Release Date: August 19, 2022
We are pleased to bring you the following new functionality in this week’s release. See details about feature enablement below.
Platform
API Tools
Configuration Management: Review & Deploy Page Notification for Pending Component Updates
This feature adds a new warning message alerting users of pending configuration changes during the Review & Deploy process. When Vault displays this message, it means that configuration changes are happening asynchronously, and users should allow the changes to complete prior to continuing the deployment.
Working with Documents
Exported Binders: Audit Trail File Naming
With this release, when users export a binder with an audit trail, Vault names the audit trail file based on the latest export version. Prior to this release, Vault based the file name on the earliest export version.
OCR Extended Language Support
With this release, Vault is extending Optical Character Recognition (OCR) support to include files containing non-English text.
Workflow
Multi-Document Viewer UI Updates
This feature provides user experience enhancements to the multi-document viewer within workflows. The dialog that collects task verdicts now supports pagination and document viewing in the background. The left sidebar now supports document list filtering and infinite scrolling.
Search
Document Content Reindex
The content of all documents is being reindexed in preparation for upgrades to the Vault search engine. This reindexing will happen following the release so the changes described below will not be immediately noticeable after the release. This process will happen over the course of several weeks.
After reindexing, Vault will add English Stemming to document content search so that plural nouns (report and reports), verb tense (injecting and injected) and comparatives (early and earliest) are automatically substituted when searching.
Older documents that have not been reindexed for a long time and need to go through the OCR process may have a different set of searchable terms based on the confidence of Vault’s newest OCR process. This means there may be more or fewer searchable terms in those documents based on the OCR confidence thresholds.
Clinical Operations
eTMF
TMF Bot: Prediction Metrics
This feature provides better in-system metrics for Prediction records. A monthly job generates a record for each classification and displays the number of uploaded Documents, Predictions, and accuracy. This allows for customers to track performance over time, and at a more granular level.
Clinical Operations Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Clinical Operations data model to support new features:
Added the the new Prediction Metrics (prediction_metrics__c) object to support the TMF Bot: Prediction Metrics feature with the following fields:
- Auto-Classification Success Rate (
auto_classification_success_rate__c) - Correct Predictions (
correct_predictions__c) - Correct Predictions Above Threshold (
correct_predictions_above_threshold__c) - Created By (
created_by__v) - Created Date (
created_date__v) - Global ID (
global_id__sys) - Link (
link__sys) - Metric Subtype (
metric_subtype__c) - Last Modified By (
modified_by__v) - Last Modified Date (
modified_date__v) - Model Performance ID (
name__v) - Number of Documents (
number_of_documents__c) - Document Subtype (
document_subtype__c) - Document Type (
document_type__c) - Performance Metric Type (
performance_metric_type__c) - Predictions Above Threshold (
predictions_above_threshold__c) - Status (
status__v) - Trained Model (
trained_model__c)
Quality
QMS
Batch Release Templates & Automation
This feature will drastically reduce the manual overhead associated with the batch release process using three new capabilities:
- Customers can now define Batch Release Templates, which they can use to define all of the documents and system checks to be performed for each batch to be released. Customers can define these templates at different granularities, such as Country or Facility.
- Once the Batch Release Template has been defined, the Create Batch / Lot Disposition Record action can be utilized to create new Batch / Lot Disposition records from a Batch Release Template. When executed, the action creates a new Batch / Lot Disposition record and the document and system checks that were defined for the Batch Release Template are automatically created.
- In addition, the batch release process has also been augmented to include a new action, Populate Related Quality Events, which can be leveraged to automatically find any Quality Events associated to the batch that is being disposed and eliminate the need for users to manually associate Quality Event records.
Quality Team Assignments: Update Audit Log to Display the User who Performed the Team Assignment Update
When performing a Quality Team assignment, the associated audit log entries on the Team Member object now specify the user which the system is creating the assignment on behalf of. Prior to this feature, the audit log only displayed System. This enhancement makes it easier to view the user which triggered the Quality Team change when viewing audit log entries of the Team Member object.
Standalone Complaints Data Model
With this feature, QMS now supports the Complaints process as a standalone data model separate from Quality Events. This will allow customers to manage their Complaints Lifecycle separate from the Quality Event Lifecycle.
New implementations are encouraged to utilize the standalone Complaints data model. Customers who are already live on the existing Complaints object type on Quality Event are encouraged to continue leveraging their existing configuration. There will be no functional differences between the two models and there is no need or functional requirement to move to the standalone Complaints data model if already leveraging the existing Complaints object type on Quality Event.
Complaints: Generate Document, Effectiveness Check, & External Notification Feature Support
This feature extends support for the three Generate Document actions, the Create Effectiveness Check, and the External Notification feature to also support the standalone Complaints data model.
QualityDocs, Station Manager, Training
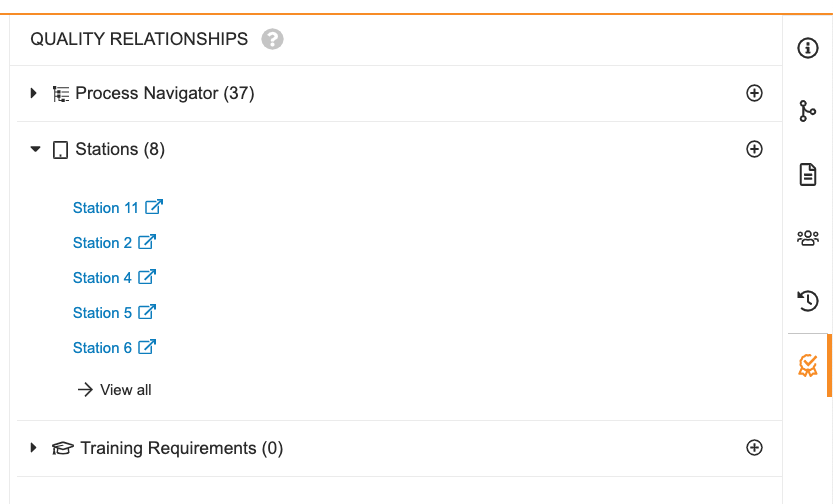
Quality Relationships Document Information Panel
The Quality Relationships Document Information Panel allows users to easily view existing Quality-specific document relationships to Process Navigator Hierarchies, Stations and Training Requirements. Users can also create or remove these relationships from this panel.
Training
Quiz: Hide Missed Questions
You can now choose whether Learners see which questions they missed on a failed Quiz attempt. Depending on your configuration, they will either see which questions were correct/incorrect, or will simply see their overall quiz score with no indication of correctness at the question level.
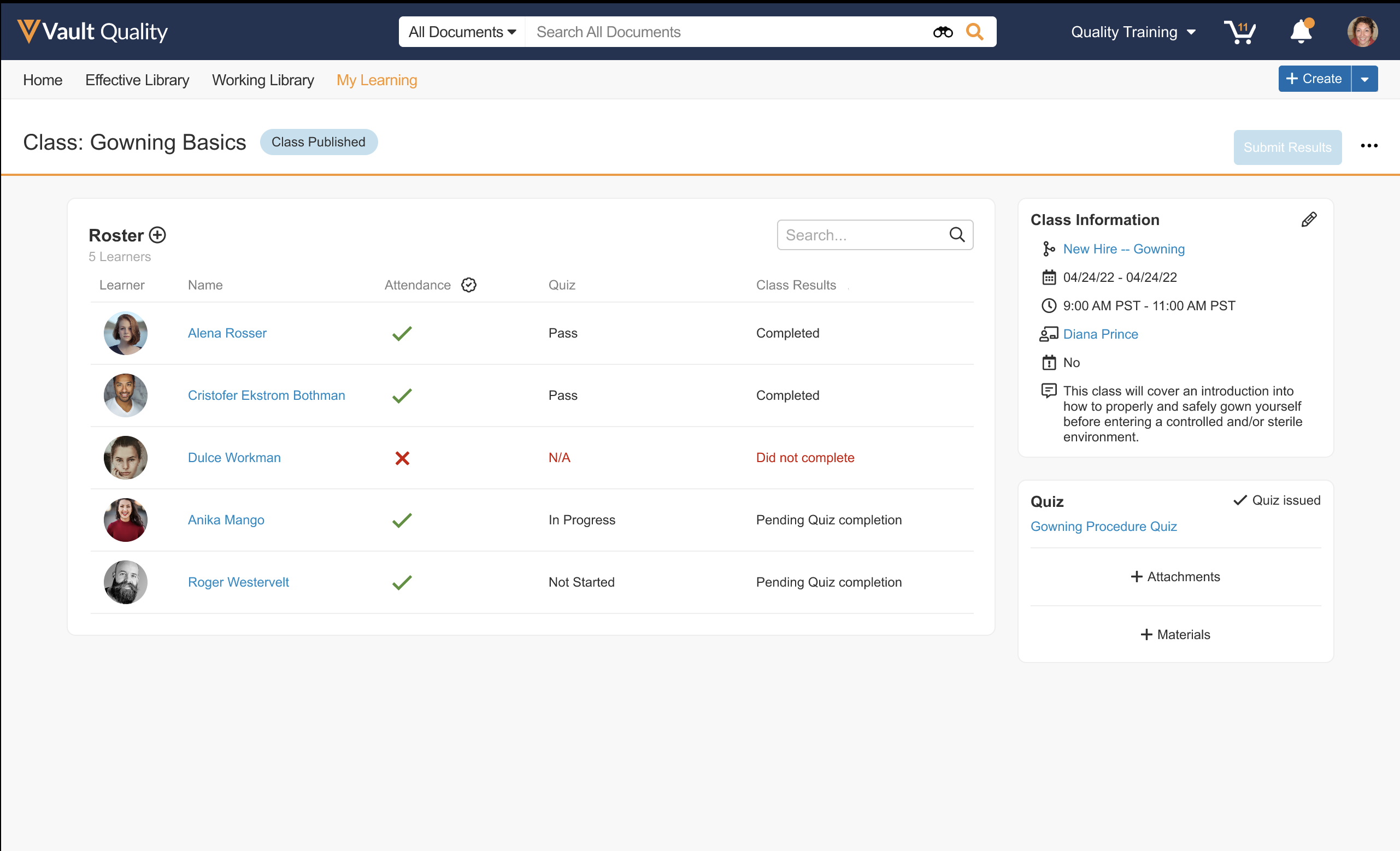
Instructor-Led Training: Simplified Experience
This new experience for Instructor-Led Training drastically simplifies the setup and administration of Classroom Training Requirements. In addition to an intuitive UI designed with Instructors in mind, it also provides a simple way to report on classes that were not run through Vault Training. Learn more about the new Instructor-Led Training experience.
Vault Validation Management
Vault Validation Management is a new application added to the Vault Quality Suite currently available to customers participating in the early adopter program. The application helps organizations manage and execute paperless validation across validation disciplines including: Computerized Systems Validation (CSV), Facility, Equipment, and Utility (FUE) commissioning and qualification, process validation, cleaning validation, and method validation.
This first release includes the core data model and automation to help manage the validation inventory, entities, and requirements (user, functional, and design specifications). Automation exists to help facilitate the creation of versions of validation entities as well as requirements. Additional objects have also been introduced to support validation activities and deliverables needed to prove an entity has been validated. Since certain types of deliverables are based on document records, customers must have QualityDocs in their Vault as a prerequisite for organizations to use Vault Validation Management.
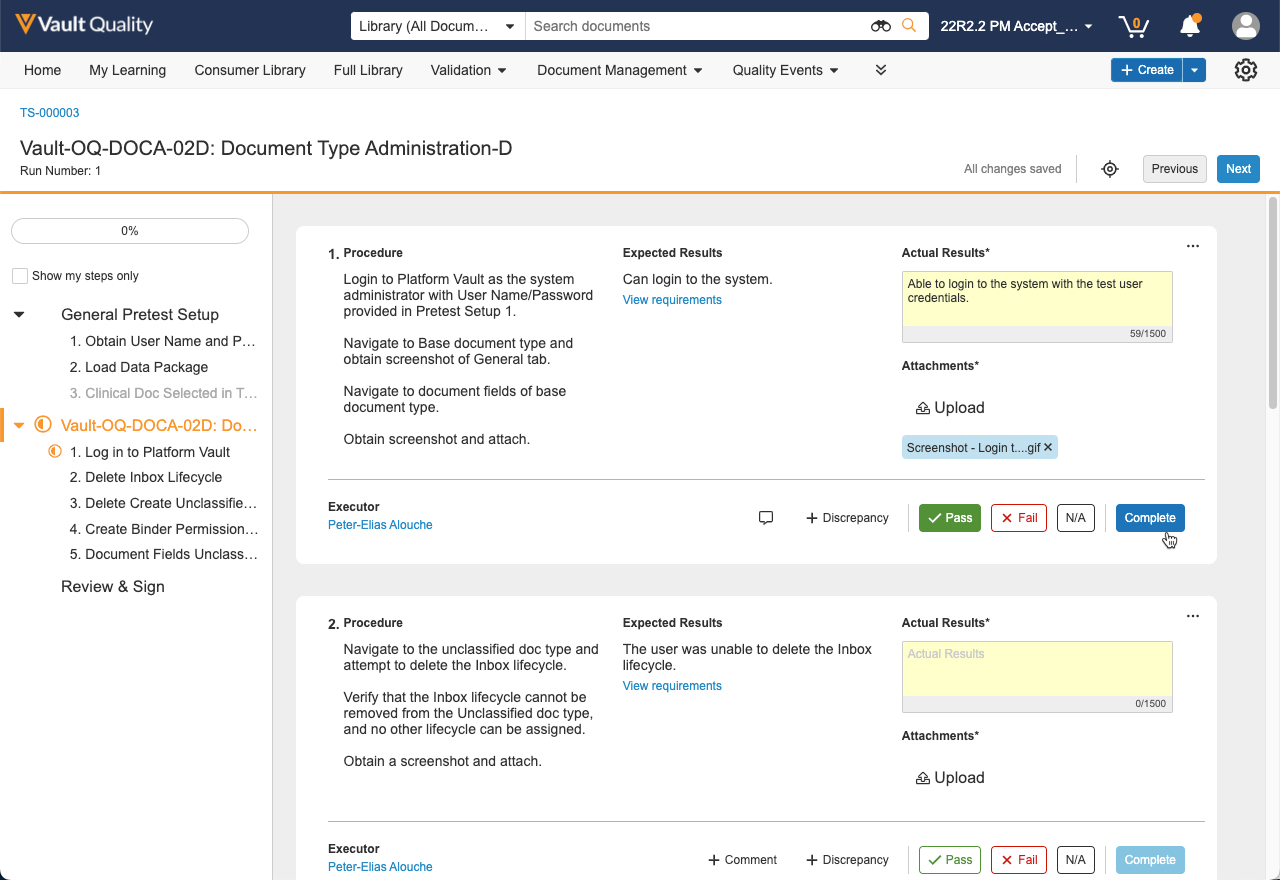
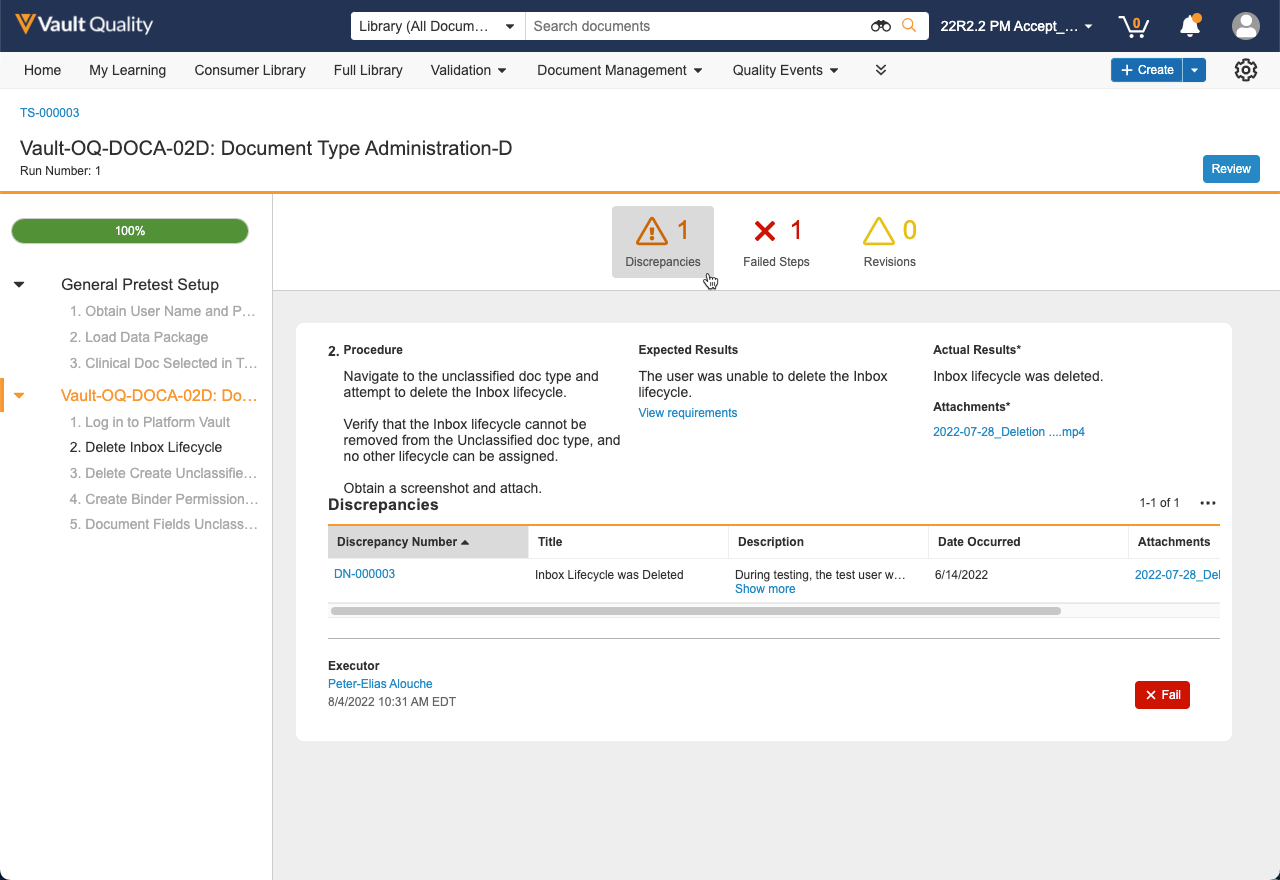
Another key feature included in the initial release is the paperless test execution experience where pre-approved test scripts consisting of setup steps and execution steps can be executed by one or more assigned executors. Executors can easily perform procedures defined by test authors, access requirements being challenged, describe their observed results, and upload attachments to provide objective evidence. Exceptions or discrepancies encountered during execution can be created directly from the test step where the issue was encountered. The discrepancy will then undergo a workflow for investigation, root cause, and corrective action.
Once executors have completed test execution, the test script undergoes independent review and required approvals. A review and approval experience has been included in the initial release to streamline the process with a review by exception capability.
Note: Validation Management is only available to customers enrolled in the Early Adopter Program. To learn more, please contact your Account Partner or Customer Success Manager.
Learn more about Vault Validation Management.
LIMS
LIMS Static Data Updates
LIMS has continued to advance the Test Execution user flow and expand Static Data. This includes Consumables and Instruments. These updates are available to customers participating in the early adopter program.
Quality Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Quality data model to support new features:
Added the following standard components to support the Batch Release Templates & Automation feature:
- Objects:
- Batch Release Template (
batch_release_template__v) - Batch Release Template Item (
batch_release_template_item__v) - Quality Event - Batch (
quality_event_batches__v) - Quality Event - Material (
quality_event_material__v) - Lot Disposition Input - Document (
lot_disposition_input_document__v)
- Batch Release Template (
- Lifecycles
- Batch Release Template Lifecycle (
batch_release_template_lifecycle__v) - Quality Event - Batch Lifecycle (
quality_event_batch_lifecycle__v) - Quality Event - Material Lifecycle (
quality_event_material_lifecycle__v)
- Batch Release Template Lifecycle (
Added the following field to both the Quiz Checklist Design object type of the Checklist Design object and the TA Checklist object to support the Quiz: Hide Missed Questions feature:
- Hide Missed Questions (
hide_missed_questions__v)
Made the following changes to support the Instructor-Led Training: Simplified Experience feature:
- New fields on Class Schedule object:
- Updated UI (
updated_ui__v) - Class Completion Date (
class_completion_date__v) - Past Class (
past_class__v)
- Updated UI (
- New field on
Class Rosterobject:- Class Completion Date (
class_completion_date__v)
- Class Completion Date (
- New picklist value for the
class_completion_status__vpicklist- Pending Quiz Completion (
pending_quiz_completion__v)
- Pending Quiz Completion (
- New Lifecycle State on
Class Scheduleobject- Published (
published_state__v)
- Published (
- New System-Provided Application Role
- Instructors (
instructor__v)
- Instructors (
Regulatory
RIM Registrations
Activity Country Dependency Enhancements
This feature allows admins to define more specific country dependencies for planned changes. A new trigger will run at the time of Activity-Registration record creation to check against these more specific rules, allowing Activity Dependency records to be created only where specific Product, Product Variant, and Packaging records are impacted.
EUDAMED UDI XML Generation Updates
EU UDI submissions are now generated using the EUDAMED XML Schema v2.0.4. Also, the Vault-generated XML now includes source values supplied for enumeration fields even if the values are invalid, which will result in validation errors and make troubleshooting easier.
XEVMPD Support for Investigational Products
This feature extends XEVMPD functionality to Investigational Medicinal Products, including the management of investigational medicinal product data, data aggregation, and gateway submission. Bulk update and submission functionalities are also extended to Investigational Medicinal Products.
RIM Submissions
Content Plan Creation Update to Set Language for Submission
The Content Plan create, update, and copy processes have been updated to set the Language for Submission field (language_for_submission__v) on Content Plan Item records in repeating language sections. This allows Language for Submission to be used for auto-matching.
Content Plan Tokenization Support of Submission Fields
This feature extends Content Plan tokenization support so that text and object reference fields on the related Submission are tokenizable. Previously, only the ${submission__v.xml_submission_id__v} token was supported.
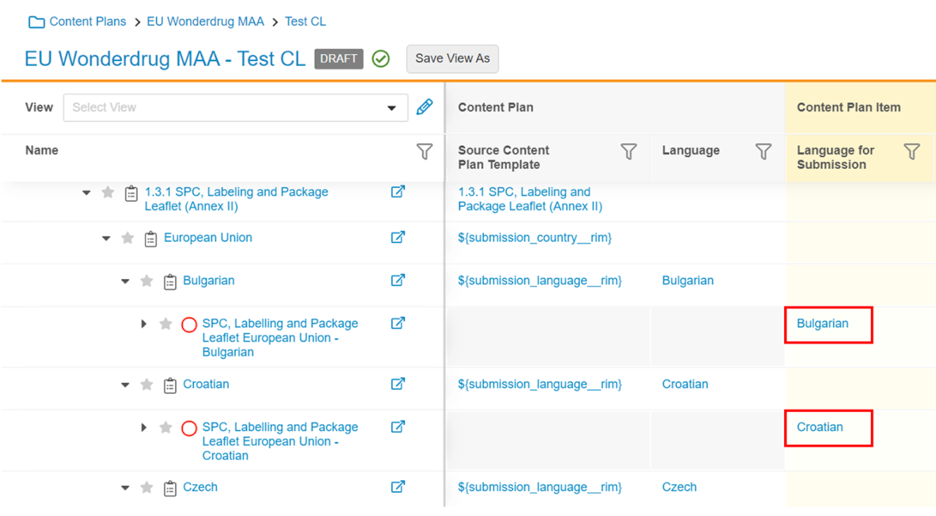
Display Document File Type Icons in the Content Plan Viewer
With this release, each matched document row in the Content Plan Hierarchy Viewer displays the icon corresponding to the document file type.
RIM Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality. With this release, we’ve added and updated the following components to the Regulatory data model to support new features:
- Added the following fields to the Country Dependency object to support Activity Country Dependency Enhancements:
- Product (
product__v) - Product Variant (
product_variant__v) - Packaging (
packaging__v)
- Product (
- Added or updated the following components to support the XEVMPD Support for Investigational Products feature:
- Added the following object types to the Product Report Item (
product_report_item__v) object:- Development Product Element (
development_product_element__v) - Development Presentation Name Element (
development_presentation_name_element__v) - Development Classification Element (
development_classification_element__v) - Development Presentation Name Element (
development_presentation_name_element__v)
- Development Product Element (
- Added the Development Product Element (
development_product_element__v) object type to the Product Report (product_report__v) object. - Added the Alias (
alias__v) field to the Medicinal Product Full Name (medicinal_product_full_name__rim) object. - Added the Authorisation Type (
authorisation_type__v) field to the Product Data Submission (product_data_submission__v) object. - Added the following fields to the Product Report Item (
product_report_item__v) object:- Other Name (
other_name__v) - Other Name Text (
other_name_text__v)
- Other Name (
- Added the following object types to the Product Report Item (
Safety
Safety features are targeted for tentative availability on August 25, 2022.
Safety
Automatic Email of Case Questionnaire for Inbox Items Configuration
Previously, Vault Safety could only send questionnaires to a primary Reporter for an AER or Case. In this release, this follow-up capability is now supported for Inbox Items as well. In addition, Vault Safety can now email questionnaires to a non-primary Reporter with their consent for Cases and Inbox Items. Previously, a Product must have also been specified to send questionnaires. Now, Vault Safety can send non-Product-specific questionnaires for Cases and Inbox Items.
Learn More
- Enablement: Enable Automatic Email of Case Questionnaire & Scheduled Reminders
- Admin Help: Set Up Scheduled Follow-Up Questionnaire Emails
- User Help: Send a Follow-Up Email Questionnaire to a Case Reporter
Cosmetics, OTC Drugs, Nutritionals, and Non-Physical Devices for Case Processing Configuration
You can now create products registered as different Product Types in different regions. You can also register Products using the following new Product Types:
- Cosmetics
- OTC Drug
- Nutritional
Learn More
- Enablement: Enable Case Processing for Over-the-Counter (OTC) Drugs, Cosmetics, Nutritionals, and Non-Physical Devices
- Admin Help: Manage Products
- User Help: Enter Case Data - Products Section
Product Hierarchy Data Model Configuration
This release introduces Product Family and Inactive Ingredients into the Product Data Model. Product Family can be optionally used to group related products. One or more Inactive Ingredients can be optionally added to Product Registrations when required for certain types of reporting.
Learn More
- Enablement: Enable Product Families and Inactive Ingredients for Business Admin Use
- Admin Help: Manage Product Families
Rule Set Administration Safeguards Auto-on
This release introduces safeguards when configuring rule parameters in custom rule sets.
Learn More
- User Help: Reporting Rule Parameter Reference
Exclude Literature and Attachment files in E2Bs by Destination Configuration
Administrators can now configure literature references and attachments to exclude them from E2B(R2) and (R3) files based on the destination of the transmission.
Learn More
- Enablement: Enable Excluding Attachments and Literature Documents
- User Help: Generate a Regulatory Report: Exclude Attachments and Literature Documents
QualityOne
QMS
COA Inspection Enhancements
This feature extends the processing capability to allow for a wider range of COA formats that can be accurately ingested. Learn more about COA Inspection enhancements.
Note: This feature is currently available only to Early Adopters. Contact your Customer Success Manager for more information.
Improved Unit Matching
This feature improves the unit matching behavior to help avoid scenarios where the unit mismatch is incorrectly determined.
Below Detectable Limit Variant Support
This feature enables Vault to accurately process Variable Specifications when the specifications are reported to be below a detectable threshold.
Analyze COA Action Update
This feature updates the Analyze COA object action by removing the need to specify a Purchase Order or Purchase Order Line Item to run the action for COA documents.
RegulatoryOne
RegulatoryOne features are targeted for tentative availability on August 30.
Registration & Dossier Management
Non-Registration Dossiers
This feature allows Admins to configure the Generate Requirements action on both the Registration and Registration Item objects. This enables Regulatory Affairs users to generate requirements from Registration Objectives for dossiers they will submit to Regulatory Authorities, in addition to generating requirements from Registration Items for internal dossiers not intended for obtaining registrations, such as those created by global teams.
Prior to this release, Admins could only configure the Generate Requirements action on either the Registration or Registration Item objects, not both. Learn more about configuring requirement generation from a dynamic template.
Select Requirement Dialog Enhancements: Sorting & Resizing
This feature enhances the Select Requirement dialog displayed when users run the Generate Requirements action, enabling users to search for root Requirement records more efficiently. Users can now filter records with the help of a filter builder and can also sort and resize the columns.
Veeva Claims
Veeva Claims features are targeted for tentative availability on August 30.
Veeva Claims
Pack Copy Reference to Panel
This feature enables customers to report better on Pack Copy elements by providing a clear relationship between Pack Copy and associated Panel records. This feature also enables customers to exclude certain fields from copying to new Pack Copy records when generating records with the Cloning Pack Copy and Localize Pack Copy actions. Learn more about Pack Copy Management.
Enablement Details
| Feature | Enablement | Application |
|---|---|---|
| API Tools | ||
| Configuration Management: Review & Deploy Page Notification for Pending Component Updates | Auto-on | Platform |
| Lifecycle & Workflow | ||
| Multi-Document Viewer UI Updates | Auto-on | Platform |
| Search | ||
| Document Content Reindex | Auto-on | Platform |
| Working with Documents | ||
| Exported Binders: Audit Trail File Naming | Auto-on | Platform |
| OCR Extended Language Support | Auto-on | Platform |
| Clinical Operations | ||
| TMF Bot: Prediction Metrics | Configuration | eTMF |
| Quality | ||
| Batch Release Templates & Automation | Configuration | QMS |
| Complaints: Generate Document, Effectiveness Check, & External Notification Feature Support | Configuration | QMS |
| Instructor-Led Training: Simplified Experience | Configuration | Training |
| LIMS Static Data Updates | Auto-on | LIMS |
| Quality Data Model Changes | Auto-on | LIMS, QMS, QualityDocs, Station Manager, Surveillance, Validation Management |
| Quality Relationships Document Information Panel | Auto-on | QualityDocs, Station Manager, Training |
| Quality Team Assignments: Update Audit Log to Display the User who Performed the Team Assignment Update | Auto-on | QMS |
| Quiz: Hide Missed Questions | Configuration | Training |
| Standalone Complaints Data Model | Auto-on | QMS |
| Vault Validation Management | Support | Validation Management |
| Regulatory | ||
| Activity Country Dependency Enhancements | Configuration | RIM Registrations |
| Content Plan Creation Update to Set Language for Submission | Auto-on | RIM Submissions |
| Content Plan Token Support for Submission Fields | Auto-on | RIM Submissions |
| Display Document File Type Icons in the Content Plan Viewer | Auto-on | RIM Submissions |
| EUDAMED UDI XML Generation Updates | Auto-on | RIM Registrations |
| XEVMPD Support for Investigational Products | Configuration | RIM Registrations |
| QualityOne | ||
| COA Inspection Enhancements | Configuration | QualityOne |
| Improved Unit Matching | Configuration | QualityOne |
| Below Detectable Limit Variant Support | Configuration | QualityOne |
| Analyze COA Action Update | Auto-on | QualityOne |
| RegulatoryOne | ||
| Non-Registration Dossiers | Configuration | RegulatoryOne Registration & Dossier Management |
| Select Requirement Dialog Enhancements: Sorting & Resizing | Auto-on | RegulatoryOne Registration & Dossier Management |
| Veeva Claims | ||
| Pack Copy Reference to Panel | Configuration | Veeva Claims |
See the following explanations for enablement options:
| Enablement | Description | Auto-On | Automatically activated and no configuration is required before using the feature; note that in some cases, a new feature is dependent on another feature that must be enabled or configured. | Admin Checkbox | Admins must turn on the feature with an Admin checkbox. Note that some "Auto-On" features have a checkbox setting that hides the feature; these will show "Auto-On." | Configuration | Admins must configure the feature (separately from an Admin checkbox) before it is available to use or is active; for example, an Admin must add document templates before users can create documents from templates. | Support | On/off option controlled by Support. |
|---|