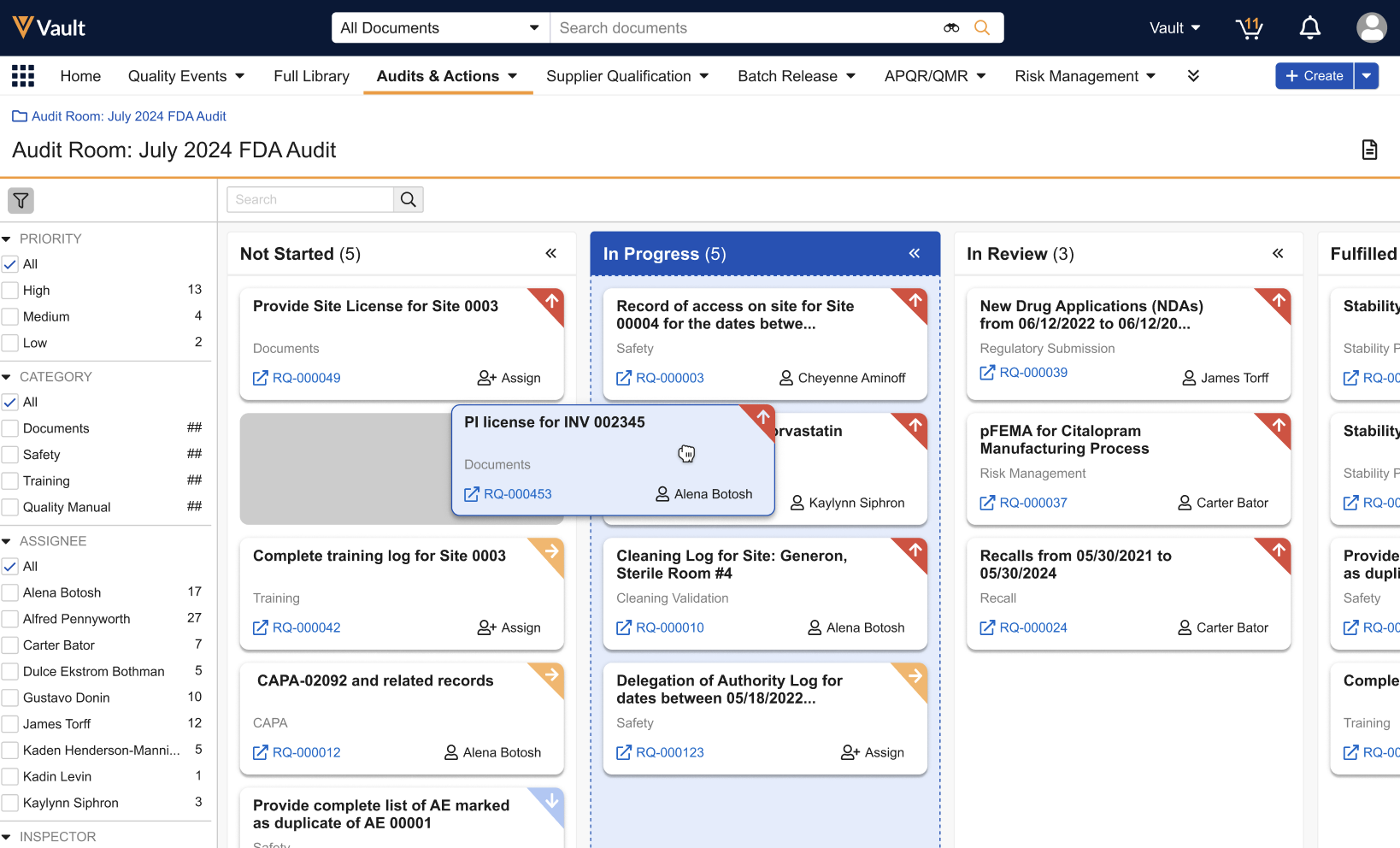
Prerelease Date: November 4, 2024 | Release Date: November 22, 2024 & December 6, 2024
The following applications may have different release dates: Safety, QualityOne client applications, RegulatoryOne, and Veeva Claims.
We are pleased to bring you Vault 24R3. Read about the new features below. You can find information on enabling new features in 24R3 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Platform
Highlights
We’re including this Highlights section in our release notes for Vault Platform to help bring your attention to the most impactful updates across all Vault applications. A summary of these major highlights is displayed below, with further information accessible from the links. The rest of the Vault Platform release notes are then categorized into thematic areas, with the minor enhancements available in the final section.
To see demos of these key features, and for knowledge articles about powerful core functionality, join the Vault Platform community on Veeva Connect.
| Feature | Description |
|---|---|
| Custom Flash Report Notification | When scheduling flash reports, you can now add your own message text to ensure that users have the necessary context for why they are receiving the report, and what action is needed. |
| Process Reporting | Vault now provides a standard way to report on key cycle time metrics, based directly on the history of records and their state changes. |
| Workflow Tasks Prompt for Participants | When completing a task, users can now be prompted to select workflow participants for later tasks in the workflow. |
| Tab for Individual Dashboard | Dashboards can now be displayed as standalone tabs, allowing users to more easily be able to access and reference key metrics. |
| Record Migration Mode Enhancements | You can now set system fields and bypass additional rules when migrating records into Vault, allowing you to have more flexibility and control in how the data is created or updated in Vault. |
| Custom WebAPI | Developers can now create custom API endpoints using the Vault Java SDK to fulfill specific business requirements. |
| SDK Profiler: Request Summary | Developers and Admins can create profiling sessions that capture all SDK requests that occur while the session is active, which allows for improved troubleshooting of custom SDK solutions and improving code quality. |
| Developer Feature: Direct Data API - Workflow Support | Direct Data API exports now include document and object workflow data, including information about each workflow, each item in the workflows, each task, and participant groups, providing an even more complete export. |
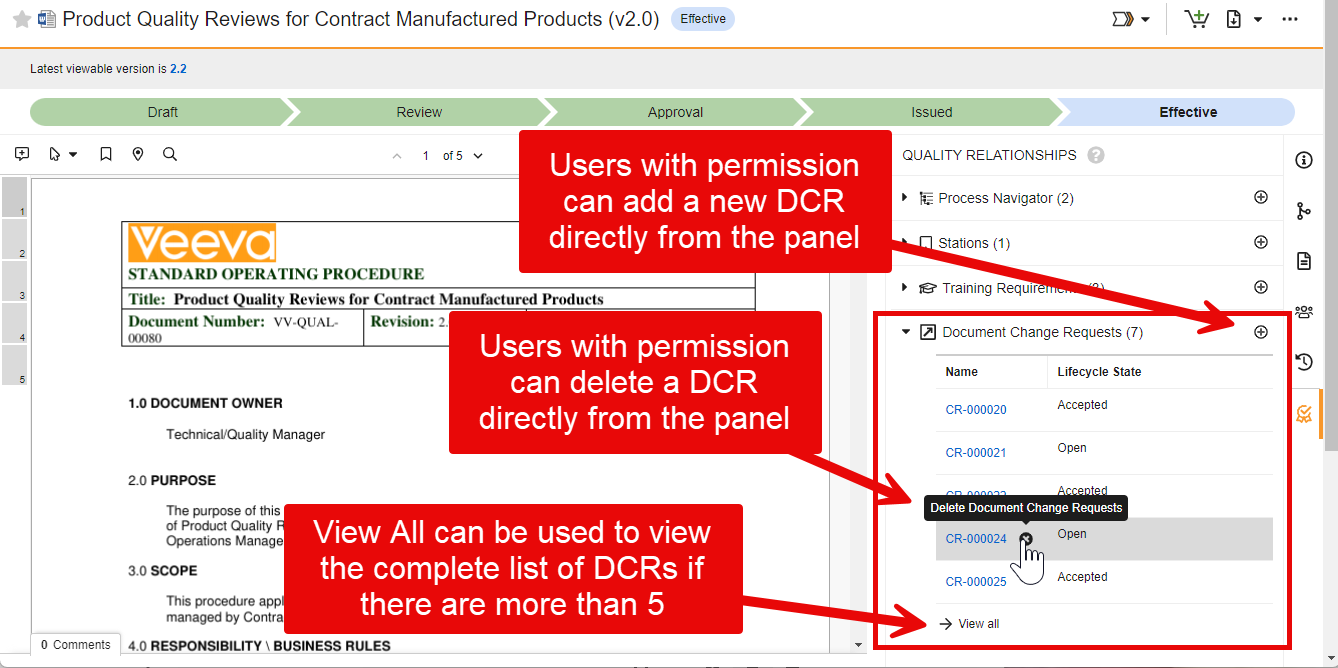
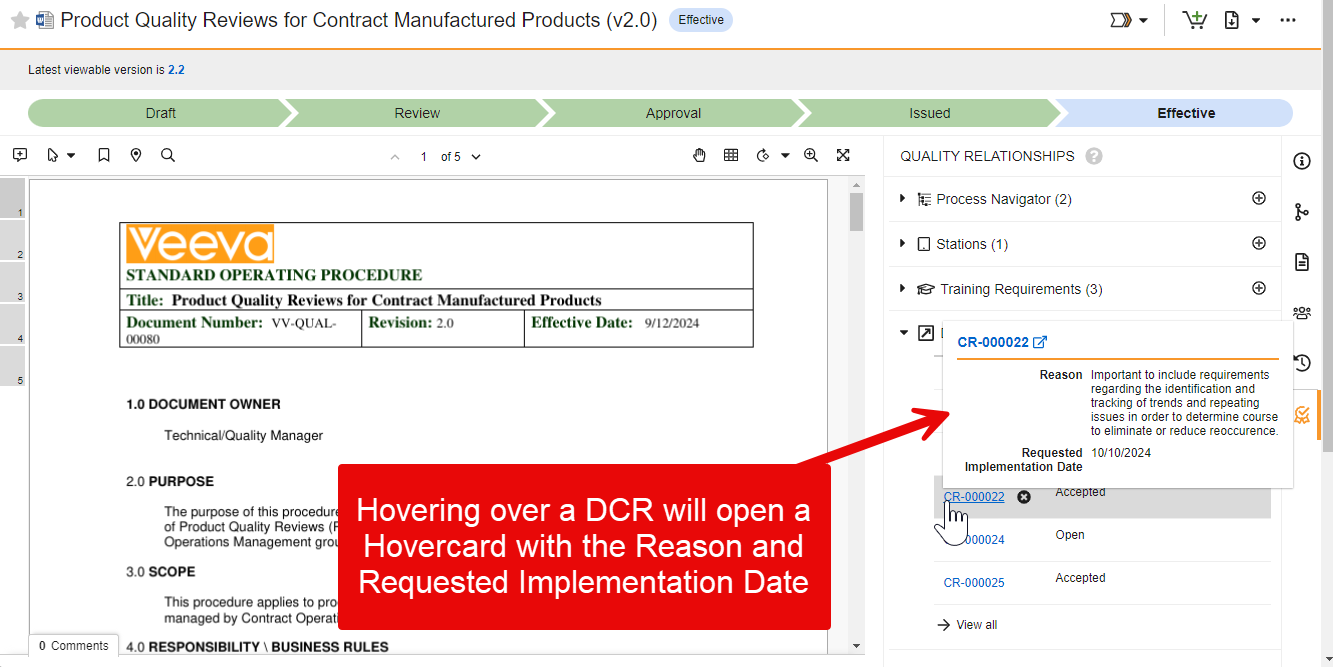
Process Reporting
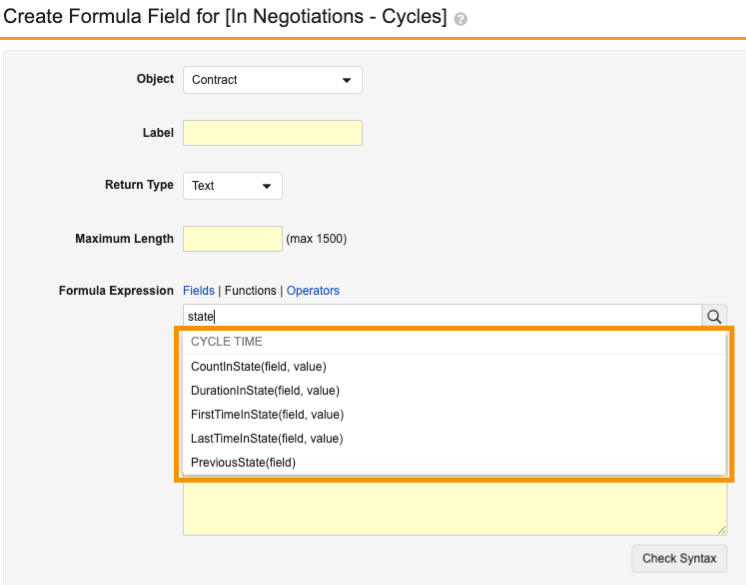
Vault now supports new functions in reports to calculate cycle time calculations in object records based on lifecycle states.
This helps users and Admins to more easily understand how long it takes to perform specific aspects of a process, when a certain state change happened, and where there are potential bottlenecks. These metrics can be used to improve the overall business process and the performance of the team.
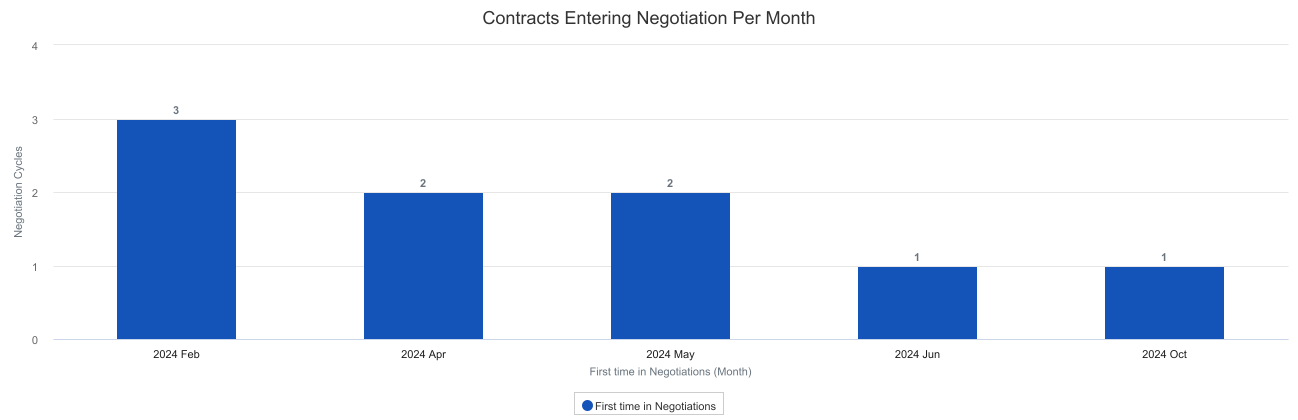
As an example, using these functions in report formulas can support a dashboard that is focused on cycles. The following example displays how many Negotiation cycles are starting each month across all contracts, including where individual contracts are going through multiple cycles:
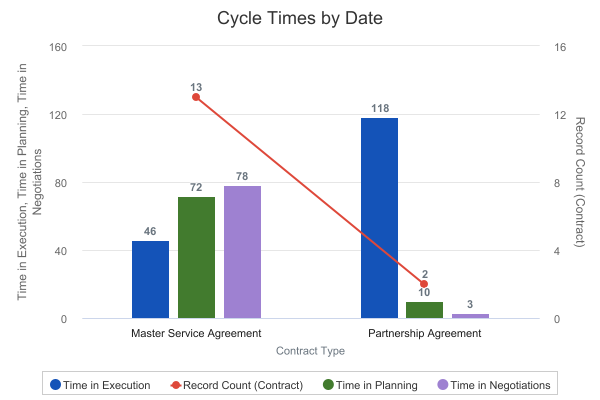
The following example displays how long each part of the process has taken across types of records:
The following new functions are available:
| Expression | Description | Parameters | Output Data Type |
|---|---|---|---|
firstTimeInState |
Reports on the date when a record went to a particular state for the first time. | Field name (for example, state_v) Value (for example, In Review) |
DateTime |
lastTimeInState |
Reports on the date when a record went to a particular state for the last time. | Field name (for example, state_v) Value (for example, Reviewed) |
DateTime |
previousState |
Reports on what was the previous state. | Value name (for example, state_v) |
Text |
durationInState |
Reports on the days a record was in a particular state. This calculates across all instances if the record went through the same state multiple times. | Field name (for example, state_v) Value (for example, In Review) |
Number with decimals |
countInState |
Reports on the number of times a record was in a particular state. | Field name (for example, state__v) Value (for example, In Edit) |
Number |
Note: Similar functions will be supported in reports for the Document report type in a future release. Currently, you can add formula fields as a Document field where similar functions are supported. However, Admins will no longer be able to define new formula fields as Document fields in the coming releases. With 24R3, customers are limited to State and State Type fields in document formula field expressions.
Learn more about Vault Formulas.
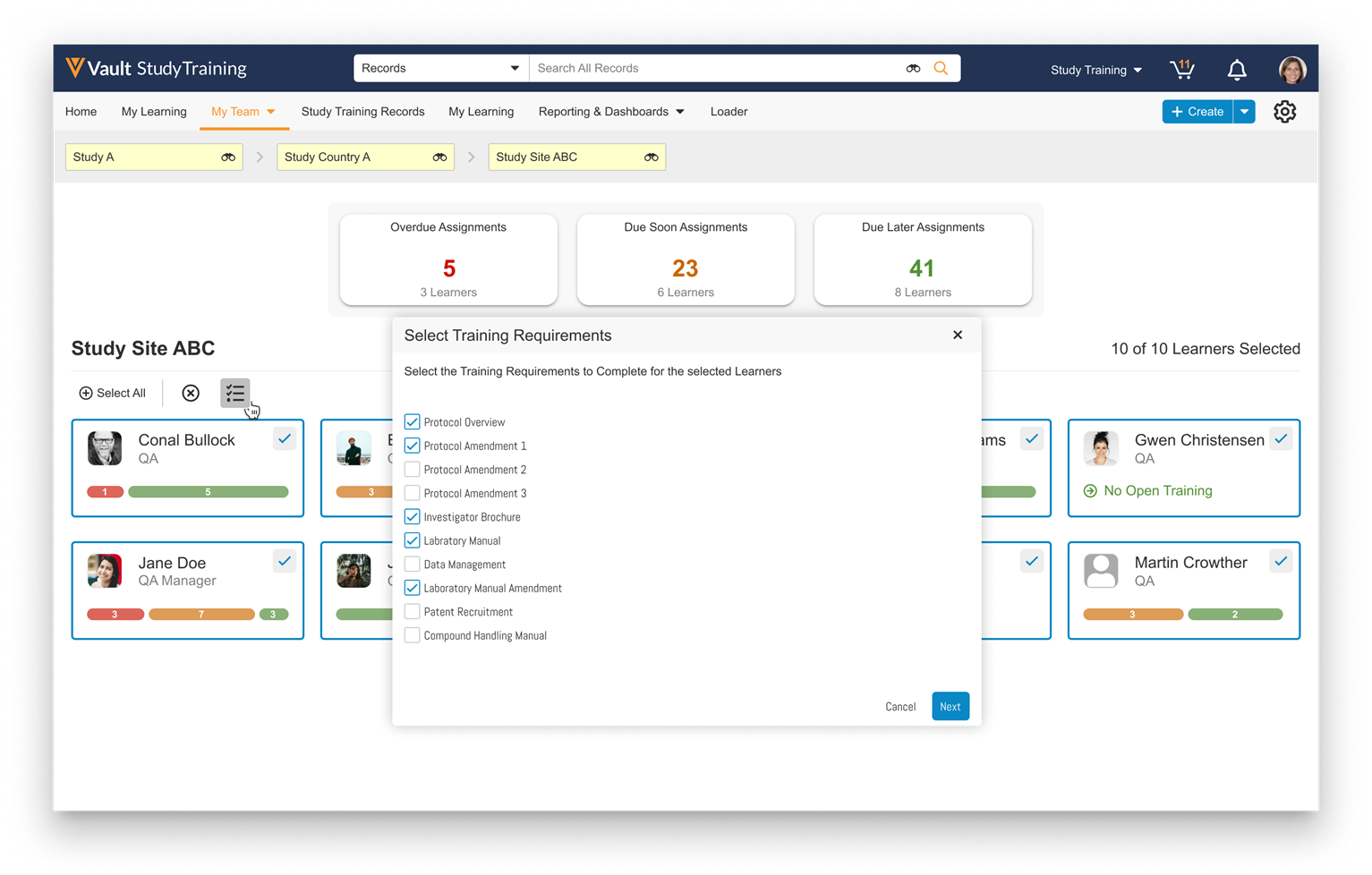
Workflow Tasks Prompt for Participants
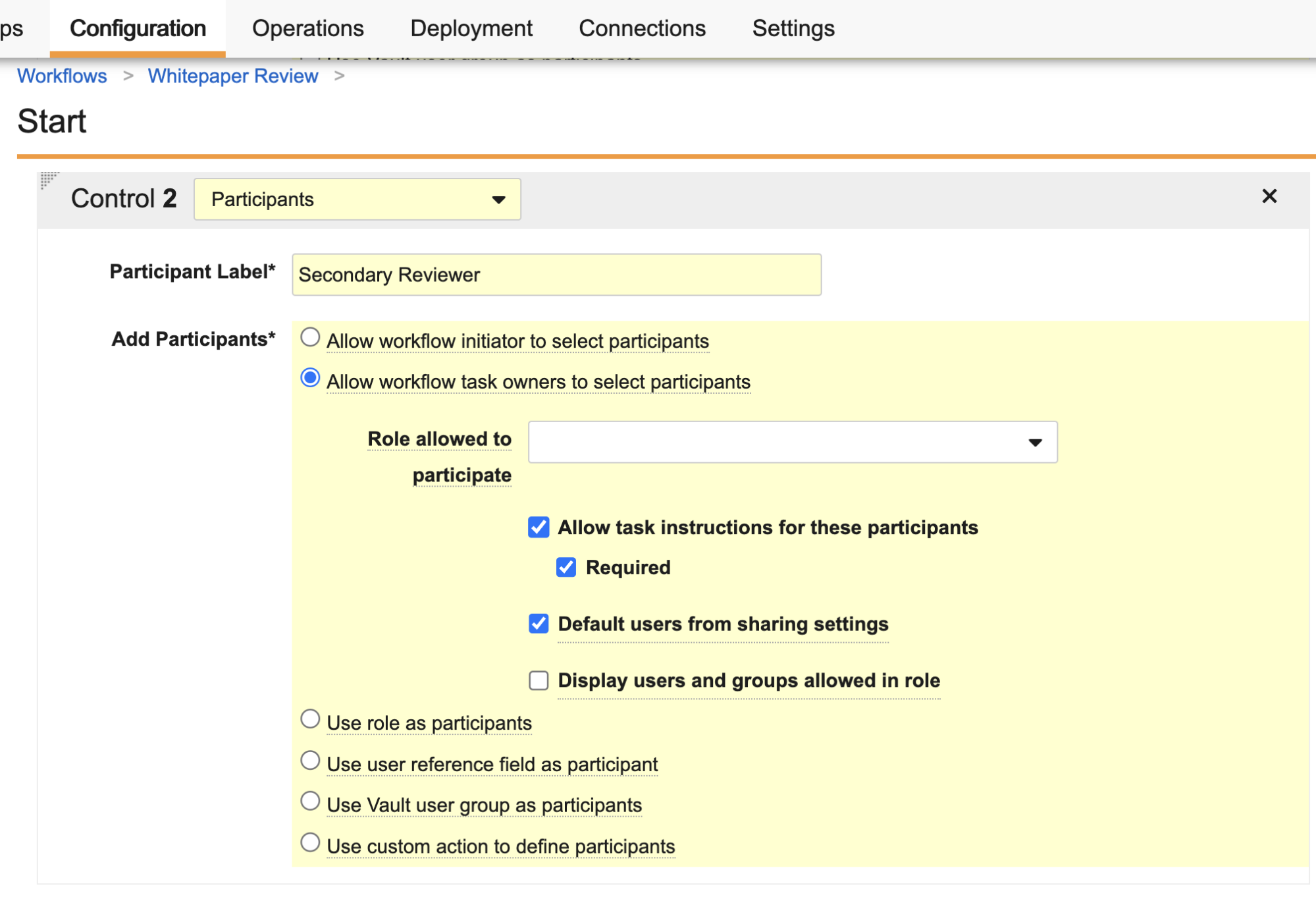
Admins can now configure workflow tasks to prompt the task owner to select a workflow participant as part of task completion. When defining a workflow participant in the workflow Start step, Admins can now select Allow workflow task owners to select participants. This option adds the workflow Participant control as a prompt when configuring a workflow task.
You can prompt for participants as part of the task, or even as part of a specific verdict (if using the Single Verdict option), which means you can now build an optional flow whereby, for example, the user can choose to send the item for a secondary review.
Workflow Participant controls configured in this way do not show to the Workflow Initiator when starting a workflow, and the prompt can contain up to 10 Participant controls per task or verdict. You cannot select the participant group assigned for the task you’re currently configuring. This feature is available for both document and object workflows.
This feature is not available for tasks completed on Vault Mobile.
See the Process Optimization section for information about the other workflow optimization features in this release.
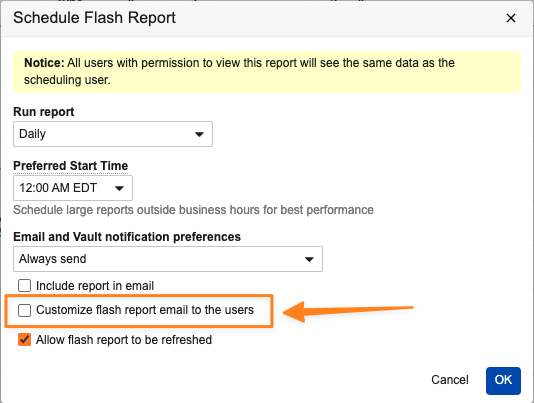
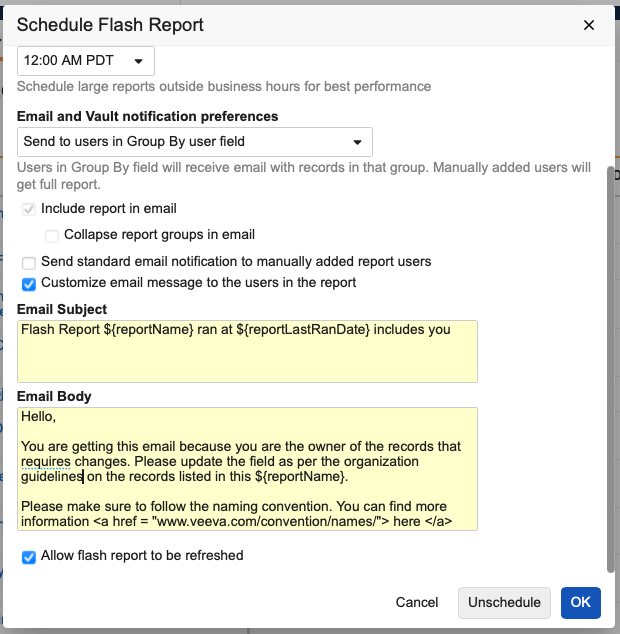
Custom Flash Report Notification
Users that schedule flash reports can now customize the email notifications for each flash report. Customizing these messages allows for inclusion of contextual information, making it easier for recipients to understand what they are receiving, why they are receiving it, and what they need to do.
Users with permission to schedule flash reports now have an option within the Schedule Flash Report dialog box to either use the standard email notification language or define custom text. When defining custom text, users can use a combination of free text, HTML tags, and report tokens, similar to how notification templates are managed in Vault configuration.
To access report tokens, users can type a dollar sign ($) or a plus sign (+) and Vault automatically displays a list of available report tokens.
When using the existing Send to users in Group By user field option, users also have the option to only send notifications to users in the report and not send any notification to users manually added to the Sharing Settings. The Send standard email notification to manually added report users option is selected by default when using the Send to users in Group By user field option.
Learn more about Flash Reports. Learn more about the developer-facing functionality of this feature.
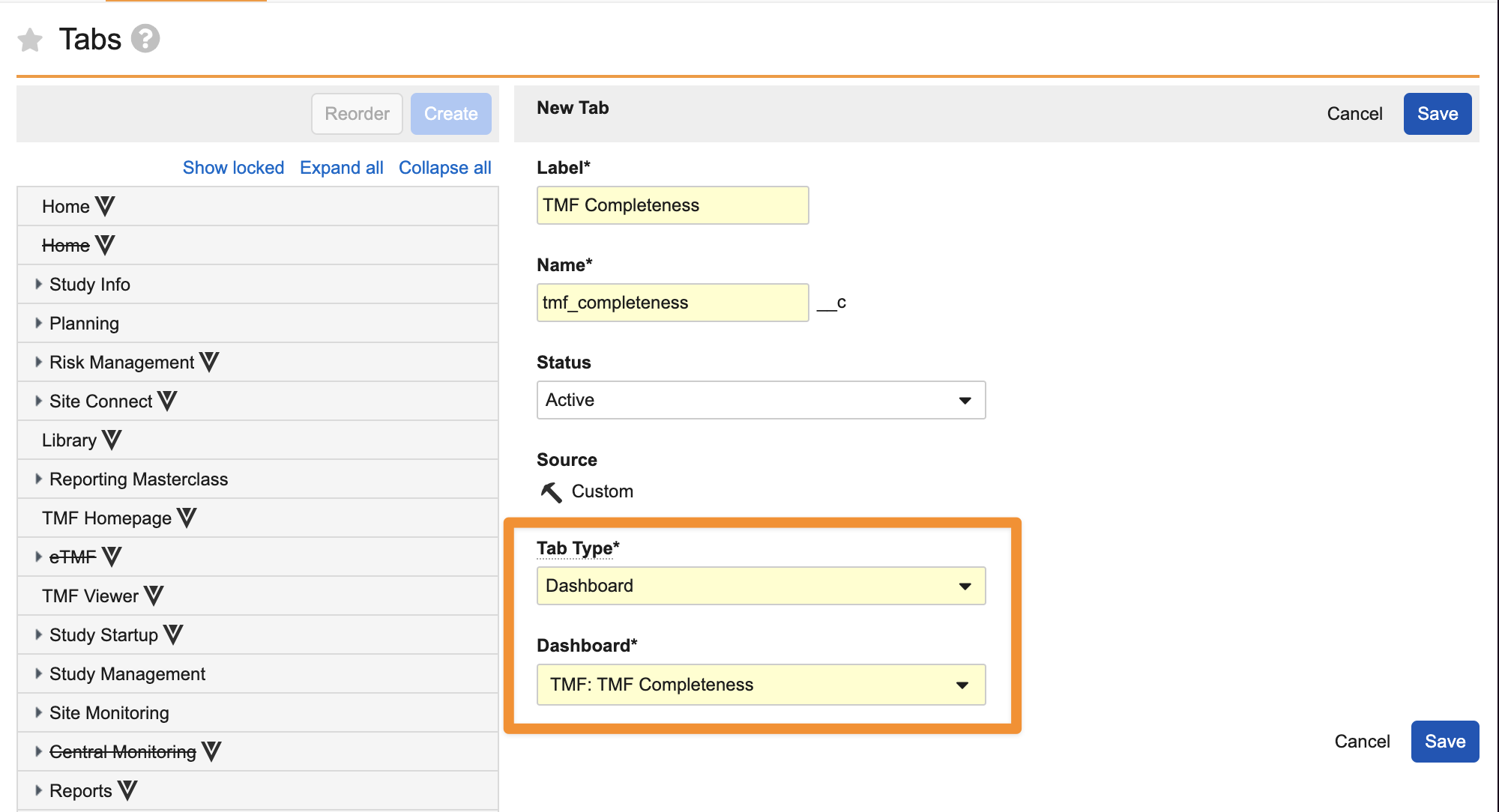
Tab for Individual Dashboard
Admins can now set specific dashboards to be standalone tabs in Vault and manage them the same as any other tab in Vault.
This enhancement allows for key dashboards and metrics to be more readily accessible to users. In some applications, there may be special home pages that are similar to dashboards, but are not configurable. Being able to define a dashboard as a tab also allows customers to opt to leverage a dashboard as a custom alternative.
Dashboard tabs cannot be used as landing tabs for users.
Learn more about Dashboards and Custom Tabs. Learn more about the developer-facing functionality of this feature.
Custom WebAPI
Developers can now create Custom WebAPIs to extend Vault with custom business logic that can be used by integrations and external tools. For example, developers can create composite APIs that return records from multiple objects, or create or update multiple object records within the same transaction. Additionally, custom WebAPIs can accept single file uploads that can be used to create documents, attach files to object records, or update attachment fields. Custom WebAPIs can also return single file responses where the content is a document, object record attachment, or attachment field.
Custom WebAPIs are assigned to a WebAPI Group that administrators define and assign to permission sets. API callers must have access to the WebAPI Group in order to access the Custom WebAPI.
Learn more about the developer-facing functionality of this feature.
SDK Profiler: Request Summary
SDK Profiler allows developers and admins to create profiling sessions for custom Vault Java SDK and inspect performance including memory used and elapsed time. This enables developers to triage production issues with custom code, performance-tune their solutions, and test changes for performance regressions. This ensures high code quality and the end-user experience is not impacted by custom code. Profiling sessions can be managed using the Vault Admin UI (Admin > Logs > Vault Java SDK) and Vault API.
User Experience
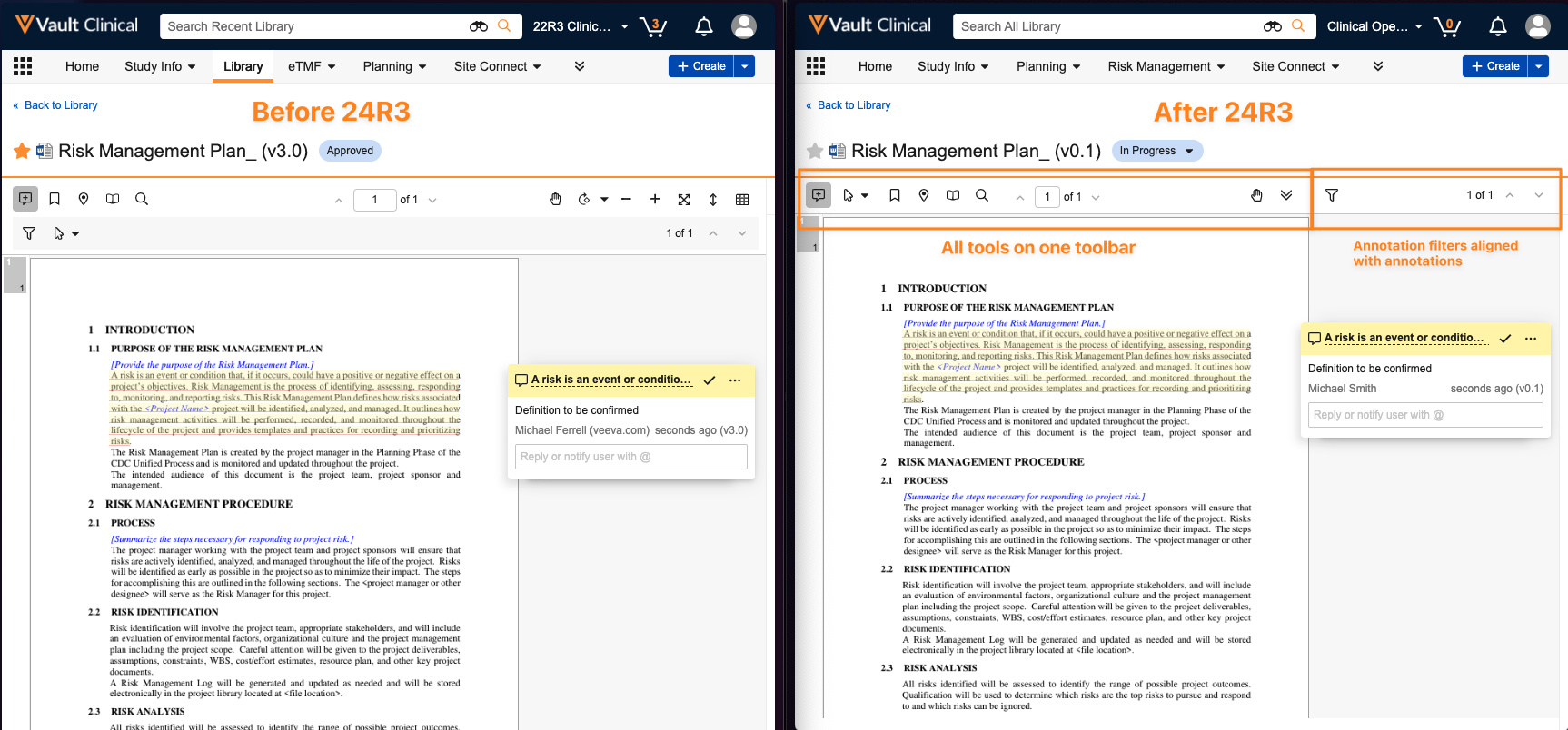
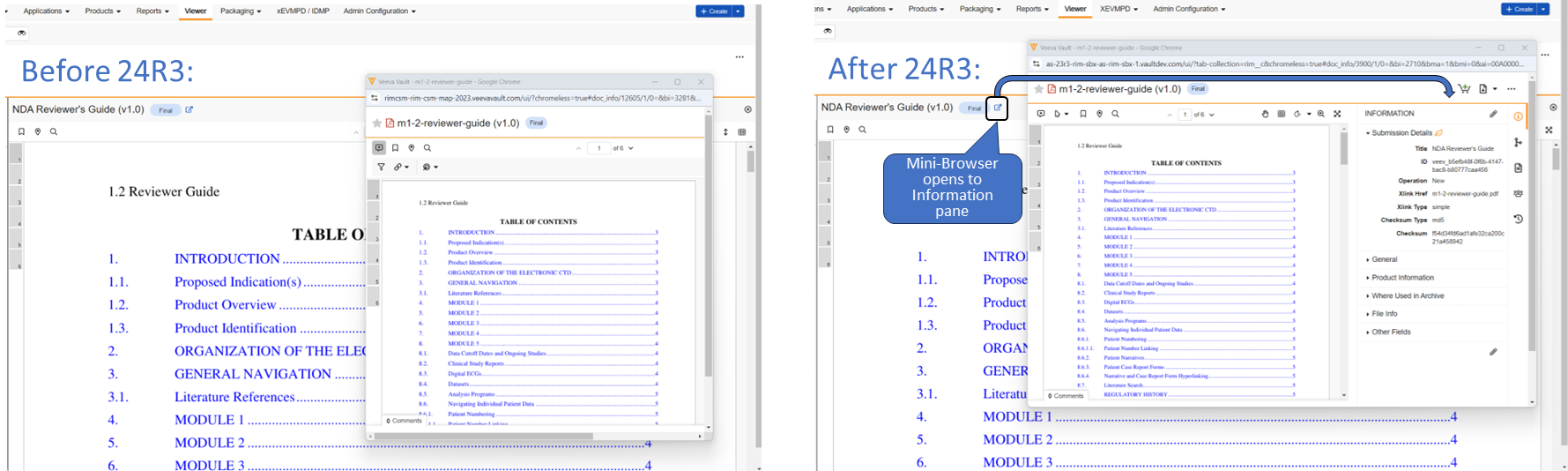
Simplified Document Viewer Toolbar
The Document Viewer experience has been simplified and optimized to ensure that all tools and options are available on a single line toolbar. This provides easier access to all tools while also minimizing document re-sizing.
Annotation Filters
The annotation filter tool is now located on the right side, placing it on the same side as all visible annotations.
Embedded Links
If you work with embedded links, all links are now clickable regardless of what mode or tool you’re using in the viewer. Previously, when Grab was unselected, users could navigate by hovering and clicking in a tooltip. Now, the tooltip is read only, and the user can navigate by clicking directly on the link itself.
Annotate Mode Defaulting
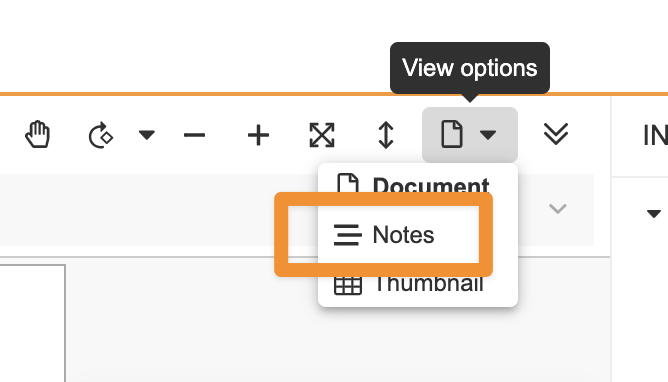
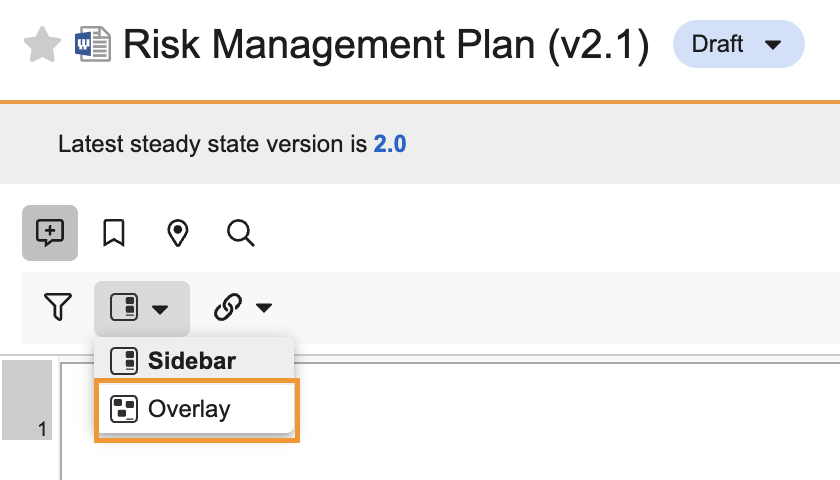
Additional enhancements to the document viewer include only defaulting to Annotate mode when annotations exist, improved video review with responsive player sizing, the ability to view videos in full screen with annotations, and deprecation of Notes view and Overlay mode.
Learn more About the Doc Info Page.
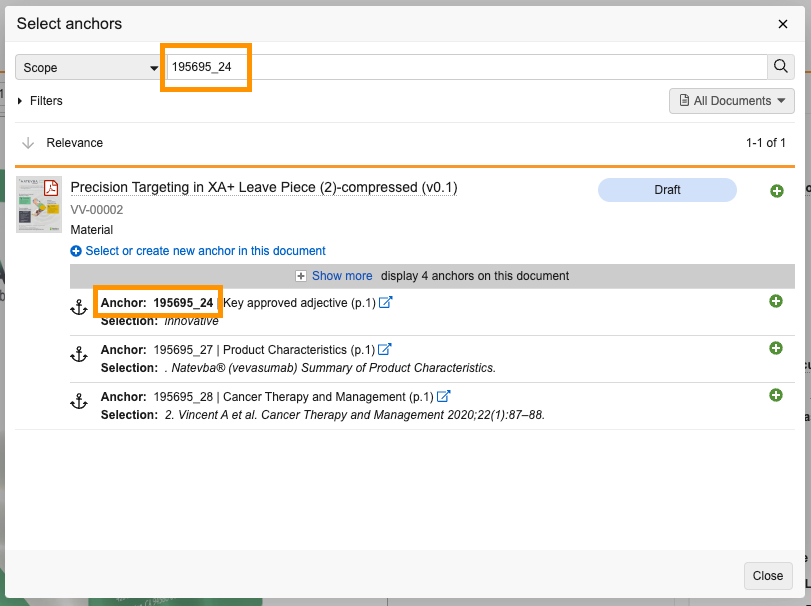
Persistent ID for Annotations
Vault now automatically creates a unique Persistent ID for all new and updated annotations. This supplemental ID is persisted across document versions when annotations are brought forward, meaning an annotation brought forward from any prior version will have the same Persistent ID in all versions.
Vault uses the Persistent ID to prevent the creation of duplicate annotations from Bring Forward Annotations. When that action is performed, Vault prevents bringing forward any individual annotation in the source version whose Persistent ID matches the Persistent ID of an existing annotation in the target version. This allows users to perform Bring Forward Annotations multiple times, from the same or different source versions, without creating redundant copies of annotations.
This ID is now displayed in the info card of anchor annotations, and can be copied to the user’s clipboard. The format of the ID will always begin with the Vault ID, for example: VaultID_###. Users can then search for this ID in the Select Anchors dialog, including when the anchor has been brought forward to a newer document version.
Learn more about Annotating Documents. Learn more about the developer-facing functionality of this feature.
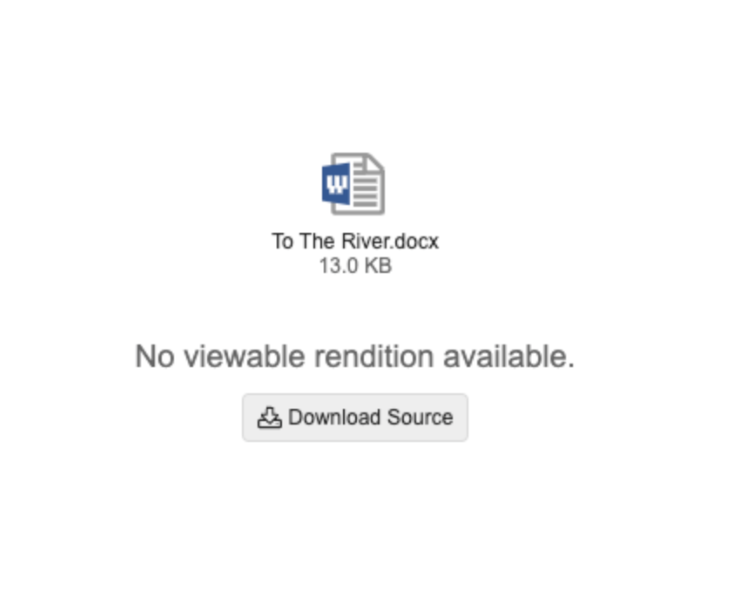
External Viewer: Enable Download Source When No Rendition is Available
For applications that leverage the External Viewer, if the document does not have a viewable rendition, and the document is configured to allow download source, users will now see a message stating that a rendition doesn’t exist, along with a button to download the source file.
This enhancement allows for use cases such as sharing Formatted Outputs in QMS for Incident Reports where the shared file is a zip file.
The External Viewer is accessed via direct URL and is often used to send documents to non-Vault users. Common uses for sending multiple documents via a direct URL to the External Viewer include:
- Sending Response Packages in MedInquiry
- Approved Email in Medical and PromoMats
- Safety Distribution via SiteConnect (for sites that do not have an active Agreement)
- Distribute PVA Documents via Email in SafetyDocs
- QMS: Sending Documents to External Parties
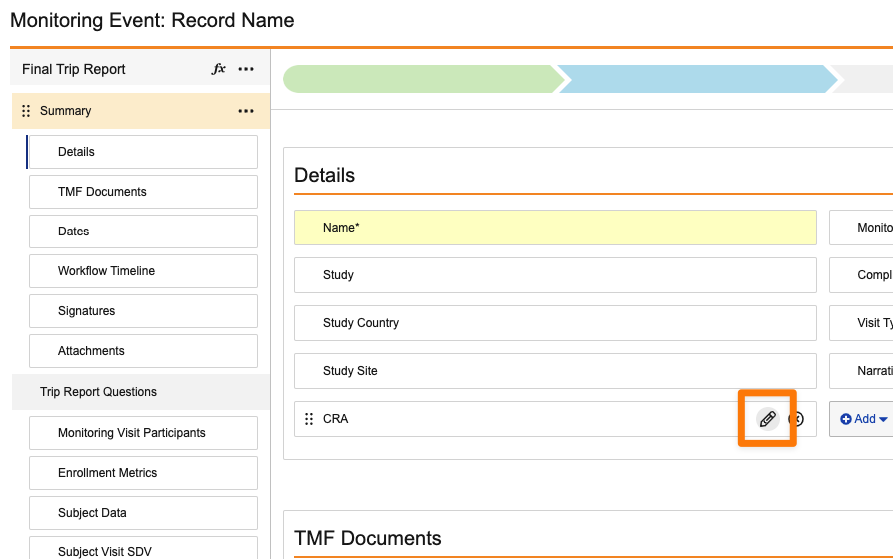
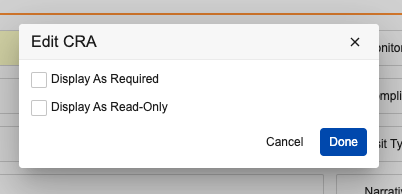
Required & Read-only Field in Layout Configuration
Prior to 24R3, Admins could only conditionally show a field as Display as Required or Display as Read Only on a given layout using layout rules. Now, Admins can easily define fields to always show as Display as Required or Display as Read-Only for a given layout without the use of layout rules.
Admins can define this directly on the field in the layout by clicking the pencil icon on any field:
Layout rules can still be leveraged to apply these effects in more conditional scenarios, using Vault’s layout expression editor, but this enhancement makes it easier to apply the effect if it should always apply on a given layout.
Learn more about configuring object layouts. Learn more about the developer-facing functionality of this feature.
Print Record Enhancements
The Workflow Timeline section is now included in the PDF generated by Print Record if available on the layout. Only the first five rows are included. Additionally, the total page count has been added to the PDF footer.
Learn more about Print Record.
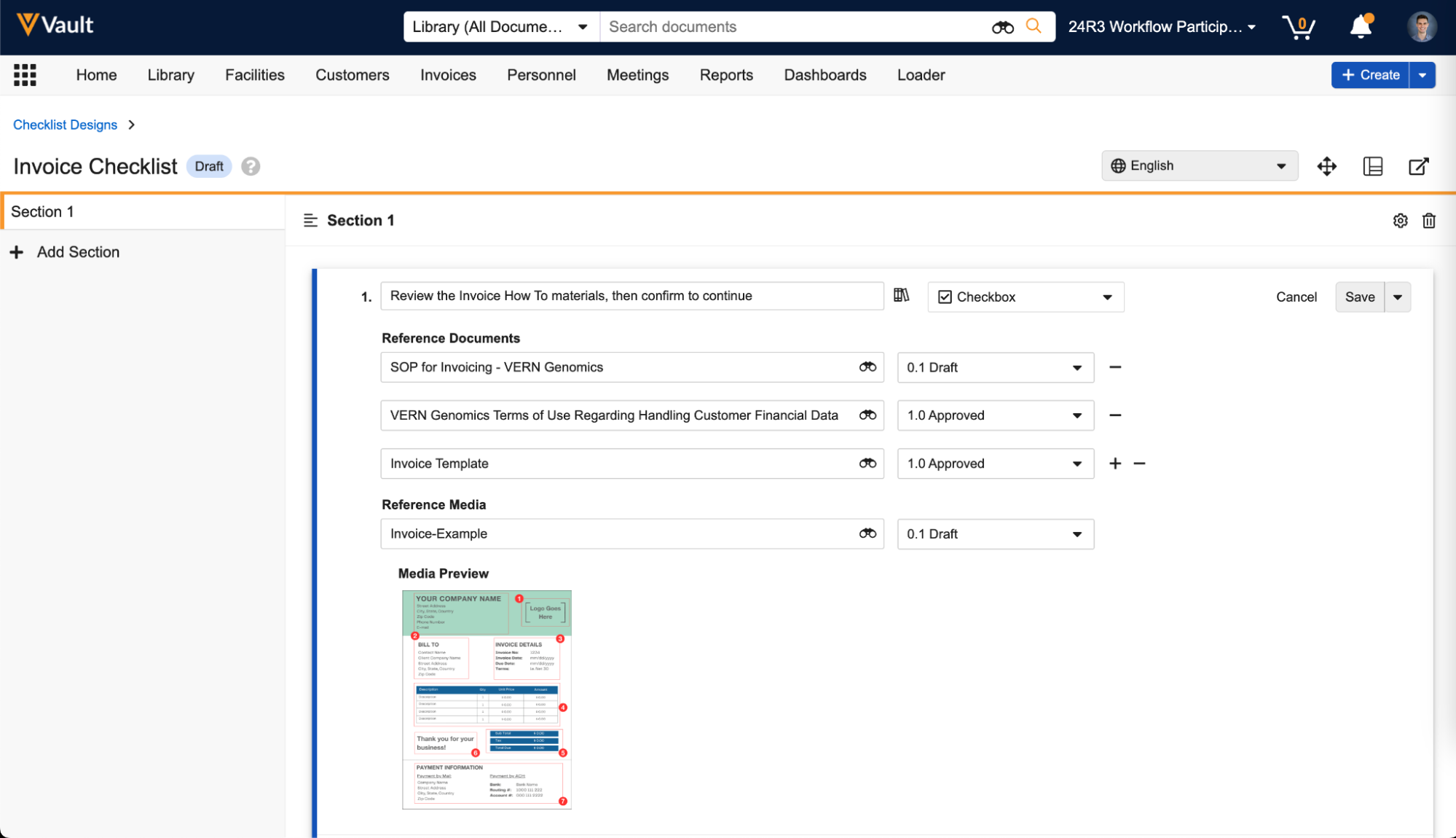
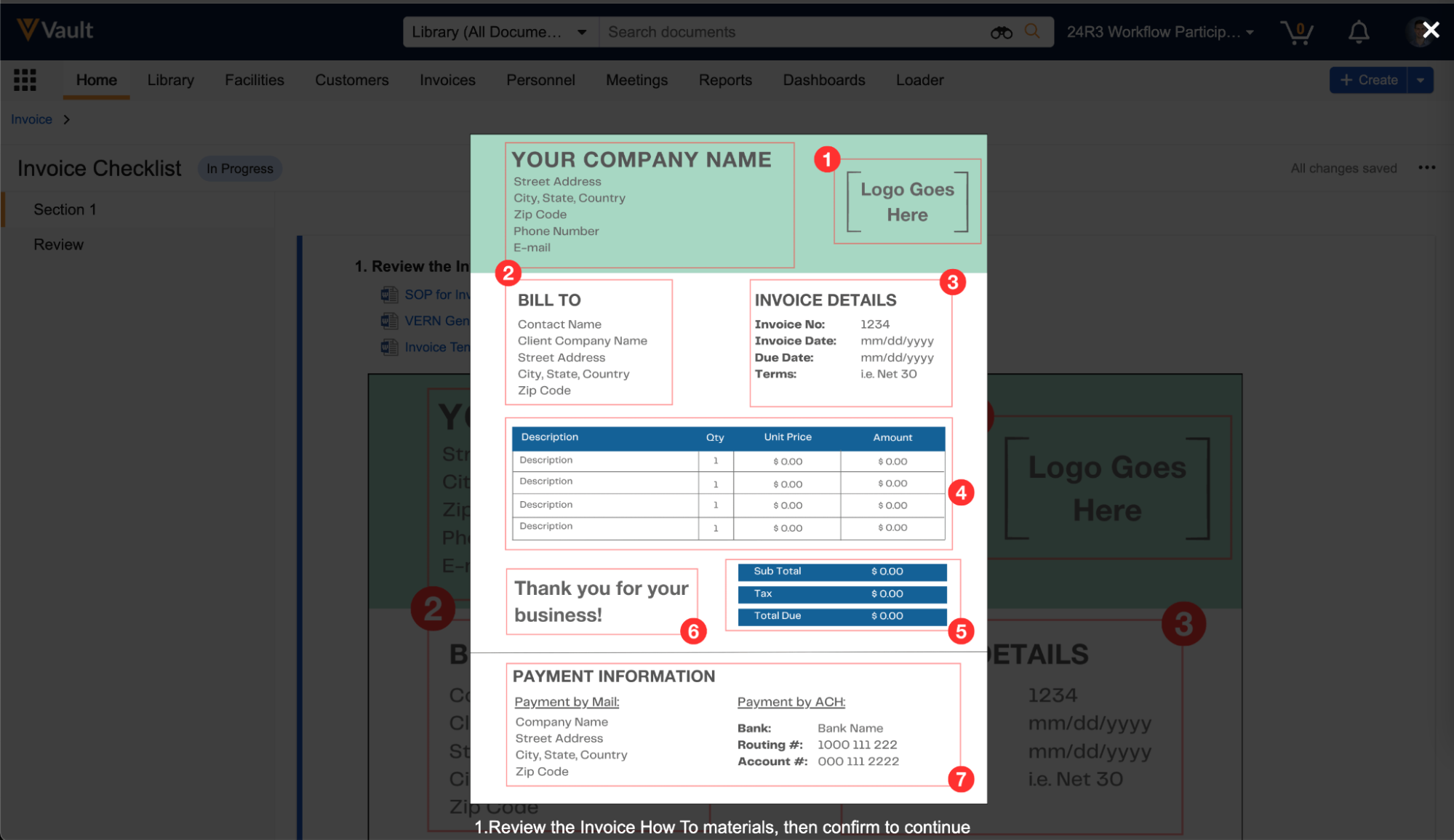
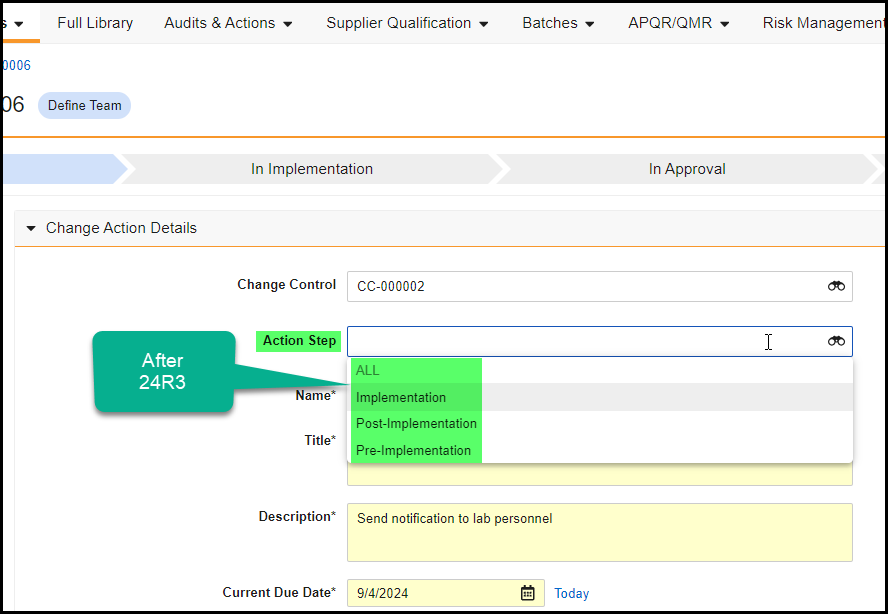
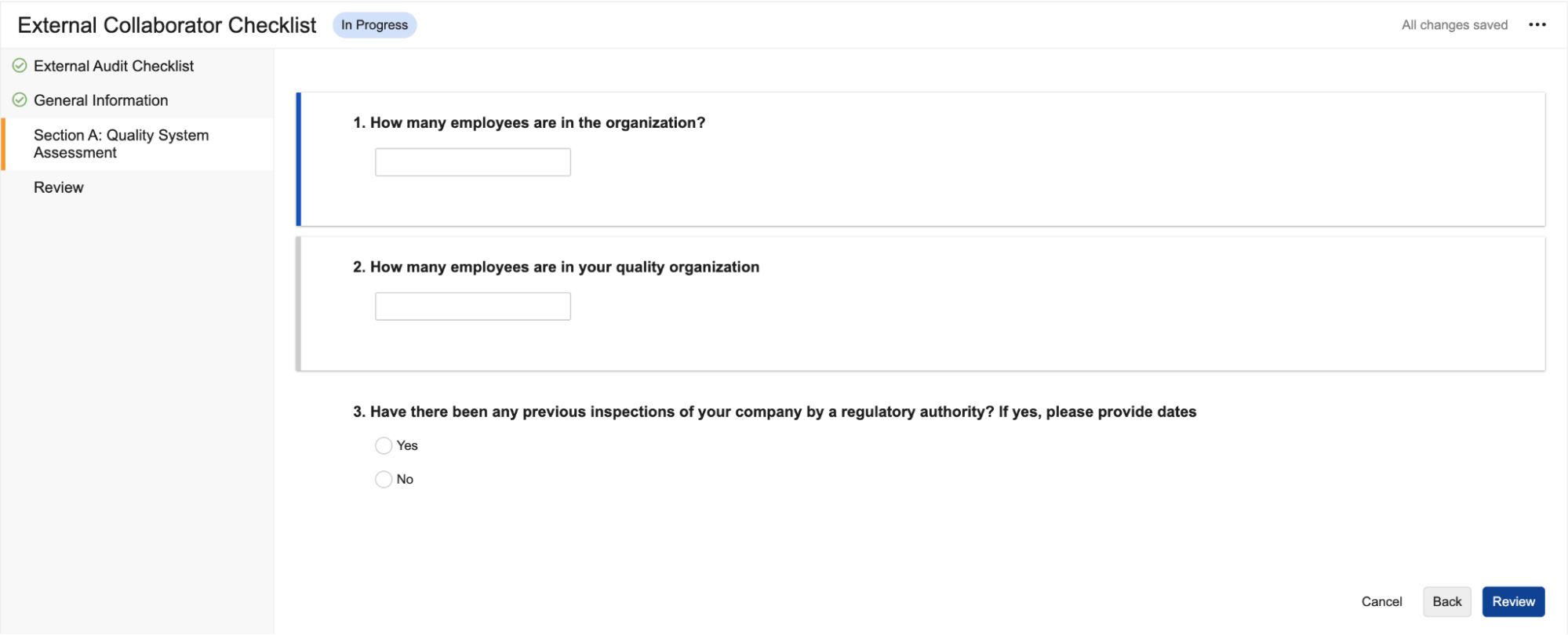
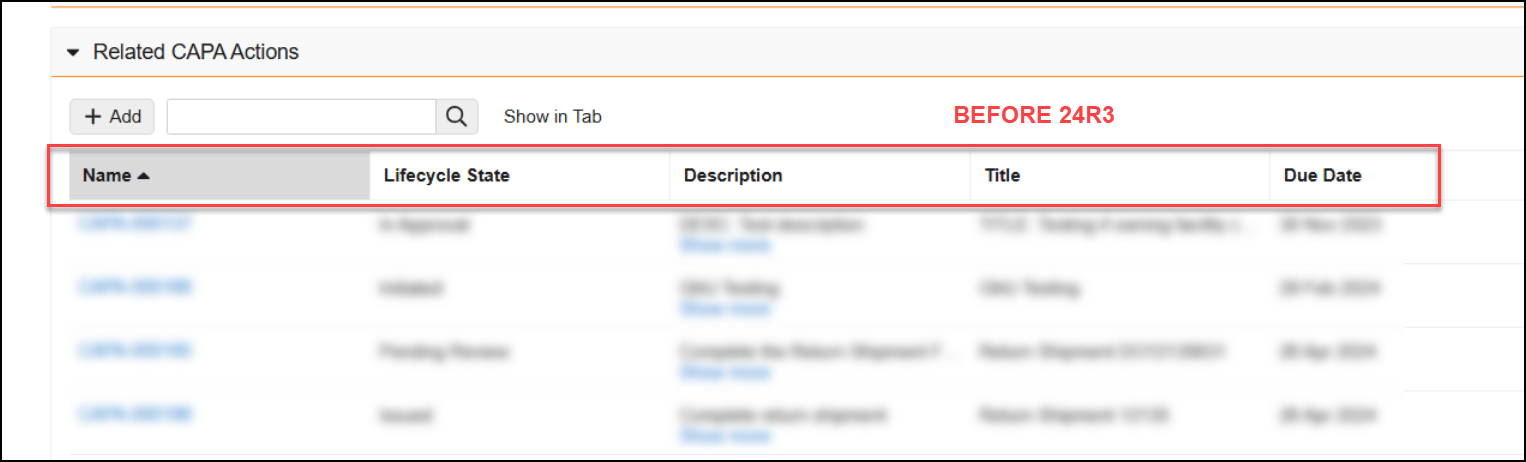
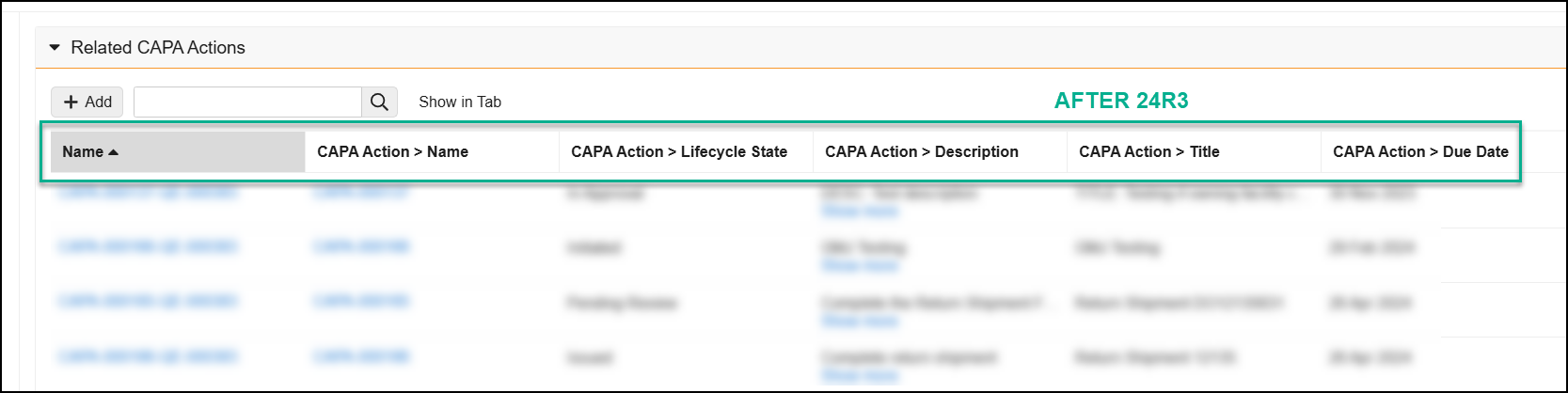
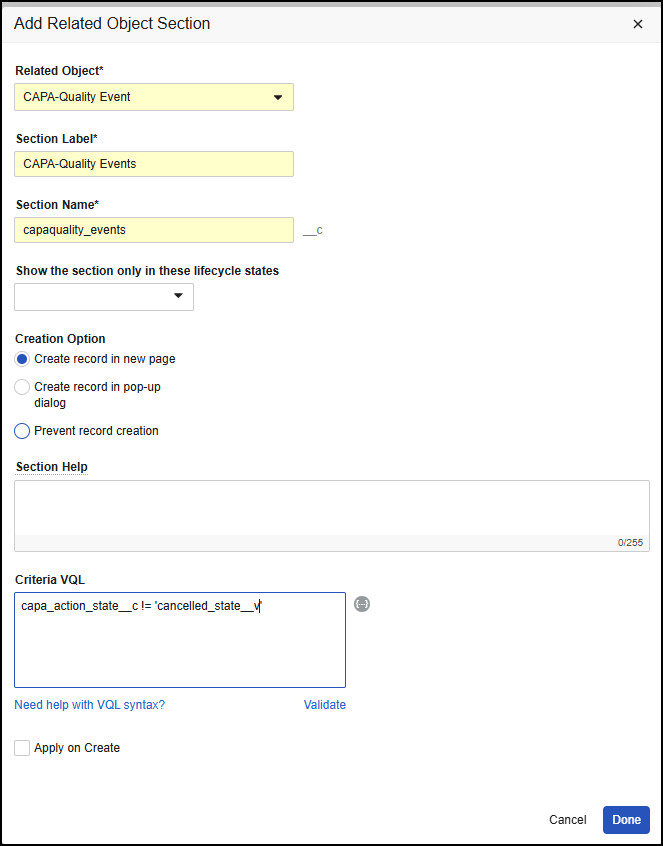
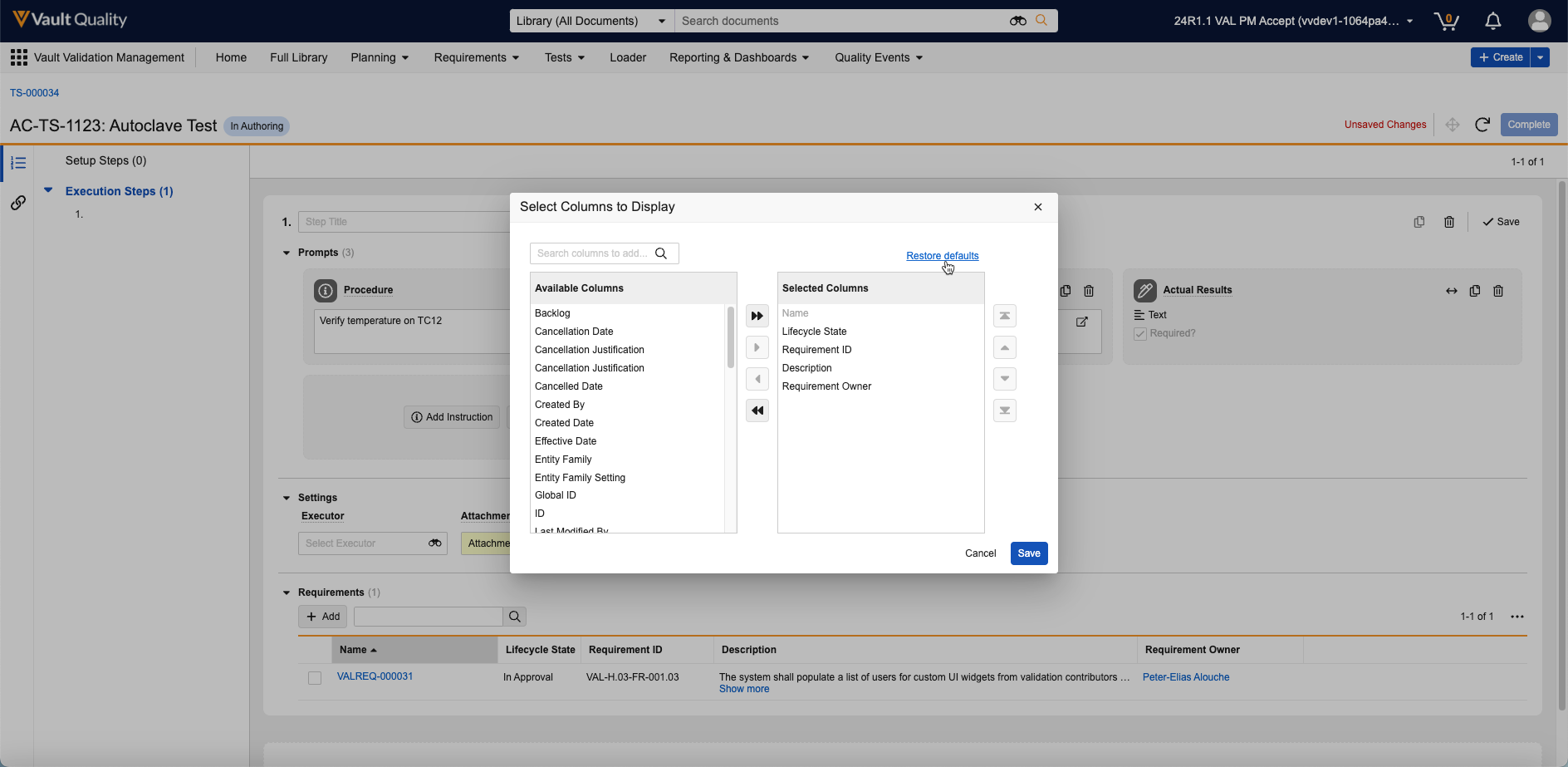
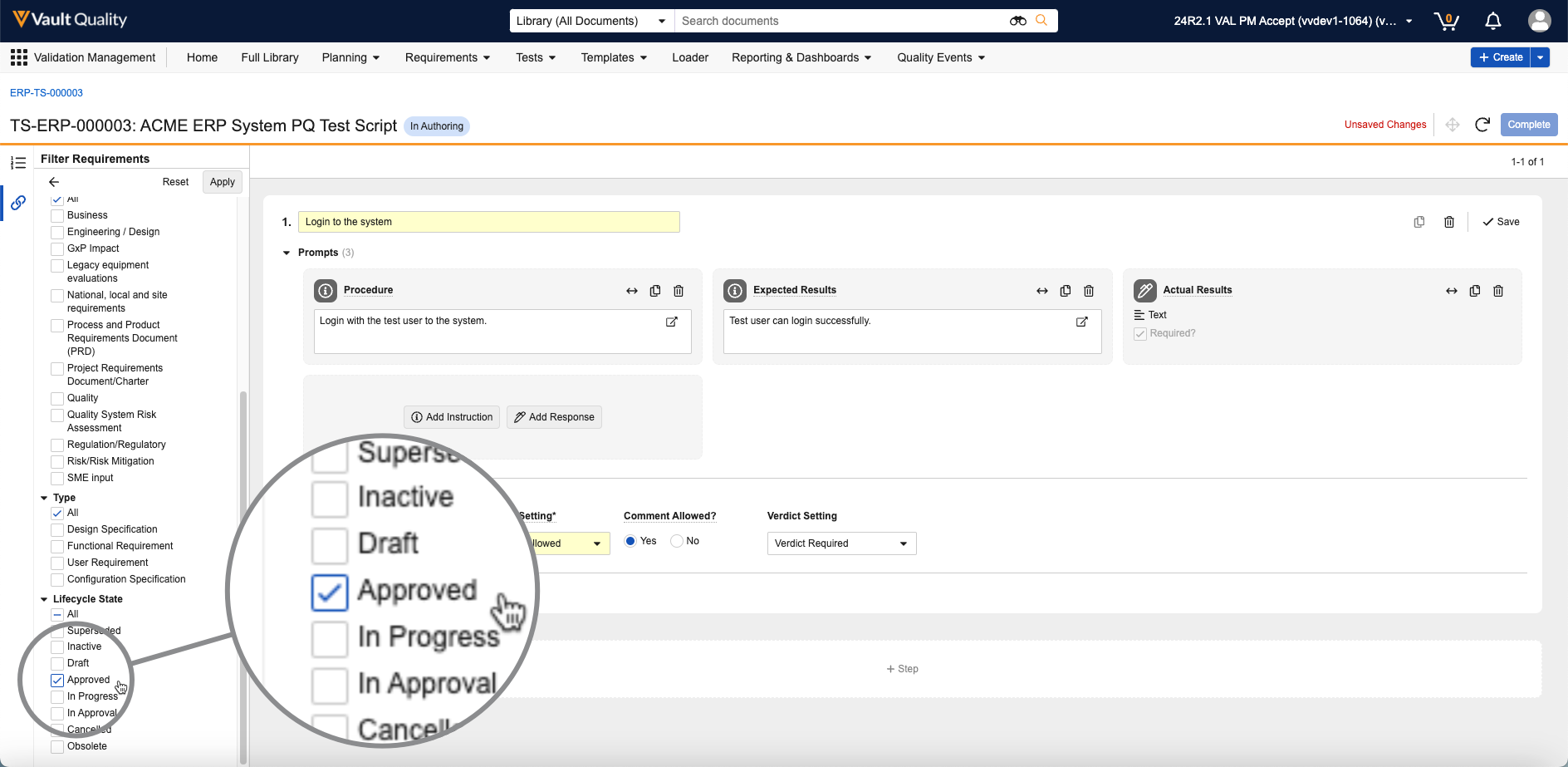
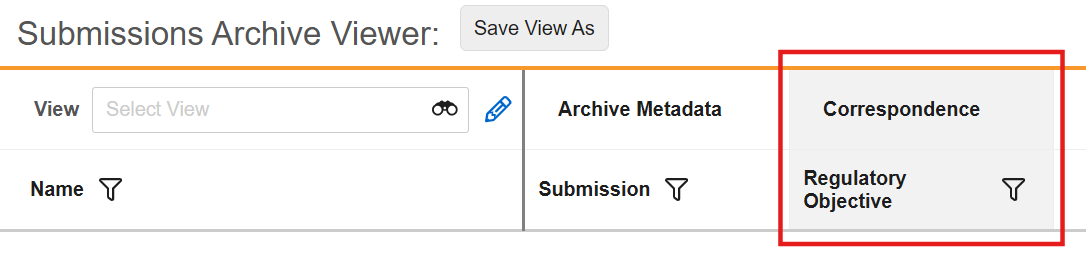
Reference Images in Checklist Questions
You can now add images to questions and multiple-choice answers in checklists via the new Reference Media field. This feature helps provide checklist respondents with richer context on questions and answers. You can also add images to Library Questions and their multiple-choice answers. Vault currently supports the following image file formats:
-
*.jpg, *.jpeg
-
*.png
-
*.gif
-
*.svg
-
*.bmp
-
*.webp
-
*.avif
In the Visual Checklist Designer, if you enable the Reference Media field in the Additional Configuration panel for a question, each question and answer displays a Reference Media field, allowing you to select an image document from your library.
After selecting the image document, you must also select a specific version you want to display. In 24R3, you can only select documents with an Image file format type. Respondents do not need to have any access to the selected document version for it to render when completing the checklist assigned to them.
The Visual Checklist Designer allows you to preview the selected image document before saving, and you can even enter Translate mode to upload a different image for the selected translation language. Users with that language selected will see the alternate image displayed instead.
When previewing the Checklist Design, or when respondents are accessing a checklist, the image is displayed up to 800px in width. You can click the image to focus it on screen.
The new reference media images are also supported in Vault Mobile.
Reference Media is designed to show the content of the selected document inline, on the page, rather than the existing Reference Document option, which provides links to documents in the library for respondents to open in a separate tab and review.
The Reference Media field is available on the Visual Checklist Designer Fields for all checklist types by default in this release.
Learn more about Configuring Checklists and Designing Checklists.
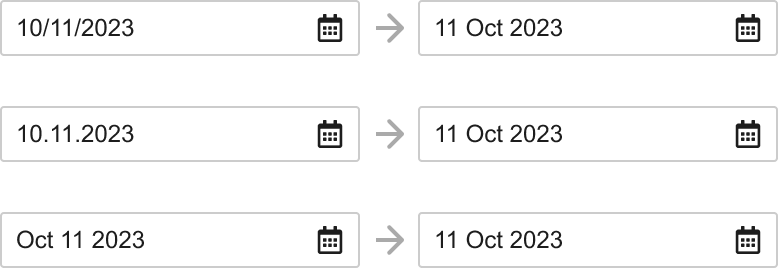
Improved Date Field Entry
When typing in a date, Vault now understands various date formats, including numeric format with slash or dot separators (or without separators), and using zeros for single-digit months and days (according to the user’s locale). Dates can also be entered in alphanumeric format, and then Vault will format the date according to the configured date format setting for the Vault.
All Date and Date/Time fields will display based on the locale for each user and all Vaults. The existing checkbox Format dates and times based on the user’s locale setting is removed.
Existing Vaults will retain the existing date format that is currently configured. New Vaults created after 24R3 will have the Alphanumeric option set as the standard default for the Vault Date Format. Alphanumeric is more readable and less ambiguous across locales, and is the setting that the majority of Vaults use currently.
For Vaults that currently have the Format dates and times based on the user’s locale option unchecked, users whose locale differs from the Vault locale will appear based on their Locale.
Learn more about Vault Date & Time Formats.
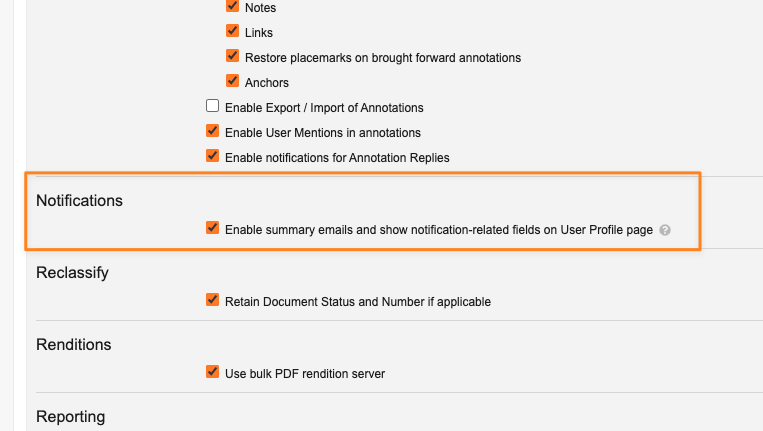
Opt-Out Setting for Future Summary Email Enhancements
In 25R1, we are introducing several enhancements to help reduce notification overload for users by using Summary Email notifications as a standard more frequently:
- Setting the Email Preference field to Summary if it was previously set to Every Occurence on many notification templates (for both Platform as well as application-specific templates)
- Updating the Delivery Interval default value to 1 hour
- Exposing some notification-related fields on the User Profile page by default, including Annotation Replies, Send As Link, Shared Views and Favorite Document notification fields, and Summary Email Interval, allowing users to adjust their own notification preferences
- Favorite Document is a new notification category that will be available in 25R1, allowing users to choose email preferences for Favorite Document notifications
- Summary Email Interval is a new field that will be available in 25R1 which will allow users to choose the interval that works best for them
This change will help all users to receive less overall emails from Vault, while still receiving the information they need in Summary Emails.
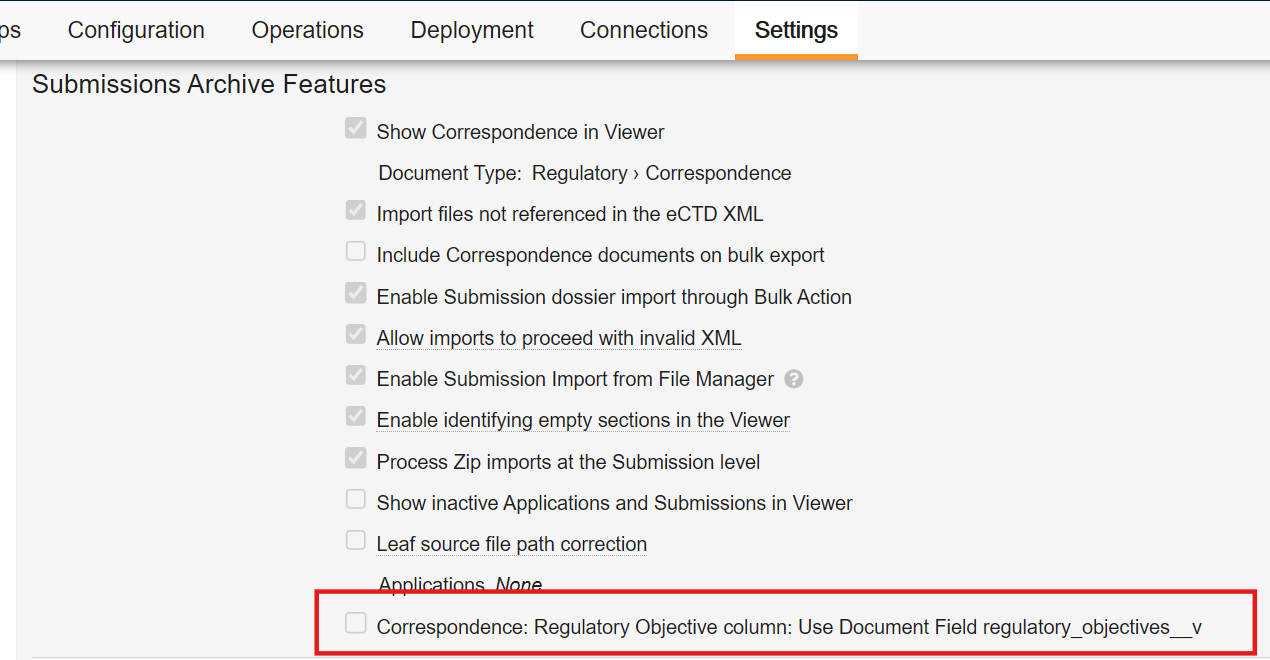
To give customers an option for additional time to prepare for these changes, in 24R3, a new checkbox is exposed in Admin > Settings > General Settings which allows customers to defer the changes in 25R1 until the 25R2 release.
Learn more about Summary Emails.
Strict Matching Adjustments
When searching for documents or object records, Vault applies rules for how many of those terms must match for an item to be a result. In this release, we are adjusting these rules to allow more results to be returned (making it slightly less strict), without allowing so many items to match that the result set is too large to be useful.
With the new rule, 70% of search terms must match for an item to be returned as a result (rounded down). For example, if your search contains:
- 2 terms, then 1 of the 2 must match
- 3 or 4 terms, then 2 of the terms must match
- 5 terms, then 3 of the 5 terms must match
- 8 terms, then 5 of the 8 terms must match.
This is a subtle difference from the previous rules, where any search containing over 5 terms required all but 2 terms to match (which in this case would be 6).
This rule that 70% of the terms must match is easier to understand than the previous ruleset, and allows a slight increase in the number of results returned, and prevents items from being unexpectedly omitted.
Learn more about strict matching and Vault search settings.
Improved Search Results for ID Patterns
Previously, searching for multiple ID patterns (such as document numbers) in Vault required using “quotes” to get accurate results. Without quotes, Vault would split the search into multiple terms, leading to too many results.
In 24R3, Vault now automatically recognizes ID patterns without needing quotes. For example, searching for ABC-123 DEF-456 will now correctly identify both IDs, regardless of hyphens, underscores, or slashes. Even if you forget the exact format, searching ABC123 DEF456 will still find ABC-123, ABC/123, or ABC_123. These changes increase the likelihood that Vault will find what you’re searching for.
Process Optimization
Workflow Uses User Reference Fields as Participants
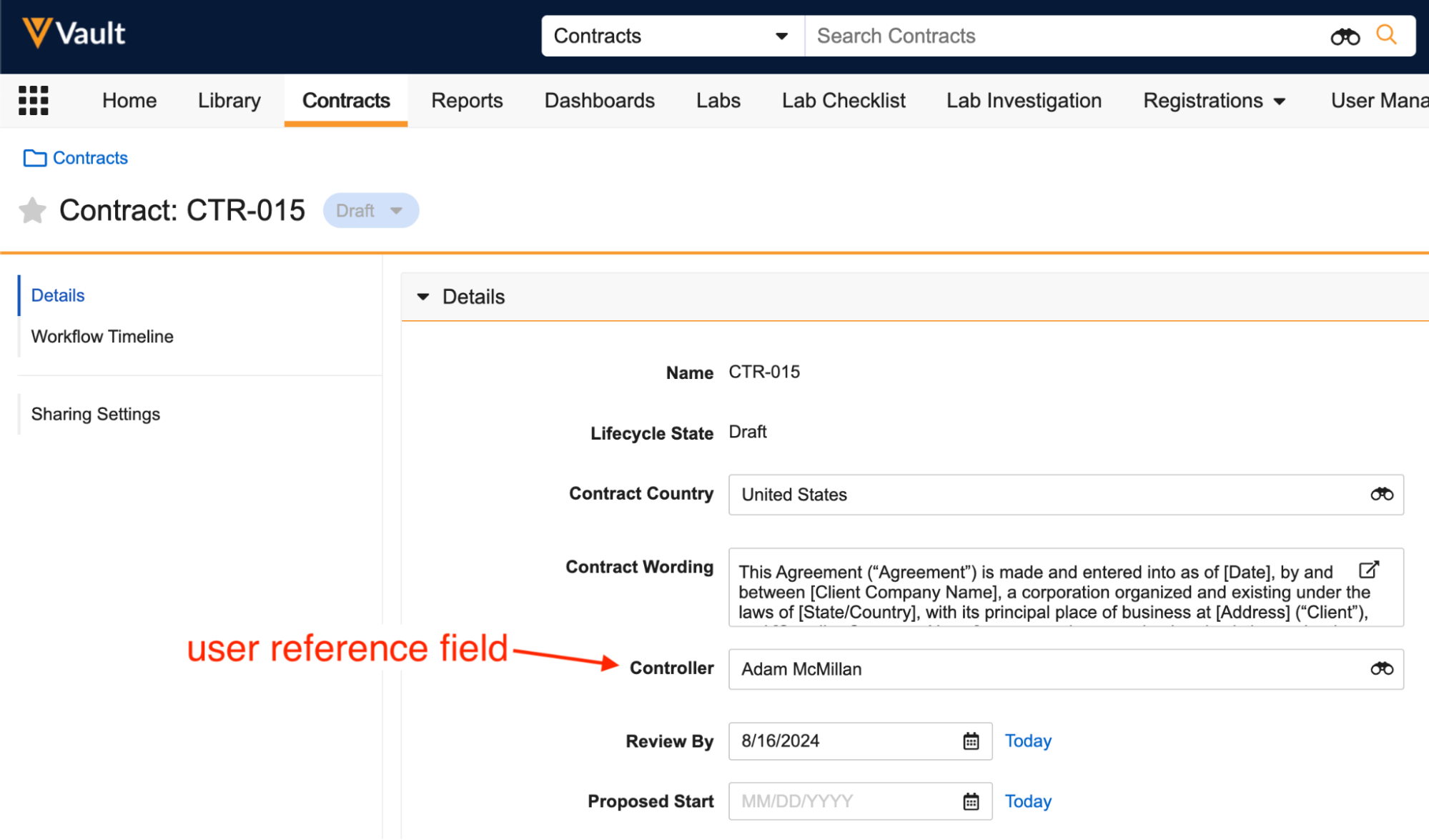
With the ability to designate specific users in fields on object records and documents, it is common to see an assignee or process owner selected, who may not have a specific role in the record’s or document’s Sharing Settings. It is now possible to assign tasks to users selected in user reference fields, along with sending them notifications, or adding them to roles via Update Sharing Settings workflow steps.
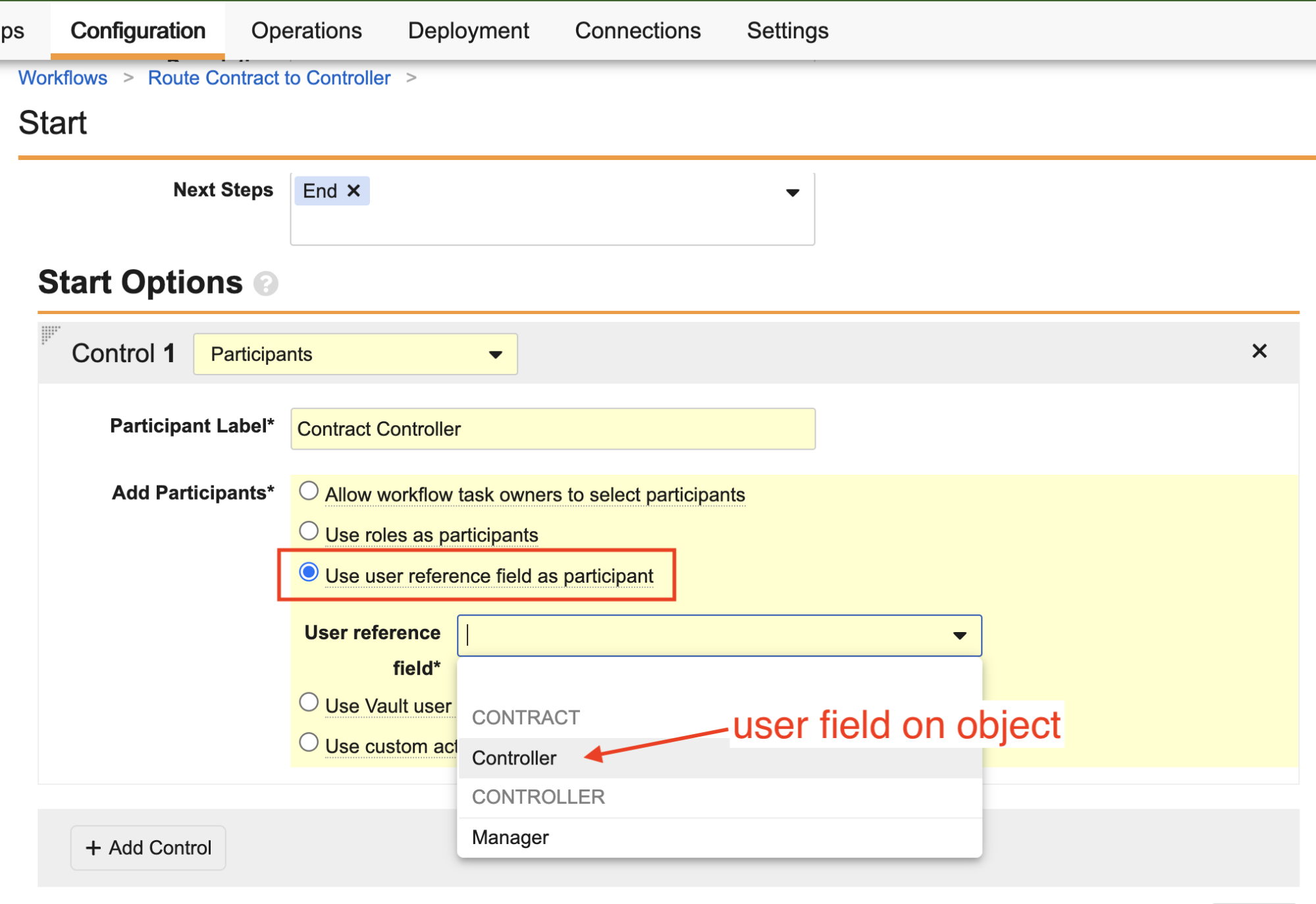
When configuring a workflow Participant on a workflow Start step, Admins can now select Use user reference field as participant, which allows you to select one of the user reference fields on the object or document.
Similar to Use roles as participants, this type of workflow Participant is not displayed in the Start step to the Workflow Initiator because Vault already knows who the user is in that field.
Typically, Vault works out who is in a workflow Participant Group when the workflow initiates. However, for these user reference participants, they are not resolved until the workflow step that uses them executes (task created, notification sent, sharing settings updated, etc.) This allows for processes where that user reference field is set earlier in the workflow, and then used as a task owner, for example, later in the workflow.
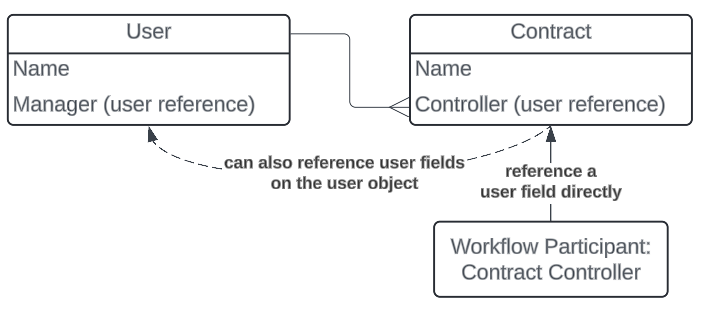
Another key aspect of this functionality is that you can configure the workflow Participant to reference another user reference field on the User object, for a specific user reference field on the object or document defined in the workflow, such as the Manager field:
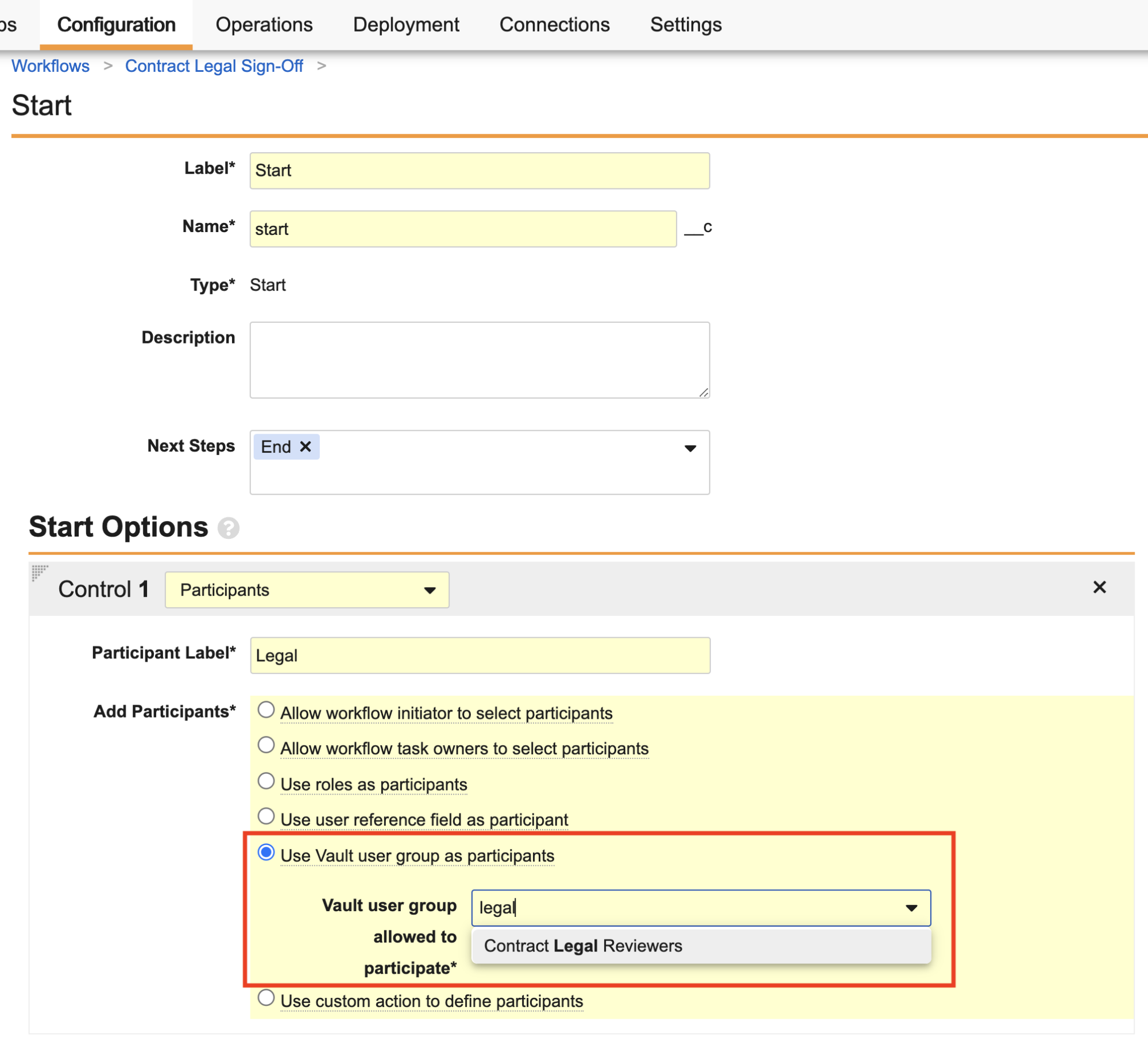
Workflow Uses Groups as Participants
Many customers utilize manual User Groups (along with auto-managed groups used by Dynamic Access Control) to share documents and data, and assign workflow tasks. In 24R3, when configuring a workflow and defining a workflow participant in the Start step, Admins can now default users from a User Group (limit of 1) by selecting Use Vault user groups as participants. When the workflow begins, Vault adds any users in that User Group at that point in time to the Workflow Participant Group (subsequently used for assigning tasks, updating sharing settings, sending notifications, etc.)
The new Use Vault user group as participants option supports System Managed, Custom, and Manager groups (if enabled for the Vault), but not auto-managed groups created through Dynamic Access Control. It works much in the same way as today’s Use roles as participants option, where that Workflow Participant field is not displayed in the Start step to the Workflow Initiator because Vault handles all the user selection.
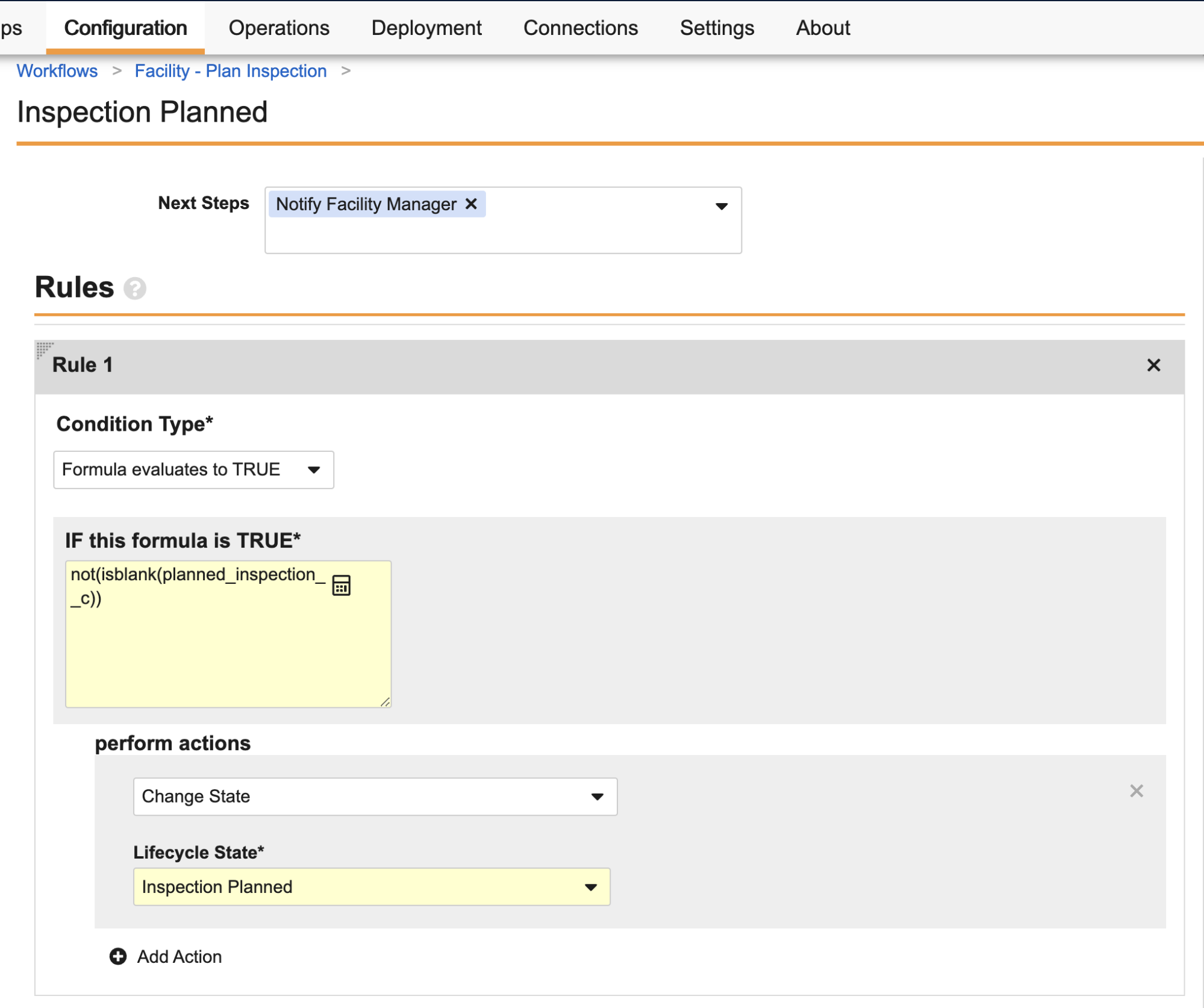
Formula Support in Lifecycle & Workflow Conditions & Entry Criteria Validation
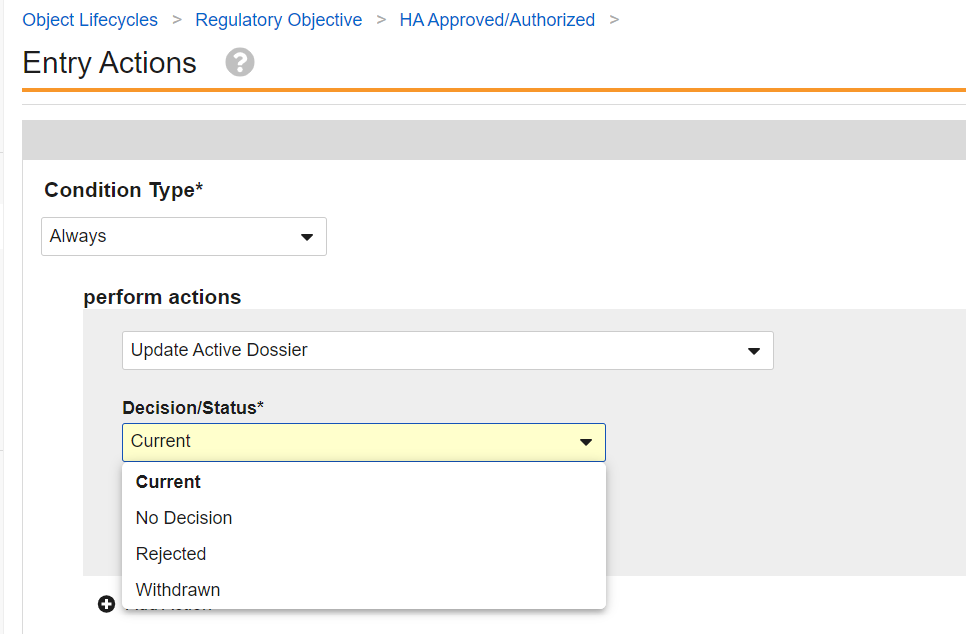
When defining lifecycle state user actions, entry actions, and entry criteria, you can now use a formula expression as the conditions for whether the action is available or is executed.
The Condition Type selector has been updated as a dropdown field rather than a radio button to select Always or Perform with conditions. In addition, the new Formula evaluates to TRUE option is available as a condition type. In the above example, you can see how this is much better for conditions based on date fields, no longer requiring configurations that say ‘within the last X days’ to achieve the effect of ‘cannot be future’, for example.
This same new capability is also available in workflows when defining Action steps (such as for field updates and state changes), and Decision steps when defining conditions for each rule.
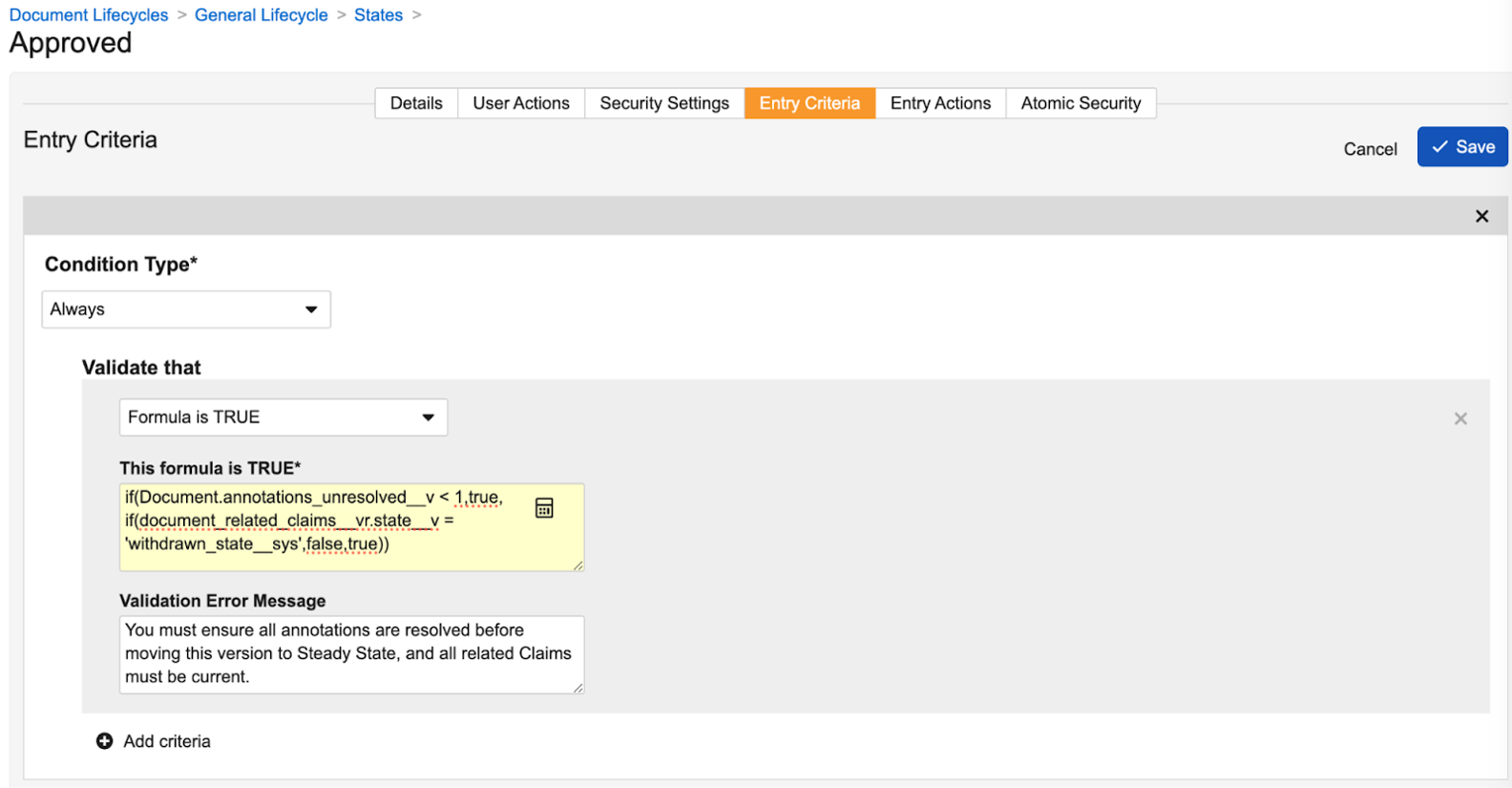
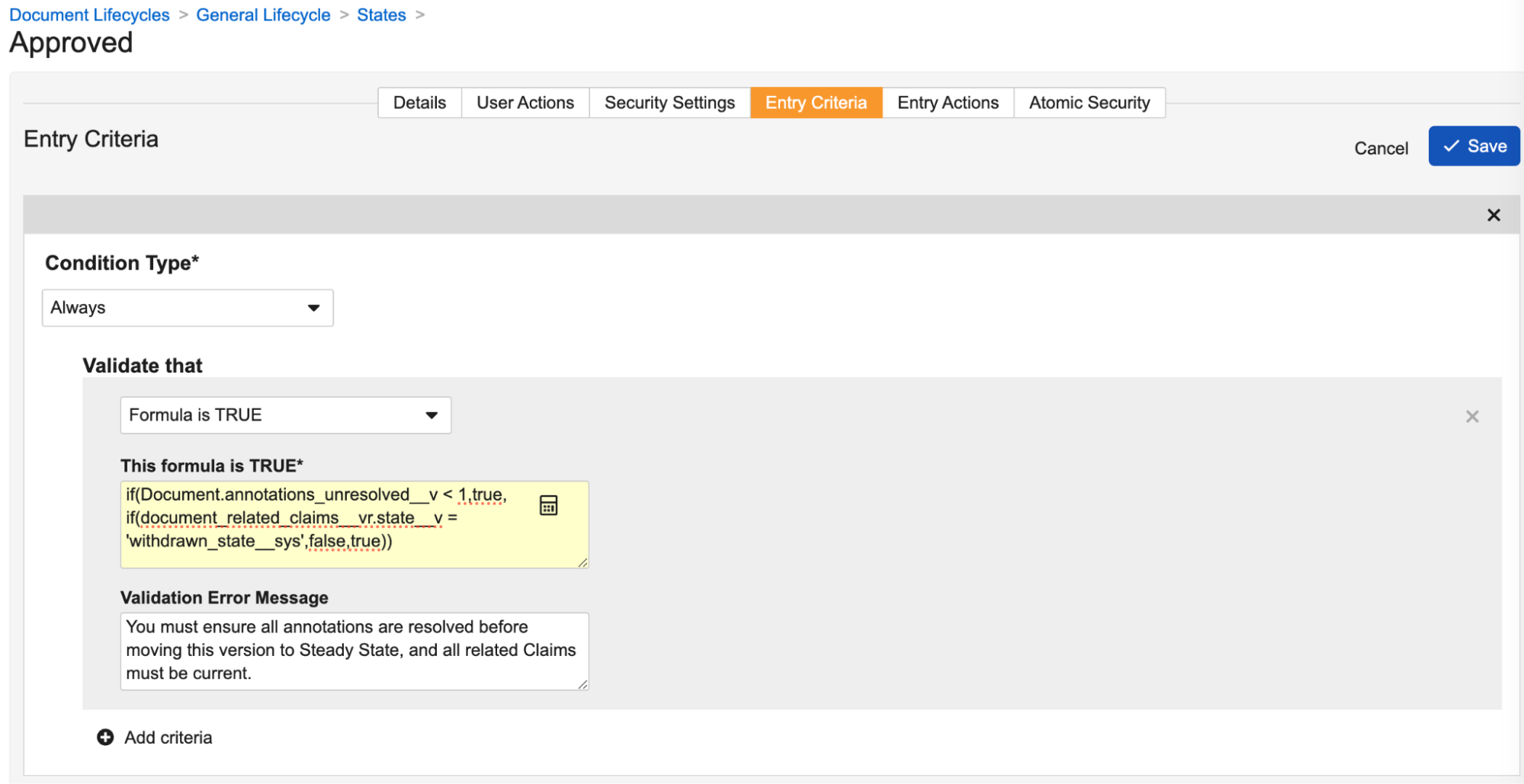
Another major part of this enhancement is to the lifecycle state entry criteria capabilities. Not only can you configure a formula (which must evaluate to ‘true’ to allow the state change), but you can also define a Validation Error Message for when the formula evaluates to ‘false’, which blocks the state change.
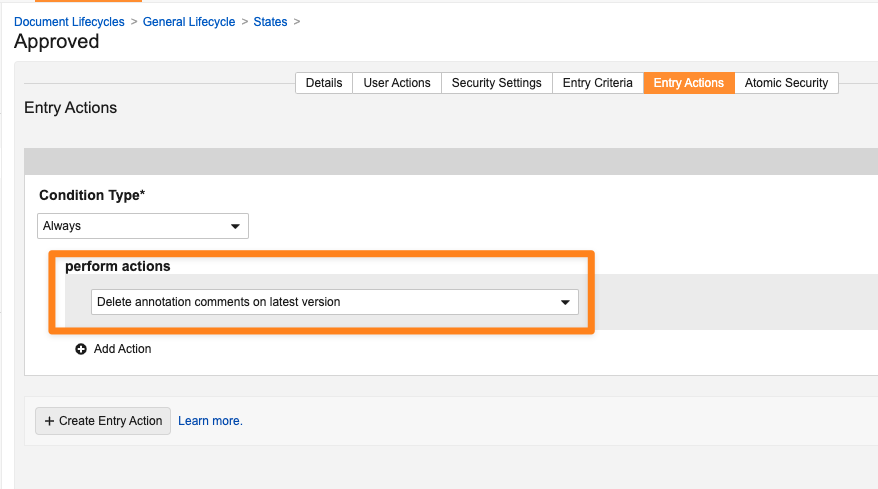
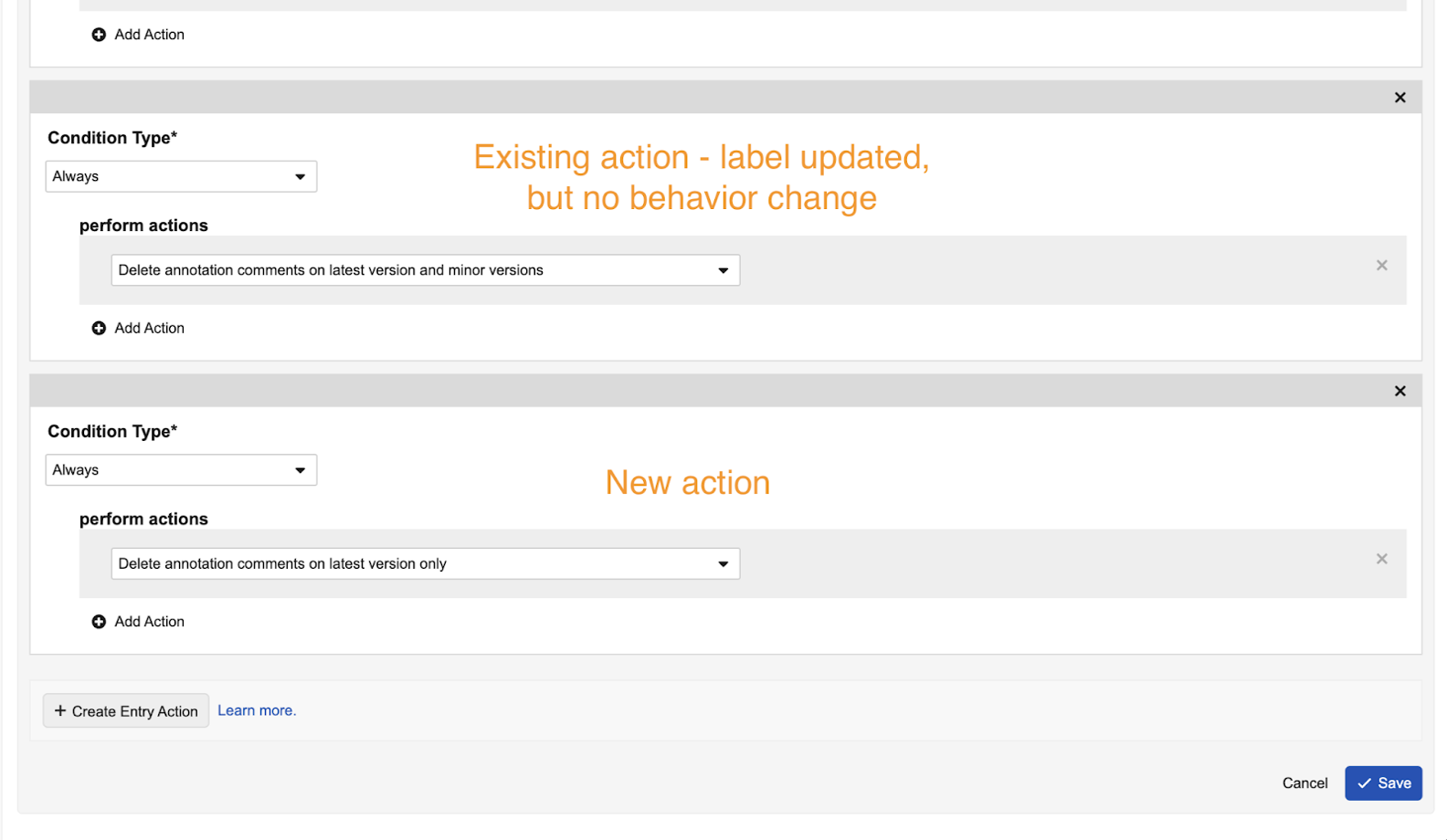
Entry Action: Delete Annotation Comments on Latest Version
This feature introduces a new lifecycle state entry action, Delete annotation comments on latest version, which deletes comment annotations and document-level comments from the latest version only.
Prior to 24R3, Admins could choose the existing Delete annotation comments entry action, which deletes comments from the latest version and all minor versions under the latest version, but cannot delete annotations from the latest version.
This enhancement provides more robust options for standardizing how annotation comments are handled upon state changes. This new entry action, as well as the existing entry action, do not affect document link or anchor annotations.
To ensure clarity for Admins, the existing entry action has been relabeled to Delete annotation comments on latest version and minor versions.
Managing Data
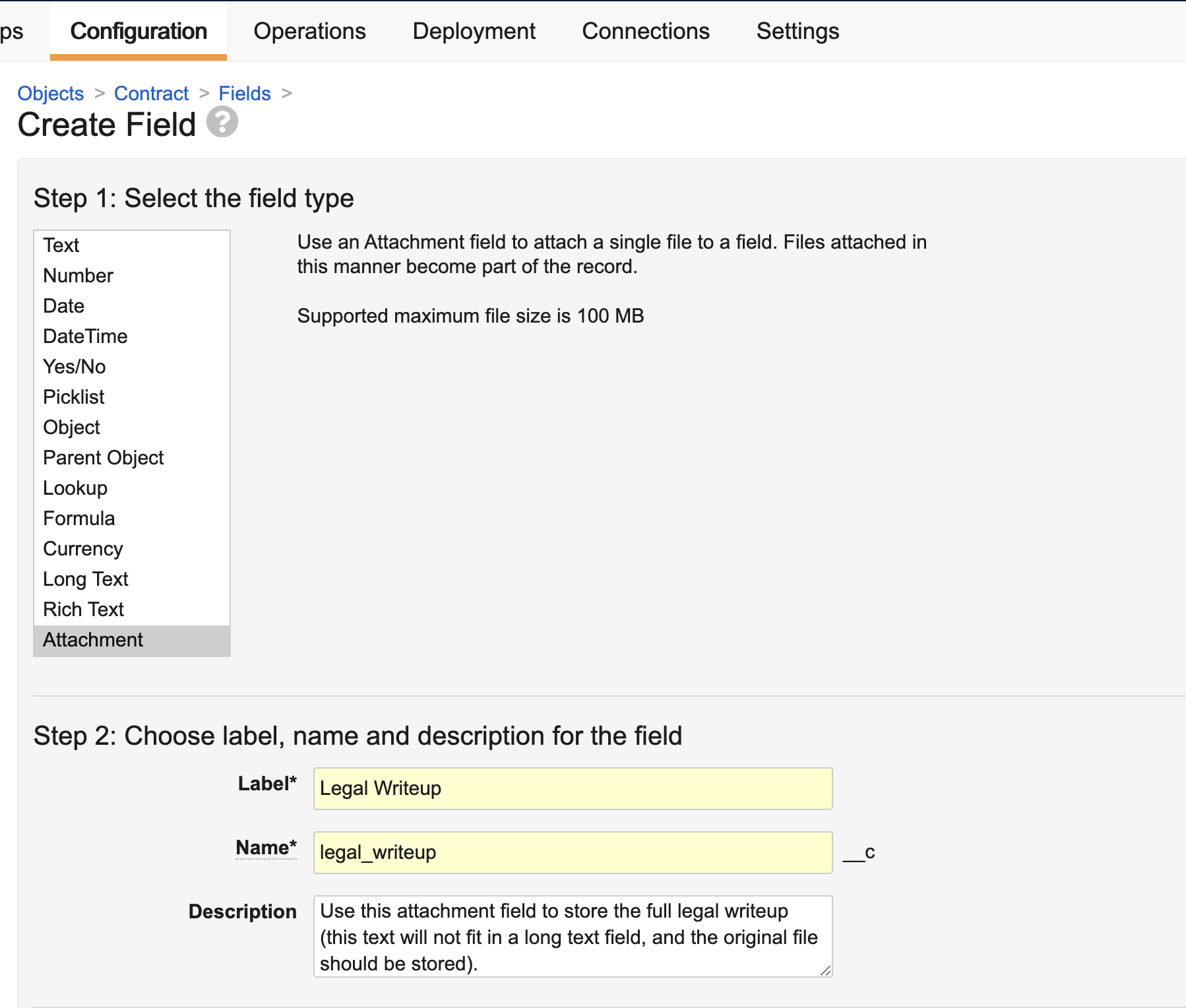
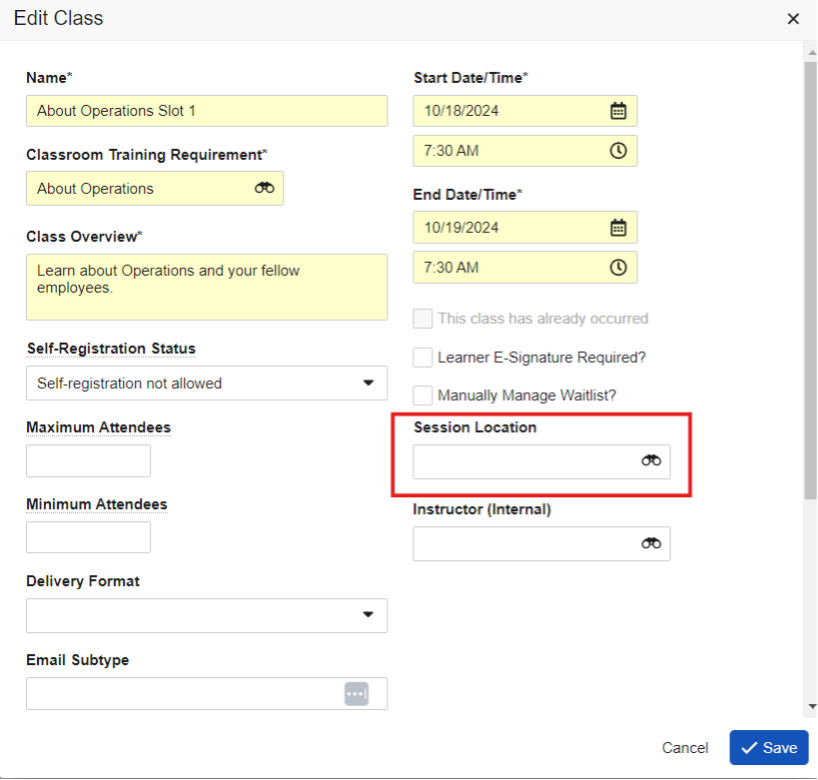
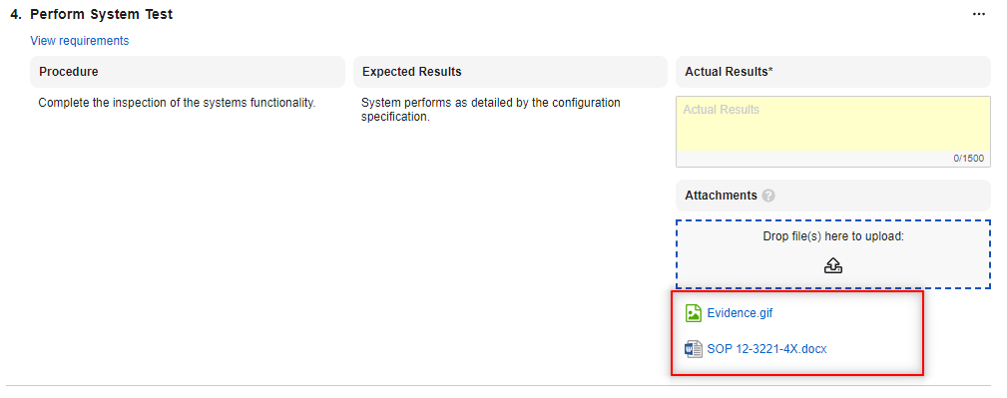
Attachment Fields
The new Attachment object field type allows users to select and upload a file to an object record. Unlike record attachments, known as Attachment sections on object records, Attachment fields allow you to configure dedicated upload slots for specific files you need included in the record.
This feature is useful in large data migrations, where pasting content into Long Text fields may not be an option, but you still want to clearly identify the purpose of the file’s contents, as opposed to a generic attachment.
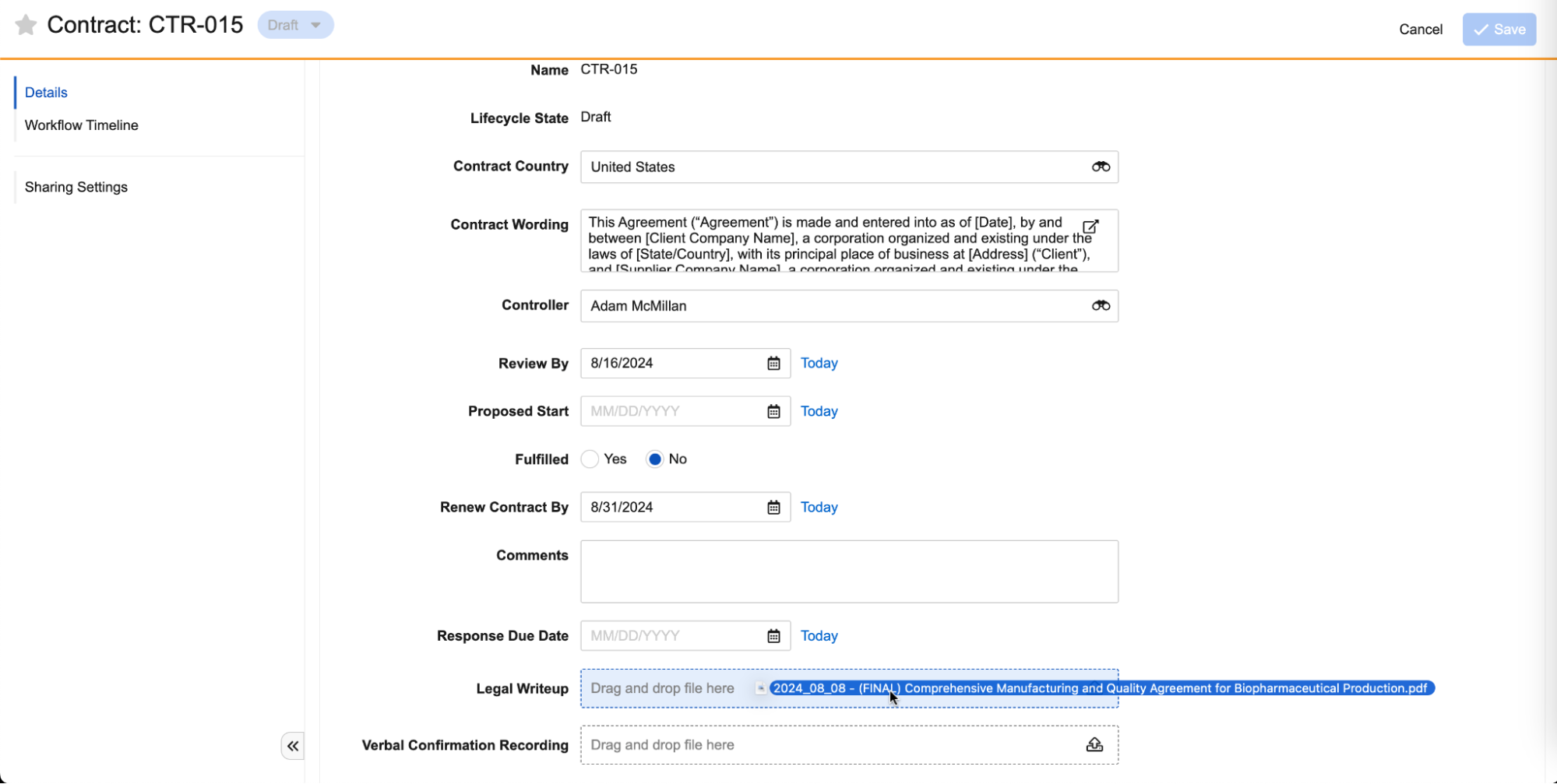
Drag and drop files directly into attachment fields:
Attachment fields can contain one file only, up to 100 MB in size:
You can use Layout Rules and Validation Rules with the isBlank() function, or lifecycle state entry criteria, to ensure these fields are populated by certain points in the lifecycle (or according to the logic you configure).
Vault does not render files uploaded to attachment fields, so you must download them in order to view them. You can control Attachment fields via Atomic Security at the Lifecycle State or Role level, and you can query these fields in VQL to retrieve the file name, MIME type, and file size. These fields are also available in reports for objects that have attachment fields configured. You can add files added to the file staging server to Attachment fields via Loader by referencing the file path (link to file staging server info). Finally, Vault cloning, including Sandboxing, supports Attachment fields, meaning if an object record is included in Vault clone, and it contains files in Attachment fields, those files are copied to the cloned Vault and record.
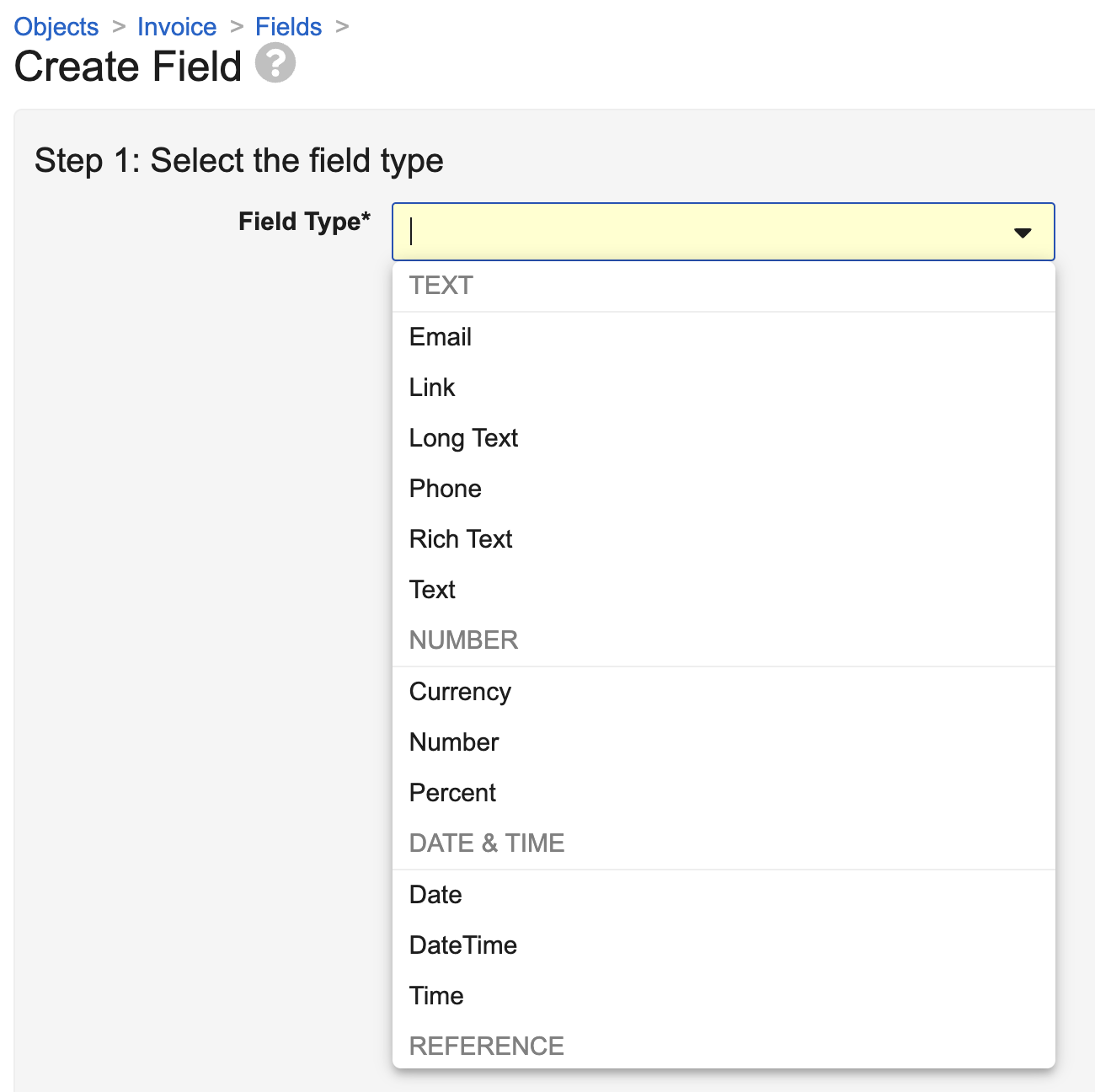
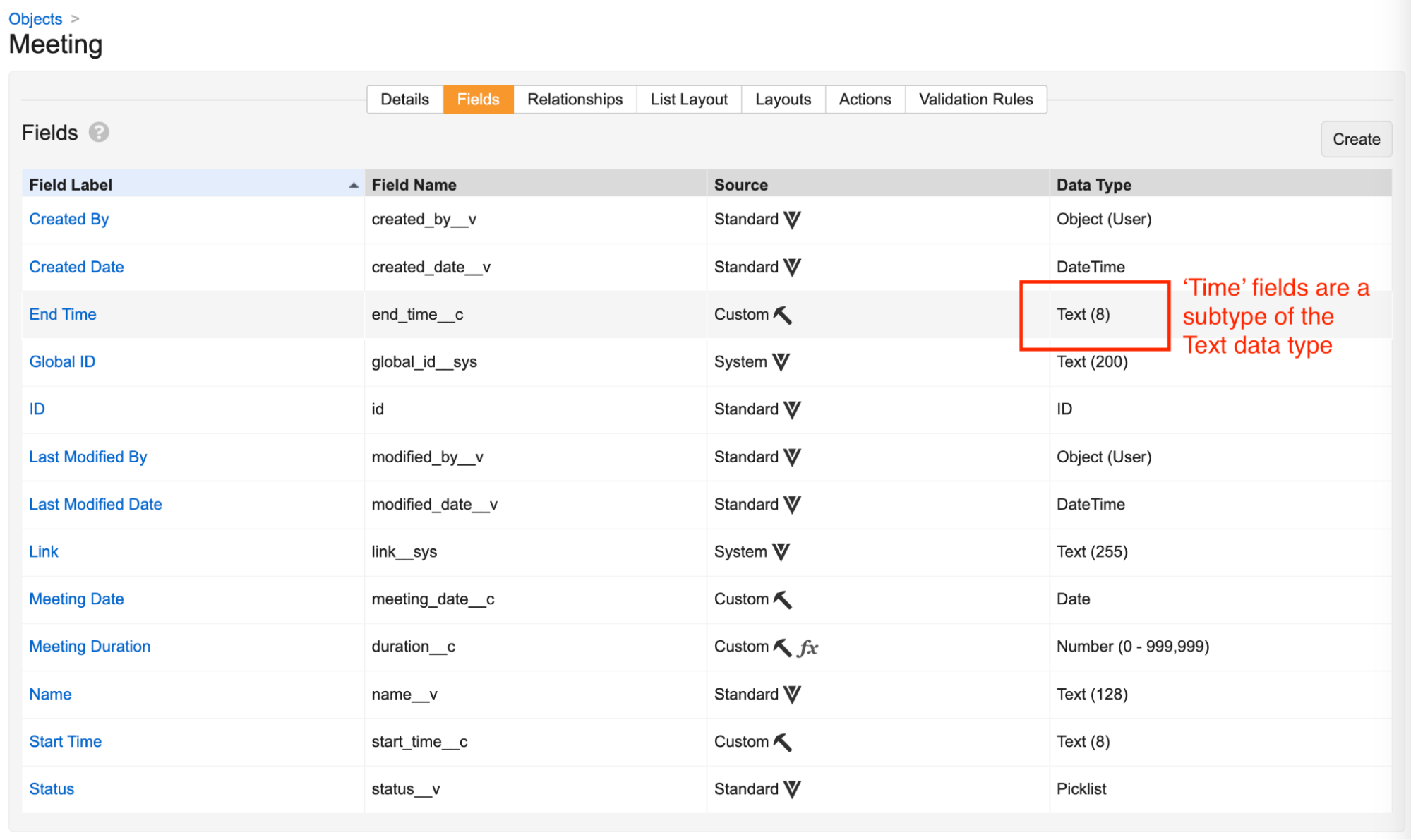
Field Subtypes
In 24R3, we are updating the UI for the Create Field page (Admin > Configuration > Objects > [Object] > Fields > Create) to accommodate new field subtypes introduced in this release.
In addition to the existing field subtypes of Link (which is a subtype of Text) and Currency (which is a subtype of Number), we are introducing the following new subtypes, which will appear as distinct new field types:
- Percent (Number)
- Email (Text) - formats as a “mailto” hyperlink (Vault does not validate if it’s a real email)
- Phone (Text) - formats as an international phone number where possible
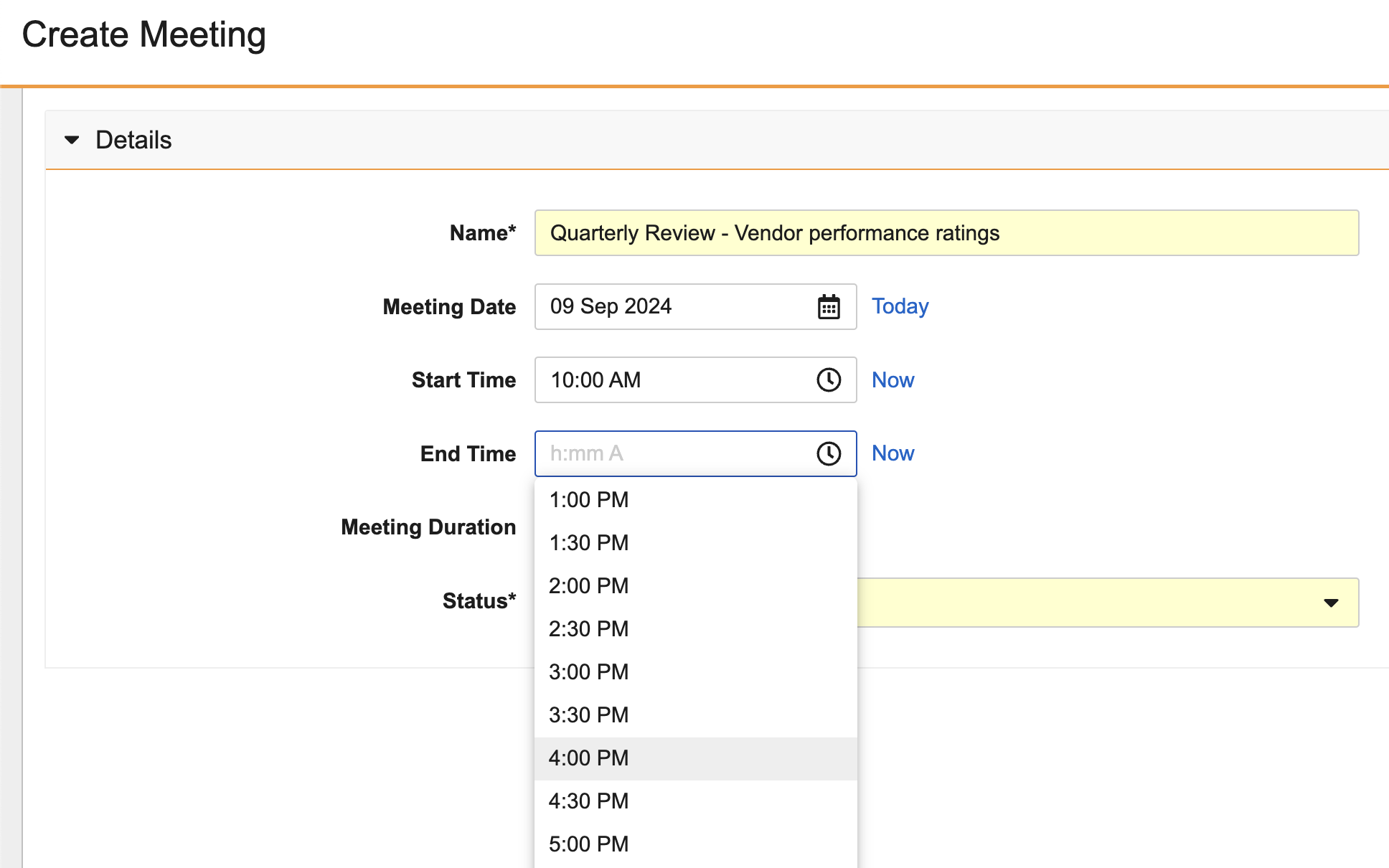
- Time (Text) - users select from a Time selector in the Vault UI, and formats according to the user’s local time
Though you can custom configure a Format Mask to act like Percent, Email, and Phone, these new standard field subtypes mean you don’t have to manage that moving forward. In addition, these standard field subtypes allow us to deliver specialized UI for easy data entry or validation.
The time closest to the current time is highlighted in the selector.
Field subtypes are specialized ways of presenting the data types of Text and Number, and when you’re reviewing an object’s fields, Vault displays the true data type in the Data Type column.
Learn more about the developer-facing functionality of this feature.
User-Person Sync Enhancements
Today, when changes are made to a User record (directly, or via the VeevaID portal), the following sync-eligible fields are automatically synced to Person records on all Vaults that reference this user: First Name, Last Name, Language, Locale, Timezone, Email, Mobile, Image, and Manager.
To aid with transparency of what changes occurred, there is a new Prior Person object related to the Person object, which captures the current values before the user-to-person sync process completes. Each time the sync occurs, the Prior Person record is updated, and the full history of these automatically-synced changes can be seen in its audit history.
In addition to this change, Vault Admins are no longer restricted from editing the sync-eligible fields on Person records. Originally, this was restricted if the associated user was a Cross-Domain or VeevaID user. However, when Person records are updated, the person-to-user sync never updates User records of Cross-Domain or VeevaID users.
Yes/No Checkbox Field Enhancement
While Yes/No fields on objects have always supported a null value, Yes/No fields configured as checkboxes have not. A Yes/No field is null if the user has not actively selected Yes or No. In 24R3, Yes/No checkbox fields also support null values, which impacts how records are created and updated.
Impact on New Record Creation and Vault Configuration
- When creating new records, if the user doesn’t make a selection, the checkbox will save as
nullif the field is not configured with a default value. Prior to 24R3, Vault saved the field as No and cleared the checkbox. - If a Yes/No checkbox field is
null, it displays the same as a checkbox set to No or cleared. However, a tooltip hover identifies the checkbox asnull. - Changing a Yes/No field to a checkbox field no longer updates
nullYes/No fields on records (to set them instead as No), because Yes/No checkbox fields now supportnullvalues. - Creating new Yes/No checkbox fields on objects or object types no longer causes updates to existing records. This happens because Yes/No checkbox fields now support
nullvalues, which is the case for records that already exist.
Automatic Configuration Update on Release Night to Preserve Behavior
Veeva will automatically preserve today’s behavior via a release-night automatic upgrade of existing configuration. Any existing custom Yes/No checkbox fields that do not have a default value configured will now have a default value of No. This matches the Create and Update record behavior prior to 24R3, where if no selection was made, Vault saved the field as No and cleared the checkbox.
Integrations and Custom SDK Code
The API now supports clearing a Yes/No checkbox field by passing in a null value via the API or SDK. Integrations and custom SDK code should be reviewed in case written with the expectation that Yes/No checkbox fields can never be null. For example:
- You are creating a record but not setting an explicit value for a Yes/No checkbox field, and expecting a No value once created.
- You are setting a Yes/No checkbox field to
nullin your code, intending it to be false (which will now benullin 24R3). - You are retrieving the value of a Yes/No checkbox field and evaluating its value to determine next lines of code, but you aren’t considering the possibility of it being
null.
Older API versions will retain their current behavior so existing integrations should see no impact unless the API version is updated.
Best Practice for Vault Configurations Referencing Yes/No Checkbox Fields
Your existing configuration will continue to work, but as a best practice, it is important to identify formula expressions, filters, reports, dynamic role assignment, etc. to always consider the null value, which is treated the same as a blank value, in all field types, including Yes/No checkbox fields.
Note: This change was originally announced when 24R2 was released. The immediate impact is limited to any integration or SDK code that assumes a Yes/No checkbox field cannot have a null value.
Analytics
Improved Color Contrast in Dashboards
In the 24R2 release, several enhancements were made to improve color contrast of the Vault user interface, in order to provide a more visually distinct and user-friendly experience.
With 24R3, we have extended these changes to Dashboards to ensure consistency in the visual experience across Vault. This changes the default colors used in Dashboard components going forward, though report creators can still use conditional fields to create custom coloring.
Learn more about Creating & Editing Dashboards and Viewing & Sharing Dashboards.
Reduce Dashboard Cache & Display Last Updated Time
Dashboards will now automatically refresh every 12 hours if not manually refreshed. Prior to 24R3, dashboard data cached and automatically refreshed every 36 hours, which often caused users to perform manual refreshes more frequently.
Additionally, Dashboard components now display an icon with the last updated time on each chart, making it easier for users to know how current the data is based on the last refresh.
Learn more about Dashboards.
Picklist Enhancements in Formulas
As part of Veeva’s work towards a common data architecture, we are introducing a new PicklistValues function, which is the recommended way to return picklists values in formula expressions for objects. The PicklistValues function supports hyphens in picklist name values, and allows you to return multiple values in your formula. For example, PicklistValues(picklist_field__c,'texas__c','new-york__c'). Though PicklistValues allows you to return multiple values, the ability to populate multi-value picklist fields with multiple values using formulas is not yet supported.
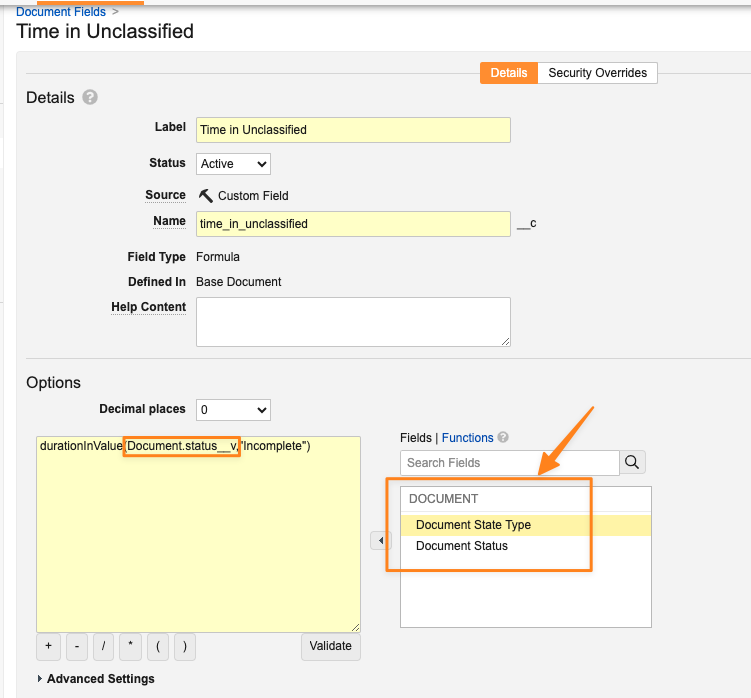
Document Formula Fields Limited to Status & State Type
When using the following document formula field functions, only the Document Status and Document State Type fields will be supported for new formula fields.
DurationInValue()NumTimesInValue()FirstTimeInValue()LastTimeInValue()PreviousValue()
These functions are commonly used to perform cycle time calculations and are rarely utilized for fields other than Document Status. Because the fields work by querying the audit trail, focusing these functions on Document Status and Document State Type will improve the scalability of Vault’s audit trail, while still supporting the main use case for these functions.
Any existing document formula fields that are using these functions and other field types will continue to work after 24R3, but new formula fields will only be able to use Document Status and Document State Type fields.
Learn more about document formula fields.
Admin Experience
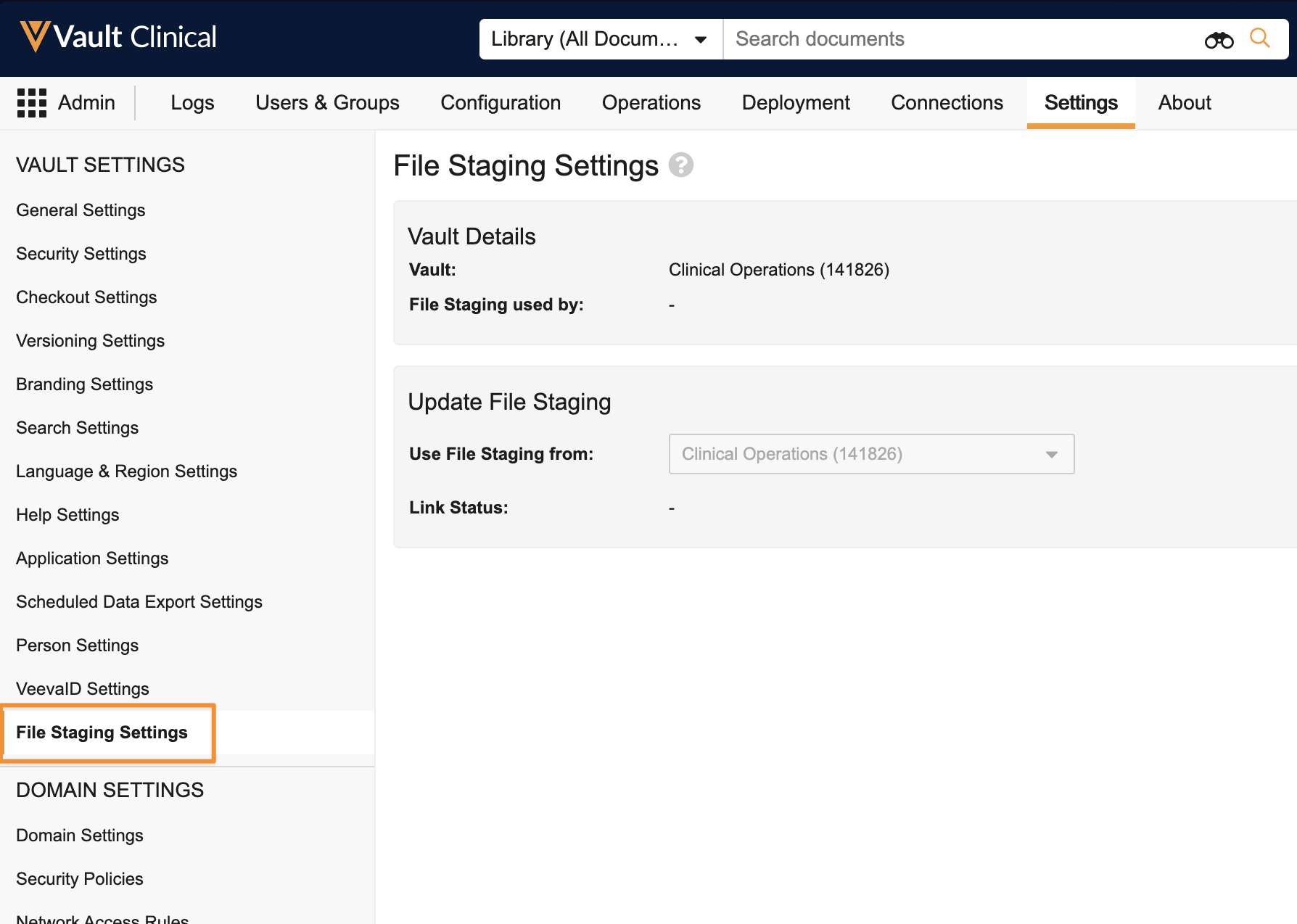
File Staging Linking
Admins will now be able to link a Vault’s File Staging server to a different Vault, without needing to rely on Product Support. Each Vault has a File Staging server that acts as a temporary storage area for importing or exporting files with Vault. In migrations, it’s common for customers to link File Staging rather than manually moving documents between different File Staging servers.
A new area is available in Admin > Settings > File Staging Settings where Admins can modify the File Staging of a Vault. Each Vault can only have one File Staging, but multiple Vaults can link to a single File Staging area.
Note: File Staging links cannot be chained together (i.e. where Vault A links to File Staging on Vault B and then Vault B links to File Staging on Vault C), nor can they be cyclical (i.e. where Vault A links to File Staging for Vault B, and then Vault B links to File Staging on Vault A).
Learn more about File Staging here.
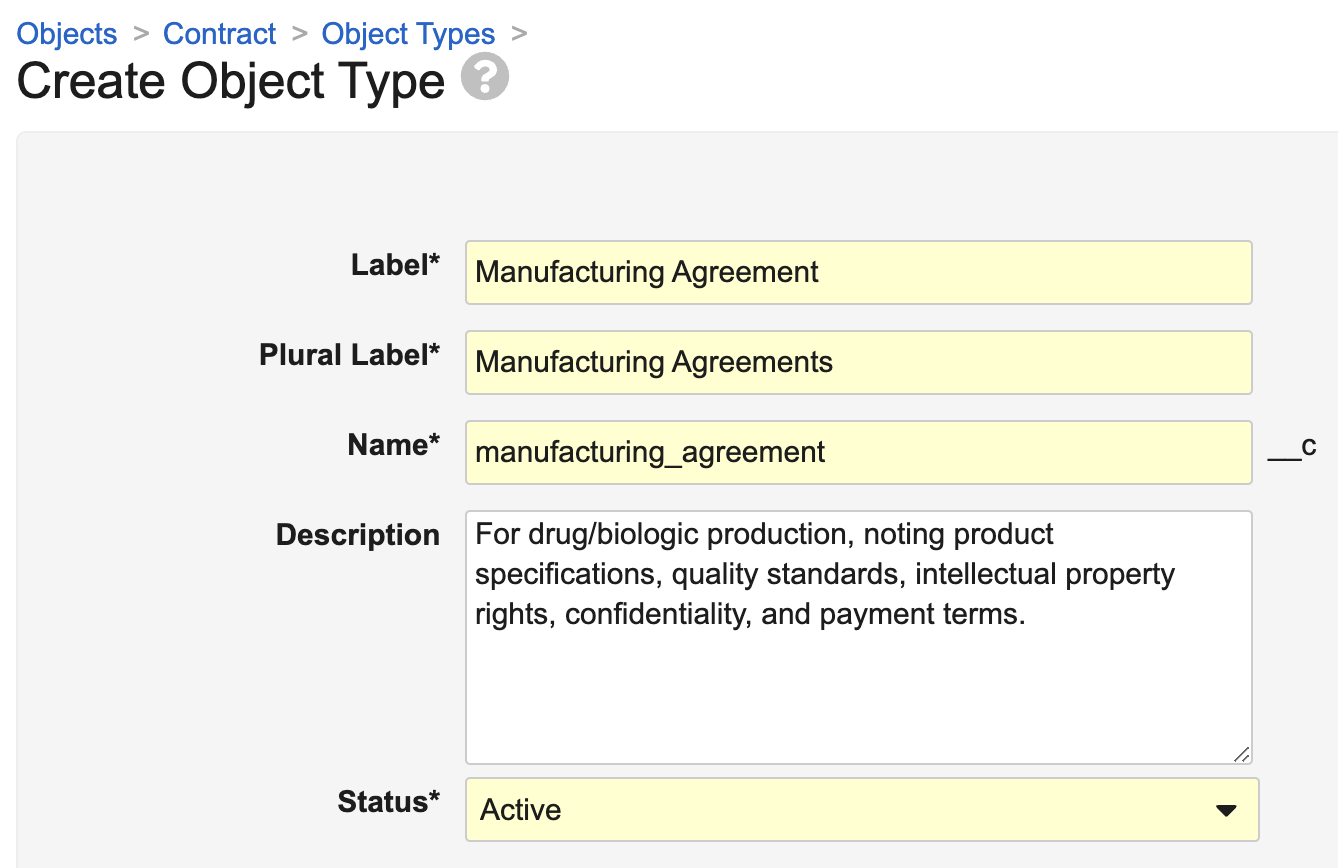
Object Type Descriptions
For objects with object types enabled, Admins may need to understand the context behind each object type. With 24R3, Admins can now populate a Description field while creating or editing an object type. The object type description is included as part of its metadata.
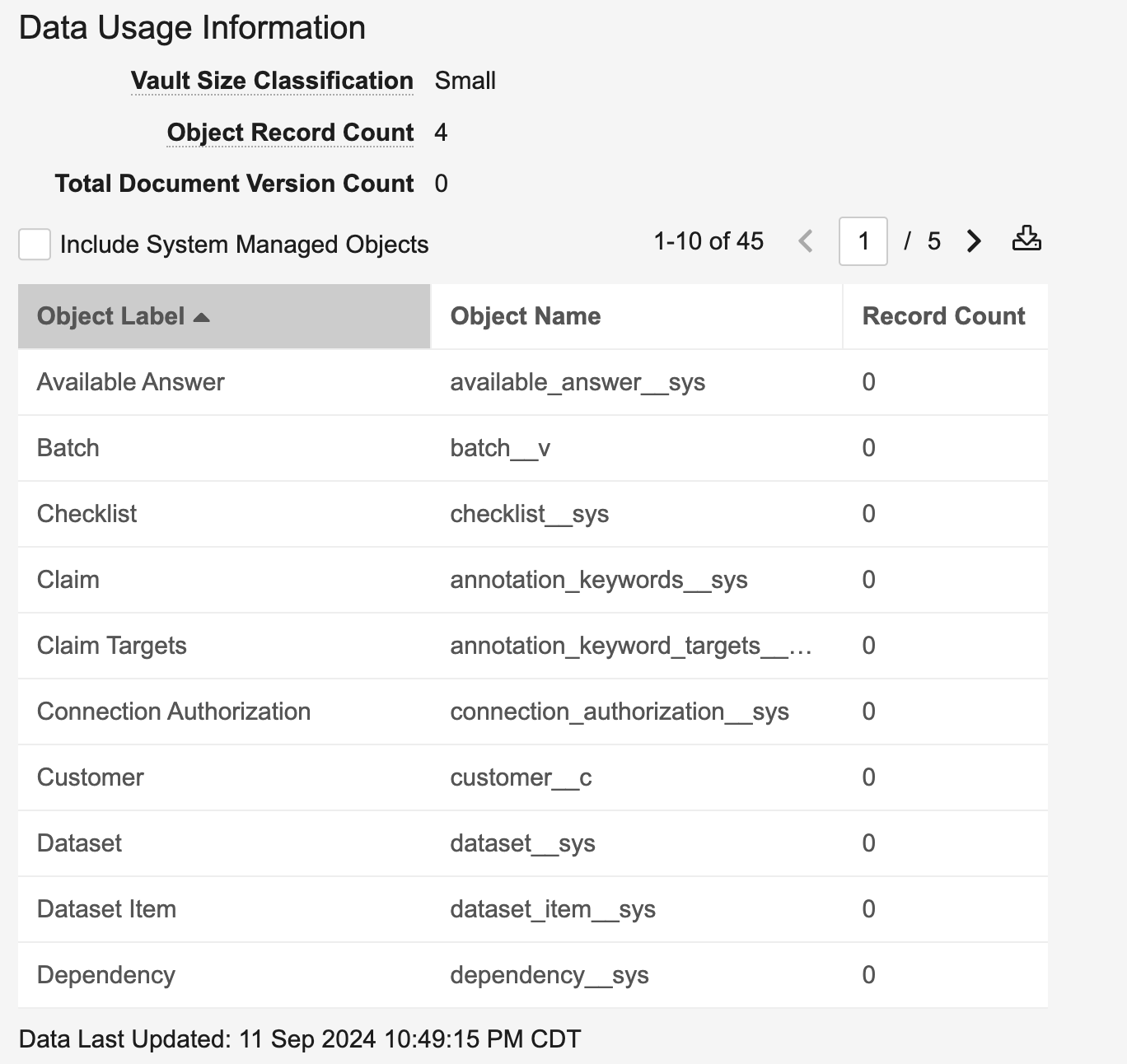
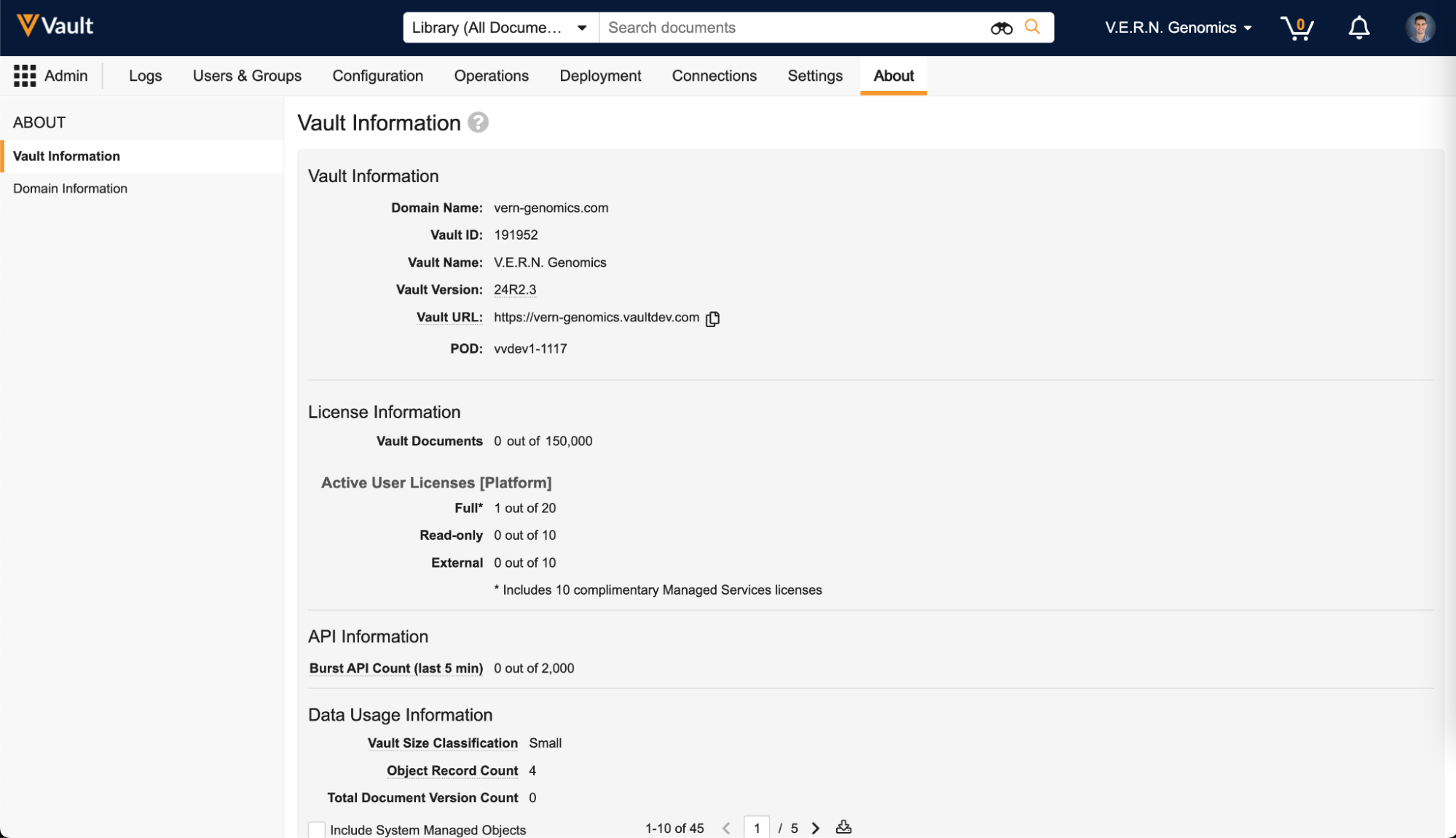
System Managed Objects Toggle for Data Usage Information
The Data Usage Information table (Admin > About > Vault Information) now excludes system-managed objects by default. This keeps the information more relevant to the customer’s data, but you can click the Include System Managed Objects checkbox to see system-managed object data.
Note: The Data Usage Information table has moved from the Settings tab to the new About tab.
Vault Loader Record Migration Mode Enhancements
Vault Loader has been enhanced to take advantage of the Record Migration Mode Enhancements feature.
Vault On-the-Go
Favorite a Document
Users can now mark and unmark documents as Favorites within Vault Mobile.
This enhancement makes it easier for users to adjust their Favorites without needing to be on the web. This can be particularly helpful if users are relying upon Favorite Document Notifications.
Learn more about Favorite Documents and using Favorite Document Notifications in Vault Mobile.
Site Selector Support (SiteVault)
SiteVault users can now view and change their site context within the Vault Mobile app. A new site selector list will be available at the top of each mobile tab, which will filter the Library results to that specific site.
SiteVault leverages a specific selector that allows users to navigate among their research organization and site(s) in SiteVault. The documents that you can access or manage are determined by the site context that’s selected. This provides a more consistent user experience between the mobile app and the web browser for SiteVault users.
Learn more about SiteVault
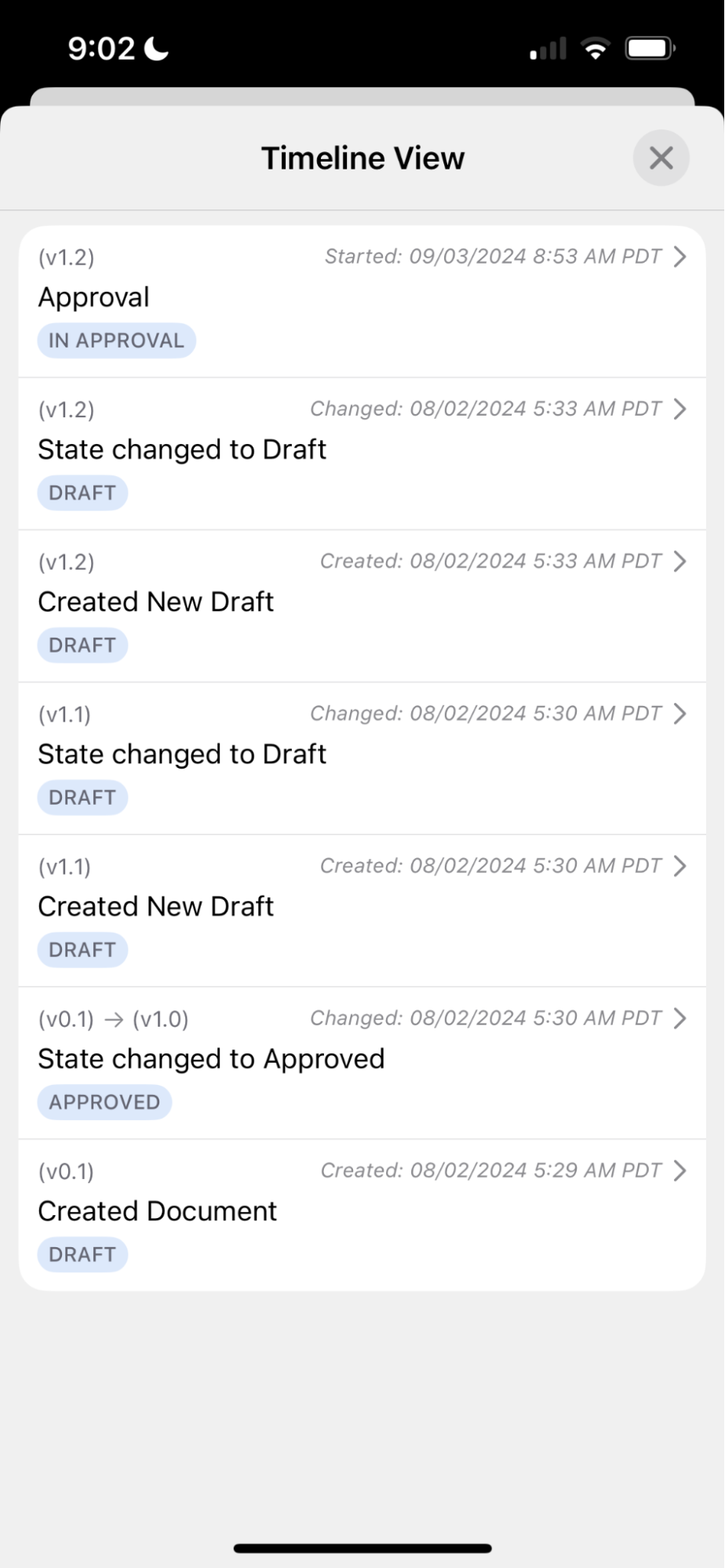
Show Timeline View
Users can now see the Timeline View for a given document within Vault Mobile. This allows them to see the history of the document, as well as view any outstanding or completed task verdicts if the document is part of a workflow.
Learn more about Vault Mobile.
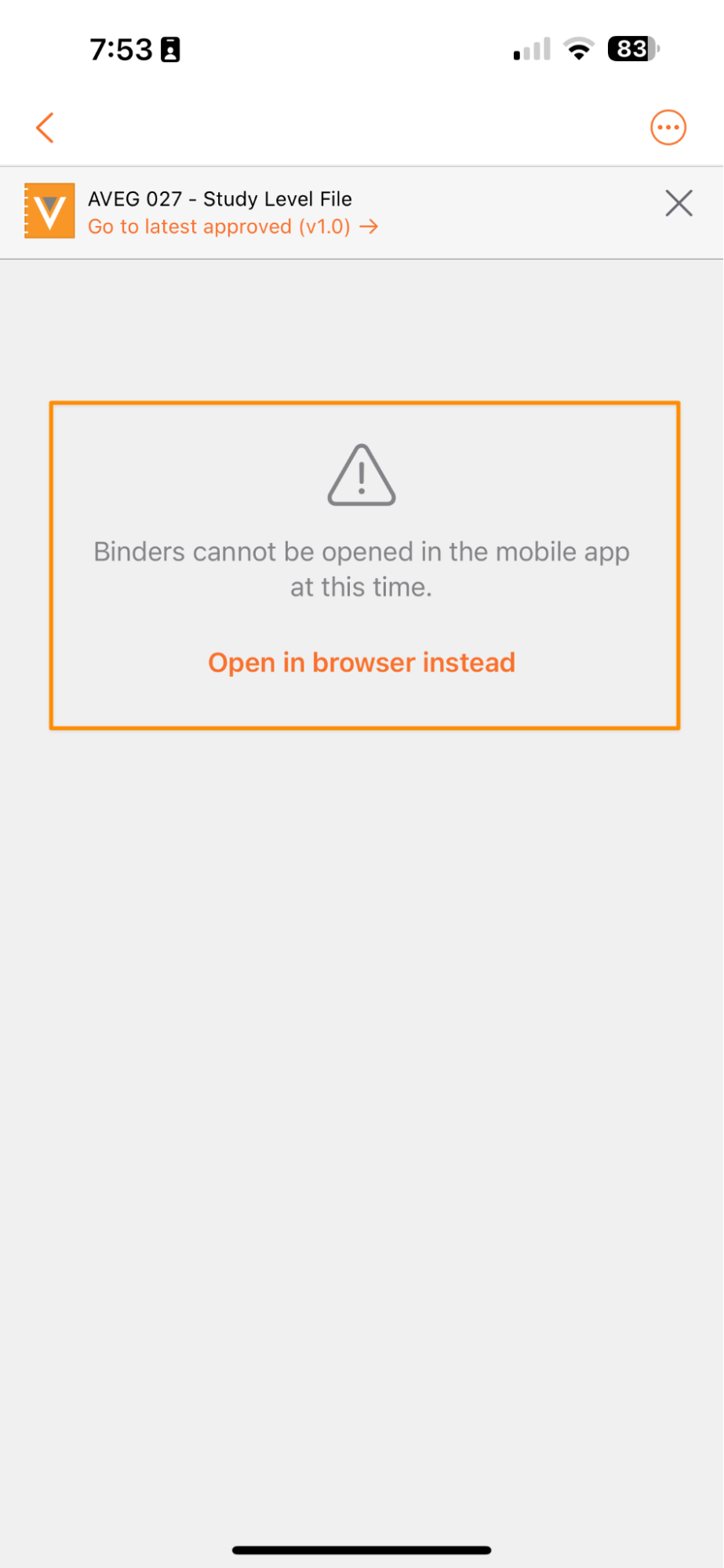
Open Binders in Web
Users that attempt to access a binder in the Vault Mobile app are now presented with an easy way to open that binder via a mobile browser.
While Vault Mobile still does not fully support navigating binders within the mobile app, this enhancement removes friction and confusion for users by allowing them to immediately open the binder in a browser to perform any needed work.
Learn more about Vault Mobile.
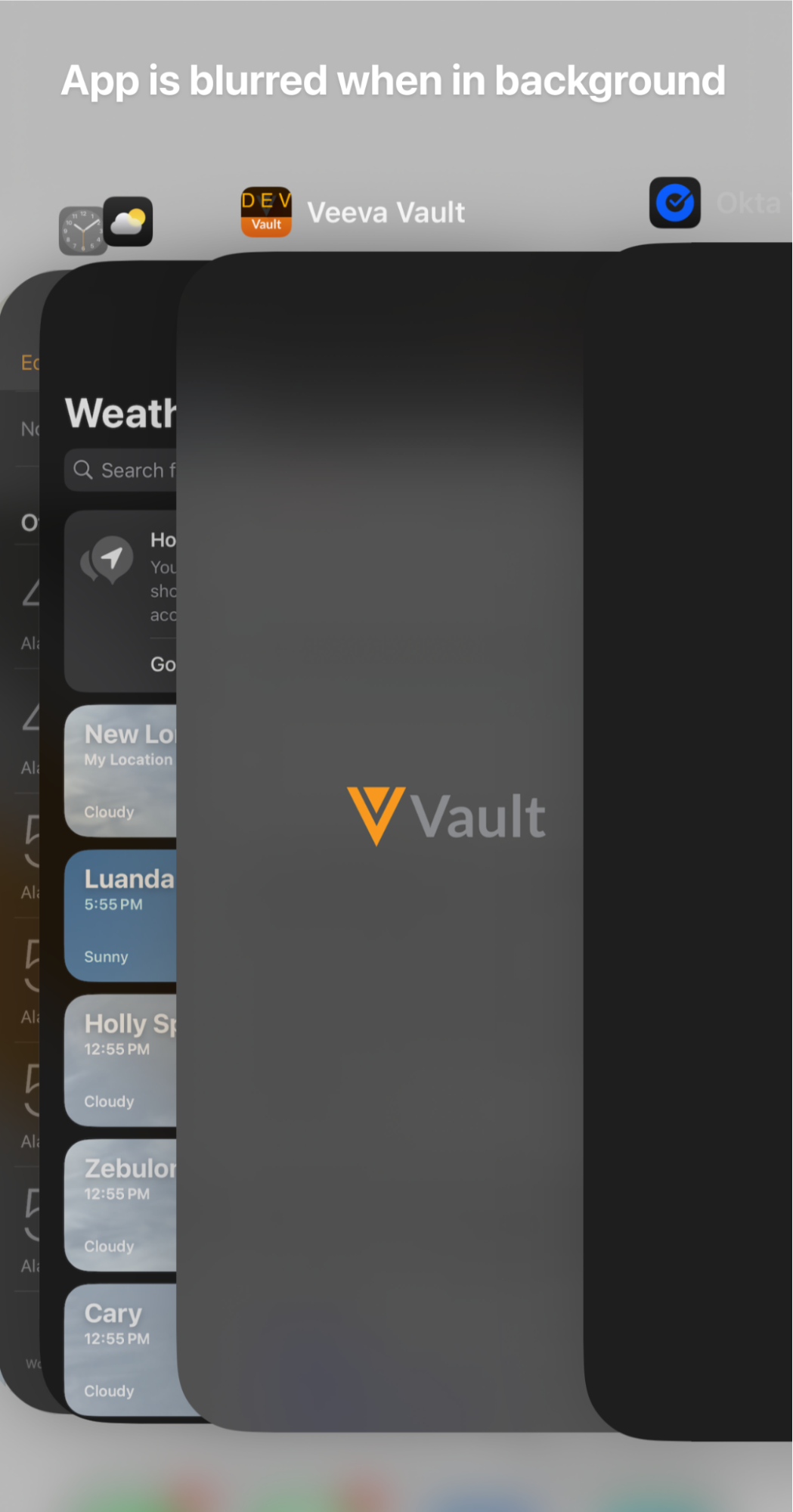
Blur Content when App is in Background
The contents of the Vault Mobile app are now blurred whenever the app is the device’s background. This enhancement provides an extra precaution to prevent any app content from being visible when a user switches between multiple apps and is consistent with how many banking and financial apps work in similar situations.
Performance & Availability
Migrate Vault Component to Raw Object
To support the generation of better configuration reports, comparison reports, and VPKs we have changed the Vault Component (vault_component__v) object to a raw object, which results in better performance for operations using this object. The generic search capability is not available for raw objects, so we recommend that users add filters such as a starts with filter on the Component Label field to perform a text search for specific components.
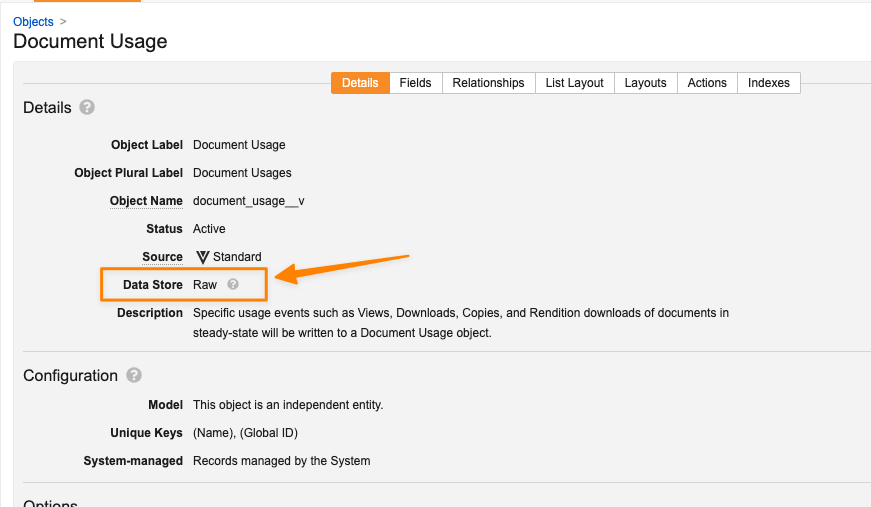
Migrate Document Usage Object to Raw Object
With this release, the Document Usage (document_usage__v) object will be migrated from a standard volume object to a raw object to provide improved scalability performance.
The Document Usage object tracks each of the following actions on steady-state documents:
- View
- Download Source
- Download Rendition
- Make a Copy
Moving this object to a raw object will help provide performance stability, as the number of records created for Document Usage can be in the millions. As this is a raw object, any filters applied on Document Usage records must be case sensitive. For reports using the Contains operator, we recommend ensuring that the filters use the appropriate case.
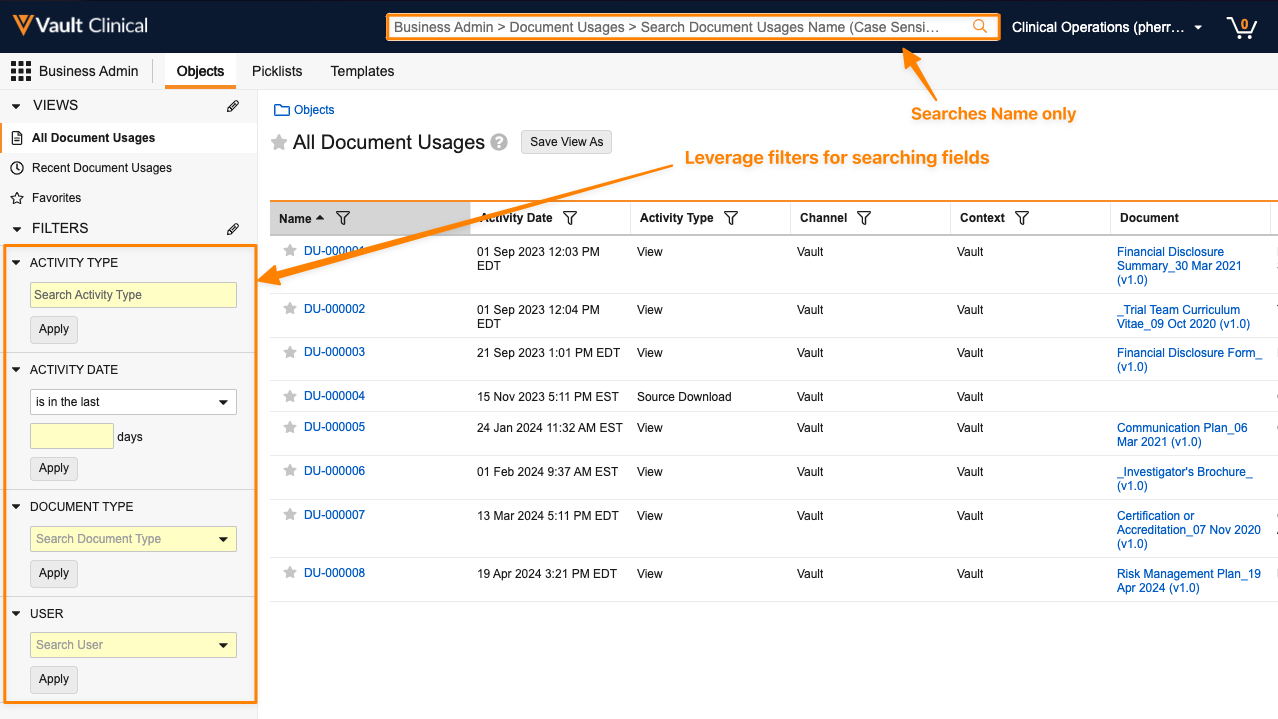
Additionally, if the Document Usage object is exposed in the Business Admin menu, the search bar only searches the Name field. Users with access must use filters on other fields instead of the search bar:
Learn more about the Document Usage object and raw objects.
Minor Enhancements
The items in this section are more minor enhancements that have been made to the Vault Platform.
Name Support in Vault Loader & Documents APIs
Prior to this release, Vault Loader only accepted label values as input for specific fields when creating documents, document versions, or binders. However, labels are not unique and may result in duplicates. To avoid encountering errors due to duplicate labels, you can now provide name values as input for the following fields when creating documents, document versions, or binders:
- Document Type (
type__v) - Subtype (
subtype__v) - Classification (
classification__v) - Lifecycle (
lifecycle__v) - Status (
status__v) - All standard and custom picklist fields
When updating multiple documents using Vault Loader, you can only provide name values for picklists. Learn more about the developer-facing functionality of this feature.
Support for XLSM Files in Collaborative Authoring
XLSM Files can now be edited with Collaborative Authoring. Collaborative Authoring has previously supported editing of XLS files, but XLSM was not initially supported in Sharepoint when Collaborative Authoring was introduced.
This enhancement allows users to work collaboratively on spreadsheets that contain macros, without needing to manage the editing of those separately from other documents.
Learn more about Collaborative Authoring.
Add Annotation ID to Link Target Object
The Link Target object now contains a new Annotation ID field to support integrations that create link targets. By populating this field, either on creation or update, Vault will automatically set Anchor ID, Anchor Title, Anchor Page, and Reference. This allows integrations to create and update link targets for anchors by updating the Annotation ID, without needing to know the specific Anchor ID.
Link Target is a Platform object, and this new field is visible on this object in all Vaults. However, the Link Target is primarily used in Commercial Vaults for Text Asset management.
Learn more about the developer-facing functionality of this feature.
Linked Documents Usability Improvements
Prior to 24R3, when a link annotation was deleted, the Linked Document relationship was not automatically deleted unless the Enable Create & Import Document Links Admin flag was unselected. With this update, those relationships are always deleted when the annotation is deleted, regardless of that flag’s status.
Also, users would only see certain link attributes (such as anchor name and page) and available actions in the Linked Documents section when Enable Create & Import Document Links was selected. With this update, those details and actions are always displayed, regardless of that flag’s setting.
Vault File Manager: File Staging Download Improvements
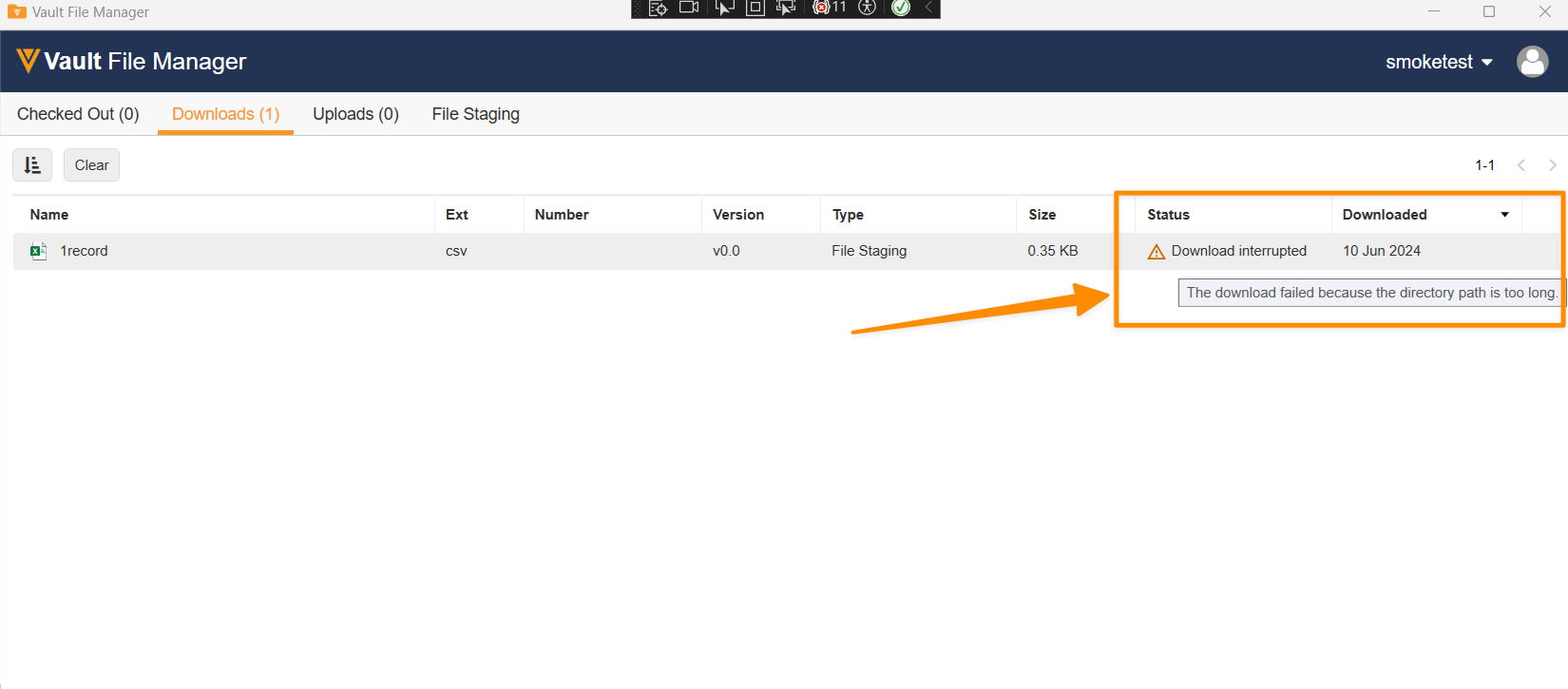
When using Vault File Manager to download content from File Staging, Admins will now see two changes that will reduce potential issues that may occur with longer filepaths.
First, Vault File Manager will display a new tooltip when hovering on the status if the filepath is longer than the Windows maximum. Prior to 24R3, hovering over the status would attempt to show the full filepath rather than note specifically that the filepath length is the issue to address. After 24R3, hovering over status will show:
Second, Vault File Manager will use a shorter naming convention for folder names when folders are downloaded to reduce the potential of hitting the Windows filepath limit. Prior to 24R3, the format was VaultID_{VaultID}__File_Staging_Download__{Datestamp}. After 24R3, the format will be {VaultID}_VFM_{Datestamp}. As an example, VaultID_1000008_File_Staging_Download 2024-05-14 16-54-29-485 will shorten to 1000008_VFM_24-05-14 16-54-29-485 after 24R3 (reducing characters from 61 to 35).
Windows has a standard limit of 256 characters for filepath length and these enhancements will help reduce the potential of downloads exceeding that and will provide clearer information to users when the filepath is exceeded.
Learn more about using Vault File Manager to work with File Staging.
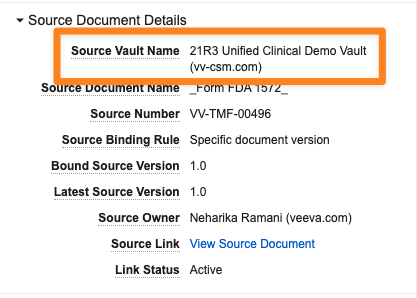
Always Display Source Vault Name for Crosslink Documents
When leveraging CrossLinks, users will now always see the name of the Source Vault (including the domain name) in the CrossLink’s metadata, regardless of whether or not they have access to that Vault. Prior to this release, the Source Vault Name for Crosslink Documents was hidden if the user viewing the Crosslink did not have access to the document’s Source Vault. This change helps improve Vault performance.
Legal Hold Search Dialog: Updated User Interface
The dialog boxes that appear for users when using Apply Legal Hold or Edit Legal Hold will have a slightly updated user interface to be more consistent with other dialog boxes in Vault. *Auto-on in Vaults with Legal Hold enabled.
Specific Document Version Fields Supported in Change Related Object Lifecycle State Action
If an object has document reference fields, the Change Related Object Lifecycle State entry action on document lifecycles allows you to automatically change the state of those object records when the document changes state. However, prior to this release, you could only configure this entry action for document reference fields configured as Latest Version on the object. In 24R3, this has been extended to allow Specific Version as well.
As an example scenario, imagine the latest version of a document is in the Draft state, but there is still a Steady State version in the Approved state. In this scenario, maybe your object has a document reference field pointing specifically to the v1.0 Approved document version. If the new Draft document version becomes Approved, moving v1.0 into the Superseded state, you may want this to cause your object record to move into an Update Document state, which could automatically start a workflow on that record, prompting someone’s review. This kind of scenario is now possible because we support Specific Version document reference fields.
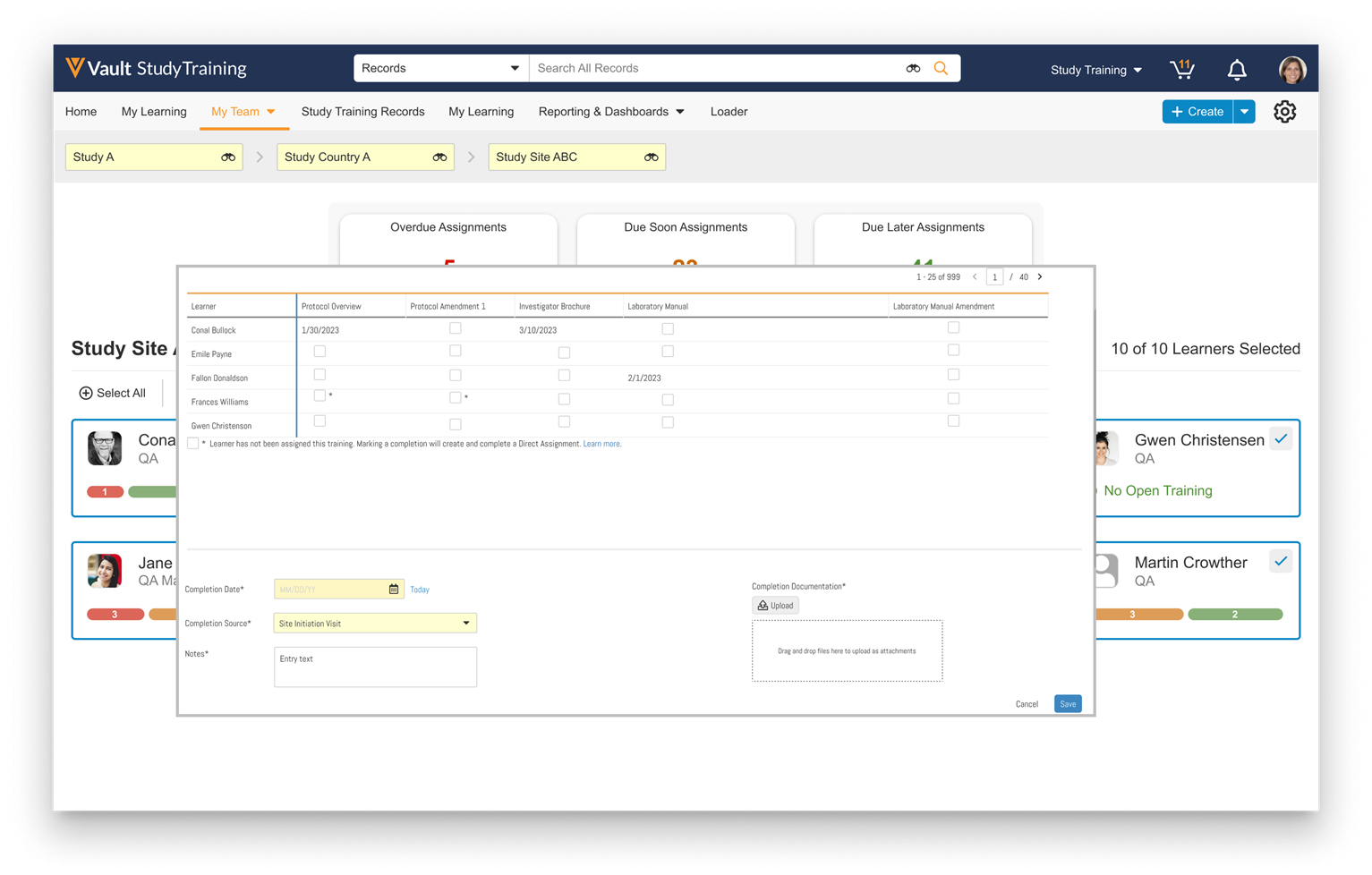
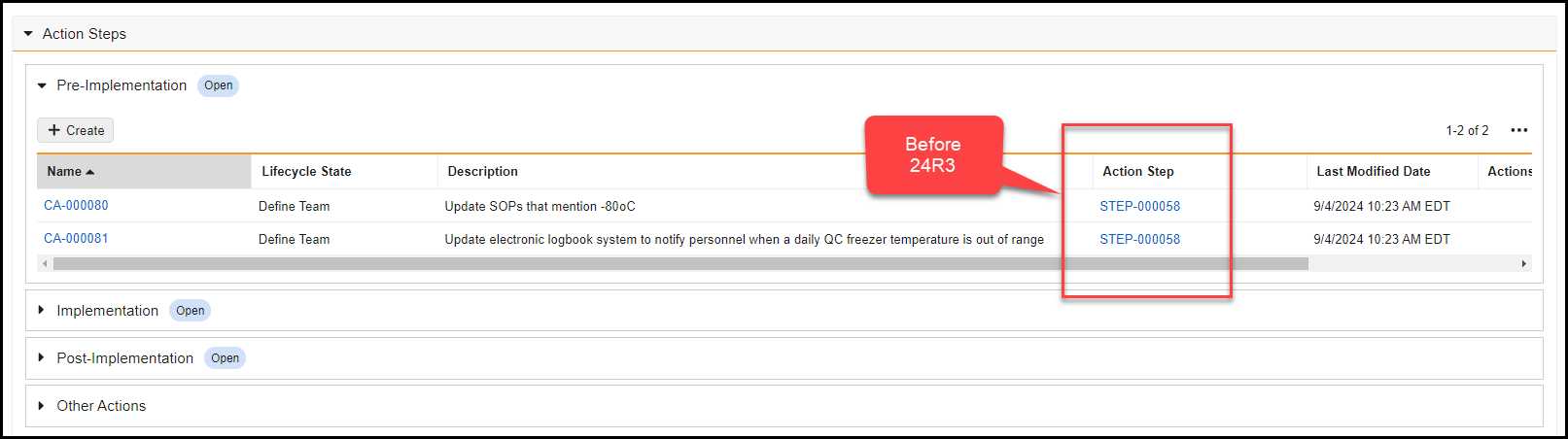
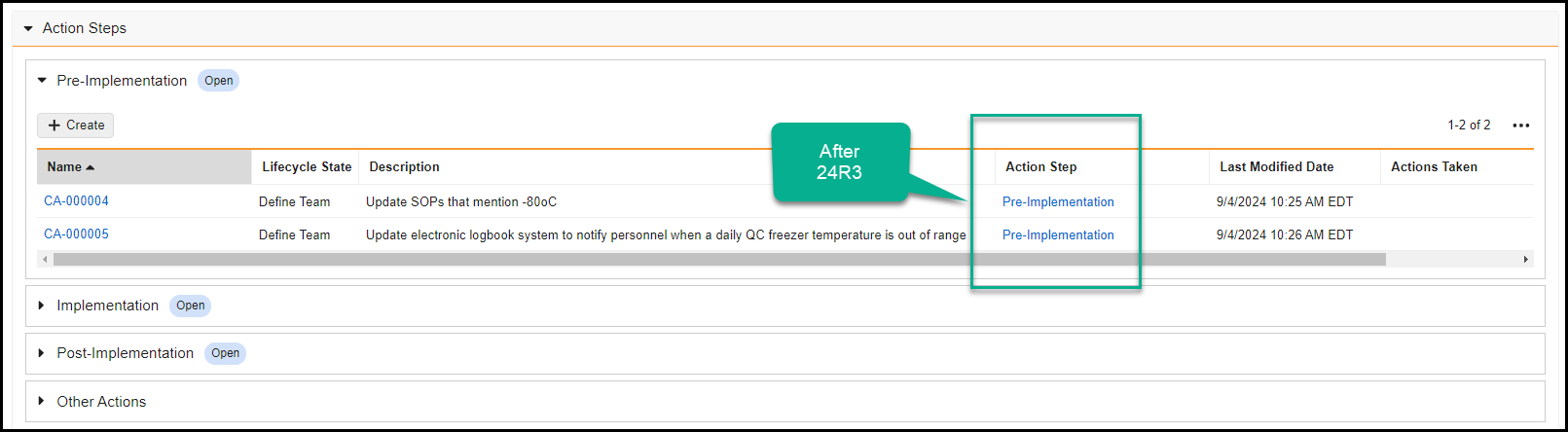
Record Migration Mode Enhancements
To improve the capabilities of record migration, we have enhanced Record Migration Mode to allow more control over creating and updating records.
When enabling Record Migration Mode, the system allows setting system fields (ID, Created By, Created Date, Modified By, Modified Date) and bypasses all rules except:
- Fields are validated against the proper data type (String, Number, Date, etc.)
- Object types and object type fields are enforced.
- Lifecycle states must exist but can be Active or Inactive. If no state or state type is specified, Vault creates records in their initial state.
- The system allows setting the ID for the record on create but not on update.
Record Migration Mode now includes the following functionality:
- A new No Triggers setting is now available. When set to Yes, it bypasses all system, standard, and custom triggers when using Record Migration Mode.
- Migrators can now bypass picklist dependencies, set object type and document references to inactive values, and change the object type of records.
- Record Migration Mode now includes support for Users (
user__sys).
Users must have Migration Mode permissions to use these headers.
Learn more about Record Migration Mode.
Email to Vault: Email Size Limit Increase
When using Email to Vault functionality, Vault now supports emails up to 40 MB in size, increased from a prior limit of 30 MB. Prior to 24R3, if an email was between 30 and 40 MB, Vault would create an email record in the Bounced state. For emails over 40 MB, the email would not be processed by Vault and a bounce email would be sent to the sender from AWS.
With this enhancement, emails up to 40 MB can be processed by Vault accordingly and not bounced due to size. If an email is over the 40 MB size limit, there is no behavior change (no Email record is created in Vault and a bounce email is sent to the sender from AWS).
Learn more about Email to Vault.
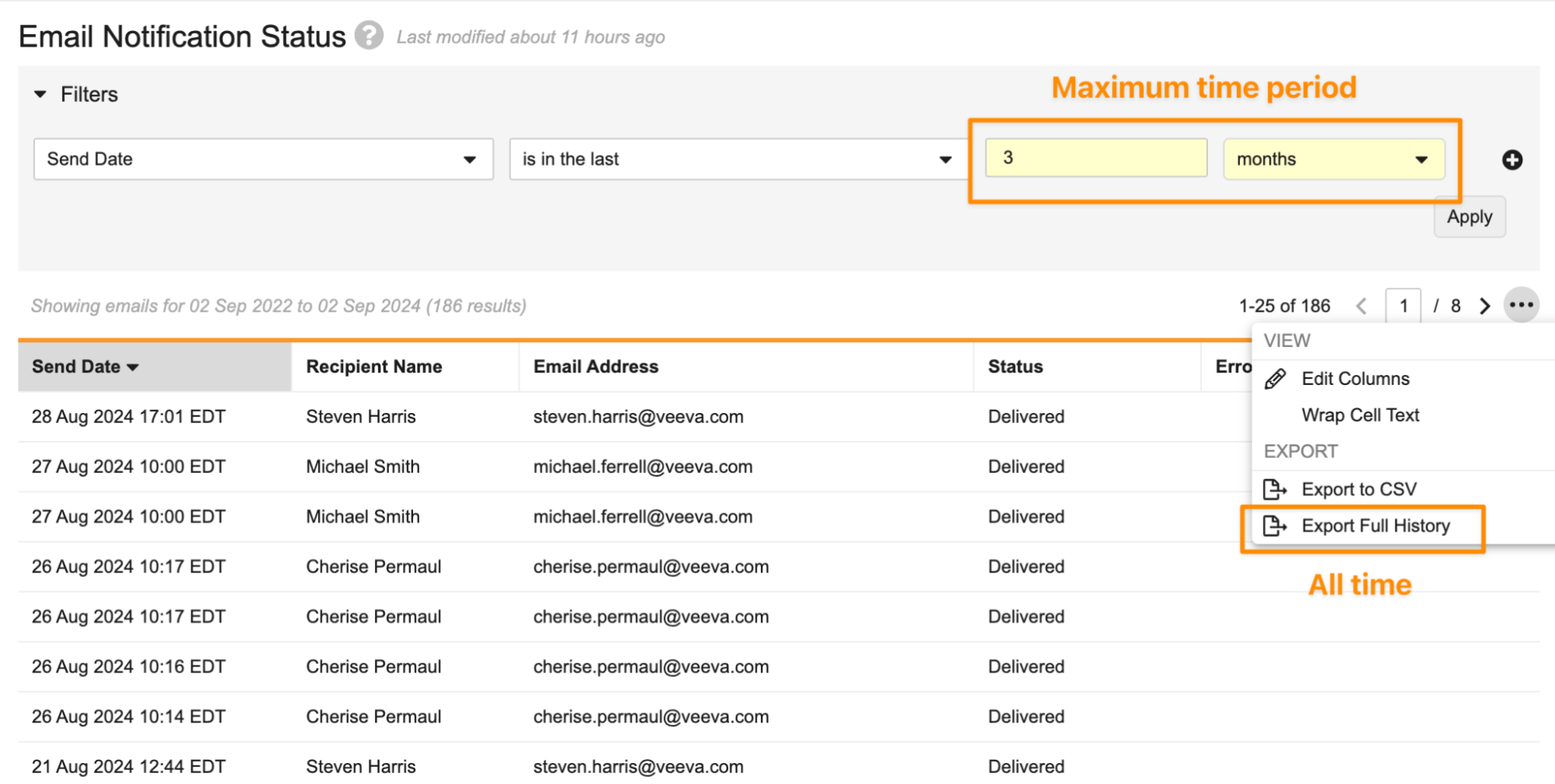
Archive Notifications After 3 Months
For end users using the All Notifications page, and for Admins using the Email Notification Status page, Vault will now show notifications over the last three (3) months, rather than over the last two (2) years.
This enhancement optimizes the performance in the Vault user interface for both end users (on the All Notifications page) and Admins (on the Email Notification Status page). Admins will still be able to access the full notification history by using the Export Full History option in the Email Notification Status page.
Learn more about the Email Notification Status page. Learn more about the developer-facing functionality of this feature.
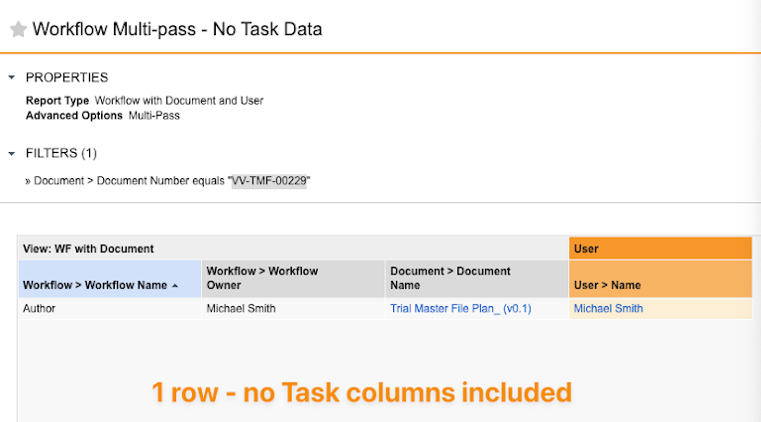
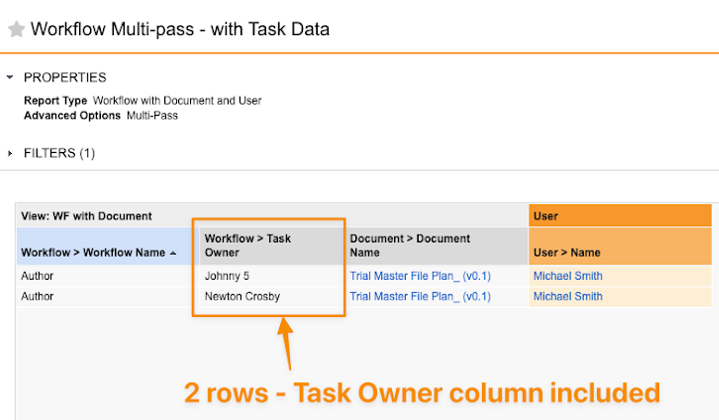
Task Exclusion in Multi-pass Workflow Reports
When using multi-pass reports that contain workflow report views, Vault will now only show multiple rows per workflow if a Task column is included in the report or if the multi-pass report type is using a Task column to join views.
Prior to 24R3, Vault would always create a row for each task within each workflow, even if the report was not using any task-related information.This would result in user confusion as it would appear that there were duplicate rows. This enhancement will help reduce the perception of duplicate rows and provide cleaner reports for users.
Learn more about Multi-Pass Reporting.
Report Cloning Enhancements
When cloning environments, such as when creating or refreshing a sandbox, Vault now uses Name (name__v) values rather than record IDs (id) for reports that reference object records. This allows Vault to provide clear messages for common report migration issues and reduce potential errors.
With this change, if an object record is present in the target system but does not have the same record ID (id) as it did in the source system, it no longer results in errors or blanked-out filters. Instead, the system establishes the link using the Name (name__v) of the object record.
Learn more about Administering Sandbox Vaults. Learn more about the developer-facing functionality of this feature.
Predictable Report Width
Prior to 24R3, report width was limited to 21,884 characters per row, and this limit could only be enforced at run-time. This could create trial-and-error scenarios for users creating reports since they would have to run the report to see if the limit was hit, and it wasn’t easy to identify what changes to make to address the limit.
Going forward, report width will now be limited by the number of columns. Reports will be able to include up to 100 columns. This change allows for reports to still maintain the ability to include a significant amount of data, while also making it easier for creators and editors to manage.
If reports already exist that exceed 100 columns but not the 21,884 character limit, these reports will continue to function as-is, but new reports will be limited by columns rather than characters.
Learn more about Creating Reports.
End VeevaID Session With Vault Log Out
When VeevaID users choose to log out of Vault, this will now log them out of both Vault and VeevaID. This enhancement will help reduce confusion for VeevaID users.
Enforce Password Validation on Password Reset from User Profile
When a user initiates a password reset through the User Profile and enters a new password, Vault now validates the password and returns an error if unsupported characters are included. Vault also displays a list of supported characters as part of the error message:
Vault passwords can only contain standard letters (A-Z, a-z), digits (0-9), the space character, and the following special characters: ! “ # $ % & ‘ ( ) * + , - . / : ; < = > ? @ [ \ ] ^ _ ` { | } \ ~ . Accented letters (like é, ñ, ü) and other non-ASCII characters are not allowed.
Prior to 24R3, Vault would allow a user to save a new password with unsupported characters, and would only enforce validation when the user attempted to use the new password.
This enhancement will provide the error more proactively and reduce user confusion.
Enforce Password Validation on Password Reset from Login Screen
When a user initiates a password reset through the Login screen and enters a new password, Vault now validates the password and returns an error if unsupported characters are included. Vault also displays a list of supported characters as part of the error message:
Vault passwords can only contain standard letters (A-Z, a-z), digits (0-9), the space character, and the following special characters: ! “ # $ % & ‘ ( ) * + , - . / : ; < = > ? @ [ \ ] ^ _ ` { | } \ ~ . Accented letters (like é, ñ, ü) and other non-ASCII characters are not allowed.
Prior to 24R3, Vault would allow a user to save a new password with unsupported characters, and would only enforce validation when the user attempted to use the new password.
This enhancement will provide the error more proactively and reduce user confusion.
Note: This enhancement applies to password resets initiated via the Vault Login page as part of the 24R3 General Release and is not available in pre-release or Limited Release Vaults.
Admin About Tab for Vault & Domain Information
There is a new About tab available to Admins, which is the new home for the Vault and domain information that used to be on the General Settings page under the Settings tab. The About tab keeps the non-configurable information such as license, API, and data usage information separate from the configurable options in the Settings tab.
Vault Time for Jobs
Job operations are now always displayed and stored in the Vault’s time zone (known as Vault Time). Underneath the selected runtime on the Vault Time field, Vault shows what this translates to in the user’s selected timezone. This feature reduces confusion and improves consistency by ensuring all background operations are using the Vault Time defined in Admin > Settings > Language & Region Settings > Vault Information.
Learn more about the developer-facing functionality of this feature.
Remove System Audit of Vault Loader Actions
Vault Loader events are no longer recorded in the System Audit History. The corresponding updates to object records and documents are still recorded in the Object Record Audit History and Document Audit History, respectively.
VQL Date Literals
VQL expressions now support date literals. Instead of providing a Date or DateTime value in the VQL query, you can supply literals such as TODAY, THIS_MONTH, and DAYS_AGO:n.
Learn more about VQL date literals on the Developer Portal.
Release Warning on Sandbox Vaults Page
A warning (UI Alert) will be shown to users on the Sandbox Vaults page while an infrastructure release event is ongoing. This message warns users that Sandboxing performance may be degraded, but no other functionality will be impacted by the backend maintenance.
Java 17 for Vault Java SDK: Optional Enablement
Over the next several releases, Veeva is upgrading the Vault Java SDK to Java 17 to allow developers to take advantage of the upgrades that come with the new version. In addition to new features, Java 17 includes optimizations that can improve performance and efficiency compared to Java 8, allowing for reduced resource usage.
In this release, Developers and Admins can optionally enable Java 17 for Vault Java SDK to test their custom code for compatibility with the new Java version.
Vault Loader CLI Java 17 Support
In order to use the latest version of the Vault Loader CLI, developers need to ensure that Java 17 is available in the environment. Developers can continue to use older versions of Vault Loader CLI with Java 8.
Scheduled Data Export Maximum Object Limit
Scheduled Data Export (SDE) will have a maximum object limit of 30 on Vaults that are not currently using SDE. Existing Vaults currently running Scheduled Data Export will continue to work as they did.
Disable Audit Trail on User Exception Messages & User Exception Items
Record changes for the User Exceptions Message (UEM) and User Exception Item (UEI) objects are no longer included in audit trails. These objects are used for information purposes to aid in integration management.
Client Credential Support for HttpService
Vault now allows the creation of Client Credentials (client_crendentials__sys) type records, which can store a Client ID and an encrypted Client Secret. Previously, users could only create Connection Authorization records of the type Basic Authentication (basic_auth__sys). Developers can use these values in the Vault Java SDK together with the HTTPService.
24R3 Platform Data Model Changes
See 24R3 Platform Data Model Changes.
Vault Connections
Clinical Operations-EDC Connection
Clinical Operations-EDC Connection: Protocol Deviations Link to CDMS
For Protocol Deviations managed by the Clinical Operations-EDC Connection, this feature introduces a standard link field on Protocol Deviations in Vault CTMS that leads directly to the Protocol Deviation review page in Vault EDC. Using this link, customers can quickly and easily navigate to the source Protocol Deviation when reviewing issues in Vault CTMS, similar to how Casebook Link is used for Subjects and Subject Visits. Learn more about other new Clinical Operations features below.
Clinical Operations-EDC: Display Protocol Deviation EDC ID
The Clinical Operations-EDC Connection now includes the EDC ID (edc_id__v) field within protocol deviations. This new field displays the corresponding EDC ID of the source Protocol Deviation, allowing users to quickly identify the source record in EDC directly from their Clinical Operations Vault when viewing, extracting, or reporting on data. Learn more about other new Clinical Operations features below.
Clinical Operations-eCOA Connection
Enhance Clinical Operations Lifecycle Transfer
This feature enhances the Clinical Operations-eCOA Connection to automatically transfer standard and custom study, study country, and study site lifecycle states. Learn more about other new Clinical Operations and eCOA features below.
RIM-PromoMats Connection
RIM-PromoMats: Product Data Transfer
To further support a single source of truth for Product- and Registration-related data records, new integrations between RIM and PromoMats Vaults allow the transfer of Product and Registration information needed within Regulatory and Commercial business teams. Brand (Trade Name) information is transferred from PromoMats Vaults to the Regulatory Text object within RIM Vaults. Indication is also transferred from RIM to PromoMats in a new integration.
Brand records are created and managed in PromoMats and used as a source for the Connection to populate the Regulatory Text object in RIM of object types Drug Trade Name and Device Trade Name. RIM Vaults remain the source of truth for all other Product-related and Registrations data needed for Commercial business processes. We have updated the PromoMats data model to include ten new objects and object relationships synced with the source data in RIM. A summary of the integrations is provided below:
| Integration | RIM Object | PromoMats Object | Data Transfer Direction |
|---|---|---|---|
| Product | Product Family, Product, Product Family Product, Product Variant, Product Component, Packaging | Product Family, Product Form, Product Family Product Form, Product Variant, Product Component, Packaging | RIM > PromoMats |
| Therapeutic Indication | Therapeutic Indication | Indication | RIM > PromoMats |
| Brand (Trade Name) | Regulatory Text (object types: Drug Trade Name, Device Trade Name) | Brand | PromoMats > RIM |
| Registrations | Registration, Registered, Regulatory Text, Registered Packaged, Medicinal Product, Registered Indication | Registration, Registered Brand, Registered Packaged Product, Registered Indication | RIM > PromoMats |
New object (see RIM and PromoMats Data Model Updates for more details)
Learn more about other new Regulatory and Commercial features below.
RIM-Clinical Operations Connection
RIM-Clinical Operations: Enhance Study Defaulting Logic on Clinical Crosslinks
When cross-linking documents from RIM to Clinical Operations Vaults, the connection now respects clinical Study records on the source document and attempts to match them to Study records in the Clinical Operations Vault, even if the Study was not created in RIM by the connection (link__sys field is blank). The previous crosslinking logic of the connection ignores the Study records on source documents in RIM if they are not mapped to Study records in Clinical (as per the link__sys field). With this enhancement, the connection displays an error message if the source document includes Study records that cannot be mapped to Study records in the Clinical Operations Vault. Learn more about other new Regulatory and Clinical Operations features below.
RIM-Clinical Operations: Update to Clinical Study Field Rules
In 24R3, Vault Clinical Operations has added three (3) new standard fields: Type of Control, Study Type, and Study Subtype. These fields already exist in Vault RIM and customers are manually populating the information in RIM or creating custom field rules. With the addition of these standard fields in Vault Clinical Operations, the RIM - Clinical Operations Connection has added new standard field rules to enable the transfer of these field values to Vault RIM. This enhancement increases productivity and data integrity as there is no longer a need to manually populate these values. Learn more about other new Regulatory and Clinical Operations features below.
RIM-Clinical Operations: EU Special Handling for Clinical Crosslinks
When cross-linking documents from RIM to Clinical Operations Vaults, if the Country on the source document is European Union, the connection maps to Study Countries in the Clinical Operations Vault based on Jurisdiction = European Union. The current crosslinking logic of the connection fails to create the crosslink since the European Union is not a Country value in Vault Clinical Operations. This enhancement ensures that the crosslinked documents are created in Vault Clinical Operations. To utilize this feature, mapping Countries to the European Union Jurisdiction using the Jurisdiction Country simple join is required in Clinical Operations Vault. Learn more about other new Regulatory and Clinical Operations features below.
Safety-Clinical Operations Connection
Safety-Clinical Operations Connection: CRO Support
With this release, the Safety-Clinical Operations Connection now supports multiple sponsor organizations for each study, allowing contract research organizations (CROs) to utilize the connection. When users create or edit Study or Study Country records in their Clinical Operations Vault, the connection creates or updates the Study or Study Registration record in their Safety Vault. CRO users can also distribute Safety Letters using Veeva Site Connect.
Learn more about configuring CRO support for the Safety-Clinical Operations Connection.
Safety-EDC Connection
Safety-EDC Connection: Send SAE Reporter Details to Safety
With this release, SAE reporter contact information transfers to Vault Safety through the Safety-EDC Connection. This enhancement facilitates direct communication between Safety users and the reporter for clarifying questions about an SAE, thereby reducing triage and processing time. EDC Admins can map reporter details directly on the Adverse Event form. In a future release, EDC Vaults will support defaulting site information to automatically set reporter details when sending SAEs to Vault Safety.
Learn more about configuring your Vaults to send SAE reporter details to Vault Safety using the Safety-EDC Connection.
Safety-EDC Connection: Multiple-EDC Connections on Vault Safety
With this release, Vault Safety now supports connections to multiple EDC Vaults through the Safety-EDC Vault Connection. Admins can use the new Copy to New action to create and configure Safety-EDC connections for each EDC Vault instance. This enhancement enables customers with multiple EDC Vaults to use the connection to transfer their SAE data to Vault Safety seamlessly.
Learn more about establishing multiple EDC Vault connections.
Safety-EDC Connection: New Fields & Error Messages
This release enhances the Safety-EDC Vault connection by expanding the data transferred between EDC Vaults and Safety Vaults. Organizations can now automatically transfer additional field values, including the Highlighted Term and Severity of Case Adverse Events, along with the Site Narrative for each Case. Additionally, when a custom field is missing in Safety Vault, a clear error message identifies the specific issue, guiding Admins to update the configuration.
Learn more about the Safety-EDC Connection.
Safety-RIM Connection
Safety-RIM Connection Enhancements
The Safety-RIM Vault connection has enhanced product data mapping from RIM to Safety. Marketed Drug Product Registration and Marketed Device Product Registration object types in RIM now set the appropriate RIM Registration Type picklist values in Vault Safety. The Medical Device Product object type in RIM now sets the Device Product object type in Vault Safety. These enhancements better support Device types of Products and accurately aligns with the RIM Product and Registration data model.
Learn more about the Safety-RIM Connection.
Clinical Operations
In addition to the below release notes, the Vault CTMS, Vault eTMF, Veeva Site Connect, Vault Study Startup, and Vault Study Training Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Clinical Operations application family:
- Clinical Operations-EDC Connection: Protocol Deviations Link to CDMS
- Clinical Operations-EDC: Display Protocol Deviation EDC ID
- RIM-Clinical Operations: Enhance Study Defaulting Logic on Clinical Crosslinks
- RIM-Clinical Operations: Update to Clinical Study Field Rules
- RIM-Clinical Operations: EU Special Handling for Clinical Crosslinks
- Safety-Clinical Operations Connection: CRO Support
All Clinical Operations Applications
Clinical Operations Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Clinical Operations checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Clinical Operations for the impacted field inventory.
Study Training customers can also reference Study Training & Vault Training Standard Checkbox Defaulting for similar changes in Study Training Vaults.
Document Deletion Log of Documents in Clinical
A new document action, Delete Document with Reason, allows users to simultaneously create a Document Deletion Log and delete a document version from the system. Deletion logs track the Deletion Reason and optional Deletion Comments along with a set of metadata from the deleted document. Deletion logs do not retain copies of the document or its audit trail. Any Quality Issues with a relationship tied to the deleted document version is removed and the Related Document field on the Quality Issue is made null. The Quality Issue records automatically reference Related Document Deletion details to maintain transparency.
Enabling document deletion logs requires a one way feature flag that cannot be reverted and is dependent on the enablement of the Disable Document Requiredness on Quality Issues setting.
Auto-Manage Number Expected on EDL Items
This feature adds flexibility to the Update Requiredness, # Expected Based on EDL Matchings feature by introducing, on an item-by-item basis, whether Expected Documents should automatically sync the # Expected with the total number of matched documents. A new checkbox field on Expected Documents and Template Expected Documents called Auto-Manage # Expected (automanage_expected__v) determines the update behavior on each Expected Document. This field can be set at the template level and then managed on a study-by-study basis by leveraging the new Update Auto-Manage # Expected system action. The default value for the field is false and configuration updates are required to leverage the feature. When enabled, end users cannot manually modify the # Expected field.
Veeva Standard Library Questions
This feature introduces Veeva Standard Library Questions and Available Answers to all Clinical Operations Vaults. Customers can reference these Standard Library Questions and Available Answers in Site Feasibility Surveys. Standard Library Questions also support the Veeva Standard Questions and Reusable Responses feature.
Customers cannot edit Standard Library Question and Available Answer records.
Clinical Subartifacts
Clinical Subartifacts further refine the Classification of a document through a standard Subartifact document field, which negates the requirement for a separate Classification for TMF Reference Model subartifacts. The new Subartifact object defines a parent Classification through a Document Type Details field. Establishing the relationship causes the Subartifact field to be filtered based on the Classification applied at the time of document upload. This new document metadata is reportable and column filtering is supported in the TMF Viewer.
To ensure that Clinical Subartifacts support completeness tracking, EDL Item matching has been extended to support matching on Subartifacts. EDL Items have a new Subartifact field where the subartifact is defined. When this value is populated, Vault matches the EDL item to the document that matches on both the Classification and Subartifact, along with other matching criteria that may be applied.
The Clinical Subartifacts data model is auto-on, but configuration is required to begin using Subartifacts on documents and EDL Items.
CTMS
CTMS Transfer Expanded Scope: Subjects & Subject Groups
As clinical trials increasingly incorporate complex designs, Sponsors are outsourcing more of these trials to Contract Research Organizations (CROs). This trend necessitates efficient and seamless subject data transfer solutions between CROs and Sponsors, enabling comprehensive oversight of complex trials and allowing Sponsors to dive deeper into enrollment of subject groups or gain greater visibility into subjects related to protocol deviations.
To meet this growing demand, this feature expands the scope of the CTMS Transfer feature to include the Subjects object and Subject Group object types, allowing customers to transfer records from CRO Vaults to Sponsor Vaults. Additionally, these new object types are available in the Outbound Clinical Mappings UI for CRO Source Vaults, allowing CROs to map their custom values as needed.
These enhancements empower organizations working in an outsourced model to manage and transfer complex clinical trial data with greater efficiency and accuracy, supporting the continued evolution of clinical research practices.
Japanese Clinical Trial Notification (CTN) Capability Removed from Vault Application Settings
Enabling the Japanese Clinical Trial Notification capabilities now requires Veeva Support in order to adequately monitor resources and support for adoption.
Migration Mode Changes for CTMS Transfer
To address challenges arising from configuration misalignment between Sponsor and CRO Vaults, we’ve introduced key improvements to CTMS Transfer. Previously, these differences in Vault configurations often led to transfer failures, as full alignment could be challenging and impact the Sponsor’s business processes. CTMS Transfer enhancements reduce the likelihood of transfer failures caused by configuration mismatches between CRO and Sponsor Vaults, ensuring more reliable transfers.
CTMS Transfer now creates and updates records when an object type is Inactive in the Sponsor Vault Target. Additionally, CTMS Transfer successfully creates and updates the records even when required fields in the Sponsor Vault are left blank by the CRO. Finally, the Sponsor’s entry actions, entry criteria, and event actions are ignored during transfers, preventing unintended triggers.
Disclosures
Introducing Vault Disclosures
We are excited to introduce Vault Disclosures, a comprehensive product designed to centralize and streamline clinical trial disclosure management. With pre-populated data and advanced automation features, Vault Disclosures accelerates registry submissions while improving compliance across global regulations.
In today’s increasingly complex regulatory environment, managing clinical trial disclosures has become more challenging than ever. Vault Disclosures directly addresses key pain points by helping organizations navigate regulatory complexity, ensuring data quality and accuracy, and eliminating the inefficiencies of disparate systems and spreadsheets.
Vault Disclosures transforms the management of clinical trial disclosures by centralizing all disclosure activities, including disclosure content authoring, review, and approval. It enhances efficiency through data and document reuse. By leveraging pre-configured rules, global country intelligence, and automated alerts for registry updates, Vault Disclosures accelerates submission timelines, ensuring faster and error-free submissions.
What makes Vault Disclosures unique is its unification with the Vault Clinical Operations Platform, which eliminates the need for third-party integrations and manual processes. Its self-service configuration allows flexibility to meet specific study or organizational needs, while reports and dashboards provide actionable insights into global submission statuses and operational progress, enabling early identification and resolution of potential issues.
Vault Disclosures is tailored for organizations of all sizes, from large enterprise sponsors to smaller biotech companies and CROs, providing a scalable and adaptable solution to meet diverse clinical trial disclosure needs. It is available as an add-on purchase to Vault CTMS.
Learn more about Vault Disclosures.
Vault Payments
Complex Trials: Payments
Complex Trial capabilities have been enhanced to support Vault Payments. Previously, only Arms could be used as Fee matching criteria during Payable Item generation. With this release, we have extended matching criteria for both Visit and Procedure Fees to support all Subject Group object types (Arm, Study Cohort, Substudy, Study Part, and Study Element). In instances where more than one Subject Group is included on a Fee, Vault matches on all Subject Groups specified (for example, if both Arm and Study Cohort are included on a Fee, only Visits/Procedures for Subjects with both Arm and Study Cohort will match).
Rate criteria have been enhanced to support Subject Group metrics. Rate criteria limit the number of Payable Items Vault generates based on the number of subjects in a particular Rate Metric, such as subjects with the Enrolled status. When a Rate Subject Group is specified, Vault uses the corresponding Subject Group Metric, such as subjects with the Enrolled status in Arm A. Subject Groups must have enrollment metrics to use Rate Subject Group criteria matching.
Automated payment adjustments have been enhanced to support changes to Subject Group values on Subjects. Changes to Subject Group fields and Subject Group Metrics that impact Fee matching criteria trigger evaluation of related Payable Items. When adjustments are made, Vault can either mark the Payable Item as Inactive or it can create an Adjustment Payable Item to reflect what has already been paid and what still needs to be paid.
Learn more about automated payment adjustments and rate criteria.
Fee Schedule Approval Automation
This feature streamlines the process for approving amendments to Fee Schedules. Previously, users were required to apply an End Date to an existing Fee Schedule before approving a new version. This was typically done using separate processes. Now, using a new action available on the Fee Schedule lifecycle, users can review and approve updates to existing related Fee Schedules as part of approving a new Fee Schedule, which saves time, reduces errors, and eliminates manual steps. Related Fee Schedules are those in an approved lifecycle state with matching values for Study Site and Primary Organization.
When users take the action to automate Fee Schedule approval, Vault proposes the following updates to existing related Fee Schedules:
- For a new subsequent Fee Schedule, Vault applies an End Date to the existing Fee Schedule.
- For a new earlier Fee Schedule, Vault applies a Start Date on the existing Fee Schedule.
- When the Start/End Dates negate or replace the date range of an existing Fee Schedule, Vault sets the existing Fee Schedule to Superseded.
For single updates, users are prompted to review the proposed changes prior to processing the change and approving the new Fee Schedule. This capability can also be included in a workflow or enabled as a bulk action to update multiple Fee Schedules with this additional automation.
Payment Adjustments: Superseded Fee Schedules
This feature provides support for automated adjustments when a Payable Item is linked to a Fee Schedule in the Superseded lifecycle state. This capability is enabled for customers that have the Enable Automated Adjustments feature active in their Vault. Previously, Vault only considered Fee Schedules in an Approved lifecycle state when adjusting Payable Items. Now, Vault also considers Fee Schedules in the Superseded lifecycle state. If Payable Items exist and the Fees are identical between an Approved and Superseded Fee Schedule, Vault updates the Payable Item’s Fee Schedule field to the Approved Fee Schedule. If there are differences, Vault adjusts the Payable Item to either mark it as Inactive or creates an adjustment Payable Item to reflect what has already been paid and what still needs to be paid based on the Approved Fee Schedule.
Payment Adjustments for Limit Decreases
Automated payment adjustments now support limit decreases to Maximum Count. For example, if the Maximum Count is reduced (for example, from 4 to 3), Vault adjusts Payable Items accordingly.
Learn more about automated payment adjustments.
Payable Event Date
A Payable Event Date (payable_event_date__v) field is now available that is populated based on the related Payable Event (for example, Visit Date, Procedure Date, or Site Fee Date). For Additional Fees, users can manually enter the Payable Event Date.
eTMF
Multilingual Models
Note: There is a known issue (DEV-802059) that prevents multi-lingual document classification.
As clinical trials operate on a global scale, and a significant portion of TMF documents are written in languages other than English, it’s not uncommon for sponsors or CROs to have more than 50% of their TMF documents in non-English languages. To date, the TMF Bot could only train on and auto-classify documents with English text.
To significantly expand the TMF Bot’s coverage, we are introducing the Multilingual Models feature. This enhancement enables the TMF Bot to train on and automatically classify and/or detect key metadata in documents that are in non-English languages. More than 30 languages are supported.
Automated Model Refresh
To ensure the TMF Bot consistently applies the latest organization’s filing requirements, it is essential that the model deployed in production is continuously updated with the latest TMF documents and data. A recent analysis of TMF Bot performance revealed that some customers with custom models had not refreshed their models for extended periods, sometimes exceeding 18 months. This lack of regular updates can lead to negative impacts on the TMF Bot’s effectiveness.
In response, this feature automatically enables the training job in all customer Vaults on release night, ensuring models are consistently refreshed with each release.
One-Click Model Retraining
To ensure the TMF Bot consistently applies the latest organization’s filing requirements, it is crucial that the model deployed in production is trained on the latest TMF document and data updates. While automated refreshes are scheduled with each new Vault release, customers may require more frequent model refreshes in cases where they update their classifications or filing guides.
This new feature introduces a streamlined one-click process for refreshing a deployed trained model. Instead of manually copying the model record and initiating a training job, customers can now use the Refresh Model action. The action automatically creates a deep copy of the current model and starts the training process. This enhancement not only reduces the number of steps required, but also prevents users from starting multiple training jobs simultaneously, ensuring optimal system performance.
Training Set Optimization
After a thorough investigation into TMF Bot performance across our customer base, we identified that a significant portion (50-70%) of documents in the training set belong to a small number of classifications, each containing over 1,000 documents. These high-volume classifications, though few in number (less than 20), consume a significant portion of the model’s training space, which is capped at 200,000. This imbalance reduced the diversity of classifications available for the TMF Bot to train on, potentially impacting its effectiveness.
To maximize the number of classifications on which a model trains, the TMF Bot’s document extraction query is refactored to execute separately for each active document classification within your Vault. This change ensures a more balanced coverage of classifications across the training set, and should improve auto-classification coverage and error rates.
Exclude from Metadata Extraction Studies Under Legal Hold
When a trial or product is involved in litigation, it is essential to preserve the integrity of related documents. In Vault, these preserved documents cannot be re-classified.
In some cases, the TMF Bot metadata extraction may detect a study that is under legal hold and set that value on the document. Since reclassification is blocked in these situations, such a metadata prediction would prevent a user from processing the document out of the inbox, thereby hindering the study’s operations and eventual archiving process.
This feature updates the metadata extraction process to address this scenario. The TMF Bot now avoids setting a Study value to any document if that Study is currently under legal hold.
Site Connect
Payments Information in Site Connect
Sites have historically struggled with limited visibility into the payment process for activities completed on a study, leading to uncertainty about if or when they will be paid. Even when payment letters are provided by Sponsors or CROs, these are not easily reportable, making the view of total payments across a study or patient challenging.
To address these challenges, Site Connect now offers Payments Information functionality by surfacing information collected in our Vault Payments application. This feature enables authorized site personnel to access their site’s payment details from a new Payment Information section where they can filter, search, and download paid or pending payment requests. Sites can also easily reconcile payments against their invoices and add notes, or navigate into a payment request to see each included payable item. This streamlined process provides greater transparency, traceability, and efficiency, significantly improving the payment experience for sites.
System Links for Sites
A new System Links section in Site Home allows site users to view Study system links shared by Sponsors and CROs. Sponsors and CROs can use the Site System Links tab under the Site Connect tab to create system links with the system name, type, URL, and an optional description.
Automated Document Exchange
Site Connect now supports automatically sending documents and document requests to study sites. Sponsors and CROs can determine which documents to auto-send by setting the new document picklist field, Auto-distribute document action to either Revise & Return or None. This field is read-only on documents by default, but Sponsors and CROs can make it editable. An hourly job picks up these documents and creates Distribution Task records as long as the related Study has Connected Study Type field set to Document Exchange and related Study Sites have the new Auto-document exchange checkbox field enabled. Documents now also support a new Sponsor/CRO Comments text field which is included in the Distribution Task that is created for the site as well.
For auto-document requests creation, Expected Document (edl_item__v) records have a new Auto-request from Site checkbox field and Sponsor/CRO Comments text field. For all site-level Required EDL records, when the Auto-request from site field is set to true and matching document(s) do not exist in the Vault, Vault creates a Provide Original document request Distribution Task record(s). The default due date is set to one week on all of the above site tasks which users can modify if needed from the Distribution Task itself. Sites receive a notification once a day on all of the documents and document requests created via auto-distribution.
Authenticated Document Links
To enhance document access security with Site Connect, Sponsors and CROs can now mandate site users to authenticate with their VeevaID when accessing document links sent via email. You can configure this requirement for each document type using the Toggle Authenticate Document Links action on the corresponding Vault Clinical Docs artifacts.
This feature is applicable to Document Exchange, Study Announcements, Safety Distribution Email notifications, as well as the Download all document link when at least one document within the link requires authentication.
Reason for Recall
To enhance clarity and transparency in the document exchange workflow process for site users, sponsors & CROs must now provide a reason (up to 255 characters) when recalling a document. Once the recall reason is saved, affected site users receive a notification detailing the recalled document, along with the reason for the recall included in the notification body.
Site Connect Licensing
Site Connect customers can now track Site Connect licenses on the Study Site License object in Clinical Operations Vaults. Admins can view license consumption in their Vault Settings. Users will see a warning message if consumption is over the licensed amount.
Additional Vault Clinical Docs Support
Using Site Connect, Sponsors and Sites can seamlessly exchange documents. With this feature, we’ve expanded the document types that can be exchanged to include the following.
| VCD Artifact | Aligned Site Document Type | What’s New |
|---|---|---|
| Relevant Communications (Site Management) | Correspondence | New VCD Artifact. Correspondence site document type now sendable from site. |
| Investigator Brochure Summary of Changes | IB Summary of Changes | Existing VCD Artifact previously mapped to Investigator Brochure site document type now mapped to new IB Summary of Changes site document type |
| IP Verification Statements | IP & Supply Shipping | New VCD Artifact. |
| Regulatory Progress Report | Regulatory Authority Submission | New VCD Artifact. |
| Import License | IP & Supply Shipping | New VCD Artifact. |
| Notification to Regulatory Authority of Safety or Trial Information | Regulatory Authority Submission | New VCD Artifact. |
| IRB or IEC Progress Report | IRB/IEC Submission | New VCD Artifact. |
| Certificate of Analysis | IP & Supply Shipping | New VCD Artifact. |
The following Document Type is now able to be sent from sites:
- Correspondence
Study Startup
Site Feasibility: Veeva Standard Questions and Reusable Responses
Research sites are often required to answer the same questions across sponsors during trial feasibility. This activity becomes taxing and often increases a site’s response time. To reduce this burden, we are introducing industry-standard survey questions within Site Feasibility. These questions and answer sets cannot be edited and the answers are stored and reused across Vaults. Veeva Standard Questions are provisioned as Library Questions with the Veeva Standard Question field set to Yes. When Standard Questions are used in Survey Templates, responses are saved in a Stored Responses object and can be viewed directly through an Organization’s Site Capabilities section.
Survey respondents (Sites) are required to enter a Universal Site Number (USN) as a unique identifier when a reusable response question that is reusing answers based on organization is included in the Feasibility Survey. The unique identifier allows for the registry to store and reuse answers across Vaults. A Site can search or sign up for a USN through a new View USN Suggestions or Sign Up banner within the Feasibility Survey.
Admins can also define Custom Standard Questions in the Question Library and use them across Survey Templates. These questions are reused in a singular Vault based on the Reuse Response field and the Standard Question Type. Vault supports custom Standard Questions related to Organizations and Personnel.
Veeva Standard Questions and Available Answers are provisioned in every Clinical Operations Vault, regardless of SSU enablement. This feature requires updates to existing Survey Templates to use.
Learn more about working with standard survey questions.
Study Training
In addition to the features described below, the Study Training application also receives enhancements from the following features described in the Quality: Training section:
New Standard Responsibility
In Clinical Operations Vaults, a new Responsibility object record is added for 24R3:
- Name: Determine eligibility criteria (inclusion/exclusion)
- Responsibility Category: Medical
In Study Training Vaults, this new responsibility will be added as new corresponding Learner Role and Clinical Mapping records.
Study Training & Vault Training Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Study Training and Vault Training checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Quality for the impacted field inventory.
Study Training customers can also reference Clinical Operations Standard Checkbox Defaulting for similar changes in Clinical Operations Vaults.
Mark Training Complete in Bulk
From the My Study Team page, a user can select one or more Learners and mark them as having completed one or more Training Assignments. This feature will be available in Study Training but not Vault Training.
Prior to this change, a training administrator used Facilitated Training to mark training as completed one Training Requirement at a time. This allowed the Training Admin to mark multiple learners complete for a single Training Requirement.
This feature allows a trusted user, such as the CRA, to mark multiple learners complete for multiple Training Requirements. Thus, the user can manually enter training completions into the system in batches.
Example Use-Case: When a CRA visits a site during the Site Initiation Visit, they may gather a set of people to train them on a set of content. This can equate to one or multiple Training Requirements. Some of the audience may stay for all of the pieces of training and some may leave before the training is complete. Mark Training Complete in Bulk allows the CRA (Manager) to enter all those training records into the system at once.
- Up to 50 learners may be selected for bulk completion.
- Up to 40 Training Requirements may be processed at once with this feature.
- Anyone with the appropriate permissions, such as administrators, may utilize this feature. However, the primary use is for the CRA.
- Proof of completion may be uploaded using this feature.
- E-signature functionality may be enabled.
- Only Training Assignments that have Consent to Share = False are included in the scope of this feature.
Learner Selection:
Training Requirement Selection:
Select Training Requirement for each Learner:
For more details and feature enablement, see My Study Team in Veeva Vault Help.
Clinical Operations Data Model Changes
See 24R3 Clinical Operations Data Model Changes.
Veeva eCOA
The Enhance Clinical Operations Lifecycle Transfer feature, listed in the Vault Connections section also affects the eCOA application.
Rename ePRO to eCOA
This feature updates the product name from ePRO to eCOA to represent the broader capabilities of the platform. The ePRO term is now only used to refer to electronic Patient Reported Outcomes and not the application we use to collect the information.
Studio
Enable Collection Approval with Incomplete Translation
This feature allows sponsor and CRO users to approve collections with incomplete translations. Users in Studio can exclude study languages from a collection if the language is not in an approved collection. Excluded languages will not prevent the collection from entering UAT and will not be included in the collection languages when the collection is approved.
Inactivate Events
This feature enables sponsor/CRO staff to inactivate events that have been used in an approved collection. Inactivating allows you to remove an event you no longer want to use in a study, while ensuring that any collected data associated with the event remains visible to site staff. The events must first be removed from all schedules, rules, and anchor events. Inactive events are removed from a participant’s event list in eCOA unless the event has any data associated with it. Data that has already been collected is included in reporting, and the event changes are tracked in the audit report. Events can also be reactivated later if needed.
Survey and Schedule Editor Enhancements and Snippet Addition
This feature adds a copy-and-paste JSON code snippets library, improving the survey and schedule creation for sponsor/CRO users. Additionally, study builders can now create schedule JSONs when drafting the first version of the survey.
The snippet library includes various blocks, from full templates to question blocks and parameters, giving study builders complete flexibility when building JSONs. Each snippet contains an easy action to copy and detailed information about its use.
Turn off Data Changes or Provide Timeframe for Changes
This feature enables sponsors to turn off the ability for site users to perform data changes on submitted surveys. Sponsor users can also set a window during which site users can change data to ensure data is changed in a timely manner. This setting can be changed throughout the course of the study if needed.
Support Blank Labels in Numeric Rating Scale Questions
This feature enables Sponsor/CRO staff to configure numeric rating scale (NRS) labels with an empty string to act as a spacer. Spacers will cause other labels to wrap and create specific label widths to meet licensing requirements.
Schedule As Needed Surveys for Specific Duration
This feature enables sponsor or CRO staff users to add a specific amount of time they want an As Needed survey to be available for. The schedule can be configured to be available for a specific number of minutes, hours, or days.
ePRO Vault
Survey Library Manager
This feature enables sponsor/CRO staff users to create and edit reusable surveys in their customer library. Translations can also be uploaded and managed for each survey. Users can control the availability of the library survey for study use by publishing, archiving, or unarchiving library surveys. Users can also create a new version of a library survey to make changes.
Email Check When Inviting Site Personnel
The following updates are available with this feature:
- When a sponsor/CRO staff user adds a site user, they receive in-line feedback about whether the email address is already in use in this eCOA Vault or other Veeva applications. This feedback helps users confirm whether they are using the email already associated with the site staff user in Veeva applications, which is helpful if a site staff user has multiple email addresses.
- The system controls the assignment of Site User security profiles to minimize errors in site user management.
Reporting
24R3 Feature Audit Events
This feature enables the system to add relevant user actions from 24R3 features as audit events on the MyVeeva audit trail.
Multiple Tables in CDB Exports
This feature enables study team members to choose whether to export to CDB in one table or multiple tables. By default, the data will still enter into CDB as a single table, and a new sponsor toggle is available to allow them to split the data into one table per survey.
API
New API for Participant IDs
Depending on how the clinical trial is executed, participants may have their IDs listed in multiple systems. This feature creates an external API that enables the participant list to be seeded from any credentialed system, such as an RTSM system, eliminating the need for site staff to manually create and update participant ID information in Veeva eCOA. The person configuring the integration will coordinate with Veeva to meet prerequisites and then integrate with Veeva eCOA and make updates as needed.
Veeva eCOA (Sites)
Study Preview
This feature allows all study users to review and explore the study schedule, including all the surveys, schedules and notifications that are configured. You can interact with the schedule to determine which surveys will become available at which event, and you can preview the surveys in your browser to experience them as the user would.
New Site Translation - Italian
This feature enables Veeva ePRO to be viewed in Italian by site users in Italy.
Deactivate Participant Who Is No Longer Participating in the Study
This feature enables site staff to deactivate a participant who is no longer participating in the survey to end data collection. Once deactivated, a participant no longer receives notifications, future surveys are canceled, and the participant can no longer be edited in eCOA. The following activities are still allowed:
- In-progress surveys are still available for completion.
- Surveys that have been completed offline will be submitted when the user reconnects.
- The participant can still log in and view their study information and resources.
A participant can also be reactivated if needed.
Removing Surveys Collected in Error
This feature enables site staff to omit survey data from being included in reports if they were collected in error. All actions are audited and available to the sponsor through the data change report. Completed ePRO and eClinRO surveys are eligible for removal, and a reason for change is collected when they are removed. A sponsor can control whether the site has the ability to remove surveys in the study settings.
Surveys can also be restored if needed.
Ask for Help Global Response Status
This feature enables site staff to categorize help requests as Open or Archived to make it easier to see which requests still need a response. Once a request is moved to Archived or back to Open, the response status is updated for all site staff members.
Site In-App Notifications Added
This feature enables site staff users to receive in-app notifications and optionally reduce the volume of email notifications from a study. The notification bell, accessed from the header, shows an indicator when unread notifications are available.
Improved Error Messages for Visits
This feature updates error messages to be more specific when a visit is not sent to the patient as expected.
Survey Data Loss Prevention
This feature enables the server to save surveys that fail back-end data checks to prevent data loss. In the rare case that a survey fails a data check, an indicator is displayed to inform the site user that the survey may contain invalid data. Veeva will reach out to the site to verify whether the data should be added to the record.
MyVeeva for Patients
Translations
24R3 Translations and New Languages
MyVeeva users can now view application text, emails, notifications, and the terms of use and privacy policy in the following languages:
- Afrikaans (South Africa)
- Assamese (India)
- Bengali (India)
- Chinese, Traditional (Hong Kong)
- Chinese, Traditional (Taiwan)
- Croatian (Croatia)
- English (Israel)
- English (Singapore)
- Gujarati (India)
- Haitian Creole (Haiti)
- Hindi (India)
- Kannada (India)
- Lithuanian (Lithuania)
- Malay (Singapore)
- Malayalam (India)
- Marathi (India)
- Odia (India)
- Punjabi (India)
- Russian (Israel)
- Russian (Lithuania)
- Russian (Ukraine)
- Serbian (Serbia, Cyrillic)
- Spanish (Argentina)
- Spanish (Chile)
- Spanish (Colombia)
- Spanish (Peru)
- Tamil (India)
- Tamil (Singapore)
- Telugu (India)
- Ukrainian (Ukraine)
- Urdu (India)
General UI
Email Notifications Sent for Accounts with No Phone Number
With this feature, unregistered participants who only have an email associated with their accounts can receive eConsent notifications. Previously, these users did not receive notifications. This update will ensure that the remote participants will be able to more easily register an account in the MyVeeva for Patients app.
Study Contact Added
This feature enables sites to add a contact phone number to the app for MyVeeva Users. They can update or remove the number as needed. In the MyVeeva app, the phone number is associated with a phone icon on the Study tab, and when a participant selects the icon, they are prompted to call the number that was added.
Patient Language Based on Site Selection
To streamline the language preference process, we’ve removed the language dropdown from the Account page in the MyVeeva app. Now, the app uses the language the site staff selects when adding the participant, participants can no longer update language preference settings. The participant has access to view which language is selected by viewing Details in the Study tab. Previously, switching between system notifications in one language and study information in another could be disorienting for participants.
Users Can Enable and Disable Biometric Settings Directly in iOS App
This feature ensures that app users can enable and disable Face ID from the Account Settings tab in the app instead of leaving the app to change them in the device settings. This change aligns with how the app works with Touch ID and on Android devices and simplifies the experience for the participant.
24R3 ePRO/eClinRO Enhancements & Fixes, Including Improved Formatting, Minor Workflow Updates, and Improved User Guidance
The following updates are available with this feature:
- The participant profile header in eCOA is updated to display minimal data in a more visually appealing way and be more scalable.
- If a participant is added to eCOA from SiteVault, site staff are not instructed to give them an activation code since it is not needed.
- A notification reminding a site staff user to activate the study version is displayed above the Participants list when a new study version is available.
- Survey buttons have minor updates to align with other site buttons so MyVeeva users have a consistent experience.
Default Phone Number Country Code Based on User Language
This feature updates the phone country code that is selected by default on empty phone number input fields to be based on the user’s language locale. Automatically selecting the participant’s country code instead of the USA country code will improve their app experience.
Improved Mobile eConsent Image View
This feature enables MyVeeva users to zoom and pan on images in eConsent documents using native device functionality. Using native device functionality simplifies the app use for participants so they can view images in more detail without leaving the context of the eConsent.
Return to SiteVault
This feature brings site users back to the SiteVault login page after using their device to consent participants. Streamlining their workflow will improve their experience.
Users Can Temporarily Delay Forced Mobile App Upgrades
This feature allows MyVeeva users to delay a mobile app upgrade for a period of time that is determined by MyVeeva instead of forcing an immediate upgrade. Upgrades will typically be delayed for 14 days, but the timeframe may be shorter for more urgent updates. After the designated timeframe has passed, a user will have to upgrade the app to log back in. This update will allow users to complete time-sensitive tasks before installing the update, and will be less intrusive on their participation in the study.
Commercial
In addition to the below release notes, the Vault PromoMats Veeva Connect community offers general release communications, release highlights, and key feature demos.
The RIM-PromoMats: Product Data Transfer feature, listed in the Vault Connections section also affects the Commercial application family.
PromoMats
Modular Content Previews: Materials (HTML)
With this release, Vault can visualize Content Modules against layout templates to demonstrate how the content module would appear within a content and are available for up to 24 hours.
This functionality aids the review and approval of Content Modules and Content Module Combinations by allowing reviewers to more easily interpret the content and provide their feedback during review.
This feature leverages HTML templates that are stored within Vault.
Modular Content: CRM Email Preview Generation Moved to Asynchronous Job
With this release, we have updated the CRM Email Module preview generation to occur asynchronously to improve overall performance. Users can now access previews generated through a notification link or email link. Previews are valid and accessible for up to 24 hours after generation.
Modular Content: Workflow Banner on Content Module App Page
With this release, reviewers can now see and complete workflow tasks directly on the Content Module app page instead of navigating to the Content Module record. Vault only displays the workflow banner on the Content Module page if single record workflows are enabled for Content Modules.
Auto-Link on Initial Upload
Admins can now enable Auto-Run of Auto-Linking in Vault to automatically link and suggest Text Assets when a user uploads the initial version of a document or through the use of Make a Copy. The process will automatically initiate for configured document types and eliminates the need for users to manually initiate the Auto-Linking action or to change the document’s state.
Auto-Update Reference Anchors for Text Assets
When a document that is used for linked annotations (e.g. a reference) is up-versioned to a new steady state, persistent text anchors that are used as substantiation on Claims automatically update to reflect the latest document version details. Vault adds the previous document version to a new section on the Claim record called Archived References.
The new Archived References section on the Claims page will allow you to remove the Archived References, by selecting the first of the two Remove options that appear. However, we strongly recommended that you do not remove Archived References from your Claims.
This feature needs to be configured as an entry action for the steady state of the referenced document’s lifecycle.
Created From Relationship Type
This release introduces the new Created From document relationship. When a user creates a copy of a document, Vault captures the original document in the Created From relationship, similar to the existing Based On relationship. However, unlike the Based On relationship, users can add additional documents to the Created From relationship via the API or manually through the UI after document creation.
Improved Logging for Make a Local Copy Job
This feature improves the level of detail provided in Vault Job logs if there is an error during the Make a Local Copy job. This supports customers in troubleshooting unexpected outcomes.
Auto-Populate CRM Slide Notes
This feature automatically populates the CLM Slide Notes field in PromoMats Vaults when using a PowerPoint (PPTX) source file as the original document to generate a multi-slide presentation with the Create Presentation feature. This eliminates the need for users to manually extract Slides notes into document metadata fields for downstream use by Veeva Vault or Vault CRM.
Commercial Data Model Changes
See 24R3 Commercial Data Model Changes.
Medical
In addition to the below release notes, the Vault MedComms and Vault MedInquiry Veeva Connect communities offer general release communications, release highlights, and key feature demos.
All Medical Applications
Created From Relationship Type
This release introduces the new Created From document relationship. When a user creates a copy of a document, Vault captures the original document in the Created From relationship, similar to the existing Based On relationship. However, unlike the Based On relationship, users can add additional documents to the Created From relationship via the API or manually through the UI after document creation.
MedComms
Auto-Generated Default Values for Copied Statements
With this release, when a user performs the Copy Record action on a Scientific Statement, the system now automatically populates the new Source Statement field on the newly created record. Vault populates the field with the ID of the Statement from which the record is copied. Additionally, a Reason for Copy field is added to the Statement, and action layouts are used to restrict the visibility of Source Statement and Reason for Copy fields to only appear during the Copy Record action.
Auto-Populate CRM Slide Notes
This feature automatically populates the CRM Slide Notes field in MedComms Vaults when using a PowerPoint (PPTX) source file as the original document to generate a multi-slide presentation with the Create Presentation feature.
Content Modules UI for CRM Emails
Vault Medical now supports an intuitive user interface for building CRM Email Modules. With this update, users can also comment directly in the Content Module UI.
MedInquiry
Medical Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Vault Medical checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Medical for the impacted field inventory.
Attribute Fulfillment Document Views & Downloads to Case Responses
This feature tracks external views and downloads of Fulfillment Documents. Each time a Response Document Link is opened externally and the Fulfillment Documents in it are viewed or downloaded, this is tracked. There are two primary drivers for the feature: to allow MedInquiry users to see that the recipient has viewed the document that was sent in the response package, and to allow customers to accurately report on views of content externally.
Tag Fulfillment Document Records That Originate From a Standard Response
With this release, if Vault automatically adds a fulfillment document from a Standard Response, Vault tags the fulfillment document as a Standard Response Document.
Enhanced Fulfillment Document Picker
This release introduces multiple usability improvements for the Fulfillment Document section used for managing documents included in the Response Package:
- Users can now reorder and remove Fulfillment Documents directly from the Case Response page.
- Users can now add documents via the standard document picker instead of a join record.
- The updated interface surfaces key information about the added documents, including tags for documents included in the Standard Response, document number, Product, document type, and external access statistics (views and downloads).
Fulfillment Documents on a Case Response Must Be Unique
This enhancement prevents users from adding duplicate fulfillment documents to a Case Response. If users attempt to add a duplicate, Vault displays an error. Additionally, when the Generate Response Package or Generate Preview Response Package action runs, Vault validates that documents included in the package are unique. If the documents are not unique, the action fails.
Add Standard Fulfillment Documents Action Does Not Add Duplicate Documents
This functionality prevents Vault from adding the same document as a Fulfillment Document record to a Case Response when the Add Standard Response Documents action is run if a document has already been added manually.
Limit Number of Fulfillment Documents per Case Response to 100
With this release, we have limited the number of fulfillment documents on a Case Response to 100.
Show Previous Cases at Intake
During Case intake, Vault displays any of the selected Case Contact’s previous cases in the Medical Inquiry User Interface.
Medical Inquiry User Interface: Display Field Updates from Create Record Actions
With this functionality, if field updates are defined in the Create Record action of a lifecycle of an object shown in the Medical Inquiry UI, those updates are reflected in the Medical Inquiry UI on save.
Telephony Support: Conversations
This feature enables MedInquiry users to answer phone calls and live chat requests within Vault with a screen pop-up that redirects users to the Case creation page. When a call or live chat request is answered in MedInquiry by a user, the following occurs:
- Users are redirected to the Case Intake UI.
- The Case Intake UI shows pre-populated Case and Case Contact information with data coming from the telephony provider.
- MedInquiry attempts to match caller info with Case Contacts in Vault and/or OpenData HCP records.
- A Conversation record is created to log the inbound call, live chat request or call transfer.
Telephony controls and functionality are provided by CTI applications like Amazon Connect, Genesys, and others. Because the call and live chat can be handled within Vault, users don’t have to use two systems to log cases while talking to HCPs on the phone or live chat.
Medical Inquiry Keyword Tracking: Cleanup Job & Reprocess Job
This release introduces a daily recurring job to delete all Case Request Medical Inquiry Keyword records for Case Requests older than three (3) years. This feature optimizes the amount of data generated in Vault by the Medical Inquiry Keyword Tracking feature.
Additionally, Admins can now configure a standard report and dashboard for Medical Inquiry Keywords in all MedInquiry Vaults.
Medical Data Model Changes
See 24R3 Medical Data Model Changes.
Quality
All Quality Applications
Remove LIMS Objects from Quality Vaults
The following LIMS-related configuration have been deleted from Quality Vaults:
- Any object whose name begins with
lims_v - The following fields:
| Object | Field to be deleted |
|---|---|
quality_batch__v |
control_specification__v field |
quality_batch__v |
release_specification__v field |
quality_batch__v |
protocol__v field |
quality_batch__v |
lims_generate_spec_execution__v action |
asset__v |
current_location__v field |
asset__v |
current_location_svo__v field |
asset__v |
label_definition__v field |
asset__v |
lims_print_asset_labels__v action |
asset_family__v |
label_definition__v field |
asset_family__v |
integration__v |
QualityDocs
QualityDocs Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard QualityDocs checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Quality for the impacted field inventory.
Document Change Control Tokens for Notification Templates
Three new tokens are available for inclusion in Document Change Control Notification Templates. These tokens allow notification recipients to view the list of documents associated with the Document Change Control and whether they are being routed for Change Authorization or are to be made Effective or Obsolete.
The new tokens are:
${mdccChangeAuthDocsList}displays a comma separated list of documents to be sent for Change Authorization.${mdccReleaseDocsList}displays a comma separated list of documents to be made Effective.${mdccObsoleteDocsList}displays a comma separated list of documents to be made Obsolete.
Quality Relationships Panel Enhancements
This feature expands a document’s Quality Relationships panel to include Document Change Requests (DCRs). Users can now view DCRs directly from this panel and are no longer required to leave the document to view related DCRs in separate tabs and/or reports. Users with appropriate permissions can also create and delete DCRs from within the panel. Hovercards include the Reason and Requested Implementation Date on the DCR record.
This feature also includes additional data in the hovercards for each of the existing relationship panes:
- Process Navigator
- Description
- Stations
- Description
- Training Requirements
- Does this Training Requirement recur? (Yes/No)
Additionally, there is a data column for each of the existing relationship panes on the Quality Relationships panel:
Process Navigator: Hierarchy Builder UI Enhancements
The 24R2 Release introduced the Hierarchy Builder for Process Navigator. This feature includes several minor enhancements to the styling, spacing, process divider lines, and other visual components on the user interface of the Hierarchy Builder to further improve the page’s appearance and usability.
Support for Auto-Start Workflows in the Document Change Control
If a document is assigned to both a manually-initiated workflow and one or more auto-start workflows, the Document Change Control record’s “Docs to be Made Effective with Workflows” and “Docs to be Made Obsolete with Workflows” sections will display both manual and auto-start workflows for the document in the workflow column.
Station Manager
Prevent Duplicate Documents in Station Manager
Previous to this release, it was possible for a Station Administrator to add the same version of a document to a Station record more than once. This feature introduces an error message and prevents a Station Administrator from adding a document version as Planned or Assigned when that document version already exists in either of these sections on the Station record.
Station Manager Login Improvements (iOS)
This feature introduces minor visual and usability updates to the login screen and improved error messaging in the case of failed logins.
Training
Note: All features apply to both Training and Study Training unless otherwise stated.
Study Training & Vault Training Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Study Training and Vault Training checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Quality for the impacted field inventory.
Study Training customers can also reference Clinical Operations Standard Checkbox Defaulting for similar changes in Clinical Operations Vaults.
E-Learning: Support Reporting on Locked Quizzes
In this change, SCORM 1.2 E-learning courses including a quiz will be able to better communicate with Vault regarding a Learner’s quiz status. An E-Learning course must be built with the correct variables to communicate with Vault.
A quiz locked within an E-learning course will now update Training Content Status and E-Learning Status Details records with the Learner’s quiz status. Once a Learner has reached the maximum number of quiz attempts, Vault indicates the quiz’s locked status on the Training Assignment page and prompts them to contact an Admin to reset it. Training Admins can then unlock the training using the Reset Learner’s Course Progress action on individual records.
In addition, Training Content Status and E-Learning Status Details records are reportable, allowing Training Admins to report on and reset locked courses. Prior to this change, it was not possible to report on locked Learn GxP training with quizzes that were based on SCORM 1.2 documents.
LearnGxP courses will be updated in the future to support this feature. Please reach out to your Learning Strategy Consultant if you have any questions.
Instructor-Led Training Enhancements
When a document is added to a class, Learners gain access to that document.
In this change, when a document is added to the Class record’s Materials section, each attendee is added to the Trainee role on the Library document.
Prior to this change, Vault did not add class attendees to a classroom material’s sharing settings, and as a result, Learners may not have been able to view them.
Prevent users from saving the End Date before the Start Date
Vault blocks the possibility of inserting an End Date that is earlier than the Start Date when creating a Class for a Classroom Training Requirement.
Prevent use of the Generate QR Code Action without Edit permission
With this change, only users with Edit permission for the Class Schedule and Session objects are able to trigger the Generate QR Code action on the Instructor-Led training class. For users without those permissions, the action is not visible.
Prior to this change, any user with access to the Class could trigger the action.
Allow Instructor to filter by Present, Absent, or Unmarked attendance
In this change, Instructors are able to filter the Class attendance list by Attendance Status.
When a Class requires the Learner’s eSignature, the Instructor can easily filter by Learners who are not present and mark their absence.
This feature is deferred to a future release.
Session Location field Added to ILT Page Class Information Section
In this change, the Session Location field is available when creating or editing a Class. Session Location will be added to the Class Information panel in a future release.
Instructor-Led Training: Maximum Attendees & Waitlisting
With this change, Instructors can now set a maximum attendee count and add Learners to the waitlist for classes that are full. When roster spots open, waitlisted Learners are added to the class automatically based on the order of registration.
This change is necessary because physical classrooms and other spaces have seat limitations. Setting a maximum attendee count ensures that everyone who attends a class has a seat, and the waitlist feature ensures that no seats are wasted.
- The Registrations field on the class shows the seat capacity.
- Maximum attendees are not enforced if the field is blank.
- Training Admins can add Learners to the waitlist.
- Instructors can move waitlisted Learners to the class roster.
- Instructors can add Learners to the class roster even when it is at maximum capacity.
For more details and configuration, see Instructor-Led Training.
Instructor-Led Training: Support More Than 100 Learners in Class
In this change, the maximum number of Learners in a single Instructor-Led Training class has been increased to allow for more than 100 Learners per class. New Custom Sharing Rules allow internal users to view classroom-related objects.
This feature enables the hosting of large-scale classes, webinars, and other sessions to support the business.
- The new maximum number of Learners per class is 1000.
- For customers using the old classroom training method prior to 22R2.2, the limit of 100 maximum Learners still applies.
- Learners and managers with access to classroom objects prior to the implementation of this feature will continue to have access to those objects.
For more details and configuration, see Instructor-Led Training.
Learner Exemption Requests
In this change, Learners can submit an exemption request for a Training Requirement. The Training Administrator receives a task to complete the request based on the submitted documentation.
Prior to this change, it was not possible to request or grant exemption from Training Assignments.
The purpose of this feature is to allow Learners to launch an exemption request from a Training Assignment, and submit the appropriate date and supporting information.
For example, a Learner who has extensive experience and education on a topic may provide evidence of their competence in order to request exemption completion for a Training Assignment.
- New fields on Training Requirement: Allow Learner Exemption
- New fields on all Training Assignment Types: Evidence Completion Date
- New object: Learner Request
- New Workflow: Review Exemption Request Workflow
- New Completion Source picklist value: Exemption
- When the Review Exemption Request Workflow is in progress, Learners are not able to self-complete the Training Assignment.
For more details and feature enablement, see Working with Facilitated Training.
My Team Page & My Study Team Page UI Updates
In this change, a Manager or CRA can toggle between Eligible and Ineligible Learners.
Prior to this change, both Eligible and Ineligible Learners were shown in the same view.
The purpose of this feature is to allow Managers or the CRA to view Eligible and Ineligible Learners separately, allowing them to better understand the data and focus their desired actions.
Require Re-Training User Action
This feature allows Training Admins to manually initiate the process of re-assignment of previously completed trainings to a Learner or group of Learners. The action to program the re-training will be available to be added on Person and Curriculum objects. When a training is reassigned, it becomes part of the Learners’ training matrix, as opposed to an ad-hoc Direct Assignment. The new assignment takes precedence over any previous recurrence dates, training matrix updates, and document updates.
Prior to this change, retraining could only be issued via Direct Assignment. This feature allows Training Admins to re-issue training for similar reasons as Direct Assignment, for example retraining as part of a CAPA, or when a Learner returns from a leave of absence.
Training Enhancements
My Learning Page: Curriculum Card Completion Percentage Value
In this change, the Curriculum Completion Percentage Value shown in My Learning > Open > Curriculum Card corresponds to data from the Training Completion Status record, which is the same location that calculates curriculum completion for the Curriculum Prerequisites feature.
Prior to this change, the completion percentage was calculated based on the My Learning UI logic. The purpose of this change is to improve system performance and unify curriculum completion across the application.
Upon completion, Training Assignments are stamped On Time or Late
In this change, the Completed Status field on the Training Assignment object will be populated with the “On Time” value when the task Due Date on the assignment is blank.
In future releases, Vault will not populate the Due Date field when a training is optional.
Clinical Mapping Change Limitations
With this change, users cannot create, update, or delete Clinical Mapping responsibility records used in Responsibility-Based Training. In addition, users cannot change the Training Type field within a Learner Role.
For example, when Training Type = Responsibility and a user attempts to create, update, or delete a record, they receive an error message.
For more information on Clinical Mappings see Veeva Vault Help.
Study Training Matrix Automation UI Polish
This change includes minor updates to the collapse and expand behaviors for the Study Training Matrix, where sections automatically collapse under certain conditions when finalizing, or when adding Training Requirements to the Study Training Matrix.
For example, when the user finalizes the Study Training Matrix, it automatically collapses when entering view mode. When a user adds a Training Requirement to the Study Training Matrix, the section remains in its original collapsed or open state.
Additionally, Training Requirement hovercards show additional details about the related Library document, including its Additional Information field. Prior to this change, Vault displayed the requirement’s name only.
For more details see Study Training Matrix Builder in Veeva Vault Help.
QMS
Vault QMS Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard QMS checkbox fields. When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Quality for the impacted field inventory.
Audit Room
This release introduces the Audit Room feature suite and workspace to Vault QMS. With Audit Room, you can manage the often time-sensitive and high-intensity activity of facilitating and responding to a live third-party audit. We’ve added purpose-built tooling for your Audit Response teams designed for speed, accuracy, collaborative teamwork and ease of use. There are two primary components to the Audit Room feature suite: the Audit Room for your Audit Response teams to triage, collaborate on, manage, and publish responses to inquiries, and the Inspector Portal for auditors and inspectors to seamlessly work with your organization and review responses to inquiries without having to resort to passing content and managing responses back-and-forth via email.
Into the Audit Room
The Audit Response team is served by the Audit Room page itself, accessible directly from the Audit record where they can file and manage inquiries (Audit Room Requests) and responses. From here, they can prioritize and route those inquiries through configurable workflows to ensure the quality and accuracy of responses before delivering those responses to the Inspectors in their portal.
The image below displays the Audit Room Response Team view with inquiry status and prioritization indicators.
Filters keep Response managers focused on the right actions at the right time. Audit Response managers can drag-and-drop inquiries through their lifecycles leveraging configurable workflows and security.
Individual inquiries can also be managed just like Vault records, with full workflow and lifecycle support. Responses consist of approved fields only, as well as references to Vault documents and/or attachments. Security for Inspectors is managed automatically. Only Published fulfilled responses will be made visible to Inspectors.
In addition to managing the formal responses through the Audit Room Requests, Audit Response teams are also afforded a less formal collaboration-focused communication tool with Scribe Notes. A Scribe Note is a live-collaboration Microsoft Word document available to any internal user within the Audit Room. Scribe Notes require Vault’s native Collaborative Authoring feature to be enabled before use.
The Inspector Portal
With the Inspector Portal, your organization can leverage Veeva ID to invite external parties directly into your Vault for the purpose of the Audit. Leveraging Veeva ID allows Auditors to need only a single login to work with multiple organizations leveraging Veeva ID-enabled solutions, regardless of which or how many specific Veeva customers they’re working with. Alternatively, Auditors may be modeled as External Users, with your organization controlling the provisioning and invitation process. Regardless of which approach is used to model your inspectors or auditors, they will only have access to security controlled pages from which they can view the Audits that they’ve been invited to participate in.
The Inspector Portal page shown below lists Audits that an Inspector has rights to, with a simplified Vault experience and no access to any other areas of your Vault.
Their next step is to enter the Audit itself to review their inquiries and responses. Vault automatically manages security to the audits and response materials (documents, attachments, etc.) as part of your Audit lifecycles.
Once in an Audit, Inspectors are presented with a listing of the Audit Room Requests that have been fulfilled and Published. Each request can be explored to see additional information. Auditors and Inspectors have simplified gestures to help keep them organized, reviewing and closing requests within the Inspector Portal.
Lastly, since all of your inquiry and response data is captured within the Vault, you can access data across Audits and Audit Rooms via Vault’s Reporting & Dashboards engine, allowing for an unprecedented type of access to responses, and response metrics to delve into for trend or insight surfacing natively available to your Audit Response teams within Vault.
Audit Rooms can be leveraged with or without inviting Inspectors or Auditors to participate directly in the process on a per-audit basis - perfect if your organization’s primary goal is a collaborative and efficient workspace for managing audit or inspection responses.
Available in QMS Vaults with this release, Audit Rooms requires configuration to use and can integrate seamlessly with your existing Audit and Inspection workflows and lifecycles. You can learn more about using and configuring the Audit Room solution in Vault Help.
Change Action Path Enhancements
This release introduces usability improvements for the Action Paths & Steps feature introduced in 24R2. As of this release, all of the usability improvements are automatically enabled.
- The name of each Action Step record will no longer be STEP-######. Instead, each Action Step record name will reflect the name of the Action Path defined by the system administrator in the QMS Action Path Configuration.
- When a user edits a Change Control or Change Plan, the Create buttons in the Action Steps section will be hidden if the user does not have permission to create Change Actions. Prior to this release, the Create button was not hidden and users without permission to create Change Actions would receive an error message.
- When editing a Change Action record, users can change the assigned Action Step. Prior to this feature, Vault did not restrict the list of available Action Steps to those associated with the Change Actions in the parent Change Control. Beginning with this release, users can only select Action Steps that are associated with Change Actions in the parent Change Control. This ensures Change Actions are always associated with Action Steps defined within the scope of the parent Change Control.
For example, the first screenshot below reflects how the system behaved before 24R3. When editing a Change Action record’s Action Step field, Vault provided a list of all Action Steps, even those that were outside the scope of the current parent Change Control or Change Plan record.
After 24R3, the list of Action Steps available for selection will be limited to those in the parent Change Control or Change Plan record, and they will be named based on their applicable Action Path.
Action Paths: Support Change Action Templates
This feature enables Vault to automatically assign a new Change Action, created from a Change Action Template, to a specific Action Step on a parent Change Control, Quality Event Change Control, or Change Plan.
Prior to this feature, whenever a Change Action was created from a Change Action Template, the new Change Action was always assigned to the parent record’s Other Actions. If the Change Action belonged in a particular Action Step, a user had to manually update the Change Action to assign the desired Action Step. This feature eliminates the need for the secondary manual Change Action update.
Creators of Change Action Templates can now assign an Action Step to a Change Action Template.
When a Change Action is created from a Change Action Template, and the parent record uses the Action Path & Action Step identified in the template, Vault automatically assigns the new Change Action to that Step.
When an Action Step (e.g. Pre-Implementation) is already In Progress or Closed, users cannot create a Change Action for that Action Step. Therefore, if a user attempts to create a Change Action from a Change Action Template associated with an Action Step that is not Open, Vault does not create the Change Action, and the user receives a notification that the Change Action could not be created because the Change Action Template is associated with an Action Step that is already In Progress or Closed.
Assuming an organization already configured the Change Action Template and Action Path & Steps features, the only additional task necessary for a system administrator to enable this feature is to add the new Change Action Template Action Path & Step control to a section in the Change Action Template object layout.
A Change Action Template can only be assigned to a single Action Path & Action Step. This feature has no impact on Change Actions created from Change Action Templates when the parent record does not use an Action Path.
External Collaboration: Supplier Questionnaires
External Collaboration was designed to allow a customer to maintain a contact list of individuals at partner organizations that they need to interact with. With this feature, these contacts can receive a task with temporary access to Vault, complete the assigned task, and then Vault will revoke their access.
In 24R3 we are expanding the External Collaboration functionality to include the ability to assign an external collaborator a task to complete a checklist that was created and approved for use by your business administrator.
With this feature, organizations use checklists to send questionnaires to their suppliers and contract manufacturers. Questionnaires can be created and sent to facilitate pre-audit assessments while onboarding new business partners. They can also be created to collect information from existing partners to conduct a risk-based assessment of need for additional action.
This feature requires step-by-step configuration to enable. Learn more on how to configure External Collaboration: Supplier Questionnaires.
Learn more about working with Checklists.
External Collaboration: Reassign Task when External Collaborator Changes
Beginning in this release, users can reassign a task to a different external collaborator by selecting the new person’s Person record from the External Collaborator field on any External Collaboration record. If the previous collaborator had a task, then the new collaborator will be activated and invited to Vault if necessary, and Vault automatically cancels the task and reassigns it to the new one chosen.
This functionality applies only when the replacement external collaborator already has an existing user account linked to their Person record.
Prior to this release, when working with a QMS record in which the user needs to change the External Collaborator assigned, Admins must either use a manual user action after updating the External Collaborator field to a new value or cancel the in-progress workflow to reassign a new External Collaborator to a task, causing an interruption to the flow, depending on their configurations. Starting with the 24R3 release, this behavior will change due to the background reassignment operation. If the replacement external collaborator does not have an existing user account, Admins can create and link a new user account to the Person record, or users can cancel the workflow, change the external collaborator, and complete a user action or move the record through its lifecycle to trigger the external collaborator activation automatically.
You can learn more about Replacing External Collaborators in Vault Help.
Field Corrective Actions: Business Process Integration & Automations
This release marks the introduction of formal management tools for Field Corrective Actions (FCAs) in Vault QMS. This feature, in conjunction with the Field Corrective Actions (Recall Management) Data Model feature, provides our MedTech customers with a new process to manage Field Corrective Actions within Vault QMS. Together, these features allow customers to identify, capture, communicate, and execute Field Corrective Actions.
Firstly, FCAs support the Create Product Tracking Records action, which will automatically assess the Lots associated with a given FCA, and create the appropriate Containment or Product Action for Consignees based on whether the consignee for that lot is internal or external to your organization. The automation includes decision logic for handling Service Request creation for Capital Equipment devices based on the Device Type of a given Product Variant.
Once the appropriate Field Corrective Action - Lot and Lot - Consignee records have been created, the automation creates the appropriate Product / Material Tracking records to store records of product return and service requests at the lot level, and outreach actions and prompt Field Action Coordinators and Owners to follow up appropriately. Once all appropriate action and tracking records have been created, the system automatically updates the FCA so Field Action Owners and Coordinators know that they’re ready to take the next steps with the FCA.
With FCAs now accessible in your QMS Vault, the following core QMS features will be expanded to support FCA management:
- External Collaborators may be directly incorporated into your HHE process via Vault QMS’ External Collaboration feature.
- External parties can be kept abreast of Product Action developments via the External Notification feature.
- The External Notification feature set has been further expanded specifically for FCA management with automation to create Product Return Outreach records automatically, which associate and store the communications’ message, timing, and recipient to the appropriate action within the Vault.
The Field Corrective Action: Automations feature requires that Admins enable this feature in their Vault from Administration > Settings > Application Settings.
Field Corrective Actions Data Model
This release introduces the ability for Med-Tech organizations to identify, capture, communicate, and execute field corrective actions and recalls. To that end, we’ve added several new standard objects, such as Field Corrective Actions (FCA) and Product / Material Tracking Outreach, with standard lifecycles to capture, manage, communicate, and track FCAs.
Additionally, we’ve incorporated enhancements to the standard data model of several existing objects like Containment, Product Return, and Product Variant to support native integration into your existing processes. You can learn more about the specific changes to the data model in the 24R3 Quality Data Model Changes. These new and altered standard object definitions are visible to Admins upon release, but require configuration and enablement (via Administration > Settings > Application Settings) to create and use FCA-related records. You can learn more about the key objects associated with Field Corrective Actions in Vault Help. Learn more about the related Field Corrective Action: Automations feature also included in this release.
Complaints Email Processor Update: Support for Complaint Intake
Organizations receive complaints from consumers and healthcare practitioners. These complaints are often captured through a website or call center where they are logged in a Customer Relationship Management (CRM) system. When a complaint requires quality assurance attention, a Complaint record is created in Vault QMS. There are multiple methods of creating the QMS Complaint record:
- Automatic creation of a Complaint record via a custom-built integration with the IT system that captures incoming complaints.
- Automatic creation of a Complaint record based on an email sent to a configured Vault Email Inbox.
- Manual creation of a Complaint record by a QMS user.
The 24R2 release introduced a new option that allows QMS customers to capture potential complaints, called Complaint Intake records, to triage in Vault before making the decision to promote them to actual Complaint records. There are also multiple methods of creating potential complaints:
- Automatic creation of a Complaint Intake record via a custom-built integration with the IT system that captures incoming complaints.
- Manual creation of a Complaint Intake record by a QMS user.
Beginning in this release, system administrators can now configure a Vault Email Inbox to receive email and automatically create Complaint Intake records from the emails received. This feature is beneficial when it is not feasible to rely solely on manual creation of potential complaints, or to build a custom integration with another system to automate potential complaint creation. Vault stores the email received, and any email attachments, in the Complaint Intake record created via this feature.
If the Vault Email Inbox receives an email about a potential complaint that already exists in Vault (for example, the sender replies to the original email), the additional email and any attachments will be captured on the original Complaint Intake record.
Recurrence Check: Editable Date & Match Field Prompts
In this release, we’re continuing our investment in Recurrence Check tools by allowing date ranges and Matching Fields to be overridden by users running recurrence checks. With the new functionality, Admins can decide if specific types of recurrence checks are intended to always run the same way when invoked, or provide greater flexibility, allowing organizations to expand the range of records returned as potential matches when executing the check on a check-by-check basis.
Prior to this feature, recurrence checks could only surface records that have, for example, awareness dates equal to or prior to a given record’s awareness date. Now, individual recurrence check actions can be expanded to account for recurrences of an issue with any preferred date range selected by the user. Similarly, this feature may enable organizations to change the timeframe of recurrence checks to focus on questions like “Have we had a recurrence of ‘this’ issue since the CAPAs were put in place on date X” by adjusting the match fields and date ranges as needed.
To take advantage of this new feature, Administrators will need to configure changes to their existing (or new) recurrence check actions. You can learn more about Recurrence Checks in Vault Help.
Dialog Layout Updates for Recurrence Check
In this release, we are introducing new layouts that will display match criteria in an updated UI design exclusively for the recurrence check functionality. The new designs apply when launching the record check, refreshing the results of an in-progress recurrence check, and clicking the View Match Criteria button on the comparison page.
Recurrence Check Data Model Changes
In preparation for upcoming Recurrence Check capabilities, we’re introducing new data model changes. Refer to the 24R3 Quality Data Model Changes where you can learn more about the affected components. Learn more about the developer-facing functionality of this feature.
Email Body Layout Section for Download to PDF Support
This release introduces a new configuration option for System Administrators: the Email Body layout section for Complaint, Complaint Intake, Quality Event Complaints, and Supplier Change Notification objects. The section displays the content of the email sent to a Vault Email Inbox and processed by the Create Complaint or Supplier Change Notification email processor, resulting in the creation of the object record. The screenshots below show examples of the Email Body layout section on Supplier Change Notification and Complaint records.
We encourage System Administrators to replace the Email Body field in object layouts with the new Email Body layout section because the Email Body layout section supports the Print Record feature introduced in 24R2. The Print Record functionality enables a user to generate a downloadable PDF file containing the record’s data as shown on the record’s detail page. Replacing the Email Body field with the Email Body layout section ensures the content of the email displayed on the record is readable in the exported PDF file. The email content exported to PDF is a text version of the original email.
Note: Although the Email Body layout section can be added to any Quality Event object type, Vault only displays the section with email content on Quality Event records where the object type is Complaint, Complaint Email, or Medtech Complaint.
Download to PDF: Support for Vault QMS Specific Sections & Fields
This release we’re expanding on 24R2’s Print Record feature, with a focus on functionality for QMS specific application controls. Most of the controls across QMS that were previously unsupported for Print Record are now supported:
- Quality Teams Section
- Related Events App Section
- Root Cause Analysis Tool Section
- Notification Recipients Section
- Risk Builder Section
- Risk Level Assignment
- Record Check Insights Link
- Quality Incident Configuration Data
These sections will now natively render when using the Print Record function on any record in Vault QMS. There are a few more application sections that are coming in future releases as we further push to provide native printing capabilities across the application, so stay tuned for more updates with our upcoming releases!
Mapping Materials to Products
In this release, we are adding changes to the data model to strengthen the mapping functionality by introducing a new object, Material-Product, to allow a material to be mapped to any level of the Product hierarchy.
This will tackle common scenarios in which users cannot easily recognize the relationship between a Material and a Product, when there are changes at the material level that affect multiple upstream products, or changes at the product level that affect multiple downstream materials.
Please refer to the 24R3 Quality Data Model Changes for more detailed information on the affected components.
Reason for Change
Health authorities expect life sciences companies to capture a reason for change when correcting data fields on records associated with certain key processes. This feature introduces the ability for a System Admin to configure Vault QMS to require a reason for change when users update specific fields on records in particular states. Vault QMS captures the reason for the change, what is changed, the user who makes the change, and when the change was made.
When a user edits fields managed by a Reason for Change configuration and saves the record, Vault QMS opens a dialog like the one shown below to collect a reason for change. The user must select one of the valid reasons defined by an organization in the Reason for Change picklist. If the selected reason is configured to require supporting information, the Comments field is mandatory.
When the user clicks the Save button in the Reason for Change dialog, the changes to the record’s fields are saved, and Vault QMS creates a related Reason for Change record as shown below.
The Reason for Change record includes what was changed, why the changes were made, who made the changes, and when the changes were made.
The example below shows how System Admins define a Reason for Change configuration for an object and object type (standard objects and object types only). An object and object type can only have one (1) Reason for Change configuration. Each Reason for Change configuration can define up to 30 fields (standard or custom) requiring a reason for change in the lifecycle states specified. System Admins can also require users to enter a free text Comment to support specific reasons in the Reason for Change picklist.
This feature adds the Reason for Change Capture (reason_for_change_capture__v) field to the following join objects, which changes them from simple joins to complex joins:
- CAPA-Quality Event (
capaquality_event__qdm) - Deviation-Batch (
deviation_batch__v) - Complaint-Batch (
complaint_batch__v) - Lab Investigation-Batch (
lab_investigation_batch__v) - Quality Event-Batch (
quality_event_batches__v)
With this change, users will notice a difference in the way related objects are displayed. The example below shows a Quality Event record’s related CAPA Actions using the CAPA-Quality Event (capaquality_event__qdm) join object before the 24R3 release.
After the 24R3 release, the same Quality Event record’s related CAPA Actions section appears like the following example.
In addition, if a section on an object’s layout uses one of the five (5) join objects identified above, and the section contains VQL Criteria, the 24R3 release has removed the VQL query from the Criteria VQL field. The example below shows a section in the Quality Event object’s layout using the CAPA-Quality Event (capaquality_event__qdm) join object before the 24R3 release. This Criteria VQL example shows related CAPA Actions whose lifecycle state is not Cancelled.
After the 24R3 release, the VQL query in the Criteria VQL field is blank. In order to replicate the behavior of the Criteria VQL configuration after the 24R3 release, a System Admin must first create a Lookup field on the CAPA-Quality Event (capaquality_event__qdm) join object that points to a CAPA Action’s lifecycle state.
Then the System Admin must use the new Lookup field in the Criteria VQL field on the section of a Quality Event object layout to display related CAPA Action records that are not in the Cancelled state. The example below shows the Criteria VQL configuration using the Lookup field.
You can learn more about the Reason for Change feature in Vault Help. Learn more about the developer-facing functionality of this feature.
24R3 QMS Standard Layout & Data Model Updates
The 24R1 release introduced Action Layouts, allowing a System Administrator to tailor the user experience viewing an object record’s fields. Layouts define how an object record’s fields are organized for display, as well as rules that determine such things as when fields are required or hidden.
The Vault QMS application built upon this capability by providing Standard Layouts across many core QMS processes in the 24R2 release. They are read-only example Layouts for standard objects included in the QMS application. Standard Layouts are not used to display object records to end users. Rather, they are provided for system administrators to copy and adapt when creating custom Layouts for their organization’s business requirements. Standard Layouts make it easier for system administrators to adopt the new Action Layout capabilities in their own implementations. System administrators cannot edit, delete, or activate Standard Layouts, or set them as a Default Layout. Note that Standard Layouts are not counted towards an organization’s custom Layout limit.
This release continues the progress made in 24R2 by introducing new Standard Layouts for additional QMS processes. It also includes updates to existing Standard Layouts that were previously deployed. In support of these changes, the Vault Quality data model was updated to include new objects, fields, and picklists.
This feature also introduces a number of updates to standard field configuration options that can be utilized by System Administrators, and picklist maintenance options for Business Administrators.
Please refer to the 24R3 Quality Data Model Changes where you can find a link to a downloadable resource that catalogs all of the Vault Quality data model changes, configuration updates, and picklist maintenance options introduced by this feature.
Surveillance
eMDR: Section F
With 24R3, organizations acting as importers of medical devices can now submit eMDRs to the FDA. In line with the general direction of Vault Product Surveillance, the standard layout for eMDR now supports working with eMDR Section F data. Existing customers can add the Section F application section to their eMDR layouts as part of adopting this feature.
New metadata fields for capturing and supporting Section F of the eMDR form are now present in Vault Product Surveillance’s Adverse Event Reports and supporting Complaints and MedTech Complaint Details objects. This feature will require configuration to use. We strongly recommend reviewing the eMDR Standard Layout example to see how to best implement this section to the eMDR process as it includes examples of how to keep users focused, for example, by ensuring the section is only presented to users when relevant.
Reportability Enhancements: Multiple Decision Trees
This feature enhances the current Reportability Assessment functionality for organizations using Vault QMS and/or Vault Product Surveillance by introducing the capability to create multiple decision trees. Organizations valuing a simple, global decision tree methodology are able to follow that practice, whereas, for organizations requiring more nuanced decision-making patterns, these Decision Trees can guide assessors through more granular assessments based on the metadata associated with a given Complaint, improving accuracy and traceability when it comes to Reportability decision making. During a Reportability Assessment, Vault automatically selects and provisions the appropriate Decision Trees for a given assessment based on metadata like the Country of Incident, Product Variant, Where the Product is Sold, Reportability Requirements of those countries, and other configurable attributes of the Decision Trees themselves.
Not only can Vault now select the appropriate decision tree to use, but it can also account for the need for multiple Decision Trees to be instantiated for a given Complaint based on metadata related to the Complaint, helping to account for Complaints regarding Countries of impact.
Vault creates individual assessments when users execute the Create Reportability Assessment action based on the metadata of the Complaint and configuration of the Decision Trees themselves. This action can also be triggered through an entry action.
Lastly, if your Vault has Vault Product Surveillance enabled, the system also automatically creates the necessary adverse event reports based on the outcomes of the Reportability Decision Trees associated with the Complaint. You can learn more about these changes to the Reportability Assessment tools within Vault QMS & Vault Product Surveillance in Vault Help.
Batch Release
Add Documents to Checks
This feature allows you to add unplanned Documents to a Check from the Batch Disposition Execution page so that they can be monitored before a disposition decision is made. To enable this functionality, set the Batch Disposition Check Requirement field Allow Add Documents to True. An Add icon is displayed on the Check, which opens the Add Document dialog to select documents to add to the Check. A Disposition Check Item is created for each document added.
Batch Release Role Assignment
This feature allows Admins and quality managers to manage who can be assigned to roles and what application role they will be granted when assigned. Disposition Role assignments are pushed down to its children Batch Disposition Checks. This capability builds on current Vault Platform security capabilities including application security and Dynamic Access Control (DAC) to make it easier to manage both eligibility and assignment and make assignments reportable.
Check Object Class Conversion
The Batch Disposition Check object has been reimplemented as a User Task object class type. The object batch_disposition_check__v has been migrated to batch_disposition_check_task__v. As a User Task object, records assigned to users will appear on their home page.
Genealogy for Reporting
The new Batch Genealogy feature will create batch relationship records when a Batch Disposition is created in a new object called Batch Genealogy (batch_genealogy__v) so that they can be used for reporting. The batch genealogy will be flattened for the batch being dispositioned so that it has a relationship to every batch in its genealogy up to 20 levels. When all Batch Disposition records are closed, after a month, a job will remove these records. Any reports configured can be used to create a PDF document upon closing of the Batch Disposition.
Validation Management
Vault Validation Management Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Vault Validation Management checkbox fields. When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Quality for the impacted field inventory.
Admin-Defined List Layouts
In 24R3, Admins can define best practice list layouts for Validation Requirements, Discrepancy, and Test Step Change records. Existing users can adopt these layouts by navigating to the applicable object data grid and clicking the Restore defaults hyperlink. This restores the default configuration for columns displayed for these objects in the test authoring, pre-approval, test execution, and post-approval UIs. New users added to your Vault after 24R3 automatically inherit the Admin-defined configuration as their default list layout configuration.
Attachment Display Enhancements for Test Steps
In 24R3, Vault Validation Management has enhanced how test step attachments display on test step cards. An icon is now displayed based on the attachment’s file type. This change impacts test step cards in the Test Script Execution interface, Test Script Review interface, and the Test Protocol Review interface.
Copy Test Protocols
In 24R3, Vault Validation Management allows users to copy a Test Protocol with selected test scripts including all child test steps, additional prompts, Co-Authors, and requirements to re-execute and re-author the Test Protocol. When a user executes the Copy action, they can select which test scripts should be copied to a new Test Protocol and select a target Validation Deliverable. This feature requires configuration to utilize.
Copy Test Script Enhancements
In 24R3, Vault Validation Management enhances the existing deep copy test script capability to support broader usage of copying a Test Script record. With this enhancement, users can copy a test script to a different Validation Deliverable and a different Test Protocol. In addition, Admins can configure an entry action to validate the states of all linked Validation Requirements for the test script before transitioning it to the next state. This feature requires configuration to utilize.
Default Requirement Burndown Filter to Approved Requirements
Prior to 24R3, all requirements (user, functional, design specifications) regardless of lifecycle state appeared in the Requirements Burndown View. In 24R3, Admins can decide whether Validation Requirements in a particular lifecycle state appear by default for Test Authors from the Requirements Burndown View when authoring test scripts.
Admins now have the option to associate a lifecycle state with the Approved state type on the Validation Requirement Lifecycle. If the Approved state type is not configured, the Requirements Burndown View displays all requirements regardless of their lifecycle state. Test Authors can still modify the default filter if they wish to view requirements and specifications in other lifecycle states. This may occur in cases where scripting is taking place to get ahead while other team members are drafting requirements concurrently.
Download to PDF: Support for Vault Validation Specific Section & Fields
In 24R3, Vault Validation Management supports the Download to PDF action to include application control fields such as Requirement Owner, Activity Owner, Deliverable Owner, Author, Executor, Witness, Discrepancy Owner, Template Requirement, and Co-Authors in various object page layouts and an application control section for Requirement Entity Version in the Validation Entity Version object page layouts. The application control fields will be printed as plain text. The application control section will be printed based on the list layout configuration for the Validation Requirement object. This is an auto-on feature.
Dry Run for Test Scripts
In 24R3, Authors are able to start dry runs for their Test Scripts to verify their accuracy prior to submitting for pre-approval. Now, authors can request members of the Validation Team to perform a dry run of the test script. Assigned executors of a dry run can provide comments, identify issues with the test script, and suggest improvements that can be made. The comments can be addressed by the author, improving the quality of the test script prior to submitting the test script for pre-approval. The feature is not visible to all Validation Management users by default and requires configuration changes to use the feature, including modifications to existing permission sets.
Optimize the Loading of Test Step Cards in the Test Authoring UI
With this feature, when Test Authors open the Test Authoring Interface, they see all steps in the actual size of all prompts instead of the loading icon for each step. This loading behavior eliminates the unnecessary scrolling after the data loads.
Requirements Burndown Search Bar
In 24R3, Test Authors can now search any field that exists in a user requirement, functional requirement, or design specification in the Validation Requirement object from the burndown panel. The new search bar appears in the Requirements Burndown View on the Test Authoring Interface. It helps Test Authors quickly find specific requirements while tracing them to test steps to ensure that they are challenged.
Validation Management: Standard Enhancements
In 24R3, the Validation Management application continues to enhance the standard data model and component design to help standardize the application. Examples include adding various standard date and cancellation related fields in various objects. In this release, we also have put controls in place to prevent Admins from enabling a Dynamic Access Control (DAC) attribute, like custom sharing rules or matching sharing rules, from any Vault Validation Management standard object. This was done because security is governed by the Validation Teams feature, and allowing Admins to enable DAC introduces unnecessary complexity that we want our customers to avoid. Since this change is automatically enabled, all customers must ensure they have not enabled DAC on any standard object (e.g. val_requirement_svo__v or val_activity__v) prior to the 24R3 release.
We are also introducing a new object type to help organizations manage Configuration Specifications on the Validation Requirement object (val_requirement_svo__v). Although the object type is inactive, customers must at a minimum grant Read permissions to this object type for users involved with authoring, executing, or reviewing/approving test scripts when 24R3 is released. To ensure additional guard rails are in place, we have also introduced a hard limit of 500 test steps per test script which will become effective automatically in the 24R3 release.
LIMS
Sample Results Entry
This feature allows Admins to set up a test used primarily for transcribing test results of a sample. These are tests that are quick to enter results for, do not require an execution method, do not require inputs to be logged, and do not have cross-test variables or aggregate results.
Limits of Detection & Quantification
This feature introduces the ability to define Limits of Detections (LOD) and Limits of Quantification (LOQ) values for Test Definitions and Test Definition Variations. It also introduces the ability to define the calculation value to use when a result entered is needed to calculate other test result values and is below the LOD. When a test result entered is below the LOD or LOQ for that test definition, the system indicates that to the user. The system also uses the calculation value defined in its test definition/variation.
Prevent Self Approval
LIMS Prevent Self Approval places users into record roles based on prescribed activity. These roles can then be configured as part of Workflows’ Segregation of Duties to disallow a particular role from participating in workflows. LIMS then does the following:
- Place users into Contributed to Test Execution role if they enter or modify a Lab Test Result or Lab Test Input
- Sharing Settings are updated via Manual role assignment on the Lab Test, Lab Sample, and Spec Execution record
- Place users into a Contributed to Test Execution role if they contribute to Peer Review of a Lab Test
- This requires additional workflow configuration to execute Custom Action: Cascade Contributor Roles
- Sharing Settings can be updated via Manual role assignment on the Lab Test, Lab Sample, and Spec Execution record
- This requires additional workflow configuration to execute Custom Action: Cascade Contributor Roles
- Place users into a Contributed to Stability role if they create or modify a Study, Study Time Point, or Study Action record
- Sharing Settings can be updated via Manual role assignment on the changed record and Study if not changed record
- Place users into a Contributed to Spec Data role if they create or modify a Test Definition or child record, Sample Plan or child record, Spec Data or child record
- Sharing Settings can be updated via manual role assignment on the changed record and Study if not changed record
To fully enable this feature, the following configuration is required:
- Use Workflow Segregation of Duties to block Contributor roles from desired Workflows (such as Contributed to Test Execution cannot participate in a Peer Review workflow)
- Use Custom Action on any Lab Test, Lab Sample, or Spec Execution Workflow to cascade workflow participants upward into Contributed to Test Execution
Test Definition Variation
This feature introduces the ability to create variations within a Test Definition based on the existing Test Definition Results and Inputs. At runtime, tests created using a Test Definition Variation have the Results and Inputs defined in the Test Definition Variation.
Three new objects are being introduced as part of this feature:
- Test Definition Variation
- Test Definition Variation Result
- Test Definition Variation Input
When a Test Definition Variation Input is created from a corresponding Test Definition Input, the following field values are copied from the Test Definition Input. The field values can be modified at the Test Definition Variation Input level, which are applied at runtime:
- Expected Amount (for consumables)
- Expected Amount Units (for consumables)
- Required
Any fields not listed above are defined based on the corresponding Test Definition Input (this includes Test Definition Step).
When a Test Definition Variation Result is created from a corresponding Test Definition Result, the following field values are copied from the Test Definition Result. The field values can be modified at the Test Definition Variation Result level, which is applied at runtime:
- Calculation Formula (for numeric type results)
- Any applicable variables must be defined at the Test Definition Result level
- Data Class
- Data Entry Method
- External Name
- Notation (for numeric type results)
- Picklist (for picklist type results)
- Precision (for numeric type results)
- Precision Type (for numeric type results)
- Required
- Rounding Rule (for numeric type results)
- Unit of Measure (for numeric type results)
Any fields not listed above are defined based on the corresponding Test Definition Result (this includes Test Definition Step).
The table below shows an example of a Test Definition for pH analysis with 3 variations. Each variation contains the same Test Definition Inputs, but the Test Definition Results vary; the cells in gray indicate the Test Definitions Result which are not included in the Variation (and therefore will not be created as results at runtime). The calculation formula for Average pH also differs between Variation 2 and Variation 3.
Creation of a Test Definition Variation is optional. However, if a Test Definition has one or more variations, runtime tests can only be created from a variation and not the base Test Definition.
In this release only, Lab Tests with the same Test Definition Variation can be combined in a Test Set.
Test Execution User Interface (UI) Enhancements
In 24R2.2 we introduced Test Definition Variations. In order to accommodate Tests with variations in the Test Execution UI, we have updated it to visually show differences in Tests due to variations (such as results with different units, results applicable to one variation but not another, or mode of entry differences between variations). UI changes are auto-on for all customers regardless of whether they are using Test Definition Variations.
In this release, Tests must have the exact same Lab Test Inputs in order to be added to a Test Set.
Some additional changes are being introduced in the Test Execution UI:
- Entry cells for Lab Test Inputs and Results are now open/in edit mode before a Lab Test Step has been completed. Required entries are displayed as yellow cells and optional entries are displayed as white cells.
- Inputs are ordered based on their defined order in the Test Definition.
- When there are Tests from more than one variation in a Lab Test Set, each variation is assigned a color within the Results grid (see screenshot below).
- When the Method Document is displayed (when applicable to the Test Definition), the list of Lab Samples is no longer compressed. Instead, users can scroll to view additional Lab Samples.
- Once a Lab Test Step has been completed, in order to make a change to a Lab Test Input or a Lab Test Result, the user can use Actions > Edit in the applicable entry cell.
- Result Override for calculations and integration results is available via Actions > Manually Override Result in the applicable entry cell as long as the user has the required role on their user account.
In order to utilize the Test Execution UI with variations, the user needs Read permission for the following objects:
- Test Definition Variation
- Test Definition Variation Result
- Test Definition Variation Input
Example of UI for a Test without variations:
Example of UI for a test with variations:
Additional Test Execution User Interface (UI) Enhancements
The following enhancements have been added to the updated Test Execution UI:
- Users can now navigate the Results grid using the keyboard’s arrow keys. They can also edit results by pressing enter on a selected cell or use Tab and Shift+Tab to navigate the grid.
- Within the Inputs grid, the arrow key navigation does not apply, but Tab and Shift+Tab can still be used to navigate the grid.
- The Alert exception icon color is now orange instead of yellow.
- The logic for consumable selection is now as follows: the consumable must be in the Available state type and either have no Expiration Date or an Expiration Date is ≧ the current date.
- When a calculation that was previously manually overridden has been subsequently recalculated (for example, due to a modification of a result which is used by the calculation), users will see the following informational exclamation mark icon within the result cell to denote “previous manual override”:
Track Change Analysis Merging
This feature allows Admins to ensure a Change Analysis has been merged before it is sent through subsequent workflows such as Review and Approval.
Track Conformance of Lab Tests
This feature adds a conformance flag on Lab Tests. If a Lab Test has any non-conforming result, the Conforms field value on the Lab Test is set to false.
Track Previously Completed on Test Steps
A Lab Test Step’s complete flag switches from true to false when a user makes a change to a result or input. This feature adds a field to clearly track if the step was previously completed by adding the following:
- New Previously Completed field on Lab Test Steps
- Logic for the system to set the Previously Completed field
- Data migration to set the Previously Completed field in existing LIMS Vaults
Stability Study Enhancements
The following changes have been made to Stability functionality to improve usability:
- Changes to Lab Study Design and Lab Study Review pages:
- Added Exit Overview icon to the top right corner
- Removed the Create Design Data Copy icon
- Removed the breadcrumb trail
- Record name (Lab Study Design or Lab Study) is now a hyperlink
- When generating a Lab Study record from a Lab Study Design record, the user will be prompted to input a name for the resultant Lab Study. If no name is inputted, the resultant Lab Study will be named the same as the Lab Study Design record.
- When a Stability Spec Execution corresponding to an initial time point (for example, time zero) has the Results Spec Execution field populated (i.e., with a batch release Spec Execution) the following changes are made to the Reevaluate Criteria logic:
- Only Lab Criteria Evaluation records which are valid (Invalid = No) within the Results Spec Execution is used when generating and evaluating the lab criteria evaluation records within the Stability Spec Execution record
- The system ensures that there is at least one valid Lab Criteria Evaluation record match, or else an error will be presented
- Any Lab Criteria Evaluation records belonging to a Criteria Set with Evaluate = “On Demand” are not generated in the Stability Spec Execution record
- When a Stability Spec Execution is created from an initiated study time point, the following fields are added to the Spec Execution and are populated based on the corresponding value from the Study Time Point record:
- Time Stored
- Time Stored Units
Support for LIMS Lifecycle User Actions as Workflow Steps
This feature adds support for configuring the following actions as workflow system actions:
- Initiate Spec Execution
- Re-evaluate Criteria
- Initiate Lab Study
- Initiate Study Timepoint
- Resample and Keep
- Generate Aliquoted Sample
Spec Execution Initiation Harmonization
Note: These changes impact all LIMS Vaults, regardless of whether the Aliquots functionality is in use or not.
This feature introduces the following changes:
-
For change analysis, a version verification/completion action check was added to Spec Data records to ensure that the total Pull/Select Count (
pull_count__v) for a given Sample Definition is less than or equal to the Sample Collection Count (sample_collection_count__v) on the Sample Definition. This check is only for Spec Data records which do not have any Aliquot or Pool type Spec Data Sample Action records. -
The Spec Execution initiation logic has been harmonized to one process with the removal of the Enable Full Sample Action Automation application setting. As a result, there is a migration effort being made to ALL LIMS Vaults to populate the following fields:
For Vaults that did not have the setting enabled (the majority of Vaults unless Aliquoting functionality was enabled):
-
Spec Data records:
- Set
initial_sample_auto_action_vto TRUE/YES
- Set
-
Spec Data Sample Action records with object of type
spec_data_pull_sample_action__v- Set
auto_complete__v= TRUE/YES - Set
auto_match__v= TRUE/YES - Set
auto_generate__v= TRUE/YES
- Set
-
Update the default value at the object level to TRUE/YES for the following objects/fields:
lims_spec_data_v-lims_spec_data_sample_action_vlims_spec_data_sample_action_v-auto_complete_vlims_spec_data_sample_action_v-auto_match_vlims_spec_data_sample_action_v-auto_generate_v
-
If any Spec Execution records are not Initiated (
initiated__v= false orinitiated__v= null/blank) AND theinitial_sample_auto_action_vfield is null/blank, then set theinitial_sample_auto_action_vfield to TRUE/YES.
For Vaults that had the setting enabled (Vaults with Aliquoting functionality enabled):
-
For any Spec Data records having
initial_sample_auto_action__v= null/blank, setinitial_sample_auto_action_vto TRUE/YES. For related Spec Data Sample Action records with object of typespec_data_pull_sample_action__v, if any of the following fields are blank/null OR false/no, update the field as follows: - Set
auto_complete__v= TRUE/YES - Set
auto_match__v= TRUE/YES -
Set
auto_generate__v= TRUE/YES -
For Any Spec Data records having
initial_sample_auto_action__vof the spec = TRUE/YES OR NO/FALSE, no updates will be made to the record or any of the related Spec Data Sample Actions. -
If any Spec Execution records are not Initiated (
initiated__v= false orinitiated__v= null/blank) AND theinitial_sample_auto_action_vfield is null/blank, then set theinitial_sample_auto_action_vfield to true/yes. -
In Vaults that did not have the Enable Full Sample Action Automation setting enabled, users now receive a different message when a Spec Execution is initiated. They will receive one of the following depending on the Spec Data setup:
- Spec Execution Auto Action has successfully completed for Spec Execution <record name>.
- Spec Execution Auto Sample Matching did not occur for Spec Execution <record name>. Reason: one or more Lab Samples could not be located to perform auto-matching.
- Spec Execution Auto Sample Matching could not be completed for Spec Execution <record name>. Reason: one or more Lab Samples could not be located to perform auto-matching. Refer to the following unmatched Spec Execution Sample Action record(s): <record name>.
View Spec Data in Mapping Section of Material Detail Page
This feature allows users to add a Spec Data column to the Spec Data Mapping related list on a Material Detail page. Admins can add the column to the Pagelayout via the Vault REST API. See the following links for more information on retrieving the Pagelayout and markup, executing and saving the new Pagelayout, and XML string configuration.
Deep Copy Spec Data Sample Action
This feature adds a Deep Copy action to unlocked Spec Data Sample actions that copies:
- A Select/Pull Spec Data Sample action and its related Test Sample actions and Spec Data Criteria records.
- A Test Sample action and its related Spec Data Criteria records.
Deep Delete Spec Data Sample Action
This feature adds a Delete Action to unlocked Spec Data Sample Actions that deletes:
- A Select/Pull Spec Data Sample action, and its related Test Sample actions and Spec Data Criteria records.
- A Test Sample action, and its related Spec Data Criteria records.
Quality Data Model Changes
See 24R3 Quality Data Model Changes
Regulatory
In addition to the below release notes, the Vault RIM Registrations and Vault RIM Submissions, Publishing, and Archive Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Regulatory application family:
- RIM-PromoMats: Product Data Transfer
- RIM-Clinical Operations: Enhance Study Defaulting Logic on Clinical Crosslinks
- RIM-Clinical Operations: Update to Clinical Study Field Rules
- RIM-Clinical Operations: EU Special Handling for Clinical Crosslinks
- Safety-RIM Connection Enhancements
All RIM Applications
Vault RIM Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Vault RIM checkbox fields: When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation.
See 24R3 Data Model Changes: Regulatory for the impacted field inventory.
RIM Bot Enhancements
Note: There is a known issue (DEV-802059) that prevents multi-lingual document classification.
With this release, a number of enhancements have been made to the RIM Bot. With Training Set Optimization, the number of documents extracted for a single classification when training and testing models is capped. This helps to ensure that the model is trained on a larger number of distinct classifications.
Automated Model Refresh supports maintaining good RIM Bot accuracy over time by retraining Trained Models automatically with each Vault General Release. An Auto-training Job that was released in 24R2 as part of RIM Bot: Auto-on in All RIM Vaults will be enabled if it has been disabled in all Production Vaults and prevented from being disabled. This automatic retraining will ensure that new Document Classifications added to the system will be considered in RIM Bot auto-classifications.
Multilingual model support extends RIM Bot coverage beyond English documents, to non-English documents as well.
Finally, a new one-click action, Retrain Model, provides a more efficient method for manually retraining models between General Releases. By reducing clicks and automating steps, customers can more easily retrain their models as desired.
RIM Registrations
Support for Drug-Led Complex Combination Products
With this release, Create Registrations is enhanced to generate Registered Product records for complex products when Co-Packaged or Integral Device is set to Yes. Updates to the RIM Data Model were completed in a previous release. This enhancement completes support of the IDMP requirement that co-packaged and integral devices be included in the submission data set.
Configurable Procedure Countries for Create Registrations
This feature provides the capability to manage Procedure Type countries using configuration (with Constraints) rather than relying on a hardcoded list of countries when creating RIM Registrations in bulk. The Health Authority field set in the Constraint’s Region Specific Entry will be set for the Registration referencing that Constraint, for example Centrally Authorised Procedures (CPs) when the Health Authority should be EMA rather than the local Health Authority.
A reason for this update includes BREXIT, for which the list of countries have required updating more than once based on regulatory updates. Making this list configurable facilitates constraining country lists for currently-supported CPs, while providing future and existing support for regions that may implement procedures that behave similarly to EU CPs.
Create Related Records: Support for Enhanced Change Management
This update to the Create Related Records wizard allows product-related changes to be tracked at a more detailed level, in a similar manner to the way labeling-related changes are tracked. Product changes can be linked to multiple events and activities, providing greater flexibility.
Impact to Existing Customers: Customers wanting to use Enhanced Change Management will need to add a Product Changes section to Event and Activity object page layouts. Once they start populating the Event Product Change records, the Create Related Records wizard supports propagating them to Activity Product Changes.
Create Related Records: Validation Enhancements
This enhancement updates existing validation when users run the Create Related Records action. It is designed to be timely and dynamic based on Event data. With a wide array of use cases supported, the local data needed to support change management can be propagated quickly and correctly.
Examples of use cases addressed by this enhancement include:
- Preventing Shelf Life details from being pushed to Regulatory Objectives or Submissions that don’t have the related Packaging
- Adding a new Packaging Site, only where the related Packaging exists
- New product approvals where Registrations do not yet exist
- Changes that require the creation of new Registrations
- Adding a new investigational product, a frequent case including comparator and reference products, without the product being in the application.
Potential data quality issues are raised in the preview to aid user review. For example, where there are multiple existing Application relationships that match an Event detail, the preview flags these for review to ensure they should be copied to the Regulatory Objective and/or Submission.
It is important to note that users must have the full set of Application relationships in place in order to use dynamic validation as intended.
Impact to Existing Customers: Validation logic is enabled via the Enable dynamic validation for Create Related Records admin setting. The Enable Application Relationships setting must be enabled as a prerequisite.
Activity Dependency Enhancements
A single Event may have multiple types of dependencies when it is determined to be cross-functional. This feature addresses a common use case and provides the ability to create labeling-specific Activity Dependency records when a labeling impact is identified on Activities for a non-labeling Event, if, for example, a labeling impact is identified from a manufacturing change. It also allows for specifying Country Dependencies at the Application level. This enables dependencies based on Applications across different markets, within the same market, and within the same Application.
Impact to Existing Customers: Enable the Rerun dependency checks when labeling impact is identified Application setting. Add the new Reference Application and Dependent Application fields to the Country Dependency and Application Dependency object page layouts.
Manage Registered Details Filtering Enhancements
The Manage Registered Details (MRD) wizard has additional filtering capabilities for text fields to narrow the search for records to facilitate updates. These filters support searching text values as well as blank (undefined) fields. This feature is also available in the Create Registrations wizard.
This feature increases the efficiency and ease of updating registered details by making it easier and quicker to identify, select, and update records with fewer clicks.
eAF Report Generation Enhancement
When generating an eAF Report from a Regulatory Objective, existing reports for the same Medicinal Product are replaced so only the most current report is available. This prevents redundant reports from building up over time.
This is automatically available in all Vaults where eAF Report generation is configured and Medicinal Product and Regulatory Objective filters are configured on reports.
Support for Multiple Review Steps in Registration Verification
This feature enhances the Registration Verification workflow to support the configuration of additional review tasks. Multi-record Registration Verification workflows that include multiple review steps will set the verdict to Rejected for records that have not been verified. Before this change, Vault set the verdict to Unknown in the CSV results file. Although the unverified data is deleted, this enhancement better represents the actual verdict.
RIM Submissions
Auto-Match RLCP Documents into GCP
With this release, document matching within CTD Module 4 and Module 5 can be constrained to documents from specific Report Level Content Plans (RCLPs).
Users can select a related RCLP when creating Event Clinical Studies and Event Nonclinical Studies. The RLCP values are propagated to the corresponding study-related sections in the Global Content Plan and used as matching criteria.
Copy Content Plan Action to Respect Use for Content Planning Field
When using the Copy Content Plan action, only relationships that are used for content planning are copied onto the target Submission and Application records and target Submission Content Plan. Previously, the Copy Content Plan action copied all relationship records from the source Submission to the target Submission and its parent Application, even those designated only for use with Registrations.
If the Use for Content Planning field is not configured or is set to No then the referenced relationship is excluded. If Use for Content Planning is Yes or is blank, the relationship is included in the target Submission. Results notifications include a message that the indicated CP sections were not copied because the relationship setting is set to No. Also, if the target Submission already has the relationship with the setting set to No, that target Submission setting is respected and that relationship is not included in the target.
This enhancement reduces the cleanup effort needed in Content Plans, Submission record relationships, and Application relationships when Copy Content Plan user action is executed. This enhancement specifically impacts the Copy Content Plan action executed from the Submission record, and does not impact dragging and dropping sections directly into an existing CP. If users want all relationships in the target, including those with Use for Content Planning set to No in the source Submission, users can copy the Submission record itself rather than copying the Content Plan.
RIM Registrations, RIM Submissions
Cloning Enabled for Content Plan Item Template Mappings
Content Plan Item Template Mapping (CPITM) (content_plan_item_template_mapping__v) records are now included when creating Sandbox Snapshots or cloning environments as part of a deployment. When configuring the Dispatch Global Content Plan Across Templates feature released in 24R2, the CPITM object records could only be migrated via Vault Loader or VPKs. This enhancement improves the ease and efficiency of adopting Dispatch Global Content Plan Across Templates.
GCP Dispatch Validations
This feature allows Admins to configure validations for the lifecycle states of a Global Content Plan’s related records. It also provides the ability to only dispatch documents from the Global Content Plan that have been version-locked to the Global Content Plan, whether dispatched by Document Set or by Country. This prevents the dispatch of documents that are not yet complete.
New Application Settings are available to validate the lifecycle state of target Submissions and Activities. A new option is available on the Dispatch Global Content Plan user action to validate that matched documents are locked prior to dispatch.
Learn more about configuring GCP dispatch validation.
RIM Submissions Archive
Submissions Archive Viewer: Filter Correspondence by Regulatory Objective
Correspondence documents displayed in the Submissions Archive Viewer can now be filtered by their associated Regulatory Objectives. Prior to this enhancement, Correspondence could be filtered only by Application or Submission. A new Correspondence subsection with a Regulatory Objective column can be added to new or existing Saved Views. This additional filtering option allows Archive consumers to review Health Authority Correspondence related to all the Submissions in the selected Regulatory Objective, or where an Application and at least one Regulatory Objective is set on the correspondence. This may be particularly useful when Correspondence documents are associated with more than one Regulatory Objective.
With this release, the Regulatory Objective association can be retrieved from either the custom Correspondence document field regulatory_objectives__c, or the standard document field regulatory_objectives__v (introduced in 24R2). A new temporary enablement option under Admin > Settings > Application Settings > Submissions Archive Features determines whether the system points to the custom or standard relationship.
RIM Vaults provisioned prior to 24R2 that are using the regulatory_objectives__c custom document field can immediately start using this new feature without migrating from the custom to standard document field. In a future release, this setting will be deprecated, at which time all customers will need to use the standard regulatory_objectives__v document field to see this attribute in Viewer. The deprecation will be communicated via the announcements in the release notes, at a future date. By providing the temporary setting, customers will have time to migrate their document field values to the standard field while still seeing immediate benefit of the Regulatory Objective filtering.
Submissions Archive Viewer: Mini-Browser Window Size Increase
When opening Submissions Archive documents using the pop-out, the width of the mini-browser window has been increased to display the Document’s Viewable Rendition and Details navigation bar at once.
- If Tab Collections are configured in your Vault and the Viewer tab exists in the Tab Collection, the mini-browser remembers the last-used state of the Details panel (open or closed).
- If Tab Collections are not configured in your Vault, the mini-browser always opens with the Details panel in the closed state.
Note: Your browser’s zoom settings impact display. Zooming in via browser zoom settings affects how much of the Details panel and navigation bar are displayed.
Import Notification Updates
Submissions Archive’s import notification is enhanced to inform users when an unsupported configuration prevents import, such as when setting a document field to Required on Submissions Archive-managed Document Types. The system displays the following message:
The system cannot complete this action due to unsupported configuration. The following system-managed Document Types have one or more Document Fields set to Required:
- Submissions Archive (
archive__v) - Submissions Archive > Content (
archive_v.content_v) - Submissions Archive > Structure (
archive_v.structure_v)
EU eCTD v3.1
With this release Submissions Archive is supporting importing, viewing, and exporting of European Union submissions using the EU Module 1 Specification, version 3.1, for eCTD submissions.
WHO eCTD v1.1
With this release Submissions Archive supports importing, viewing, and exporting of WHO submissions using the WHO-PQT Specification, version 1.1, for eCTD submissions.
ECOWAS eCTD v1.0
With this release Submissions Archive supports importing, viewing, and exporting of ECOWAS submissions using the ECOWAS Specification, version 1.0, for eCTD submissions.
RIM Submissions, RIM Submissions Archive
Active Dossier Additional Status Tracking
This feature adds support for tracking additional regulatory approval scenarios within the Active Dossier:
- New Active Dossier Statuses:
- Dispatched, Not Submitted
- Rejected
- Withdrawn
- No Decision
- New Approval Type field that captures if the document is associated with an Explicit, Implicit or Partial approval.
- Documents that have been dispatched but not submitted are set to Dispatched, Not Submitted instead of a blank status.
- New configurable Entry Action on the Regulatory Objective lifecycle state that automates Active Dossier status change for the new statuses and setting of the Approval Type.
- Updated population of the Active Dossier from the Global Content Plan. The Active Dossier Item Detail (ADID) is created/updated for Activities whose Local Dispositions are in Dispositions requiring submission after implementation or Dispositions for immediate implementation without additional activity. ADIDs created for these Activities will be set with Approval Type = Implicit Approval.
All the above are Auto-on except for the new Entry Action which requires configuration. Furthermore, the new Approval Type field is not yet supported in Active Dossier Loader. This will be added in a future release.
Active Dossier Calculation Update: Narrow Scope of Pending Current Change
With this release, an update has been made to the Active Dossier auto-calculation logic triggered from Regulatory Objective approval to narrow the scope of records that are moved during the pending current calculation. Instead of proposing candidate records from the related Regulatory Objective in any status to be set to Pending Current, Vault will only update those that are in a Submitted status to Pending Current. This will prevent unexpected proposed changes of Active Dossier Item Details to Pending Current in some scenarios.
Active Dossier Hovercard Update: Needs Submission
The Item Detail hovercard in the Active Dossier Viewer has been updated to include the Needs Submission field. This allows users to see if a document needs to be submitted when the Global Content Plan is dispatched to the Submission Content Plan.
Active Dossier Translation Handling Update
This enhancement to the Active Dossier generation supports more relevant translation tracking when multiple translations of the same source document are submitted in the same submission. It sets the Translation Document field on each new ADID record based on the ADID’s Country and the Vault’s Country Language records. In the case where there are multiple translations if the country has multiple languages, Vault sets the first instance on the ADID.
Active Dossier Viewer & Editor Enhancements
This feature enhances the Active Dossier Viewer and Editor to improve navigation and provide a simpler way for users to perform routine updates and corrections to the Active Dossier.
The following auto-on changes have been made to the Active Dossier Viewer:
- Introduction of two layouts: Country Overview and Item Detail.
- The Edit Active Dossier action on Submission, Regulatory Objective, and Event will navigate users to the Item Detail layout.
- Introduction of a new edit button in the Item Detail Layout to navigate to the Active Dossier Editor.
- The show Current/All/Pending toggle has been replaced with a status selector to allow for a more targeted selection of which Active Dossier Statuses to display.
A revamped Active Dossier Editor has also been introduced that includes the following:
- Supports the following individual or bulk updates to the Active Dossier Item Details records: Editing of editable fields, Deletion, Confirm Pending (for pending status changes).
- The Deletion and Confirm Pending actions as well as updates to the Active Dossier Status in the Editor will update the country status icon automatically so that the Apply Pending Status Changes action no longer needs to be additionally run
- The progress and results of each update can be tracked.
- A reason and optional comment is enforced when users perform an edit or deletion (does not include confirm pending action).
- Saved views are no longer supported for the Active Dossier Editor. It is recommended to create these saved views in the Active Dossier Viewer.
In order to fully leverage the Edit and Delete actions in the new Active Dossier Editor, users must be assigned a permission set in which they can Edit the new Reason for Change and Latest Change Comments fields within the Active Dossier Item Detail object. If a user’s assigned permission set already has Edit permission for All Object Fields (under Object Field Permissions) for this object, this feature is automatically enabled for them. Otherwise, these permissions must be configured.
RIM Publishing
FDA Sync Gateway Profile Deprecation
All FDA Sync Gateway Profiles have been removed from RIM Vaults and can no longer be used to transmit submissions via ESG. This is a followup to the 23R3 Announcement around deprecation. All FDA Gateway Profiles should be migrated to the FDA Async Gateway Profile.
Support Source Document Overlay Resolution
Customers now have additional flexibility when publishing with Overlays. Currently, the Publishing Element resolves tokens based on the Published document metadata, and would result in the values being the same across all pages of merged documents, such as Document Number.
Now, a new Picklist Field on the Publishing Element, Source of Published Overlay, allows users to populate Overlay token values based on Source Document values rather than Published document values. In particular when merging published output, this will resolve the Overlay tokens with different values per Source matched to the Content Plan Item(s). Implementing this feature provides more traceability from Source Documents to merged Published Output, and may support more accurate navigation of merged documents. For example:
By default Vault will always use the Source Document as the source of metadata information, unless a Publishing Elements Source of Published Overlay picklist field is set to Published Document.
Publishing Pre-Validation Updates
An additional pre-validation check has been added before proceeding to publish.
Publishing and Table of Contents generation will fail when encountering more than 2500 rows in the system generated Table of Contents. The user-facing exception CSV can be downloaded via the Publishing Status icon.
Validation Results Archival Job Update
The Validation Results Archival Job will create an archive package ZIP file 90 days after the Actual Submission Date. Previously, this package was created 365 days after the Submission Date. This change will reduce unnecessary records in customer Vaults, while still providing easy access to Validation Results from previous submissions. The Validation Results Archival job will be made Active on all Vaults with the 25R1 release.
Update Best Practice Validation Rules Logic for Filenames
The following regional rules’ Validation Criteria Rule IDs have been updated to more accurately validate acceptable filenames across regions:
- CH: RuleCH15.BP3 (15.BP3)
- EU: Rule15.BP3 (15.BP3)
- GCC: RuleGCC13 (13)
- JO: RuleJO13 (13)
- TH: RuleTH15.BP3 (15.BP3)
- TW: RuleTWO.BP2 (OBP2)
The rule logic changes are auto-on for the above mentioned Validation Criteria Rule IDs.
Update Health Canada Rules to Report Warning on Optional Sections
Two new Validation Criteria have been created to better support the Health Canada requirements for Validation Rules G.06 and G.08. The Validation Criteria will check the Index leafs for optional attributes, and if not present report as a Warning.
The existing Validation Rules G.06 and G.08 have been updated to only report on mandatory Leaf attributes.
This capability requires updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages. If no updates are made to customer Vaults only the new logic of Rule G.06 and G.08 will take effect.
NeeS Canada Validation
Customers publishing non-eCTD submissions (NeeS) for Health Canada can now validate the publishing output based on the HC NeeS v5.2 validation criteria.
This capability requires updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
All validation rules and data model updates to support HC NeeS v5.2 can be provided to customer support teams via VPK packages.
Publishing EU eCTD v3.1
With this release Vault Submission Publishing supports publishing and validating EU dossiers based on EMA eCTD guidance v3.1 and validation v8.0.
This capability requires updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
Publishing WHO eCTD Version 1.1
With this release Vault Submission Publishing supports publishing and validating WHO dossiers based on WHO eCTD guidance v1.1 and validation v1.1.
This capability requires updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
NeeS EAEU Publishing Support
With this release Vault Submission Publishing supports publishing EAEU Non-eCTD based on the v.1.1.0 of the EAEU R0.22 specification. See the RIM Data Model updates for additional object and field creation to support EAEU Publishing.
This capability requires updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
RIM Publishing, RIM Submissions
Update Table of Contents Page Numbers When Merging
Table of Contents generation will now respect the order of the Table of Contents Content Plan Item within the Content Plan structure when generating Table of Contents for merge scenarios.
If a Table of Contents is placed at the top, in the middle, or at the bottom of a Content Plan section with Content Plan Items that will be merged, the overall page numbering of the Table of Contents will be accurately reflected on the top level Content Plan section row of the Table of Contents. Previously, the overall page numbering did not always appear correctly when the Table of Contents was not the first Content Plan Item included in the merge.
Regulatory Data Model Changes
See 24R3 Regulatory Data Model Changes.
Safety
The Safety 24R3 release, including all Platform features, is scheduled for tentative availability on November 27, 2024 & December 13, 2024. For the latest central dictionary updates for your Safety Vault, see Safety Central Dictionary Updates.
Several features listed in the Vault Connections section also affect the Safety application family.
Safety
Sender-Based Email Inbox Item Pre-Population
With this release, Vault Safety can now automatically set the Country and Report Type fields on an Inbox Item based on the sender’s email. This feature allows Admins to pre-populate these fields for Transmission Profiles, reducing redundant data entry. Vault Safety creates Inbox Items with these mapped values, helping users triage and assign them more efficiently. This improvement accelerates the triage of inbound emails and eliminates repetitive data entry.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable Sender-Based Email Attachment Handling
- User Help: Email to Inbox Item
Inbox Item Fields: Report Type & Seriousness
In this release, Report Type and Seriousness fields are added to the Inbox Item object to support filtering and prioritization. This data is often included when Vault generates Inbox Items from structured data sourced from E2B or JSON integrations or Vault to Vault Connections (such as the EDC-Safety Connection). Report Type (Clinical versus Post-Marketing) and Seriousness impact reporting timelines. Priority and Seriousness are calculated when users add new information to an Inbox Item and can now drive the priorities of triaging Inbox Items to ensure compliance.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable Inbox Items
- User Help: Inbox Item Overview
Duplicate E2B XML File Detection
With this release, Vault Safety can detect E2B XML files already imported into Inbox Items. Admins can enable this setting on Transmission Profiles, and users can apply it to manually uploaded files. When a duplicate E2B XML file is detected, Vault Safety marks the Inbox Item as Duplicate and links the source document to the original. Additionally, Vault Safety retains the original file name of the ZIP file’s individual E2B XML files, enhancing traceability. This enhancement automates duplicate E2B XML file detection when importing multi-E2B files from health authorities such as MHRA and EMA.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable E2B Import to Inbox Item
- User Help: Import an Inbox Item
Duplicate Inbox Item Linking
When an Inbox Item is marked as a duplicate after performing duplicate detection, Vault links the matching Inbox Item record on the duplicate Inbox Item in the new Linked Inbox Item field. This feature makes it easy to identify and track duplicate Inbox Items that are associated with an existing Inbox Item.
Learn More
- User Help:
Import Online Questionnaire Responses to Follow-Up Inbox Items
With this release, when creating an Inbox Item after online questionnaire completion, Vault Safety imports the online questionnaire responses into the Inbox Item. Vault Safety provisions standard question field mappings that Admins can apply to multiple questionnaire designs, speeding up configuration for pre-population and importing of responses. Users can review these responses directly on the Inbox Item or on the Inbox Item to Case Compare page. This enhancement simplifies the follow-up process and minimizes manual data entry, improving efficiency when handling online questionnaire responses.
Learn More
- Enablement: Enable Import Online Questionnaire Responses to Follow-Up Inbox Items
- Admin Help: Set Up Follow-Up Online Questionnaires: Create Question Field Mappings
- User Help: Send a Follow-Up Online Questionnaire to Case Contacts: Post-Questionnaire Completion Steps
Case Access Group Security: Study Type Update for Inbox Items
When an Inbox Item does not specify a Study but does reference a Study Type, Vault now uses the Study Type as part of the criteria for assigning a Case Access Group. This enhancement supports such scenarios as postmarket studies for a partner’s study that is not set up in your Vault’s Study library. By considering the Study Type for both Inbox Items and Cases, Vault provides more consistent Case Access Group assignments.
Learn More
Case Access Groups on CDMS Subject Information
With this release, Vault Safety now supports the ability to restrict access to EDC subject information object data received from the Safety-EDC Vault Connection based on Case Access Groups. Previously, users had access to subject information across all access groups. This feature significantly improves security by providing more granular control over personally identifiable information (PII), thereby enhancing compliance.
Learn more about configuring the Safety-EDC Connection.
Duplicate Detection During Case Processing
With this release, users can perform duplicate detection during Case processing to compare the current Case record against other Cases and Inbox Items. Case Processors can identify duplicates after data entry. This enhancement reduces manual duplicate detection effort and mitigates overreporting.
Learn More
- Enablement: Enable Duplicate Detection During Case Processing
- User Help: Duplicate Detection for Cases
Latest Study Registration Data on Follow-Up Cases
Vault Safety now snapshots the latest Study Registrations from the Study library to Follow-up Case versions. The Case Study Registrations section automatically reflects the updated information. Previously, when using the Create Follow-up action, Case Follow-up records did not include Study Registration records and details that were added after the initial Case creation. This feature enhances compliance and ensures that Case Processors have the latest data to make decisions.
Learn More
- User Help:
Follow-Ups to Domestic Cases from Global Inbox Items
With this release, Vault Safety allows users to create Follow-ups to a Domestic Case from Global Inbox Items. Now, for Inbox Items with a Global type or blank localization, after selecting a matching Domestic Case on the Inbox Item, users can create a Follow-up Case to the Domestic Case on the Inbox Item to Case Compare page. This enhancement supports users in efficiently processing follow-ups received in English for Domestic Cases.
Learn More
- User Help: Inbox Item Follow-Up: Localized Data
Update Localized Case Narrative Language Based on Follow-Up Information
With this release, when merging an Inbox Item with an updated Localization value to an existing domestic Case, Vault Safety now updates the ISO language of the Narrative document to the new value. Previously, when Localization was updated in a follow-up, Narrative documents remained linked to the Localization of the initial Case. This enhancement ensures accuracy and consistency in case documentation.
Learn More
- User Help: Inbox Item Follow-Up: Merge Behavior
Localized Cases: Global Narrative Override on Follow-Ups
With this release, Admins can configure an action on Localized Cases that overrides existing Localized Narratives based on related global Case Narratives. After running the Sync Global Narrative to Local Narrative action, Vault up-versions the Localized Narrative document and then replaces any existing content with content from the global Narrative document. This action removes the manual work of copying content from global to localized narratives for follow-ups. This time-saving feature streamlines case-processing workflows and improves localized submission compliance.
Learn More
- Enablement: Enable Localized Cases: Global Narrative Override on Follow-ups
- User Help: Prepare a Localized Case
Localized Cases: Domestic Narrative Override on Follow-Ups
This feature extends the functionality in Localized Cases: Global Narrative Override on Follow-Ups to domestic Case Narratives. With the Sync Global Narrative to Local Narrative action on Localized Cases, users can also override existing Localized Narratives based on related domestic Case Narratives. After running the action, Vault up-versions the Localized Narrative document and then replaces any existing content with content from the domestic Narrative document. This action removes the manual work of copying content from domestic to localized narratives for follow-ups. This time-saving feature streamlines case-processing workflows and improves localized submission compliance.
This feature is Auto-on in Vaults with Localized Cases: Global Narrative Override on Follow-ups enabled.
Learn More
- User Help: Prepare a Localized Case
Global Field Sync to Foreign Localized Case Follow-Ups
Vault Safety now includes an option to overwrite all text fields on Localized Cases with the latest text from the related global Case when a Transmission is created for a foreign follow-up Localized Case. Previously, Vault copied global text field values only to blank localized text fields on Localized Follow-up Cases.
Learn More
- User Help: Prepare a Localized Case
Case Version Compare IDs
With this release, Vault Safety introduces an ID to identify Case children records, such as Case Adverse Event and Case Product, across Case versions. This feature enhances matching precision during Case Version Compare.
Learn More
- User Help: Case Version Compare
Case Copy with Linking
Vault Safety now supports the creation of up to 100 copies of a Case. Case copies include all parent and child records, along with custom fields from the source Case. These copies maintain links to the original Case documents, and audit logs clearly indicate when Cases were created by copying. On the source Case, Vault displays the number of copies made. This feature will significantly boost case processing efficiency, particularly for scenarios such as literature and legal cases, as well as other customer-specific needs.
Learn More
- Enablement: Enable Case Copy with Linking
- User Help: Copy a Case
Bulk Unblind Studies with Isolate Blinded Product Information
With this release, Vault Safety supports bulk unblinding clinical trial Cases for Studies without Study Arms in Vaults with the Isolate Blinded Product Information feature configured. When a study concludes, users can unblind associated Cases efficiently. This feature removes blind protection from previously unblinded Cases and populates Study Product information for blinded Cases while leaving in-flight Cases unmodified. This enhancement streamlines the unblinding process at the end of a study.
Learn More
- Enablement: Enable Bulk Unblind Studies with Isolate Blinded Product Information
- Admin Help: Bulk Unblind Isolated Blinded Product Information
Datasheet Expectedness for Blinded Study Products
With this release, Vault Safety can generate Expectedness records for blinded Study Products on blinded Study Cases. This feature enables Admins to associate Datasheets with Study Product Placeholders when configuring double-blinded Studies without Study Arms. Automatically generating Expectedness records allows Medical Reviewers to access all relevant values from associated Datasheets, supporting comprehensive assessments during blinded Case reviews.
Learn More
- Enablement: Enable Datasheet Expectedness for Blinded Study Products
- Admin Help: Manage Datasheets & Auto-Expectedness
- User Help: Prepare a Localized Case
Copy Datasheet Expectedness Justification to Case Assessments
With this release, Vault Safety can copy rationales for adverse event expectedness from Datasheets to Case Assessments. When you populate the new Expectedness Justification field on Datasheets, Vault maps the text to Case Assessment Expectedness and Localized Case Assessments. Previously, searching Datasheets for rationales was time-consuming. Mapping the rationale instead makes it easier to enter the correct value to be exported to the J2.11 Other References data element of PMDA E2B(R3) reports. This enhancement meets PMDA regulatory requirements and increases the efficiency of case assessments. This feature can also be used to populate Expectedness Justification values on global Case Assessment Expectedness records.
Learn More
- Enablement: Enable Copy Datasheet Expectedness Justification to Case Assessments
- Admin Help: Manage Datasheets & Auto-Expectedness
- User Help: How Case Assessment Expectedness is Generated
Conditional Age by Decade Expectedness Evaluation Update
In Vaults with conditional expectedness by age configured, when evaluating expectedness based on a patient age entered in decades, Vault now evaluates expectedness considering both the upper and lower bounds of decade ranges. For example, if the patient’s age is entered using 2 in the Number field and Decade in the Unit field, indicating they are between 10–20 years old, and the adverse event on the Datasheet is expected only for patients with an age range of 2–12 years old, Vault now considers this adverse event unexpected since the upper bound of the decade is outside the age range.
Learn More
- Admin Help: Manage Datasheets and Auto-Expectedness
- User Help: How Case Assessment Expectedness is Generated
MedDRA Bulk Recode Without Case Upversioning
With this release, when performing MedDRA Bulk Recode, Vault Safety now recodes non-current terms with current MedDRA terms as defined by your organization directly on the latest Case version, and optionally on all Case versions. Previously, Vault Safety created minor versions and reopened Cases, which users would need to close. This feature streamlines the MedDRA Bulk Recode process.
This feature is Auto-on, but you can configure your Vault to bulk recode all Cases rather than latest Cases.
Learn More
- Enablement: Enable MedDRA Bulk Recode Cases
- Admin Help: Bulk Recode MedDRA-Coded Terms on Cases
Multi-Product Selector for Study Products on Cases
To facilitate Study Product selection after Case promotion, the Specify Study Products action is now available on Cases, extending the feature released for Inbox Items in 23R2. Case Processors can now add multiple Study Products to a Case at once. This enhancement ensures consistency in Study Product entry across Inbox Items and Cases, improving case processing efficiency, especially for complex studies involving numerous phases or products.
Learn More
- Enablement: Enable Multi-Product Selector for Study Products on Cases
- User Help: Code and Browse Study Products
Automatically Set MedDRA Code When Product Indication is NullFlavor
With this release, Vault Safety automatically sets the Name (MedDRA) field to Drug use for unknown indication (10057097) when users select a nullFlavor for the Reason Omitted field on Product Indication. This enhancement ensures compliance with the business rule related to G.k.7.r.2b in the ICH E2B(R3) specifications, streamlining data entry and improving the accuracy of adverse event reporting.
Learn More
- User Help: Enter Case Data
Licensing Check for MedDRA
In this release, Vault Safety now includes validation of your company’s MedDRA credentials as a prerequisite to the MedDRA dictionary update process. When upgrading the MedDRA dictionary in your Vault, you can use the Validate Dictionary License action to verify your MedDRA license information. If the credentials are invalid, Vault displays an error message and you will not be able to proceed with the upgrade process. This enhancement ensures that your organization remains fully compliant with MSSO licensing requirements, preventing unauthorized updates and helping to maintain regulatory standards.
Learn More
- Enablement: Enable Licensing Check for MedDRA
- Admin Help: Manage the MedDRA Dictionary
JDrug and WHODrug Cross Reference Tool
In Vaults with WHODrug and JDrug dictionaries configured, Vault Safety provides WHODrug Cross Reference Tool license holders with automatic coding of WHODrug products when the corresponding JDrug External Product is coded, and vice versa. This feature eliminates time-consuming double-coding by directly matching IDF and WHODrug codes and supports more accurate coding.
Note: The Iyakuhinmei Data File (IDF) may also be referred to as Japan Drug Dictionary (JDD) or JDrug.
Learn More
- Enablement: Enable JDrug and WHODrug Cross Reference Tool
- Admin Help: Manage the WHODrug Cross Reference Tool (CRT) Japan
- User Help:
DME Watchlist Update: Immune thrombocytopenic purpura (10074667)
With this release, the term Immune thrombocytopenic purpura (10074667) on the DME list is updated to reflect the most recent term information from MedDRA, Immune thombocytopenia (10083842). This enhancement removes the deprecated version of the term in the DME list and ensures compliance with reporting requirements.
To utilize this feature, you must run the Upgrade to Latest action. You can run this action even when your Vault is already on the latest MedDRA version.
QC Checklist Updates
With this release, Vault Safety introduces several enhancements to Case Checklists. Admins can now configure a Checklist rules action in custom and non-standard states. Users can generate in-process Checklists as part of the Checklist generation workflow or using a record action. Admins can generate ad-hoc post-closure QC Checklists for testing purposes. Additionally, with this release, the quarterly and yearly intervals for post-closure QC Checklist generation are deprecated. This feature significantly improves flexibility and user control during the QC Checklist generation process.
Learn More
- Enablement: Enable QC Checklist Generation
- Admin Help: Configure QC Checklist Creation Rules: Create a Checklist Creation Rule
- User Help: Generate a QC Checklist: Generate QC Checklists
Improved FDA One Last Time Reporting
With this release, Vault Safety supports a more robust evaluation of One Last Time reporting to the United States Food and Drug Administration (FDA) to avoid situations where Transmission Profiles are switched between versions. Previously, if a Case contained an approved Biologic and an approved Drug product (both owned by your company), Vault generated duplicate Transmissions.
Learn More
- User Help: One Last Time Reporting
Configurable PII and E2B Masking for NullFlavor Fields
With this release, Vault Safety expands the Exceptions to PII Masking Safety rule set parameter to include nullFlavors. When users generating masked reports select Null Flavors in the Exception to PII Masking Content Protection field, Vault does not mask nullFlavor values for Personal Identifiable Information (PII) fields in generated reports. Instead, Vault populates the nullFlavor values from the Case on reports. Previously, Vault exported “MSK/Privacy” in place of nullFlavors on masked reports. This feature addresses compliance and clarity because masking a field that contains no information can imply that there is information in that field.
Learn More
- Enablement: Enable Configurable PII and E2B Masking for NullFlavor Fields
- User Help:
Case-Level Reportability Indicator
To help streamline Case workload prioritization, Vault Safety introduces Case Reportability classification with this release. Admins can now add the new Case Reportability field to the Case page. This field displays Health Authority, Business Partner, Health Authority & Business Partner, or Not Reportable, depending on the outcome of the most recent rule engine run for the Case, allowing users to easily see the Case’s reportability status. This streamlines prioritization by indicating whether the Case is not reportable, or is reportable to at least one Health Authority or Business Partner.
Learn More
- Enablement: Enable Case-Level Reportability Indicator
- User Help: Enter Case Data: Case Reportability
Country Specific IMP ICSR Reporting
Vault now supports enhanced selection of Investigational Medicinal Products (IMPs) on a country-specific basis. When an investigational product is used exclusively in a particular study country, Vault can now evaluate reportability on a Product-Country basis. This feature introduces a new Study Product Country object and a new Study Product Role reporting rule parameter, which determine country-specific evaluations for Study Products. Previously, Vault considered any Study Product within a Case as reportable across all jurisdictions where the Study was registered. This enhancement addresses the issue of over-reporting to health authorities and ensures regulatory compliance.
Learn More
- Enablement: Enable Country Specific IMP ICSR Reporting
- Admin Help: Manage Studies: Studies with Country-Specific Investigational Medicinal Products
- User Help: Reporting Rule Parameter Reference: Study Product Role
FDA MedWatch 3500A: August 2024 Version
With this release, Vault Safety supports the generation of the latest version of the 3500A MedWatch form, published by the FDA in August 2024. The new version is available for preview, submission, and distribution of ICSRs in Vault Safety for drug, biologic, medical device, and clinical trial cases.
Learn More
- Enablement: Enable FDA MedWatch 3500A: August 2024 Version
- User Help: FDA MedWatch 3500A (August 2024 Version) Generation Data Mapping
EU Convention E2B(R2)
With this release, Vault Safety supports the generation, submission, and distribution of EU Convention E2B(R2) individual case safety reports. This feature enables users to include regional elements required for agencies such as Swissmedic and the Therapeutic Goods Administration (TGA) of Australia, facilitating international submissions. By standardizing this functionality, Vault Safety eliminates the need for custom SDK implementations, streamlining the submission process for global agencies and partners.
Generating EU Convention E2B(R2) format reports is Auto-on. The document type is automatically available in the Document Types picklist during Transmission generation. To configure your Vault for transmitting electronic submissions to a regulatory agency, you must set up a custom Transmission Profile.
Learn More
E2B Readable Renditions
With this release, Vault Safety can generate E2B(R2) and (R3) reports in a readable format with clear language and structure available for download in PDF format. This enhancement streamlines and improves the review process of E2B files during submissions, distributions, inspections, audits, and other routine quality checks.
Learn More
- Enablement: Enable E2B Readable Renditions
- User Help: Generate E2B Readable Renditions
MFDS Regional E2B Fields in E2B Readable Renditions
With this release, E2B Readable Renditions support regional MFDS E2B data elements. This enhancement streamlines and improves the review process of MFDS E2B(R3) files during submissions, distributions, inspections, audits, and other routine quality checks.
This feature is Auto-on in Vaults with E2B Readable Renditions enabled.
Learn More
- User Help: Generate E2B Readable Renditions
MFDS E2B(R3)
With this release, Vault Safety supports generating and submitting individual case safety reports in the MFDS E2B(R3) format. To support transmissions to the Republic of Korea’s Ministry of Food and Drug Safety (MFDS) in MFDS E2B(R3) format, this feature also includes the new MFDS Transmission Profile.
Learn More
- Enablement: Enable MFDS E2B(R3)
- Admin Help: Standard Agency ICSR Submissions
- User Help: E2B Generation: MFDS E2B(R3) Mapping
Select Registrations on Submissions
With this release, Vault Safety enhances support for aggregate reports by including the appropriate product registration details used to generate the ICSR Submission. This enhancement allows users to track the registration used when generating ICSR Submission and allows for more accurate aggregate reporting.
When generating a general report to the FDA, Vault selects the reportable Product from the passing Case Product or combination Product and selects the reportable Study from the Study Registration associated with the Study Product. For non-study Cases, Vault prioritizes the earliest registration date and the lowest registration number, with fallback logic applied based on the Product. For study Cases, Vault prioritizes the lowest registration number, with fallback logic based on the earliest created registration. This ensures Vault identifies the most relevant Product or Study for reporting purposes.
Although this feature is Auto-on, some components may require configuration.
Learn More
- Enablement: Enable Select Registrations on Submissions
- User Help: Understand the Reporting Rules Engine
PMDA Post-Market Reports: Filter by Local Awareness Date
With this release, Vault Safety supports filtering Cases on PMDA Post-Market aggregate reports by Local Awareness Date. Previously, users could filter Cases by Case Receipt Date / New Info Date or Case Approval Date only. The additional filtering option is useful when generating reports for localized Cases because the date local affiliates become aware of Cases may differ from the receipt or new information date on the global Case. This enhancement is available on all PMDA Post-Market tabulations.
Learn More
Additional Filters for CIOMS II Aggregate Report
With this release, the CIOMS II report provides enhanced flexibility to generate a wider variety of reports with the results presented in the standard CIOMS II line listing format. The following optional filters are now available:
- Report Type
- Case Seriousness
- Case Relatedness
- Case Expectedness
- Expectedness Source
- Country inclusion and exclusion
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable CIOMS II Line Listings
- User Help: Create CIOMS II Reports
Gateway Support for Multiple Transmission Profiles
To enhance support for sponsors, Vault Gateway and AS2 Connection now allow inbound transmissions for gateways with multiple transmission profile assignments.
AS2 Connection: Restore AS2 Connections
Vault Safety now allows the AS2 Connections of a Vault to be restored after the Vault has been refreshed. This makes it easier to start AS2 Connections earlier in the development cycle without having to reconfigure them once the Vault is refreshed. This feature introduces a new Admin action, Refresh AS2 Connections to streamline sandbox refreshes and management of AS2 Connections.
Learn More
- Admin Help: Restore AS2 Connections
AS2 Connection: Transfer AS2 Connections (Environment Management)
With this release, Admins can transfer AS2 Connections between Vaults that are owned by the same customer and that are in the same deployment region and on the same release type (LR to Limited Release to Limited Release, or General Release to General Release). Once the required AS2 Connections have been created and tested in one Vault, Admins can easily migrate them, including their entire connection details (AS2 settings, URLs, Allowed IP list, and certificates) to the new Vault environment by using the new Transfer AS2 Connections user action. This feature eases implementation effort and reduces potential AS2 Connection misconfigurations when switching between Vaults.
Learn More
- Admin Help: Transfer AS2 Connections
Auto-Generation of Product Family Product Records
Vault Safety automatically generates Product Family Product records when users create new Product records. This increases efficiency.
Vault Expressions: Custom Safety Rule Parameters
With this release, Vault Safety extends the Vault Expression Engine to the Reporting Rules Engine. Admins can now configure custom Reporting Rule Parameters for evaluating Case data using expressions. This enhancement provides greater flexibility in reportability decisions, allowing for tailored evaluation criteria without the need for custom SDK development.
Learn More
- User Help:
Safety Standardization 24R3
With this release, Vault Safety adds several standard components to ensure customers can easily adopt best practices for major business processes. Additional Standardization capabilities will be available in upcoming releases.
Safety Workbench
New Application: Vault Safety Workbench
The Safety 24R3 release introduces the Vault Safety Workbench application as part of the Veeva Vault Safety Suite. To use this application, a Vault Safety Workbench and a Vault Safety license are required. For information on installing and configuring Vault Safety Workbench for your Vault, contact your Veeva Representative.
Vault Safety Workbench can analyze high volumes of Vault Safety data for ad-hoc and periodic reporting. This application also allows more flexible and configurable reporting depending on your organization’s business processes. See the Vault Safety Workbench Overview to learn more.
Note: Vault Safety Workbench is available for Early Adopters only. To learn more, contact your Veeva Representative.
Safety Signal
New Application: Vault Safety Signal
The Safety 24R3 release introduces the Vault Safety Signal application as part of the Veeva Vault Safety Suite. To use this application, a Vault Safety Signal and a Vault Safety license are required. For information on installing and configuring Vault Safety Signal for your Vault, contact your Veeva Representative.
Vault Safety Signal uses data from your existing Vault Safety database to detect new, unexpected adverse events or changes in existing adverse events to a medicinal product. The detection can be applied to post-marketing or clinical trial Cases. See the Vault Safety Signal Overview to learn more.
Note: Vault Safety Signal is available for Early Adopters only. To learn more, contact your Veeva Representative.
Learn More
- Enablement: Enable Safety Signal
SafetyDocs
Update Notification Template for PSMF Periodic Review
The notification template for the PSMF Periodic Review workflow provides more information to recipients about what task they have been assigned. This improvement provides the user with additional context to boost productivity.
Learn More
- Enablement: Enable PSMF Periodic Reviews
- Admin Help: Configure the PSMF Periodic Review Job
- User Help: Set up PSMF Periodic Reviews
Create Inbox Items from Literature Articles in CRO Vaults
To better support Contract Research Organization (CRO) customers who perform literature reviews for different sponsors, Vault SafetyDocs has added an Organization field to the Literature Article and Literature Review objects. When creating an Inbox Item from a Literature Article, Vault considers the Organization field. If the field is populated on the Literature Article, Vault sets the Organization field on the Inbox Item to the same Organization value as the Literature Article it was created from. If the field is not populated on the Literature Article, then there is no change to the current behavior. These additional fields allow CRO customers with multi-sponsor Vaults to specify a separate sponsor/organization for each review and article.
Learn More
- Enablement: Enable Create Inbox Items from Literature Articles in CRO Vaults
- User Help:
Literature Coding to Product Family
To support literature searches completed at the Product Family level, Vault SafetyDocs now supports coding Literature Articles to Product Families, Products, or both. The Product Family field has been added to the Search Term, Literature Standard Search Term, Literature Review, and Literature Article objects. By eliminating the need to select a specific Product, this feature allows for more precise coding of Literature Articles and broader signal detection.
Learn More
- Enablement: Enable Literature Coding to Product Family
- Admin Help: Create Literature Standard Search Terms
- User Help: Manage the Literature Review Process
Literature Multi-Product Support
Vault SafetyDocs now supports independent verdicts for each product in a literature article. Literature articles may contain multiple company products. Searching for each product in an article and identifying possible interactions between drugs is critical to ICSR product evaluation and signal analysis. To support this, Vault SafetyDocs has introduced the Literature Article Product object. When users set a Product on a Literature Article record, Vault creates a Literature Article Product record. Additionally, Vault SafetyDocs creates Inbox Items for each Literature Article Product record with a verdict. This better supports scenarios that include multiple products, leading to enhanced compliance.
Learn More
- Enablement: Enable Literature Multi-Product Support
- User Help: Manage the Literature Review Process
Customize Sender Email Address for PVA Document Distribution
Vault SafetyDocs can be configured to send Vault emails using the customer’s email domain. Prior to this, emails sent from Vault used the domain “@veevavault.com”. In scenarios such as Pharmacovigilance Agreement (PVA) distribution where Vault sends emails to external partners, the emails may be considered suspect (and go to the spam folder) if they do not come from the customer’s email domain. The ability to configure the “From” domain addresses this challenge.
Learn More
- Enablement: Enable Customize Sender Email Address for PVA Document Distribution
- User Help:
Pharmacovigilance Agreements Usability Improvements
With this release, Vault SafetyDocs displays meaningful values when a PVA object is referenced in an object field rather than the auto-generated name. For example, PVA Contact references now display the user’s name and PVA Obligation references now display the Clause of Title field value. This improves the usability of PVA-related records.
Learn More
- Enablement: Enable PVA Management
Risk Management Automation
The Vault SafetyDocs Risk Management functionality now automates the creation of local Risk Management Plans (RMPs), supports versioning and localization of Risk Management Measures (RMMs), and manages the effective versions of RMPs and RMMs. Vault can generate local RMPs and RMMs automatically, based on the global/core RMP and RMM respectively, in all markets where the Product is registered. New actions have been introduced that set the version number and the Lifecycle state to Effective. Risk Management automation enhances user efficiency in this process.
Learn More
- Enablement: Enable Risk Management
- Admin Help: Configure Countries for Local Risk Management Plans
- User Help: Track Risk Management Plans
Custom Translation Connections for Literature Abstracts
Vault now supports Literature Abstract translation through custom Translation Connections. Previously, Vault only supported Amazon Translate. Using custom Translation Connections you can connect SafetyDocs to any external translation service to meet your organizational needs.
Learn More
- User Help: Translate Literature Abstracts
Capture Additional Information for Safety Investigations
With this release, users can capture more detailed information on Safety Investigations. Users can now enter a description, track a due date, and document validation and disposition rationales for a Safety Investigation.
Learn More
Add MedDRA Terms and Queries for Safety Investigation
With this release, users can now add MedDRA Terms and MedDRA Queries directly to Safety Investigations. Using the MedDRA browser, users can select multiple MedDRA Terms at any hierarchical level and select MedDRA Queries. This enhancement allows for a more detailed and consistent representation of the events under investigation, which better aligns with health authority requests and improves the clarity of Safety Investigations.
Learn More
- Enablement: Enable Adding MedDRA Terms and Queries for Safety Investigations
- User Help: Signal Management: Add a MedDRA Term or MedDRA Query to a Safety Investigation
Open Safety Investigations from Literature Articles
With this release, users can open Safety Investigations directly from Literature Articles. Users can select the Open Investigation action to create a Safety Investigation and associated Literature Data records. This feature enhances the signal management process by efficiently creating Safety Investigations for Literature Article Products resulting in the Investigation Review Outcome of New Safety Information.
Learn More
- User Help:
Product-Event Disposition Updates
With this release, the Product-Event Disposition label is updated to PEC Period to better align with industry terminology. Vault SafetyDocs enhances uniqueness rules for PEC Period records. For a PEC Period record, the Reporting Period field must be unique to this record. Additionally, when auto-naming PEC Period records, Vault uses a random number instead of the Detection Date for better record differentiation. These changes align with industry standards and enhance accuracy during signal detection and reporting processes.
SafetyDocs Standardization 24R3
With this release, Vault SafetyDocs adds a number of standard components to ensure customers can easily adopt best practices for major business processes. Additional Standardization capabilities will be available in upcoming releases.
Safety & SafetyDocs
E2B Generation: Literature Retransmission
To support reporting on cases created from literature articles in SafetyDocs, when generating E2B files, Vault Safety can now retransmit literature documents that are attached to Literature Article records. This feature eliminates the need to copy and reclassify documents manually after Inbox Item promotion. Flexible retransmission of literature documents in E2B transmissions enhances compliance and usability.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable Literature E2B Retransmission
- User Help:
Safety Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard Safety checkbox fields. When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: Safety for a list of impacted fields.
Safety Data Model Changes
See 24R3 Safety Data Model Changes.
QualityOne
In addition to the below release notes, the QualityOne Veeva Connect community offers general release communications, release highlights, and key feature demos.
QualityOne
Teams Enhancement: Inherit Team from Multiple Source Objects
With this release, QualityOne Teams now supports inheriting role memberships for Team Roles in a single Team from multiple parent objects’ Teams. In prior releases, in shared-parentage scenarios where a subprocess may be related to multiple parent processes (like an action item, for example) the subprocess may have needed one Team definition per object type to account for each type of parent process.
For example, in shared-parentage scenarios like Extension Requests, which may be derived from CAPAs, Change Controls, or Audits, a single Extension Request Team definition can now be used for all types of Extension Requests, intelligently inheriting its role memberships from the appropriate parent process record, with no interaction from the user during day-to-day activities.
This feature reduces the complexity of Teams configurations by allowing for reusability across different Team-enabled objects.
Learn more about inheriting Team role memberships.
Teams Enhancement: Global Limit Increase
This feature introduces enhancements to the performance and scalability of QualityOne Teams, increasing several previously enforced global limits. Admins will no longer need to contact their Veeva Representative regarding configurations approaching these published values.
Teams configurations can now globally support:
- Up to 100 teams per Vault
- Up to 10 roles per Team
- Up to 20 team members per Team Role
Learn more about configuring Teams and working with Teams.
Teams Enhancement: Manage Team Assignments
This developer feature supports the addition and removal of team members from a Team Role in batches. This feature additionally adds a notification template to the Vault UI; Vault sends the notification to inform the user making these changes if the additions and removals were successful or not.
Learn more about the developer-facing functionality of this feature.
Learn more about working with Teams.
Related Record Automation Enhancement for Event Actions
This feature enhances the Create Related Record action to improve its performance as an event action. The Create Related Record action now runs synchronously when configured as an event action, enabling at-scale record creations to be more robust and performant.
Learn more about configuring the Create Related Record action.
QualityOne Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard QualityOne checkbox fields. When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: QualityOne for a list of impacted fields.
QMS (QualityOne)
Generate Documents for Additional QMS Processes
This feature extends the previously introduced Generate Documents using Object Record Actions for Audits and Generate Documents for Standard QMS Processes features by adding the following objects:
- Lab Investigation
- Continuous Improvement Initiatives
- Inspection (to generate COA)
This feature allows users to generate a document from a Formatted Output or a Document Template using an Admin-configured entry or user action. By generating documents, users can leverage Vault’s full Document Management functionalities, removing dependency on manually configuring and uploading documents to the Vault Library. Generating documents using a 1-click solution also automatically generates a link between the object record and the document in the Vault Library, ensuring users retain the context of the generated document.
Learn more about generating documents and configuring Document Generation.
Periodic Reviews: APQRs for Internal Facilities
This feature enhances the end-to-end functionality of the Periodic Review feature by enabling users to conduct Annual Product Quality Reviews (APQRs) for internal facilities. Prior to this release, users were only able to conduct APQRs for supplier organizations.
Learn more about Periodic Reviews and performing APQR.
Inspection Sample Test Result Moving to Raw Object
This feature improves the scalability and performance of the Inspection Sample Test Result object by migrating it from a standard Vault object (SVO) to a raw object. Contact your Veeva Representative for more information about this change.
Learn more about setting up Inspections and defining Object Application Security.
COA Inspections: Improved COA Variant Matching
This feature introduces an improved matching strategy to COA Matching Rules when extracting data from the header section of a COA file, increasing the likelihood of finding a match for fields associated with key data in COA files.
Learn more about COA Matching Rules and working with COA Inspections.
Note: The COA Inspections feature is currently available only to Early Adopters. Contact your Veeva Representative for more information about this add-on feature.
Reuse CCPs & OPRPs within the Same HACCP Plan
This feature allows users to more efficiently manage critical controls applied across multiple hazard analyses within the same HACCP plan. Previously, users had to create separate CCPs and OPRPs to add to each Process Hazard Analysis in a HACCP Plan. With this release, users can add CCPs and OPRPs previously defined in another Process Hazard Analysis to additional Process Hazard Analyses in the same HACCP Plan, saving time and improving the consistency of hazard control practices.
Learn more about performing hazard analysis.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Generate HACCP Flow Diagram & Data Documents
This feature allows users to generate documents from a HACCP Plan record by running an action from the HACCP Flow Diagram. When users run the action, Vault generates a PDF rendition of the HACCP Flow Diagram and another document containing data from the associated HACCP Plan, and stores both of these documents in a binder in the Library. Admins can configure different document templates to extract specific field data from HACCP Plans, and leverage Vault’s Document Management functionality to manage generated HACCP documents.
Learn more about generating HACCP documents.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Hazard Analysis Completeness Check Improvement
This feature builds on the Hazard Analysis Completeness Check functionality introduced in 24R2, offering the following improvements:
- The hazard analysis completeness status now displays on Process Steps through a single click of a toggle icon, without the need to refresh the HACCP Flow Diagram.
- The hazard analysis completeness status now displays on individual Process Hazard Analysis records in the HACCP Flow Diagram’s Information panel.
- Customers can configure Vault to automatically check and update the hazard analysis completeness status for each Process Step each time a user edits an element on the HACCP Flow Diagram. Automating this process enhances efficiency, accuracy, consistency, and transparency in risk management practices and ensures thorough and timely evaluations of food safety hazards.
Learn more about assessing hazard analysis completeness.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Adjust Connection Line Position
With this release, users can now adjust the position of Connection lines between Process Steps on the HACCP Flow Diagram.
Previously, users relied on Vault’s auto-positioning functionality to determine the position of a Connection’s line on the HACCP Flow Diagram and could not adjust it if needed. Now, users can reposition all line segments of a Connection.
This feature offers enhanced visual clarity of process flow layouts and more granular control over the structure and organization of information on the HACCP Flow Diagram, as well as increased flexibility to accommodate process changes or reflect alternative workflows without the need to redesign the HACCP Flow Diagram.
Learn more about working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: PRP & Control Measure Enhancement
This feature enables users to view a brief summary of PRPs and Control Measures listed in the Hazard Analysis section of the Information panel, and then expand each PRP and Control Measure to see the full details. Previously, the Information panel displayed only the name of the reference record for the PRP or Control Measure. This enhancement provides users with context for each PRP and Control Measure associated with a Process Step or Process Step Group on the HACCP Flow Diagram without the need to navigate to the individual records, improving the efficiency and accuracy of hazard analysis.
Learn more about performing hazard analysis.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Comparison IDs for HACCP Plans
This feature introduces data model updates to the HACCP Plan object and its related objects to provide improved traceability between factory HACCP Plans and their originating HACCP Plan Designs. The new Comparison ID field contains the unique system ID of the original HACCP Plan from which each factory plan is created, and can be used to implement comparison and traceability functionality.
Learn more about Comparison IDs.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Atomic Security Standardization for Secured Relationships
This feature enhances the HACCP Flow Diagram UI to reflect atomic security user permissions for secured relationships, providing a consistent user experience across Vault. Previously, when users did not have Edit permission for a secured relationship between two objects, they were offered user actions that they were not permitted to take, and received an error as a result. With this release, the HACCP Flow Diagram UI no longer displays options to add records that rely on the secured relationship.
Learn more about working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Plan Translation
With this release, Vault now supports the process of creating translated HACCP Plans. This feature enables organizations to create copies of a predefined HACCP Plan Design and translate them for local manufacturing site use, eliminating the need for local sites to maintain their own translated versions of HACCP Plans that may not align with organizational best practices.
This feature introduces the following functionality:
- A new user action available on the HACCP Plan object supports the generation of a translation copy of a HACCP Plan that includes all standard related records in the HACCP Plan such as HACCP Plan Process Steps and Process Hazard Analyses, as well as any custom objects used in HACCP Plans.
- New user actions on the HACCP Plan object support the export of translatable HACCP Plan field data from the translation copy of a HACCP Plan and the subsequent import of translated data. Upon import, Vault updates the HACCP Plan to display the translated data.
- Admins can configure Vault to display translated field data in Details and related object sections on object records and on the HACCP Flow Diagram.
- Organizations can define translated reference data for both custom and standard reference objects to reuse when creating HACCP Plans.
- When creating records for a HACCP Plan, translated reference records are available for selection depending on the user’s language settings. If VQL criteria is configured to constrain the reference records available to select, Admins can configure criteria to filter translated records.
This feature simplifies the translation process for HACCP Plans, helping to ensure consistency in food safety practices for geographically dispersed organizations.
Learn more about configuring HACCP Plan translation and translating HACCP Plans.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
QMS (QualityOne) & HSE
Auto-Create Proposed Audit Enhancements
This feature enhances the logic for duplicate Proposed Audit creation on the Create Proposed Audits action. With this release, duplicate Proposed Audit creation is limited to the Audit Program the action runs on, rather than all Audit Programs in a Vault.
Learn more about using the Create Proposed Audits action.
HSE
Generate Documents for Standard HSE Processes
This feature makes the Generate Documents using Object Record Actions for Audits and Generate Documents for Standard QMS Processes features previously introduced in QMS available in HSE for the following objects:
- Audit
- HSE Event
- Permit to Work
- Risk Register
- Risk Study
This feature allows users to generate a document from a Formatted Output or a Document Template using an Admin-configured entry or user action. By generating documents, users can leverage Vault’s full Document Management functionalities, removing dependency on manually configuring and uploading documents to the Vault Library. Generating documents using a 1-click solution also automatically generates a link between the object record and the document in the Vault Library, ensuring users retain the context of the generated document.
Learn more about generating documents and configuring Document Generation.
QualityOne Data Model Changes
See 24R3 QualityOne Data Model Changes.
RegulatoryOne
The RegulatoryOne 24R3 release, including all Platform features, is scheduled for tentative availability on December 17, 2024.
In addition to the below release notes, the RegulatoryOne Veeva Connect community offers general release communications, release highlights, and key feature demos.
RegulatoryOne
Application License Warning
This feature enhances the security and integrity of our application. This feature is aimed to enforce the use of a user’s application license to access different parts of the application. Vault now checks the validity of a user’s license before granting access to various application features and displays a warning if the user tries to access features not supported by their license. In future releases, users will not be able to access features not applicable to their licenses.
RegulatoryOne & Veeva Claims Data Model Standardization
This feature introduces data model updates to RegulatoryOne and Veeva Claims applications to support evolving needs to allow for harmonization between the applications so users can leverage Veeva’s evolving best practices.
RegulatoryOne Standard Checkbox Defaulting
With the release of the Yes/No Checkbox Field Enhancement Vault Platform feature, we’ve updated the default behavior for certain standard RegulatoryOne checkbox fields. When these checkbox fields are configured without a default value, Vault automatically sets the field to No (false) upon record creation. See 24R3 Data Model Changes: RegulatoryOne & Veeva Claims for a list of impacted fields.
Compliance Management
Formulation Questionnaires: Import Attachments
This feature allows users to upload and automatically classify Formulation Questionnaire attachments in bulk, helping users to quickly add supplier documents to Vault.
Learn more about importing attachments from Formulation Questionnaires.
Formulation Questionnaires: Questionnaire Name Tokens
This feature allows Admins to add the names of the Formulation Questionnaire and questionnaire type from which it was generated to the email sent to suppliers using custom tokens, making it easy for suppliers to distinguish between multiple questionnaire emails received for the same Formulation.
Learn more about configuring notifications for Formulation Questionnaires.
Registration & Dossier Management
Local Impact Assessment Enhancement: Override Assessment Results
This feature provides users with flexibility to override the matching Registration found by running the Local Impact Assessment action. Users can now search for and select a different Registration or create a new record for the Registration Item.
Learn more about selecting a Registration after running the Local Impact Assessment action.
Local Impact Assessment Enhancement: Multiple Registration Items in a Registration
This feature enhances the reuse of existing registration data by allowing users to run the Local Impact Assessment action regardless of the sequence of the actions they run. The action now identifies Registration Item records for the appropriate Registration and Registration Objective without having to first run the Generate Registration Data action.
Learn more about Local Impact Assessments.
Local Impact Assessment Enhancement: Ignore Cancelled & Withdrawn Objectives
This feature allows Admins to configure Cancelled and Withdrawn Registration Objectives so that the Local Impact Assessment action considers only valid Registration Objectives in specified lifecycle states, such as Active and In Progress, eliminating irrelevant data from assessments.
Learn more about configuring the Local Impact Assessment action.
Admin UI to Manage Registration Attributes & Relational Token Settings
This feature provides Admins with the capability to configure Registration Attribute and Relational Token Setting components through application configurations on the Configurations tab and eliminates the need to run MDLs through Postman to manage application settings for supported actions such as Generate Registration Data, Generate Requirements, and Local Impact Assessment.
Learn more about creating Registration Attributes and Relational Token Settings in your Vault.
Create Document from Template Action: Limit Increase
This feature increases the number of documents users can generate with the Create Document from Template action from 50 to 100.
Learn more about automatically creating dossier documents from templates.
RegulatoryOne Data Model Changes
See 24R3 RegulatoryOne & Veeva Claims Data Model Changes.
Veeva Claims
The Veeva Claims 24R3 release, including all Platform features, is scheduled for tentative availability on December 17, 2024.
In addition to the below release notes, the Claims Veeva Connect community offers general release communications, release highlights, and key feature demos.
Veeva Claims
Simplified Substantiations Enhancement
This feature enhances the existing Upload field on Substantiation records to allow users to upload a reference document after the creation of a new Substantiation record. Users can now use the field to upload a reference document when editing an existing Substantiation record.
Learn more about creating and modifying Substantiations.
Bulk Add Existing Claims to a Project
This feature allows users to add multiple existing Claim records directly from the Claims library to a new or existing Project by utilizing a bulk record action, saving users time by using a more efficient method of record creation.
Learn more about adding Claims to a Project.
24R3 Veeva Claims Enhancements
This feature enables Admins to define Lifecycle States to exclude for the Bulk Create Local Adaptations (Project) action on a new page in the Application Configurations section of the Admin > Configuration page.
Learn more about configuring the Bulk Create Local Adaptations (Project) action.
Application License Warning
See feature description.
RegulatoryOne & Veeva Claims Data Model Standardization
See feature description.