Release Date: September 23, 2022
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
Working with Documents
Glossary Terms
This feature allows users to select text in a document and search for approved Glossary definitions. Users can also copy the term and definition to the clipboard for easy sharing. Admins can view and report on Glossary activity using the Glossary Events object. Admins can also configure an external search engine that users can search. Learn more about the glossary.
Freeze Headers and Columns in Document Grid Layout
When viewing document search results in the grid layout, the column headers are now frozen so they are always visible when scrolling vertically. Users also have the option of freezing columns to make them always visible when scrolling horizontally.
Vault Objects
HVO Lookup Fields
High Volume Objects (HVOs) now support lookup fields with limitations on faceting, filtering and sorting in comparison to Standard Volume Objects (SVOs).
Lifecycle & Workflow
Multi-Record Workflows
This feature allows users to send multiple records on the same workflow, increasing productivity and improving user experience for task participants. Admins may now define workflows that support up to 100 records of the same object. Additionally, admins may enable existing workflows to support multiple records.
Limit Workflow Task Participants
This feature allows Admins to define a maximum number of participants that may be selected for each participant control defined in the workflow’s start step. When enabled, this prevents users from accidentally assigning a task to a large group of users, for example, All Internal Users.
Pre-Populated Values in Document Workflow Field Prompts
Vault now pre-populates document field values in Start and Task step field prompts and single verdict prompts in workflows on a single document when values are present in the related document field.
Reporting & Dashboards
Document with Object Report Types
This feature allows customers to create reports on documents and their associated object records. When Admins define Document as the primary object in a report type, they can now add additional objects to the report type with an up or down relationship. Vault supports the addition of single- and multi-value outbound relationships to Document report types. For objects referenced in document fields, Vault shows the object relationship as an up object if the field is single-value and as a down object if the field is multi-value.
Control Charts
This feature allows users to visualize report data as a control chart. Similar to other dashboard charts, users can generate control charts using reports that contain at least one grouping. Vault completes sigma calculations automatically and highlights them in the chart. Users can also manually add a target line to compare with historical data.
Formulas
Object Reference Fields in Expressions
This feature allows Vault expressions to use object reference fields. Users may wrap an object reference field in the Text() function or the ID() function to return the record label or ID as text respectively. Object reference fields that are not wrapped in Text() or ID() will return the object reference directly. Existing uses of object reference fields that return the record ID or the record label as text will continue to return the same value.
Usability & UI Updates
Enterprise Home Page: New Grid View with Updated Card View
With this release, in addition to the current card view, a new grid view for the Vault Enterprise Home page (aka the My Vaults page) is available. Users can toggle between the new grid view and updated card views, which has an enhanced user interface that allows users to quickly find the right Vault to open. The grid view provides search and sort capabilities for users with access to a large number of Vaults. Additionally, the Vault selector picklist and the Enterprise Home Page card and grid views now indicate if a Vault is in maintenance or configuration mode when not available.
The new Enterprise Home Page is only visible to users with access to limited release Vaults. It will be visible to all users when the My Vaults page is enabled on their home domain in 22R3.0.
Search
Precision Highlighter
Highlighting search terms within search results now bolds matching search terms beyond the exact terms typed into the search box. When a result is matched due to stemming, the inflected form of the word is now highlighted in the results. For example, if a search for “early” matched a document with the term “earlier”, “earlier” will now be bolded in the results.
This update also highlights terms that match because of synonyms. In this case, a search for “IB”, will bold “investigative brochure” in the results if there is an entry for that synonym in the thesaurus.
Access Control
Add Delegates to a User: Performance Optimization
This feature optimizes the performance of the drop-down selection for adding new delegates to a user, which is especially useful for Vaults with a large number of users. A banner displays on the Active Delegations page when delegate selection optimization is in progress.
Delegate Access Feature: Enablement Available in all Vaults
This release, we made the Delegate Access feature available in all limited release Vaults. It will be enabled in general release Vaults in 22R3.0.
If not previously enabled, the feature is turned off by default, and can be turned on by an Administrator in Admin > Settings > General Settings.
Domain Users: UI Enhancements
The domain administration user interface (Admin > Users & Groups > Domain Users) has been enhanced to scale on domain with hundreds of Vaults. New capabilities include inline edit of Vault memberships, an enhanced add membership flow, and the ability to export a user’s Vault memberships.
Administration
Editable Vault Name
With this release, administrators can edit the Vault Name field on the Vault General Settings page (Admin > Settings > General Settings). Additionally, the General Settings page now displays Vault URL.
Query Governor
To ensure Vault stability and performance, VQL now enforces constraints on expensive VQL queries. These changes affect API version 22.3 and higher, and existing integrations are not affected. Learn more in the Developer Release Notes.
Person Object: Duplicate Person Record Detection
This feature helps detect the creation of duplicate Person records by alerting users of potential duplicates when Person records are created in Business Admin or a standalone Person tab. Admins may review and edit duplicate matching criteria in the Settings tab.
Notifications: Email Notification Status
This feature allows admins to view the delivery status of email notifications on a new page in the Operations tab. Admins can filter the list of email notifications by date, email address, and status as well as export it to CSV.
Notifications are now only retained within the Vault database for 2 years. Notifications older than 2 years are available for download as zipped CSV files.
Notifications: Read & Unread status
This feature adds a Read and Unread status for each web notification. Users can mark unread notifications as Read from the Notifications panel and Notifications page.
Document Related Configuration Settings Added to Audit Trail
With this release, changes to the following document-related configuration settings now have audit events that display in the System Audit History:
- Maximum characters for exported file name (including extension)
- Maximum characters for exported folder name
- Maximum characters for exported document path
This functionality was released in 22R2.2 without documentation.
Scheduled Data Exports: Update Scheduled Start Time
In this release, there is the added ability for an Admin to change and set the start time of a Scheduled Data Exports job.
Scheduled Data Exports: Document Metadata in Default Alphabetic Order
Scheduled Data Exports of document and document version metadata now have a default alphabetic order for column headers.
Scheduled Data Exports: Export Picklist Labels and Names
Scheduled Data Exports of document, document version, and object metadata now include picklist names and labels.
Test Data Packages: Override Default Reference Lookup Fields
When creating Test Data packages, Admins can now override the default reference lookup to an object with any one of the unique fields available for the object. Previously, this was only available for Migration Packages.
Configuration Migration
Outbound Packages: Support Migrating User Group Data
In this release, there is added support for including User Groups in Configuration Migration Packages and Test Data Packages. Configuration can reference Groups and you can now include Groups as Data Steps. Groups will also be processed and deployed according to dependencies.
Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Platform data model to support new features:
- Added the Outbound Email Status (
outbound_email_status__sys) field to the Person (person__sys) object to support the Outbound Email Address for Persons feature - Added the following objects to support the Glossary Terms feature:
- Definition (
glossary_definition__sys) - Glossary Event (
glossary_event__sys)
- Definition (
Vault Connections
RIM to Clinical Operations: Transfer of All Document Versions to Clinical
This feature adds the ability to transfer all steady state and superseded versions of a document that occur within a 5-minute job run window instead of only the latest steady state version when the connection job runs. This will be implemented on the Clinical side of the connection only. This feature is enabled by an Admin setting.
Customers should consider this feature only if they have cases where document versions are created in such rapid succession (e.g. less than five minutes) that earlier steady state or superseded versions are in scope for transfer.
Clinical Operations
Study Startup, eTMF
Clinical Creation of Documents from Email Attachments
This feature leverages platform email ingestion functionality to support inbound email addresses that create an unclassified Vault document for each attachment included in the email that is sent to Vault.
TMF Bot: Auto-Classification Enhancements
Every release we target enhancements to TMF Bot Auto-classification functionality. This feature includes adding support for Documents created from Email Attachments. Learn more about email processing.
Vault Payments
Automate Payment Adjustments
With this feature, Vault Payments customers can now automate support for adjusting Payable Items. When enabled, Subject Visits, Procedures, or Site Fee changes will now trigger an automated adjustment process. Additionally, existing Payable Items can be adjusted when a new Fee Schedule is Approved in the system. Learn more about Vault payments and configuring Vault payments.
Payment Limits evaluate across Fee Schedules with Date Effectivity
With this feature, Vault Payments now evaluates Fees with Payment Limits, e.g. Maximum or Rate limits, across multiple Approved Fee Schedules at a Study Site. Payable Items related to Fees with the same name, regardless of Fee Schedule, are now considered in evaluating and determining the eligibility of generating additional Payable Items. Learn more about Vault payments.
Generate Payable Items available in Fee Schedule Workflows
Vault Payments customers can now initiate the “Generate Payable Items” functionality via a workflow on a Fee Schedule record. Learn more about Vault payments.
SiteConnect
Site Connect to Inbox
With this feature, Site Connect customers can designate that certain document artifacts received from SiteVault are always created as Unclassified documents. Additionally, when the Clinical Operations vault is missing a mapping to the necessary Vault Clinical Docs artifact any documents received from SiteVault will now get created as Unclassified documents. Learn more about Learn more about configuring Site Connect.
Site Connect Single-Study docs
With this feature, Site Connect Admins can ensure that any documents received from SiteVault are always created with a single Study value. An incoming document related to multiple studies in SiteVault will be created as multiple documents in Clinical Operations, each with a single Study value. Learn more about Learn more about configuring Site Connect.
Site Connect: Additional Vault Clinical Docs Support
This feature adds support to transfer new document types to SiteVault via Veeva Site Connect.
- SAE Report Form Template
- Pregnancy Report Form Template
- Special Events of Interest Form Template
- Participant Adverse Event Log
- Protocol Deviations
- Investigator Brochure Summary of Changes
Clinical Operations Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Clinical Operations data model to support new features:
Commercial
PromoMats
Add Supporting Documents to eCTD Compliance Packages
This feature allows Vault to automatically pull in supporting documents while generating a Pre Clearance or Post Marketing eCTD Compliance Package. Supporting documents must be one of the new standard Supporting Documents document subtypes and must be linked to the promotional material through the new document relationship eCTD Support Documents. Users can also add additional Clean Materials without duplicate lines on the FDA 2253 form using a new document field.
Add Promotional Documents to eCTD Compliance Packages
With this release, users can add promotional materials to an existing eCTD compliance package. This release also introduces a new user action that allows users to select one or many documents from the library to add to the Compliance Package. Vault regenerates the Compliance Package to include submission ready copies of the new documents, new supporting documents, and linked references when applicable.
Modular Content & Claims Data Model Change
This feature adds new configuration to PromoMats to make the creation, management, and usage of Text Assets (Claims & Reusable Text records) and Content Modules simpler and more intuitive.
Enhanced Suggest Links
This feature improves the Suggest Links matching functionality and introduces the ability to build the Suggest Links action into the lifecycle process. This feature also introduces support for matching Text Assets related to Multiple Countries & Products while using Suggest Links. Learn more about Enhanced Suggest Links functionality.
Copy Source of Text Assets
This feature introduces a new standard field, Source Text Asset, on the annotation_keywords__sys object. This field automatically populates on the newly created record with the source record when it is created via a Copy Record action.
Multi:Product & Multi:Country Support for Text Assets (Claims and Reusable Text)
This feature introduces two (2) standard join objects, Text Asset Country and Text Asset Product, that allow users to relate multiple countries and products to their Text Asset object records. This feature also introduces two (2) new Admin checkboxes to enable automatic join record creation. When enabled, Vault automatically creates join records when creating a new Text Asset record or editing an existing Text Asset record.
Multi:Product & Multi:Country Support for Content Modules
This feature introduces two (2) standard join objects, Content Module Country and Content Module Product, that allow users to relate multiple countries and products to their Content Module object records. This feature also introduces two (2) new Admin checkboxes to enable automatic join record creation. When enabled, Vault automatically creates join records when creating a new Content Module record or editing an existing Content Module record.
Multichannel
Asynchronous Auto Publish to CRM for Approved Email Fragments
With this release, Vault automatically creates email fragments asynchronously, enhancing the Auto Publish to CRM for Approved Email Fragments feature. Vault notifies users when fragments are created and available for use within Vault.
Automated Image Rendition Setting Removal
With this release, we have removed the Application Setting checkbox for “Enable Automated Image Renditions”. The Automated Image Renditions feature continues to provide high quality Image Renditions via configuration of Rendition Types without any changes to the existing feature or configuration. In addition, the “Enable enhanced PSD file image conversion” Application Setting is now available for use without the prior enablement of the “Enable Automated Image Renditions” setting. The maximum number of Automated Image Rendition Rendition Type that you can create is also increased to 25.
High Resolution Presentation Slides for CRM & Engage
In this release, Vault allows high resolution generation of CLM Presentation Slides for use with CRM and Engage. A new Application Setting to “Render Presentation Slides in High Resolution” allows Vault to render newly created CLM presentation slides as 96 DPI.
Product & Country Become Shared Fields
The Product (product__v) and Country (country__v) document fields are now shared document fields. Admins can remove the fields from the Base document and assign them to explicit document types, subtypes, and classifications. Assigning these document fields to explicit document types increases the flexibility to leverage VQL constraints.
This feature was postponed to a future release.
Commercial Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added the following components to the Commercial data model to support new features:
Medical
MedComms
Related ISI and Related PI Relationships added to Base Document Type
This feature adds the new standard Related ISI and Related PI relationship types to the Base document type and makes them available for use on all document types. For Vaults where these standard Relationship Types do not exist, we have added the ability to activate these standard Relationship Types and add them to the base document type. Learn more about the Related ISI and Related PI relationship types.
Product & Country Become Shared Fields
The Product (product__v) and Country (country__v) document fields are now shared document fields. Admins can remove the fields from the Base document and assign them to explicit document types, subtypes, and classifications. Assigning these document fields to explicit document types increases the flexibility to leverage VQL constraints.
Multichannel
Asynchronous Auto Publish to CRM for Approved Email Fragments
With this release, Vault automatically creates email fragments asynchronously, enhancing the Auto Publish to CRM for Approved Email Fragments feature. Vault notifies users when fragments are created and available for use within Vault.
Automated Image Rendition Setting Removal
With this release, we have removed the Application Setting checkbox for “Enable Automated Image Renditions”. The Automated Image Renditions feature continues to provide high quality Image Renditions via configuration of Rendition Types without any changes to the existing feature or configuration. In addition, the “Enable enhanced PSD file image conversion” Application Setting is now available for use without the prior enablement of the “Enable Automated Image Renditions” setting. The maximum number of Automated Image Rendition Rendition Type that you can create is also increased to 25.
High Resolution Presentation Slides for CRM & Engage
In this release, Vault allows high resolution generation of CLM Presentation Slides for use with CRM and Engage. A new Application Setting to “Render Presentation Slides in High Resolution” allows Vault to render newly created CLM presentation slides as 96 DPI.
Product & Country Become Shared Fields
The Product (product__v) and Country (country__v) document fields are now shared document fields. Admins can remove the fields from the Base document and assign them to explicit document types, subtypes, and classifications. Assigning these document fields to explicit document types increases the flexibility to leverage VQL constraints.
Medical Data Model Changes
The following changes were made to MedComms Vaults:
- The Country (
country__v) document field is now a shared document field - The Product (
product__v) document field is now a shared document field - Added the Related ISI (
related_isi__v) relationship type to the Base document type - Added the Related PI (
related_pi__v) relationship type to the Base document type
Training
Study Training receives functionality and data model updates in parallel with the Quality Suite: Vault Training application.
Study Training
Training Matrix Builder
This feature allows an authorized user to rapidly assemble the training needs for a given Study by specifying the training materials that each study team member needs to complete training on.
Using an intuitive, guided process, the user can build and publish a study-specific training matrix. After the training matrix is published, the application will automatically assign training tasks to each study team member. Learn more about the matrix builder.
Quality
QualityDocs
Process Navigator: Show Associated Process Documents
This enhancement adds a new checkbox option in the Documents section of the Process Navigator detail page that expands the section view to include documents associated with the current process navigator hierarchy as well as its immediate parent and children processes. This feature introduces a new Associated Processes column in the Documents section that displays the process navigator hierarchies that a document is associated with. Additionally, the default non-editable columns that display in the Documents section have been pared down to the Name and Associated Processes columns only.
Note: With this release, any previously saved column display settings in the Documents section of the Process Navigator detail page will be removed due to the change in the default columns that display. To add desired columns, a user can click the ellipsis (…) above the Documents section and select Edit Columns.
QMS
Set Role Permissions on Related Object
This feature enhances existing QMS security to allow Admins to configure an entry or user action that assigns the appropriate application role, such as Viewer, on a related object record to the user(s) assigned to a defined role on an associated object record. This ensures that the user(s) who are assigned to the object record, such as a CAPA action, will be able to access and view the related object record, such as the related Deviation, to assist in performing their assigned tasks while still preventing them from being able to access and view unrelated records. Learn more about configuring this action.
Duplicate Detection Check Data Model
This feature provides the data model required to support the upcoming Duplicate Detection Check feature that will be available for Quality Event: Complaints and MedTech Complaints as well as standalone Complaints.This data model update consists of the following new components:
- A standard Record Check Rule Detail object
- A Complaint object reference field on the Record Check Result object
- A Complaint object reference field, a Record Check Rule Detail object reference field, and an Is Original boolean field to the Record Check Match Record object
Set From Email Attributes on Complaint Emails
External Notifications sent for Complaints or Medtech Complaints on the Quality Event or Complaint object now support customizable From: email addresses. For example, instead of emails appearing like they are sent from Vault, customers have the ability to utilize a From: email address that appears that the email was sent from Verteo Complaints.
This feature also automatically enables the Outbound Email Address feature which allows Vault Admins to configure Outbound Email Addresses.
External Notification: Support for Standard & Custom Object Types on Quality Event & Audit Objects
With this enhancement, the existing External Notification feature now supports all standard and custom object types on the Quality Event and Audit objects, and allows users to send notifications as well as share documents to Vault Users as well as non-Vault users through the Send External Notification action.
Complaints: Relationship Automation, Recurrence Check, & Checklist Support
This feature extends support for Relationship Automation, Recurrence Check, and Checklists features to also support the standalone Complaints data model. This feature automatically provisions objects to support Checklists on the Complaint object.
Part, Asset & Service Data Model Updates
New standard objects have been created to replace the object types of the context object. The following standard objects are now available:
- Part (
part__v) - Asset (
asset__v) - Service (
service__v) - Quality Event-Asset (
quality_event_asset__v) - Quality Event-Part (
quality_event_part__v) - Quality Event-Service (
quality_event_service__v) - SCAR-Asset (
scar_asset__v) - SCAR-Part (
scar_part__v) - SCAR-Service (
scar_service__v) - SCAR-Material (
scar_material__v)
Training
Prevent Delegate Completion
Delegate users will no longer be able to complete training on behalf of a user.
Option Not to Issue Quiz for Document Revision Training
This feature allows Admins to decide whether a quiz, if configured on the Training Requirement, is included when a Training Assignment is created due to document revision. Training Assignments created due to reasons other than document revision will always receive the quiz.
Disallow Quiz Design Population for Classroom Training Requirements
Users are now prevented from misconfiguring Classroom Training Requirements by populating the Quiz Design field on a Classroom Training Requirement.
Note: This feature does notremove the ability to add a Quiz to a Classroom Training Requirement. It simply enforces that a Quiz is added in the correct place, rather than through the Quiz Design field.
Vault LIMS
Vault LIMS continues to expand its available features in this release, including the following additional functionality:
- Continued advancement to creating static data.
- Users can manage in-process Material and Batches, including automated logging via Lab Protocols.
- Lab Tests can now capture Consumables and Instruments.
- Users can now track shipping details for Lab Samples.
- Calculations can be created and executed in testing.
Learn more about Vault LIMS.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Quality data model to support new features:
The following components were added to support the Complaints: Relationship Automation, Recurrence Check, & Checklist Support feature:
- Complaint Checklist (
complaint_checklist__sys) - Complaint Dependency (
complaint_dependency__sys) - Complaint Sub-Checklist (
complaint_subchecklist__sys) - Complaint Checklist Design (
complaint_checklist_design__sys) object type on Checklist Design Object
The following components were added to support the Set From Email Attributes on Complaint Emails feature:
- From (
from__v) on Complaint Sent Notification object - From (
from__v) on Quality Event Sent Notification object - Complaint (
complaint__v) field on Record Check Result object - Related Complaint (
related_complaint__v) field on Record Check Match Record object - From Email Address (
from_email_address__v) object type on Person object
Regulatory
RIM Submissions
Drag & Drop Documents from Desktop to Content Plan Item
With this release, users can drag and drop one or more files from a file explorer to a Content Plan Item in the Content Plan Viewer to upload and create documents in Vault and match to the Content Plan Item. The document creation and matching progress is tracked in real time with a toast dialog, allowing users to navigate to other pages within Vault while the process completes. Additionally, when duplicate detection is enabled, existing documents with the same checksum as a dropped file will be matched instead of creating duplicate documents.
Create Content Plan as Inactive
With this feature, a new configurable checkbox is available on the Create Content Plan and Content Plan Items system action. When configured, all Content Plan and Content Plan Item records are created in the Inactive lifecycle state, except for Content Plan Items where the source Content Plan Item Template record field Create as Active is set to true. Starting with an entirely inactive Content Plan and activating the records which need to be submitted allows small/daily submissions to be assembled more quickly.
Inactive Content Plan Records Cleanup Job
With this release, a new daily job, Delete Inactive Content Plans, is available for RIM customers. The job deletes inactive Content Plan and Content Plan Item records which meet the following criteria:
- Status is “Inactive”
- The Content Plan Item’s All Document Count (
all_doc_count__v) equals zero (0) - For submission content plans, the Submission record’s Actual Submission Date (
actual_submission_date__rim) is at least 365 days earlier than the current date - For report level content plans, the Last Published Date (
last_published_date__v) is at least 365 days earlier than the current date - For Global Content Plans, the Event Complete Date (
event_complete_date__v) is at least 365 days earlier than the current date
Dispatch Global Content Plan Harmonization: Application Relationships
With this release, the Dispatch Global Content Plan action has been updated to be compatible with the new data flow introduced with the Submission Wizard in the 22R1 release. In Vaults where the Enable Application Relationships setting is enabled, this process will leverage the Application relationships as the source data for creating Submission relationships.
Update Set Version Locking on Matched Documents Action
With this release, the Include Latest Lockable State Version configuration option is available for the locking action types on the Set Version Locking on Matched Documents user action. When configured, these actions lock the latest lockable-state version of a matched document instead of only the latest steady state version.
Deprecate FDA Form 2253
The FDA Form 2253 feature is now deprecated in Vault RIM. The form should be generated in PromoMats and CrossLinked to RIM via the RIM to PromoMats Vault Connection.
RIM Submissions, RIM Submissions Archive
Set Blank Active Dossier Item Country Status
With this release, the Set Active Dossier Item Country Status system action has been updated to allow for setting a blank value. This can be leveraged to clear out the Submitted status for a country on Active Dossier Item records when the related regulatory objective has been withdrawn or rejected.
RIM Submissions Archive
EAEU Import Updates
This update to the RIM Submissions Archive EAEU dossier type submission display supports the latest amendments to the Rules for Registration and Examination of Medicinal Products for Medical Use in EAEU.
RIM Registrations
Bulk Create Activities, Submissions, and Regulatory Objectives Wizard Linking Enhancement
This feature allows users to follow a two-step process in their execution of change controls and corresponding usage of the Create Related Records wizard. They can create an Event and corresponding Activities to first assess the extent of changes needed and finalize related data. Then, they can use the Create Related Records wizard to create Regulatory Objectives and Submissions linked to Activities with impact identified to facilitate regulatory actions and cascade finalized supporting data from the Event.
EUDAMED Business Rule Validation
With this release, UDI submissions can be validated against business rules published by the European Commission to surface issues that would result in a rejection by EUDAMED.
IDMP/DADI Data Model Updates
This feature updates source objects, output objects, and the data model algorithm supporting IDMP per EU IG v2.1 and the DADI form for human variations.
RIM Publishing
Taiwan eCTD DTD 1.0 Publishing & Validation
With this release, RIM Submissions Publishing supports the TW FDA v1.1 (DTD 1.0) specification. Users can now create Content Plans and generate submissions that are compliant with TW FDA v1.1 specification. Vault validates these submissions based on the corresponding TW FDA v1.0 Validation Criteria Version.
US FDA Validation Criteria v4.4 Support
With this release, Vault Submissions Publishing supports validation of US submissions against the US FDA validation criteria version 4.4.
Reference Leaf UI
With this release, users are provided with an interface to set a reference leaf. The user action shows a hierarchical view of published submissions containing only eligible documents for the user to select.
RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model to support new features:
Safety
Safety features are targeted for tentative availability on September 29, 2022.
Safety
Disable Auto-Calculations on Case Promotion Admin Checkbox
You can now bypass the calculations that occur automatically during Case promotion for Inbox Items received through AS2 gateway transmissions or Vault REST API. A new setting is introduced on the Transmission Profile, which administrators can use to control this functionality.
Learn More
- Enablement: Enable Inbox Item Follow-Up Case Compare: (22R3) Disable Auto-Calculations
- User Help: Inbox Item Follow-Up: Auto-Calculations
Automated Case Promotion for API Transmissions Configuration
Support for Automated Case Promotion is extended to Safety Intake API (E2B and JSON) sources by leveraging Transmission Profiles for settings.
Learn More
- Enablement: Automated Case Promotion
- User Help: Manage Transmission Profiles
IMDRF: Dictionary Maintenance, Case Processing, and Submissions Configuration
Vault Safety now supports all code types for the International Medical Device Regulators Forum (IMDRF) and the management of dictionary updates. When entering a Case Product Device Problem, you can select the Device Code Type and then search for the Device Code from the IMDRF Dictionary. In addition, when generating FDA E2B(R2), FDA VAERS(R3), and FDA MedWatch 3500A forms, the system can export multiple IMDRF device codes, prioritizing the lowest-level Medical Device Problem codes.
Learn More
- Enablement: Enable the IMDRF Dictionary for Maintenance, Case Processing, and Submissions
- Admin Help: Manage the IMDRF Dictionary
- User Help: Enter Case Data: Case Product Device Code
Remedial Actions and Malfunction for Combination Products Configuration
Vault Safety now supports data entry for Remedial Actions and Malfunction at the device-level, as well as device codes for Cases that involve multiple Combination Products or a Combination Product that has multiple device constituents. Case Product Remedial Action and Malfunction can be exported in Medwatch 3500A, FDA E2B(R2), and VAERS E2B(R3) formats.
Learn More
- Enablement: Enable Remedial Actions and Malfunction for Combination Products
- User Help:
Localized Case Child and Grandchild Record Deletion from Case Support
Previously users were required to delete localized children manually before deleting their global versions (i.e. Local Dosage prior to removing a Dosage record). With this enhancement enabled, Localized children will be deleted when global case children are deleted. Contact Veeva Support to enable this behavior in your Vault. In the 22R3 release (December 2022), administrators will be able to enable this behavior through configuration.
Learn More
- Enablement: Enable Localized Record Deletion
Domestic Case Processing and Submission: Report Type-Based Localization Support
Vault Safety now supports the ability to configure localizations by Case Type. For example, this enhancement allows for the localization of Postmarket Cases for the following countries:
- Switzerland
- Czech Republic
- Spain
- Canada
Contact Veeva Support to enable this enhancement in your Vault. In the 22R3 release (December 2022), administrators will be able to enable this enhancement through configuration.
Learn More
- Enablement: Enable Domestic Case Processing for Agency Jurisdictions
- User Help:
MedDRA Bulk Recode Cases Configuration
Vault Safety now supports bulk recoding of MedDRA-coded terms on Cases. When the MedDRA Dictionary is updated to the latest version, admins can now use the Dictionary Bulk Recode feature to upload replacement terms and preview impacted terms in Cases prior to the bulk recode. Bulk recode can be applied to open and closed Cases, where changes are tracked in the audit trail.
Learn More
- Enablement: Enable MedDRA Bulk Recode Cases
- User Help: Bulk Recode MedDRA-Coded Terms
Cross Reporting Most Conservative Submission Support
With this feature, Vault Safety will support the evaluation of additional cross-reporting scenarios for situations where there are multiple cross-reporting obligations within the same jurisdiction.
EMA PII Masking Exceptions Auto-on
With this release, Vault Safety now automatically supports EMA-compliant PII Masking specific to when Patient Content Protection is used for an EMA Submission.
Learn More
- User Help: Generate Masked Safety Reports
Distribution Reporting Obligation Evaluation for Study Cases with Non-Study Co-Suspect Products Auto-on
This release ensures the “Most Conservative Case Product and Case Assessment” reporting rules evaluation methods correctly generate Distributions for Study Cases with suspect or interacting marketed Case Products, in addition to suspect Study Product(s).
Learn More
- User Help: Configure Reporting Rules Product Selection: Most Conservative Case Product and Case Assessment
Generate Transmissions and Auto-Submit Enhancements Admin Checkbox
Administrators can now configure the system to suppress document generation for transmissions eligible for auto-submit. This resolves a possible race condition if the Evaluate Submission Obligations and Submit to Gateway actions are triggered in rapid succession.
Learn More
EDQM Dictionary Updates
The European Directorate for the Quality of Medicines & HealthCare (EDQM) has updated its standard terminology for the Dose Form and Routes of Administration (RoA) data elements on E2B(R3) Submissions. As a result, Vault Safety has implemented the following changes to its standard EDQM Mappings.
New Records:
|
Updated Record:
|
QualityOne
QMS
Multiple COA Attachment Support for Automated Email Intake
This feature extends External Collaboration to allow external users, such as suppliers, to submit multiple Certificate of Analysis (COA) files to Vault. The External Collaborator can reply to a Vault email notification with multiple attachments for upload and analysis for a given COA Inspection object record. Learn more about COA email intake.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Enhancing In-Process Inspection Object Actions to Exclude Samples
This feature allows users to exclude Inspection Samples from In-Process Inspection analysis when users trigger the Analyze Inspection and Analyze Inspection Sample actions. For In-Process Inspections, whenever new Inspection Samples are generated using the Generate Samples action, Inspection Samples created and generated previously will be marked for analysis exclusion. For other Inspection object types, users can manually exclude Inspection Samples by selecting the “Yes” value in the Exclude from Analysis field. Learn more about excluding samples.
Object Sharing Rule Record Uniqueness
This feature improves maintenance of Object Sharing Rule records by preventing the creation of duplicate records containing the same combination of User, Organization, and Application Role values. Learn more about COA Inspections.
COA Inspection Enhancements
These features are part of our ongoing and continual investment into being able to reliably ingest a wider range of COA formats. Learn more about COA Inspections.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Table Cell Consistency & Format
This feature enhances Vault to more reliably process COA files when there is no explicit demarcation between rows of data.
HACCP Management Data Model Enhancement
We’ve introduced the Process Step Connection and HACCP Plan Process Step Connection objects to allow users to define the flow between the process steps of a particular process, and store similar information for HACCP Plan process steps. The flow can be managed by defining the input steps and output steps of individual process steps.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Document Control
QualityOne Process Navigator
Process Navigator provides a visual interface for users to navigate through processes and supporting documents in QualityOne. Hierarchies will allow users to create and define customized visual hierarchies using a standard data model, and to associate documents to various levels of hierarchies. Learn more about Process Navigator.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on October 4.
Registration & Dossier Management
Local Impact Assessment Registration Selection Enhancements: Resize & Edit Columns
This feature enhances the Search Registration dialog to allow Admins to add and remove columns displayed to users when they run the Local Impact Assessment action. Users can also resize columns on the list of Registrations they see in the dialog.
Global Change Impact Assessment
This feature enables users to see a list of potential Registration Items that must be assessed after a global operational change, such as a supplier change or a label change.
Users can select a set of registration attributes for an event and define a change from one value to another. Vault displays a list of existing Registration Items that that are potentially impacted by the operational change and may require registration activities such as a submission for a new registration or an amendment to an existing Registration. Learn more about Global Change Impact Assessments.
Copy & Amend Dossier
With this feature, users can re-use existing dossiers instead of creating new ones every time. This can be beneficial when you must create new local dossiers from a global dossier or when you want to copy a previously submitted dossier and re-submit it with modifications, while keeping track of both versions. Learn more about reusing shared requirements.
Veeva Claims
Veeva Claims features are targeted for tentative availability on October 4.
Veeva Claims
Bulk Add Elements to Pack Copy
This feature enables users to add multiple Element records to multiple Panels of an existing Pack Copy using a bulk object record action. When configured, users can select Add to Pack Copy after selecting the option to perform a bulk action from the All Actions menu. Learn more about adding Elements to pack copy.
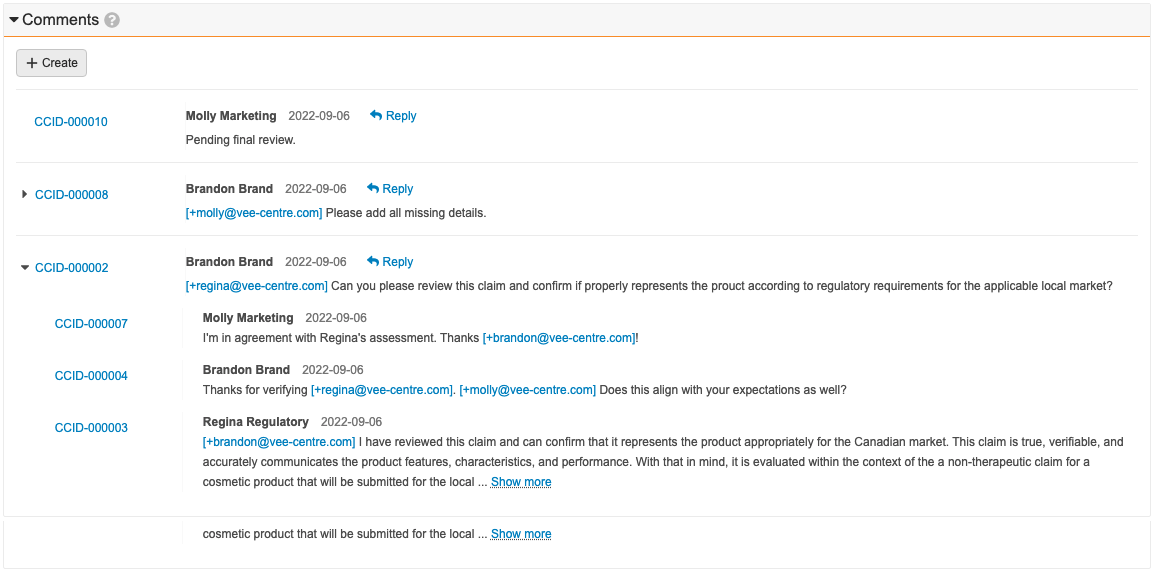
Comment Threads for Claims & Projects
This feature enables users to capture and visualize discussions and decisions on Claims and Projects in a new single-level threaded comment view. This new section allows users to create new comments and reply to existing comments. Users can use existing comment functionality to mention and tag other users. Learn more about comment threads for Claims and Projects.
Comment Threads for Objects
This feature enables users to capture and visualize discussions and decisions on Local Adaptations and Pack Copies in a new single-level threaded comment view. This new section allows users to create new comments and reply to existing comments. Users can use existing comment functionality to mention and tag other users. Learn more about configuring comment threads for objects.
Panel Element Hierarchical Copy
In this release, we have added the ability for Admins to enable the Allow hierarchical copy attribute for the Element (element__v) and Panel (panel__v) field on the Panel Element (panel_element_join__v) object.
Project Hierarchy Enhancements
This enhancement enables users to filter the project hierarchy only on the Claim > Statement values, which are joined with the Project. Learn more about the Project Hierarchy Viewer.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| Working with Documents | ||
| Glossary Terms | Auto-on | Platform |
| Freeze Headers and Columns in Document Grid Layout | Auto-on | Platform |
| Vault Objects | ||
| HVO Lookup Fields | Configuration | Platform |
| Lifecycle & Workflow | ||
| Multi-Record Workflows | Configuration | Platform |
| Limit Workflow Task Participants | Admin Checkbox | Platform |
| Pre-Populated Values in Document Workflow Field Prompts | Auto-on | Platform |
| Reporting & Dashboards | ||
| Document with Object Report Types | Configuration | Platform |
| Control Charts | Auto-on | Platform |
| Formulas | ||
| Object Reference Fields in Expressions | Auto-on | Platform |
| Usability & UI Updates | ||
| Enterprise Home Page: New Grid View with Updated Card View | Auto-on | Platform |
| Search | ||
| Precision Highlighter | Auto-on | Platform |
| Access Control | ||
| Add Delegates to a User: Performance Optimization | Auto-on | Platform |
| Delegate Access Feature: Enablement Available in all Vaults | Auto-on | Platform |
| Domain Users: UI Enhancements | Auto-on | Platform |
| Administration | ||
| Editable Vault Name | Configuration | Platform |
| Query Governor | Auto-on | Platform |
| Person Object: Duplicate Person Record Detection | Auto-on | Platform |
| Notifications: Email Notification Status | Auto-on | Platform |
| Notifications: Read & Unread status | Auto-on | Platform |
| Scheduled Data Exports: Update Scheduled Start Time | Auto-on | Platform |
| Scheduled Data Exports: Document Metadata in Default Alphabetic Order | Auto-on | Platform |
| Scheduled Data Exports: Export Picklist Labels and Names | Auto-on | Platform |
| Test Data Packages: Override Default Reference Lookup Fields | Auto-on | Platform |
| Configuration Migration | ||
| Outbound Packages: Support Migrating Group Data | Auto-on | Platform |
| Platform Data Model Changes | Auto-on | Platform |
| Vault Connections | ||
| RIM to Clinical Operations: Transfer of All Document Versions to Clinical | Admin Checkbox | Vault Connections |
| Clinical Operations | ||
| Clinical Creation of Documents from Email Attachments | Configuration | Study Startup, eTMF |
| TMF Bot: Auto-Classification Enhancements | Auto-on | eTMF |
| Automate Payment Adjustments | Admin Checkbox | Vault Payments |
| Payment Limits evaluate across Fee Schedules with Date Effectivity | Auto-on | Vault Payments |
| Generate Payable Items available in Fee Schedule Workflows | Configuration | Vault Payments |
| Site Connect to Inbox | Auto-on | Site Connect |
| Site Connect Single-Study docs | Admin Checkbox | Site Connect |
| Site Connect: Additional Vault Clinical Docs Support | Configuration | Site Connect |
| Clinical Operations Data Model Changes | Auto-on | CTMS, Site Connect, Study Startup, Vault Payments, eTMF |
| Commercial | ||
| Add Supporting Documents to eCTD Compliance Packages | Configuration | PromoMats |
| Add Promotional Documents to eCTD Compliance Packages | Configuration | PromoMats |
| Modular Content & Claims Data Model Change | Configuration | PromoMats |
| Enhanced Suggest Links | Admin Checkbox | PromoMats |
| Copy Source of Text Assets | Auto-on | PromoMats |
| Multi:Product & Multi:Country Support for Text Assets (Claims and Reusable Text) | Auto-on | PromoMats |
| Multi:Product & Multi:Country Support for Content Modules | Auto-on | PromoMats |
| Asynchronous Auto Publish to CRM for Approved Email Fragments | Auto-on | MedComms, PromoMats |
| Automated Image Rendition Setting Removal | Auto-on | MedComms, PromoMats |
| High Resolution Presentation Slides for CRM & Engage | Admin Checkbox | MedComms, PromoMats |
| Product & Country Become Shared Fields | Auto-on | Multichannel, PromoMats |
| Commercial Data Model Changes | Auto-on | MedComms, PromoMats |
| Medical | ||
| Related ISI and Related PI Relationships added to Base Document Type | Auto-on | MedComms, PromoMats |
| Product & Country Become Shared Fields | Auto-on | Multichannel, PromoMats |
| Asynchronous Auto Publish to CRM for Approved Email Fragments | Auto-on | MedComms, PromoMats |
| Automated Image Rendition Setting Removal | Auto-on | MedComms, PromoMats |
| High Resolution Presentation Slides for CRM & Engage | Admin Checkbox | MedComms, PromoMats |
| Product & Country Become Shared Fields | Auto-on | Multichannel, PromoMats |
| Medical Data Model Changes | Auto-on | MedComms |
| Training | ||
| Training Matrix Builder | Configuration | Study Training |
| Quality | ||
| Process Navigator: Show Associated Process Documents | Auto-on | QualityDocs |
| Set Role Permissions on Related Object | Configuration | QMS |
| Duplicate Detection Check Data Model | Auto-on | QMS |
| Set From Email Attributes on Complaint Emails | Configuration | QMS |
| External Notification: Support for Standard & Custom Object Types on Quality Event & Audit Objects | Configuration | QMS |
| Complaints: Relationship Automation, Recurrence Check, & Checklist Support | Configuration | QMS |
| Part, Asset & Service Data Model Updates | Auto-on | QMS |
| Prevent Delegate Completion | Auto-on | Training |
| Option Not to Issue Quiz for Document Revision Training | Auto-on | Training |
| Disallow Quiz Design Population for Classroom Training Requirements | Auto-on | Training |
| Vault LIMS | Auto-on | LIMS |
| Quality Data Model Changes | Auto-on | LIMS, QMS, QualityDocs, Station Manager, Surveillance, Validation Management |
| Regulatory | ||
| Drag & Drop Documents from Desktop to Content Plan Item | Auto-on | RIM Submissions |
| Create Content Plan as Inactive | Configuration | RIM Submissions |
| Inactive Content Plan Records Cleanup Job | Configuration | RIM Submissions |
| Dispatch Global Content Plan Harmonization: Application Relationships | Auto-on | RIM Submissions |
| Update Set Version Locking on Matched Documents Action | Admin Checkbox | RIM Submissions |
| Deprecate FDA Form 2253 | Auto-on | RIM Submissions |
| Set Blank Active Dossier Item Country Status | Configuration | RIM Submissions, RIM Submissions Archive |
| EAEU Import Updates | Auto-on | RIM Submissions Archive |
| Bulk Create Activities, Submissions, and Regulatory Objectives Wizard Linking Enhancement | Auto-on | RIM Registrations |
| EUDAMED UDI Business Rule Validation | Configuration | RIM Registrations |
| IDMP / DADI Data Model Updates | Auto-on | RIM Registrations |
| Taiwan eCTD DTD 1.0 Publishing & Validation | Configuration | RIM Publishing |
| US FDA Validation Criteria v4.4 Support | Configuration | RIM Publishing |
| Reference Leaf UI | Configuration | RIM Publishing |
| RIM Data Model Changes | Auto-on | RIM Publishing, RIM Registrations, RIM Submissions, RIM Submissions Archive |
| QualityOne | ||
| Multiple COA Attachment Support for Automated Email Intake | Configuration | QualityOne |
| Enhancing In-Process Inspection Object Actions to Exclude Samples | Auto-on | QualityOne |
| Object Sharing Rule Record Uniqueness | Auto-on | QualityOne |
| COA Inspection Enhancements | Configuration | QualityOne |
| Table Cell Consistency & Format | Auto-on | QualityOne |
| HACCP Management Data Model Enhancement | Configuration | QualityOne |
| QualityOne Process Navigator | Configuration | QualityOne |
| RegulatoryOne | ||
| Local Impact Assessment Registration Selection Enhancements: Resize & Edit Columns | Auto-on | RegulatoryOne Registration & Dossier Management |
| Global Change Impact Assessment | Configuration | RegulatoryOne Registration & Dossier Management |
| Copy & Amend Dossier | Configuration | RegulatoryOne Registration & Dossier Management |
| Veeva Claims | ||
| Bulk Add Elements to Pack Copy | Configuration | Veeva Claims |
| Comment Threads for Claims & Projects | Configuration | Veeva Claims |
| Comment Thread for Objects | Configuration | Veeva Claims |
| Panel Element Hierarchical Copy | Configuration | Veeva Claims |
| Project Hierarchy Enhancements | Auto-on | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some "Auto-On" features have a checkbox setting that hides the feature; these will show "Auto-On." | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|