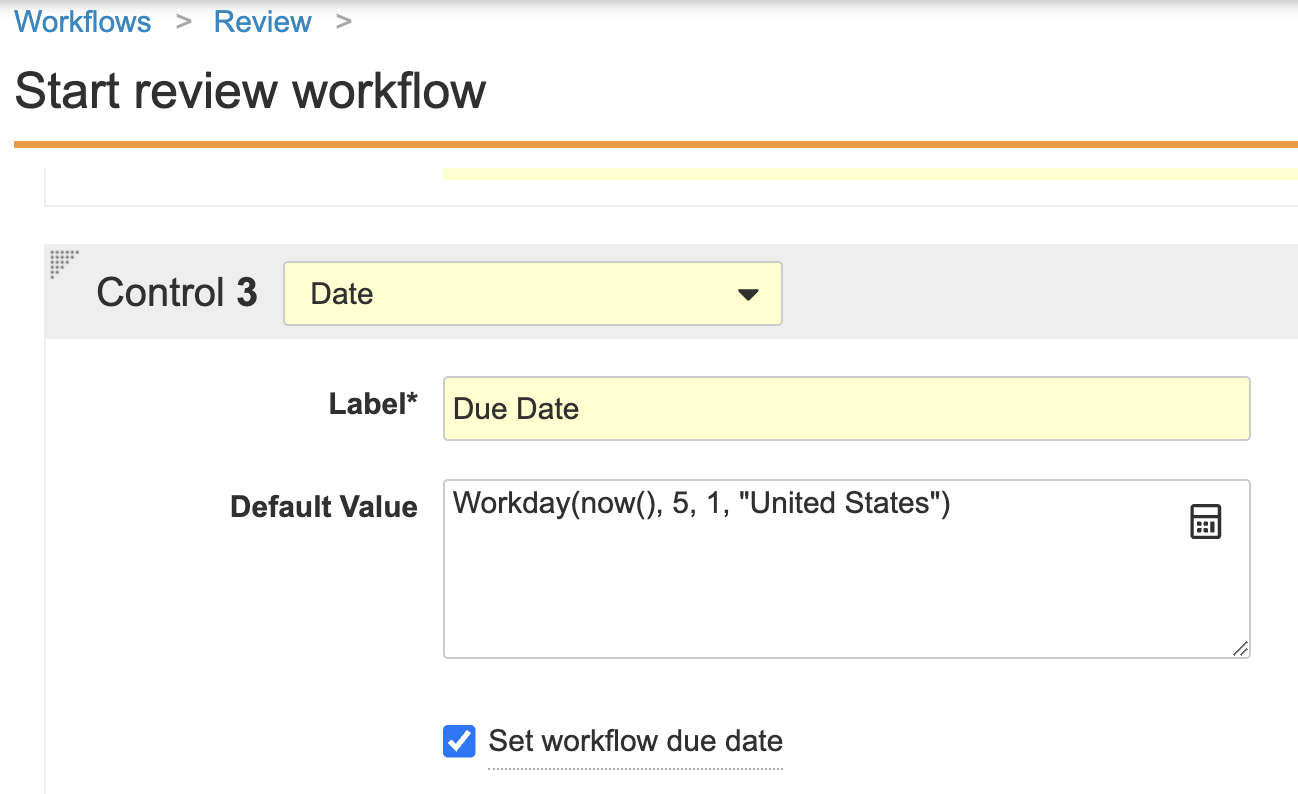
Formulas for Workflow DatesConfiguration23R3.4
Vault now allows formula expressions for Task Due Dates similar to many other areas of Vault. This allows for greater flexibility and control over how the due date is captured, including the today() function, and the ability to calculate a number of days based on one or more other fields on the object record or document.
This also applies to date prompts in the workflow Start step, which means you can also set the workflow due date using a formula.
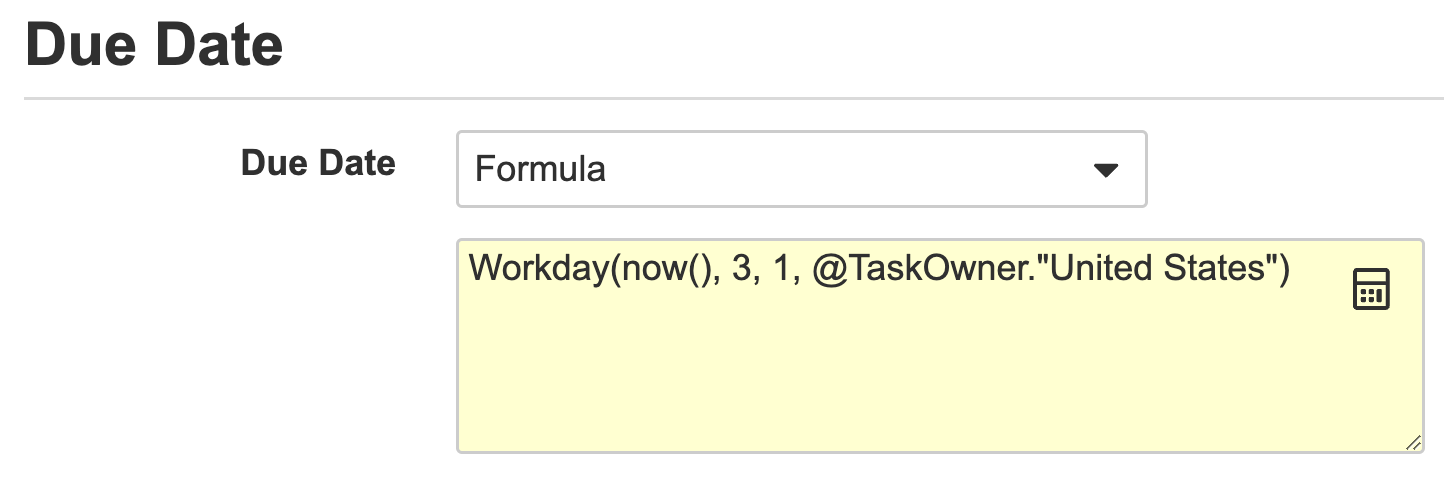
Workflow Owner & Task Owner Formula System VariablesConfiguration23R3.4
As part of the new formula abilities for Task Due Dates, you can now also base those due dates on the holiday schedule of the Workflow Owner or Task Owner. For example if you use the @WorkflowOwner variable in the following way, the task due date will be set to 10 working days from today based on the holiday schedule of the workflow initiators locale/country:
Workday(Today(),10, 1, @WorkflowOwner.holiday_schedule__sys)
This gets especially interesting when using the @TaskOwner variable, as Vault will set task due dates for each task assignee based on their locale and holiday schedule.
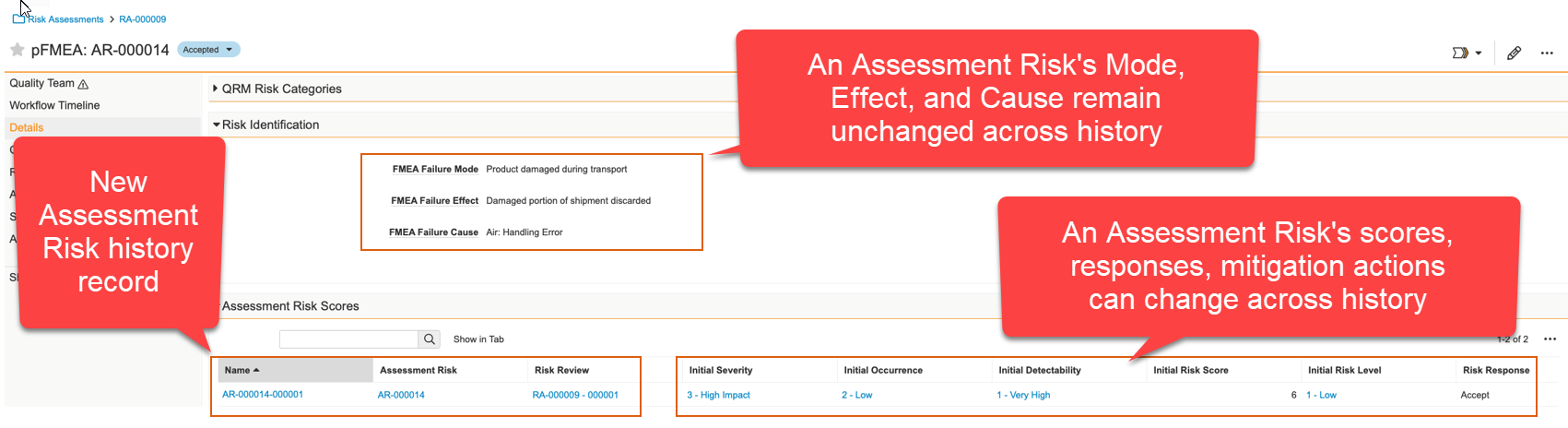
Consistent Timeline View for Superseded DocumentsAuto-on23R3.4
Within the Timeline View for documents, Vault now consistently displays events for Superseded documents. Prior to 24R1, if a version became Superseded as the result of a workflow rather than a state change, the Timeline View would show the most recent version as Superseded rather than the prior version.
If a document became Superseded through a state change, this was appropriately reflected in the Timeline View on the correct version.
This enhancement ensures that the Timeline View is accurate and consistent regardless of what action causes a version to become Superseded.
Learn more about the Document Timeline View.
Objects
Pre-default Object ReferenceConfiguration23R3.5
When creating or copying object records, often there are object reference fields that only have 1 (one) value available for selection due to configuration, such as reference constraints or controlling fields (like parent-child or sibling) - or if there really is only one (1) record in that referenced object. To enable more efficient object record creation, there is a new attribute for object reference fields called Pre-default on non-required field when only one reference record is available.
If enabled, when creating or copying object records, Vault will pre-populate object reference fields if there is only 1 (one) record available for selection. Of course, if the object reference field is Required, then Vault will always pre-populate if there is only 1 (one) record available for selection (regardless of whether this new attribute is enabled or not).
Note: This pre-defaulting behavior is only when creating records via the record details page UI; it is not a defaulting behavior for API or other background processes.
Merge Records APIConfiguration23R3.4
Duplicate records in Vault can happen due to migrations, integrations, or day-to-day activities. These duplicate records can be difficult to correct because of the many ways an object record can be referenced. For example, an object record may be referenced in both configuration and document and record relationships. This problem is now greatly simplified through a new Merge Records API which allows you to merge a Main record with a Duplicate record. The merging process updates all inbound references (including attachments) from other objects that point to the Duplicate Record and moves those over to the Main record. Field values on the Main record are not changed, and when the process is complete, the Duplicate record is deleted.
The Merge Records API will only work on objects where Enable Merges is configured, and the user performing the API call has the Application: Object: Merge Records permission. Enable Merges can only be turned on for custom objects.
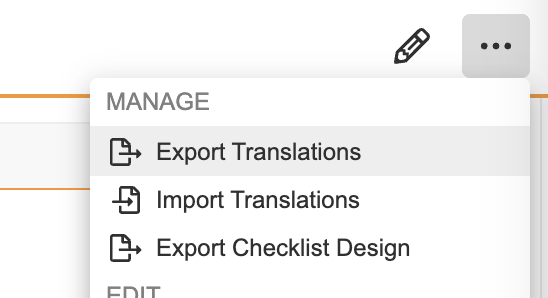
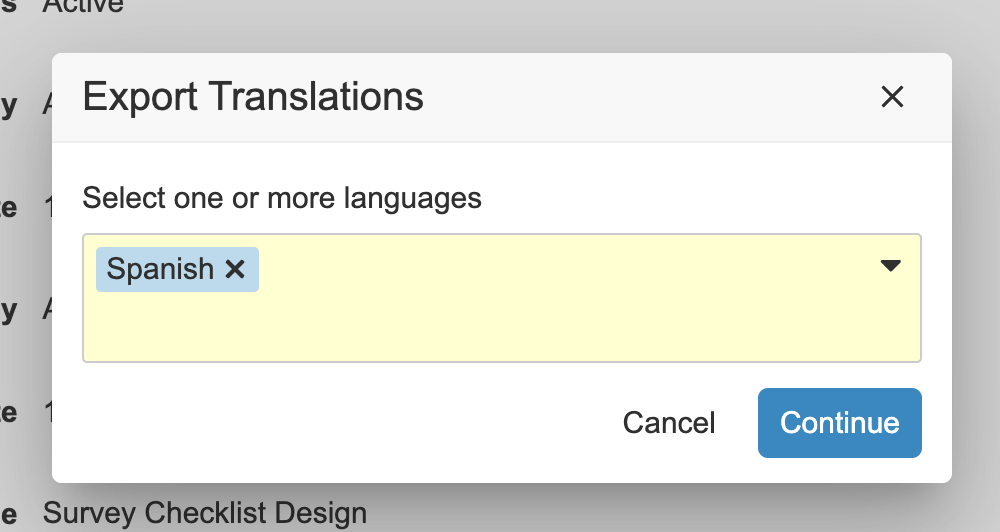
Checklist Design TranslationConfiguration23R3.4
Vault now supports translations of Checklist Designs. Rather than creating one Checklist Design per language, admins can now import multiple translations for each Checklist Design, including translations for any Sections, Questions, and Answers.
This enhancement allows customers that leverage checklists to more efficiently manage Checklist Designs in global organizations by reducing the number of separate Checklist Designs that need to be created/maintained. This also provides better traceability in reporting by having everything maintained in a single Checklist Design across languages.
Admins can maintain one CSV file per language that includes translations across multiple checklist designs for that language. A user action has been added to the Checklist Design object to allow Admins to import and export the translation files.
Remember that these actions must be configured on the Checklist Design object lifecycle as user actions, and users will require access to those actions in their permission sets.
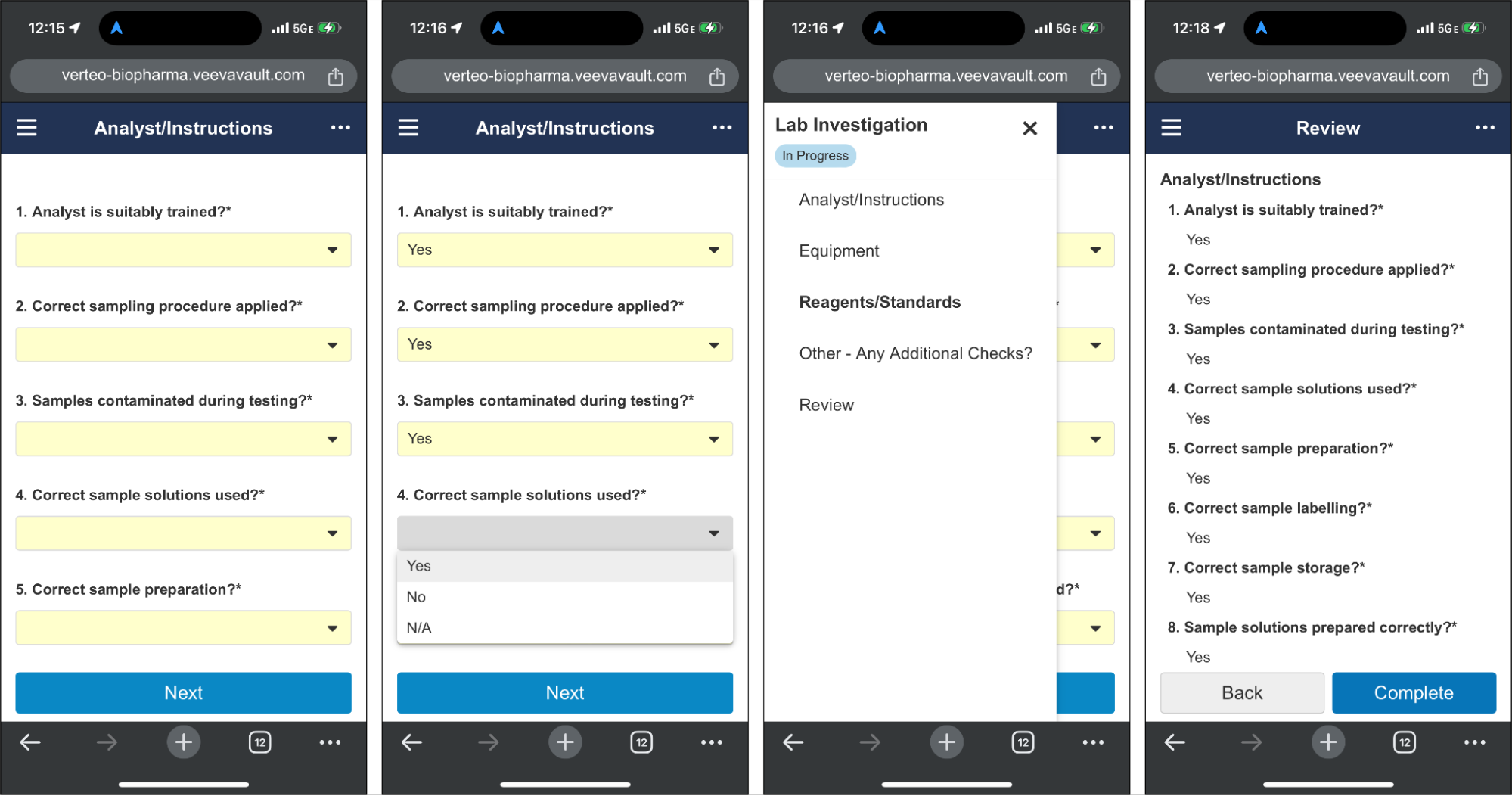
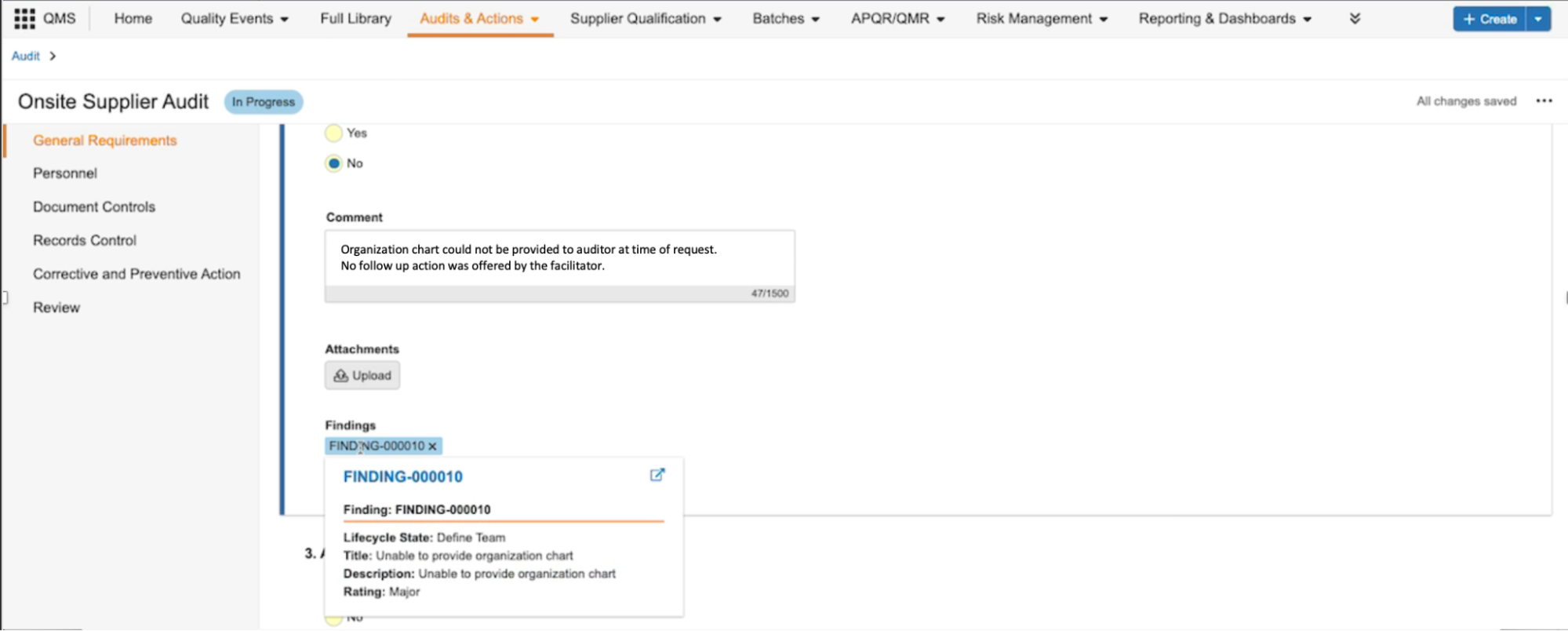
Mobile-Optimized Checklist UI for RespondentsAuto-on23R3.4
Checklist respondents who are away from their computer can now access their checklist via their phone’s web browser. This new, mobile-optimized view allows users to easily answer any question type, upload attachments, add a related Vault document, and review their answers before completing.
The following checklists do not support the mobile-optimized view with this release:
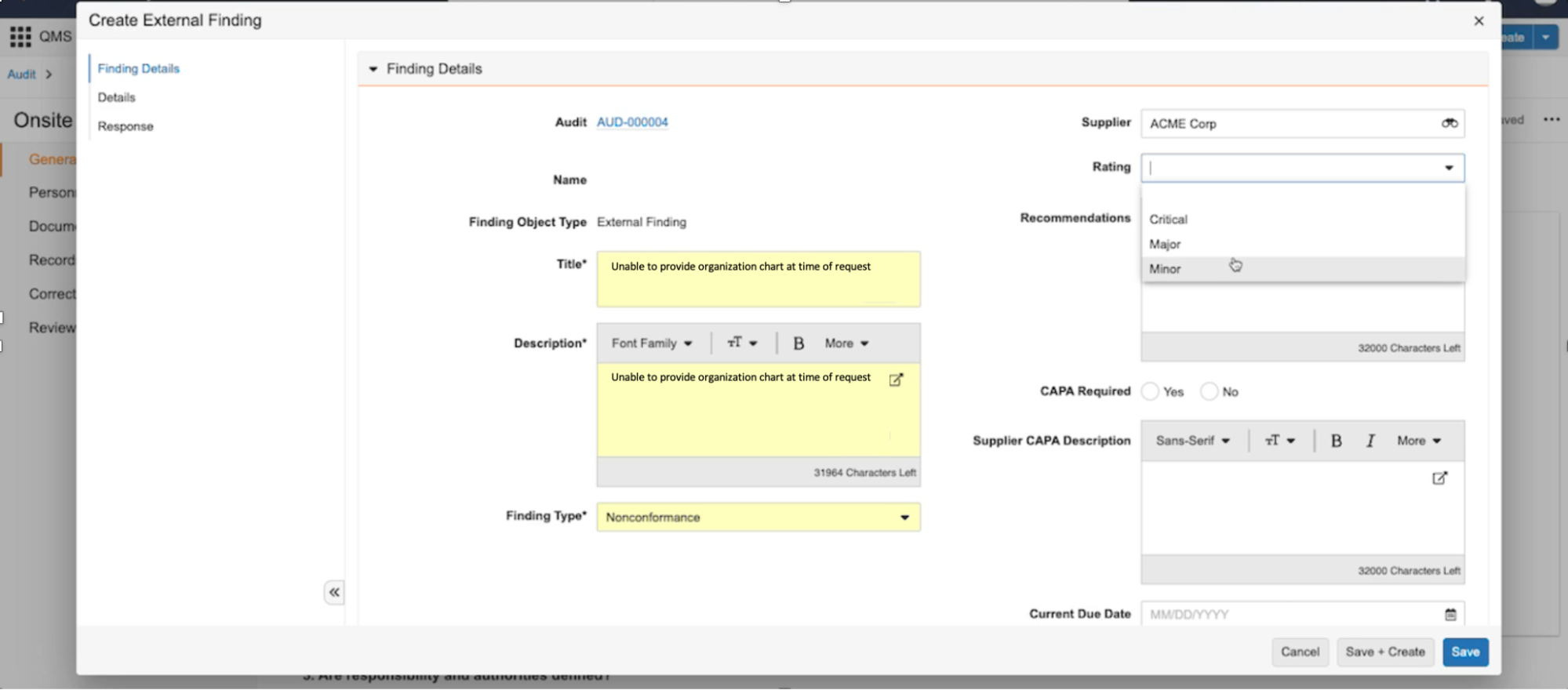
- QualityOne:
- Quality Event Checklists
- Audit Checklists
- Quizzes
- Quality
- Audit Checklists with Findings
Note: This feature affects checklists viewed in a mobile web browser. The Vault Mobile app does not support checklists in this release.
Support Library Questions in Visual Checklist DesignerAuto-on23R3.4
Admins can now create questions in the new Visual Checklist Designer from Questions that have been added to the library.
Admins can also now directly add questions to the library from a Question created in the Visual Checklist Designer.
This enhancement provides customers the ability to leverage the usability of Visual Checklist Designer without needing to manually re-create questions that may be applicable across multiple Checklist Designs.
Note: Quizzes (used in Vault Training and Study Training) do not support Library Questions.
Learn more about Visual Checklist Designer and Library Questions.
Checklists: Increased Character Limit for Answer Text in Answer Library DesignAuto-on23R3.4
The character limit for Answer Text fields (on the Answer Library Design and Available Answer objects) has been increased from 128 characters to 255 characters, for consistency with the character limits on the Available Answer Design object.
Related Record Audit LimitsAuto-on23R3.4
We have made significant performance improvements to the related record audit trail functionality (which provides a comprehensive audit trail for where there are closely-related processes being reviewed). As part of these changes, we have limited the number of related objects you can select to ten (10), and the default date range is now set to be within the last one (1) month.
Expression Support for Long & Rich Text FieldsConfiguration23R3.4
Long Text and Rich Text fields on objects can now be used in all expressions and with expression functions that deal with text values, where applicable.
As an example, with this enhancement, in a MedInqury Vault, a value from an existing Long Text or Rich Text field on Standard Response could be copied to Case Response using an expression in an object lifecycle entry action.
Prior to 24R1, Long Text and Rich Text fields were supported in some expressions (for example, report formula fields), but this enhancement ensures that they are supported in all areas that leverage expressions.
High Volume Object (HVO) Renamed to Raw ObjectAuto-on23R3.2
High volume objects are now called raw objects. As we look to increase the scalability of our standard objects, we will begin treating high volume objects more like regular database tables, used for storing raw data.
To make the purpose of these objects clearer, we are updating the terminology across our user and developer documentation and in the Vault UI itself, referring to this class of objects instead as raw objects.
There is now a limit of 1 billion records for raw objects. In addition, the data_store attribute for the Object MDL component now includes a value of raw. This change is backward compatible, and high_volume is still supported as an input value.
New Object Audit Fields in CSV Exports & APIAuto-on23R3.2
The object audit API (CSV and JSON) and data exports from the Vault UI (CSV) now include new fields that display the API names for fields, objects, new values, and old values.
These new fields make it easier to join audit data with other Vault data and to perform additional analysis of audit data in external systems.
Common UI & Search
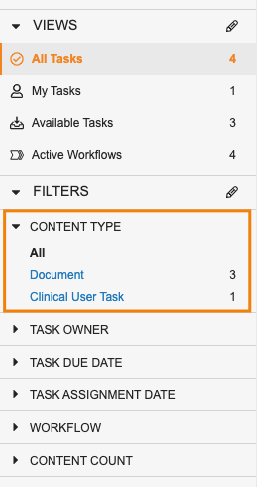
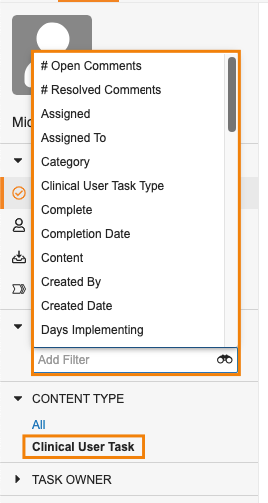
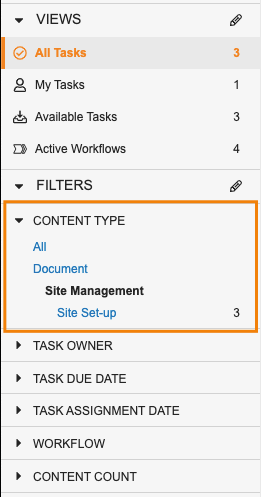
Task Filtering EnhancementsAuto-on23R3.4
Users can now much more easily distinguish Muti Record and Multi Document workflow tasks in the Home tab.
Using the new Content Type filter, users can look at just Document tasks, or tasks for a specific Object.
When filtered by the Content Type to a specific object or to Document, users are also able to select other fields from that object/document to continue filtering the view further.
When filtering for Document in Content Type, Vault also allows users to filter further based on specific Document Types.
In addition, users can filter by Workflow Name, and we have made all this information available as columns in the task grid view.
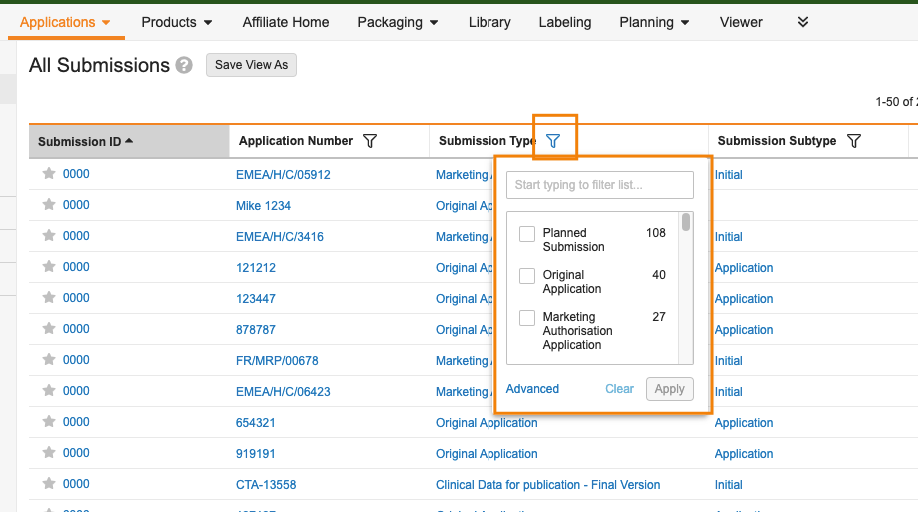
Column Header FiltersAuto-on23R3.2
Users are now able to filter documents and records directly from the column header, similar to other modern web applications.
Column Header Filters are available on picklists, object references, numbers, dates, Yes/No, User, and standard fields such as Document Type, Status, Lifecycle, and can be used on:
- Object tabs
- Document tabs (in the grid view)
- Objects within Business Admin
- Home tab
- Expanded Search Sections
This enhancement works in conjunction with the filter options on the left-hand side of Vault. Users can now more easily apply filters based on displayed columns, while the left-hand filters are useful in applying filters on information that is not displayed as a column, or using special filters that aren’t truly columns that can be displayed (such as Role and Steady State Only).
Learn more about filtering in Vault on Vault Help here: Filtering on Fields & Roles
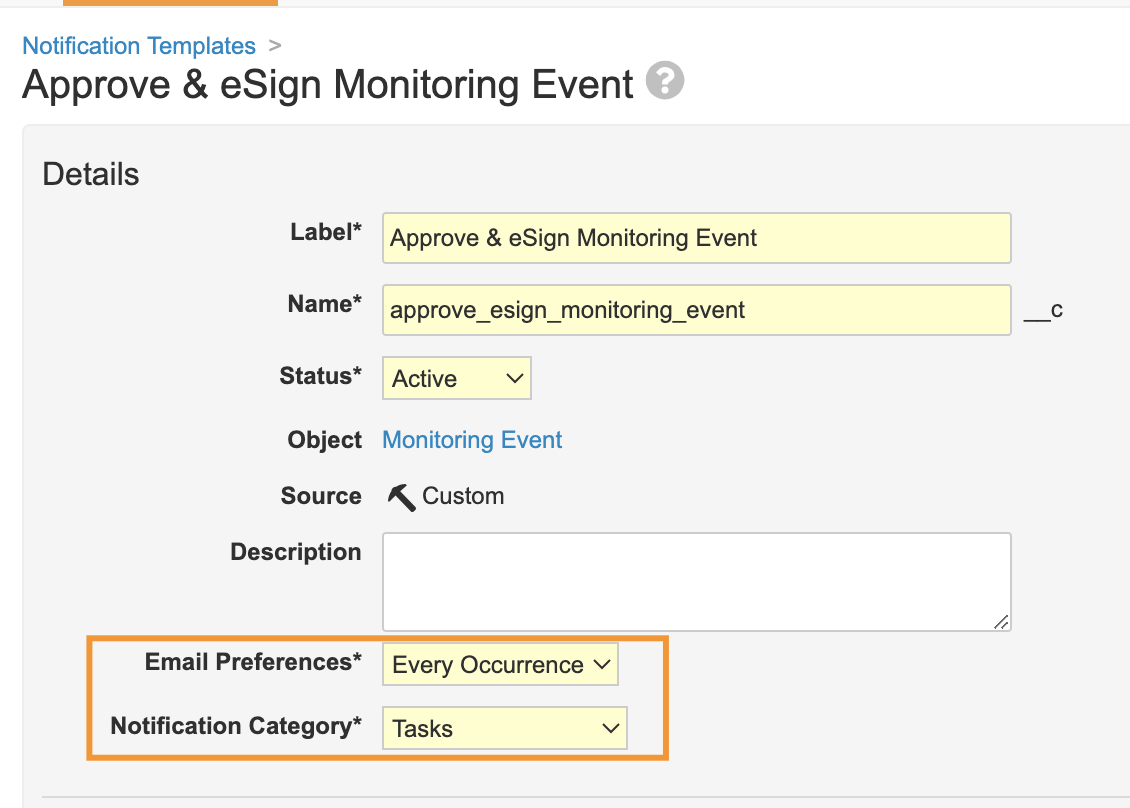
Notification Categories & Email PreferencesAuto-on23R3.2
As part of the notification template configuration, Admins can now define the frequency that these notifications are sent by email, specifically. Notifications can be configured to send an email on Every Occurrence, as a Summary, or Never (meaning they are treated like in-app notifications only).
This is also an enhancement to Vault Notifications to allow Notification Categories - these can control how notifications are grouped on the Notifications page in Vault and how notifications are grouped within summary emails. Admins can now define their own notification categories, and assign a category to their notification templates.
These changes will help users receive the right notifications, in the right way, at the right time, while reducing notification noise, without compromising on critical and time-sensitive notifications.
Version Created By & Date FiltersAuto-on23R3.4
When filtering documents by Created Date or Created By, Vault uses the information from the latest version you can see. If you want to have more specific versions returned, you can now also filter by Version Created By and Version Created Date. Remember that an icon will indicate if the returned results are not the latest version of the document.
Rename All Tabs to AllAuto-on23R3.2
Admins who have configured Tab Collections likely want users to access those options first rather than selecting the option to view all tabs.
As such, we have moved that option to the bottom of the list of Tab Collections, relabeled it simply as All, and given it a ‘global’ icon to the left to help users distinguish it from your configured Tab Collections.
The Business Admin and Admin options (which only show for a smaller set of users) remain below a dividing line, which now appears below the ‘All’ option.
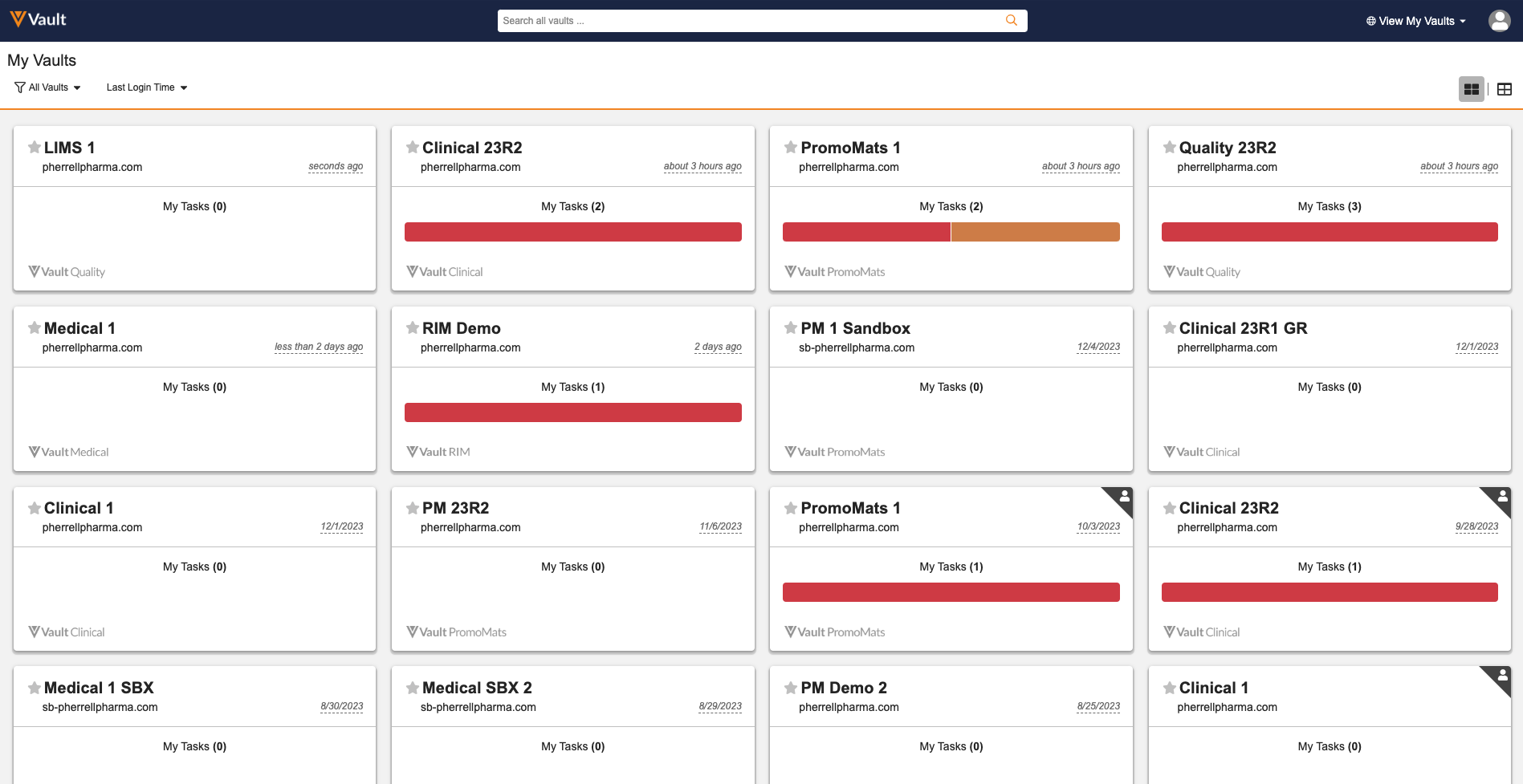
My Vaults Card Layout EnhancementsAuto-on23R3.2
Users leveraging the My Vaults page to access multiple Vaults from a single account will now see that the Card layout supports up to 50 Vaults on a single scrollable page. Prior to 24R1, if a user had access to more than 12 Vaults, the My Vaults page would only display 12 Vaults per page.
By allowing up to 50 Vaults on a single page, users are better able to navigate to the Vault they need without needing to traverse multiple pages.
Additionally, Vault will automatically display as many cards per row as possible based on the size of a user’s window and widen cards to ensure the entire screen is leveraged.
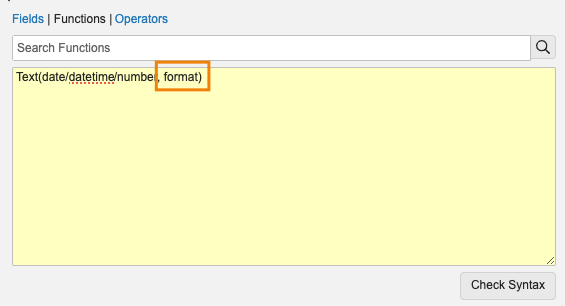
Add Spaces and Characters to Text with Text() FunctionConfiguration23R3.2
With 24R1, we are enhancing how text fields can be formatted using Vault formulas by allowing the Text() function to accept the Text data type and apply simple formatting such as inserting characters or adding spaces.
Today, if a text field is passed into the Text() function, the same text will always be returned.
With 24R1, Admins will be able to add an optional second parameter to Text(). For example, Text("1800FLOWERS", "A-AAA-AAAAAAA") will now return “1-800-FLOWERS”.
Additional Supported Time ZonesAuto-on23R3.4
Vault supports 229 additional time zones according to the latest standards. Users around the world can select the most precise time zone for their location so that dates and times display correctly in the user interface.
Seven (7) time zone labels have been updated to conform with the latest standards.
Outbound Email Domains: Support Multiple Root DomainsConfiguration23R3.2
Vault allows Outbound Emails to be sent to external recipients using a non-Veeva email address like medinfo@verteo.com. This functionality is available and configurable within MedInquiry Vaults (for Case Response Emails), QMS Vaults (for Sending External Notifications), and Study Startup Vaults (for sending feasibility surveys).
With this release, Admins can create up to three (3) root domains (for example, verteo.com) and five (5) email domains. Many companies leverage multiple domains within their organization, particularly in scenarios where there are subsidiary organizations within the same organizational umbrella. This enhancement allows customers to leverage outbound email with these different domains.
To facilitate this change, there is a new Email Domains section in Outbound Email Addresses to allow companies to setup additional subdomains if needed
Action Layouts
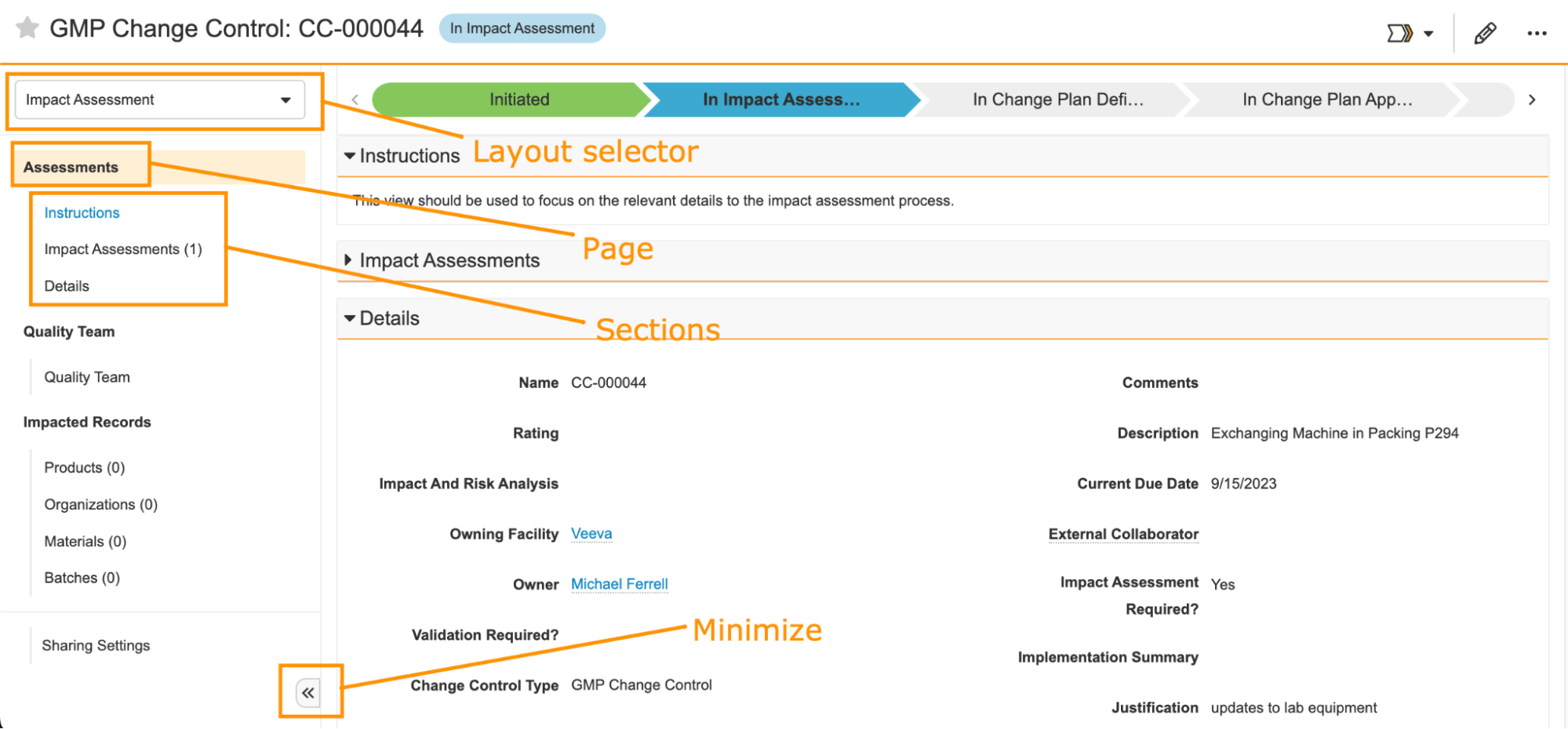
With 24R1, we are introducing Action Layouts to provide new and powerful ways to customize user experience on the Record Details Page. Action Layout is anchored by three new features: Pages, Multiple Layouts, and Layout Profiles, along with a number of enhancements in visual styling, Layout Editor, and Layout Rules. Admins can create different layouts and target them for specific use cases and/or specific users, allowing different users to view a record in a more streamlined layout with just the right amount of information at the right time. The enhancements are detailed below and allow customers to create better organized and more contextual, focused layouts for different users.
Action Layouts: PagesConfiguration23R3.2
Currently, the Record Details Page layout only contains sections. In this release, Admins can now configure up to 10 separate pages in a layout to better organize sections. A page is used to group relevant sections together so that users can easily navigate to them and focus on the right data needed for a task. By default, all existing layouts will remain unchanged with just sections, but the user interface for the left-hand navigation will appear slightly cleaner, and users will have the option to minimize the menu, allowing for a full-screen view.
In addition, we have enhanced the Layout Editor that allows for:
- Creating new pages
- Dragging and dropping sections reordering
- Inserting pages and sections
- Adding custom Help Content both within sections and as standalone sections
- Updated Layout Rules editor
Action Layouts: Layout ProfilesConfiguration23R3.2
Admins can now configure up to 20 different layouts per Object and per Object Type. This allows for a much more sophisticated configuration that supports multiple layouts built for specific use cases, rather than one layout that must fit all use cases. After the release, users will continue to see the same layout as before. In order to take advantage of Layout Profile to assign different layouts to users, Admins would have to create new layouts and associate them to a Layout Profile. Admins can use a Layout Profile to assign a single layout to a user or give more than one layout for a user to view a record.
This allows the added focus supported by Layout Pages to be flexible based on the user and the role that they have. For instance, in QMS, a member of the QA team may be able to perform their work more efficiently by having a Layout tailored to their role, while a Subject Matter Expert may benefit from a completely different Layout.
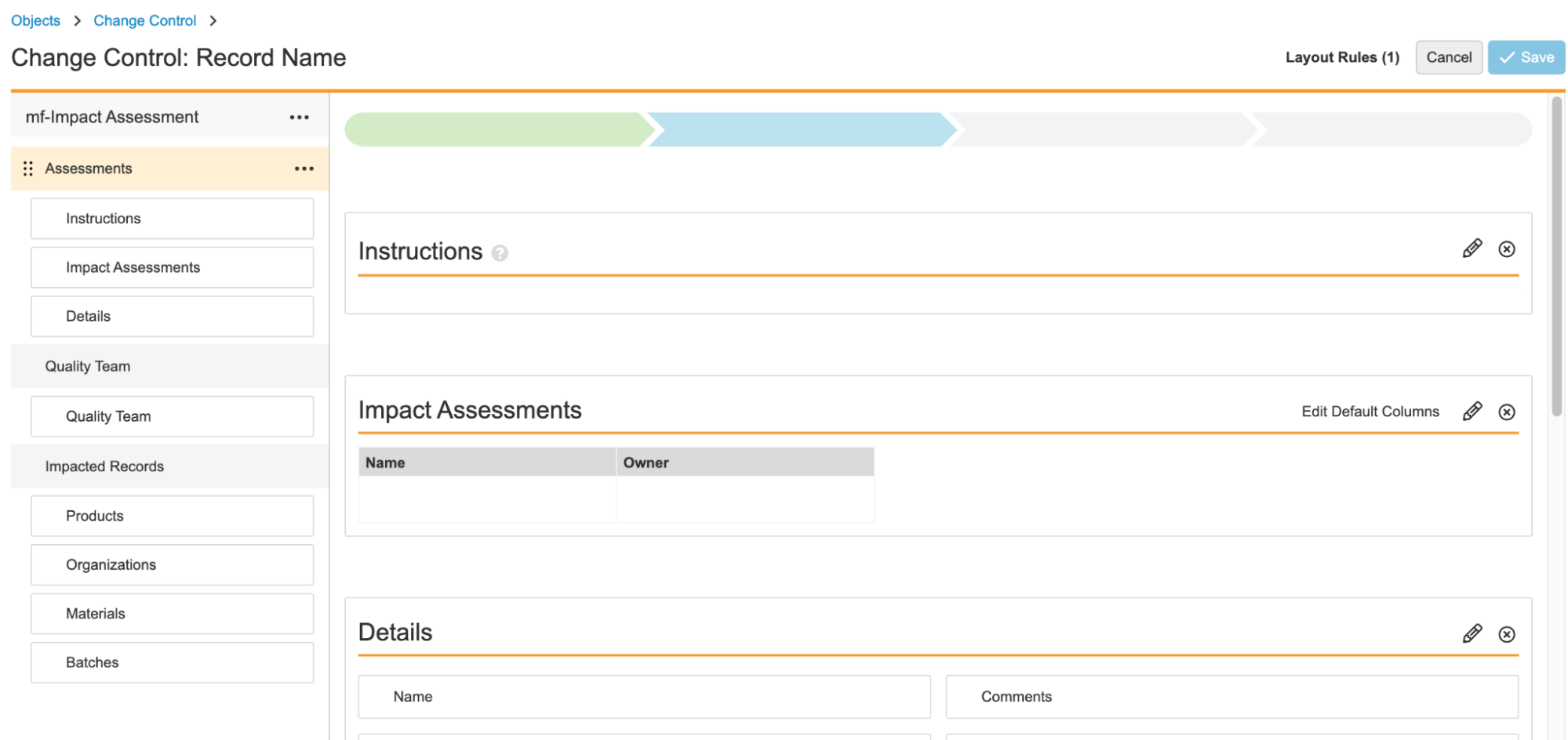
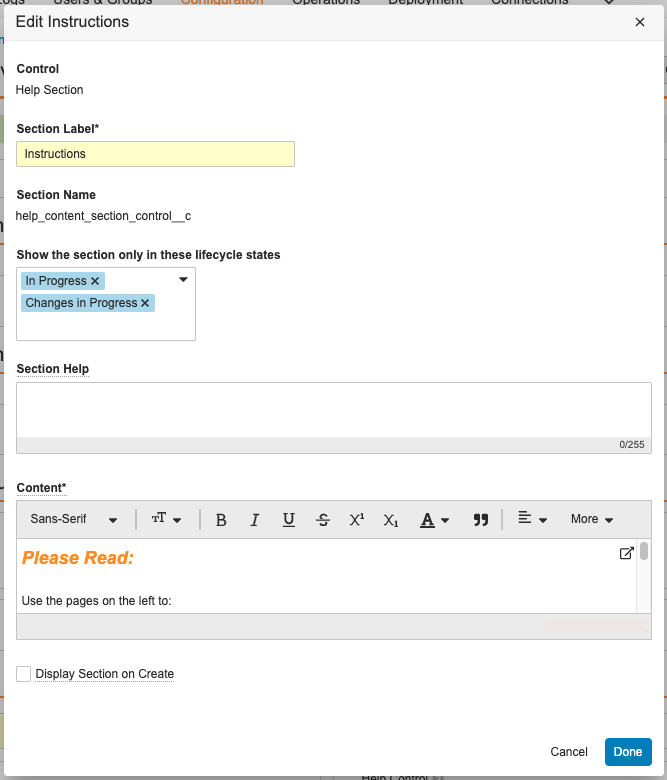
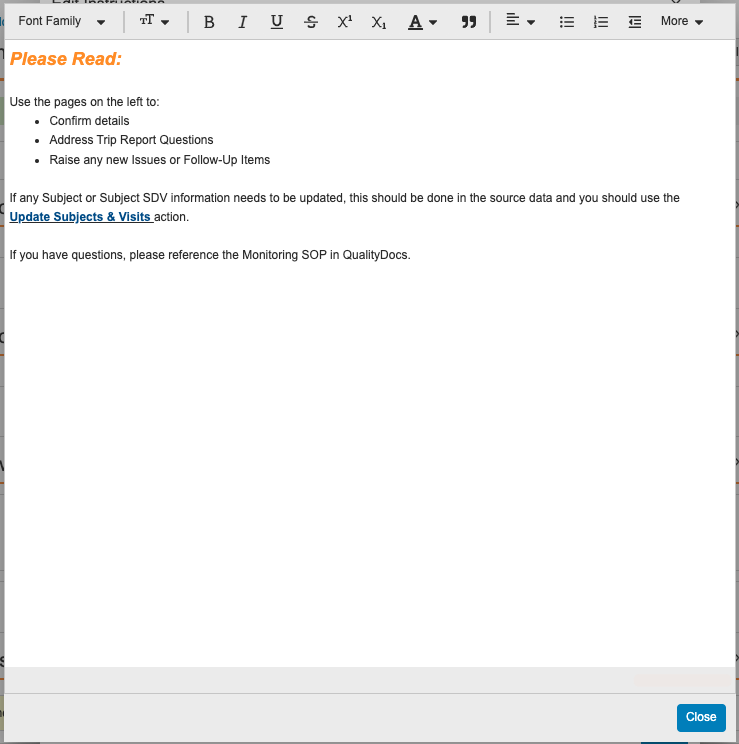
Help Section/ControlConfiguration23R3.4
With Action Layouts, Admins can configure Help Sections and Help Controls to add additional help text within a given section. This allows users to recieve detailed and contextual information to help guide them on what they are expected to do on a given record, at a given time.
This feature enhances this functionality to leverage a rich text editor, providing Admins more flexibility and ease-of-use in defining formatting of Help Sections and Help Controls.
Learn more about Help Sections and Help Controls.
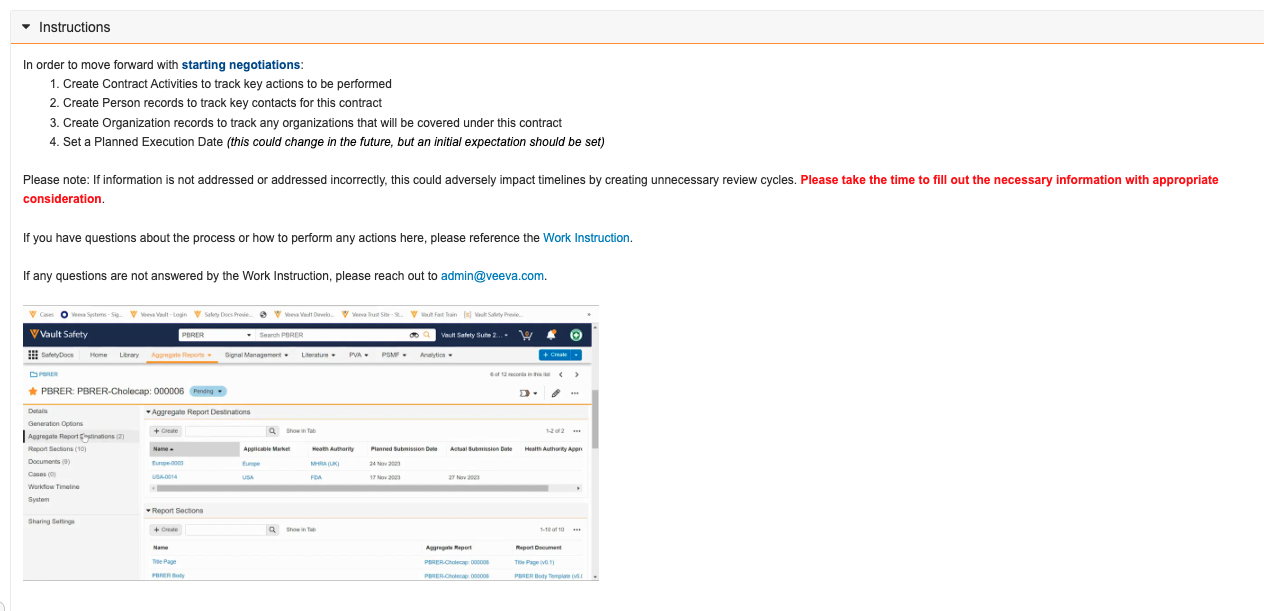
Help Section/Control: External Image SupportConfiguration23R3.5
Within the Rich Text Editor for Help Sections and Help Controls, Admins can also use an external image URL to embed an image. For instance, if you are configuring a Help Section to provide instructions to users for how to fill out a record, you could also embed a screenshot of what a properly filled out record looks like.
This enhancement adds further flexibility to the Help Section and Help Control functionality for Action Layouts to ensure the right information is provided to the right users at the right time.
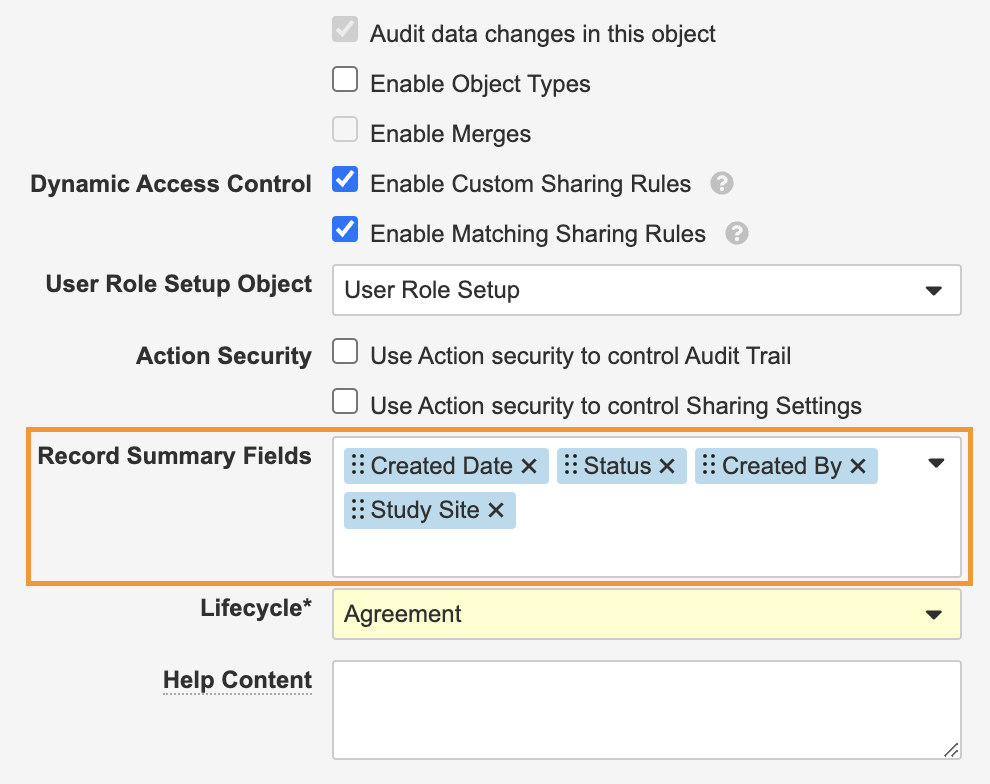
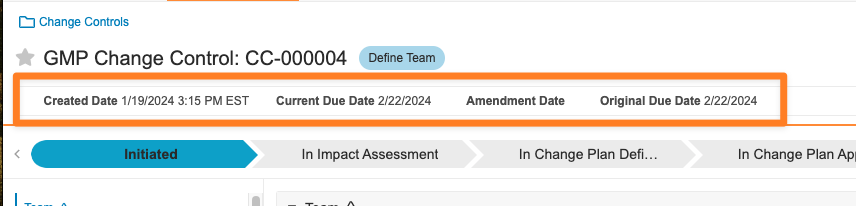
Action Layouts: Record Summary FieldsConfiguration23R3.4
With Action Layouts, Admins can configure up to six (6) fields by Object or Object Type to always display at the top of a record page as Record Summary Fields. This allows key information to display at all times for users while interacting with a record.
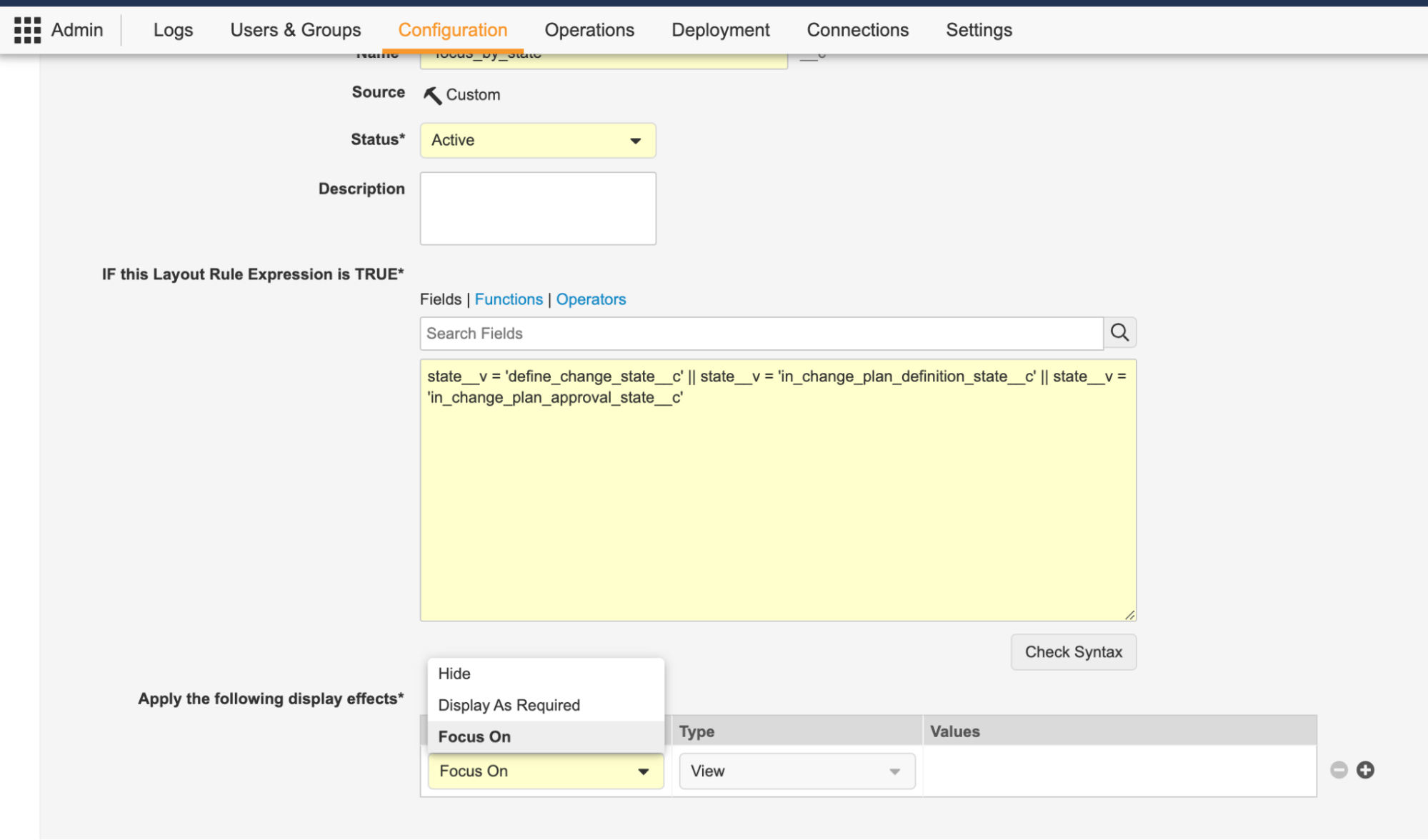
Action Layouts: Layout Rules EnhancementConfiguration23R3.2
To further enable the flexibility in these Layout changes, Layout Rules have also been enhanced. Prior to 24R1, Admins could use Layout Rules to hide or show Sections or Fields based on business logic conditions, such as the lifecycle state of a record.
With the 24R1 release that introduces Pages in a layout, Layout Rules can now also hide or show pages within layouts. Additionally, since a user may have multiple layouts assigned to them to view a record, a new effect called Focus On allows Admins to define criteria to pick a specific layout to show first. For instance, perhaps when a Change Control in QMS is in the In Implementation state, it might make sense to display a different Layout by default based on where that record is in its process.
Lastly, there is also a new Display as Required effect, which allows fields to be displayed as required fields based on the Layout Rule Expression - this can reduce the potential for users to encounter Entry Criteria errors by ensuring that fields display as required in the right circumstances.
These Layout Rules Enhancements build in further flexibility with the Action Layout changes to help ensure that users are focused on the right information at the right time.
Layout Rule: Display As Read-Only EffectConfiguration23R3.4
To further enhance Action Layouts, Admins can now use a new Effect option in Layout Rules - Display as Read-Only.
This feature allows greater flexibility in how individual fields are displayed to users based on the different layouts available. For instance, a Layout Rule could make a field appear as read-only based on a Lifecycle State, such as Draft.
This enhancement doesn’t change the underlying security on the fields, but provides the flexibility to display non-editable fields for users on specific layouts.
Learn more about Layout Rules.
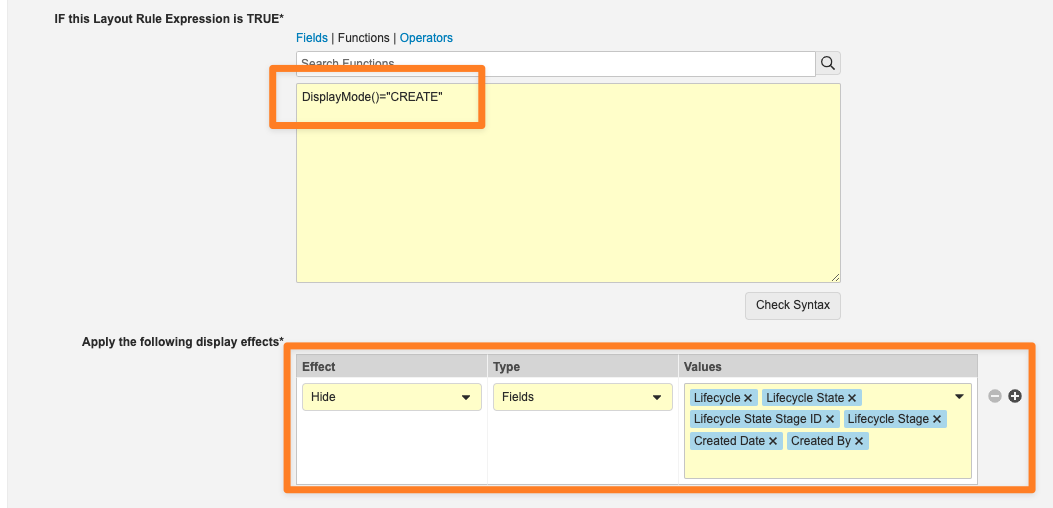
Layout Rules: DisplayMode() FunctionConfiguration23R3.4
The new DisplayMode() function allows Admins to configure Layout Rules to apply effects based on how a user is interacting with the Object Record. There are four (4) display modes: VIEW, CREATE, EDIT, or COPY.
For example, this function is used to hide certain system-managed fields when a user is creating a record:
This enhancement further extends the flexibility of Action Layouts by defining variations based on the actual activity a user is performing on a record.
Action Layouts: Additional ImprovementsConfiguration23R3.4
With 24R1, additional enhancements for Action Layouts functionality include:
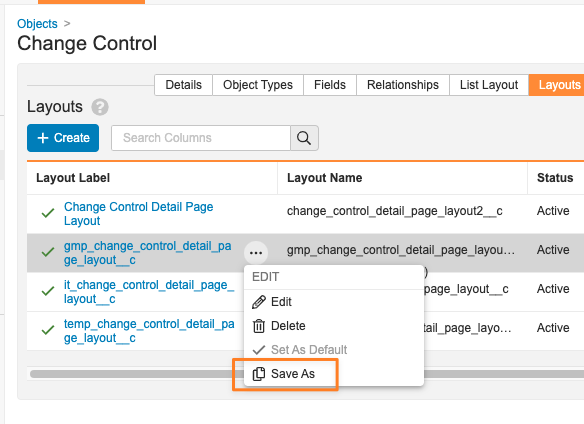
Save As (creates a new layout from an existing one):
When configuring layouts, Admins will now have the ability to choose an existing layout and save it as a new one. This enhancement streamlines the process for creating and managing Layouts and enables Admins to take advantage of Standard Layouts.
Standard Layouts:
To enable adoption of Action Layouts, Vault now supports Standard Layouts to be deployed by application teams. Standard Layouts are read-only, and users can save them as a new Custom Layout instead. Standard Layouts are not counted towards the Custom Layout limit.
Custom Layout Limit Increase:
The maximum number of Custom Layouts per Object Type has been increased from 20 to 50.
Layout Editor - Multi-Select Drag-and-Drop:
By holding Command (on Mac) or Control (on Windows), Admins can select multiple sections within the Layout Editor and drag them to reorder sections. This allows Admins to more easily make adjustments when certain sections should stay together.
Layout Editor - Layout Rules Filtering
Elements impacted by layout rules such as fields or sections are indicated by a fx icon. These icons are now clickable and will navigate you to a filtered list of layout rules that impact that element only. This allows Admins to manage layout rules in a more targeted manner.
Layout Profile - Add Base Layouts
When configuring layouts for a particular Object Type, Admins will now have the option to add base layouts instead of being limited to layouts on that Object Type only. If a base layout can be used for multiple object types, this allows Admins to leverage Action Layouts with less layouts in total.
Reporting & Expressions
Limit Increases for ReportsAuto-on23R3.5
Vault now supports a greater number of flash reports overall and a greater number of formula fields for multipass reports.
The limit for the total number of flash reports is being increased to 400 (from 200).
The limit for the total number of formula fields permitted in a multipass report is being increased to 10 (from 3).
Learn more about Flash Reports and Multipass Reports.
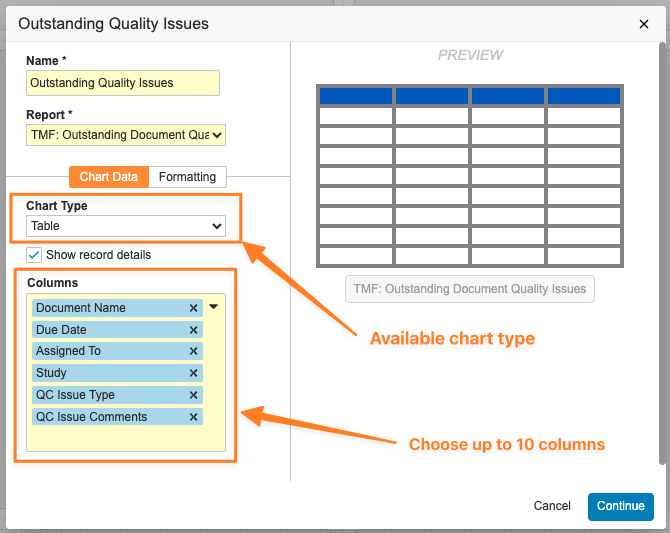
Table ChartAuto-on23R3.5
Vault now supports a new Table Chart component type for Dashboards. Any report that has no grouping or just one level of grouping can now be turned into a Table Chart.
This enhancement extends Vault’s Dashboard options for customers to be able to visualize information - Table Charts in particular allow more data points to be shown from the underlying report on the dashboard, without a user needing to click into the report to see more detail.
The sorting order and formatting from the underlying report will drive the sorting and formatting on the Table Chart.
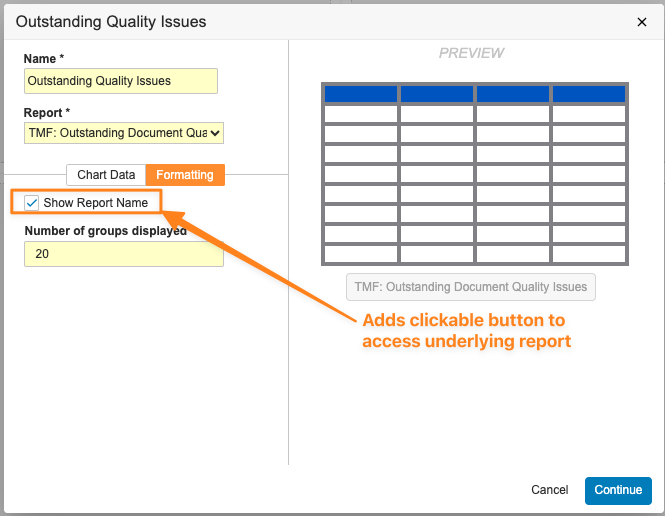
Table Charts will support a maximum of 200 rows and groups. When users are creating Table Charts, they will be able to include up to 10 fields as columns. Users will also be able to add a clickable report name to allow viewers to more easily access the underlying report.
Learn more about Dashboards
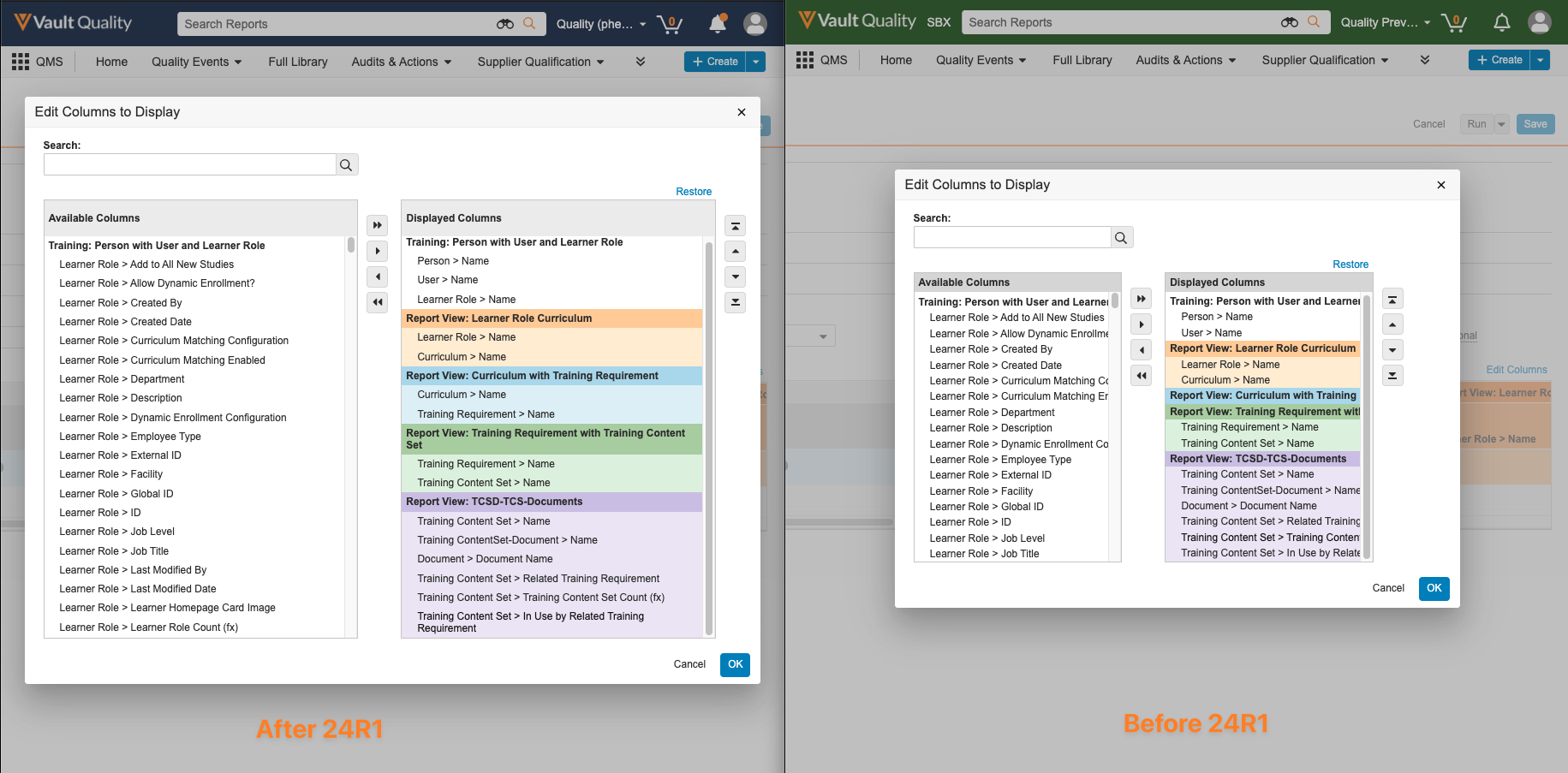
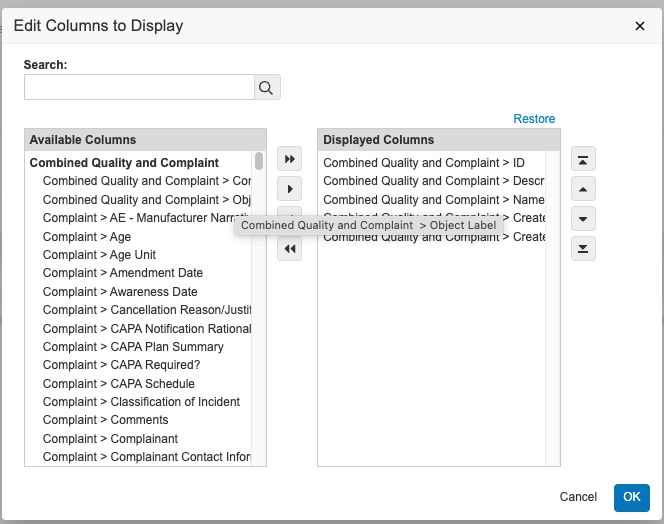
Report Edit Columns Modal EnhancementAuto-on23R3.5
When using the Edit Columns dialog box in editing reports, users will now see an improved user interface including:
- A larger dialog box
- Wrapped text for longer field names
- Darker background when the mouse pointer is hovered over a field name
Prior to 24R1, the combination of a smaller dialog box and long field names would make it difficult for users to identify the right information to include. This enhancement will make it easier and quicker for users to be able to identify and apply the right information in reports.
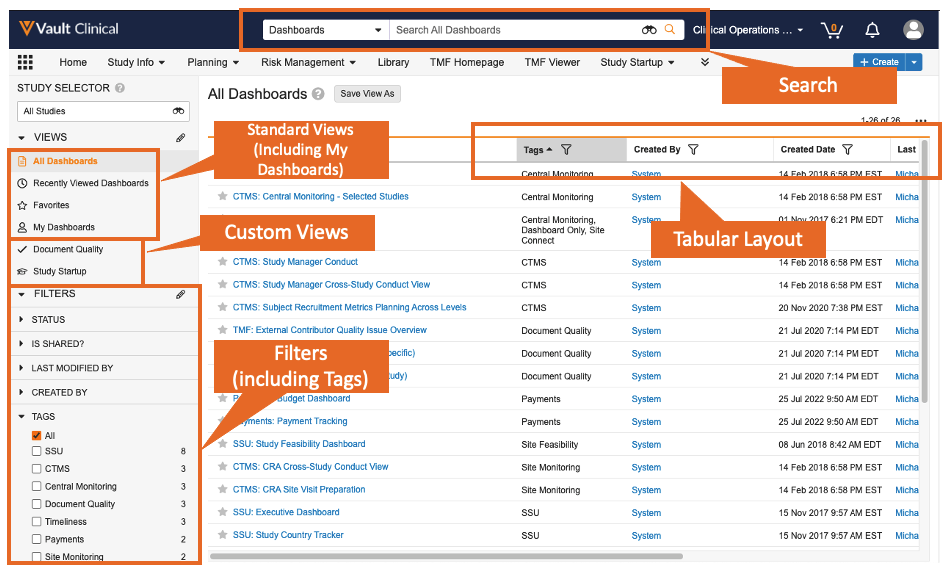
Enhanced Dashboards TabAuto-on23R3.4
Users will now have access to search and filter the Dashboards tab, create and save Custom Views, and leverage a sortable tabular layout. This feature provides users with an improved experience in accessing dashboards by providing them familiar options that are available in other areas of Vault to find and organize dashboards.
Several new fields on dashboards are now exposed to help customers identify and sort the dashboard, including the ability to add Tags to better organize dashboards.
To enable this change, all existing Dashboards will be migrated to objects, there is no change to existing dashboards from a user experience standpoint.
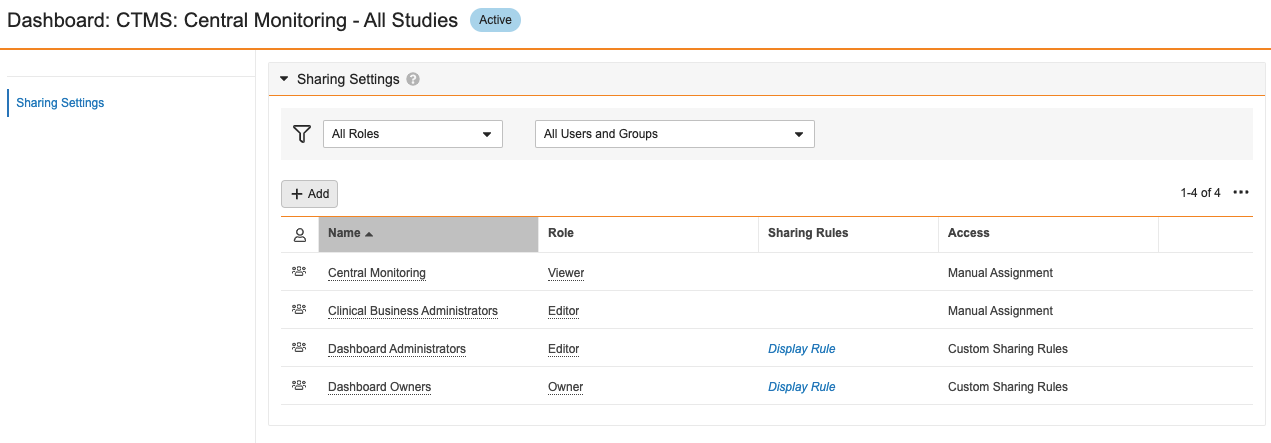
This enhancement will also bring dashboard security/sharing consistent with reporting, with a standard Sharing Settings section and standard Dashboard Administrators and Dashboard Owners groups:
The Dashboard Administrators group contains System Administrators, Business Administrators, and any custom Security Profiles that have the Administer Dashboard permission.
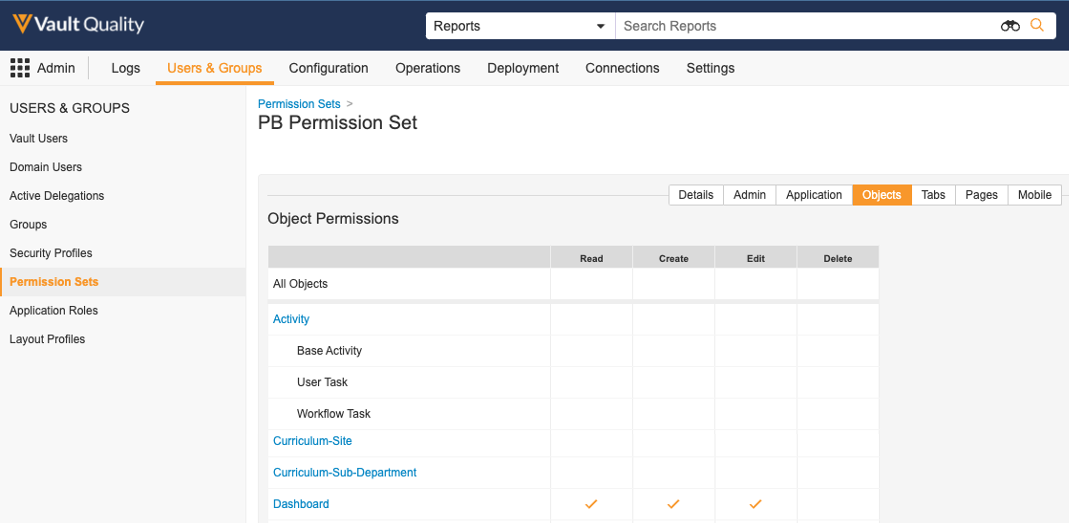
A new Object Permission will also be available in Permission Sets to control Read, Create, Edit and Delete for Dashboards:
Going forward, these Object Permissions should be used for new permission sets when granting users Read, Create, Edit, or Delete access for Dashboards.
All existing Dashboard permissions will be maintained; for instance, if a permission set has Read Dashboards & Reports checked under the Application tab, that permission set will automatically have Read checked for the Dashboard object.
The Dashboard Owners group contains Vault Owners.
Learn more about Creating & Editing Dashboards and Viewing & Sharing Dashboards.
Union Report Type EnhancementAuto-on23R3.4
When using Union-All reports, Vault now supports accessing all object fields for any objects that are unioned. This allows Admins to build Union-All report types without needing to union every field in order to provide users access to all relevant metadata and allows for flexibility by having both fields treated as unioned and some fields as unique by object.
Union-All report views now support formula fields. With this support, customers can combine fields from different data types and create a new column for the final report. With this, Admins can combine text with picklists, different picklists, and different objects. Combining different IDs or record names in a single column will enable us to join them with another view in a multipass report type.
Additional enhancements to Union-All Report Types with 24R1 also include:
- Object and document reference fields that are unioned will now display as hyperlinks.
- Object label is added as a field to make it easier to understand which object it belongs to.
- Union-All report views that contain grouping can now be combined with other views in Multi-Pass reporting.
- Union-All report views can now have results exported to Excel.
- Long Text fields and Rich Text fields are now supported when exporting Union-All reports to Excel.
Learn more about Union-All Report Types.
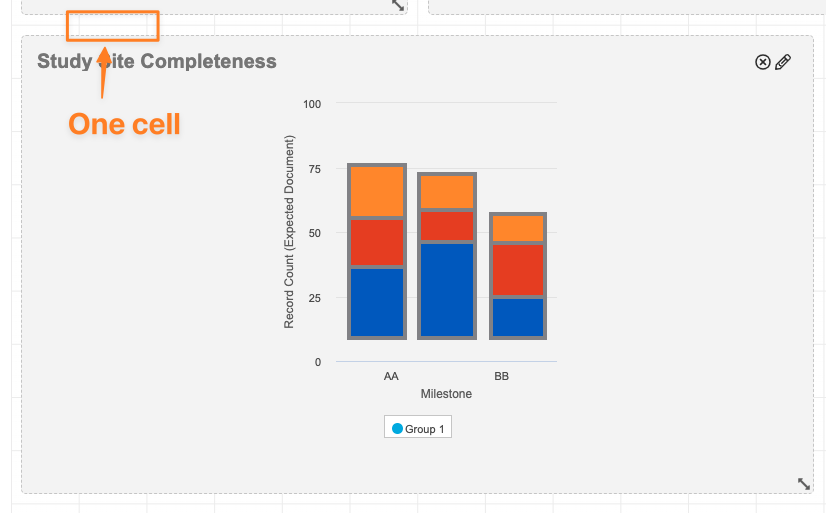
Expand Chart EnhancementAuto-on23R3.4
Column, Line and Control Charts will now show one data point per dashboard cell, allowing more information to be readily displayed to users based on the size of the dashboard component. For example, a chart expanded to a width of 7 cells will see 7 data points, and a chart expanded to 11 cells will see 11 data points.
Prior to 24R1, these charts would always show 5 data points if not expanded across the entire width of the page, regardless of the number of cells the chart covered.
There will be no change in behavior for charts expanded across the entire width of the page.
Security
Increased Field Limit for User Role Setup ObjectsAuto-on23R3.5
For objects of the class “User Role Setup” (this is the kind of object you use for auto-assigning users to roles on objects through dynamic access control), we have increased the maximum number of matching fields allowed from 5 (five) to 6 (six). This is in response to many customer requests where auto-matching users to roles needs to be more complex, or where the same user role setup configuration is used across many objects with different matching fields.
Note: Standard fields do not count towards this limit.
Tools
Sandbox Allowance WarningAuto-on23R3.5
For Production Vaults where we have granted customers additional temporary sandboxes on top of their Sandbox limit (often for projects that only need these vaults for a specific period of time), we are updating our terms of use to allow Veeva to delete sandboxes that have surpassed their expiration date, starting in September 2024.
After the 24R1 release, this more proactive approach to Sandbox cleanup will be displayed in a message when admin users access the Sandbox management page, giving everyone the chance to plan around these changes.
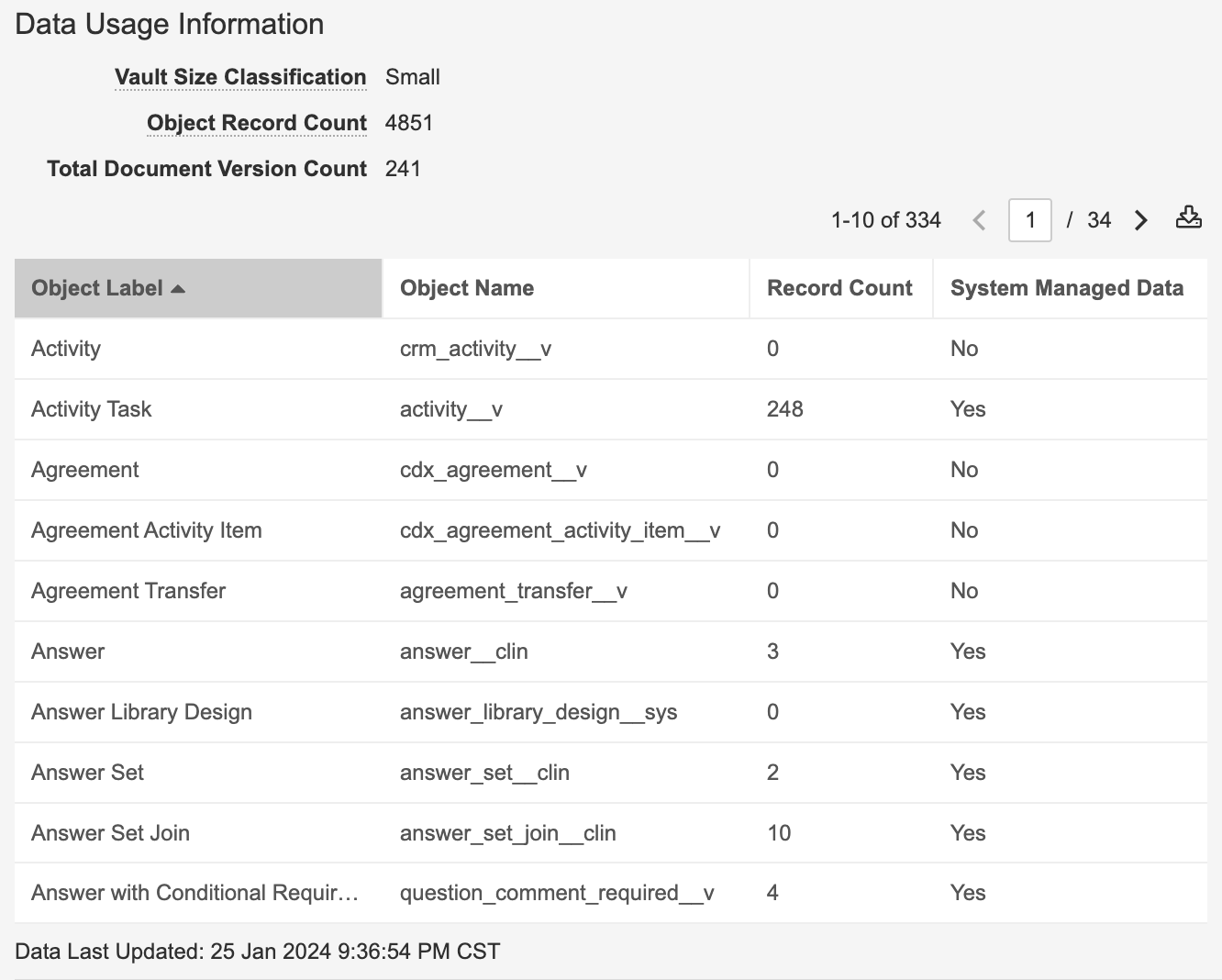
Vault Data Usage InformationAuto-on23R3.4
In Admin > Settings > General Settings, Vault now provides a Data Usage Information section:
This provides Admins an easy view of the size of their Vault, including an exportable breakdown of object records. Vault also displays the Object Record Count, excluding system-managed data, and Total Document Version Count. The information displayed in Data Usage Information is updated for object record counts on an ongoing basis and Total Document Version Count is updated on a daily basis.
System Managed Data is a classification that indicates that the data does not count towards limits, such as with Sandbox Sizes - differentiating user-managed data from configuration data that is included in Vault cloning. For instance, in RIM, Controlled Vocabulary is considered configuration data, as it is part of the core functionality of RIM (i.e. is not counted in Sandbox Sizes). Vault no longer counted configuration data as of our 23R3 release, though it was not readily visible what was considered user-managed and what wasn’t.
Data Usage Information also provides a Vault Size Classification, which categorizes the size of the Vault based on the amount of documents and data. The sizes here are the same as what is used for Sandbox Sizes.
For scenarios where the size of a sandbox may need to be adjusted, this enhancement makes it easier for Admins to identify details of what may need to be addressed.
Learn more about Sandbox Sizes.
External URL Reverse IP Lookup ChangeAuto-on23R3.4
External URL Jobs are now sent from an IP address associated with Veeva Vault. Customers who are currently allowing listing by domain will need to update their rules to support the *.veevavault.com domains.
Vault Loader Document Download Limit Increase to 10,000 23R3.2
When exporting document source files and rendition files using Vault Loader, the limit has now been increased from 2,000 files to 10,000 files. This change has been applied to all vaults, and includes no other changes in functionality.
Migration Packages Support for Record Migration Mode Update & UpsertConfiguration23R3.2
Our customers often adopt Vault applications in stages, meaning that for a time there is some key, shared data that exists both in Vault and in external systems. During later implementations where those systems are consolidated into Vault applications, there is a need to make configuration and data changes to that shared data. This process is handled as a data migration, and to support that we are extending the Migration Mode capabilities from only object record creation to also now cover update and upsert.
Enhancements to Query Field TypeConfiguration23R3.2
Field rules now allow using a different SELECT field than the Query field in the field rule. This will be used by app developers/customers to get required field value without overloading the Query Field.
To achieve this, we’re introducing a new Query Field Select attribute (query_field_select).
The attribute will be recommended on Field Rules used in Vault Connections, but will not be required for field rules on local or external connections.
Learn more about Query Object Rules here.
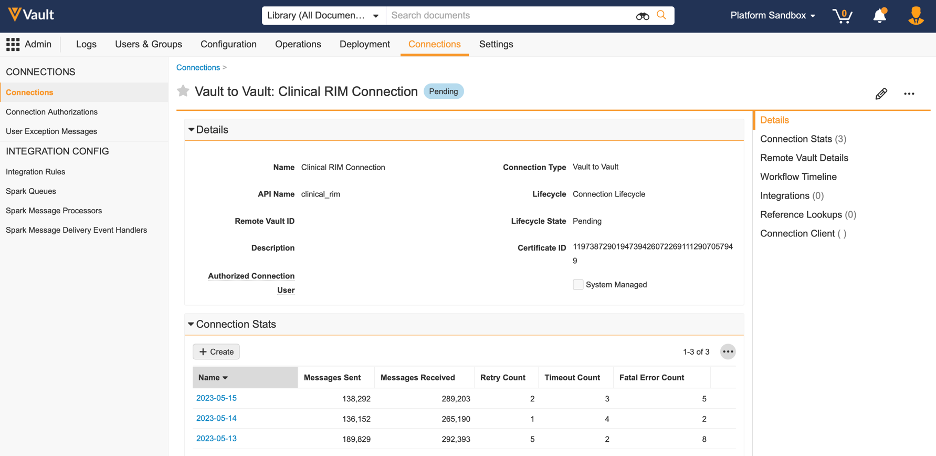
Connection StatsAuto-on23R3.2
Admins can now access daily performance metrics for connections (Vault-to-Vault or external) including messages sent or received, failures, retries, and elapsed time.
In previous releases, Vault already provided the total number of connection messages (inbound or outbound) as part of daily Performance Statistics. However, more granular details are often needed per connection to troubleshoot and monitor connections and integrations.
With this release, we have introduced a new object that stores daily stats per connection and per integration. This object does not support custom fields, triggers, and customization in general.
This object is available in Business Admin by default, but customers can also create a new report type to monitor connection details.
A new related section for Connection Stats is available by default on Connections as well to allow these stats to be easily viewed per connection:
Learn more about Vault Connections on Vault Help here: Creating & Managing Connections
Scheduled Data Export Support for AWS Bucket PolicyConfiguration23R3.2
The Scheduled Data Export functionality used Access Control Lists (ACL) prior to 24R1, but we now support the recommended AWS Bucket Policy approach for S3 buckets instead.
With ACLs disabled, you can use policies to more easily control access to every object in your bucket, regardless of who uploaded the objects in your bucket. See the AWS S3 Documentation for more information on bucket ownership.
Errors and Warnings in API Usage LogsConfiguration23R3.2
API Usage Logs will now include error and warning messages that appear in the CSV file output. New columns will include:
api_response_warning_messageapi_response_error_message
Additionally, we will include the X-VaultAPI-ReferenceId header in the API Usage Logs with column:
reference_id
Invalid Operators & Fields on Legacy Users Query TargetAuto-on23R3.5
When using Vault REST API v24.1+ on the legacy users query target (users), VQL no longer supports the created_by__v or modified_by__v fields in the ORDER BY clause and in WHERE clauses that use a comparison (>, <, >=, <=), BETWEEN, or LIKE operator. Previous versions are unaffected by these limits but may return invalid results. These limitations do not apply to the user__sys object query target.
Vault Mobile
Note: Vault Mobile does not have a Limited Release, and only releases with Vault General Releases. Features listed here will be available for testing and assessment during the pre-release period for the next General Release. Learn more about the mobile pre-release here.
Apply Study Information to New Documents (Clinical Vaults Only)Auto-on23R3.2
Users in Clinical Operations Vaults now have the option to be able to apply Study, Study Country and Study Site metadata on documents scanned via Vault Mobile.
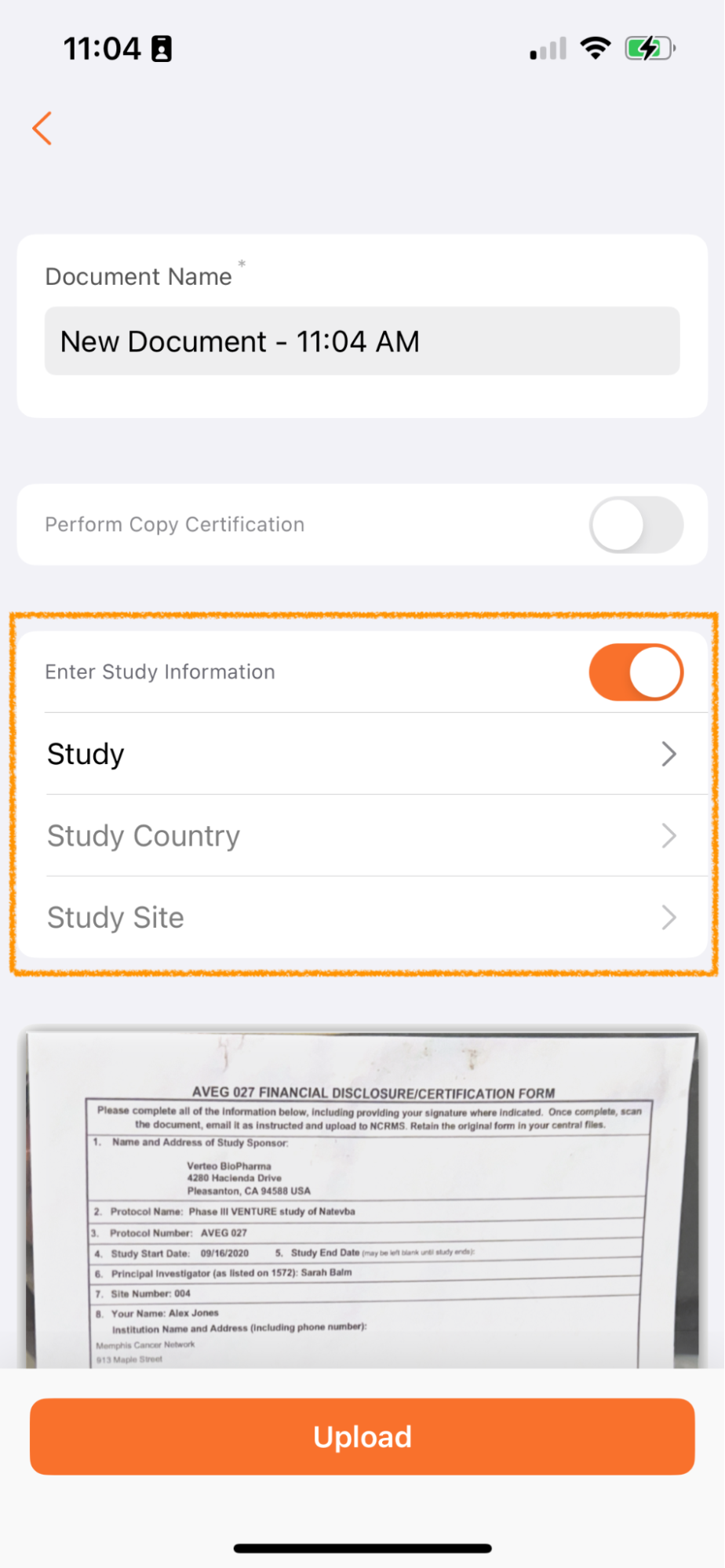
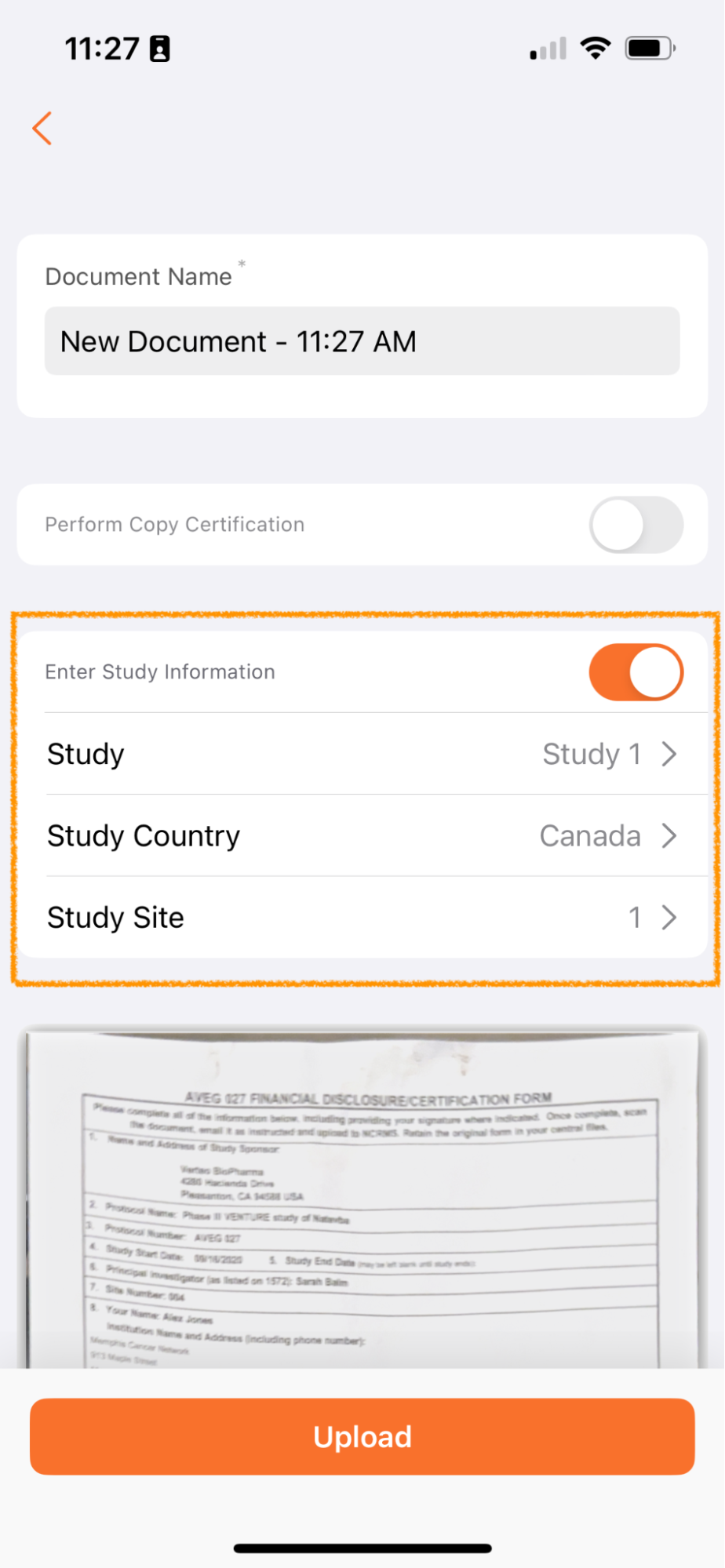
Documents uploaded via Vault Mobile are unclassified and added to the Document Inbox. Many customers manage the processing of documents in the Inbox by study - for example, there may be a team focused on processing documents for Study ABC and another focused on processing documents for Study XYZ.
By allowing users to add this metadata when uploading, users managing the Document Inbox will be able to more easily split duties and focus on the right documents for their role and study assignment.
This feature only applies when using the Scan function and does not apply to files uploaded to Vault Mobile through the device.
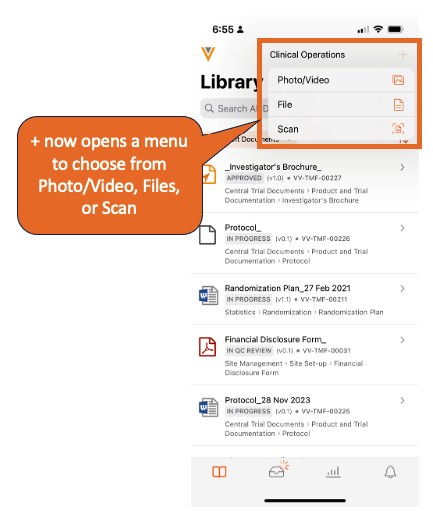
Add Content to PlaceholdersAuto-on23R3.2
Vault Mobile now allows users to scan or upload documents into existing placeholders in Vault, further extending the capabilities for users to be able to contribute content on-the-go.
As an example, a user could send a task to another user requesting a document using a placeholder; the user who receives the task could scan a document to fulfill that placeholder and complete the task.
This also provides a mechanism for users to contribute content as classified (since the placeholder would already have a classification assigned).
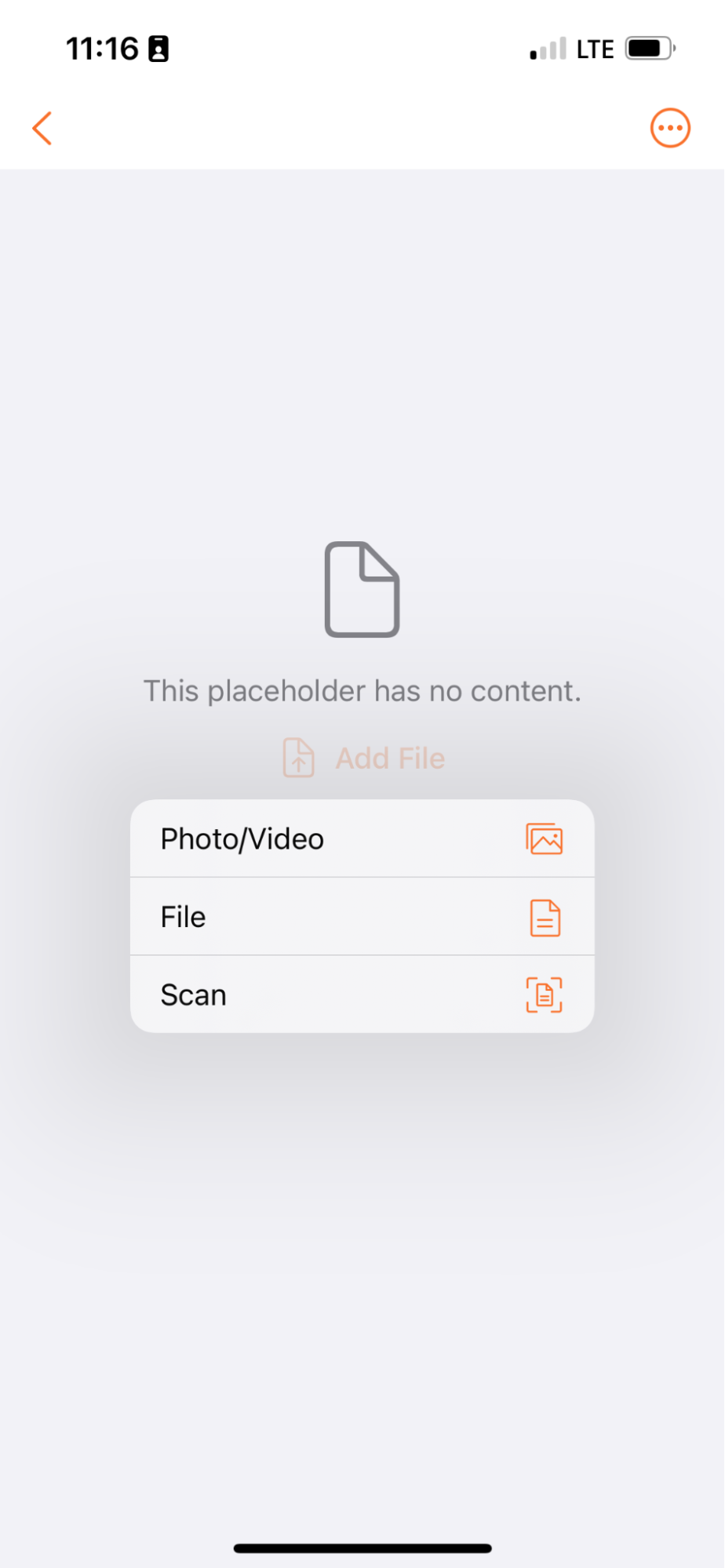
Select a File for UploadAuto-on23R3.4
When creating a new document in Vault Mobile, users now have a choice to select an existing file/photo from their device in addition to scanning a new document.
Prior to 24R1, Vault Mobile users typically created new unclassified documents by scanning document pages from within the mobile app. For some users who might already have a file on their phone (and don’t need to scan anything), there is not an easy way to select an existing file for upload from inside the app.
There is an existing Share to Vault function that does allow users to share files from another app into Vault, but it requires users to log into Vault Mobile before attempting to share from another app which makes it a less seamless user experience.
Allowing users to select a file from their phone rounds out the document creation experience on Vault Mobile and provides a user experience that is more comparable with the Web user experience.
This enhancement is auto-on but Admins do have the option to disable this in Admin > General Settings > Settings:
Disabling this blocks the ability to upload from Photos or Files while still allowing users to scan new files.
Language SupportAuto-on23R3.4
Text in the Vault Mobile app now reflects the user’s Vault Language and Locale settings, rather than always displaying in English.
This enhancement allows Vault Mobile to be better utilized by users globally, and makes the mobile app user experience consistent with the web user experience.
Learn more about Language & Region Settings.
Platform Data Model Changes
See 24R1 Platform Data Model Changes.
Vault Connections
Clinical Operations-CDMS Connection
Clinical Operations-EDC Connection: Complex Clinical TrialsAuto-on23R3.2
This feature enhances the Clinical Operations to CDMS Connection to support the introduction of Complex Clinical Trials in Vault CTMS. With Complex Clinical Trials, customers can create a variety of subject groupings (such as arms, cohorts, and sub-studies) that can be used for Milestone, Metrics, and Subject Enrollment tracking.
Using a new field rule, subject grouping assignments are transferred from CDMS to Vault CTMS. Once the subject groupings are manually linked between the two systems, when a subject in CDMS has a subject grouping assignment or an arm, cohort or substudy, the connection matches the subject grouping to a subject grouping assignment for the same study in Vault CTMS. If there is a match, the subject grouping assignment on the Vault CTMS record is updated. If there is no match, the Connection creates a User Exception record and leaves the subject grouping assignment reference on the Vault CTMS record with no change. Learn more about other new Clinical Operations features.
Clinical Operations-EDC Connection: Subject Visit MethodAuto-on23R3.2
In response to increased trial decentralization, the Subject Visit Method field was added to Clinical Operations Vaults in 23R2 and captures additional information about how the visit is performed (i.e., on-site or remotely). Subject Visit Method is used in Fee Schedules to support differing payment amounts and is also tracked on Monitored Subject Visits to provide CRAs insight into the expected documentation to monitor.
With this feature, the Clinical Operations to CDMS Connection is enhanced to support transfer of Subject Visit Method from CDMS to Vault Clinical.
Learn more about the Clinical Operations to CDMS Connection. Learn more about other new Clinical Operations features.
LIMS-Quality Connection: Document Exchange
LIMS-Quality Connection: Document ExchangeConfiguration23R3.5
This feature will add new connection and integration points between the QualityDocs and LIMS Vaults to support the automated creation of crosslinks when a specified document type reaches a steady state. Document crosslinks can be sent from QualityDocs to LIMS and also from LIMS to QualityDocs as part of the connection. The connection will support automating steady state document crosslink versioning and metadata updates between both Vaults.
Quality - RIM Connection
Quality-RIM Connection: Document ExchangeConfiguration23R3.5
This feature introduces the automated crosslinking of documents between Quality and Regulatory Vaults when documents of specified type/subtype/classification reach a steady state. This allows documents that are authored in a Quality Vault, but need to be included in submissions, to be automatically transferred to a RIM Vault as a crosslink. This connection also allows documents that originate in RIM Vaults, such as Product Specifications, Labeling/Artwork Documents and CMC Documents, for example, to be automatically transferred to Quality Vaults. This helps to ensure that there is one source of truth and eliminates the need to create crosslinks manually. The crosslink is created by the system when the initial version of a document reaches steady state and then is automatically upversioned as new major versions of the document reach steady state. Existing crosslinks become automatically obsolete if the existing document is made obsolete.
Quality-RIM Connection: Updated Error Message when Executing UpdateRimChangeDetails ActionAuto-on23R3.2
When the Update RIM Change Details record action is configured as a user action for Impact Assessment records, the action will now always be visible. Previously, this record action was only visible if the RIM Event ID field on the Impact Assessment record was populated. Users will now receive an error if both the RIM Event ID and Quality Event or Change Control fields are not populated. Learn more about other new Quality and Regulatory features.
RIM-PromoMats Connection
RIM-PromoMats Connection: CrossLink Document Transfer IntegrationConfiguration23R3.4
The RIM to PromoMats Connection can now transfer Steady State documents to support AdPromo submissions. Once configured, customers using the Connection will no longer need to download Current-in-Use Labeling documents for RIM for upload into PromoMats. This improves end-user efficiency and reduces the risk of including outdated Labeling Documents in their AdPromo submissions.
Admins can configure the Document Types to be transferred based on Document Type Groups and Integration Rules (Query Object Rules, Field Rules, and Reference Lookups). By default, the integration only initiates the Steady State document transfer once they reach their next steady state after the feature is configured. Learn more about other new Regulatory and Commercial features.
RIM-PromoMats Connection Performance ImprovementsAuto-on23R3.4
This feature introduces performance improvements to the RIM-PromoMats connection so that validation errors on one submission do not block other submissions. Learn more about other new Regulatory and Commercial features.
Medical-Safety Connection
Medical-Safety Connection: Adverse Event ReportsConfiguration23R3.4
This feature will allow customers to share potential Adverse Events raised in Vault Medical - MedInquiry with Vault Safety via a Vault to Vault connection.
In this connection, Adverse Events records in MedInquiry are shared to Vault Safety with required patient, reporter, product, and event information, where they are created as Inbox Items records for Safety Case processing and reporting. After Inbox Item creation, reconciliation information is shared from Vault Safety back to MedInquiry. Further action on the Inbox Item in Vault Safety will trigger the sharing of Case information from Safety back to MedInquiry (including the Case Number and outcome of the Inbox Item in Safety). Learn more about other new Medical and Safety features.
Learn more about the Medical-Safety Vault Connection, using the Medical-Safety Vault Connection, and enabling this Vault Connection.
PromoMats-Medical Connection
PromoMats-Medical ConnectionConfiguration23R3.4
This feature will allow the transfer of steady state documents and their related anchors between Medical Vaults and PromoMats Vaults. For example, medical literature references stored in Medical Vaults can be transferred to PromoMats Vaults to help support claims made in promotional / marketing materials.
Upon release, this bi-directional connection will include a default configuration that customers can add to, modify or disable. Configurable elements include Document Types with the PromoMats Medical Connection Document Type Group and Integration Rules (Query Object Rules, Field Rules, and Reference Lookups).
The transfer of these documents and data between the Medical and PromoMats Vaults will make it so that it is no longer required for documents to be downloaded from one Vault and imported to another leading to errors and compliance risks.
The creation of reference documents will be automated via crosslinks and will allow key documents to have a single source of truth within the organization and reduce the potential of duplicate document creation. Learn more about other new Commercial and Medical features.
Safety-Clinical Operations Connection
Safety-Clinical Operations Connection: Safety LettersAuto-on23R3.5
The Safety-Clinical Operations Connection now supports Safety Letter distribution from Vault Safety to Vault Clinical Operations. Prior to this, Safety Letter distribution from Pharmacovigilance to Clinical Operations systems was manual and error-prone. With this feature, Safety Letter distribution is done in an automatic and compliant manner.
This feature is Auto-On in Vaults with the Safety-Clinical Operations Connection enabled.
Learn more about other new Safety features.
Learn more about the Safety-Clinical Operations Vault Connection and enabling this Vault Connection.
Safety-EDC Connection
Safety-EDC Connection: Serious Adverse Event ReportsConfiguration23R3.4
The Safety-EDC Vault Connection supports the automated transfer of Serious Adverse Events (SAEs) from Vault EDC to Vault Safety along with related subject Case information. SAEs are discovered by study sites and entered in EDC Vaults by site users as SAE Assessments, which automatically generate Inbox Items in Vault Safety for Case processing and reporting. This feature helps users avoid delays and compliance issues. Learn more about other new Safety features.
Learn more about the Safety-EDC Vault Connection and enabling this Vault Connection.
Safety-EDC Connection: Subject Information Selection UIConfiguration23R3.5
The Safety-EDC Vault Connection allows Admins to configure access to the EDC subject information and selection UI after running the Add Relevant Subject Information action, providing valuable insights for Safety users with appropriate permissions. Within the Case view, Safety users can seamlessly access and interact with critical subject information, enhancing their ability to make informed decisions. This includes visibility into EDC Subject Adverse Events, Case Products (such as Study Drugs and ConMeds), Medical and Drug History, and Test Results. By facilitating this access, the feature supports efficient information retrieval and reconciliation between EDC and Safety Vaults, optimizing workflow processes. Learn more about other new Safety features.
Learn more about the Safety-EDC Vault Connection and enabling this Vault Connection.
Safety-RIM Connection
Safety-RIM Vault Connection: Product Trade NamesAuto-on23R3.4
The Safety-RIM Product Connection now transfers details of associated Product Trade Names from Vault RIM to Vault Safety. Visibility of Trade Name details helps users select the correct Product during the intake process. This feature enhances the Safety-RIM Vault connection by ensuring alignment of Trade Names between RIM and Safety.
This feature is Auto-On in Vaults with the Safety-RIM Connection enabled. Learn more about other new Safety and Regulatory features.
Learn more about the Safety-RIM Vault Connection.
Clinical Operations
Several features listed in the Vault Connections section also affect the Clinical Operations application family.
CTMS
Complex Trials: Subject Metrics and Recruitment PlanningAuto-on23R3.5
In this release, updates to the Clinical Operations data model introduce the ability for customers to track complex study designs in Vault Clinical using Subject Groups. This feature further expands on Subject Groups by allowing customers to easily specify where subject metrics and recruitment planning are needed on a group-by-group basis.
Two new fields Create Recruitment Plan and Track Enrollment Metrics allow customers to select if Metrics over Time and Subject Metrics, respectively, are needed for a specific Subject Group. Metric types are generated based on the values set at the Study level. For example, any metrics listed in Metrics Not In Use are not created for the Study or any Subject Group.
Metrics and Metrics Over Time calculation behavior is updated to calculate Subject Group counts and Study counts independently. For example, if a subject is enrolled with a Subject Group specified, the subject will be counted in the Total Enrolled metric for Subject Group and Study separately. If a subject does not have a Subject Group, the subject is only counted at the Study level. Metric calculations for Subject Groups are only applicable when date-based metric calculation is selected.
Roll-up behavior also functions independently. When Subject Groups are specified, site level metrics roll up to the corresponding Subject Group country metric, and country values roll up to the corresponding Subject Group study metric. Similarly, for non-Subject Group metrics, site level values roll up to the Country level, which roll up to the Study level.
Complex Trials: Subject MilestonesConfiguration23R3.5
This feature enhances Automated Enrollment Milestones functionality to support Complex Clinical Trial designs. When configured, Subject Group Enrollment Milestones (i.e., First Subject In for a Substudy) are automatically populated with Actual Finish Dates based on the corresponding dates entered for related Subject records with a Subject Group specified. Study and Subject Group Enrollment Milestones are independently populated.
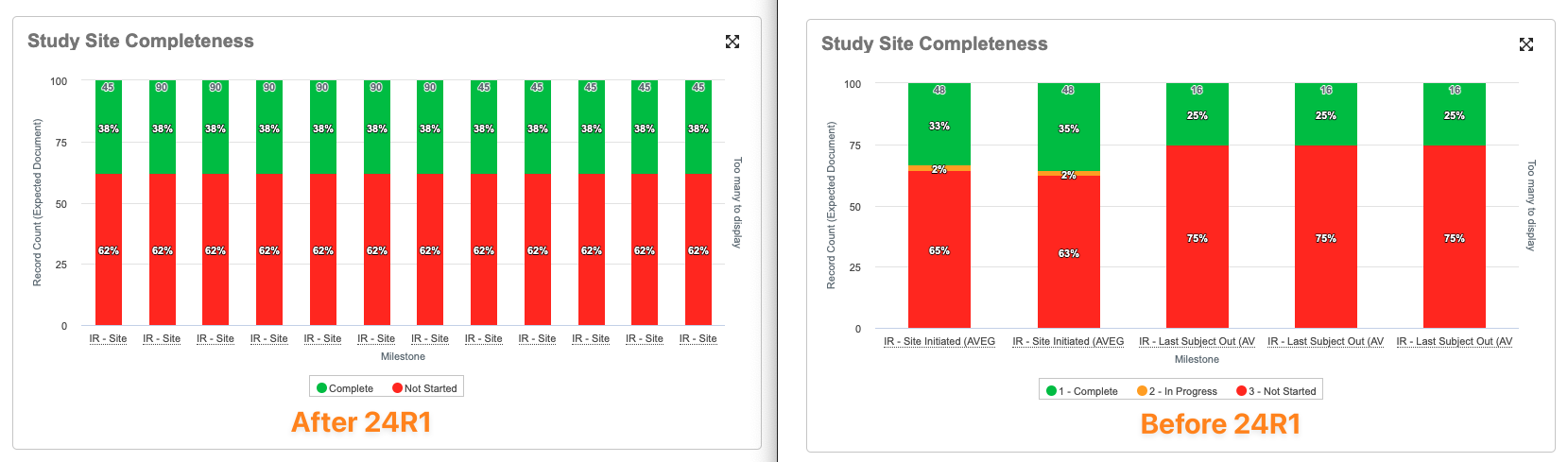
Last Subject Milestones: Lost to Follow-Up Dates EvaluatedAuto-on23R3.5
With this feature, Vault now evaluates Lost to Follow-up dates on Subject records when populating Actual Finish Dates on Last Subject Enrollment Milestones (i.e., Last Subject Out).
CTMS TransferConfiguration23R3.4
Sponsors involved in clinical research often contract the expertise of Clinical Research Organizations (CROs) to manage their studies. In these situations, the CRO’s preferred operational model involves tracking and managing study data within their proprietary Clinical Trial Management System (CTMS), leveraging their established processes to enhance trial efficiency.
Despite this delegation of responsibilities, Sponsors retain the ultimate accountability for the Subjects’ safety, as well as the quality and integrity of the trial data. They have to closely oversee CRO activities such as trial enrollment, status, compliance, and issues resolution. Documenting evidence of this oversight (data review, and recording oversight issues) is crucial for regulatory inspections. This leads the Sponsor to implement costly custom solutions with their CRO partners to access oversight data from their CTMS.
To simplify data transfers between the CRO and the Sponsor systems, and to empower Sponsors with seamless access to their trial data, we are introducing CTMS Transfer. This feature automates the daily transfer of Study data from the CRO to the Sponsor when both organizations utilize Vault CTMS. The transfer includes critical elements such as Study Countries, Sites, Arms, Enrollment Metrics, Milestones, Issues, and Trip Report documents. It’s important to note that the transfer is unidirectional, strictly occurring from a designated Source Vault to a Target Vault. The transfer is limited to specific standard fields and values. Any custom value in the Source Vault must be mapped to the Clinical Standard Fields. Furthermore, the transferred data remains read-only in the Target Vault.
Japanese Clinical Trial Notification Validation ReviewAuto-on23R3.4
Vault CTMS offers a robust set of capabilities designed to support clinical trials in Japan. This includes generating Clinical Trial Notifications (CTN) for the Japanese Pharmaceuticals & Medical Devices Agency (PMDA) in a specific XML format. These CTNs need to be submitted on a regular basis and within certain timeframes to maintain compliance.
In the dynamic landscape of regulatory requirements, it’s not uncommon for the XML format or content rules of CTNs to undergo changes with short notice. In such cases, it remains imperative for our customers to swiftly adapt and comply with these new requirements within the same time constraints. It’s critical that our customers’ processes remain agile and are not prevented from generating CTNs.
To empower our customers in promptly aligning with the latest regulatory standards, we are introducing a CTN Validation Review feature. Vault CTMS users will have the option to proceed with generating a CTN document and XML file after reviewing the validation checks currently in place. This ensures a flexible and responsive approach, enabling our customers to adapt swiftly to regulatory changes without compromising on efficiency.
Oversight Issue Tracking for Outsourced StudiesAuto-on23R3.4
In recent years, the Clinical Trials Outsourcing market has experienced substantial growth, and this trend is anticipated to persist in the coming years. Various outsourcing models, including Full Services Outsourcing (FSO), Specific Services Outsourcing (FSP), and hybrid models, address diverse industry needs.
Regardless of the chosen outsourcing model, ICH E6 (R2) emphatically asserts that Sponsors maintain ultimate responsibility for outsourced trials and should maintain appropriate oversight. Consequently, Sponsors bear the responsibility for ensuring the safety and well-being of trial participants, as well as the accuracy and quality of data collected by the Contract Research Organization (CRO) during the trial. Experience has shown that during inspections, Sponsors are required to provide evidence of their oversight activities to inspectors, particularly regarding issues identified through the oversight process and their communication to the CRO.
To standardize the documentation of oversight activities, we are introducing a new Oversight Issue object in Vault CTMS. This feature enables the tracking of Oversight Issues for Monitoring Events, Milestones, and Issue records. Furthermore, CTMS users can be guided through their oversight activities thanks to Recurring Oversight Milestones and Clinical User Tasks. In this case, Oversight Issues could be directly tracked from and be associated with these Clinical User Tasks. Documenting these activities in Vault CTMS provides a comprehensive context for the tracking of Oversight Issues and helps to illustrate the end-to-end story.
Vault Payments
Payments Adjustment ReasonsAuto-on23R3.4
With Payment Adjustments, Vault can automatically update Payable Items when changes are made to corresponding Visits, Procedures, Site Fees, or Fee Schedules. With this feature, Vault will now automatically populate the Payment Adjustment Reason from a predefined picklist, providing additional information to users and streamlining communication with sites about the adjustment.
Learn more about Vault Payment Adjustments.
Payment Adjustment OverrideConfiguration23R3.4
Users can now choose to exclude Payable Items from automatic updates. This allows for easier adoption by existing customers that may need to separate out items previously managed manually. It also allows customers to approve deviations for individual Payable Items while keeping them associated with the corresponding Fee in Vault Payments.
Learn more about Vault Payment Adjustments.
eTMF
Study Country & Site Metadata ExtractionAuto-on23R3.5
The TMF Bot’s capabilities continue to expand. In addition to document classification and the Study field, it now supports Study Country and Study Site metadata. These values are automatically populated upon document upload to the Document Inbox of Vault. This enhancement works closely with Study Metadata Extraction, and favors Study Site matches where a Study match is found.
This enhancement further streamlines the processing of documents within eTMF. From our surveys and research, we project that the automated inclusion of Study, Country, and Site information by the TMF Bot will lead to an average time saving of one minute per document.
TMF Standard Document FieldsConfiguration23R3.4
To support industry standard concepts, we are introducing six (6) new standard document fields and triggers to Clinical Operations Vaults. This feature adds triggers on the new fields and two (2) existing fields, Primary and Secondary QC Status. The new fields are provisioned as Shared Fields, and must be configured on document types to be used. Below is a breakdown of the new fields, their behaviors, and descriptions of their intended use:
|
Field Label |
Field Name |
Editable |
Field Type |
Field Description |
Field Behavior |
|
Effective Date |
|
Yes |
Date |
The date on which this document is considered ready for use. |
|
|
Collected Date |
|
Yes |
Date |
The date on which this document version is received from the source. |
|
|
System Created Date |
|
No |
Date |
The date on which this document version is uploaded to Vault. |
|
|
Inspection Ready Date |
|
Yes |
Date |
The date on which this document version is considered inspection ready and has passed all quality control checks. |
|
|
System Approved Date |
|
No |
Date |
The date on which this document version is approved in Vault. |
|
|
External Document Version |
|
Yes |
Number |
The version number for this document version as it is tracked outside of Vault, independent of the Vault Version. |
N/A |
|
Primary QC Status |
|
Yes |
Already exists |
Already exists |
|
|
Secondary QC Status |
|
Yes |
Already exists |
Already exists |
|
Study Startup
Survey Reminder NotificationsConfiguration23R3.5
Feasibility teams no longer need to rely on email or phone communication to remind sites to complete outstanding surveys because Vault Study Startup now supports Survey Reminder Notifications. With this feature, feasibility teams can rely on Vault to trigger reminders at a set frequency. Users can also leverage a new action for ad hoc notifications, which Vault can send individually or in bulk to the survey respondent. Site and Outreach Target invitations will track the Last and Next Reminder Date, Notification Frequency, and Reminder Delivery Status. This feature will also increase the PAL link expiration from 30 days to 60 days.
Learn more about Feasibility Surveys and Designing Checklists.
Study Startup, eTMF
Milestone Workspace & EDL Template Scalability EnhancementsAuto-on23R3.5
As a part of the 23R2 Release, Vault Clinical introduced EDL Override Templates, a new template structure that increases flexibility and decreases the overall maintenance effort for Country Specific EDL Templates (Country Intelligence). This 24R1 feature includes several components, including building on the existing functionality and introducing new capabilities.
The feature includes the following enhancements:
- EDL Automation, Person, Organization or Product specific Expected Documents, is now better supported with EDL Overrides, because of updates to EDL Hierarchy logic.
- Override Templates can now be configured with a Template Expected Document (
edl_item__v) record having a milestone type value as blank, if no extra milestone types are needed for the Country Specific Expected Document. - Now when a Milestone Item is deleted, only the related Milestone Document will be deleted.
The feature includes the following new capabilities:
- For migration purposes, Milestone Documents can now be created in bulk via API.
- To support Study deletion, Admins will now have the ability to delete Milestone Documents within Archived Studies.
The following are PSAs for this feature:
- Prior to 24R1, EDL Overrides could be disabled in some Clinical Vaults with Country Intelligence. Now, EDL Overrides will be enabled in all Clinical Vaults.
- Vault will follow EDL Override logic for Country Intelligence and EDL Automation if any EDL Templates within one Template Hierarchy (existing under the Study EDL Template) use overrides, i.e. the Country Override field is set to ‘yes.’
Recurring Milestone SchedulesConfiguration23R3.4
Existing Milestones functionality effectively manages one-time activities; however, today there is no easy option in Vault to create Milestones for processes that must be planned and executed at regular, repeating intervals during certain periods of a Study. A common example use case is the execution of Document QC every “x” days during a long-running trial. The Recurring Milestones Schedules feature bridges this gap.
This configurable feature introduces two new objects: Recurring Milestone Schedules and Recurring Milestone Schedule Templates. These provide the ability to manage creation of Milestones at regular, date-based intervals.
Users can define Events (for example, completion of Study one-time Milestones) to activate and inactivate these Schedules. While a Schedule is active, a daily job will create Milestones for a specified Study, Study Country, or Site on the Schedule’s Next Create Date.
For example, imagine you have a Study where Document QC will be tracked at Study level but not until the Protocol Approved Milestone is Complete. You can set up a Recurring Milestone Schedule for Document QC where Protocol Approved Milestone is the Activation Event.
When the Protocol Approved Milestone is completed, a system action will activate your Recurring Milestone Schedule and set the Next Create Date when the next Document QC Milestone should be created.
Recurring Milestones allow Study teams to plan and manage these repeated processes.
Disable Related Document Requiredness on Quality IssuesAdmin Checkbox23R3.4
In some cases, users may need to “Hard Delete” a document in Vault due to user errors (such as uploading sensitive content or PHI to the TMF). This feature increases flexibility for how a user can log Quality Issues (QIs) by allowing the Related Document field to be optional, rather than required. Previously, to delete a document, a user would have to redirect a QI to a document like the TMF Plan. With the feature flag enabled, a user no longer needs to repoint the Related Document field before hard deleting the document, streamlining the document deletion process if a Quality Issues exists on a document.
This feature is enabled by a one way feature flag that cannot be reverted. Once the flag is enabled, all Quality Issues will be re-indexed, so it is recommended to enable this feature after working hours.
Site Connect
Send Investigator Brochure with Safety DistributionAuto-on23R3.5
With this auto-on enhancement Site Connect customers can utilize the Site Connect’s Safety Distribution functionality to also send Investigator Brochures to their Study Sites. Investigator Brochures can be associated with a Safety Distribution as the main Safety Document or added as a supporting document.
Mark Document Recalled TrackingAuto-on23R3.4
With this auto-on enhancement, Site Connect customers can now include a comment when marking documents as Recalled. Users in Clinical Operations Vaults will also be able to track and report on which Study Sites they have marked a document as recalled.
Prior to this feature, Sponsors/CROs were unable to report on which Study Sites they had recalled a document from after taking the action.
Note: A new Recalled by Sponsor/CRO field is added to the Distribution Task object as a checkbox. This will set a value of FALSE on all existing Distribution Task records, update the Last Modified Date field, and an event will not be recorded in the record audit trail for this update.
Additional Vault Clinical Docs SupportConfiguration23R3.4
Site Connect customers can now exchange the following document types with Sites:
- IP Treatment Decoding Documentation
- IP Re-labeling Documentation
- Recruitment Plan
- System Account Management
- Safety Report Supporting Document
The configuration for any relevant document types must be updated so that they map to the new Vault Clinical Docs artifacts.
Site Connect Data Model Update on Distribution TaskAuto-on23R3.5
With this release we are introducing the following new fields to the Distribution Task object:
- Last Downloaded Date (
last_downloaded_date__v) - SiteVault Document Type (
sitevault_document_type__v) - Last Filed to SiteVault Date (
last_filed_to_sitevault_date__v) - SiteVault Document Number (
sitevault_document_number__v) - Recalled by Sponsor/CRO (
recalled_by_sponsorcro__v) - Reason for Recall (
reason_for_recall__v)
This auto-on feature does not provide new functionality to end users, but is instead preparation for the larger changes coming in 24R2 (which will bring the Site experience of Site Connect into the Clinical Operations Vault).
All Clinical Operations Applications
Complex Trials: Tracking Country & Site ParticipationConfiguration23R3.4
In recent years, clinical study designs have become increasingly complex, building in flexibility to test multiple treatments, different dosages, and many patient populations under a single master protocol using a common core of sites, investigators, and personnel. While these designs offer efficiency in operational time and cost, they also introduce the need for more complex up-front planning processes. This feature adds support for managing more complex clinical studies in Clinical Operations Vaults.
Key elements include:
- The existing Study Arm object now includes object types for Study Arm, Cohort, Study Part, Substudy, and Study Element
- A new object reference field for all of the Study Arm object types on Subject, Monitored Subject, and Issues objects
- New join objects between Study Arm and Study Country, Study Site, and Study Product are added to track the utilization of these Study design elements
- Study Country Subject Group
- Study Site Subject Group
- Study Product Subject Group
- A new action, Seed Subject Group Records, that will create the needed records for tracking Study Counties, Study Sites, and Study Products
The Seed Subject Group Records action creates a record for each active Study Country, Study Site, and Study Product on the Study at the time the action is taken. Once taken, if new Study Country, Study Site, or Study Product records are added, Vault automatically creates the corresponding Study Group join records. The join records have standard lifecycle states that match the standard lifecycle states of the related record (Study Country, Study Site, Study Product).
With these updates, customers can now define Study Subject groupings in Clinical Operations Vaults and track the products being investigated as well as the countries and sites participating in each group.
Clinical Operations License Tracking by ApplicationAuto-on23R3.4
This new auto-on feature will extend the existing eTMF license tracking in Clinical Operations Vaults to include license tracking for additional Clinical Operations applications: CTMS, Payments, and SSU.
License consumption across applications is visible to admins in Vault Settings and within a new object (Study Site License) that tracks Clinical Operations licenses based on Vault data. Customers will see a warning message banner when consumption is over the licensed amount in Production Vaults.
This new feature will ignore over consumption count warnings on Sandbox environments, but the tracking will be visible.
Additionally, to ensure data cleanliness, this feature prevents Study Countries from moving to a Lifecycle State with a Status of Inactive if the Study Country’s Study Site records have a Status of Active, In Migration, or Archived. All Study Sites must have a Lifecycle State with a Status of Inactive before a Study Country can be moved to a Lifecycle State with a Status of Inactive.
Note: For any questions about your contracted organization’s licenses, any changes to your licensing needs, or any discrepancy about licensing, please reach out to your Veeva Account Partner.
Re-trigger EDL AutomationConfiguration23R3.4
At times (for example, when new EDL template items are added), customers may need to recreate Expected Documents for existing Study Person, Study Product, and Study Organization records. Previously, this was only possible through entry actions on lifecycle state changes. This feature introduces a new record action that triggers the EDL Automation Creation job without requiring a state change. This action can be executed on individual records and in bulk.
Email to Vault: Subaddress Case InsensitiveAuto-on23R3.4
With this feature, the Clinical Email Processor will be case insensitive when parsing the subaddress from an inbound email and setting the Study field. Any subaddress used for creating a document from an email will be processed as lowercase letters. When a Study is created, the Email Subaddress field will be copied as all lowercase letters from the Study Number. Additionally, any edits made to the Study after the release will result in an update to the Email Subaddress field to ensure lowercase letters.
Cycle Time: Omission of Study Part MilestonesAuto-on23R3.4
To support Master Protocols, Vault will now omit Cycle Time calculations for Study Arm related milestones. As a part of the data model for Master Protocols, milestones will allow for enrollment tracking by Study Subject groupings. Milestones that include a value for Study Arm (arm__v), will be excluded from the nightly Cycle Time job and will not skew cycle time metrics.
Clinical Operations Data Model Changes
See 24R1 Clinical Operations Data Model Changes.
Commercial
Several features listed in the Vault Connections section also affect the Commercial application family.
PromoMats
Improved OCR for ImagesAuto-on23R3.5
Vault’s Optical Character Recognition (OCR) functionality has been improved to better detect text from images, resulting in more recognized text that can be used with other Vault features. This includes search, annotations, Auto-Linking, and more.
Prior to 24R1, Vault’s OCR capabilities have been primarily optimized for scanned PDFs, which has impacted applications such as PromoMats where image files are heavily utilized. Improving OCR for these image files will ensure a more consistent experience for users in leveraging features that rely upon recognized text.
Learn more about Optical Character Recognition.
Commercial Action LayoutsConfiguration23R3.5
Standard commercial action layouts will be available in PromoMats for you to copy and use as a base for custom action layouts. Objects and object types have many fields available, however not all of them are relevant during specific lifecycle states or for specific users. Action Layouts allow you to increase efficiency by tailoring the object record input screens for specific lifecycle states and/or user security profiles.
Automated Image Tagging for Audits: Behavior UpdatesAuto-on23R3.5
With this release, we have added additional details to the document audit trail for Automated Image Tagging to support user troubleshooting when no tags are returned.
PromoMats Text Asset Object: Remove Custom Field LimitAuto-on23R3.2
Text Asset Objects can now have the maximum number of custom fields standard for objects.
Auto-On Auto-Linking: Match Text Variations MaximumAuto-on23R3.2
In 24R1 as part of Auto-Linking all PromoMats Vaults will be allotted the maximum number of match text variations, which is nine (9).
Automated Image TaggingConfiguration23R3.4
Vault PromoMats now supports automated tagging on uploaded image content. This functionality populates document metadata fields with predefined tags set by an Admin. Automated Image Tagging is critical in supporting process efficiencies and standardizing tagging, making it easier for end-users to find digital assets through improved searchability.
Automated Image Tagging leverages the viewable rendition created by Vault, supporting the following file formats:
- Image Formats: .eps, .ai, .indd, .psd, .avif, .bmp, .jpg, .jpeg, .png, .svg, .tif, .tiff, .webP, .heic
- Creative Cloud: .ai, .indd, .psd, and InDesign packages (zip)
- PowerPoint Presentations: .ppt, .pptx
- Other: .pdf
Automated Image Tagging does not support audio or video files.
This feature leverages the AWS Rekognition Service allowing customers to benefit from Amazon’s powerful Rekognition engine to improve tagging consistency.
Automated Image Tagging is included with the PromoMats user license with up to 250,000 tagging requests per Vault per month.
Modular Content CombinationsAuto-on23R3.4
Vault now allows users to create Combinations for Content Modules. Combinations allow users to group assets within a Content Module for specific channels, messaging, or personas.
When Content Module Combinations are used within CRM Email modules, the combination behaves as a business rule for allowed assets for the CRM email builder.
Claims Linking: Auto-On Auto-LinkingAuto-on23R3.4
To offer a more targeted experience for Claims Linking, Auto-Linking is now the default for all PromoMats Vaults. When users click the Suggest Link icon, the new Auto-Linking functionality runs in the background to provide a better matching experience with increased flexibility and suggestions.
Commercial Application Settings OptimizationAuto-on23R3.4
The PromoMats Application Settings has been updated to remove flags that are no longer necessary, and align setting labels with feature names. Flags required at the launch of a feature may no longer be relevant or are now manageable in configuration.
Auto-On Multichannel Features: Enable Engage IntegrationAuto-on23R3.4
Engage Portals features are automatically enabled for use in Commercial Vaults that are licensed for Multichannel App.
Auto-On Multichannel Features: Enable Approved EmailAuto-on23R3.4
Approved Email features are automatically enabled for use in Commercial Vaults that are licensed for Multichannel App. This includes the Auto-Publishing for Email Fragments feature.
eCTD: Annotate Submission Ready ReferencesAdmin Checkbox23R3.4
In this release, annotated references and annotated labeling documents that are pulled into eCTD compliance packages can have relevant substantiating content highlighted. Anchors that are relevant to included PDF links are outlined in a red box on submission ready copies.
Claims Linking: Lightbulb Icon for Auto-LinkingAuto-on23R3.4
The lightbulb icon appears for all document states if the corresponding lifecycle has no Suggest Links user actions added to any state. If any state has a User Action configured for Suggest Links, then only the applicable states will have the lightbulb icon appear. Configuration is not required; the lightbulb icon is available for document types based on the enablement action found under Document Type Details.
Claims Linking: Language Filter Behavior ChangeAuto-on23R3.4
With this release, Auto-Linking filtering is altered for the Language field. If Language is available on a document when Auto-Linking is performed, the system attempts to match to all Text Assets matched with the document’s Language value but also match to all Text Assets that have no Language value set.
Text Assets: Functionality ChangesAuto-on23R3.4
Due to Auto-Linking being enabled for all PromoMats vaults, the 500 record creation limit will be removed, Text Assets can now have more than 9 custom fields, and Match Text Variations will have a maximum of 9.
Text Assets: Duplicate Record ChangeAuto-on23R3.4
Multiple text assets can now exist with the same field values and match text values. However, only one Text Asset with the same field and match text values can be in the Approved state to be identified when using Auto-Linking or manual text asset linking.
Portals: Layouts Available in the Portal LibraryAuto-on23R3.4
Portal end-users will now have additional options when viewing content in the Portal Library. The standard layouts in the Document Library will now be available in the Portal Library. This provides end-users with a consistent user experience in the portal as they are accustomed to in their day-to-day use of Vault.
The layouts that are now available include Compact, Thumbnail, Detail and Grid. The Grid view will reflect the existing layout that has been used in the Portal Library prior to 24R1.
The Compact layout will be the default view for viewing content in the Portal Library.
Allow Multi-Select for Standard Audience FieldConfiguration23R3.4
Administrators now have the ability to make the professional_consumer__v document field multi-select. For promotional materials with both consumer and professional selected, the eCTD bulk generate feature will generate a consumer compliance package and will add a note to the comments field of the 2253: “This submission contains materials for both professional and consumer audiences.”
Best Effort Mapping for Standard Metrics DurationsAuto-on23R3.4
PromoMats will perform best effort mapping for Vaults that do not have any states configured on the Standard Metrics State Mappings configuration page. If a Vault does have at least one state already mapped, PromoMats will make no changes to the mapping configuration. The best effort mapping that will be performed can be found here.
Vault Digital Publishing: AWS Bucket Policy SupportConfiguration23R3.4
Vault Digital Publishing now supports the use of bucket policies for configuring the integration between Vault and the customer’s Amazon Web Services S3 Bucket.
eCTD: Improved Error MessagingAuto-on23R3.4
We have made improvements to error handling for eCTD compliance packages that make it faster to support actionable error messages. We also introduced an error message informing users of corrupt submission package renditions when trying to generate eCTD compliance packages. Please note that new notification templates will be used for eCTD compliance package generation after release night.
eCTD: Create as SystemAuto-on23R3.4
eCTD compliance packages and the documents created within are now generated by the System. Previously, the documents displayed as being generated by the user. This enhancement simplifies the permission requirements for users creating eCTD compliance packages; now, the user only requires Create Binder permissions on the Submission Ready Compliance Package document type and view permissions on the Center object and Promotional Material Document Type object.
eCTD: Submission Package EnhancementsAuto-on23R3.2
eCTD Compliance Packages now support submission packages created with the following compression methods: Deflate64, Deflate, and Stored. In addition, documents created from submission package contents are renamed using the same naming convention as other submission ready copies in the binder. Previously, documents created from the submission package were not renamed.
eCTD: Update Correspondence Letter on RegenerateAuto-on23R3.2
For Pre Clearance eCTD compliance packages, the correspondence letter is now automatically regenerated on package regeneration.
Auto-On Multichannel Features: Enable CLM Integration with Auto-PublishingAuto-on23R3.2
The CLM features are automatically enabled for use in Commercial Vaults licensed for the Multichannel App.
Set State for Previous Email Fragment VersionsConfiguration23R3.2
Admins can configure a new entry action to automatically set email fragments to a selected state, even when they are not the latest version of the email fragment.
Commercial Data Model Changes
See 24R1 Commercial Data Model Changes.
Medical
Several features listed in the Vault Connections section also affect the Medical application family.
MedComms, Multichannel, PromoMats
Auto-On Multichannel Features: Enable CLM Integration with Auto-PublishingAuto-on23R3.2
The CLM features are automatically enabled for use in Medical Vaults that are licensed for Multichannel App
Set State for Previous Email Fragment VersionsConfiguration23R3.2
Admins can configure a new entry action to automatically set email fragments to a selected state, even when they are not the latest version of the email fragment.
Auto-On Multichannel Features: Enable Approved EmailAuto-on23R3.4
Approved Email features are automatically enabled for use in Medical Vaults that are licensed for Multichannel App. This includes the Auto-Publishing for Email Fragments feature.
Multichannel Performance ImprovementsAuto-on23R3.4
This feature delivers performance improvements to increase the speed of distributing Multichannel content.
MedComms
Auto-On Multichannel Features: Enable Engage IntegrationAuto-on23R3.4
Engage Portals features are automatically enabled for use in Medical Vaults that are licensed for Multichannel App
Portals: Layouts Available in the Portal LibraryAuto-on23R3.4
Portal end-users will now have additional options when viewing content in the Portal Library. The standard layouts in the Document Library will now be available in the Portal Library. This provides end-users with a consistent user experience in the portal as they are accustomed to in their day-to-day use of Vault.
The layouts that are now available include Compact, Thumbnail, Detail and Grid. The Grid view will reflect the existing layout that has been used in the Portal Library prior to 24R1.
The Compact layout will be the default view for viewing content in the Portal Library.
Vault Digital Publishing: AWS Bucket Policy SupportConfiguration23R3.4
Vault Digital Publishing now supports the use of bucket policies for configuring the integration between Vault and the customer’s Amazon Web Services S3 Bucket.
Set State for Previous Email Fragment VersionsConfiguration23R3.2
Admins can configure a new entry action to automatically set email fragments to a selected state, even when they are not the latest version of the email fragment.
MedInquiry
Standard Case Owner & Enhanced Escalation for MedInquiryConfiguration23R3.2
This feature adds a new Owner field to the Case, Case Request and Event objects that can be kept in sync with the owner of the record. The Owner field defaults to the current users in the Medical Inquiry UI.
Using Workflow Actions, the value of the Owner field can be filled with the user that completes a Task that has been assigned to them.
This feature enables users to build a view to see the Cases they have been assigned to work on.
Automatically Add Email Attachments onto MedInquiry Object RecordsConfiguration23R3.4
Currently, upon ingestion of an email with an attachment, the user must download the attached files to their local machine and re-upload as object record attachments. This configurable feature enables automatic transfer of attachments from Ingested Emails to the attachment section of Case Request, Case Response or Event object records.
Administrators can add the Pull Email Attachments onto Record action to either an Entry Action on Case Request and Case Response or a User Action on Event, Case Request and Case Response.
Allow Case Responses to be Created AutomaticallyConfiguration23R3.4
This feature adds an Action to the Case and Case Request object lifecycle to allow a Case Response to be automatically created.
Create Related Case Response action enables automatic creation of a record that can be used as a Case Acknowledgement Email. The record will be created automatically and sent to the HCP via an Entry Action.
Allow Case Response Emails to be Sent AutomaticallyConfiguration23R3.4
Send Response Package action can now be configured as an Entry Action as well as a User Action. This feature allows Case Responses to be automatically sent to the HCP when the record enters a certain lifecycle state.
When configured, it could be used to address requirements for automatically issued Case Acknowledgement, Case Status Update and Case Closed emails.
Learn more about Responding to a Case.
Automatic Case Contact Creation for Email IngestionAdmin Checkbox23R3.4
With this feature, a new Case Contact will be created when an email is ingested and no email address match exists among Case Contact details. This automation ensures Case Contact field is never left blank upon the initial inquiry ingestion.
Today, when an email is ingested where no Case Contact with that email address exists, the Case Contact field on the Case is left blank, so the user is required to create a Case Contact manually before a Case Response can be sent in reply to the original inquiry.
Learn more about Creating a New Case Contact.
MedInquiry OpenData Search: Pull HCP Country & Show Only Local HCPsAdmin Checkbox23R3.4
With this feature, a Case Contact that is created via the MedInquiry OpenData Connection will have its Country automatically populated.
Additionally, admin configuration can constrain OpenData search to only show HCP records for the country in which the MedInquiry user operates. User’s operating country is defined by the Locale field on their user record.
Learn more about Adding Case Contacts with OpenData.
Support for Criteria VQL on OpenData Search Case Contact fieldAuto-on23R3.4
The OpenData Case Contact field will now support Criteria VQL. This functionality enables handling of criteria dependencies, for example, Country and Contact Source.
CRM Data Sharing: Pull Custom Response DetailsConfiguration23R3.4
The CRM Data Sharing feature in MedInquiry has been modified to allow the Case Response to be sent to the email address specified in the Veeva CRM Medical Inquiry record.
The CRM Data Sharing feature previously only supported sending the email response to the email address defined in Vault’s Case Contact record, which is usually synced from Veeva CRM’s Account record.
If an HCP is out of the office, for example, and wishes that the Case Response be sent to an email address different from the one on their Account record, CRM users are able to specify the new email address in the CRM Medical Inquiry record. Should the email field in the CRM Medical Inquiry record be mapped to the Case email address field in MedInquiry, the Case Response uses the specified email address as the response recipient. .
MedComms, MedInquiry
Medical Provision Action LayoutsConfiguration23R3.5
Standard medical action layouts will be available for you to copy and use as a base for custom action layouts. Objects and object types have many fields available, however not all of them are relevant during specific lifecycle states or for specific users. Action Layouts allow you to increase efficiency by tailoring the object record input screens for specific lifecycle states and/or user security profiles.
MedComms, PromoMats
Modular Content: CRM Email & Combinations Data Model in Vault MedcommsConfiguration23R3.4
MedComms now supports Modular Content. The use case supported is authoring CRM Email Modules for use with the CRM Email Builder.
Medical Data Model Changes
See 24R1 Medical Data Model Changes.
Training
Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
LearnGxP ContentDirectConfiguration23R3.5
This feature introduces seamless automation to the delivery of courses and updates for customers subscribed to LearnGxP’s course library. As courses are updated by the LearnGxP team, changes are automatically reflected in a customer Vault, eliminating the need for manual intervention and saving valuable time.\
Quiz Support for Mobile Web & Mobile AppAuto-on23R3.5
With this update, quizzes have a mobile-friendly display for the web and application. Previously, users completing a quiz on mobile would see the desktop version. Now, the user interface is optimized for a mobile device.
Standard Action Layouts for Training ObjectsConfiguration23R3.5
Standard Action Layouts are now provided for the Learner Role, Curriculum, and Training Requirement objects. These layouts contain best practices for organizing data into sections and fields for these training matrix objects. Admins can copy these templates and customize for their own use.
Study Training
Study Training: Training Matrix AutomationAuto-on23R3.5
The Training Matrix Builder Automation is a new version of the Training Matrix Builder. Vault now auto-creates records related to Training Materials, Learner Roles, and Studies using clinical data and the Training Matrix Builder. Automation creates Curricula based on the Training Requirements assigned to Learner Roles. Overall this feature will reduce by 70% the number of clicks it takes to create a Training Matrix for a study.
Auto-Create Security Records for Study Country & SiteConfiguration23R3.4
This feature builds upon existing automation by extending the creation of User Role Setup records to include Study Country and Study Site. By automating this process, the feature eliminates the need for manual creation of User Role Setup records for each user and study. This not only reduces the administrative burden on IT, but also mitigates the risk of user error or unauthorized document access in the Clinical Operations Vault.
Quality
A feature listed in the Vault Connections section also affects the Quality application family.
QualityDocs
Enable Launching Document Workflows from the Document Change ControlConfiguration23R3.2
This feature allows users to start workflows on documents directly from the Document Change Control (DCC) record via new Documents to be Made Effective and Documents to be Made Obsolete sections. Users will also be able to view the status of the workflows and click on workflow envelope links from the DCC. This feature is not compatible with Legacy Workflows.
Document Change Control: Document Association Governance LimitAuto-on23R3.2
A governance limit now restricts the number of documents that can be added to a single Document Change Control (DCC) to a maximum total of 100 across all three sections: Change Authorization, Documents to be made Effective and Documents to be Made Obsolete. The limit applies to all Document Change Control records created after the 24R1 release. Any Document Change Control records created before the release with 100 or more associated documents can continue to be processed and are not impacted.
Station Manager
Document Page Thumbnail View in Android Station ManagerAuto-on23R3.4
Users of the Station Manager Android application now have the option to view all pages of a document in a thumbnail viewer. This allows the user to much more quickly and easily navigate to the desired pages in a document. This feature was released for users of the Station Manager iOS application in the 23R3 release.
Training
Add to Calendar Link: Training Assignment Link in Calendar InviteAuto-on23R3.2
Learners who add classes to their Google or Outlook calendar using the Add to Calendar link can now access the related Classroom Training Assignment directly from the meeting invitation.
Curriculum Page: User Interface UpdatesAuto-on23R3.2
The Curriculum page contains several user interface updates for usability, including Learners’ ability to unenroll from self-enrolled Training Assignments from the Self-Enrollment Curriculum page, Card view (Open tab), or Explore tab.
Users can now begin training by clicking a hyperlink to the Training Assignment, instead of clicking the Begin Training button, which has been removed. Additionally, a new Curriculum page breadcrumb and All Open icon allow for easier navigation.
Curriculum PrerequisitesConfiguration23R3.2
This feature allows Training Admins to create Prerequisite Training Rules among curricula indicating which Curricula must be completed before gaining access to another given Curriculum. It also introduces the ability to offset due dates so that locked curricula do not become overdue while the prerequisite is incomplete. After a prerequisite Curriculum is complete, Vault sets the Due Date on the now-unlocked Curriculum’s Training Assignments. This feature was initially released in 23R3.2, and is enhanced in 23R3.4 to allow a prerequisite rules to be defined for Curricula containing substitute Training Requirements. Enhancements also include support for migrating prerequisite rules using Vault Loader or the API.
Oftentimes, skills build on one another and should be completed in a particular order, also known as mastery- or competency-based training. For example, Learners might need to take Safety 101 before moving onto a more demanding process training. Curriculum Prerequisites enforce a given learning approach and decrease manual assignment time.
Curriculum SmartMatch Renamed to Curriculum MatchingAuto-on23R3.2
In this release, the feature previously known as Curriculum SmartMatch is relabeled to Curriculum Matching in the Vault UI, including Quality application configurations, object fields, and application controls.
See Configuring Curriculum Matching for more information on assigning subsets of Curricula to Learner Roles based on Curriculum Matching Rules.
Document Training Assignments: Show Additional Document Toolbar OptionsAuto-on23R3.2
To maintain a consistent user experience when viewing a document from a Training Assignment or from the Library, the icons for Full Screen, View Annotations, and View Options are all now available from Vault Document Training Assignments.
Learner Homepage: User Interface UpdatesAuto-on23R3.2
The Begin Training button label on the Learner Homepage’s Card View now displays as View Training in the following cases:
- When an Evaluation Training Assignment is in the In Evaluation or In Evaluation Preparation state
- When an External Training Assignment is in the In Verification state
Additionally, for Classroom Training Assignments where a Learner is not added to a roster and self-registration is not allowed, Classroom Training Assignments were previously hyperlinked in List View, and in the Learner Role and Curriculum pages. These types of Classroom Training Assignments are visible but are no longer hyperlinked.
Training Assignment State Change: Ignore State Of Evaluation Checklist DesignAuto-on23R3.2
When a Training Assignment state is updated (for example, Canceled), this feature allows Vault to ignore the related Evaluation Checklist Design record’s lifecycle state. Previously, Training Assignment records could not change state when the Evaluation Checklist Design record was in draft.
Training Requirement Prerequisites: User Interface UpdatesAuto-on23R3.2
The user experience for navigating and completing Training Assignments with prerequisites is improved in the following ways:
- Learner Homepage’s Curriculum and Learner Role views: When a Training Assignment has a prerequisite Training Requirement, Vault displays the Due Date in a color according to its status, rather than gray. Additionally, the blue hyperlink to locked Training Assignments is disabled.
- Learner Role Page: When a Training Assignment has an incomplete prerequisite Training Requirement, Vault displays it with a lock icon. Additionally, the blue hyperlink to locked Training Assignments is disabled.
- Learner Homepage’s List View: When a Training Assignment has an incomplete prerequisite Training Requirement, the blue hyperlink to locked Training Assignments is disabled.
- All Training Assignments locked as the result of a prerequisite Training Requirement or Curriculum display in black text. Additionally, when users hover over these assignments, the text is updated to “This course is locked due to incomplete prerequisites”.
In lists of Training Assignments with prerequisites, Vault displays them in completion order, with the last assignment to be completed at the bottom of the list. Previously, Vault displayed the current assignment as last in the list.
My Team Page (Manager Homepage)Configuration23R3.4
Managers now have immediate access to real-time insights into the state of their direct team’s training progress. With a quick glance, managers can evaluate both individual and overall team performance without relying on a central Training Admin team. This enhancement will not only help scale organizations, it also addresses the crucial role Managers play in their team’s training by allowing them to actively track training status and make recommendations or assignments without Training Admin assistance or intervention.
Curriculum Completion Outcomes: Grant Additional PermissionsConfiguration23R3.4
This feature introduces the capability for Admins to create rules that automatically grant additional permissions to a Learner upon the completion of a Curriculum. Often, access to systems and business processes (for example, access to QualityDocs for approving documents) is based on training completion. In this release, Vault Training can automatically create a User Role record for a Learner upon completing a certain Curriculum.
Supplemental MaterialsConfiguration23R3.4
Supplemental Materials are optional reference training materials that can be added to a Training Requirement to complement the required materials. For example, training materials such as job aids, job manuals, forms, and work instructions can be supplemental to required procedures and policies. Updates to Supplemental Materials do not require retraining or Training Requirement Impact Assessment.
Training Requirement Impact Assessment: Copy Training Impact from DocumentConfiguration23R3.4
This feature automatically copies the Training Impact field value from the document to the Training Requirement Impact Assessment (TRIA) record. This allows for TRIA records to be automatically closed based on business processes when Training Impact = No.
Assign, Pause, or Cancel Training Assignments Using Learner Role-Person LifecycleConfiguration23R3.4
With Learner Role-Person lifecycle and other configurations, Vault Training can now use a Learner Role-Person record’s lifecycle state to determine whether to assign, pause, or cancel Training Assignments. For example, a New Hire Start Date field can trigger lifecycle actions to stagger Learner Role assignment, or a Clinical Study’s Start Date and End Date can determine when assignments should begin and end.
Instructor-Led Training: Improved Error Message when Adding InstructorAuto-on23R3.4
When an Instructor with an inactive user account is added to a Class, the error message is clear and actionable.
Instructor-Led Training: Auto-Cancel Workflow Tasks when Instructor is UpdatedAuto-on23R3.4
This feature automatically cancels a workflow task for a previous Instructor in the event the instructor has been updated. Before this feature, these workflow tasks were required to be manually cancelled.
Evaluation Training Assignment: Create Checklists for Learners without a Vault UserAuto-on23R3.4
Evaluation Checklists can now be created for Training Assignments for Learners who are not Vault users, as indicated by the Person is not a Vault user field on the Learner’s Person record.
Auto-Manage Persons: Exclude System Users from Auto-CreationAuto-on23R3.4
System user accounts, such as the Java SDK Service Account and Application Owner, will be excluded from being processed by the Auto-Manage Persons feature.
Training Matrix & Other Limits UpdatedAuto-on23R3.4
In order to maintain Vault’s performance, most training matrix objects within the Vault Training data model have historically been subject to default limits, with the option to request select limit increases from Veeva Support. Based on customer feature adoption, usage, and feedback, this feature raises the limits on most training matrix objects and relationships across all Training Vaults, and any future increases to these limits will be through Vault releases. For example, the maximum number of Prerequisite Training Rules which could be defined for Training Requirements within a single Curriculum record was 25. In 24R1, this limit is removed, and Training Admins can define prerequisite rules for any number of Training Requirements in a given Curriculum.
Data Model: New Fields & ObjectsAuto-on23R3.4
Updates to the Training data model are included in this release to standardize commonly-used fields and harmonize objects and their limitations with the Platform data model. These new objects, object types, fields, and picklists allow Vault Training and Study Training customers to be consistent with Veeva best practice. See 24R1 Quality Data Model Changes for details.
QMS
Standard Action Layout on Deviation ObjectConfiguration23R3.5
This release introduced the Vault Action Layouts functionality, a huge suite of features all targeted at empowering Vault customers with creating robust, dynamic and flexible user interface experiences for working with Vault records. To enable Vault QMS Administrators eager to learn about these powerful features, your QMS Vault now has two new, standard action layouts on the Deviation object: GMP Deviation Detail Page (Full View) and GMP Deviation State Layout. These action layouts are deployed in an inactive status so as not to interfere with your configuration or end users. These action layouts have been preconfigured to act as guiding best practices and examples of how Admins can be sure that their Vault record-based solutions present only relevant metadata to users at the right times with Layout Rules, Layout Expressions and Display Effects. These example layouts are just the beginning of what you can accomplish with Action Layouts in Vault QMS.
To get started, Admins may make a copy of these inactive Action Layouts, and use that copy to explore and see some of the possibilities available with Action Layouts right in the Vault itself. These layouts are provisioned to all vaults, but require configuration before being visible to any audiences other than configuration Admins.
QRM: Risk Builder: Columns to DisplayConfiguration23R3.4
This feature enables greater flexibility in the way organizations choose to display Assessment Risk columns in Risk Builder.
System administrators now have the ability to configure Risk Builder’s default columns, and the column order. The Assessment Risk control’s new dialog window includes a field picker at the bottom of the screen to specify which Assessment Risk fields display by default for the selected Risk Assessment object type.
System Administrators can also configure whether the Assessment Risk section allows users to modify the columns displayed using the Allow users to override columns checkbox. When this option is checked, users have access to the Edit Columns menu, allowing them to change the columns and column order they see in Risk Builder.
Learn more about configuring the Assessment Risk control that displays Assessment Risks in Risk Builder.
QRM: Risk Builder EnhancementsAuto-on23R3.4
This release introduces several new Risk Builder enhancements based on customer feedback.
Manage Assessment Risk Mitigations
Users can now view, assign, and remove records that mitigate Assessment Risks directly within Risk Builder. Records that can be used to mitigate risks include:
- Mitigation Actions
- Quality Events
- CAPA Actions
- Change Controls
- Continuous Improvements
- MedTech CAPAs
The screenshot below shows an Assessment Risk with multiple records assigned to mitigate the risk. Clicking X next to an assigned risk mitigation record removes it from the Assessment Risk. Clicking the plus (+) allows a user to assign one or more existing records, or create a new record, to mitigate the risk.
Delete Assessment Risk Deletes Associated Quality Team
As of 23R3, users cannot delete an Assessment Risk record if it has a Quality Team with assigned team members. Beginning with this release, Vault will allow users to delete an Assessment Risk record from Risk Builder, even if it has a defined Quality Team.
Additional UI Enhancements
- Vault now automatically wraps the labels for the Severity/Occurrence and Detectability axes in a Risk Assessment’s Heatmap to prevent the labels from becoming unreadable.
- The label for the Criticality axis in a Risk Assessment’s Heatmap will use the name defined in the label field for the Criticality object.
QRM: Periodic Risk ReviewConfiguration23R3.4
Organizations use the Vault QMS application’s Quality Risk Management capabilities to proactively identify risks in their enterprise and operational processes. Risk Assessments are used to identify and characterize risks based on several common risk methodologies, such as a Process Failure Mode and Effects Analysis (pFMEA). Each Risk Assessment contains risks, called Assessment Risks, that describe failure mode, effect, cause, control and several other characteristics of risk based on the assessment methodology. Assessment Risks are scored with pre- and post-assessment fields to capture Severity, probability of Occurrence, and Detectability scores based on a Risk Matrix.
As an organization’s business environment changes, so do the risks posed to its enterprise and operational processes. Companies need to periodically review their Risk Assessments to ensure they are addressing risk appropriately based on the current state.
When performing periodic reviews, organizations want to capture a snapshot of a Risk Assessment and its Assessment Risks. This enables companies to understand how risk changed over a period of time, providing insight into how they might continue to address risk. The current approach to capture historical information is through the use of the Vault Formatted Output feature, which stores snapshots of a Risk Assessment in PDF format as attachments on Risk Assessment record. Storing historical risk data in PDF attachments limits an organization’s ability to:
- See which mitigating quality processes (CAPAs, for example) were used to address risks at a specific point in time.
- Effectively see how a Risk Assessment’s risks have changed over a period of time.
The 24R1 release addresses these limitations by introducing new configurable capabilities that a system administrator can enable to allow organizations to store historical data about a Risk Assessment and its Assessment Risks (including risk scores and mitigation actions) in a structured format rather than a PDF. As a result, users can easily view previously approved Risk Assessments and their Assessment Risks at points in time, including:
- Risk Assessment Summary
- Risk Assessment attachments
- Risk Assessment approvals and E-signatures
- Assessment Risks
- Assessment Risk Scores
- Assessment Risk Mitigation Actions
Companies will benefit from easier access to historical risk information when they periodically review and revise Risk Assessments.
Beginning in 24R1, when a Risk Assessment is approved, Vault will automatically capture snapshot information.
Over time, as periodic reviews are conducted and Risk Assessments change, users can view its history from the Risk Assessment record and from Risk Builder.
In addition to historical risk data, this release introduces a new capability for the periodic review process. Users can already manually start an approved Risk Assessment’s periodic review workflow. Beginning in 24R1, Vault can also automatically start an approved Risk Assessment’s periodic review workflow based on its Next Review Date field.
QRM: Recompute Risk MatrixConfiguration23R3.2
Quality Risk Management (QRM) enables organizations to manage risks associated with enterprise and operational processes using Risk Assessments. A key component of QRM is the Risk Matrix, which defines key risk dimensions: Severity (for example, Minor, Moderate, Major), Occurrence (for example, Rare, Occasional, Likely), and Detectability (for example, Low, Medium, High).
A Risk Matrix is either quantitative or qualitative. Quantitative Risk Matrices assign numeric values to the risk dimensions, and automatically calculate Risk Scores for each possible combination of the risk dimensions. The calculated Risk Scores are the product of the risk dimensions. A quantitative Risk Matrix also allows you to define threshold Risk Levels so that Risk Scores can be categorized. Qualitative Risk Matrices do not calculate Risk Scores, but they do support manual assignment of Risk Levels.
Once a Risk Matrix is approved, it is used in Risk Assessments to assign Initial and Residual Risk Scores and Risk Levels. Customers using QRM prior to 22R2 may have Risk Assessments that do not populate Initial and Residual Risk Scores (standard fields) from the associated Risk Matrix. Additionally, Risk Assessments created before the 23R3 release do not populate Criticality Scores and Levels defined in the Risk Matrix. This is problematic when customers want to graphically visualize a Risk Assessment using the heatmap feature introduced in 23R3. The heatmap feature depends on Risk Assessments containing Assessment Risk records that use the Risk Level and Criticality Level fields. But updating a Risk Matrix and all of the Risk Assessments using that matrix is difficult. This feature eliminates the difficulty by providing a way for organizations to automate the steps.
Admins can now configure a user action on Risk Matrix lifecycle states to asynchronously update Risk and Criticality fields in a Risk Matrix, and all the Assessment Risk records within Risk Assessments where the Risk Matrix is used. The screenshot below shows what the user action automates for Risk Scores and Risk Levels, populating Assessment Risk records’ Risk Score and Risk Level fields based on the Risk Scores and Risk Levels defined in the applicable Risk Matrix.
Enhanced Checklists for Audit ExecutionConfiguration23R3.4
With this release, Vault QMS introduces enhancements to checklists specific to the support of executing an Audit. Users can now leverage the new Audit Checklist type of checklist to define reusable, structured questionnaires which allow the capture of detailed responses, attachments, and support direct filing of findings or observations. Responses to the questions and findings related to those responses will be automatically linked up to the Audit through the checklist or directly as findings.
This direct entry can eliminate or strongly mitigate any need for auditors to copy and paste the results of their audits into Vault QMS by supporting the audit itself as the checklist within Vault QMS, reducing or eliminating the need for Word-style documents in the audit’s execution.
Further, as auditors capture audit executions within these checklists, organizations can additionally benefit by analyzing or reporting on which audits, or even which specific questions or steps within their audits, are most effective at surfacing potential findings.
Users familiar with Vault’s checklist feature should find the experience straight forward, with simple, intuitive new actions added in place:
Users may start an Audit Checklist if an Audit Checklist is configured for the Audit Type, Method and Category of the audit record. A task can be created to ensure the user does not forget or get distracted from completing their checklist.
Audit Checklists are associated to the Audit directly, and available to viewers of the Audit or reporting users.
Findings may be filed directly from any question in the checklist with simple actions.
Filing of findings is performed within the checklist, and is dynamic based on your organization’s definition of a finding, associated metadata, and configuration. Multiple findings per question or step are supported.
Finally, all findings associated with each question or step within the checklist are saved and displayed both within the checklist interface itself and in Vault.
Use of this feature requires configuration of the Audit Checklist and update of the relevant page layouts for Audits and Findings to ensure the new entities are visible to your users.
Related Event App SectionConfiguration23R3.4
This release introduces a single-stop-shop for managing and viewing related quality event records, regardless of which types of Quality Events or standalone quality events (Deviations or Complaints, for example) those records are from. With this new app section, Vault QMS is now smart enough to show key information such as name, title, type, lifecycle state and record check results from related records in a concise, detailed format. This enhancement is especially valuable for any organization which has adopted, is in the process of adopting, or plans to adopt the standalone data model for quality events. For example:
Before this feature, with the basic Vault object record details page related record sections, the best possible layout for visualizing related records resulted in a sparsely filled table, requiring horizontal scrolling to view the complete data.
With this feature, the data is collected and condensed. Just a few columns show the critical data about related records regardless of which type of quality event those records are.
From a single section on a page layout, users with appropriate rights can now identify and manage which quality event records, regardless of data model, are related to the record they’re looking at.
The new section allows for creation, deletion or modification of linked records. The Related Events application section can be added to any page layout for the Quality Event object or standalone quality event objects, and will visualize and allow interaction with any records of those objects related to the current record, or vice versa. Users who link records via this application section need only create a single relationship record, and Vault will create (and manage, in the case of deletion) the inverse linking record, such that, after linking, regardless of which record a user is viewing, they will see the relationship in this section.
To support rendering in the Related Events grid and align with other Event types, the Change Plan object has a new standard field Title which has been added to the object.
This feature requires configuration, as an Admin must add the application section to desired page layouts before any changes will appear for users.
Enable Adverse Event Reportability Assessment in QMSConfiguration23R3.2
Vault QMS customers can now assess if a Complaint must be reported to health authorities by completing a Reportability Assessment, functionality that was previously only available to Vault Product Surveillance customers. Using the Reportability Assessment object connected to either Pharma Complaint or MedTech Complaint object types of the Complaint object, organizations can now set up questions to determine the Severity of a complaint and thus determine the reportability of the Adverse Event to health authorities. The severity and reportability are determined based on the answers in the Reportability Assessment provided by Complaint handling users.
This feature enables QMS customers to determine if an event is reportable and to which health authorities based on severity of the Complaint, Reporting Decision Rules, and where the Product is marketed. If your organization is interested in automating the generation of the adverse event documentation, or in tools supporting adverse event submission, you may wish to review Vault QMS’ companion product, the Vault Product Surveillance application.
Quality Incidents: Create a Quality Incident from a QR CodeConfiguration23R3.4
Organizations work hard to establish a culture of quality for their employees and contractors as they perform day-to-day tasks. This includes emphasizing the need to report incidents that could affect the quality of products or processes. This is especially important in areas that participate in manufacturing operations.
Vault QMS enables users to create Quality Incident records that capture information known at the time the incident is encountered, and includes a lightweight process that allows the appropriate individuals to triage the incident and determine if a full-fledged quality event process (for example, Deviation, Nonconformance, or others) is necessary. Ensuring employees have a quick and easy way to create Quality Incidents is imperative to ensure they are reported. Prior to this release, Quality Incidents could only be created through the standard Vault QMS user interface. This release introduces an easier, low-friction method of creating Quality Incidents using a mobile device.
System administrators can now configure QR codes to be printed and placed anywhere employees or contractors work. Vault QMS users can scan QR codes with their mobile device’s camera to fill out a streamlined page that collects required information about the incident they encountered. Individuals must have a Vault QMS license, and they will be prompted to log into a Vault if they don’t have an active session established. This feature utilizes a mobile device’s browser. Learn more about supported browsers.
This is an example of a QR code in a PDF file that was automatically generated and downloaded from Verteo Biopharma’s Vault. The company printed the PDF file and posted it in a prominent location within the manufacturing area at their Dublin facility:
After a QMS user scans the QR code with their mobile device and logs into the Vault, a screen like the following is displayed. The Organization, Facility, and Department values are defaulted from the QR code. The user enters values for the mandatory Date Occurred and Title fields, and optionally provides a Summary. In addition to filling out the Quality Incident fields, a user can add up to five (5) attachments (up to 20 MB per attachment). Quality Incident attachments would typically include pictures or short videos demonstrating the incident encountered.
After the Quality Incident information is provided, the user taps Submit. The screenshot below shows how the page refreshes to indicate the Quality Incident was created in Vault, and enables the user to create another Quality Incident if desired.
Now that the Quality Incident record exists in Vault, users participate in its triage process using the standard Vault QMS user interface. The screenshot below shows the Quality Incident record in Vault just after it was created. Learn more about using Quality Incidents
System administrators configure a Quality Incident Intake Layout to specify up to 10 Quality Incident fields that appear on the page, and the order in which they appear.
Then business administrators create Quality Incident Configuration Data records that represent QR codes associated with different areas of an organization’s operations. Each Quality Incident Configuration Data record references a Quality Incident Intake Layout configuration. A QR code can be associated with specific values for any combination of the following fields on a Quality Incident record:
- Country (not shown in the sample screenshots above)
- Organization
- Facility
- Department
In the example above, Verteo’s system administrator created a Quality Incident Intake Layout that includes the Organization, Facility, Department, Date Occurred, Title, and Summary fields. Verteo’s business administrator used the layout to create a QR code for use in Verteo’s manufacturing area at the Dublin facility by specifying default values for the following fields:
- Organization = Verteo Biopharma
- Facility = Dublin
- Department = Manufacturing
When a business administrator executes the user action to generate a QR code, Vault displays the QR code in a dialog. The dialog screen enables the business administrator to copy a static URL for the QR code. The static URL can be shared with users to access the same Quality Incident creation page via a desktop browser.
Learn more about configuring and managing Quality Incident QR Codes.
External Notifications Enhancements: Multiple Recipients & Add Document from LibraryConfiguration23R3.4
Organizations often use External Notifications to correspond with stakeholders outside their companies about product complaints through email. The correspondence often involves multiple people identified on a Complaint record, like the initial reporter, the complainant, and healthcare providers.
Prior to this release, users were required to initiate one External Notification for each Person identified on a record. With the 24R1 release, Admins can configure an External Notification that allows users to send one email to multiple Persons on a record. For example, the screenshot below shows an External Notification configuration that enables a Vault user to send an acknowledgement notification that a complaint was received to individuals identified on the Complaint record’s Complainant and Reporter fields.
The external notification configuration above means that a user working with a Complaint record sees the following screen when sending the external notification, allowing the sender to select multiple external individuals.
Correspondence with external stakeholders often requires attaching multiple documents that are created, reviewed and approved in Vault. Prior to this release, an External Notification could only be configured to include documents identified in fields on a record. With 24R1, system administrators can enable users to send documents that reference the record via a field on the document.
For example, consider an approved correspondence document written for a specific Complaint record identified in a document field:
If enabled by a system administrator, users sending an external notification can include documents that reference a record. For example, the document above can be included in an external notification for the Complaint record:
This enhancement enables system administrators to consolidate the number of External Notification configurations required to handle the various combinations of required Recipients and document attachments. As a result, organizations can provide users with fewer External Notification menu options that offer greater flexibility to handle all of their correspondence requirements.
Learn more about setting up External Notifications.
5 Whys Analysis: Support for Dynamic Reference ConstraintsAuto-on23R3.4
In 23R2, enhancements were made to support static reference constraints for 5 Whys Analysis.
In this release, we are further improving the 5 Whys Analysis tool to support dynamic reference constraints which allows users to filter object reference fields based on the selection of another field on the Root Cause object.
The tool has also been enhanced and now supports rich text, number and lookup data types. In addition, security has been enhanced to allow customers to define whether Why cards can be added or deleted based on the Atomic Security Relationships configuration on the Root Cause Analysis Lifecycle and what the user can see and do within the tool itself is further driven by user permissions on the 5 Whys analysis related objects (Root Cause Analysis, Root Cause Analysis Item, and Root Cause).
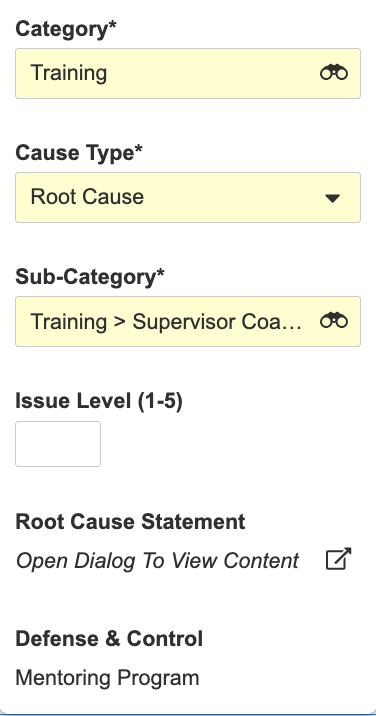
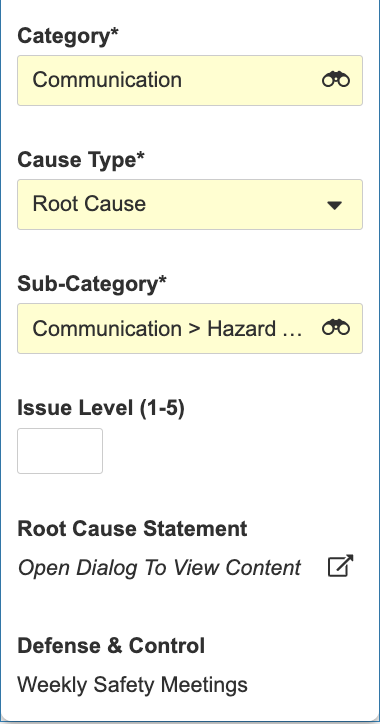
Duplicate Check: Check Lifecycle State Processing EnhancementConfiguration23R3.2
In this release, QMS Duplicate Check types of Record Checks have been enhanced to now indicate a set of states in which identifying a record as a duplicate should not change the state of the identified record. This configurable field, “Do not transition complaints in the following lifecycle states” allows organizations to, for any given Duplicate Check configuration, preserve previously canceled, closed records, or, if desired, mostly processed records in their current lifecycle states, while still capturing and linking the identified records as duplicates.
This feature requires configuration to take effect, and is only applicable to Duplicate Check types of Record Checks.
Duplicate Check: Update Summary Page to Accurately Reflect Lifecycle TransitionsAuto-on23R3.4
This release introduces enhancements to the summary page of Duplicate Checks based on feedback from our customer base. These automatically-enabled enhancements aim to add clarity as to what operations will affect which records as a result of the actions taken during the Duplicate Check.
Record Check Enhancements: Capture Suggested Terms & UI EnhancementsAuto-on23R3.2
In this release, QMS Record Checks have been enhanced with better support around matching and suggested terms. For Record Checks leveraging matching terms (seen in Admin as “Fields on object to compare in text search”), suggested terms used during the Check are now captured and saved with the Record Check Result and Record Check Rule Detail records generated as part of running the Record Check. This will provide greater historical clarity on exactly which terms were used in confirming matches.
While this behavior is automatically enabled for Record Checks already leveraging matching terms, these new suggested term captures will only be available on new Record Checks performed after this release.
Quality Teams Related Object SecurityAuto-on23R3.4
In QMS, Quality Teams are used to manually assign users to roles for a specific process record. A process record also has relationships to other objects that are not quality team-enabled. Currently, there is no way to secure who can modify those related records if they were created by a Quality Team member, only the user who created them can edit or delete them.
If the user is no longer a member of the Quality Team, no other user can remove or edit that related record unless a more open security model is implemented that allows any user to make modifications to the object.
For example, in a Deviation record, the Owner adds a related asset record to the Deviation - Asset section, two days later, and while the deviation is still in revision, the Owner leaves the company.
If a new assigned Owner in the Quality Team will then have to delete or edit the related asset record, they will not be able to, as they do not have a role in the sharing settings of that Deviation - Asset record.
This new feature will leverage record role overrides to provide dynamic access to records that are not governed by a Quality Team. It will give Quality Team members the ability to dynamically edit or delete related records associated with a Quality Team-enabled process in a secure and scalable way.
In the example mentioned above, since the Admin has configured the record role override in the configuration of the Quality Team, the new owner will automatically be assigned the role for the related object record. This gives them the possibility to edit or delete the old related record created by the previous owner who left the company.
Cascade Delete Quality Team MembersAuto-on23R3.4
This automatically-enabled feature streamlines the need for record deletion in configurations with Quality Teams, typically in a Sandbox or QA environment, by enabling single-action delete for team-enabled records. Prior to this feature, record deletion may have been impossible if the record to be deleted had associated Quality Team members, requiring first that the Power Delete Quality Team Members record action be executed, then deletion of the record in question attempted. This two-step process could be cumbersome when attempting to set up testing data in QA or Sandbox environments, especially at scale. Further, such operations would be complicated in configurations where inheritance of Team Members is configured for child records, requiring analysis all the way down the ‘tree’ of records to be deleted, execution of the Power Delete action on each, then finally deletion of the records themselves.
This feature eliminates that deep-dive analysis requirement and introduces a reliance on the configuration itself to govern how deletion for team-enabled records works. Users with the rights to delete the team-enabled records shall now not be blocked by the existence of team members associated with them. Child records which inherit their team membership from the to-be-deleted record can also be deleted, if the relationship’s configuration indicates the relationship deletion rule of Cascade delete children records. In the event that a top-level team-enabled record is deleted, and subprocess records exist with a configured relationship deletion rule of Set field to blank when related record is deleted, those subprocess records’ assignments (and the records themselves - with the exception of the relationship field being set to null) will not be affected by the deletion.
This feature only impacts configurations and users where the deletion of team-enabled records is permitted by configuration.
Quality Components Configuration Migration ImprovementsAuto-on23R3.4
This feature should be a welcome, automatically-enabled, administrator facing enhancement to QMS Vaults. Vault administrators familiar with performing Vault Package (VPK) migrations may be also familiar with some of the challenges around Actions like the Sibling Check Related Record State Change, specifically where it, as an action belonging to a state, could reference another lifecycle state, creating a circular dependency and making VPK migration very challenging to unwind.
This feature introduces new VPK functionality to account for those dependencies in Vault QMS’ component actions. Using the existing Allow deployment of blocked steps parameter during VPK import will now allow Vault to create appropriate placeholder components such that the import of VPKs containing this kind of entry action can succeed without error.
Additionally, changes have been introduced to support the automated creation of placeholder components with appropriate names which can then be updated via subsequent VPK or manual configuration, streamlining VPK operations containing any of the following components:
qualityrecordcheck__vqualityrelatedrecordmatchfield__vqualitymatchtier__vqualityrelationshipstatemap__vqualityrecordcheckrule__vqualityteam__sysqualityteamrole__sysqualityteamrolemembershiprestriction__sysqualityteamrelatedobjectsecurity__sys
Surveillance
Regulatory Updates for eMDRAuto-on23R3.4
Given recent (August/September 2023) changes that the FDA has made to the eMDR submission process, Vault Product Surveillance has introduced several changes to ensure customers’ uninterrupted and compliant eMDR and form 3500A generation. Summary of changes and impacts:
| Regulatory Change | Impact |
|---|---|
| Form Code no longer accepted by FDA | Form Code has been inactivated on the Adverse Event object & removed from form generations. |
| Patient Gender and Patient Sex updated & distinguished in 3500A | Fields updated on Complaint object to accommodate distinguishing Gender and Sex, and to provide values for relevant picklists. |
| FDA Action Code length now supports up to 50 characters | FDA Action Code maximum character length increased to 50 characters on AER object. |
| Support added for 3500A section H10: This field will allow submitters to identify one or more other MDRs concerning the same event, such as user facility or voluntary MDRs that reference the same event as a manufacturer MDR | New Related Adverse Event Reports object added and Related eMDR section added, and mapped to form generation. |
| Support for manufacturer and importer MDRs with 7-digit sequence numbers | Maximum number of digits supported for Report Number increased. |
| Section H3 has been simplified | Reduced section H3 from four parts to one. |
UI Enhancements for Adverse Event ReportingAuto-on23R3.4
This release includes continued investment into our Adverse Event Reports (AER) functionality, allowing for more clarity and less redundancy when editing fields related to adverse events in Vault Product Surveillance. As a general summary, more fields from related records have been added to the AER pages within VPS, and can be added or managed directly from that page. In general, many of these impacted sections revolve around support for relating multiple entries for related data, activities or contexts to the AER. Several changes are available now in eMDR and in EU MIR reports.
eMDR AERs have received mostly changes revolving around the capture and management of this data in various sections. For example:
Section B6 (emphasized above) now supports the addition of multiple tests and supports rendering of those tests inline with the AER. Similar treatments can also be found for D10’s Concomitant Medical Products and Therapy Dates, and H6’s Adverse Event Codes.
EU MIR AERs have also received updates ranging from visualization of related previously buried relevant lists of information, to presentation and management of related items akin to the eMDR experience, to a new and improved way of handling Time Range definition for Similar Incidents in section 4.3.c. Here are several examples:
Sections 2.3 p (above) and 2.5 (not shown) both now display relevant information from the Notified Body Product (based on Product selected) and Product Marketed table (based on Product Variant from Complaint) respectively. The columns are based on the fields on the health authority forms.
Sections 2.6, 3.2 a, 3.3 a, 4.2 e and f, all now support the inline display, addition and management of multiple values for their respective fields.
Section 4.3.c of EU MIR has also received a functional facelift, reducing the number of clicks required to define Similar Incident timeframes and increasing clarity of how many similar incidents per region surfaced in each timeframe.
Similar Incident timeframes and summaries of similar incidents are presented in tabular formats for ease of consumption and management.
Similar Incident timeframes can be individually edited in a new and improved pop-up UI from directly within the AER itself.
Feedback-Based EnhancementsAuto-on23R3.4
This release includes a smattering of enhancements based directly upon feedback from the Vault Product Surveillance Early Adopter program participants. These changes harden the existing processes, and remove several pain points and redundant elements from the solution, resulting in a more simple and reliable management process for Adverse Event Reports.
Firstly, certain eMDR transmission scenarios in which ACK3 messages are received from the FDA before their associated ACK2 messages are now more clearly handled, with the ACK3s being saved regardless of whether or not an ACK2 is present, with ‘orphaned’ ACK3s being processed by a new Vault job to ensure clarity of the documentation trail is achieved.
Secondly, reportability assessment and reassessment logic has been updated. In prior releases, the transition of an Adverse Event Report from Complete to the Closed state type would automatically trigger a follow-up report. In this release, follow-up reports are only triggered during this transition if there is a reportability reassessment performed while the AER was in flight. This change results in fewer extraneous or low-value follow up reports requiring to be processed during AER closures.
Additionally, within section G3 of eMDR, Reporter details have been consolidated from being shared across the Complaint and MedTech Complaint objects, such that users can now find all relevant Reporter details (such as their Occupation, Contact Information or Site) on the Complaint object. As a result, the Date Received by Mfg (date_received_mfg__v) on the AER object has been activated, and is used for section G3.
Validation Management
Family ValidationAdmin Checkbox23R3.5
With 24R1, Vault Validation Management provides a way to group entities together into an entity family. This allows common requirements for the family to be enforced and automatically shared across all entities within the same family and as new entities are added to the family. Additional enhancements include the ability to associate an entity with an asset from the asset object (asset__v) as well as a new Validation Deliverable Entity Version object that allows deliverables to be shared across entities in the same family. Additional fields have been added to the Validation Requirement object (val_requirement_svo__v) that must be enabled on object types as part of adopting this feature in a Vault.
Standard Action Layouts for Validation ManagementConfiguration23R3.5
With 24R1, standard Action Layouts and layout rules are now provided for Validation Management objects and object types. These Action Layouts contain best practices for organizing data into sections and fields for standard validation management objects with standard fields. Admins can copy these templates and customize it for their own use. Note that all standard action layouts are inactive by default.
Printable View for Test ScriptsConfiguration23R3.4
With 24R1, Vault Validation Management will provide a way to generate a printable view of a test script that will include related object records and evidence collected from executors, such as screenshots, that are stored as test step file attachments. When the printable view is initiated from a user action, information about the test script, test steps, related object records such as discrepancies will be included so that it can be printed to a PDF from a browser and shared with an external stakeholder, like an auditor.
A new printable view configuration is available to allow Admins to define what printable views are available for configuration on a test script lifecycle state. In an XML template configuration file, Admins can configure the order that related object records will appear in the printable view as well as their fields that will display in table, one-column, or two-column formats. Additional configurable options will include a header logo image, header text, footer text, and watermark text that will be displayed at a 45 degree angle. Tokens are available for the header and footer text to include attributes such as the record number (name__v), user name that generated the printable view, and date/time the printable view was created. Free text can be added to the header and footer text.
Template RequirementsConfiguration23R3.4
In 24R1, Vault Validation Management is introducing templating capabilities for requirements and set of requirements. When enabled, user requirements, functional requirements, and design specifications can be created from a single template requirement record or import a set of multiple template requirements. This will help organizations to harmonize practices across departments and global sites and establish a library of commonly used requirements and specifications and imported as new systems, equipment, processes, and other GxP systems and processes are introduced in production.
This feature will help organizations establish a center of excellence for requirements that can be used globally in their business and have an approved repository of template requirements available for use in different types of entities regardless of validation discipline.
Re-order & Copy Support for Additional PromptsAuto-on23R3.4
With 24R1, Vault Validation Management extends support for additional prompts to allow the order of the prompts to be manipulated directly from the test script authoring UI using drag and drop. Setup Steps and Execution Steps that have additional prompts are now also included when the Setup Step or Execution Step is copied.
LIMS
Modifying Test InputsAuto-on23R3.5
Test Input values are now saved immediately upon leaving the cell, and can be modified even after Test Step completion through a sign-off flow very similar to the Result Change flow.
Aggregate TestingAuto-on23R3.4
This feature allows for retention of the original Lab Test when retesting and adds new aggregate testing functionality. This includes the Sum, Average, Min, Max, Median, Mode, CountA, StDevP and StDevS calculation functions applied to a selected result from many Lab Tests. These results are collected from all the Lab Tests associated with a separate object, specifically: Lab Sample and Spec Execution.
Stability Study ManagementAdmin Checkbox23R3.4
This feature adds the ability to manage stability studies to the LIMS application, including:
- Study design including detailing storage conditions, planning timepoints, setting orientations, testing and evaluating specifications.
- The ability to initiate multiple studies from approved study design templates.
- Awareness of upcoming timepoints across one or all active and planned studies to best manage individual studies and laboratory resources.
- Integration with existing test execution functionality for the analysis of stability samples
- The ability to use batch release testing results in the evaluation of stability study time point zero criteria.
Quality Data Model Changes
See 24R1 Quality Data Model Changes.
Regulatory
Several features listed in the Vault Connections section also affect the Regulatory application family.
RIM
RIM Standard Action LayoutsConfiguration23R3.5
With this release, standard Action Layouts are provided for the Application, Regulatory Objective, and Submission objects. Standard Action Layouts can be copied to create customer-specific layouts, allowing for a best practice starting point for your layout designs. Standard Action Layouts should be reviewed against our recommended list of configurations and based on customer-specific use cases as part of enablement.
RIM Registrations
Bulk Create & Manage Event DetailsConfiguration23R3.4
In this release, Administrators can configure Change Types, such as a Shelf Life Change or a Manufacturing Site Change, to simplify Event detail creation for users. Users can run the Create Event Details action from an Event record, select one or more Change Types that apply to that Event, and populate the information needed for those types of changes. Once done, users can generate a preview to refine the proposed Event details before creating them. Additionally, users can run the Manage Event Details action from an Event record to generate a preview to refine the Event’s existing details without adding new ones.
Event creation is the recommended first step for regulatory processes, as an Event serves as the initial entry point for regulatory data that gets carried across impacted markets via Regulatory Objectives and Submissions. Events can require the creation of dozens or even hundreds of Event details. This feature streamlines the creation and management of these details.
EUDAMED XSD Version 2.0.11 SupportAuto-on23R3.4
This feature will update EUDAMED UDI Submission Generation & Validation in alignment with the latest Production update, which uses XSD version 2.0.11.
Enhancements to Product-Based Activity Scope LevelsAuto-on23R3.4
When Application Relationships are enabled (via the Enable Application Relationships setting), and users run the Create Related Records wizard from an Event, the Application and Product and Country, Application and Product Activity Scope Levels are updated to account for the products in the Application Product records to determine which Activities to create for each impacted Application. This auto-on enhancement provides greater data integrity.
Identification of Global Site Role Values That Could Not Be MappedAuto-on23R3.4
When mapping global and local Site Roles in Bundling, Vault creates records with a placeholder value from the Controlled Vocabulary record if the global Site Role value from the Event-related records cannot be matched to local values on the new Regulatory Objective-related records. This enhancement to the Global to Local Site Role Mapping in Bundling feature (23R3.2) ensures records are created and users can update the value.
Enhanced User Messaging When Bundling Regulatory ObjectivesAuto-on23R3.4
When bundling from a Regulatory Objective, updated messaging within the wizard clarifies to users that they can only select relationships from the Events that are associated with the selected Activities, while all relationships on the bundled Regulatory Objectives will also be carried forward.
eAF Report Generation Enhancement
When generating eAF Reports from a Regulatory Objective, Vault now replaces any existing reports for the same medicinal product, preventing redundant reports from building up over time.
This feature is deferred to a future release.
External ID to be Unique on UDI Attribute SetAuto-on23R3.4
As part of the RIM Registrations data model updates (23R3.2), the Values must be unique configuration is enabled for the UDI Attribute Set object’s External ID field.
Enhanced Support for Packaging Shelf Life FieldAuto-on23R3.4
With this feature, the Shelf Life field on Packaging join objects is now supported by the Registrations wizards when the Submission Wizard is enabled. This field will also continue to be supported without the Submission Wizard.
Global-to-Local Site Role Mapping in BundlingAuto-on23R3.2
In this release, we have enhanced the Bundling action to respect the global-to-local mappings of the Site Role values.
Now, when users perform Bundling from an Activity or Regulatory Objective, Vault converts the global Site Role value from the Event-related records to the appropriate local values on the new Regulatory Objective-related records.
This applies to the following objects:
- Regulatory Objective Product
- Regulatory Objective Active Substance
- Regulatory Objective Inactive Ingredient
- Regulatory Objective Packaging
This improved functionality is available after a Business Admin performs the global-to-local mapping of the Site Role values.
With this capability, users will save time from manually updating the Site Role values from the default value to the local value.
This enhancement builds on the global-to-local mapping of Site Role values for the Create Related Records action introduced in the 23R2 Release.
Relate Bundled & Split Regulatory Objectives & Submissions to EventsAdmin Checkbox23R3.2
With this release, Regulatory Objectives and Submissions created with the Bundling or Splitting wizards are related directly to the Events of any selected Activities, enhancing end-to-end visibility.
Wizard Support for IDMP Manufacturing DetailsAuto-on23R3.2
We have expanded the Vault RIM data model to better support updates to existing manufacturers and the addition of new manufacturers. The following fields have been added:
- Authorisation Effective Date (
authorisation_effective_date__v) - Confidentiality Indicator (
confidentiality_indicator__v) - Health Authority (
health_authority__v) - Manufacturing Authorisation Number (
manufacturing_authorisation_number__v) - Manufacturing Operation Start Date (
manufacturing_operation_start_date__v) - Manufacturing Operation Stop Date (
manufacturing_operation_stop_date__v)
These new fields can be found in the following Event, Application, Submission, and Regulatory Objective relationship objects (for example, Event Product or Application Packaging): Product, Active Substance, Inactive Ingredient, and Packaging. They are fully supported by the Vault RIM bulk create and update actions.
This expansion allows organizations to capture accurate manufacturing details and easily manage better manufacturing changes.
RIM Registrations Data Model UpdatesAuto-on23R3.2
In this release, we’ve expanded the Vault RIM data model to better capture product and device details. Here’s what’s new:
-
Regulatory Text document field for trade names: The new Regulatory Text (
regulatory_text__v) field can be added to Labeling (labeling__c) document subtypes or other regulatory documents to allow users to tag documents with one or more trade names. - Applicable Product Type object field: We added the Applicable Product Type (
applicable_product_type__v) field to the following objects to ease the configuration of output records related to product data reporting:- Medicinal Product (
medicinal_product__rim) - Registered Packaged Medicinal Product (
registered_packaged_medicinal_product__v) - Administered Product Route of Administration (
administered_product_route_of_admin__rim) - Product Cross Reference (
product_cross_reference__v)
- Medicinal Product (
-
Updated RIM Object Mapping Rules: The Use for Registrations field for Event-to-Application data generation has been updated to prevent potential duplicates when users run the Create Related Records action while the Submission Wizard is inactive.
-
Use for UDI object field: The Application Shelf Life or Condition (
application_shelf_life_storage__v) object has been updated to include the Use for UDI (use_for_udi__v) field. The field will be considered when creating new Submissions and Regulatory Objectives with the Submission Wizard. -
Identifier Type picklist value: We’ve added the Master UDI-DI (
master_udidi__v) value to the Identifier Type (identifier_type__v) picklist. - Container object type: We added the Container (
container__v) object type to the following objects to allow organizations to capture more details and manage changes related to packaging containers:- Application Shelf Life (
application_shelf_life_storage__v) - Submission Shelf Life (
submission_shelf_life_storage__v) - Regulatory Shelf Life (
regulatory_objective_shelf_life_storage__v) - Event Shelf Life (
event_shelf_life_and_storage__v) - Registered Shelf Life (
registered_shelf_life_and_storage__v)
- Application Shelf Life (
- SPOR Term object field length: The length of the SPOR Term (
spor_term__v) field on the Controlled Vocabulary (controlled_vocabulary__rim) object has been increased to 1500 characters to account for SPOR values with very long names. This also allows for including the full term name in the FHIR message for IDMP.
RIM Registrations, RIM Submissions
Global Content Plan Dispatch by CountryAuto-on23R3.4
This feature allows the Global Content Plan to be dispatched to individual countries. The dispatch dialog will support selection of either Countries, Document Sets (Submission Groupings), or All. When Countries are selected in the dispatch dialog, the dispatch acts on those Countries’ Activity records.
Generate Active Dossier from Global Content Plan DispatchConfiguration23R3.4
This feature supports automatic generation of Active Dossier records upon dispatch of the Global Content Plan based on the documents dispatched. The target Activities’ Local Disposition determines whether Active Dossier records are generated as Dispatched or Pending Current. Generation upon dispatch allows for a more accurate Active Dossier for regulatory use cases outside of the normal submission and approval cycle, such as changes that can be implemented before submission or without a submission. Furthermore, this feature provides earlier visibility for those documents that are dispatched and internally ready for use to build the next document version from when there are concurrent changes.
Global Content Plan Synchronization UpdatesAuto-on23R3.4
This feature consists of enhancements for synchronizing Global Content Plans:
- The Comparison Viewer help is updated to state user selections persist when navigating away without accepting or rejecting within the comparison viewer.
- There is now validation on missing permissions when matching target Submission Content Plan sections.
- For target Submission Content Plans, newly-added Content Plan and Content Plan Item records that are created from a dispatch will be ordered based on the order in the template after synchronization.
- The Comparison Viewer retains users’ column width preferences.
Global Content Plan Dispatch Update: Notify Submission Owners Upon Initial Submission Content Plan CreationAuto-on23R3.2
A new notification, SCP Created from Dispatch, alerts Submission Owners when a new Submission Content Plan is initially created from a Global Content Plan dispatch. This is similar to the Comparison Ready notification that alerts owners when a comparison is complete and ready for their review. The new notification provides convenience to end-users, preventing Submission Owners from having to monitor the system and dispatch messages for newly-created Submission Content Plans requiring attention.
Support Reattachment of a Global Content Plan to its Event RecordAuto-on23R3.2
Global Content Plans (GCPs) that are removed from an Event record can now be added back. Previously, if the Global Content Plan field on an Event record was cleared, accidentally or purposefully, users could not reattach the GCP back to the Event record. Now, when the field is cleared, users can select the appropriate GCP on the Event field to associate it back to the original Event record.
RIM Registrations, RIM Submissions, RIM Submissions Archive
Active Dossier Hovercard Update and Viewer Support for Dispatched RecordsAuto-on23R3.2
The Dispatched Active Dossier Status picklist value was implemented in 23R2. With this release, the Active Dossier Viewer is updated to display the Dispatched icon () for Active Dossier Item Detail records with a Dispatched status. The Active Dossier hovercard is also reformatted to display the Event and Activity fields.
RIM Submissions, RIM Submissions Archive
Active Dossier Update to Support Common CountriesConfiguration23R3.2
Country codes must be explicitly defined in order for countries to be supported by Active Dossier. The Country Code for the Common (EU) and Common (GCC) countries that are used for content planning purposes are now set explicitly to common_eu and common_gcc, respectively.
Previously, Common (GCC) was identified by a Country Code of common and Common (EU) was identified by a blank Country Code. Admins will need to update the country_code__rim field on Common Country records for proper Active Dossier support for these countries.
Active Dossier Consolidated Sections (Enablement Update)Auto-on23R3.4
In a previous release (23R3), the Active Dossier Consolidated Sections feature was introduced to remove certain product-replated tokens from Active Dossier Template records. These tokens for Product Variant and Manufacturer (and in 3.2.A.1, Product, Product Variant, and Active Substance) were previously responsible for creating repeating sibling record sections. Prior to 24R1, only customer Vaults without existing Active Dossier records were automatically updated, and customers with active Active Dossier records were given the option to enable record migration with an Admin setting.
With 24R1, all Vaults will be migrated to the updated Active Dossier Template, and the Admin setting will be removed. These actions will occur automatically, regardless of whether the Vault already contains Active Dossier records. Upon completion of the record updates, end-users will see the condensed Active Dossier Viewer layout for new and existing Active Dossiers.
Cloning Enabled for RIM ViewsAuto-on23R3.4
Saved Views for Content Plans, Active Dossier, or Submissions Archive are managed by the system-managed RIM View (rim_view__v) object. To support administrative efficiency, the RIM View object is now set to be cloned when a RIM Vault is cloned.
Saved views will be inherited from parent Vaults upon deployment. Sharing settings and Users/Groups will not be inherited. Vault Owners will have access to the cloned views to share them as needed. Filters on object records that are not cloned in the saved view will not be persisted.
RIM Submissions
Improve Match Document Mode PerformanceAuto-on23R3.5
To provide a permanent solution to the slow performance sometimes seen with Suggested Matching, Match Document Mode will now display approximate document counts and page numbers, similar to what is used by the Vault Library. When more than 25 potential matches are returned in the document picker, Vault displays the count as ‘1-25 of many’ instead of calculating the exact total number. The approximate counts significantly reduce the time spent loading potential matches while still providing users with the ability to navigate the pages of results. Additional filtering may still be used to reduce high numbers of suggested matches.
Suggested Matching Enhancements: Reorder, Split, Update Expected Counts, & Bulk MatchAuto-on23R3.4
When working in Submission Content Plans, users can now execute Content Plan Item actions from within Match Document Mode, including:
- Reorder matched documents on a Content Plan Item
- Split a Content Plan Item
- Update the Expected Steady State Count for a Content Plan Item
- Match documents in bulk using Select All or Deselect All
These enhancements support a smooth and efficient flow for managing and manually matching documents within the Content Plan Viewer. Users can now perform all the steps needed to manually manage documents for a Submission Content Plan within one convenient interface.
Expand Content Plan to Matched Document LevelAuto-on23R3.4
Content Plans will now expand to the match document level (up to a maximum of 2000 documents) instead of stopping at the Content Plan Item. This enhancement reduces the time and manual effort of expanding each Content Plan Item individually to see the matched documents when executing the Expand user action at the Content Plan level.
Vault-Wide PDF StandardizationConfiguration23R3.4
Admins can now configure PDF Standardization as a Vault-wide setting in the default Rendition Profile. Prior to 24R1, this setting was only available on custom Rendition Profiles.
Extending this to the default Rendition Profile allows for standardization to be applied more holistically without the need for additional configuration to leverage custom Rendition Profiles.
When enabled, all new documents will be standardized when new versions are created. Existing documents can be re-rendered using Vault Loader.
Improved Inactive Bookmark Handling During PDF StandardizationConfiguration23R3.4
Inactive bookmarks in source files will function based on the first child action bookmark in the hierarchy. Some customers leverage inactive bookmarks to create groupings of bookmarks within source files.
Prior to 24R1, Vault would adjust inactive bookmarks up a level, which would remove the grouping. Matching inactive bookmark behavior to the first child bookmark will ensure consistency for users when working with PDF source files as well as in the document viewer.
If an inactive bookmark has no child bookmarks that are active, they will be removed in the standardized source PDF as well as in the document viewer.
Content Plan Creation to Respect DTD/XSD VersionConfiguration23R3.2
This feature introduces two new fields on the Content Plan related objects: XML ICH DTD / XSD Version and XML Regional DTD / XSD Version, along with updates to the Submission Content Plan creation processes to respect the Submission’s XML Version fields. This allows RIM customers to generate a more relevant content plan for Content Plan Templates that apply to multiple different XML versions.
This feature enables the option to set the XML ICH DTD / XSD Version on the eCTD-only Content Plan Item Templates, such as Index and STF, so that these Content Plan Items are not created for non-eCTDs.
RIM Submissions Archive
Leaf Source File Path Display in the Submissions Archive ViewerAuto-on23R3.4
With this feature, RIM Submissions Archive users are able to view and filter the leaf source file path field metadata in the viewer grid.
Embedded Doc Viewer: Select ModeAuto-on23R3.4
With the 24R1 Platform feature Doc Viewer: Improved User Defaults, Vault will open all documents (including those in the Submissions Archive Viewer) with Select mode automatically enabled and Grab mode automatically disabled.
For Submissions Archive, this change allows for videos to be rendered in the Viewer and allow users to copy text from the Viewer. This also changes how Submissions Archive users navigate through documents, particularly documents with hyperlinks: With the Select mode enabled by default, users who wish to click on a hyperlink in the Viewer can hover over the link, then select the resulting pop-up to open the link. Users can also enable Grab mode, allowing them to directly click the link in the document. TIP: you can switch modes with the keyboard shortcut “CTRL + Shift” on a PC, or “Command + Shift” on a Mac.
Enable Grab mode to click on hyperlinks directly:
Hover over link and click to open hyperlinks in Select mode (new default):
Update Viewer Document HeaderAuto-on23R3.4
With this release the document viewer header in the Submissions Archive Viewer is updated to display the document version and the document lifecycle state.
Sharing Submission Selection in the ViewerAuto-on23R3.2
Submissions Archive Viewer users can now share URLs to one or many specific Submissions in an Application. Previously, the URL only contained the Application ID, regardless of any applied Submission filters, and so sharing was limited to the Application view. Now, the viewer URL includes Application ID and Submission IDs.
As users filter their desired Submissions in the Submission column, the Submission ID is automatically added to the URL string. If a filtered Submission is removed from view, its Submission ID is automatically removed from the URL. Users can copy and paste the URL for other users to open or paste into their web browser. The full URL length cannot exceed 2083 characters. Users are warned when the length is exceeded, and any Submission IDs added beyond the maximum are excluded from the URL string.
RIM Publishing
Link Evaluator EnhancementsAuto-on23R3.5
Additional functionality for Link Evaluator will allow users to perform more tasks directly from the Link Evaluator interface. As these tasks can be completed from a single screen rather than returning to each source document, users will see improved efficiency and productivity when creating and reviewing hyperlinks. Improvements include the following:
- Link Evaluator interface: Page navigation has been improved. Users can also more easily return to the Submission Content Plan or access Submissions Archive from the Link Evaluator page.
- Link Deletion: A new Suppress Links action allows users to delete valid or invalid hyperlinks, individually or in bulk. Upon marking for deletion, Vault Submissions Publishing will suppress the hyperlink and change blue font to black in the Published Output. Suppressed and retargeted links can now also be filtered.
- Link Retargeting: When using the Retarget action, users have the option to choose a specific rendition for the target document, such as an Imported Rendition.
- Link Overrides: Users can undo the Retarget or Suppress link actions with a new override functionality, individually or in bulk. When applying an override (Suppress or Retarget) users have the option to apply them at the Submission or Application level: Applying an override at the Submission level changes the published link within the current submission, and applying an Application override changes the published link on all Publishing Active Submissions within the application (including the current Submission). This includes any future submissions with the same matched document and same link.
Section Level Merge for RLCPConfiguration23R3.4
This feature enables users to merge all documents from Content Plan Items within a Content Plan section into a single PDF upon publishing.
This feature does not currently support defining a document type for the RLCP Published Output. Any sections merged with Section Merge will be outputted as Submission Ready document types.
Document Type Definition for RLCP Published OutputConfiguration23R3.4
With this feature, users can define the document type for published Report Level Content Plan (RLCP) content on the Content Plan Item, Content Plan Item Template, or Report Level Content Plan record, allowing for better classification and auto-matching within Submission Content Plans after publishing the RLCP.
Jordan Multiple Application Number SupportAuto-on23R3.4
Users Publishing in JO now have the ability to add or remove overrides for eJDWS Application Number (Application Number) and Application Number (Number) via the Update Administrative Information action from the Submission record.
Disable Auto Reference Leaf for Non-eCTD SubmissionsAuto-on23R3.4
Vault Publishing will no longer automatically create Reference Leafs when the same document is matched multiple times in a non-eCTD Submission. Vault Publishing will disable the Reference Leaf functionality for any non-eCTD submissions (where ICH/DTD is not equal to 3.0 or 3.2), allowing the same document to be published multiple times.
When the same document is matched to multiple Content Plan Items within the same Submission Content Plan, current publishing behavior is to automatically create a Reference Leaf to prevent duplicate publishing and submission of a single document, per eCTD guidelines. For non-eCTD submissions lacking an XML backbone, the same document matched multiple times must still be published and submitted within its expected section.
RIM Publishing, RIM Submissions Archive
Single Submission Package Potential Incomplete Export NotificationAuto-on23R3.2
With this feature, Submissions Archive export messaging is updated for clarity in cases when a Submission is set for continuous publishing and the exported dossier package may be incomplete.
Increase RIM Vault Max File Size to 500GBAuto-on23R3.4
To better support customers publishing and submitting very large files, the maximum file size limit has been increased to 500GB on all RIM Vaults.
RIM Data Model Changes
See 24R1 RIM Data Model Changes.
Safety
The Safety 23R3.5 release, including all Platform features, is scheduled for tentative availability on March 14, 2024. For the latest central dictionary updates for your Safety Vault, see Safety Central Dictionary Updates.
Note: Beginning with 23R3.2 and for all subsequent releases, the new Vault Safety Help site is the official site for Vault Safety Limited Release Help content.
Several features listed in the Vault Connections section also affect the Safety application family.
Safety
Update to Seriousness PicklistConfiguration23R3.2
To support Device and Combination Product Cases, Admins can now update the Seriousness picklist to include “Required Intervention to Prevent Permanent Impairment/Damage (Devices)”. Previously, Vault Safety did not have this value. This feature includes the Seriousness picklist value only. Future enhancements will include mapping the value for report generation.
Learn More
- Enablement: Enable Update to Seriousness Picklist
- Admin Help: Migrate External Cases
Manage Custom Dose Forms, Routes of Administration, and Units of MeasurementConfiguration23R3.2
With this release, Admins can manage custom dose forms, routes of administration, and units of measurement values within Vault Safety. A flexible extension to system-provided values, Admins can add, edit, and delete custom values as needed. Using the Coding Dictionary Loader, available in the same Business Admin area where the MedDRA, WHODrug, and IMDRF dictionaries are managed, makes the process of updating custom values simple and efficient. Once loaded, custom values are immediately available for intake, Case processing, and EDQM mapping.
Learn More
- Enablement: Enable Manage Custom Dose Forms, Routes of Administration, and Units of Measurement
- Admin Help: Manage Custom Dose Forms, Routes of Administration, and Units of Measurement
Rule Engine Update for Safety Measure and Research ReportsAuto-on23R3.2
This release significantly enhances the Safety reporting rules for PMDA Safety Measure and Research reports. The reporting rule engine now excludes Cases with a Special Report Classification unless specified in the evaluated rule, effectively addressing the issue of over-reporting to non-PMDA agencies. Previously, Submissions were generated for non-reportable Cases, requiring manual intervention to suppress them. This update streamlines the reporting process, reduces manual effort, and supports more accurate and efficient Submissions, saving time and resources while enhancing compliance.
Learn More
Japan: Substance Matching for CPR Generation (Cross Report)Auto-on23R3.2
Vault Safety has expanded its support for Case Product Registration (CPR) generation for Japan PMDA Submissions to eliminate issues of over-reporting and under-reporting. This enhancement revises the CPR generation logic to prioritize exact Substance matching for investigational Product Registrations, while resorting to partial matching for post-marketed Product Registrations when an exact match isn’t available. Moreover, the system generates CPRs for Compound Products (Cases with multiple Substances) only when the Case Product matches these multiple Substances precisely. This update significantly enhances Case Processing efficiency and ensures compliance by eliminating over-reporting and under-reporting of PMDA Submissions, resulting in the proper generation of CPRs and more accurate reporting practices.
This feature is Auto-On in Vaults with PMDA Local Case Processing Automations enabled.
Learn More
- User Help: Complete Intake and Process Cases for the PMDA: Localized Case Product Registration Generation
Safety Rule Engine Troubleshooting ToolAuto-on23R3.2
Vault Safety Admins can now diagnose Submission and Distribution Rule issues by using the Safety Rules Troubleshooting tool. Admins can use the tool to execute the Vault Safety Rule Engine for a specific Case ID. They can then obtain a detailed log of the Rule Engine’s execution for that Case, including which Rules passed and which did not. Previously, Vault Safety created Transmission messages that explained why each of the Submissions and Distributions was generated. However, if no rules passed the system did not log any information. With this additional transparency, Vault Safety Admins can perform additional configuration verification, troubleshooting, and validation of Vault Safety Reporting Rules.
Learn More
- Admin Help: Troubleshooting Safety Rules
Reporting Rules Configuration for Non-Vault OwnersAuto-on23R3.2
Vault Safety users with the necessary permissions can now edit custom Reporting Rules. Previously, only users designated as Vault Owners were able to modify Reporting Rules. This improvement provides Admins increased flexibility in managing custom Reporting Rules.
Learn More
Sender-Based Inbound ValidationsConfiguration23R3.4
Vault Safety will allow Admins to assign specific groups of validation criteria (such as ICH E2B(R3)) for individual Transmission Profiles (Business Partners). Moreover, when an E2B Transmission is imported to Inbox Item through the AS2 Gateway or Vault API, the Safety system will validate the imported data against the rules on the Transmission Profile. Subsequently, it will automatically generate an acknowledgment in the E2B format indicating if the Case is accepted or rejected. In addition, the results of this validation will be included in the acknowledgement and in the Inbox Item Validation Results section. Prior to this update, Vault Safety employed a fixed collection of ICH E2B validation rules. This feature greatly enhances the accuracy of data received in E2B format, particularly those originating from partners. As a result, Safety customers can extensively automate the comprehensive process of Case handling.
Learn More
- Enablement: Enable Sender-Based Inbound Validations
- Admin Help: Manage Transmission Profiles: Add Validation Criteria
- User Help: Receive an E2B Transmission
Safety Inbox Loader: Multi-CSV ZIP Data Import for VAERS, KAERS, and Custom FormatsConfiguration23R3.4
In 23R3, support for multi-Case CSV import to Inbox Items was added to Vault Safety. With this release, Vault Safety Inbox Loader now supports bulk import to Inbox Items from multi-CSV ZIP files. Vault Safety automatically extracts and aggregates the data from all zipped CSV files and creates an Inbox Item for each Case. This feature allows Admins to specify format metadata, such as encoding and country. This enhancement improves Case processing efficiency and data quality for Cases distributed from multi-CSV ZIP files, for example, FDA in the US for VAERS Cases or KAERS in Korea.
Learn More
- Enablement: Enable Safety Inbox Loader Multi-Case Tabular Data Import
- Admin Help: Manage Safety Inbox Loader Multi-Case Tabular Data Import
- User Help: Import an Inbox Item: About Multi-Case Tabular Data Import
Case Parent Information Support for JSON Intake 23R3.4
With this release, Vault Safety now supports Case parent information import for JSON intake.
Learn More
Two-Way Relationships Between Multi-Case and Single-Case FilesAuto-on23R3.4
Vault Safety now facilitates a bidirectional relationship between the multi-Case files (XML, CSV, and ZIP) and its individual single-Case documents. This enhancement enables the seamless linking of Inbox Items to their source documents and vice versa. In the past, accomplishing this relationship was challenging, making it difficult to associate the Inbox Item with the source document.
Learn More
- User Help: Import an Inbox Item
Product Ranking Suggestions for Imported Inbox ItemsAuto-on23R3.4
With this release, Vault Safety enhances product ranking suggestions for imported Inbox Items from any source (E2B, JSON, Inbox Loader, or Connections). If there is a Study Product match, Vault Safety will assign rank 1 to the first match with a Suspect drug role. Otherwise, if there is a Study Product Placeholder match (a Blinded Name exists), Vault Safety will assign rank 1 to the first match with a Suspect drug role. Lastly, if there is no Study Product or Study Product Placeholder match, the system attempts to assign rank 1 to the first matched Company Product with Suspect drug role. Rank 1 will also be displayed in the Source Data panel of products if it was present in the source data.
Learn More
- User Help: Inbox Item Study and Product Matching
Prevent New Info Date Update During Merge to CurrentAuto-on23R3.4
When merging Inbox Items to In-flight Cases, the system no longer updates the Case New Info Date. This change supports compliant Transmission Due Date generation.
Learn More
- User Help: Merge an Inbox Item to an In-Flight Case
Document References on Domestic and Localized Follow-Up CasesAuto-on23R3.4
When creating Follow-ups to Domestic and Localized Cases, Vault Safety now copies document links from the previous Case version. This includes populating links in the Datasheet Document field on Case Assessment Expectedness and Localized Case Assessment records and in the Document field on Localized Case Document records.
Non Re-Transmittable Document ClassificationAuto-on23R3.4
Vault Safety introduces a new document classification, Case > Source > Non Re-Transmittable Document for Case documents that must not be included in E2B Transmissions. On Initial and Follow-up Case creation, the newly created Case version will be added to the Case field on all documents with the Case > Source > Non Re-Transmittable Document classification.
Combination Product Updates Applied to Case NamesAuto-on23R3.4
Updates to Case Combination Products are now reflected in Case names generated by Vault Safety. Previously, Combination Product updates did not impact Case names. More accurate and descriptive Case names enable faster browsing and identification of Cases.
Learn More
- Admin Help: About Default Case Object Naming Conventions
QC Checklist GenerationConfiguration23R3.4
With this release, Vault Safety can leverage Vault Checklists, which can be generated automatically for in-process and post-closure quality control (QC) activities. In Vault Safety, these checklists are typically used to provide QC guidance and specific field verifications that need to be performed on the Case (usually during the QC process). Admins can configure specific Checklist Designs for different use cases (for example, spontaneous, clinical, or Product-specific Cases). The appropriate Checklist Design can be automatically selected based on checklist rules, which are based on Case attributes such as Report Type, Product, Seriousness, Data Entry User, and Source. The rules can also control how often checklists are generated, according to a sampling rate (for example, every Case or randomly selected Cases based on a date range).
Quality Control is a significant contributor to Case processing times. Automatic QC Checklist Generation optimizes QC activities, enhancing processing quality and efficiency for open and closed Cases.
Learn More
- Enablement: Enable QC Checklist Generation
- Admin Help: Configure QC Checklist Creation Rules
- User Help: Generate a QC Checklist
Inactivate Product & Study RegistrationsConfiguration23R3.4
With this release, Vault Safety enables setting Product and Study Registrations to a Deleted state type so the Safety Rule Engine no longer considers them when determining a Case’s reportability. Previously, registrations were transitioned to the Inactive lifecycle state, which sometimes caused issues in follow-up situations. This new Product and Study Registration capability facilitates more precise downstream ICSR reporting.
Learn More
- Enablement: Enable Inactivate Product & Study Registrations
- Admin Help:
Datasheet Expectedness by Age and SexConfiguration23R3.4
Expectedness evaluations in Vault Safety now support age range and sex criteria. This feature enables the creation of more precise Datasheets for adverse events that affect specific demographics. This eliminates the need to override auto-calculated Expectedness values, offering time savings and greater accuracy to Case Processors.
Learn More
- Enablement: Enable Datasheet Expectedness by Age and Sex
- Admin Help:
MedDRA Browser and Search ImprovementsAuto-on23R3.4
With this release, Vault Safety enhances MedDRA search to return better results. When searching for single- or multi-word terms, Lowest Level Terms (LLTs) that are an exact match for or contain the searched term appear in the results. In addition, the limit to search terms returned by the MedDRA Browser is increased to 5000 results. These improvements increase the likelihood that users searching with the MedDRA Browser can code the correct MedDRA term.
Learn More
- Admin Help:
Standard MedDRA Query (SMQ) Hierarchy UpdateAuto-on23R3.4
When you import Standard MedDRA Queries (SMQs) into Vault Safety, the system now creates references to MedDRA terms at the Preferred Terms (PT) level. Previously, the system created references to Lowest Level Term (LLT) records. This update addresses possible under-reporting to destinations that use SMQs to determine reportability.
Learn More
- Admin Help: Import Standard MedDRA Queries
Medical History Label UpdateAuto-on23R3.4
With this release, Vault Safety updates the label of the deprecated Medical History Text field to “Medical History*”. This change will assist Admins in confirming they have the current Medical History Text field configured in their Vaults. The up-to-date Medical History Text field includes a picklist of Reason Omitted values that are valid for E2B submissions. If your Case page layouts use the deprecated Medical History* field, remove it, then add the compliant Medical History Text field.
Learn More
- Admin Help: Configure the Medical History Text Field
Create and Export Case and Safety Report CollectionsConfiguration23R3.4
To support reviewing and reporting on groups of Cases, Vault Safety introduces Case Collections and the export of multiple E2B and CIOMS I forms at once. Upon export, forms are organized into binders, facilitating easy downloading and sharing of safety reports with partners or relevant health authorities. Additionally, Vault Safety can automatically generate CIOMS I forms for all closed Cases daily. Previously, reviewing a group of Cases and generating multiple forms at once required a manual process. This feature significantly enhances efficiency by streamlining the form-generation process and provides a more cohesive approach to Case management.
Learn More
- Enablement: Enable Create and Export Case and Safety Report Collections
- Admin Help: Configure the Auto-Generate Case Forms with Case Closure Job
- User Help: Manage Case and Safety Report Collections
FDA E2B(R3) Reports
This feature has been moved to a future release.
Transmission Profiles for FDA E2B(R3) Submissions and DistributionsConfiguration23R3.4
To support Transmissions to the FDA in FDA E2B(R3) format, Vault Safety introduces three (3) new Transmission Profiles. These are CDER Study, CBER Study, and CDER IND Exempt. This feature is a building block for expanding future FDA E2B(R3) capabilities in Vault Safety.
Support Device Seriousness on MedWatch 3500A, CIOMS I, and E2BAuto-on23R3.4
When generating Case forms, Vault Safety now maps the Seriousness picklist value “Required Intervention to Prevent Permanent Impairment/Damage (Devices)” to the following reports and sections:
- MedWatch 3500A: B.2 Outcome Attributed to Adverse Event
- CIOMS I:
- I. 7 + 13 Describe Reactions
- I. 8-12 Check All
- E2B:
- E2B(R3): E.i.3.2f Other Medically Important Condition
- E2B(R2):
- A.1.5.1 Serious
- A.1.5.2 Seriousness Criteria
Learn More
- User Help:
Japan: One Last Time ReportingAuto-on23R3.4
To comply with One Last Time reporting requirements, Vault Safety supports the evaluation of reportability changes for Japan PMDA submissions.
This feature is Auto-On for customers using Case Product Registration-based Due Date Calculations for Japan. This feature provides Admins with additional options to configure Japan One Last Time Reporting in your Vault.
Learn More
- Enablement: Enable Japan One Last Time Reporting
- User Help: One Last Time Reporting for Japan (PMDA)
Japan Periodic Reporting: Post-Market Non-Serious (NUPR)Configuration23R3.4
With this release, Vault Safety introduces the first Pharmaceuticals and Medical Devices Agency (PMDA) aggregate report capability with the Post-Market Non-serious Unlisted Periodic Report (NUPR).
Learn More
- Enablement: Enable Japan Periodic Reporting - Post-Market Non-Serious (NUPR)
- User Help: Create PMDA Post-Market Aggregate Reports
DSUR and PBRER: Cumulative Tabulations for all IMP, Comparator, Placebo and Blinded Case Study ProductsAuto-on23R3.4
With this release, Vault Safety enhances the “DSUR and PBRER Investigational Product Causality” feature (released in 23R2) by extending its scope to include all Investigational, Placebo, Active Comparator, and Blinded Study Products related to a Case in the context of Serious Adverse Reactions (SAR), rather than solely focusing on the primary Case Study Product. Furthermore, when determining the appropriate column for Case counting in the Serious Adverse Events (SAE) tabulation, consideration will be given to the causality of all Investigational, Placebo, Active Comparator, and Blinded Study Products associated with a Case, not limited to the primary Case Study Product.
This feature is Auto-On in Vaults with DSUR and PBRER Investigational Product Causality enabled.
Learn More
- User Help:
- Create DSUR Aggregate Reports: Cumulative Tabulation of Serious Adverse Events from Clinical Trials
- Create DSUR Aggregate Reports: Appendix: Cumulative Summary Tabulation of Serious Adverse Reactions From Clinical Trials
- Create PBRER Aggregate Reports: Cumulative Tabulation of Serious Adverse Events from Clinical Trials
Safety Data Validation Exception HandlingConfiguration23R3.4
To help Vault Safety Admins diagnose any errors encountered during the evaluation of Safety Data Validations, Vault Safety now provides Validation Error logs for individual Cases and Submissions, and for individual Validation Criteria. Along with the existing Validation Summary Result record, this provides Admins with the information required to configure, troubleshoot, and manage custom Safety Data Validations without having to submit support requests.
Learn More
- Enablement: Enable Safety Data Validation Exception Handling
- Admin Help: Troubleshooting Validation Criteria
Validation Query Builder: Inbound Object Reference ExpressionsConfiguration23R3.4
Custom Validation Criteria that use the Vault Expression Engine can now validate multiple object records within the same check through the use of Inbound Object References. Also, the Expression Engine can validate Case documents. Previously, this feature supported the validation of data only on a single object record. With this enhancement, Veeva Professional Services and customers can administer their Safety Data Validations without requiring assistance from Veeva Technical Services.
Learn More
- Admin Help: Writing Custom Validation Criteria Rule Formulas
Safety-Clinical Operations Vault Connection: Safety LettersAuto-on23R3.4
The Safety-Clinical Operations Connection now supports Safety Letter distribution from Vault Safety to Vault Clinical Operations. Prior to this, Safety Letter distribution from Pharmacovigilance to Clinical Operations systems was manual and error-prone. With this feature, Safety Letter distribution is done in an automatic and compliant manner.
This feature is Auto-On in Vaults with the Safety-Clinical Operations Connection enabled.
Learn More
- Enablement: Enable the Safety-Clinical Operations Vault Connection: Safety Letters
- User Help: Create a Distribution
Supporting AS2 Connection AdoptionAuto-on23R3.4
With this release, the Safety Gateway includes performance and stability enhancements to support AS2 Connection adoption by all partners and supported health authorities.
This feature was released in 23R3.4 without documentation.
Case Compare: Informative Message for Custom Objects with Duplicate NamesAuto-on23R3.5
When Inbox Items are merged to In-flight Cases or promoted to Follow-up Cases, if any Custom objects have duplicate names when unique names are required, a clear and informative message appears on the Case Compare page. The message lists the records that cannot be merged or copied. Case Processors can choose to cancel the process and correct the records or continue by excluding the custom records from the action. Previously, this setup created a system error that did not offer information on which custom records Admins had configured to require unique values.
Learn More
- User Help: Complete the Inbox Item to Case Compare Page
Case Access Group Security: Inbox Items by CountryAuto-on23R3.5
With this update, you can use Case Access Group Security to restrict viewers of Inbox Items by country. When a User Access Group Assignment record specifies a country, users within that group can see Inbox Items for only that country. Previously, users had the ability to see all Inbox Items, regardless of Reporter Country. This enhancement aligns control of Inbox Item views with Case views.
Learn More
- Admin Help: Manage Case Access Group Security
Case Access Group Security: Support for Localization on Inbound TransmissionsAuto-on23R3.5
Vault Safety now supports Case Access Group security for Inbound Transmissions based on the localization of the associated Case or Inbox Item. This extends existing security controls, ensuring that user access to Inbound Transmissions is localization-specific.
Learn More
Clinical Trials: Isolate Blinded Product InformationSupport23R3.5
This feature lays the foundation for Vault Safety to fully isolate all blinded Product information for Clinical Trial Cases, supporting independent assessment of unblinded Case Products. This approach promotes clinical trial integrity when distributing SUSARs to ethics committees and study sites. Additionally, isolating unblinded Product information facilitates accurate reporting for customers in their downstream reporting hubs.
Note: This feature is currently available to customers only for testing purposes. Contact your Veeva Representative for more information.
Learn More
- Enablement: Enable Clinical Trials: Isolate Blinded Product Information
- User Help: Isolate Blinded Clinical Trial Information
Safety Feature Enablement Updates
Enablement updates apply to the following feature in this release:
Enablement Update: PMDA Local Reporting Details Generation and Submission LinkingAuto-on23R3.4
With this release, the PMDA Local Reporting Details Generation and Submission Linking feature is Auto-on Configuration-enablement. This feature was originally introduced in 23R3 with Support-enablement. Although this feature is Auto-on, some components require additional configuration.
See the following article to learn more about this feature:
Safety & SafetyDocs
Redacted Document TrackingAuto-on23R3.4
With this release, a new standard picklist Redaction field and Redacted-Unredacted relationship have been added to Vault Safety. This allows users to identify documents where Personally Identifiable Information (PII) data has been redacted and relate them to the unredacted source documents. Redacted document tracking facilitates compliance with data privacy regulations.
Learn More
- User Help: Navigate Vault Safety: Library
Create Inbox Item from Literature ArticleConfiguration23R3.4
With this release, Vault SafetyDocs makes literature case processing simpler and more integrated with Safety Management. Literature articles that are processed in Vault SafetyDocs and contain ICSRs can be sent to the Vault Safety Inbox to be processed as cases. This eliminates the need for manual data entry, reducing the possibility of errors and providing an efficient, end-to-end solution for literature case processing.
Note: Your Vault must be licensed for both Vault Safety and Vault SafetyDocs to use this feature.
Learn More
- Enablement: Enable Creation of Inbox Items from Literature Articles
- User Help:
Aggregate Reporting: Combine Tabulations and AuthoringConfiguration23R3.4
With this release, aggregate report line listings and tabulations can be generated in Vault Safety at the Aggregate Report Section level, streamlining the report authoring process. This feature integrates the tabulation generation capabilities of Vault Safety with the report authoring capabilities of Vault SafetyDocs, allowing customers to manage their end-to-end aggregate reporting process from planning to data collection, authoring, approval, and submission tracking in a single system.
Note: Your Vault must be licensed for Vault Safety to generate line listings and tabulations and Vault SafetyDocs to author aggregate reports.
Learn More
- Enablement: Enable Generating Tabulations Using Aggregate Report Sections
- User Help:
SafetyDocs
PSMF: Document DeletionConfiguration23R3.2
When users delete a PSMF Content document, Vault SafetyDocs can now automatically delete the corresponding PSMF logbook and any associated logbook entries. Prior to this release, deleting a document would cause an error because it was referenced in an associated logbook record, requiring users to first manually delete the record. With this feature, Vault SafetyDocs prevents the need for manual tasks and boosts productivity by deleting the related PSMF logbook records together with the PSMF Content documents.
Learn More
- Enablement: Enable PSMF Document Deletion
- User Help: Manage PSMF Binders and Logbooks: PSMF Logbook
PVA: Document Distribution via EmailConfiguration23R3.2
With this release, Vault SafetyDocs extends tracking of document distribution (Aggregate Reports, PSMFs, RMPs, Literature Reviews, Signal Reports, etc.) to include distribution via email. This feature adds a new action to dispatch documents from a PVA Activity or PVA Action Item record. Once this action is initiated, the system generates and sends a Vault-tracked email message with a link that the receiver can use to view the associated documents. This feature helps demonstrate compliance with PVA mandates.
Learn More
- Enablement: Enable PVA Document Distribution via Email
- User Help:
Auto-Translation of Imported Literature AbstractsAuto-on23R3.2
With this release, Vault SafetyDocs can automatically translate non-English literature abstracts that have been imported in bulk from a database. Previously, users could manually send abstracts for translation once they had been bulk imported, but now this process has been automated for greater efficiency.
This feature is Auto-On in Vaults with Literature Abstract Auto-Translation enabled.
Learn More
- User Help: Import Literature References from Database Files: Translate Imported Literature Abstracts
MedDRA Hierarchical Browser for Product-Event CombinationsAuto-on23R3.2
With this release, the MedDRA Browser has been enhanced to permit the coding of MedDRA terms for Product-Event Combinations at any level of the MedDRA hierarchy. Previously, the MedDRA hierarchical term browser for Product-Event Combinations restricted users from coding at any hierarchy level. The MedDRA Browser now features a new picklist that enables Admins to specify the MedDRA hierarchy level in which the system should search, streamlining and improving the MedDRA coding process and providing greater granularity when tracking possible signals.
Learn More
Aggregate Report Task Creation EnhancementsAuto-on23R3.2
When creating Aggregate Report Tasks, the documents attached to them now follow a new naming convention that combines parts of the names of the associated Aggregate Report and Aggregate Report Task. This logic prevents errors on document creation resulting from names exceeding the maximum character limit.
Note: This feature is a building block for expanding future Aggregate Reporting capabilities in Vault SafetyDocs, which will be available for full configuration in an upcoming release. For more information, contact your Veeva representative.
Multiple Logbook Entries per Major Version of PSMF DocumentsAuto-on23R3.4
With this release, Vault SafetyDocs supports the creation and display of up to ten (10) logbook entries for every major version of a PSMF document. Prior to this enhancement, Vault SafetyDocs supported and displayed a single logbook entry per major document version. This enhancement complies with EMA regulations (GVP Module II) and better supports the migration of logbook records from external systems.
Learn More
Exclude Obsolete Documents from the PSMF PDFAuto-on23R3.4
Vault SafetyDocs now excludes obsolete documents with the PSMF Content or PSMF Generated Document subtypes from the merged PSMF PDF. Obsolete PSMF documents are often included in the PSMF binder to retain traceability to their logbook and logbook entries. With this feature, users can include the associated logbook and logbook entries in the PSMF PDF without including the obsolete document. Exclusion of obsolete documents from the merged PDF presents a cleaner and more compliant PSMF.
Learn More
- Enablement: Enable Exclusion of Obsolete Documents from the PSMF PDF
- User Help: Manage PSMF Binders and Logbooks: Manually Generate a PSMF PDF
Document Distribution to Partners via Vault TaskConfiguration23R3.4
Vault SafetyDocs now supports document distribution to partners with Vault Tasks. This type of distribution ensures that the correct version of the document is sent and reduces manual tasks, enhancing traceability and demonstrating compliance with PVA mandates.
Note: The partner must have access to the Vault in which the task is assigned.
Learn More
- Enablement: Enable PVA Document Distribution via Vault Task
- User Help: Distribute PVA Documents via Vault Task
PVA Document Distribution Steady State VerificationAuto-on23R3.4
This update enhances document distribution features by improving the way in which the system verifies that documents are in a steady state before distribution. This change improves the accuracy of document distribution, furthering compliance with PVA mandates.
This feature is Auto-On in Vaults with PVA: Document Distribution via Email enabled.
Aggregate Reporting Feature ConfigurationConfiguration23R3.4
With this release, Aggregate Reporting is now available for full configuration in Vault SafetyDocs. This includes the following previously released features:
- Aggregate Report Authoring
- Aggregate Report Template Management
- Aggregate Reporting: Extract Report to Document
Learn More
- Enablement: Enable Aggregate Report Management
Aggregate Reporting: Section Role AssignmentAuto-on23R3.4
Vault SafetyDocs users are now able to assign and reassign Aggregate Report Sections to other users. The system ensures that the assigned user has access to the section record and associated report document. This feature makes collaboration easier and increases the productivity of aggregate report contributors.
Learn More
Update Label of Generate Aggregate Report Section Creation DialogAuto-on23R3.4
In this release, Aggregate Report Tasks have been renamed to Aggregate Report Sections to better represent their function. The Aggregate Report Task Generation dialog has been updated to the Aggregate Report Section Generation dialog. Other Aggregate Report Task labels are configurable and may be updated by customers directly to align with this terminology.
QualityOne
Document Control
External Collaboration: External Review & Approval of DocumentsConfiguration23R3.4
This feature extends pre-existing QMS External Collaboration functionality to Document Control, allowing users to assign document review and approval tasks to an external person. This feature helps streamline the collaboration process between organizations and external stakeholders, such as suppliers, and can replace less efficient collaboration mechanisms such as email or shared drives.
The External Collaboration process for document review and approval might look something like the following:
- A user indicates on a document whether the document will be sent to an external person for review or approval. If yes, the user selects an organization and can then select an External Collaborator from authorized contacts at that organization.
- When the user starts the relevant document workflow, Vault creates or activates a user account, provisions appropriate permissions, and claims an external user license.
- Vault sends the External Collaborator the applicable Welcome email with information and login details.
- The External Collaborator can add annotations to the document if needed, and provide a verdict and eSignature.
- When the External Collaborator has completed their open tasks in Vault and when the document has reached steady state, the External Collaborator receives a Goodbye email and the user account is automatically inactivated. The external license becomes available again.
This feature is intended for external stakeholders who need infrequent access to review and approve agreements and other documents.
Learn more about working with External Collaboration for Document Review & Approval.
QMS (QualityOne)
HACCP Flow Diagram EnhancementsAuto-on23R3.5
This feature offers multiple improvements to the HACCP Flow Diagram, including:
- New grid view display option to help users more easily align Process Steps and Process Step Connections.
- Ability to display Critical Controls on the diagram.
- Ability to add Ingredients to Material Process Steps on the diagram.
- New Actions menu on Process Steps with options to edit, delete, and mark Process Steps as optional or required.
- Process Step Connection labels now display on the diagram.
- Ability to select the input and output handles of Process Step Connections.
Learn more about the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Supplier Periodic Review (SPR)Configuration23R3.4
With this release, QualityOne QMS extends Periodic Reviews functionality to support Supplier Periodic Reviews (SPR), adding to pre-existing support for the Annual Product Quality Review (APQR) process.
The Supplier Periodic Review (SPR) feature allows users to capture data and complete processes related to a periodic evaluation of a supplier. As part of the SPR process, customers can assign data compilation tasks, set supplier organization attributes such as risk and audit dates, automatically generate documents from reports, and generate a Final SPR Report based on the data collected and stored in the record.
With the QMS Periodic Reviews feature, users can now manage regular evaluations of products (APQR) and suppliers (SPR) in Vault using shared functionality with custom capabilities tailored to each Periodic Review process.
Learn more about Periodic Reviews and performing SPR.
HACCP Flow Diagram: Grouping Process StepsConfiguration23R3.4
This feature enables users to create groups of process steps within a HACCP Plan that achieve a specific business or operational goal. Once created, Process Step Groups display on the HACCP Flow Diagram.
Learn more about the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Preliminary COA Variant Setup PanelConfiguration23R3.4
This feature lays the foundation to offer users a consolidated view for setting up COA Variants directly from the document Library, helping to streamline the user experience and reduce the time required to prepare a COA file for ingestion. The full Variant setup experience will be delivered across multiple releases.
Learn more about defining variants for COA file analysis.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
COA Email Intake EnhancementsAuto-on23R3.4
This feature improves email intake for COA files by preventing multiple users from responding to the same email notification with a COA file. These enhancements ensure that Vault creates the appropriate number of COA Inspection records and notifies users if a COA file has already been successfully received when using Vault Interaction with Email Intake.
This feature is Auto-on in Vaults with Automated Email Intake for Certificate of Analysis already configured.
Learn more about uploading COA files by email intake.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
QMS (QualityOne) & HSE
Auditor Profile & Qualification ManagementConfiguration23R3.4
This feature enables users to capture key demographic information about auditors, manage the qualifications required for auditors to perform any given role on various types of audits, and track auditors’ audit history and progress towards meeting qualifications for an audit role, all within Vault. The resulting benefits are the following:
- Only authorized and qualified auditors are available to select for an audit role on a Team.
- Track an auditor’s progress towards requisite qualifications.
- Better clarity on auditor qualifications, and improved oversight of the pool of qualified auditors within an organization.
- Automatically grant specialized access and eligibility for roles to auditors who meet the qualifications.
Learn more about managing Auditor Profiles & Qualifications.
RegulatoryOne
The RegulatoryOne 23R3.5 release, including all Platform features, is scheduled for tentative availability on March 19, 2024.
Compliance Management
Formulation Composition Viewer: Bulk Create Qualitative AssessmentsConfiguration23R3.4
This feature enables users to create Qualitative Assessments faster and with fewer clicks by allowing them to create up to 15 Qualitative Assessments at the same time for a Formulation from within the Formulation Composition Viewer. This reduces formulation approval time and time-to-market for organizations.
Learn more about the Formulation Composition Viewer.
Regulatory Documents & Registration & Dossier Management
Track Formulation ConcentrationsAuto-on23R3.4
This feature adds the Formulation Concentration object, giving users the ability to track the substance concentrations of a formulation.
Registration & Dossier Management
Requirement Hierarchy Viewer: Display EDL Item DataAuto-on23R3.2
This feature gives users the ability to view EDL Item data, such as the number of expected documents and the number of documents in a steady state, from within the Requirement Hierarchy Viewer. This saves users time by allowing them to view more data directly in the viewer without navigating to various EDL Item records.
Learn more about the Requirement Hierarchy Viewer.
Requirement Hierarchy Viewer: Run Record ActionsAuto-on23R3.2
This feature gives users the ability to run Requirement record actions from within the Requirement Hierarchy Viewer, allowing them to complete more tasks directly in the viewer without navigating to each record’s details page.
Learn more about the Requirement Hierarchy Viewer.
Automatic Document Creation from Template for DossiersConfiguration23R3.4
This feature allows users to automatically create documents from a specified template for all applicable EDL Items in a dossier.
Learn more about automatically creating dossier documents from templates.
Requirement Hierarchy Viewer: Add Matched DocumentsAuto-on23R3.4
This feature gives users the ability to add matched documents to EDL Items in a dossier directly in the Requirement Hierarchy Viewer, without the need to navigate to the EDL Item record.
Learn more about the Requirement Hierarchy Viewer.
Requirement Hierarchy Viewer: Upload DocumentsConfiguration23R3.4
This feature gives users the ability to upload documents and create documents from a template for EDL Items in a dossier directly in the Requirement Hierarchy Viewer, without the need to navigate to the EDL Items record.
Learn more about the Requirement Hierarchy Viewer.
Requirement Hierarchy Viewer: Lock & Unlock Matched DocumentsAuto-on23R3.4
This feature gives users the ability to lock and unlock matched documents to a document version for EDL Items in a dossier directly in the Requirement Hierarchy Viewer, without the need to navigate to the EDL Items record.
Learn more about the Requirement Hierarchy Viewer.
Requirement Hierarchy Viewer: Remove & Exclude Matched DocumentsAuto-on23R3.4
This feature gives users the ability to remove and exclude matched documents from EDL Items in a dossier directly in the Registration Hierarchy Viewer, without the need to navigate to the EDL Items record.
Learn more about the Requirement Hierarchy Viewer.
Generate Requirements Enhancement: Default Field Values from Requirement LibraryConfiguration23R3.4
This feature allows Admins to define object and field mappings from the Requirement Library record to dossier requirement details defined in the Requirement, EDL, and EDL Item objects, and link the Object Mappings to the Generate Requirements action. When the action runs, Vault automatically populates the default field values mapped in the Object Mappings in the generated records, saving users effort in populating those field values.
Learn more about configuring the Generate Requirements action.
Veeva Claims
The Veeva Claims 23R3.5 release, including all Platform features, is scheduled for tentative availability on March 19, 2024.
Veeva Claims
Comment Thread Viewer Enhancement: Timestamp & SortAuto-on23R3.2
This feature improves the overall experience of using the Comment Thread Viewer by introducing two (2) new improvements. Firstly, each comment now displays its creation time and date, providing valuable context to the conversation. Secondly, users can sort comments together with their replies based on the original comment creation date or the most recent comment and reply creation date, providing a more personalized and efficient experience.
Learn more about using comments.
Element Validation for Pack CopyAuto-on23R3.2
This feature introduces validation checks to restrict users from adding local Elements to global Pack Copies and vice versa. Additionally, we provide a configurable job for Admins to help identify any Pack Copies that may violate these new validations so that they can easily rectify those records. This enhancement allows organizations to safeguard data integrity and prevent the inadvertent creation of incorrect information, ensuring a higher level of accuracy and reliability when developing Pack Copies.
Learn more about Pack Copy Management and reviewing mismatched Panel Element records.
Unify Admin Experience for Selective Project AutomationsConfiguration23R3.2
As of this release, we are deprecating the Selectively Create Claims via User Input and Create Local Adaptations via User Input actions, which we no longer support and do not recommend configuring them in your Vault. We have replaced these actions with the Create Claims Run As System and Create Local Adaptations Run As System actions introduced in 23R2 with the Selective Project Automation Enhancements: Actions Run as System feature. The new actions allow users to generate records without requiring Admins to assign specific field-level permissions.
Learn more about configuring selective Claim creation and selective Local Adaptation creation.
Preview Claims & Local Adaptations Before Generating Selective RecordsAuto-on23R3.4
This enhancement makes the process of selectively generating Claims and Local Adaptations less error-prone by allowing users to review their selections for the Selectively Create Claims and Create Selective Local Adaptation actions on a new confirmation page.
Previously, these actions allowed users to selectively create Claims and Local Adaptations of their choice, but users could not review their selections in a consolidated view before generating new records. This process allowed users to accidentally generate records by clicking Continue before reviewing and finalizing their selections. This feature reduces confusion and eliminates unwanted record generation.
Learn more about selective Claim creation and selective Local Adaptation creation.
Dynamic Deep Copy Project Enhancement: Record SelectionAuto-on23R3.4
This feature enhances the Clone Project action to allow users to select records from each related record section to copy over to the new cloned project. Additionally, users can review their selections on a confirmation page before creating the new Project record.
Copying project records to initiate new projects is a common use case among Veeva Claim users. Previously, the Clone Project allowed users to create a new project and automatically copy all records for specified object groups. However, that may include copying unwanted records within those groups. This feature allows users to speed up their project cloning process without creating unwanted records.
Learn more about cloning Projects.
Remove Statement & Associated Records from Project: UI EnhancementsAuto-on23R3.4
This feature introduces several enhancements to improve the overall user experience of the Remove Statement and Associated Records from Project action. Users can now select all records on all pages for each related records section and see the number of records they’ve selected in each section header.
Learn more about removing Statements and related records from a Project.