Prerelease Date: March 17, 2025 | Release Date: April 4, 2025 & April 11, 2025
The following applications may have different release dates: Safety, QualityOne Client Applications, RegulatoryOne, and Veeva Claims.
We are pleased to bring you Vault 25R1. Read about the new features below. You can find information on enabling new features in 25R1 Release Impact Assessment. Information on developer features (API, VQL, etc.) is in the Developer Portal.
Platform
Highlights
A summary of the major highlights for Vault Platform are displayed below, with further information accessible from the links. The rest of the Vault Platform release notes are then categorized into thematic areas, with the minor enhancements available in the final section.
To see demos for some of these key features and to read knowledge articles about other core platform functionality, join the Vault Platform community on Veeva Connect.
| Feature | Description |
|---|---|
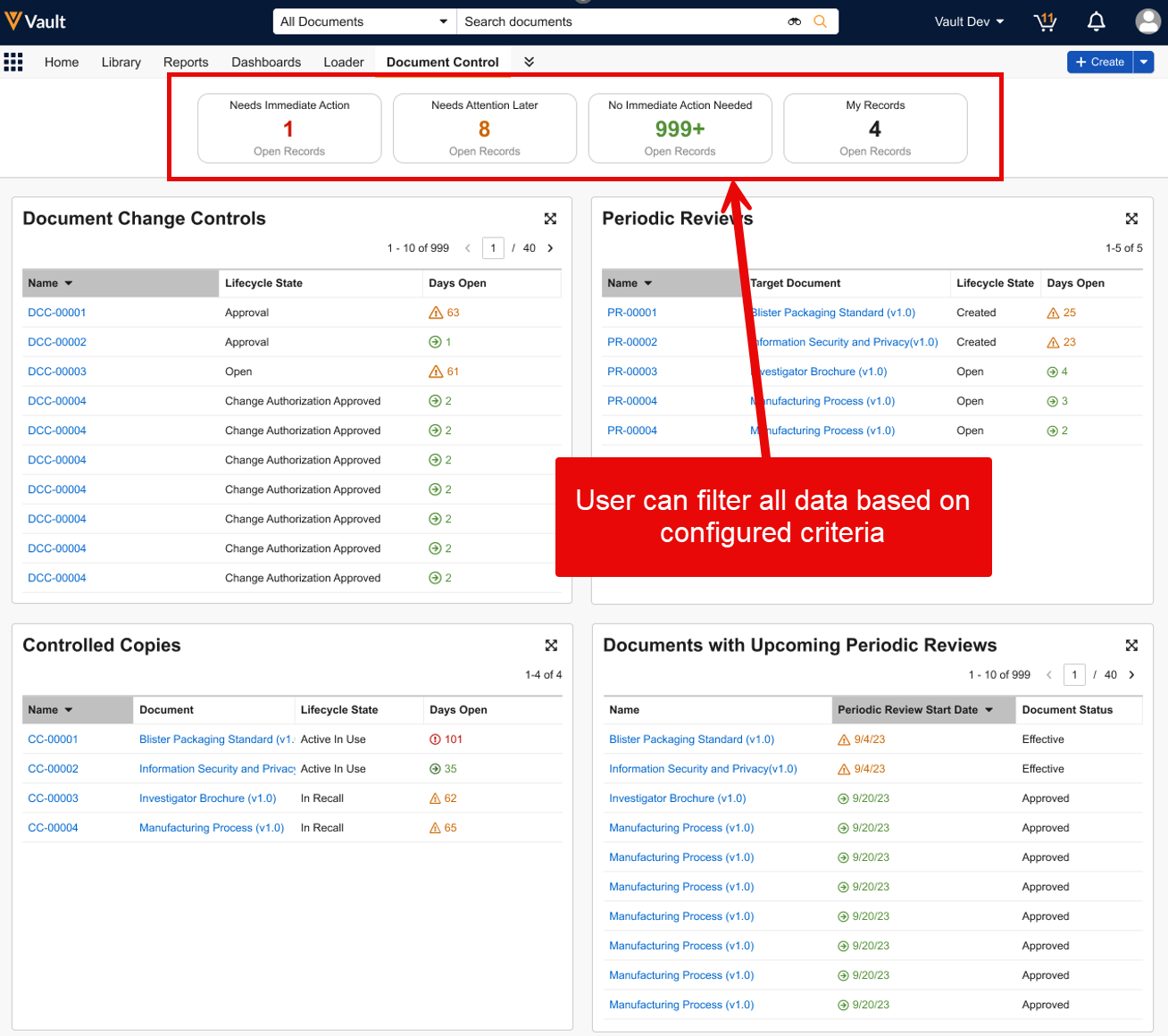
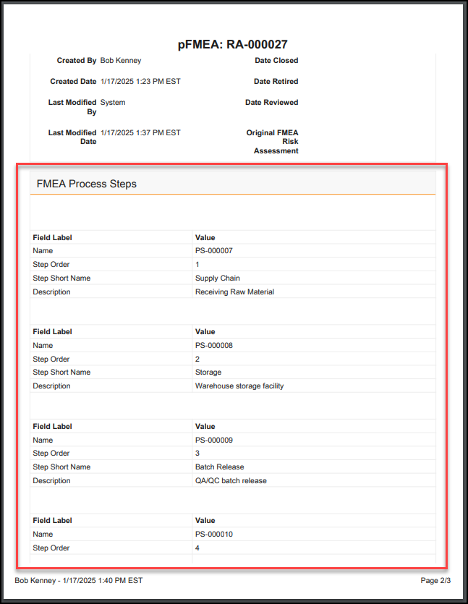
| Process Reporting on Documents | Vault now provides a standard way to report on your document-based business processes based directly on the audit history of the document’s state changes. |
| Automatic Mentioning in Collaborative Authoring | In Collaborative Authoring, you can now @mention other authors even before they’ve joined you in the editing session. |
| Custom Pages | Developers can now add custom UI to Vault using JavaScript and their preferred frameworks or libraries to write the client code. |
| Word Formatted Outputs | Admins can now configure Formatted Output templates using Microsoft Word rather than using Adobe XFA, reducing the effort to create and maintain templates. |
| Enhanced Formatting Options for Signature Page and Overlay Templates | When creating Overlay and Signature Page templates, Admins can now customize the page format, adjust the font styling, select text alignment, and add logos using intuitive rich text editors. |
Process Reporting on Documents
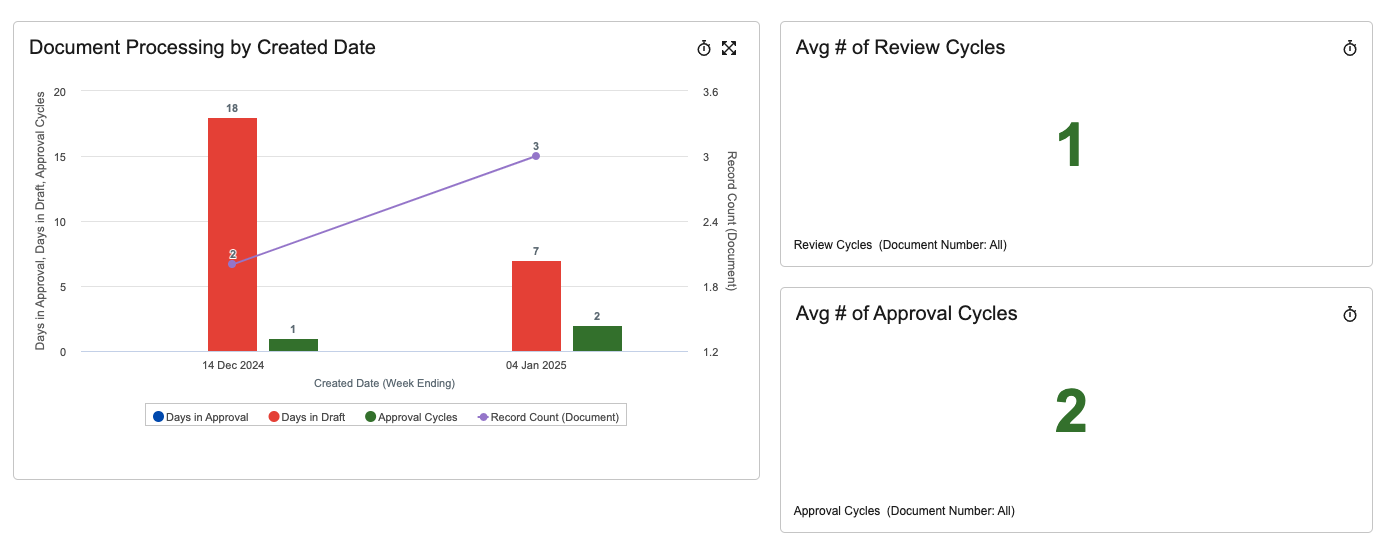
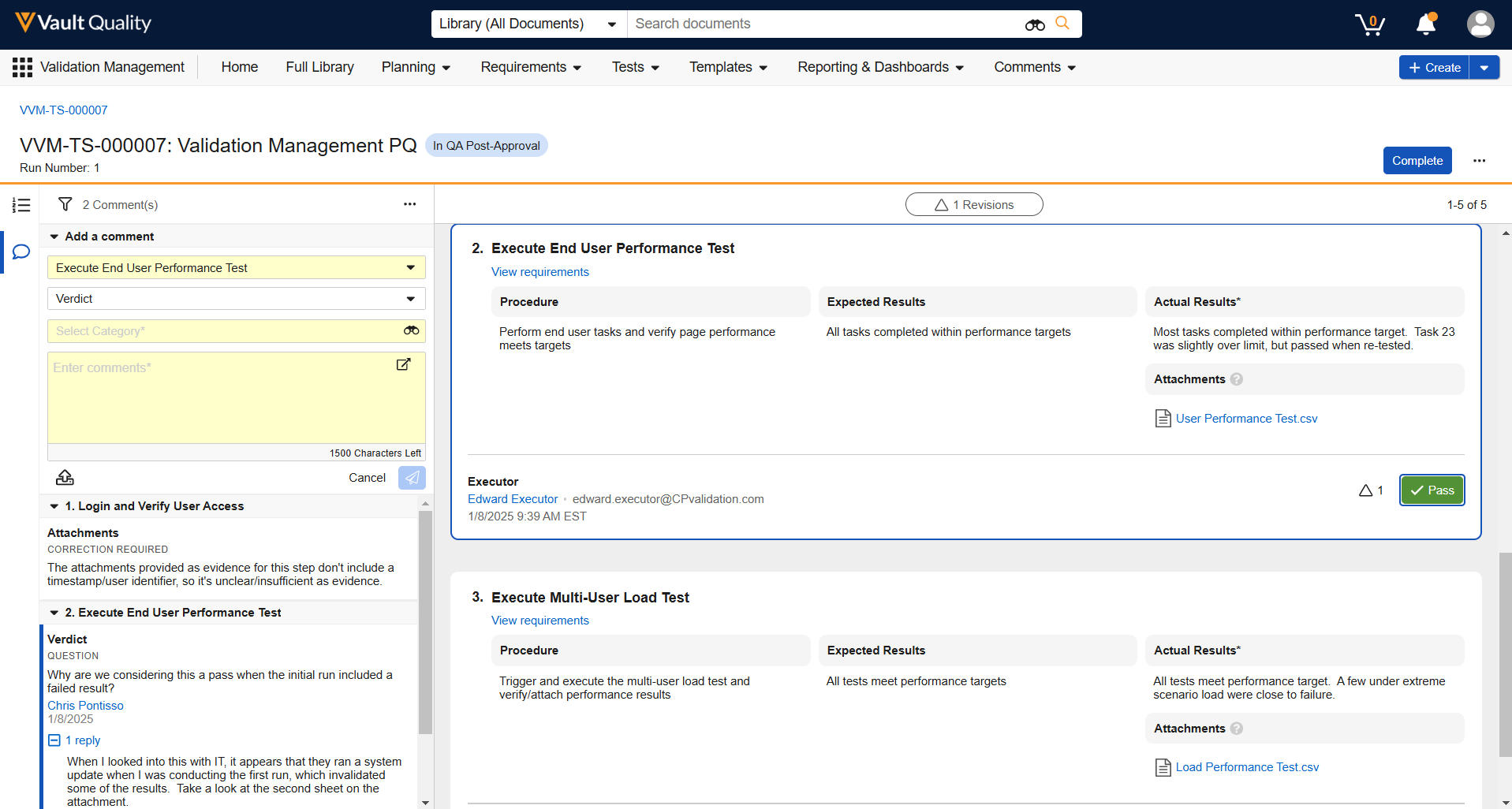
In the 24R3 release, we introduced a new Process Reporting feature for objects that allows customers to easily report on business processes based on Lifecycle State information. This helps users and Admins easily understand how long certain processes take to complete, when certain state changes occur, and identify bottlenecks. These metrics are used to improve business processes and team performance.
With the 25R1 release, this is now available for Documents as well:
Object Reports and Document Reports share the same supported formula field functions:
| Expression | Description | Parameters | Output Data Type |
|---|---|---|---|
FirstTimeInState |
Reports on the date when a document went to a particular state for the first time. | Field name (for example, status__v) Value (for example, In Review) |
DateTime |
LastTimeInState |
Reports on the date when a document went to a particular state for the last time. | Field name (for example, status__v) Value (for example, Reviewed) |
DateTime |
PreviousState |
Reports on what was the previous state. | Value name (for example, status__v) |
Text |
DurationInState |
Reports on the days a document was in a particular state. This calculates across all instances if the document went through the same state multiple times. | Field name (for example, status__v) Value (for example, In Review) |
Number with decimals |
CountInState |
Reports on the number of times a document was in a particular state. | Field name (for example, status__v) Value (for example, In Edit) |
Number |
Document-level Reporting
The initial release of these functions supports calculating data across all document versions (and does not yet support calculating this information on specific document versions). For instance, if the DurationInState function is used to calculate how many days a document was in the In Review state, the result is based on all versions that went through In Review.
Limited Support for Document Fields of Type Formula Field
Now that these state-based functions are available in report formula fields for both objects and documents, there is now a consistent and easy way for customers to report on these metrics without the need for Admin configuration. Historically, for Documents, Admins would need to add Formula Fields to their configuration in order to achieve similar results.
With parity now in formula fields you can create in reports, Admins will no longer be able to create the legacy document field type of Formula Field in Admin > Configuration > Document Fields. Any existing document formula fields using Document Status or State Type will continue to function.
End of Support for Document Fields of Type Formula Field that Reference Picklists
With 25R2, only Document fields of type Formula Field that reference Document Status will be supported. Those that reference Picklists will no longer return a value.
Learn more about Process Reporting functions.
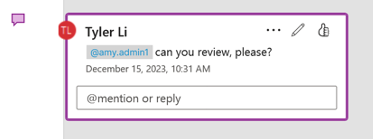
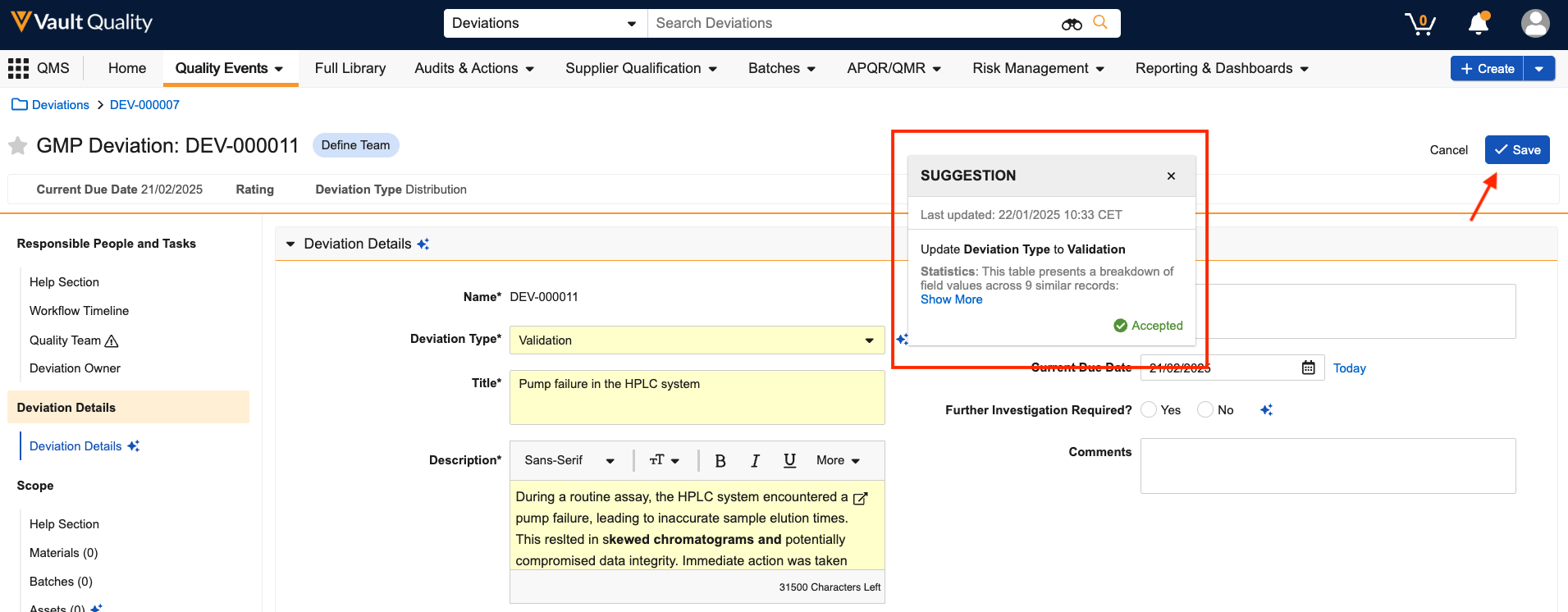
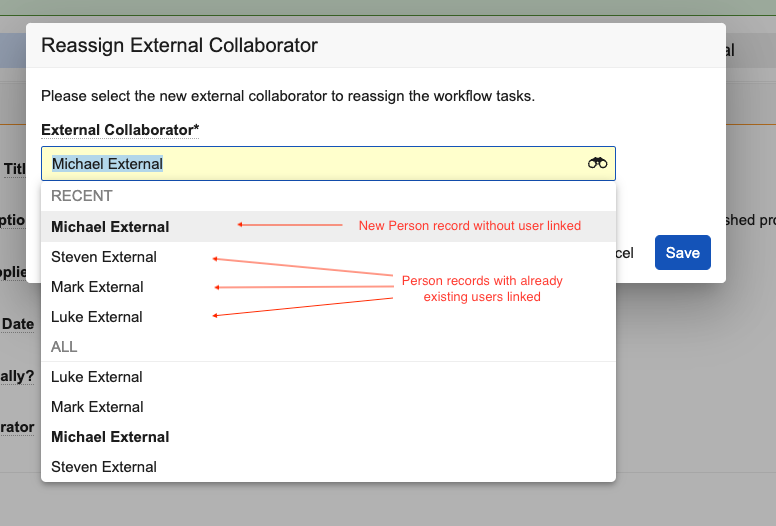
Automatic Mentioning in Collaborative Authoring
You can now @mention other authors who were assigned a task (for documents automatically checked out using workflow actions) even before they’ve joined you in the editing session.
Prior to 25R1, you could only @mention users who had already clicked the Edit in Microsoft Office action on the document in Vault. Now Vault automatically adds the workflow participants to the document on SharePoint upon checkout so that they can be immediately @mentioned.
This greatly streamlines the authoring process and reduces confusion for authors by ensuring they can immediately start communicating with other authors when editing the document.
To configure this functionality, add the User.ReadBasic.All permission to the Microsoft Graph permission in the Entra ID app configuration. Learn more about configuring automatic mentioning in the enhanced (current) and legacy collaborative authoring configuration.
If SharePoint Cannot Add a User
If a user cannot be added to the SharePoint document when starting the workflow, this is logged in the .CSV response file that is sent via notification (depending upon the workflow configuration):
This behaviour does not apply to external or guest accounts.
Learn more about Collaborative Authoring with Microsoft Office.
Custom Pages
Customers can now create custom user interfaces hosted in Vault. Custom Pages are written using JavaScript, allowing you to choose from various frameworks and libraries, as well as the Java SDK. This allows you to create specialized user interfaces for specific use cases, such as purpose-built forms, without standing up external infrastructure.
Custom Pages can be accessed by users in a Tab and are secured with Permission Sets. Custom Page code and configuration is stored and managed in Vault using the same model Vault Applications use to create Pages on the Vault Platform.
Using the Vault Admin UI in Admin > Configuration > Pages, Vault Admins can now configure Custom Pages and edit Custom Page attributes, including the custom URL path and UI header display. Vault Admins can also delete and inactivate existing Custom Pages, allowing quick access removal for all users.
Additionally, Vault Admins can create tabs (Admin > Configuration > Tabs) and page links (Admin > Configuration > Page Links) that point to Custom Pages.
Learn more about Custom Pages in the Developer Portal.
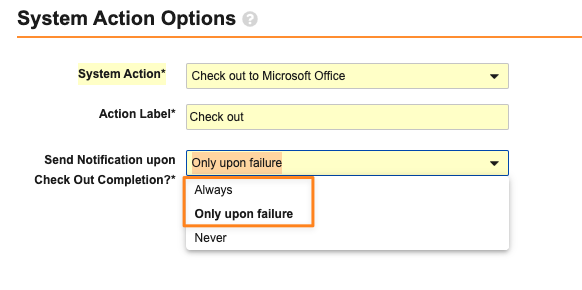
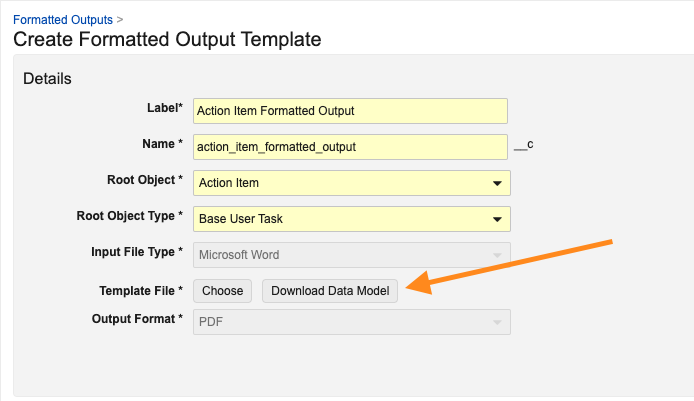
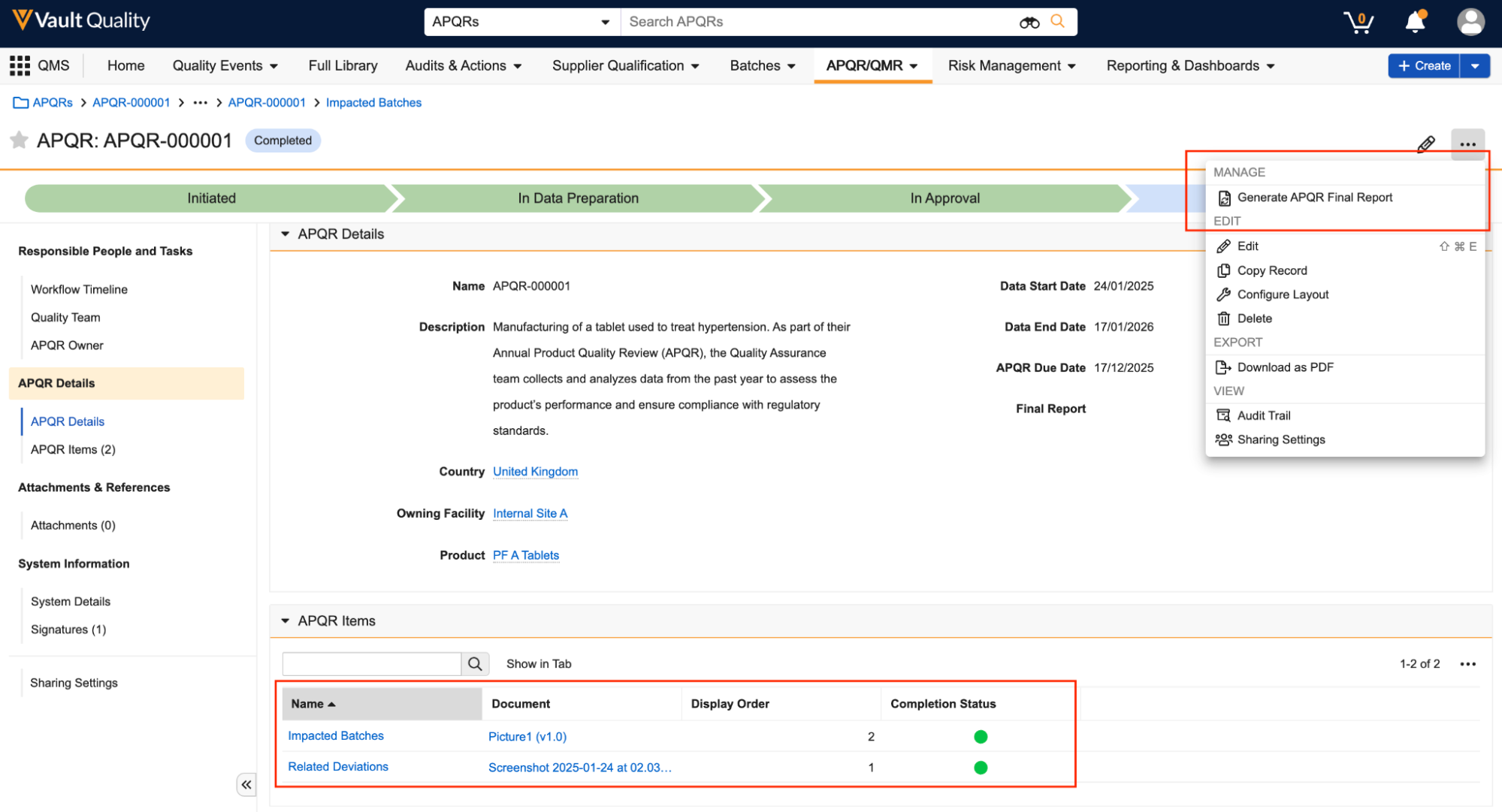
Word Formatted Outputs
Admins can now define formatted outputs for objects using Microsoft Word. Admins can use Vault-specific syntax and tokens in the Microsoft Word file to control how the data is outputted, while also managing general formatting in a much easier and user-friendly way.
Prior to 25R1, defining formatted outputs required the use of Adobe products such as LiveCycle or EAM Forms Designer, both of which are costly and require extensive knowledge to use. Moving to Microsoft Word streamlines the process for creating and maintaining formatted outputs, and allows customers more flexibility to manage their own templates.
Important: Between 25R1 and 25R2, Admins can begin working on new Word formatted outputs; however, certain application-specific functionalities (such as generating documents from formatted outputs in QMS, and generating trip reports in workflows in CTMS) will not work with Word formatted outputs until a future release.
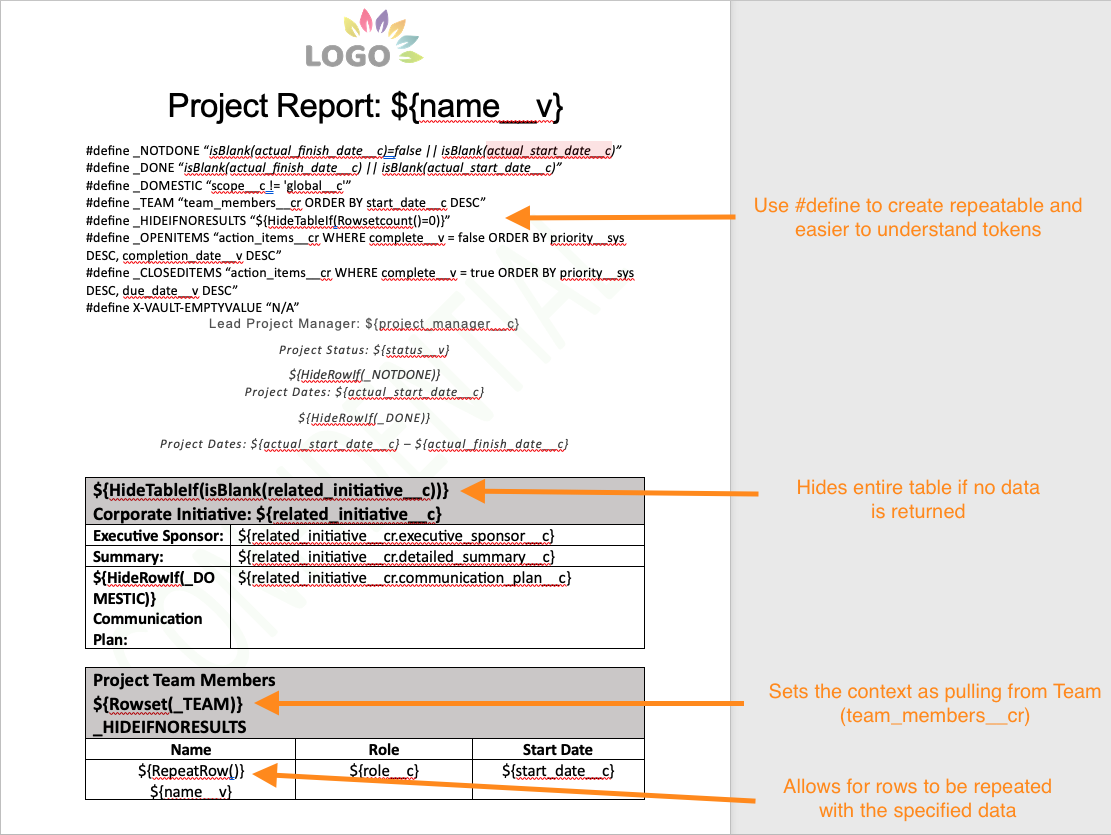
The key syntax items that users can leverage are:
-
Rowset(...): This syntax can contain the relationship name and any necessary VQL criteria, and sets the context for the table. For example,${Rowset(user__sysr WHERE status__v = 'Active')}permits a table to print information from related users on the template -
RepeatRow(),RepeatTable(): Allows a specific row or table to be repeated for each record that was retrieved in a Rowset -
HideRowIf(...),HideTableIf(...): Allows a specific row or table to be hidden based on an expression. For example,${HideRowIf(state__v = 'Inactive')}hides the given row based on the value of thestate__vfield. -
#define: An existing function in Vault formulas that can be used in Word formatted outputs to simplify the syntax and maintain the document’s readability
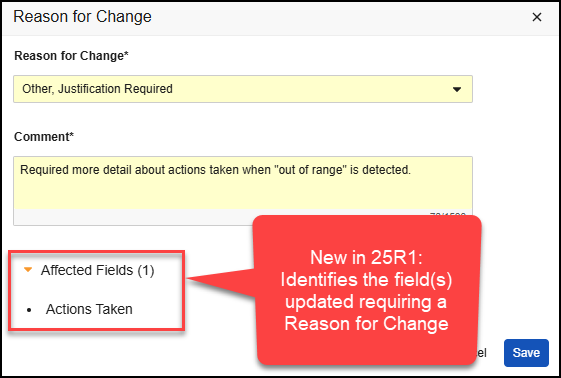
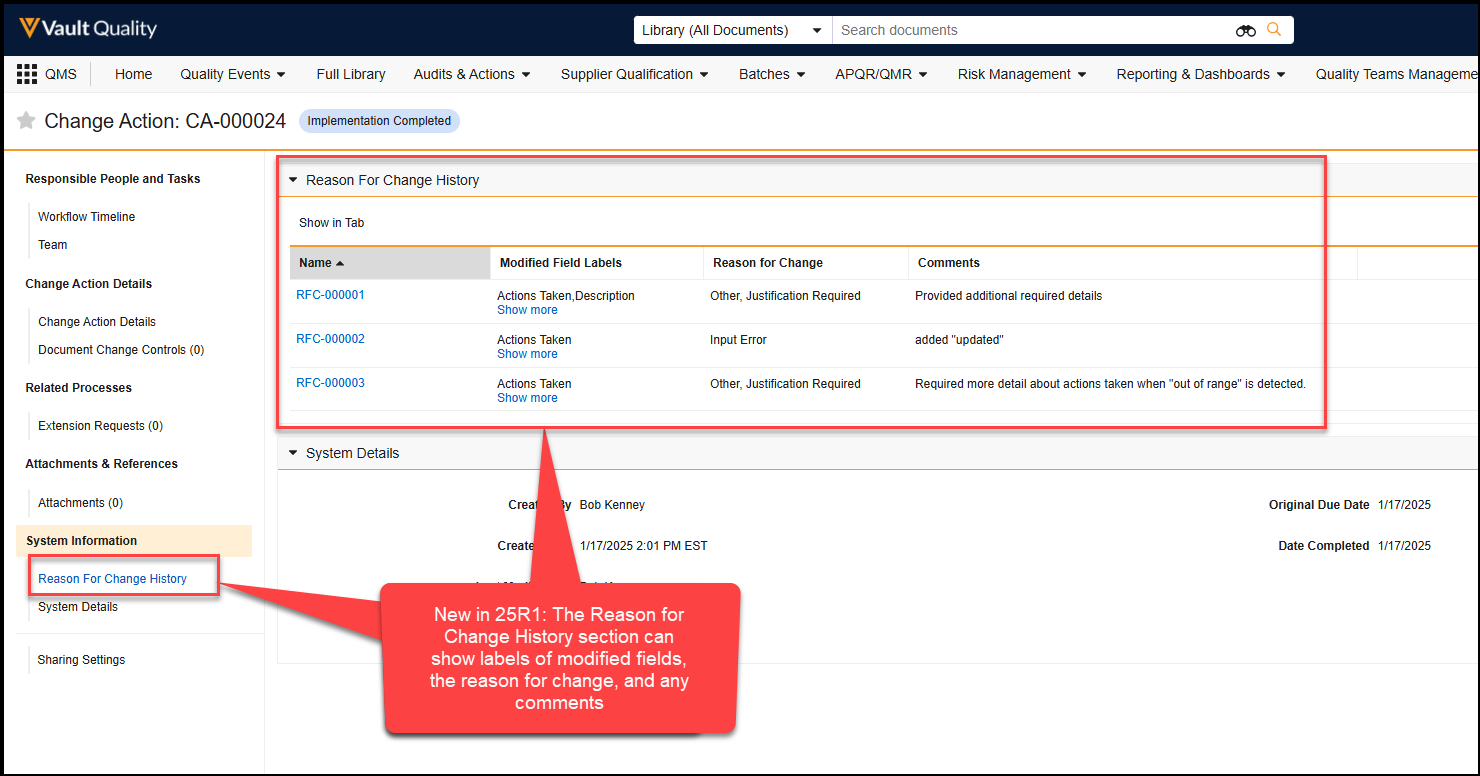
You must wrap each syntax in the following notation brackets when adding them to your template: ${..}. For example, ${HideRowIf(state__v = 'Inactive')} is a valid syntax. Once the template is validated and executed, Vault deletes the notation brackets from the document.
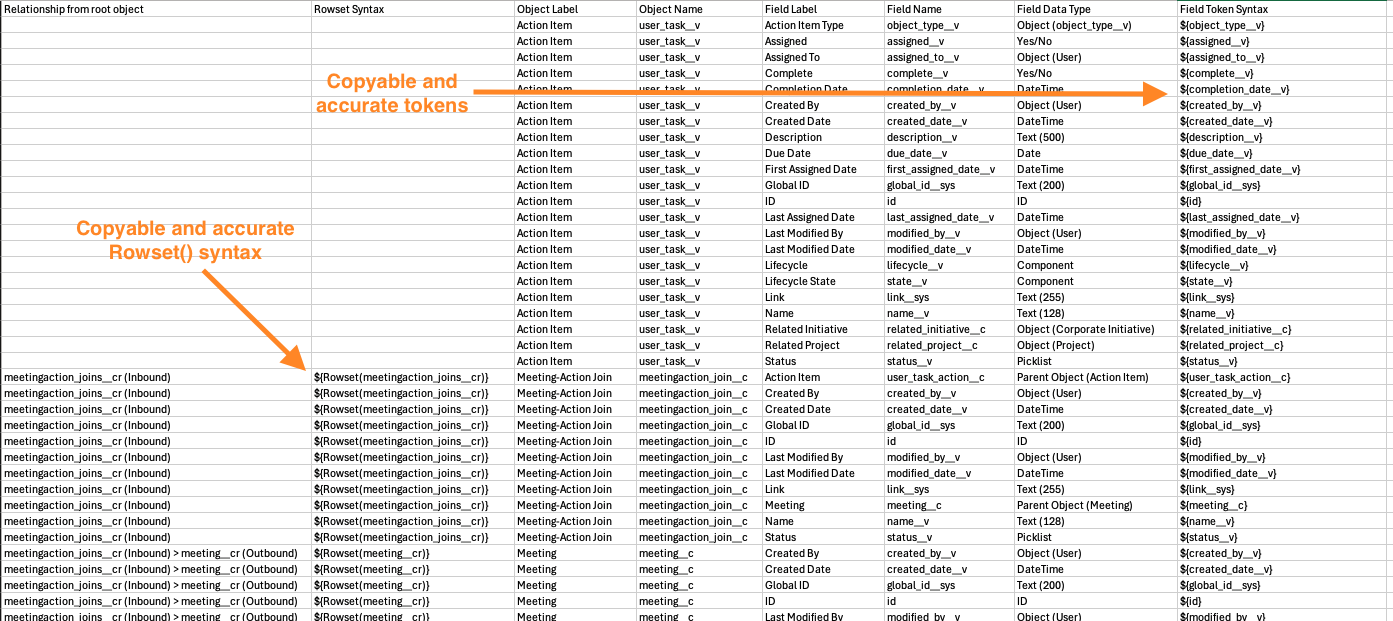
When creating new Word formatted output templates in Vault, Admins can also download a data model CSV file by clicking Download Data Model. This file allows them to easily identify available relationships and fields. The CSV also provides the relevant token syntax to leverage in the Microsoft Word template.
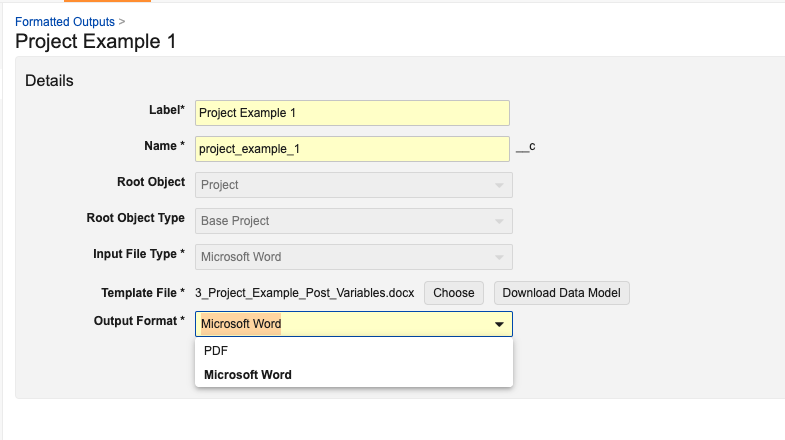
Admins can also choose PDF or Word as the output for the new Microsoft Word formatted output template. A PDF formatted output takes longer to generate than a Microsoft Word formatted output.
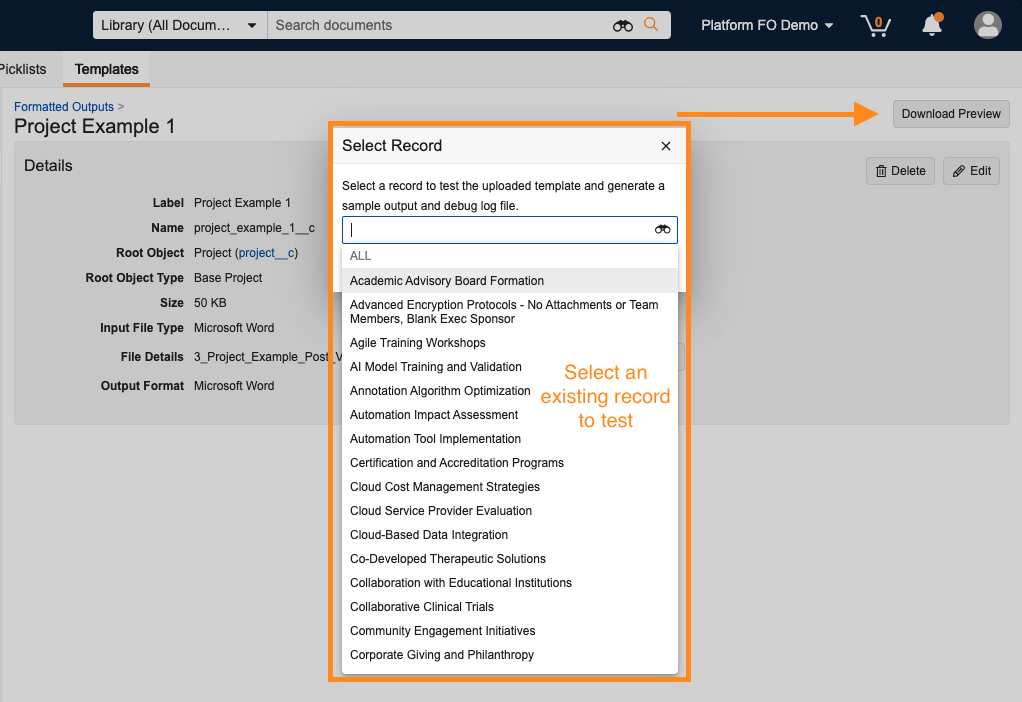
Admins can also easily test their uploaded templates by using a new Download Preview button and selecting an existing record in the system.
With 25R1, new formatted outputs can use either Microsoft Word or Adobe as input files; however, in 25R2, you must create new formatted outputs using Microsoft Word (no new Adobe formatted outputs can be created in a Vault). Though, you can still edit and use any existing Adobe formatted outputs.
Learn more about Word Formatted Outputs.
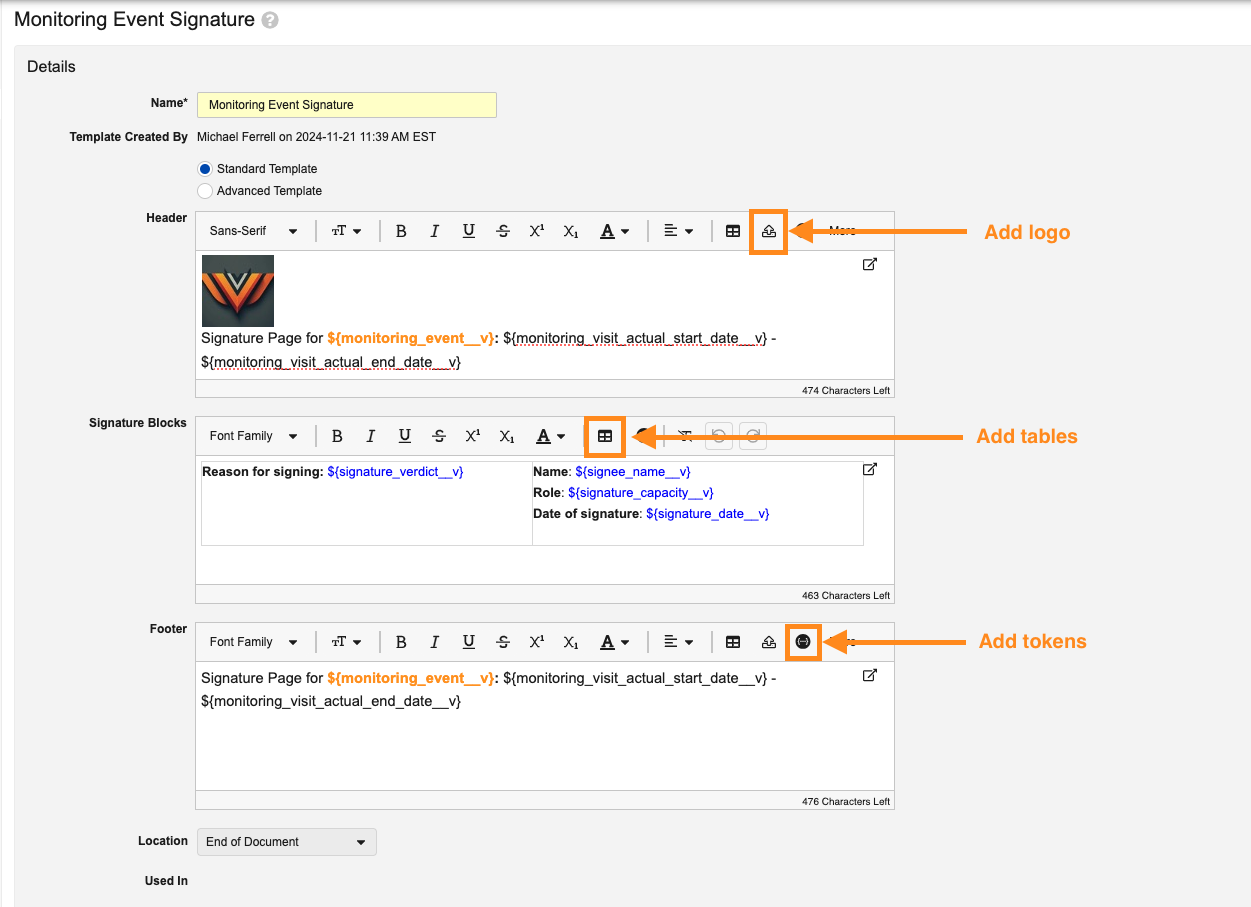
Enhanced Formatting Options for Signature Page Templates
With this release, Admins can now customize page format, adjust the font styling, select text alignment, and add logos when creating Standard Signature Page templates that will apply to viewable renditions upon download. These new options will be based on using an intuitive rich text editor for the Header, Signature Block, and Footer.
In addition to standard rich text editor options, Signature Pages also include options for adding logo(s) (based on logos added in Admin > Settings > Branding Settings), adding tables, and accessing tokens.
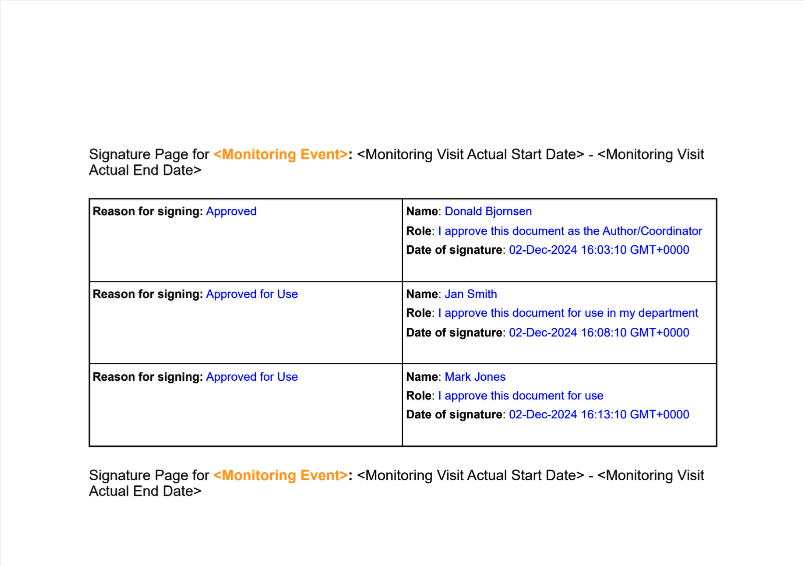
Admins will also have a Download Preview option to be able to preview the formatted Signature page:
Prior to 25R1, customers needed to leverage XFA templates to apply customizations like these to their signature page templates. Adding new formatting options allows Admins to create customized signature pages without relying upon external software.
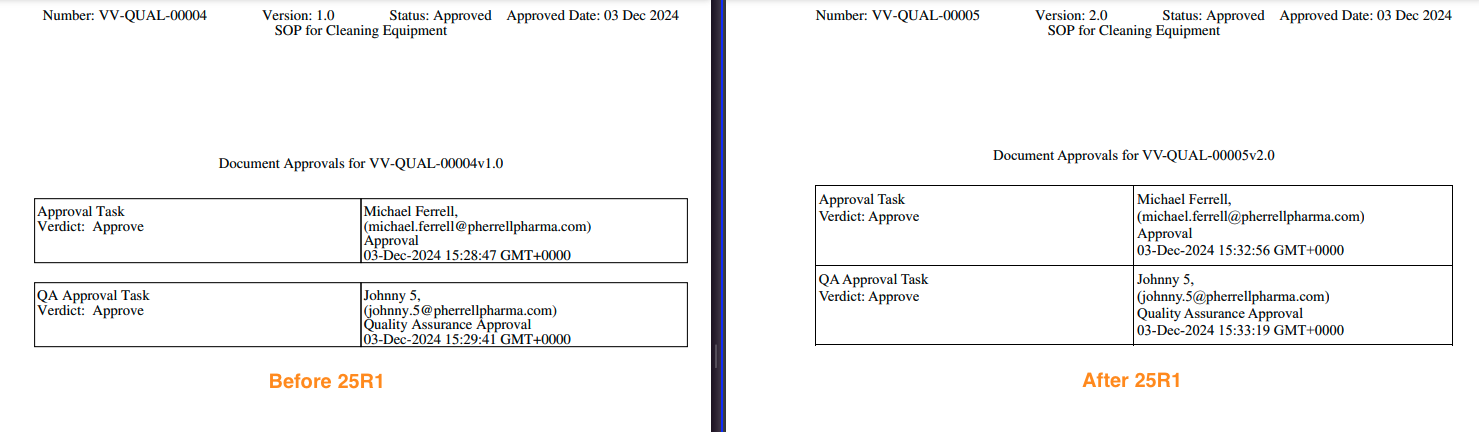
For existing Signature Pages, no change will occur as part of the 25R1 deployment. Once an existing Signature Page template has been edited and saved, the new template will apply going forward.
If an existing Signature Page template is edited and saved with no changes, there will still be minor changes to how the signature is displayed on documents going forward:
With 25R1, all new Signature Pages created in Vault will leverage this new functionality and no new XFA-based Signature templates can be created (though existing XFA templates can still be edited through MDL).
Learn more about Managing Document eSignature Templates.
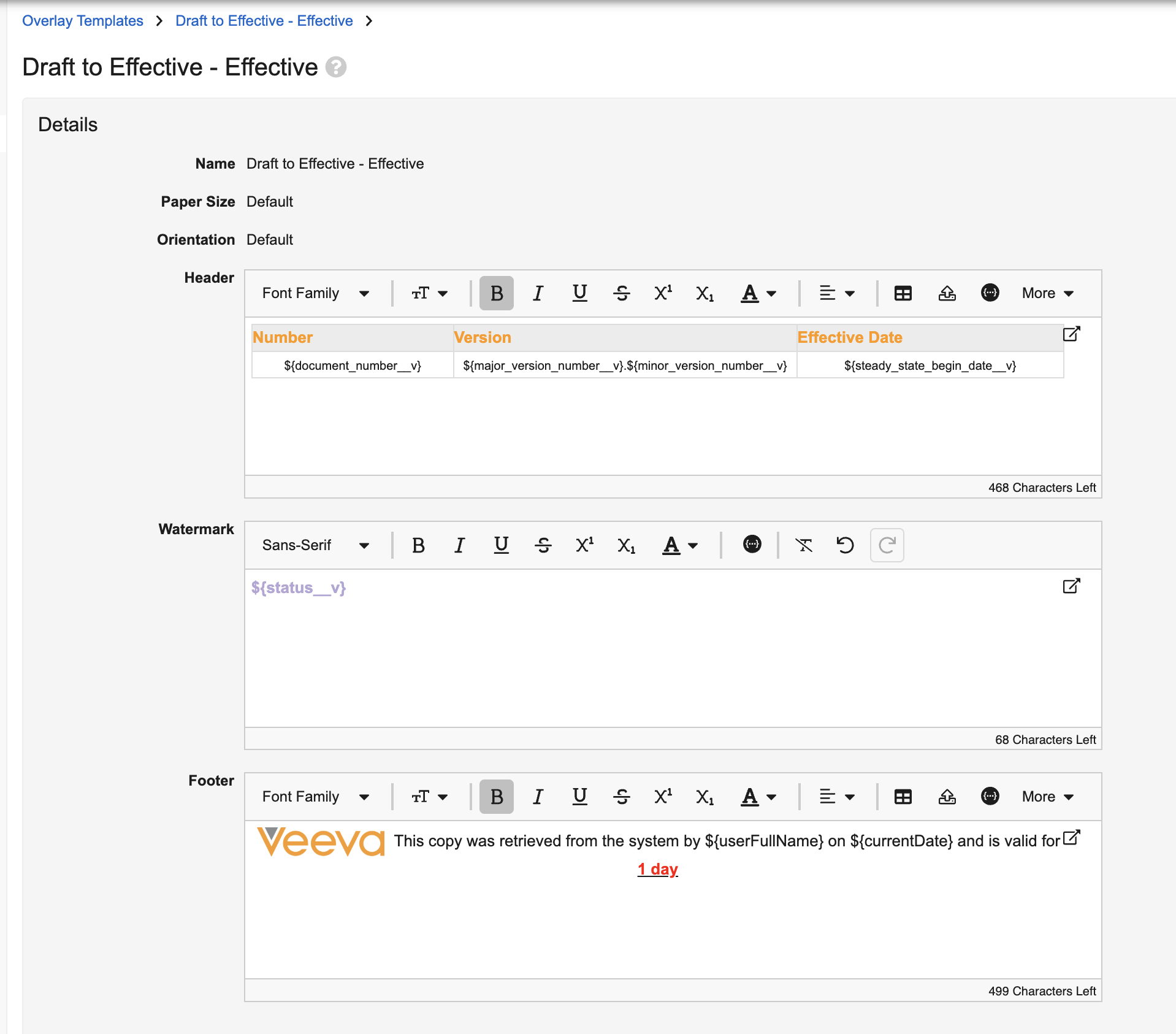
Enhanced Formatting Options for Overlay Templates
With this release, Admins can now customize formatting, adjust the font styling and coloring, and select text alignment when creating Overlay templates that apply to viewable renditions on download. These new options are based on using an intuitive rich text editor for the Header, Watermark and Footer.
In addition to standard rich text editor options, Overlay templates also include options for adding logos (based on logos added in Admin > Settings > Branding Settings), adding tables in Headers and Footers, and accessing tokens.
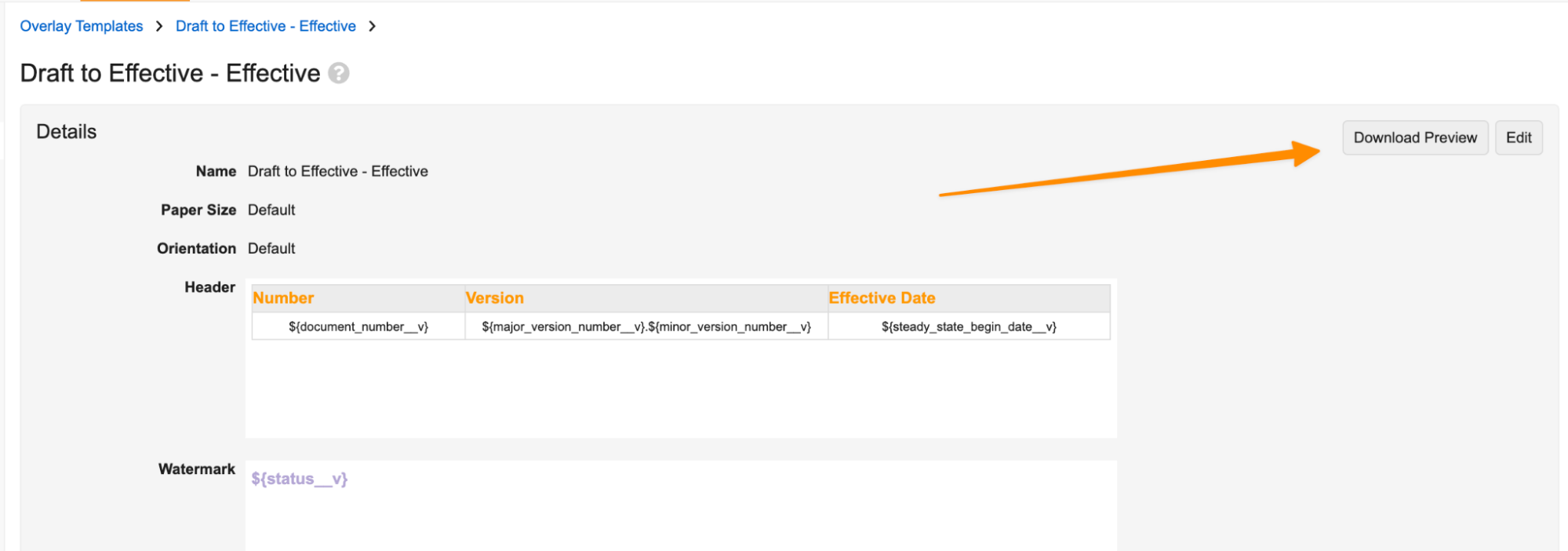
Admins also have a Download Preview option to preview the formatted Overlay:
Prior to 25R1, customers needed to use XFA templates to apply customizations like these to their Overlay templates. Adding new formatting options allows Admins to create customized Overlay templates without relying on external software.
For existing Overlay templates, no change will occur automatically as part of the 25R1 deployment. Once an existing Overlay template has been edited and saved, the new template will apply going forward.
With 25R1, all new Overlay templates created in Vault can use this new functionality and no new XFA-based Overlay templates can be created (though existing XFA templates can still be edited).
Learn more about Managing Overlays.
Vault Mobile
Support for Workflow Task Prompts for Participants
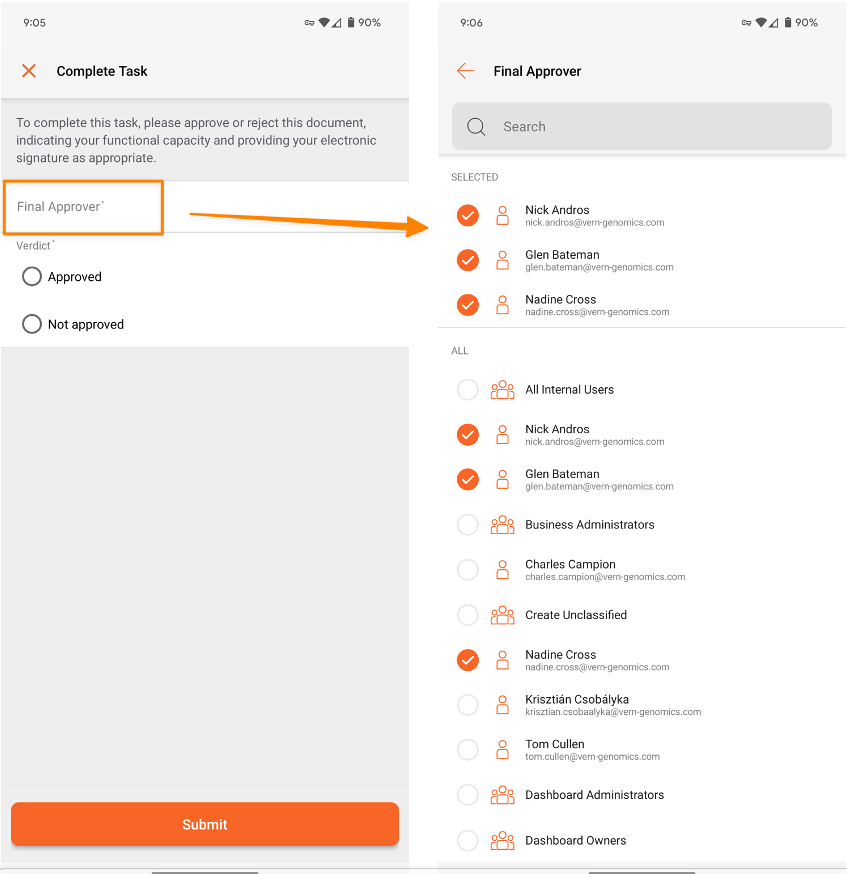
In our 24R3 release, we introduced an enhancement allowing Admins to configure workflow tasks to prompt the task owner to select a workflow participant as part of task completion. This change streamlines multi-step workflows so that the user who starts the workflow does not have to enter participants for all tasks in the workflow at the beginning.
With 25R1, this functionality is now supported when completing document workflow tasks in Vault Mobile as well. If a task is configured to prompt for additional participants, users will now be able to see and complete this prompt when completing tasks on-the-go:
Learn more about Vault Mobile.
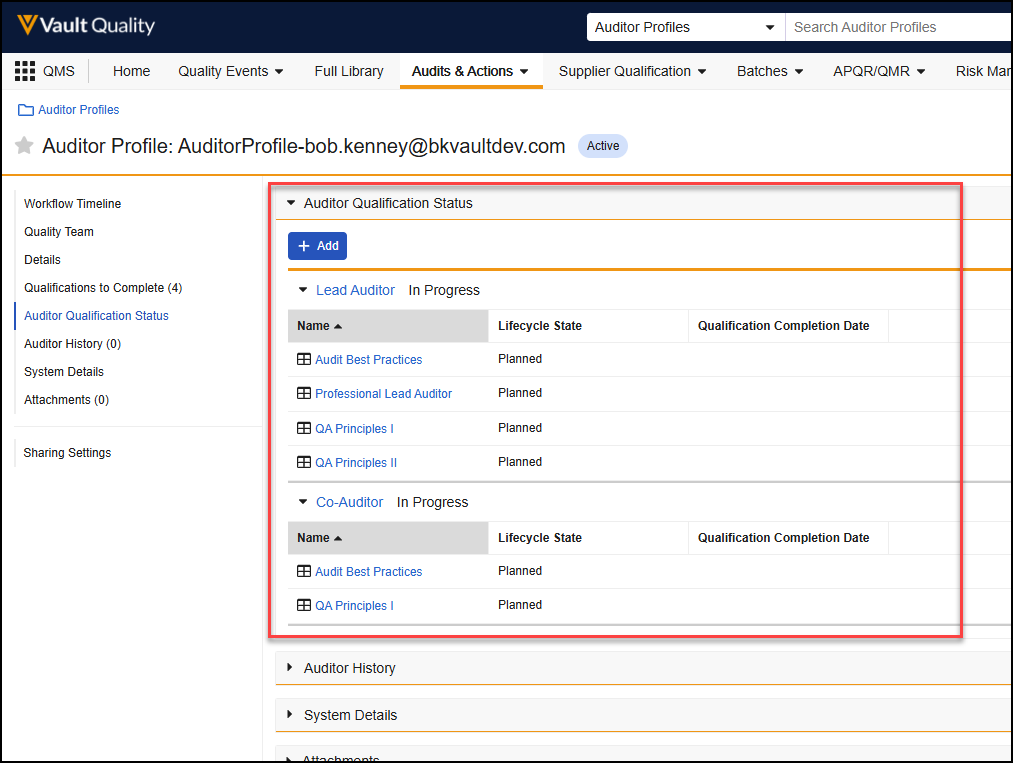
Managing Documents
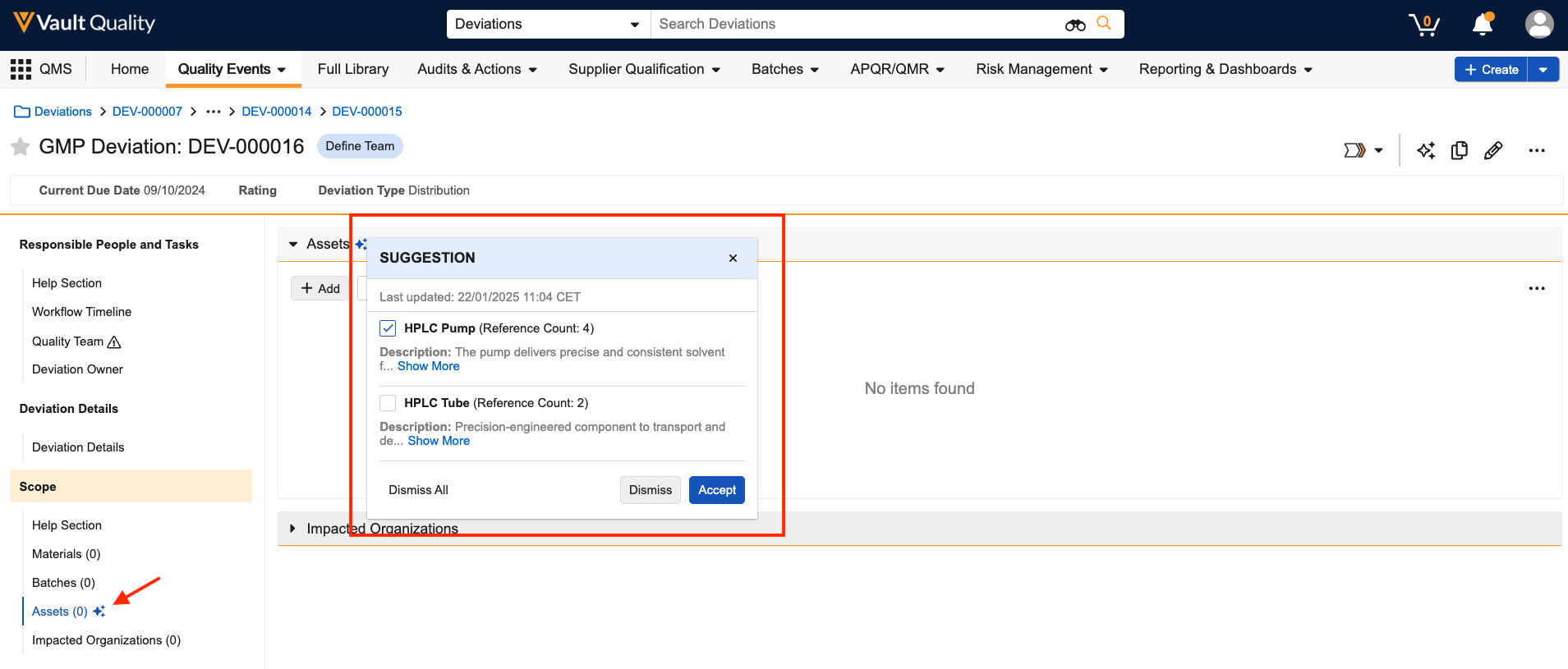
Auto Bring Forward Annotations
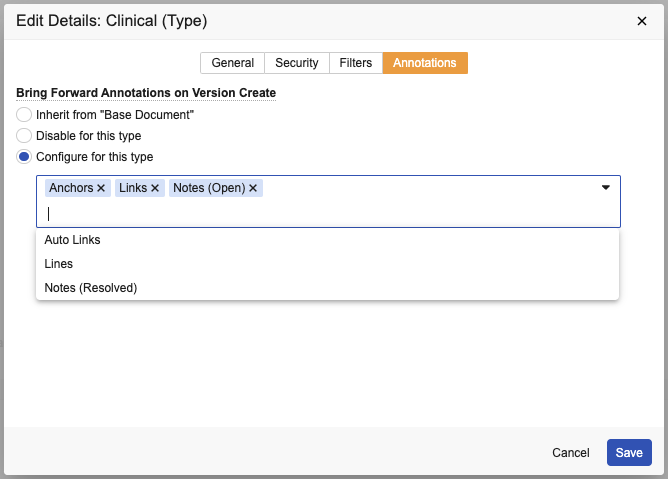
There is now an automatic way to bring forward annotations when a new version is created (using the Create Draft, Upload New Version, or Check In actions), via a new Document Type configuration in admin. This enhancement reduces the end-user effort to manually bring annotations forward to the new version, and provides a more standardized and automated process. This functionality applies to both video and non-video documents.
Users can now also include Resolved annotations when bringing forward annotations, and there’s even a way to now automatically include them, via a document type configuration. To allow users to choose this when manually bringing forward annotations in the Bring Forward Annotations dialog, this must first be enabled in Admin > Settings > Annotations > Allow users to bring forward annotations > Notes (Resolved).
Admins must specify the types of annotations that can be brought forward automatically for each document type in Admin > Configuration > Document Types.
As with other Document Type settings, the default is to inherit settings from the Parent Document Type in the hierarchy, though you can override the inheritance by either disabling it for a given document type, or by adjusting what is brought forward for a document type.
Automatically brought forward annotations will always bring forward all annotations of the configured types on all pages from the latest eligible version.
This new Document Type configuration is distinct from the existing Allow Users to Bring Forward Annotations setting (Admin > Settings > General Settings), which only determines if users themselves can execute the Bring Forward Annotations action.
Removing Automatically Brought Forward Annotations
Users with Annotate and Remove Annotations permission can remove any annotations that were automatically brought forward.
OCR Required
Automatically bringing forward annotations is dependent on completion of Optical Character Recognition (OCR) after the new version is created. If there is a delay, Vault will display a message to users (who have Annotate permissions) to keep them informed of the progress:
Learn more about Bring Forward Annotations.
Enhanced Configuration for Collaborative Authoring
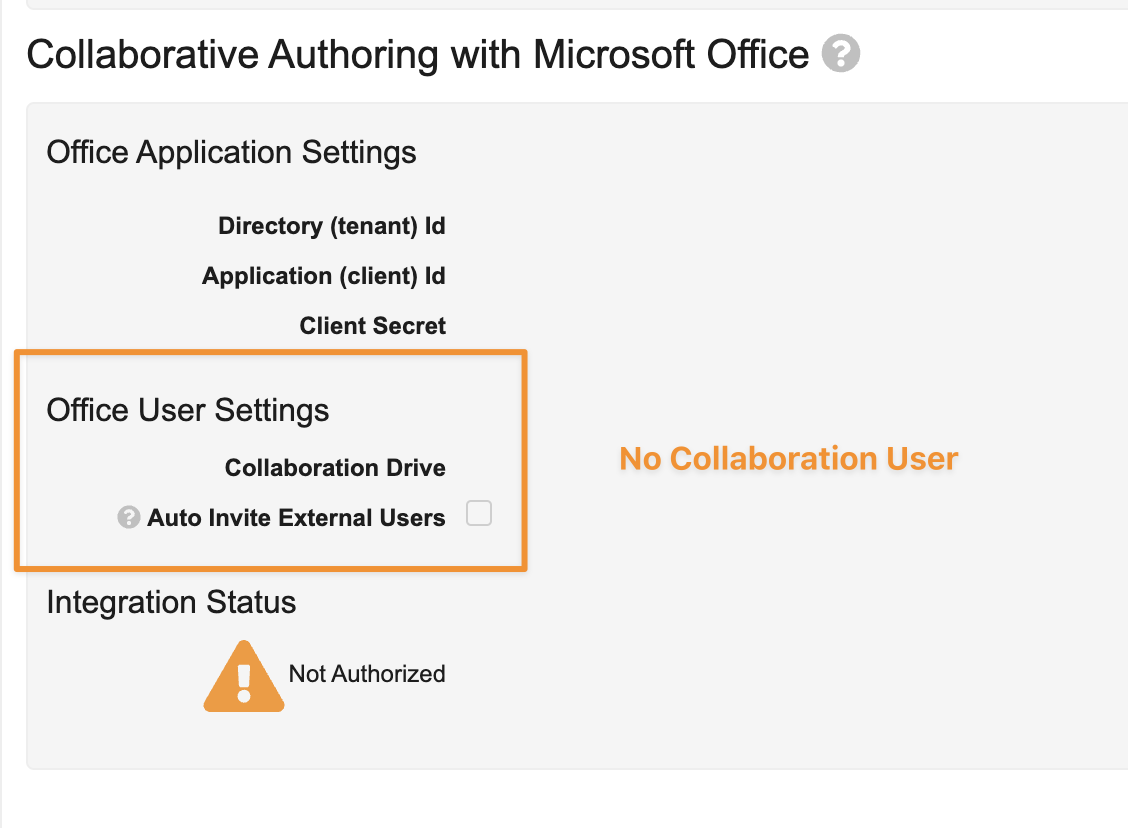
Configuring Collaborative Authoring in Vault no longer requires a Service Account (Collaboration User) to connect Vault and SharePoint, reducing the complexity of initial setup, and enabling it in Sandbox Vaults.
Note: In 26R1, this change will require customers to update their collaborative authoring configuration. There is no immediate impact for 25R1.
When Collaborative Authoring was introduced, SharePoint did not support managing the permissions at the application level, which necessitated the use of a Service Account. Now that SharePoint supports this, we no longer need the Service Account user, which simplifies the integration and brings the configuration in line with typical best practices.
25R1: No Change for Existing Vault Configurations
The editing experience for end-users does not change with the new configuration.
For Vaults that already have Collaborative Authoring configured, there is no change to the configuration, though any Vault that does not currently have Collaborative Authoring set up at the time of the 25R1 release must use the new configuration.
26R1: All Configurations Must Be Updated
Existing Vaults with Collaborative Authoring will need to update the configuration by the 26R1 release, when support for the Service Account configuration will be fully deprecated.
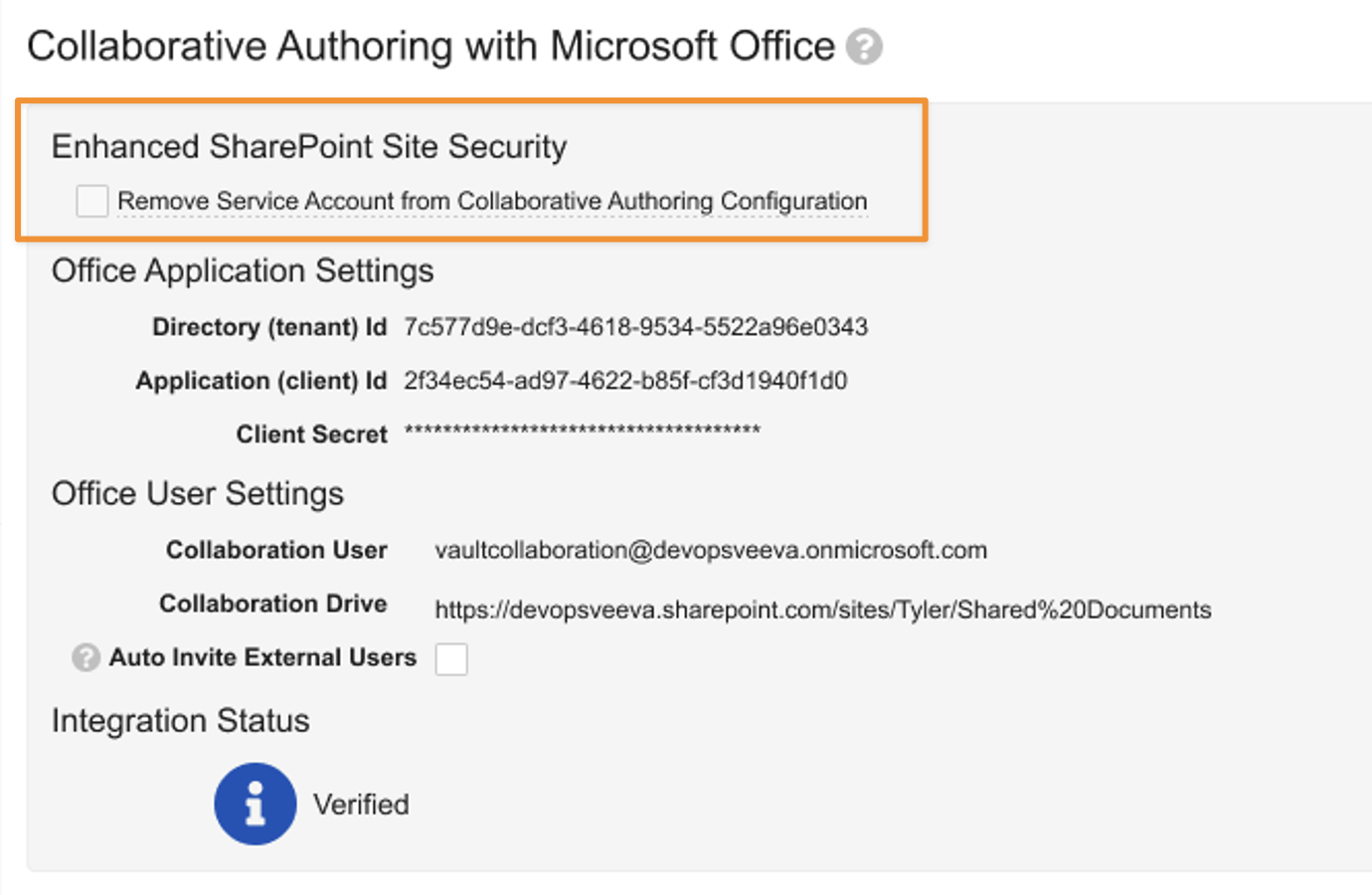
Updating this configuration is primarily done in Microsoft Entra ID (previously Azure Active Directory). Once the necessary updates are made in Microsoft Entra ID, Admins can disable the Service Account in Admin > Settings > Checkout Settings:
Learn more about Collaborative Authoring.
Managing Data
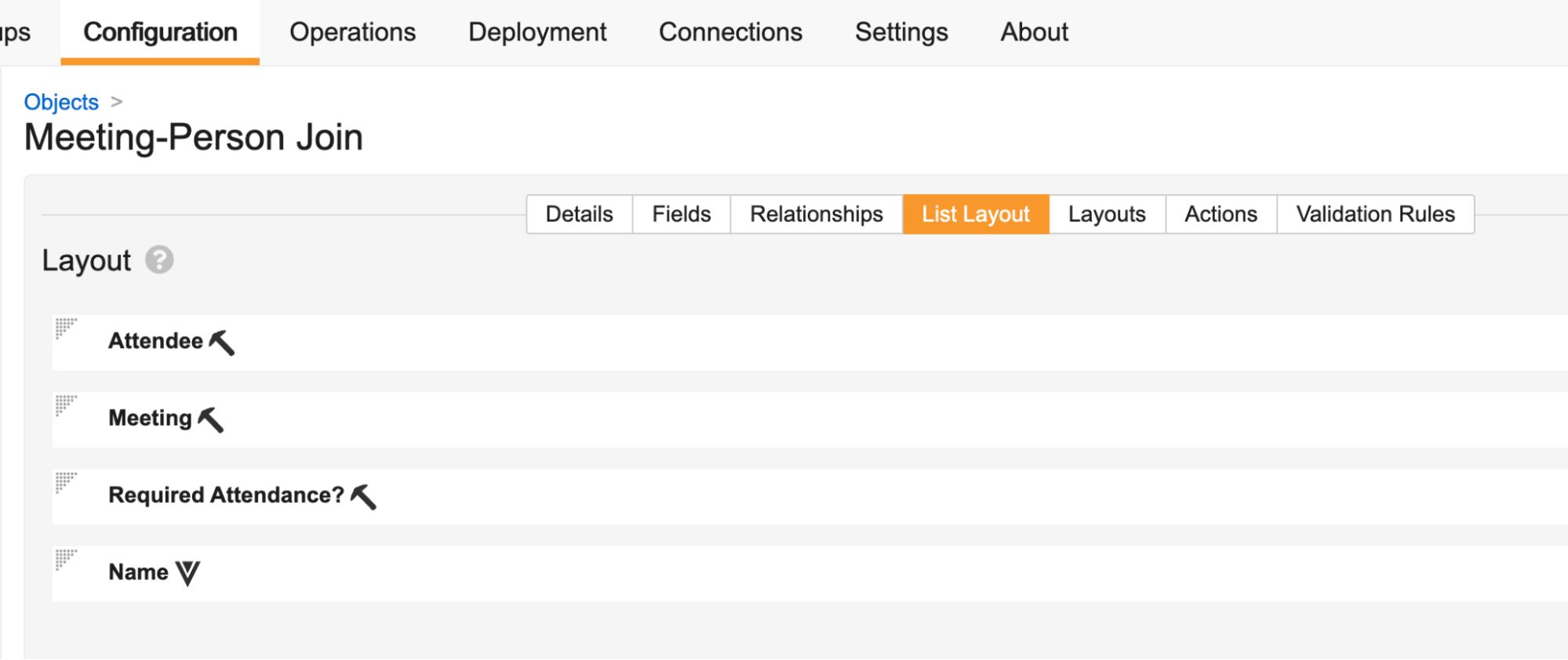
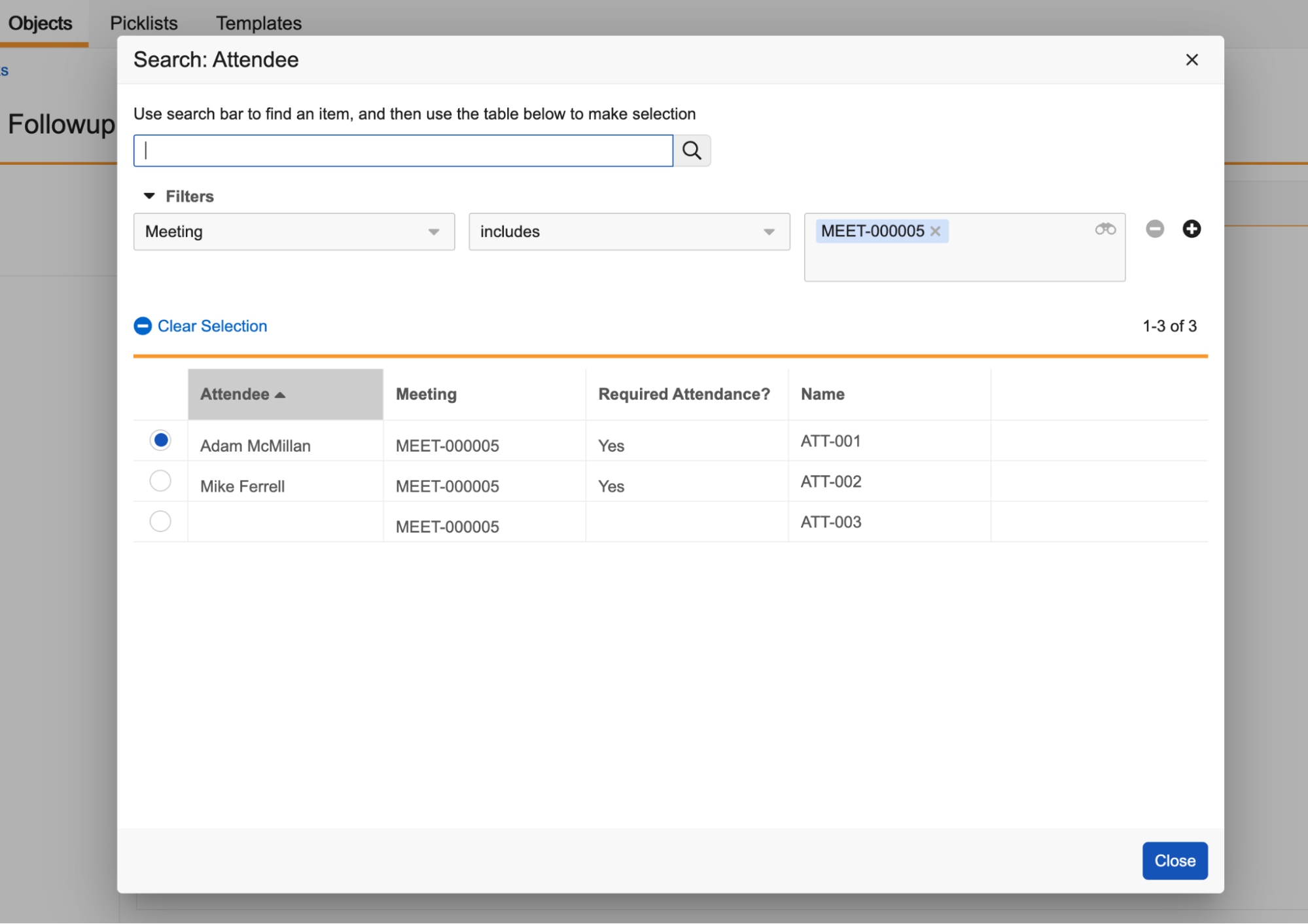
Record List: Flexible First Column
You can now choose any field to be the first column in record list views (including tabs, related object sections, and record selection dialogs), making that field’s value the clickable link to access the record. Historically, the Name field was always enforced as the first column in any record grids at runtime.
If the field you choose as the first column is blank for some records, Vault displays [Link to Record] as the clickable link text.
Records in Object Reference Fields Still Display as the Name Value
The feature described here does not change how the selected object record appears in object reference fields on documents and records. The selected records still display as the Name value.
Configuring the Default List Layout and Column Order
Prior to this release, even if admins had ordered an object’s List Layout so that the Name field was not the top field, Name was always displayed as the first column in record selection dialogs and list views. After this release, Vault will now properly respect the position of the Name field in your List Layout, so it’s important to review your objects to prevent possible confusion after release.
Icons and Yes/No Checkbox Fields
If an Icon formula field or a Yes/No checkbox field is set as the first column, the Name field will continue to be the clickable link to the record.
Attachment Fields
Attachment fields are the only exception to this feature. You cannot move Attachment fields into the first column position, in all contexts.
Formulas Support Referencing Multiple Object Levels
Vault formulas will now be able to traverse up to three relationships. For example, in QMS, the Finding object references Audit, Audit references Facility and Facility references Country. Formulas will now allow you to evaluate fields on Country when defining formulas on Findings.
For example, the following formula allows you to return the value from the Abbreviation field on the Country object: audit__vr.facility__cr.country__cr.abbreviation__vs
This change applies to object validation rules, object formula fields, and object field defaults.
Prior to 25R1, it was only possible to pull in either the ID or name of a related object more than one (1) level away in a formula expression; this enhancement provides significantly more flexibility in formula logic.
The list of fields in the formula editor will not be updated to list all fields on related objects, but the use of relationship notation will be accepted and valid.
Learn more about Creating Formulas in Vault.
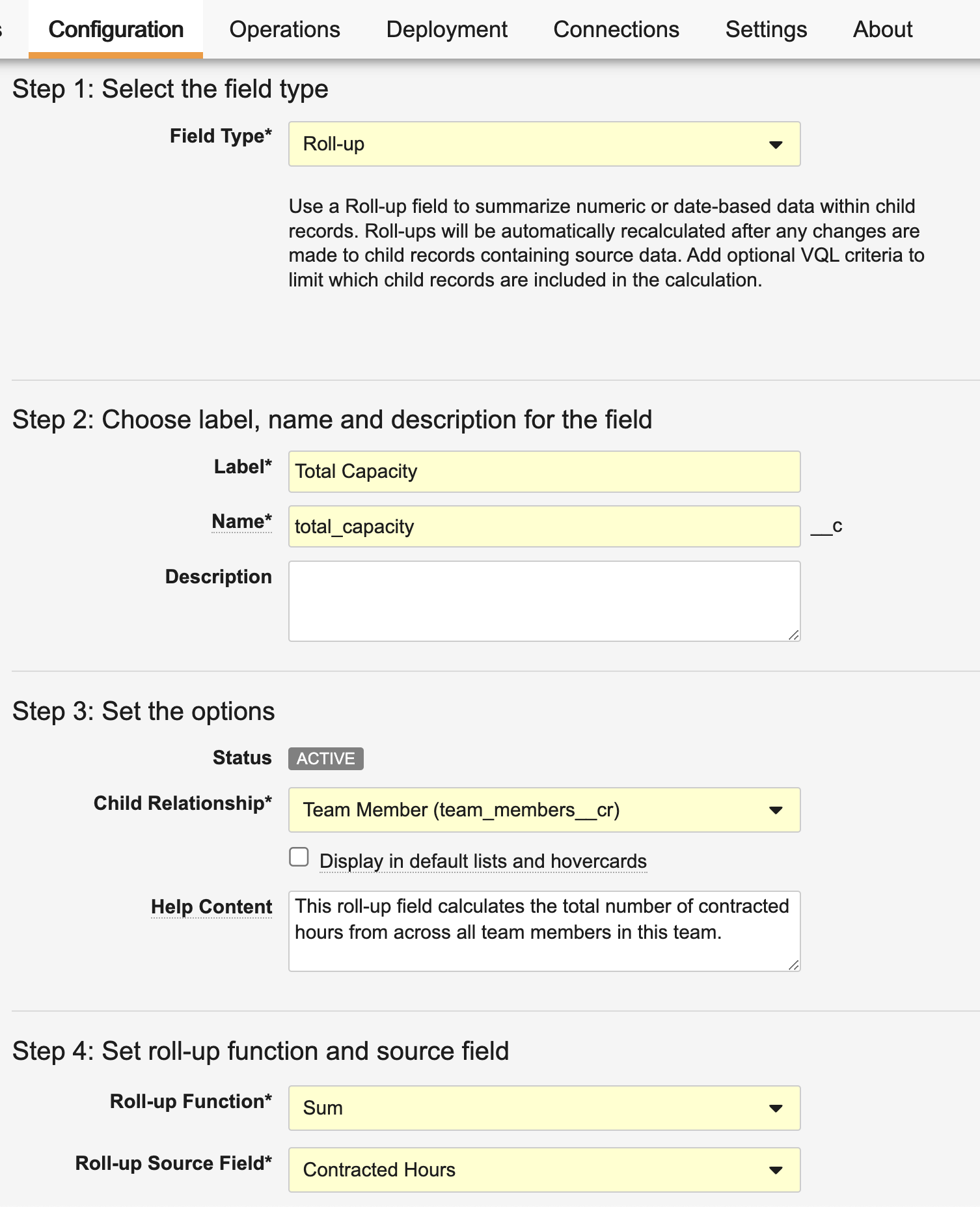
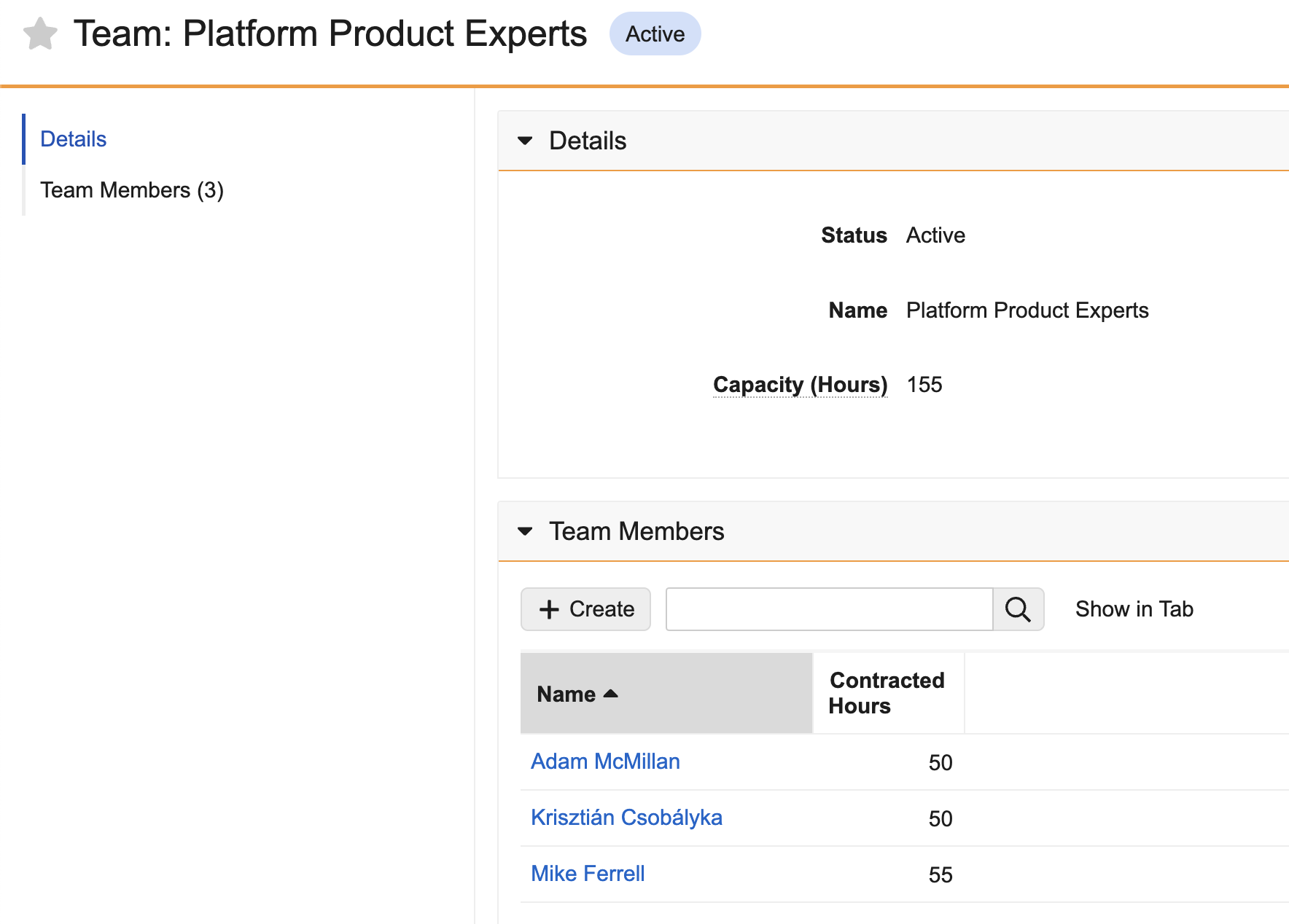
Roll-up Fields
Roll-up fields offer a powerful way to aggregate values from child records associated with a parent object via a parent-child object relationship. Without any manual effort or calculation, Roll-up fields constantly deliver these calculated values over time.
You can define up to 25 Roll-up fields on parent objects to aggregate a value (count, sum, max, or min) from child records. The aggregated value is calculated when a child record is created, updated, or deleted. You can also use a filter expression to determine which child records to include in the roll-up function.
Roll-up fields are best for use cases where you need to generate and store a calculated value at a specific point-in-time, such as when child records are created, updated or deleted. They do not support use cases where the value could be different based on something dynamic such as the current user or current date.
Limits
Roll-up fields do not support multiple levels of aggregation (a Roll-up field of Roll-up fields). Parent objects with 100 or fewer child records calculate Roll-up fields synchronously, upon child record changes.
Asynchronous Calculation
Parent objects with over 100 child records calculate Roll-up fields asynchronously, due to performance considerations. We do not recommend using Roll-up fields in criteria or conditions that are enforced as part of a record save operation, such as validation or layout rules. Similar to all system-driven calculations that execute on record save, the more Roll-up fields on a parent object, the longer it may take the record to save. We recommend taking careful consideration before creating Roll-up fields.
New Field Type in Admin
Admins will now see a new Roll-up field type for parent objects that exist within a parent-child object relationship. Once selected, Vault prompts Admins to specify the specific child relationship, roll-up function, and field before saving.
Auditing Roll-up Field Changes
Updates to Roll-up fields on parent records are considered a change on the record, captured as System on behalf of the user that triggered the roll-up calculation, or System, in the case of synchronous and asynchronous calculations respectively within the Object Record Audit History. The Last Modified By field displays the value System in both of these scenarios. In addition, the Last Modified Date and Audit Trail will update accordingly; however, it will not cause Vault Java SDK triggers to execute.
Recalculating Roll-up Fields
Lastly, there are situations where an Admin may need to recalculate all Roll-up fields on all records in an object. One such situation is when you add a new Roll-up field to an object, as this will only calculate values on a go-forward basis when adding, updating, or deleting child records in the specified child relationship. If you need historical records to populate the roll-up value, use the Recalculate Roll-Up Fields button on the object’s Details tab. Alternatively, you can populate Roll-up fields via record migration mode. In all other instances, Roll-up fields are read-only.
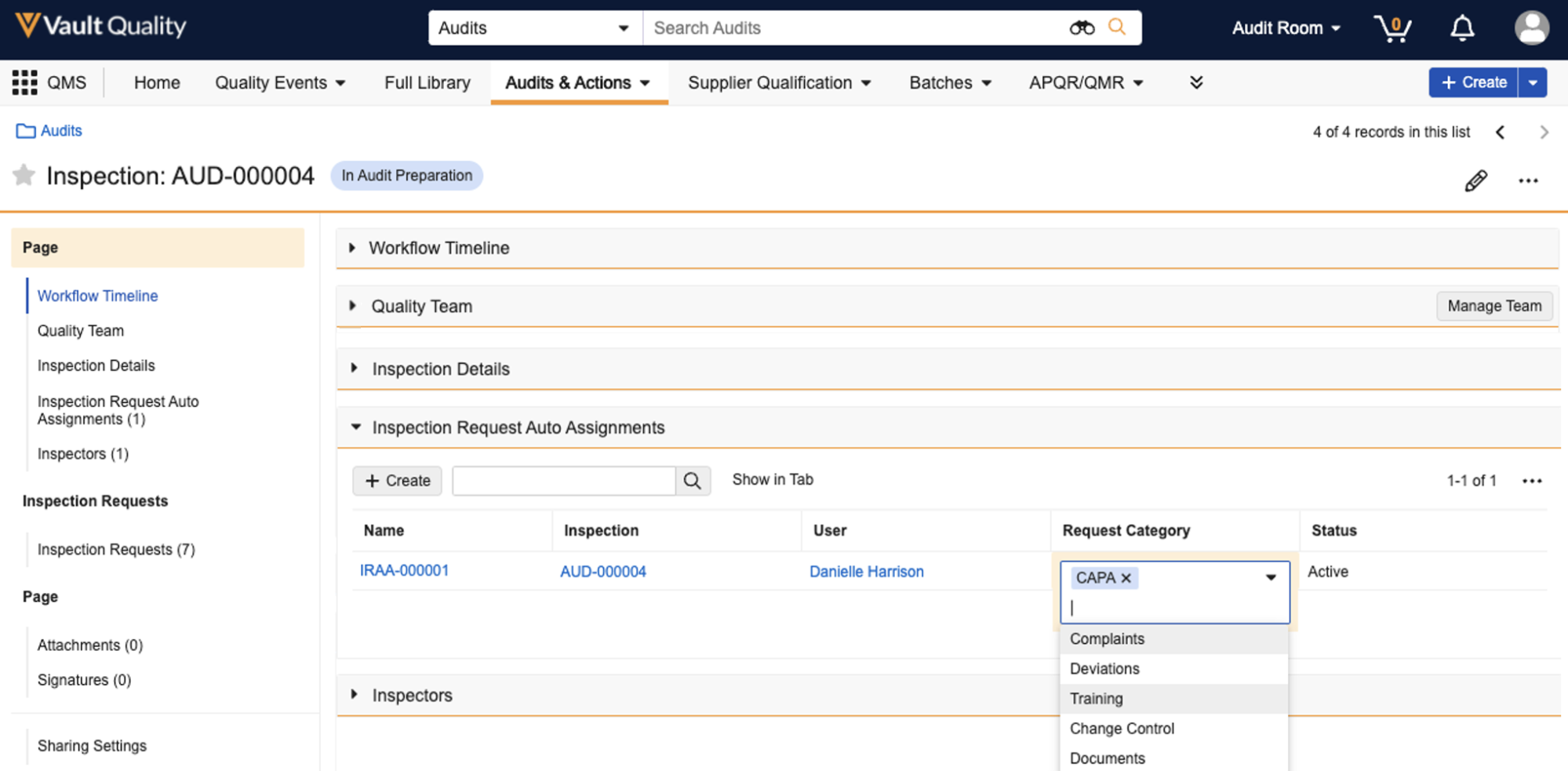
Role Filter for Objects
You can now filter object records by users in specific roles, much like you can today for document filtering, including the ability to use a dynamic Current User option. Sharing a saved view with the Current User selected is a useful tool for providing personalized saved views for users in your Vault.
Learn more about filtering by Role Assignments.
User Experience
Identifiable Browser Tabs
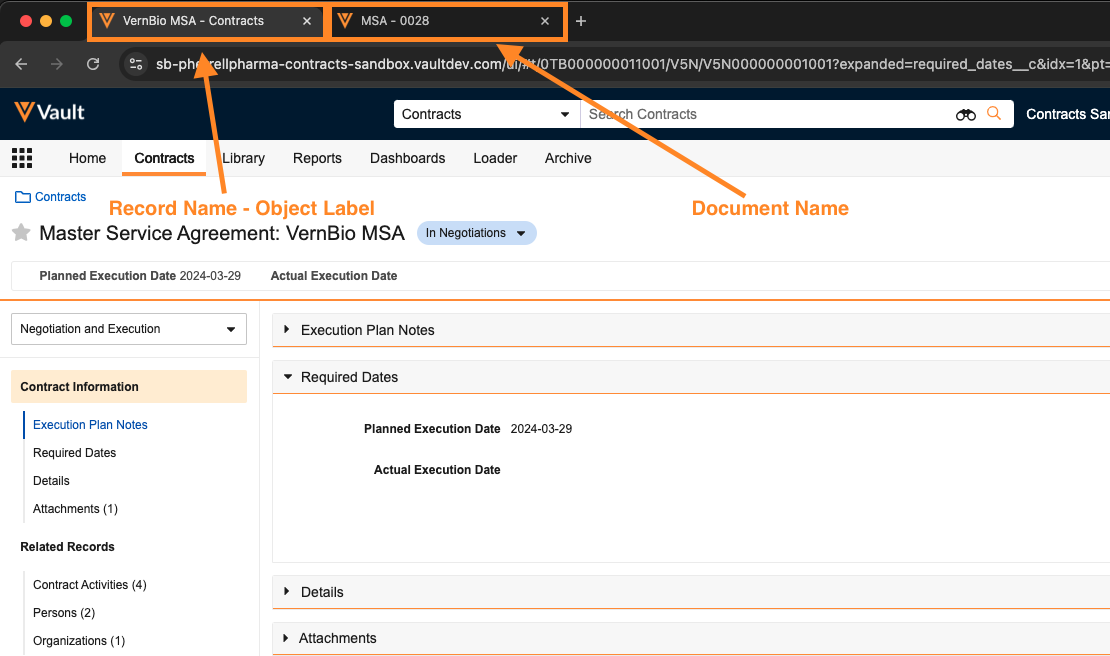
Browser tabs for Vault are now named in a more meaningful way that makes it easier for users to identify which tabs are open. Key identifiers in the browser tab names are formatted from most specific to least specific. For instance, when viewing a specific object record, the browser tab is formatted to show the record name first, followed by the object label. When viewing a specific document, the tab shows the document name.
Prior to 25R1, all browser tabs for Vault began with Veeva Vault, which made it difficult to find the specific tab you needed when multiple Vault tabs were open.
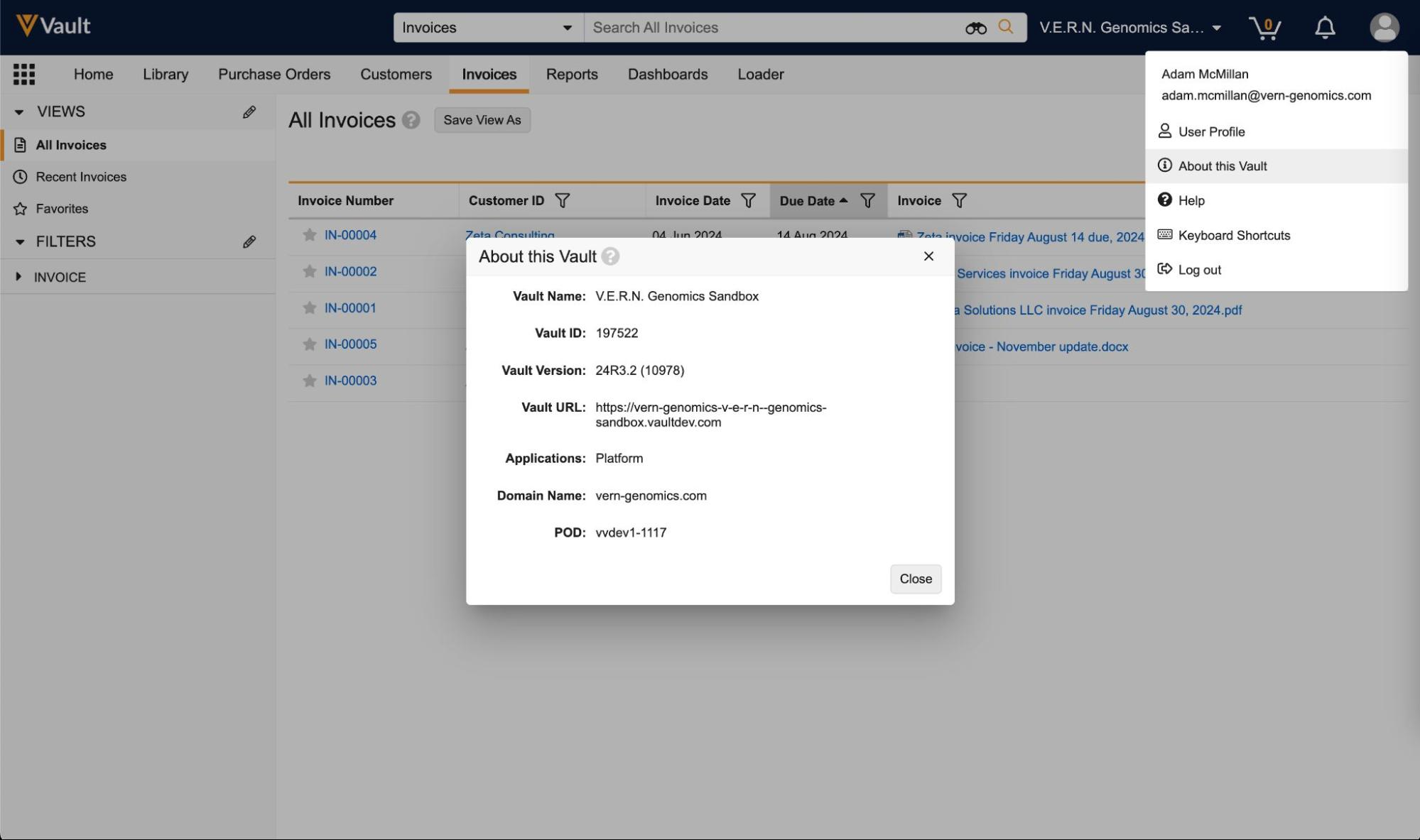
Access Vault Information from User Profile Menu
A new About this Vault option is available from the User Profile menu. This option opens a dialog containing information about the Vault such as the Vault Name, Vault ID, URL, Version, Applications, Domain Name, and POD. This is particularly helpful when providing Vault information to Veeva’s Product Support team.
Keyboard Shortcut Hints
Actions that have keyboard shortcuts assigned will now show the keyboard shortcut combination in the Actions menu, and also when hovering over actions in the Action Bar, making it easier to discover keyboard shortcuts for everyday actions.
Additional Document Viewer Keyboard Shortcuts
To further streamline the experience of viewing documents, users now have access to the following keyboard shortcuts when working with documents in the Document Viewer:
| Action | Windows Shortcut | MacOS Shortcut | Notes |
|---|---|---|---|
| Zoom In | CTRL + + (CTRL + plus sign) | CMD + + (CMD + plus sign) | Zooms in the document view. |
| Zoom Out | CTRL + - (CTRL + minus) | CMD + - (CMD + minus) | Zooms out the document view. |
| Fit Height/Fit Width | CTRL + 0 (CTRL + zero) | CMD + 0 (CMD + zero) | Toggles the document view between fitting the document height or document width in the document viewer. |
Additionally, to avoid conflicts with other keyboard shortcuts, the shortcut to toggle Grab & Pan mode has been updated to Shift + Space.
Learn more about Vault Keyboard Shortcuts.
Summary Email Enhancements & User-Driven Notifications
Vault now includes several enhancements to help reduce notification overload for users by using Summary Email notifications as a standard more frequently:
-
Reduce overall email notifications without configuration, specifically by:
-
Setting the Email Preference field to Summary if it was previously set to Every Occurrence on many notification templates (for both Platform and Application-specific templates).
-
Setting the Notification Category field to Tasks for Task Reminder notifications (
object_task_reminder_notification__vandtask_reminder_notification__v) if they were set to None. -
Updating the Delivery Interval default value to 1 hour.
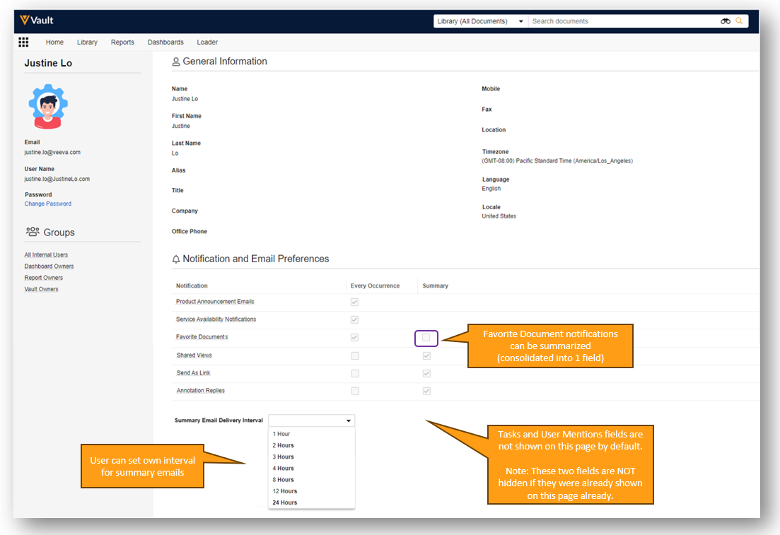
- Exposing some notification-related fields on the User Profile page by default, including Annotation Replies, Send As Link, Shared Views and Favorite Document notification fields, and a Summary Email Interval picklist, allowing users to adjust their own notification preferences.
- Tasks and User Mentions are not added by default, though if they were already added previously, they will not be removed.
-
Defaulting the Email Preferences value on new notification templates to Summary.
- Increasing the character limit for User Account Emails from 1,500 to 2,000.
-
-
Provide new options for users to control their own preferences further:
-
Favorite Document is a new notification category that will be available in 25R1, allowing users to choose email preferences for Favorite Document notifications
-
The existing Favorite Document notification options will be removed, consolidating those options into the new notification category field.
-
For users who have selected an existing Favorite Document notification, such as Favorite Document New Status, we will migrate those preferences to the new field as Summary. If no selections were made previously for Favorite Document notifications, the field will show Never.
-
-
Summary Email Interval is a new field that will be available in 25R1 which will allow users to choose the interval that works best for them.
-
This change helps all users receive less emails from Vault, while still receiving the information they need in Summary Emails, and reduces effort for Admins.
Admins can still perform additional customization, such as creating custom Notification Categories, adjusting preferences by template, adjusting the Email Summary settings, and adjusting the fields to show on the User Profile page.
All of these changes will be available in 25R1, but customers do have an option to defer the following changes to 25R2:
-
Setting the Email Preference field to Summary if it was previously set to Every Occurrence on many notification templates (for both Platform as well as application-specific templates).
-
Setting the Notification Category field to Tasks for Task Reminder notifications (
object_task_reminder_notification__vandtask_reminder_notification__v) if they were set to None. -
Updating the Delivery Interval default value to 1 hour.
-
Exposing some notification-related fields on the User Profile page by default, including Annotation Replies, Send As Link, Shared Views and Favorite Document notification fields, and Summary Email Interval, allowing users to adjust their own notification preferences.
-
Tasks and User Mentions are not added by default.
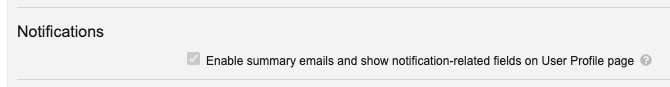
To defer these changes to 25R2, Admins must clear the following option in Admin > Settings > General Settings before the 25R1 release goes live:
Learn more about Summary Emails.
User Locale Not Restricted by User Language
Users can now have any combination of Locale and Language values, depending on their needs. Prior to this release, the available locales a user could choose from were constrained by the Language selected.
In 24R3, the Improved Date Field Entry feature was introduced, allowing Vault to accept and convert numeric date field entries based on a user’s Locale. In some instances, it was not possible to assign the preferred Locale to a user without updating their Language.
Now in 25R1, we are removing that constraint, and providing users the freedom to have any preferred combination of Language and Locale.
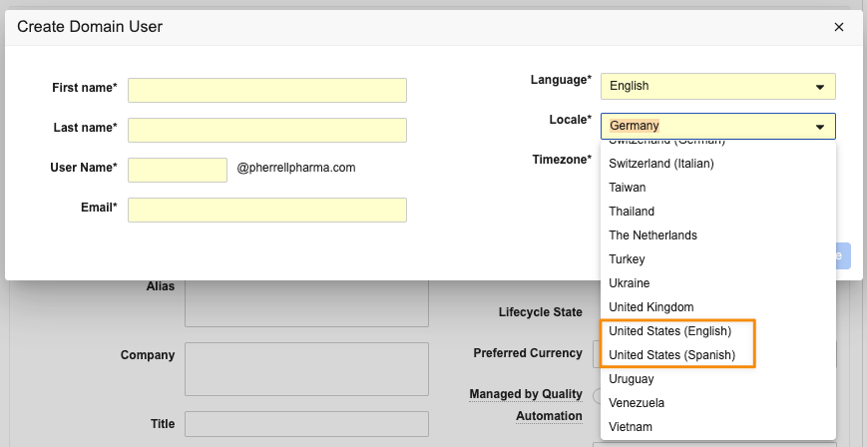
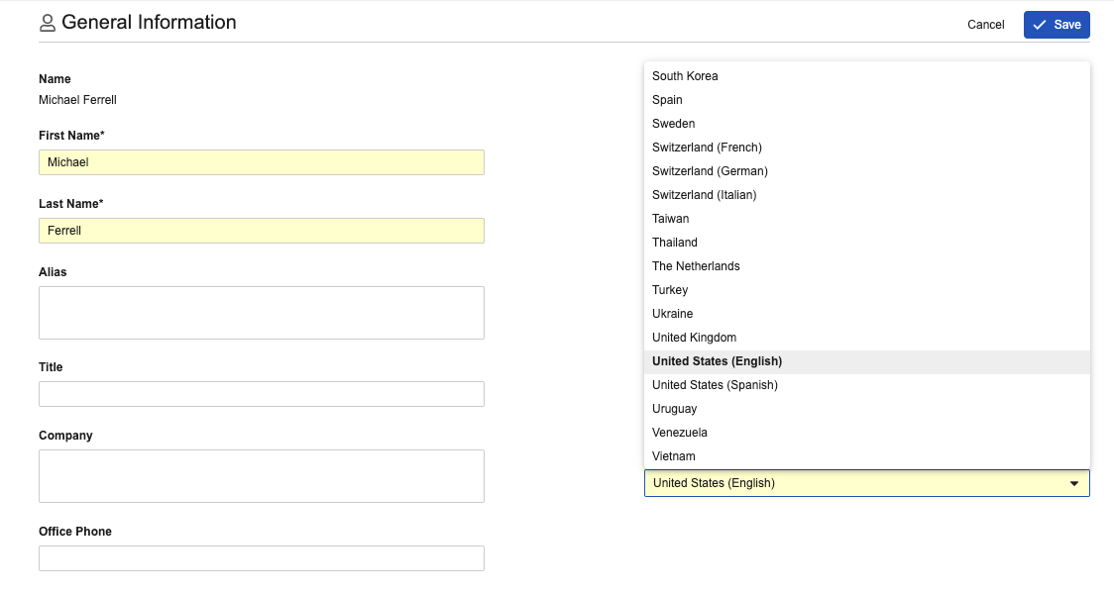
In prerelease Vaults, Locale names that are duplicated include the Language in parentheses (for example, United States (Spanish)) wherever the Locale field is visible. To ensure there is no undue impact to potential integrations, the name changes will be reverted on object pages, which means the parentheses will be displayed when creating a new Domain User or editing Locale via the User Profile, but will not be visible when editing an existing User or Person record (in Admin > Users & Groups > Vault Users or Business Admin). It also would not be visible on other objects that may reference the Locale object (if configured).
Domain User (parentheses included):
User Profile (parentheses included):
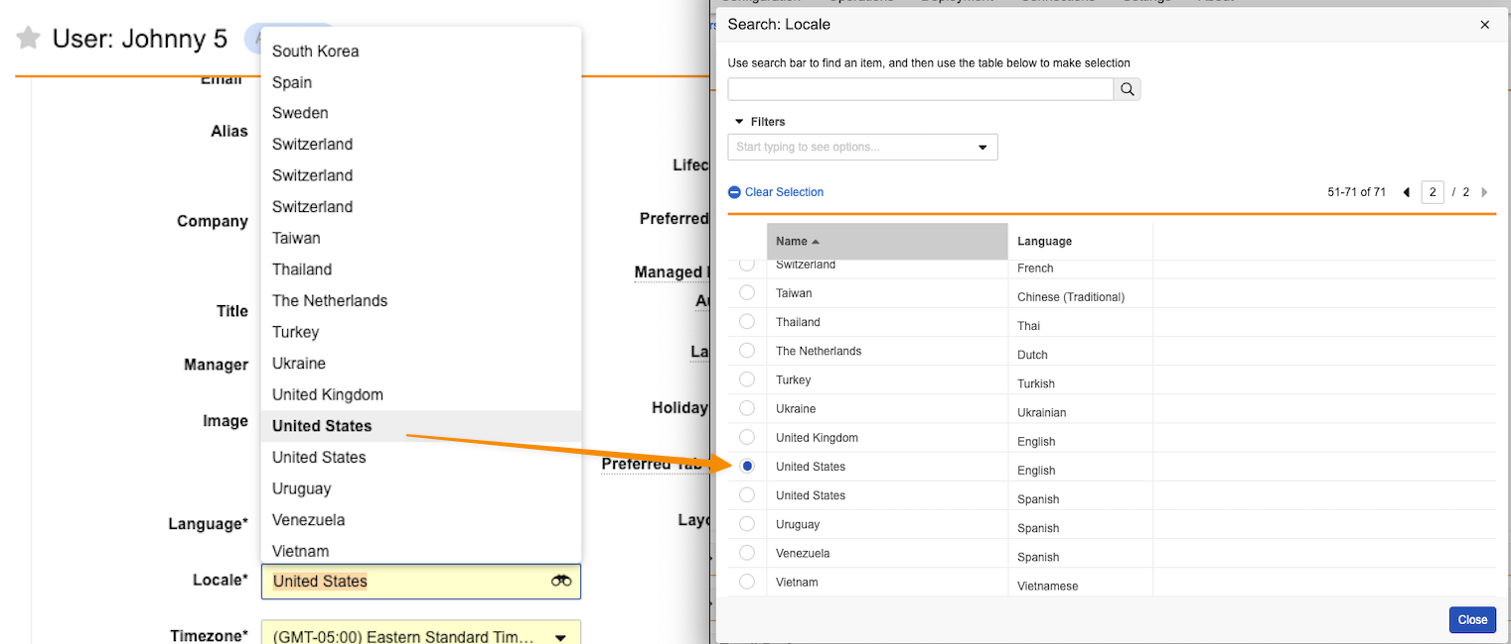
Editing User Records
When editing a User record (from Admin > Users & Groups > Vault Users or via Business Admin), Admins will need to use the binoculars in the Locale field on User to ensure they are selecting the Locale with the right Language when duplicate Locales exist.
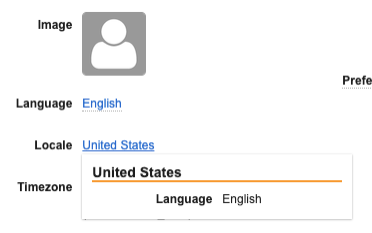
The associated Language will also display when hovering over a Locale value:
Note: Locale will continue to be constrained by Language for VeevaID users until the 25R1.3 Limited Release. At that time, VeevaID users will be able to choose any combination of Locale and Language.
Add Self-Registered VeevaID Users to Vault
When a potential VeevaID user attempts to register for the first time, Vault now presents them with any pending invites they have received, provided those invites have not expired. Then, they can click the link to accept the invite and be added to the Vault(s).
The pending invite links will display the company name and Vault name as defined in the VeevaID settings.
Prior to 25R1, if a VeevaID user registered directly at id.veeva.com, rather than through an invitation link, they had no mechanism to accept invites they previously received. This enhancement allows them to move forward directly, without needing external communication with the sponsor organization(s) that sent the invitations.
This feature is not available in prerelease Vaults and will be delivered as part of the full 25R1 release.
Learn more About VeevaID.
Analytics
Dashboard Prompt Enhancements
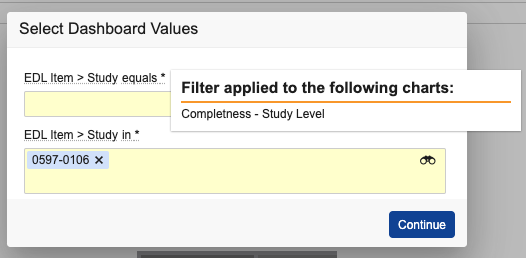
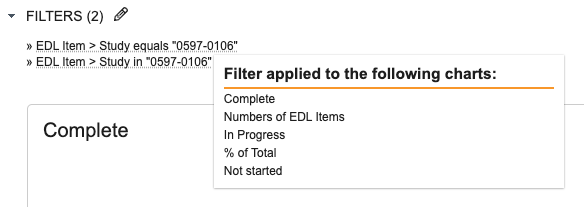
When adjusting dashboard filter prompts, it is now clear which dashboard component each filter prompt controls. The filter prompts are now shown in a pop-up dialog similar to report prompts, and users will be able to hover over prompts to easily identify which charts a prompt is associated with.
You can also hover over the selected filters to see this information when the dashboard is in view mode.
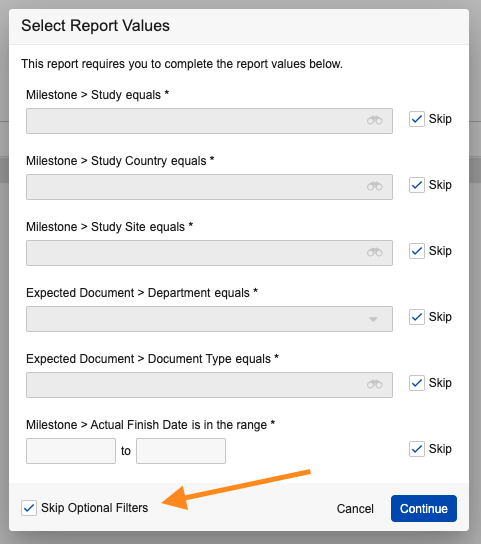
Skip All Optional Prompts
When a report or dashboard contains multiple optional prompts, users can now use Skip Optional Filters to skip all prompts.
For reports and dashboards that may contain a lot of optional prompts, this provides users an easy option to skip all prompts and see the full report results without having to click to skip each prompt individually.
Learn more about Optional Prompts.
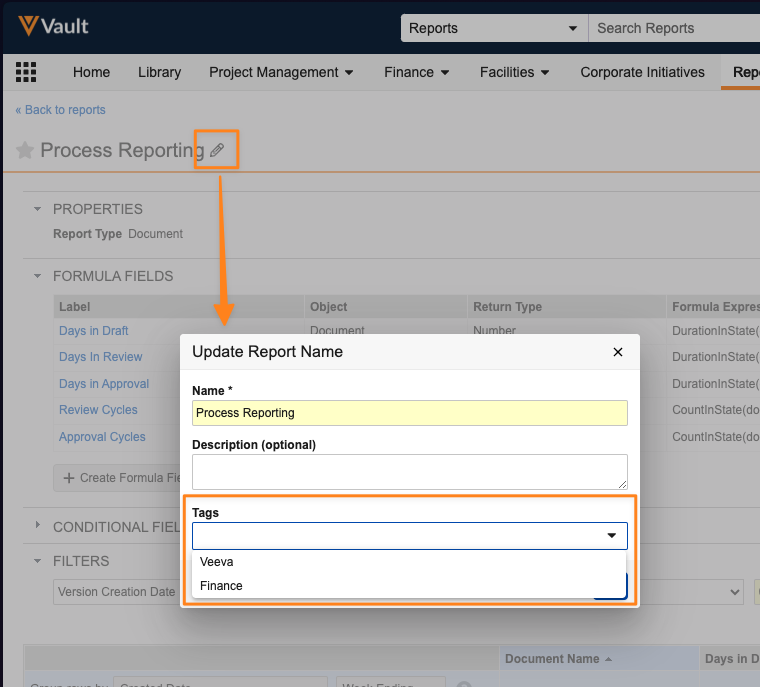
Allow Editing of Tags in Description Dialog
When editing a report’s name and description, users now have the option to also apply or modify Tags.
Prior to 25R1, report Tags could only be added from the list view on the Reports tab. Including Tags in the Name and Description dialog helps ensure that users remember to associate the appropriate tags while building the report without needing to remember to go back to the full list of reports to add the tags.
Learn more about Report Tags.
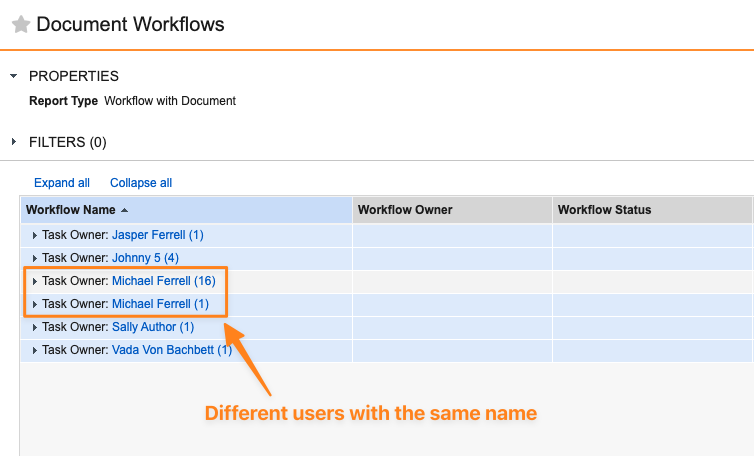
Group By Unique User in Reports
When a report is grouped on a User object field, Vault will now ensure that users with the same first name and last name are grouped separately, based on their unique user IDs. Prior to 24R3, if two users had the same first and last name, Vault would group all results as if they were the same user. Grouping based on user ID ensures that reports grouped by User fields accurately reflect information for each user without combining results based on names.
This also helps ensure that flash reports that are sent based on a grouped User field send the right results to the right recipients when individuals have the same first and last name.
Support for Additional Fields on Object Types in Formula Expressions
With 25R1, you can reference the API Name (api_name__v) and ID of an Object Type in formula expressions, and whether it is the default Object Type (default_type__v). Prior to this release, you could only reference the label of an Object Type.
This update is available everywhere that uses formulas, including formula fields in objects, field defaults, validation rules, and expression-based conditions on lifecycle and workflow configuration.
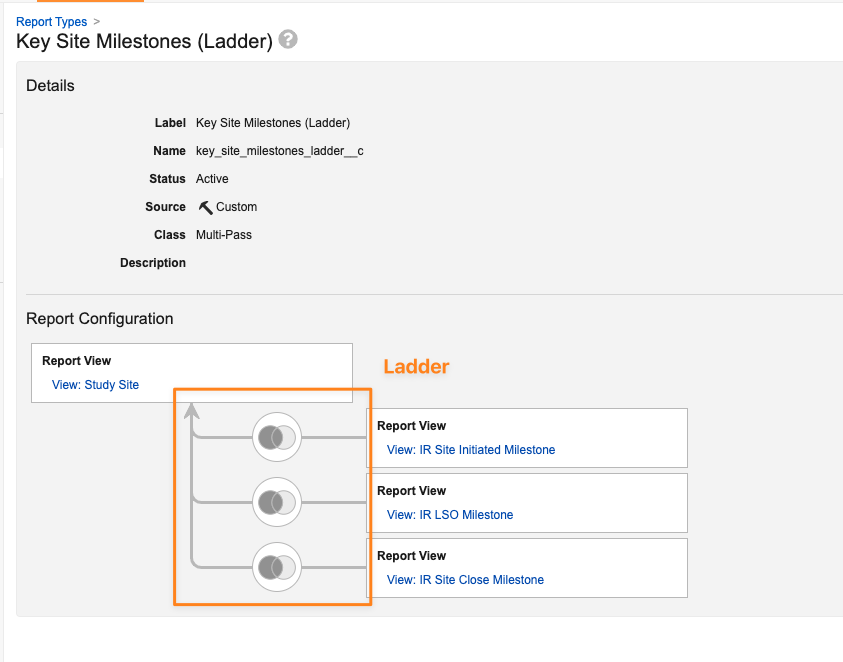
Relationship Constraint Filters for Ladder Multi-Pass Reports
Report users can now use relationship constraint filters in ladder multi-pass reports, which allows for filtering a primary Report View based on whether there are matching records in a child Report View.
A ladder multi-pass report is a report type that joins each secondary Report View directly back to the primary Report View:
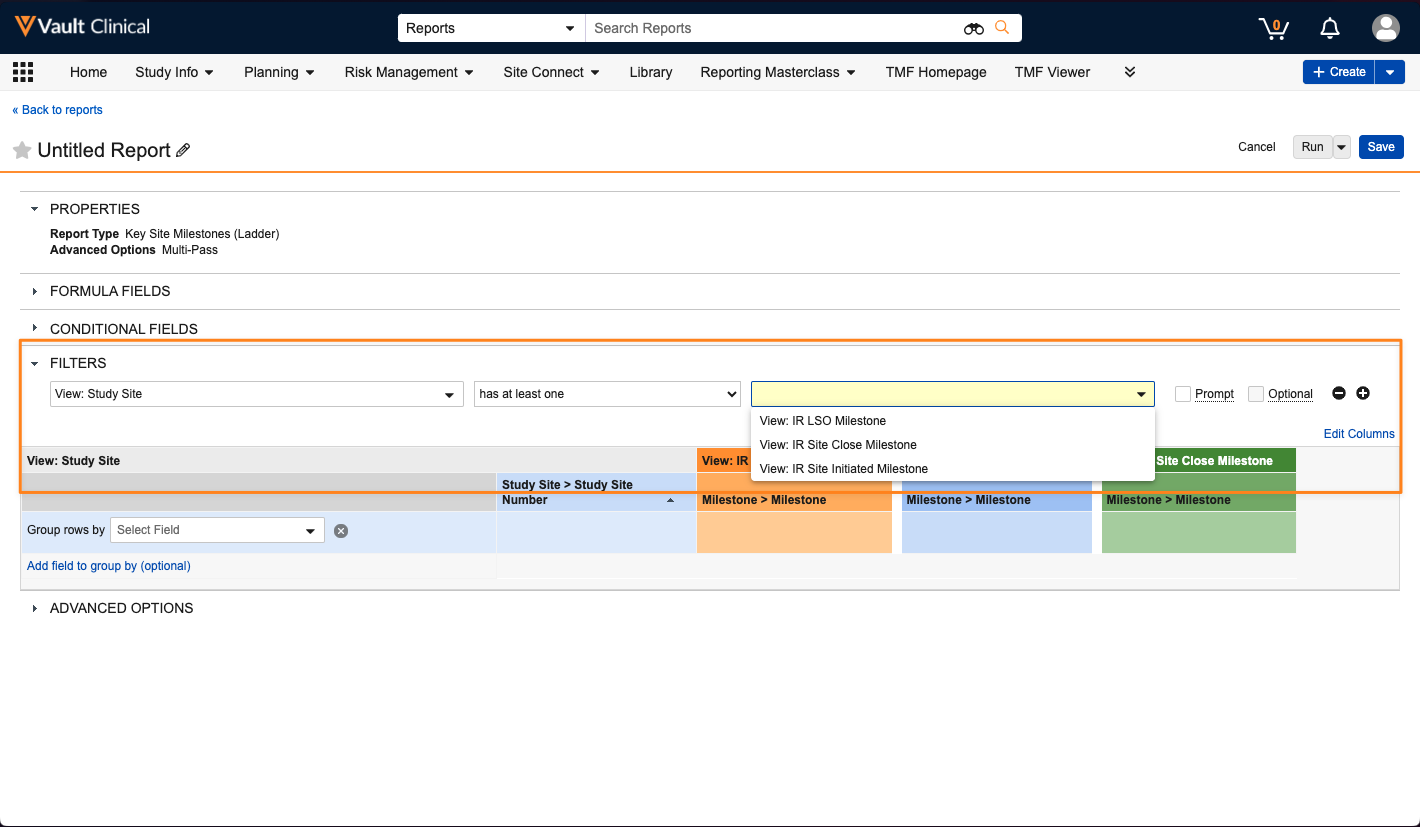
With 25R1, when a user creates or edits a report using a ladder multi-pass report type, they can apply has at least one or does not have any filters between the primary Report View and any secondary Report Views:
Prior to 25R1, a user could apply filters to secondary views like Name is not blank, but this would only filter the secondary view and would not filter the overall report results. Using these relationship constraint filters provides the ability for a report to be more focused in its results based on whether there are matching records in a secondary view.
Learn more about Multi-Pass Reporting.
Report PDF Export Enhancement
When exporting a report to PDF that contains long text or rich text fields, and the report is set to fit PDF to page, Vault will overflow text onto additional pages as needed to ensure that all information is appropriately included in the PDF output. Prior to 25R1, there could be instances where report PDFs truncated long text or rich text fields in the output, but this enhancement ensures this truncation does not occur.
This enhancement specifically applies when a report includes columns for long text or rich text fields, has the Fit PDF export columns to one page option checked in Advanced Options, and has Include full text for rich text fields or Include full text long text fields checked.
Learn more about Exporting Reports.
Admin Experience
Migration Package Deployment Enhancements
We have changed the way Migration Package (VPK) logs are attached to a package. Previously, each log file was a separate attachment. From this release, there is a single attachment, with each new log added as a new version.
Additionally, the Retrieve Package Deploy Results API now includes a URL to download a ZIP file containing all data deployment success and failure logs.
Enhanced Report MDL Migration
Customers can migrate reports with report filters on reference fields even if the referenced object, user, or document is not in the target Vault. Customers can then create the referenced value on the target Vault after the migration, and the report engine will pick this new value for the next report run.
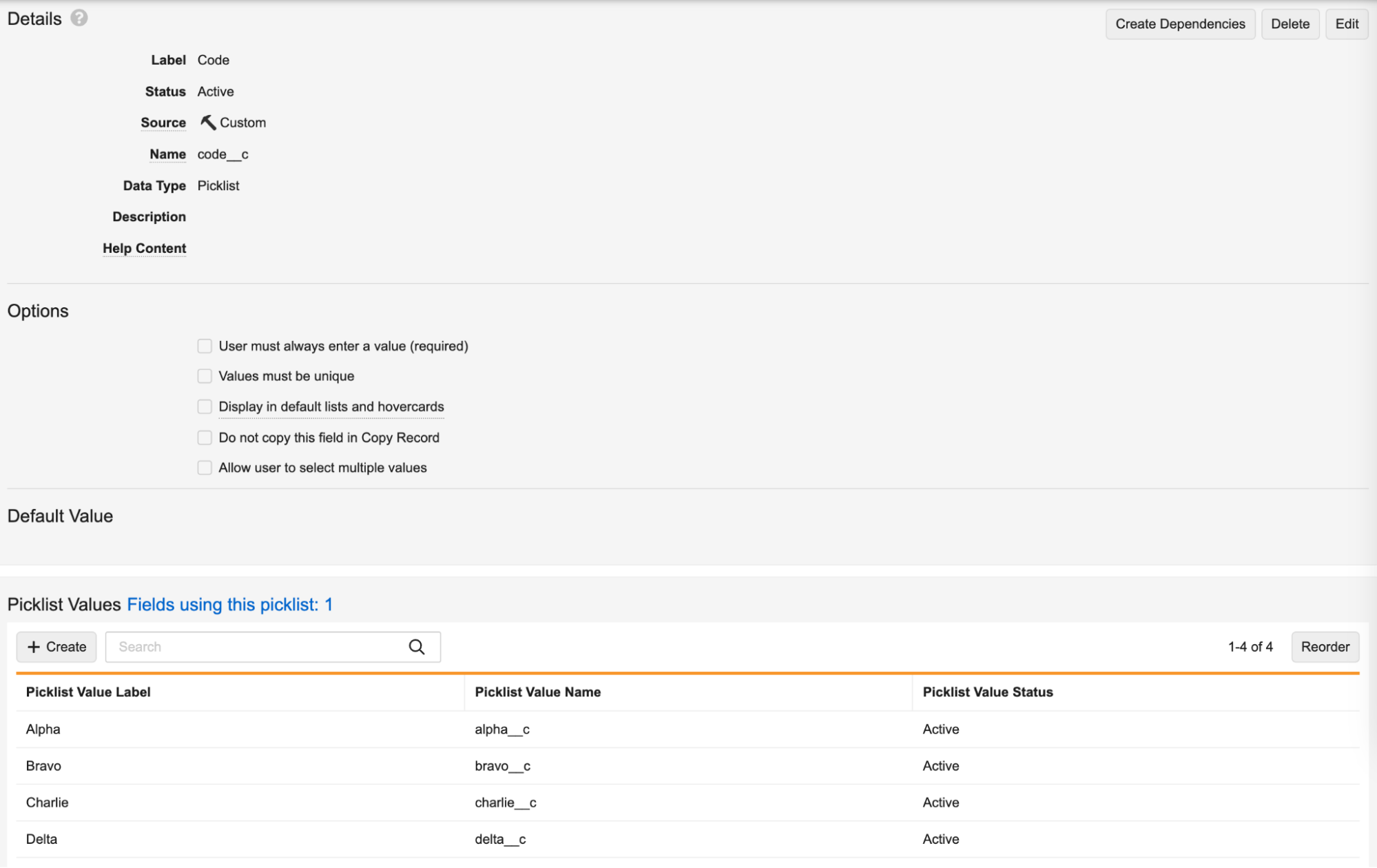
Enhanced Picklist Administration
This release includes many UI updates for picklists, including the ability to add and manage picklist values directly from the field using the picklist, the ability to search for values, picklist value pagination, improved reordering, underscore and special character matching, and inline editing capabilities.
Picklist Administration
New Picklist Values search:
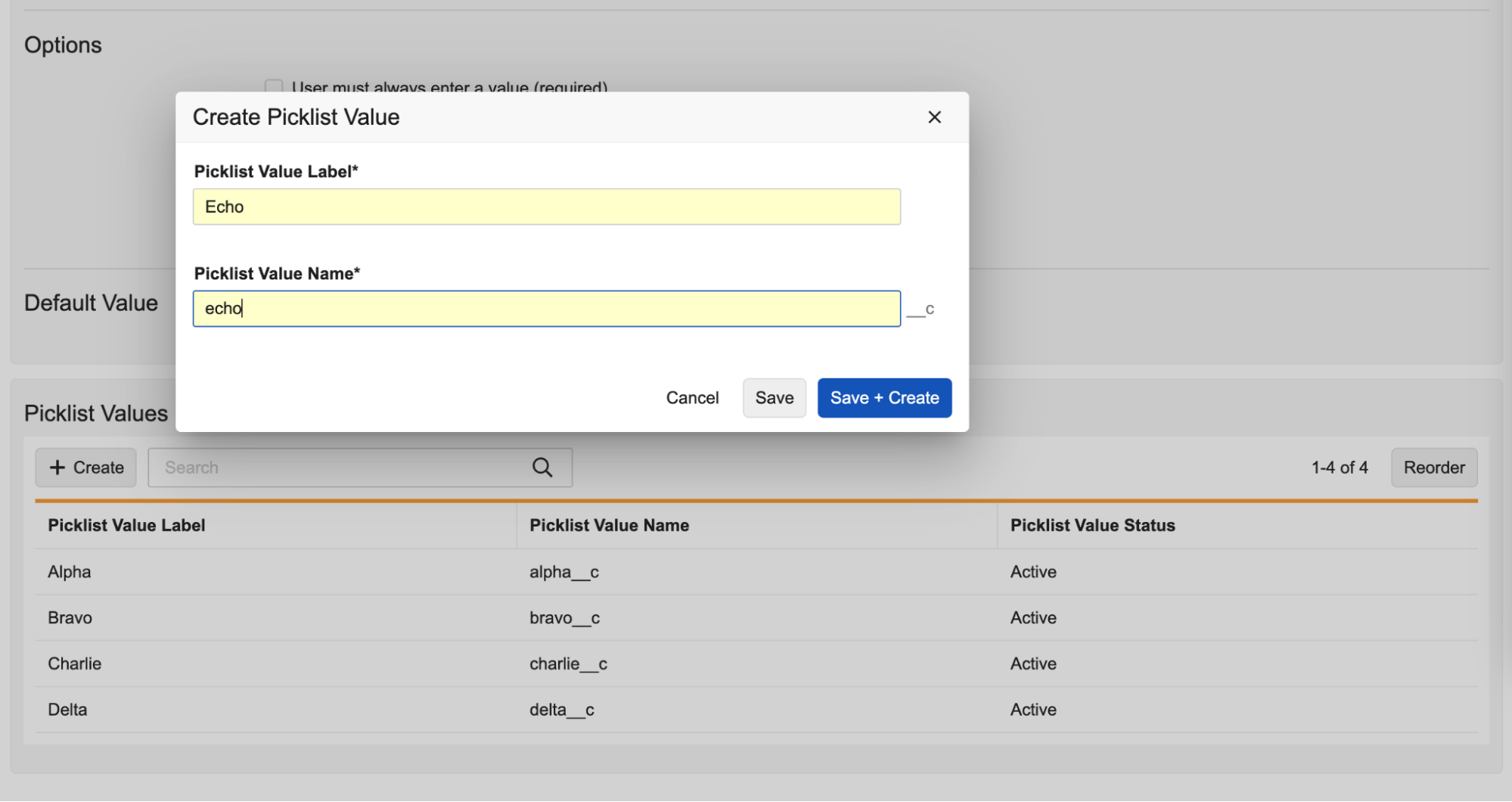
Create new picklist values directly from the Picklist field on the object:
Pressing the Enter key in the Create Picklist Value dialog executes the Save + Create action, allowing efficient entry of multiple values.
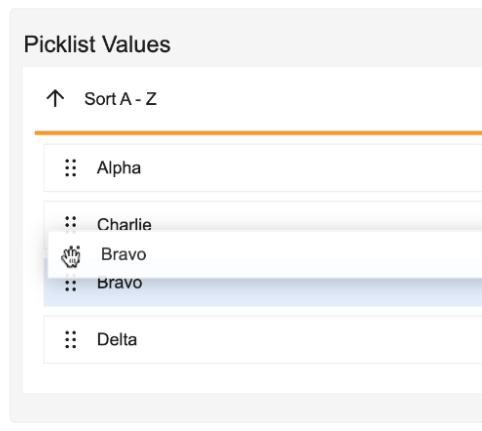
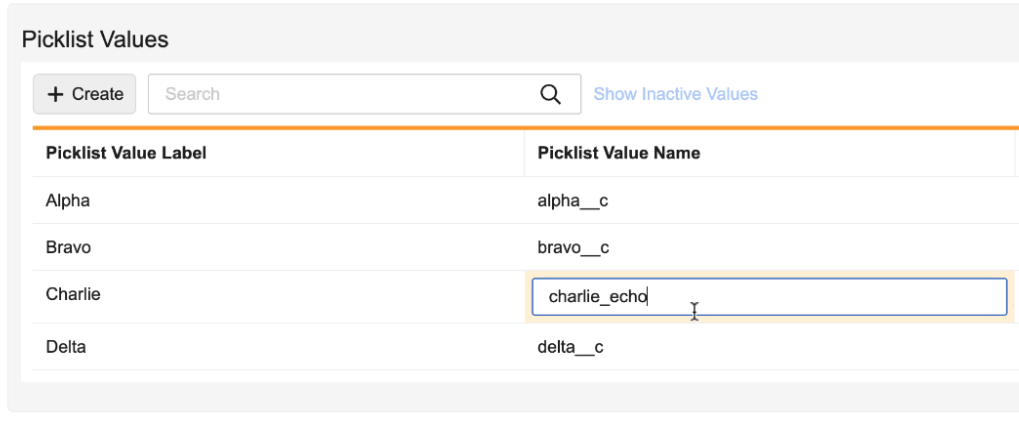
Manage, reorder, and inline edit picklist values directly from the object field using this picklist:
Picklist Dependencies
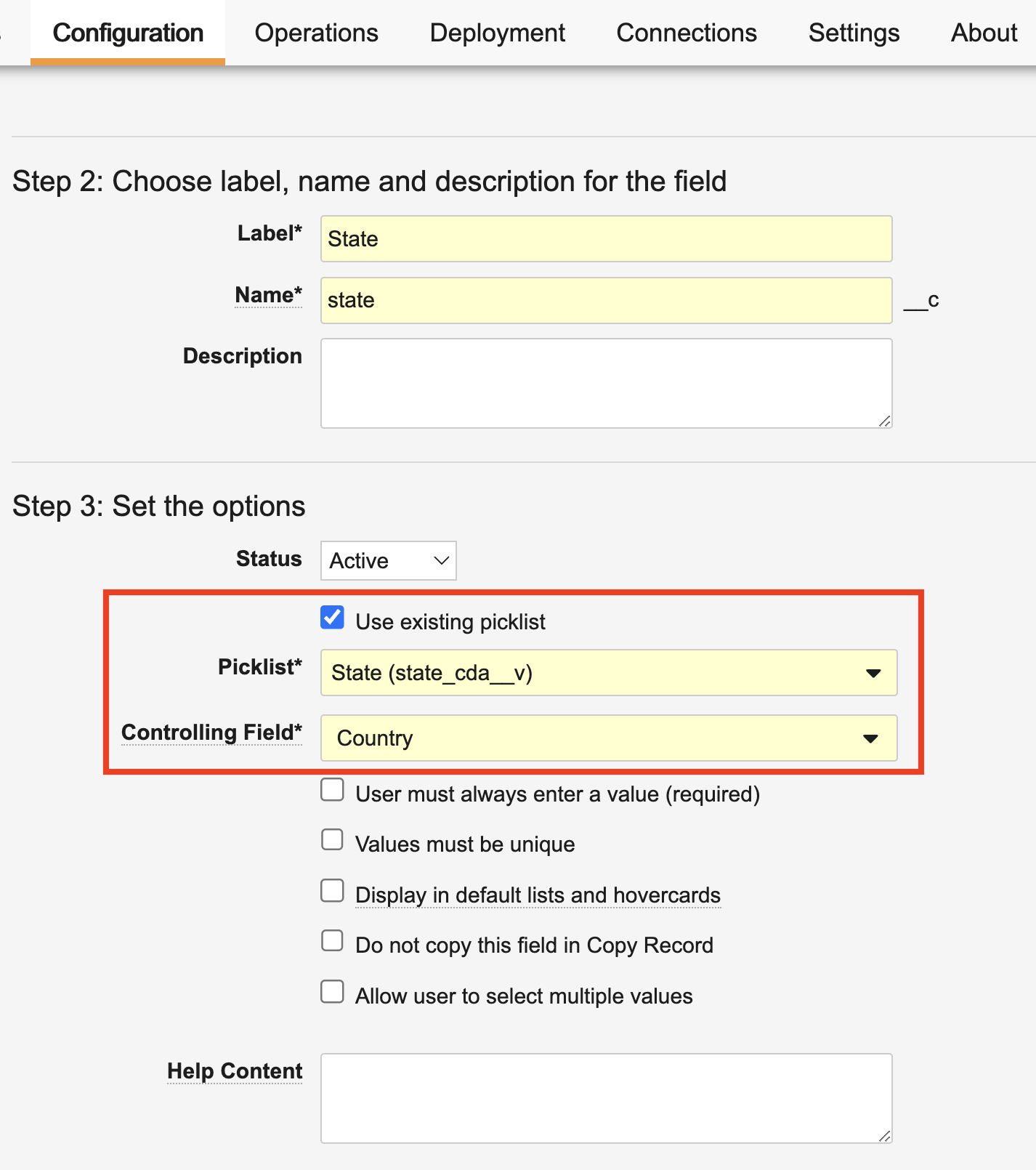
With 25R1, along with Admin users defining picklist dependencies on a specific Picklist object field, some standard and system-managed picklists can now have dependencies at a Vault level. These Vault level dependencies can only be configured by Vault Platform and application teams, and these dependencies are enforced for any Picklist object fields that use these picklists.
When reusing a dependent picklist on a Picklist object field, Vault requires you to also select the controlling Picklist object field, which must use the controlling picklist. For example, if you reuse the new State (state_cda__v) picklist in a Picklist object field, you must have another field on the object referencing the controlling Country (country_cda__v) picklist:
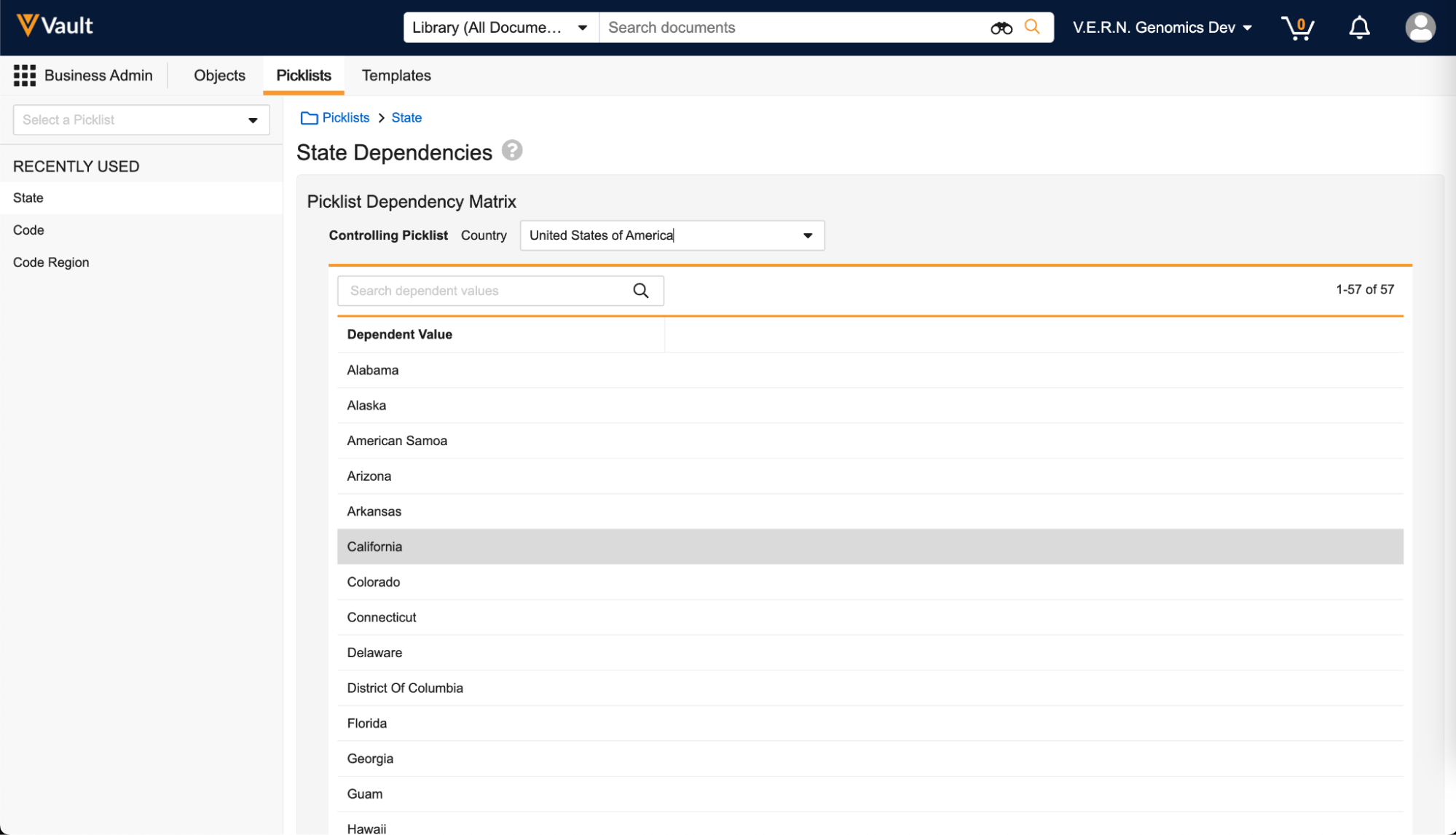
You can view standard and system-managed picklist dependencies within a new dependencies interface accessible from the picklist Admin UI (Business Admin > Picklists > View Dependencies):
Picklists can only be controlled by either Vault level or object field level dependencies, not both. Dependencies on standard and system-managed picklists are delivered by Platform and Vault application teams, and cannot be created by customers.
These changes improve the picklist administration experience, and help support picklists that contain larger sets of values. For large picklists, the View Dependencies interface provides pagination in increments of 200 values. As part of these enhancements, and to ensure optimal performance, picklists with over 200 values may only be reordered via API.
Learn more about picklist dependencies.
Data Model Documentation
Data Model Documentation for Vault applications and the Vault Platform will now be available directly within Vault, making it easier for customer Admins to access information about both the standard data model for their Vault, as well as any changes to the data model in the most recent General Release.
Users will be able to access this by going to the Help page from the upper-right dropdown.
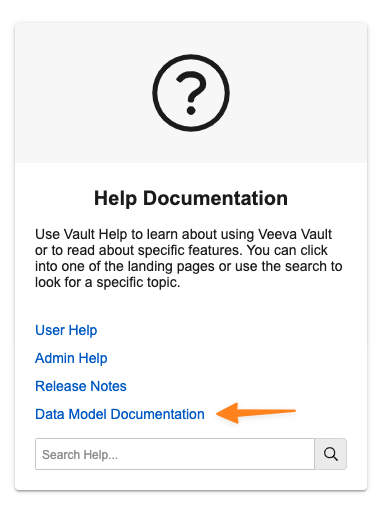
A Data Model Documentation hyperlink is available in the Help Documentation section:
This hyperlink will route to a Veeva Connect post where registered users can download an Excel file that contains:
-
An Overview sheet: This sheet contains a high-level data model diagram for the given application.
-
A “Delivered Model” sheet: This sheet contains all standard components in the data model, including what application each is associated with (which can be used to help troubleshoot licensing exceptions where a user has access to an object related to an application for which they aren’t licensed).
-
An “XXRX Updates” sheet: This sheet contains a list of all data model changes made for the latest release related to that Vault’s application family (RIM, Quality, etc.) as well as Platform changes.
Layout Profiles: Save As Action
You can now create copies of existing layout profiles by using the new Save As action. This is helpful for creating variations of layout profiles that already have many layouts selected across many objects, allowing for efficient administration. This new action is not available for the All Layouts layout profile.
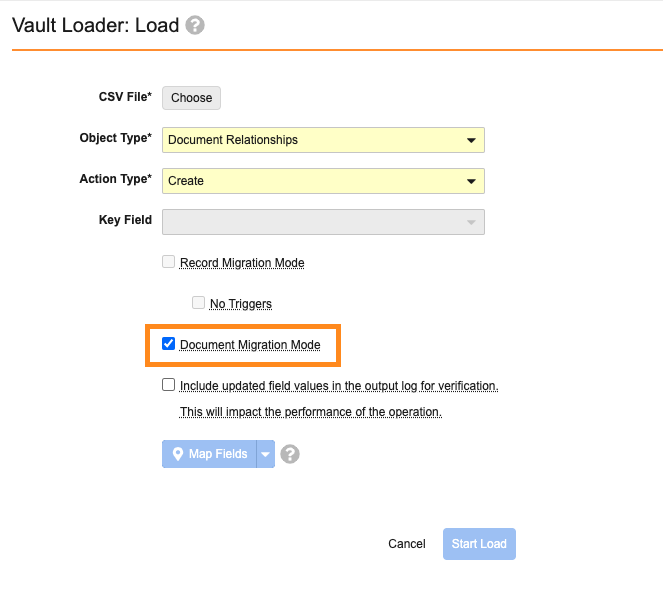
Document Migration Mode Support for Document Relationships
In order to support Similarity Scores in PromoMats (a new feature coming in 25R1), Document Migration Mode now supports creating Document Relationships using Vault Loader or the API.
In PromoMats Vaults, enabling Document Migration Mode when loading Document Relationships allows Vault to skip creating Content Similarity records, if needed. This allows for faster bulk creation when migrating Created From relationships that don’t require leveraging the Similarity Scores feature.
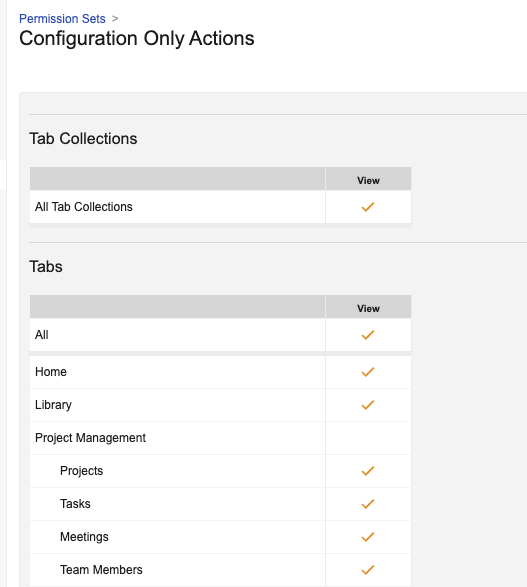
Standard Configuration Only Security Profile
Vaults now include a new standard security profile called Configuration Only to support roles that can manage configuration without access to data and documents. The Configuration Only security profile contains a standard permission set called Configuration Only Actions, which provides:
- Admin tab permissions allowing configuration tasks only (Data and Documents Audit log access is not included)
- All permissions (except Vault Owner) in the Application tab
- Limited object permissions (for objects supporting configuration)
-
All tab permissions under Tabs
- Functionally, these users would only see the Home, Library, and Loader tabs by default. All other tabs are restricted because they don’t have access to the objects.
This security profile only has Read access for users, but can be associated with user roles to provide the ability to perform user management actions, as well as to provide additional, select data access where needed.
Learn more About Standard & Custom Profiles & Permission Sets.
VeevaID SSO Option
When working with VeevaID users, organizations will now have the option to provide those users with an existing Single Sign-On (SSO) option, in addition to the standard VeevaID login. All site users are required to have a VeevaID for Veeva applications, though some sponsors have also created SSO solutions for working with site users. This option allows VeevaID users to choose, when authenticating, whether they want to login using their standard VeevaID credentials or the sponsor organization’s SSO.
Note: This feature will be completed as part of the 25R1 release, though it will not be available as part of the initial 25R1 release deployment. The feature will be available in all Vaults the week of May 5, 2025, following the initial release go-live. Once available, the existing VeevaID security policy will be visible to Admins, and Domain Administrators will be able to edit and assign an SSO profile.
Learn more about Administering VeevaID.
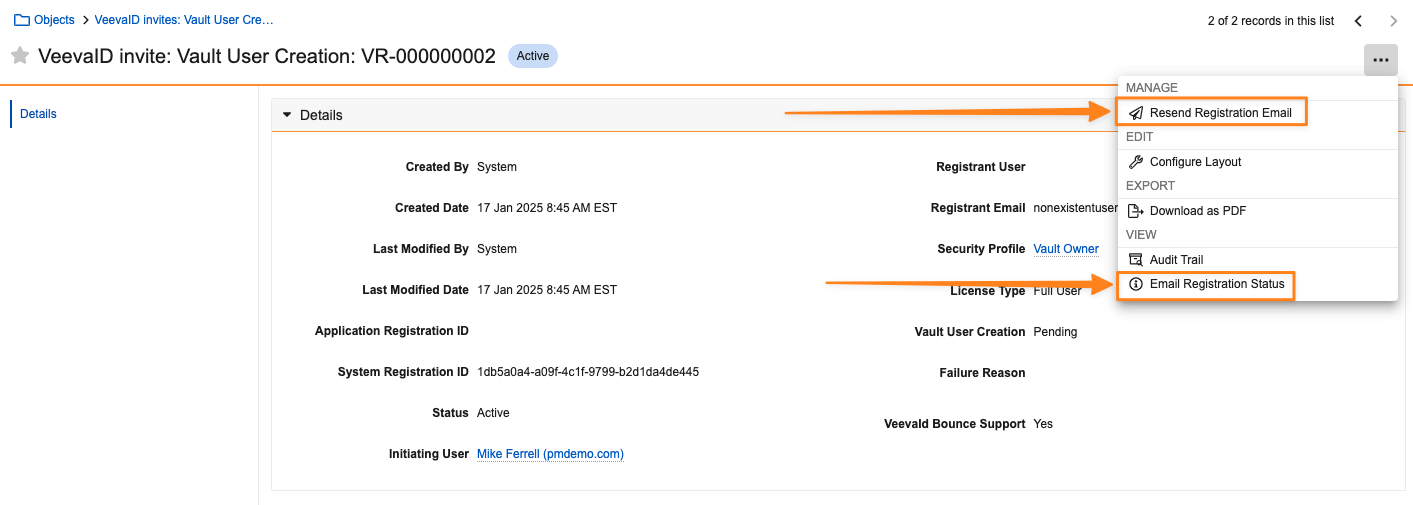
VeevaID Bounce Support
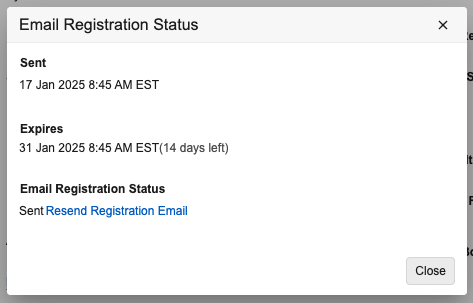
Admins now have increased visibility into VeevaID registration emails that are generated from Vault, including the ability to see if an email was successfully sent and attempt to resend the registration email if needed.
Prior to 25R1, Admins could see invitations in the VeevaID Invites: Vault User Creations object in the Business Admin, but could not see information on the actual status of the invitation email nor resend the invitation.
When on a VeevaID Invites: Vault User Creation record, Admins will now have two options:
- Resend Registration Email resends the registration email to the user. The existing VeevaID Invite: Vault User Creation record will also be updated.
- Email Registration Status opens a dialog that contains details on the VeevaID registration email’s status, including the date and time it was sent, when it expires, and the status of the email. A hyperlink will also be available to resend the registration email here as well.
Learn more About VeevaID.
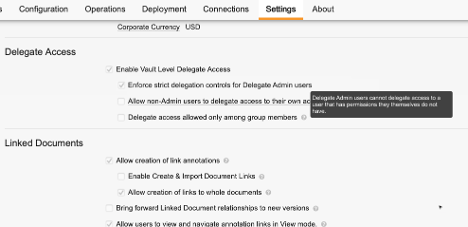
Additional Delegate Access Validation
For Vaults using Delegate Access, additional controls are now available that can prevent an Admin from delegating access to another user’s account if that action would delegate permissions they themselves do not have.
A new setting is available in all Vaults in Admin > Settings > General Settings called Enforce strict delegation controls for Delegate Admin Users.
This new setting is enabled by default on all Vaults where Delegate Access is enabled, and allows Vault to validate that an Admin is not granting permissions they don’t have. This is similar to the validation that occurs when an Admin attempts to assign a Security Profile to a user that grants permissions the Admin doesn’t have.
For example, the following three users are involved:
- Vault Owner
- System Admin
- Business Admin
If the System Admin attempts to assign the Business Admin as a delegate for the Vault Owner, Vault prevents this and displays an error message.
The same behavior also applies if the System Admin attempts to edit an existing delegation.
There is no change to behavior when a user is delegating their own account via their User Profile. For instance, the System Admin can still delegate their account to the Business Admin.
Learn more about Enabling Delegated Access.
Minor Enhancements
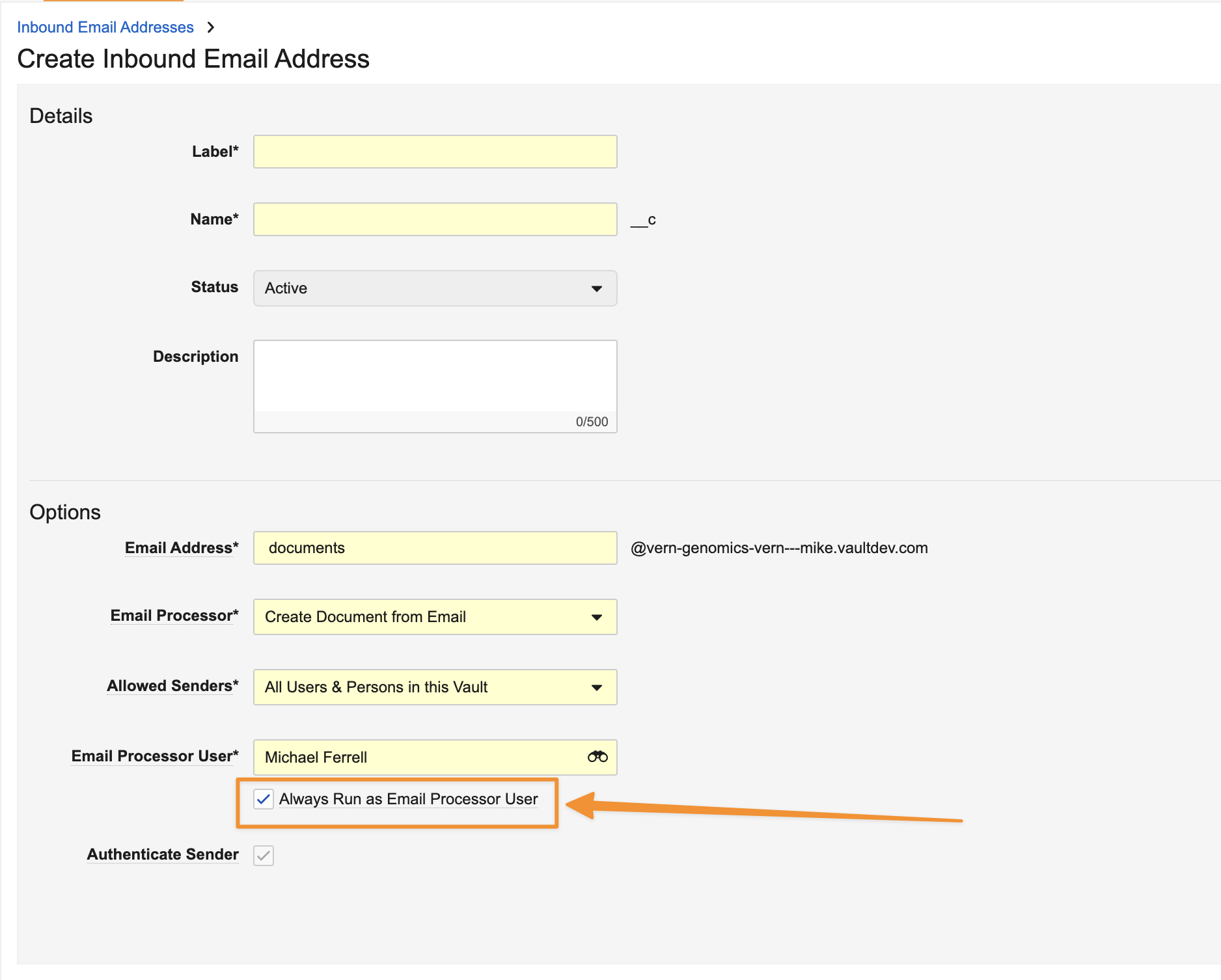
Email to Vault: Option to Always Run As Email Processor User
When leveraging Email to Vault with email processors that allow for emails to be sent by Persons or Anyone, Admins can now specify that the processor always runs as the Email Processor User, even if the sender is a Vault user. Prior to 25R1, an Admin could define an Email Processor User for these processors, but this would only apply if the sender was not a Vault user.
This enhancement specifically benefits the Medical Inquiry Email Processor. This processor is intended to always come from individuals that are not Vault users, and prior to 25R1, if an email was received that matched a Vault user, it failed. Allowing the processor to always run as the Email Processor User ensures emails are consistently processed.
Learn more about Configuring Email to Vault.
Searchable Object Fields Enablement
Filter and Search Documents by Related Object Fields
As a reminder about this existing functionality, when searching or filtering documents in a document tab, you can only search or filter on the Name (name__v) field of the records selected in object reference fields by default. In Admin > Configuration > Searchable Object Fields, you can configure additional fields from those objects to be searchable and filterable. For example, if an Organization object has an Abbreviation field and a Website field, you might want to search for documents according to the Abbreviation or Website of the records selected in the Organizations document field. You can also achieve this today by adding a Lookup Field, but there are a limited set of supported field types for that functionality.
To make it clearer which fields are configured, Vault no longer shows the Name (name__v) field for each object on the Searchable Object Fields page (because Name is always searchable and filterable by default). This makes it easier to view and manage any additional searchable object fields you have configured. You can configure up to 50 searchable object fields in total, across all objects.
Searchable Object Fields Enabled in All Vaults
Today, there are some Vaults where the Searchable Object Fields functionality was never enabled. On those Vaults, every single object field was searchable and filterable when searching for documents. This functionality caused significant performance issues for the document search functionality and made it difficult for users to find the filters they wanted to apply, since every object field appears in the filter list. In this release, the Searchable Object Fields functionality is enabled for all Vaults, and cannot be disabled.
Given the limit of 50 fields, we have performed analysis on these Vaults to pre-configure the most utilized object fields which will preserve all existing saved view criteria. We also preserved the fields that were most frequently selected by users when performing document searches to ensure minimal impact, if any.
Change Owner on Unclassified Documents
When working with unclassified documents or documents in the Staged lifecycle state, users with the Change Owner permission can now replace the document Owner on documents prior to classifying them.
This functionality has already been available in documents, but until now it was only able to be performed on Unclassified documents via the API.
Learn more about Unclassified Documents.
Saved Filters in Create Claim Link Select Target Dialog
Vault now saves and reapplies user-defined filter settings in Create Claim Link’s Select Target dialog. These filter settings persist across documents, browsers, and sessions as a saved user preference, which are also updated by modifying filters while editing an existing Claim Link’s references. This enables more efficient manual claims linking on documents where auto linking does not provide all the required referencing for any reason.
Latest Steady State Document Reference Consistency
If an object field of type Document Reference is configured to target the latest steady state of a document, it will now update with the latest document information even if the document’s state changes without creating a new version.
Consistent Last Modified By & Last Modified Date for Deleting Latest Document Version
When the latest version of a document is deleted, while maintaining the prior versions, Vault will now update both the Last Modified By and Last Modified Date fields on the new latest version..
Prior to 25R1, only the Last Modified Date was updated, which could lead to confusion about who performed the last modified action.
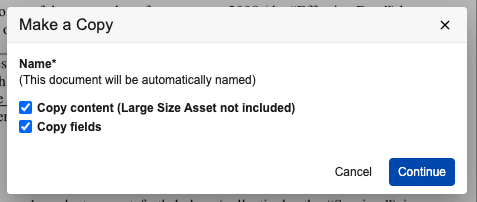
Make a Copy Dialog Indicates That Autonaming is Enabled
Vault will no longer prompt users for a new document name when using the Make a Copy action if the document name will be auto-named by the system. Prior to 25R1, the Make a Copy dialog would allow the user to input a new document name even though their entry would be disregarded for auto-named document types.
Learn more about Copying Documents.
Ignore Locked Objects During Deep Copy
When performing a deep copy on an object record (for parent-child relationships), Vault now ignores any locked child objects (for example, objects that are disabled in Vault; typically based on which Vault Applications are enabled in the Vault). Before, deep copy was prevented on parent objects with locked child objects.
Vault Name & Logo Changes
Veeva recently updated Vault application branding from Vault [Application] to Veeva [Application]. The 25R1 release will incorporate these changes in the user interface, specifically the logos visible in the banners in the upper left, the standard login page, the MyVaults page (if enabled), and the logo used in email notifications.
For the following exceptions, an updated logo will be applied and communicated at a later date:
- Logos that currently display BrandOne, Claims, SiteVault Enterprise, or Training
- Logos that contain a unique customized name
25R1 Platform Data Model Changes
With every release, we update the data model to better support evolving needs and new feature functionality.
Access the 25R1 data model documentation on Veeva Connect:
Performance & Availability
Vault Platform Configuration Limits
As a reminder, you can always review the Vault Platform configuration limits.
Limit Increases for Objects
Vault Admins can now create more custom fields across all objects, including a higher number of custom unique fields, custom long and rich text fields, custom formula fields, along with more custom object types per object.
Note: The new limits for custom fields and unique fields exclude raw objects.
| Per Object Limit | Old Limit | New Limit |
|---|---|---|
| Custom Fields | 500 | 800 |
| Unique Fields | 10 | 25 |
| Long / Rich Text | 10 | 50 |
| Formula Fields | 25 | 50 |
| Object Types | 30 | 50 |
These limit increases are necessary as the needs of our customers grow, but configuring close to the maximum comes with performance costs. Special attention should be paid to any Vault Java SDK that interacts with these components, as unoptimized code could now have a greater impact on system and UI performance.
Long and Rich Text Fields
If your object has lots of long and rich text fields, this will have a significant impact on the time to create and update records. We recommend keeping it to under 10 per object type.
Document Usage Storage Policy
To improve overall performance, Vault automatically deletes records from the Document Usage object after 36 months. In addition, Vault deletes any Document Usage records that reference deleted documents, even if they are newer than 36 months. Any deleted Document Usage records will not be recoverable. The Document Usage object is a system-managed object that automatically tracks when a user views, downloads (either the source or rendition), or copies a steady-state document. Over time, this has resulted in tens of millions of records, which can impact search performance.
For customers that regularly report on document usage, any existing reports that are not already constrained by a time period will now only pull records for the previous 36 months.
Learn more about Document Usage.
Picklist Limit Updates
In multi-value picklist fields on objects, users can select a maximum of 100 values. Prior to this change, users could select more than 100 values, which resulted in a less optimal user experience and performance.
For existing object records with more than 100 values selected, users can still edit those records and the values in that field, as long as the number of values selected is less than or equal to the quantity currently selected.This limit will also be applied to picklist fields on documents in a future release.
While limited to 100 values on a given record, the overall limit for the number of possible entries that can exist in a picklist (whether applied to objects or documents) has been raised from 1,204 to 2,000.
Selected Entity Limit for Scheduled Data Exports
In 26R3, the Veeva Vault platform Scheduled Data Exports functionality will be disabled in favor of our more robust and scalable alternatives, Direct Data API and Vault Loader APIs. As we prepare to deprecate this functionality, planned limitations will be put in place over the next few general releases.
In 25R1, the maximum number of entities that can be included in Scheduled Data Exports is now limited to 30. This limit will not be enforced for Vaults that are already over this limit.
Admin Audit Export Feedback & Limit
When exporting object and document audit logs, Vault queries the audit tables for up to ten (10) minutes to preserve Vault-wide performance. Prior to this release, Admins could select a large time range that could attempt to export hundreds of millions of rows of data, and that process would eventually time out. This feature provides clear feedback that a limit has been reached instead of failing silently.
Email to Vault: Increase Inbound Email Address Limit
When leveraging Email to Vault functionality, which allows Vault to receive email directly, a single Vault can now have up to 250 inbound email addresses configured. Prior to 25R1, the limit per Vault was 50. While this is a change to the overall Platform limit, the main use case for this limit change is to enable MedInquiry use cases, where a separate address is needed per Country.
Learn more about Configuring Email to Vault.
Vault Connections
Clinical Operations-eCOA Connection
Enhance Clinical Operations Lifecycle Transfer for Sites
This feature enhances the Clinical Operations-eCOA Connection to automatically transfer standard and custom study site lifecycle states.
Learn more about other new Clinical Operations features and eCOA features below.
Clinical Operations-EDC Connection
Clinical Operations-EDC Connection: Enhanced Review Summaries for EDC Log Events
Vault EDC uses Log Events to capture data or details outside the scheduled activities in a clinical trial. These events, such as Adverse Events or Concomitant Medications, are not tied to subject visits pre-defined per the protocol but are critical for monitoring subject health and ensuring compliance. However, when these events resulted in corresponding subject visits in Vault Clinical Operations, the associated Source Data Verification (SDV) and Data Management Review (DMR) information was previously not transferred, leading to a lack of visibility, manual effort, and potential inconsistencies.
This enhancement improves the connection between Vault EDC and Vault Clinical Operations by automatically transferring SDV and DMR summary information to subject visits created for corresponding Log Events.
This functionality only applies to new or updated Log Events, ensuring consistent and accurate data synchronization and reducing manual updates while leaving unaffected the transfer behavior of scheduled, protocol-based subject visits, as already covered.
Learn more about other new Clinical Operations features below.
Clinical Operations-EDC Connection: Improved User Exception Message Processing
It is crucial to optimize the handling of failed records to ensure the Clinical Operations-EDC connection efficiently processes data and minimizes unnecessary resource consumption. This new feature introduces a more efficient processing mechanism for the Clinical Operations-EDC connection. The system now skips reprocessing successful records and focuses exclusively on new, changed, or failed records. Failed records are tracked using the User Exception mechanism and automatically included in subsequent processing. Additionally, the connection now maintains records of the Last Successful Run Time (LSRT) and Last Run Time (LRT) for each integration point, providing better visibility into data processing.
This enhancement is auto-enabled for all ClinOps-EDC Connection customers, enabling faster, more efficient, and resource-conscious data integration.
Learn more about other new Clinical Operations features below.
Clinical Operations-EDC Connection: Originating Site
The latest updates to EDC introduce improvements in handling subject transfers between sites during a trial. An Originating Site (Source Site) field was added to the EDC data model for managing subject-related data in case of a subject transfer, such as Protocol Deviations, Events (“Visits” in Clinical Operations), and Procedures. This field helps identify the site where these activities occurred.
Clinical Operations will keep existing Visits, Procedures, and Protocol Deviations at the originating site for subjects transferred within EDC, with a new field added to monitor the subject’s current site. Any new Visits, Procedures, and Protocol Deviations created after the transfer will be associated with the new site.
When a subject transfer occurs in EDC, the Connection will use the new Originating Site field value to retain the current site for Visits, Protocol Deviations, and Procedures.
This feature is auto-on and does not impact performance in the Connection. This feature only applies to subject activities created after the release. Subject activities created prior to the release will not include a value for the originating site.
Learn more about other new Clinical Operations features below.
Clinical Operations-EDC Connection: Visit Filtering
Previously, users could set Query Object Rules (QORs) to apply filters across all integration rules in the ClinOps-EDC Connection, except Visit data. This inability to exclude or include Visits from transfers can limit the overall filtering capabilities in some scenarios, given that visits are related to Subjects and Sites. This enhancement resolves such previous limitations and aims to provide a more complete and consistent filtering experience. The new feature allows Admins to define filtering for the Clinical Operations-EDC Connection when transferring visits from EDC.
This feature, available only through the Vault Admin section, enables Admins to configure visit filtering settings within the integration configuration interface, using Vault Query Object Rules.
Query Object Rules for visits must define the Event Deltas (event_deltas__vr) object as the query object. Filtering is supported for data points that are available in this object, such as Study, Subject ID, and Visit Definition.
Learn more about other new Clinical Operations features below.
Medical-Safety Connection
Medical-Safety Connection: Support Case Product Indication, Case Cause of Death
Reported Adverse Events may contain information about Product Indication and/or Cause of Death. These are now supported by new standard fields in Vault Medical on the Event Product and Event objects, respectively. The Medical-Safety Connection now creates the corresponding Case Product Indication and Case Cause of Death records in Safety when applicable.
Learn more about other new Medical and Safety features below.
PromoMats-Medical Connection
PromoMats-Medical Connection: Anchor Persistent ID Support
With this release, the PromoMats-Medical Connection supports Persistent IDs. Anchors created on a Crosslink by the PromoMats-Medical Connection now have the same anchor Persistent ID as the source Vault for traceability, identified by the Vault ID prefix. Connection-created anchors are not duplicated by the Bring Forward Annotations action and are always created by the connection anchor job.
Learn more about other new Commercial and Medical features below.
Quality-RIM Connection
Quality-RIM Connection: Enhanced Change Control
For customers using the Quality-RIM Connection to manage Change Controls, the new Enhanced Change Control feature is the next generation of Variation Management, providing added flexibility when users complete assessments and determine an appropriate filing strategy. Building on the 24R3 RIM feature, Support for Enhanced Change Management, the connection will now recognize a many-to-many relationship between Event records in RIM and Change Control records in QMS. The previous limitation of one Event per one Change Control did not allow for flexibility for submissions or tracking partial health authority decisions. With this enhancement, two new standard objects and lifecycles in QMS (Regulatory Change Item and Regulatory Activity Item) will interact with the standard RIM objects added in 24R3 (Change Item and Activity Change Item).
When QMS team members create Change Controls where regulatory assessments are needed, these are tied to Products and Product Variants though the manual creation of Regulatory Change Item records. The connection will create Change Item records in RIM, and Regulatory team members will then associate the appropriate Event records in RIM. Optional configurations also allow for the automatic creation of Events with Event Change Items in RIM. Regulatory team members now have the ability to bundle and split Activity Change Items to support their needed ways of working. For more details, see Splitting Support for Enhanced Change Management. In turn, the connection will create Regulatory Activity Items in QMS, which provides the Quality team visibility into the status of each product in each affected market.
Following Activity disposition and submission completion, and upon receipt of health authority decisions (if required), the connection will update the status of Activity Change Items (RIM) and Regulatory Activity Item records (QMS).
The changes above benefit Quality teams by allowing for individual assessments on each item affected by a Change Control. This also allows Quality to request reassessments of individual items if the Change Control scope changes. Regulatory teams will benefit from the additional granularity that supports appropriate filing strategies based on the impact of each change that is part of a single Change Control record.
Enhanced Change Control requires the harmonization of product data between RIM and QMS.
A typical connection process flow can be found below:
Learn more about other new Quality and Regulatory features below.
Quality-Safety Connection
Quality-Safety Connection: Transfer PQCs Found in Veeva Safety to Veeva Quality
Adverse Events (AEs) often include physical issues with the product, which are referred to as Product Quality Complaints (PQCs). Adverse Events that include Product Quality Complaints are common, and this creates the need for good communication between an organization’s Safety and Quality teams. Safety and Quality or Manufacturing departments typically rely upon email to communicate about AEs and PQCs, but the unstructured nature of email often leads to missing information. Incomplete data about AEs and PQCs lead to superfluous email exchange, extending the time required to address safety issues. Life sciences companies report that their Safety and Quality teams expend hundreds or even thousands of hours a year on back-and-forth email exchange, depending upon their volume of AEs and PQCs.
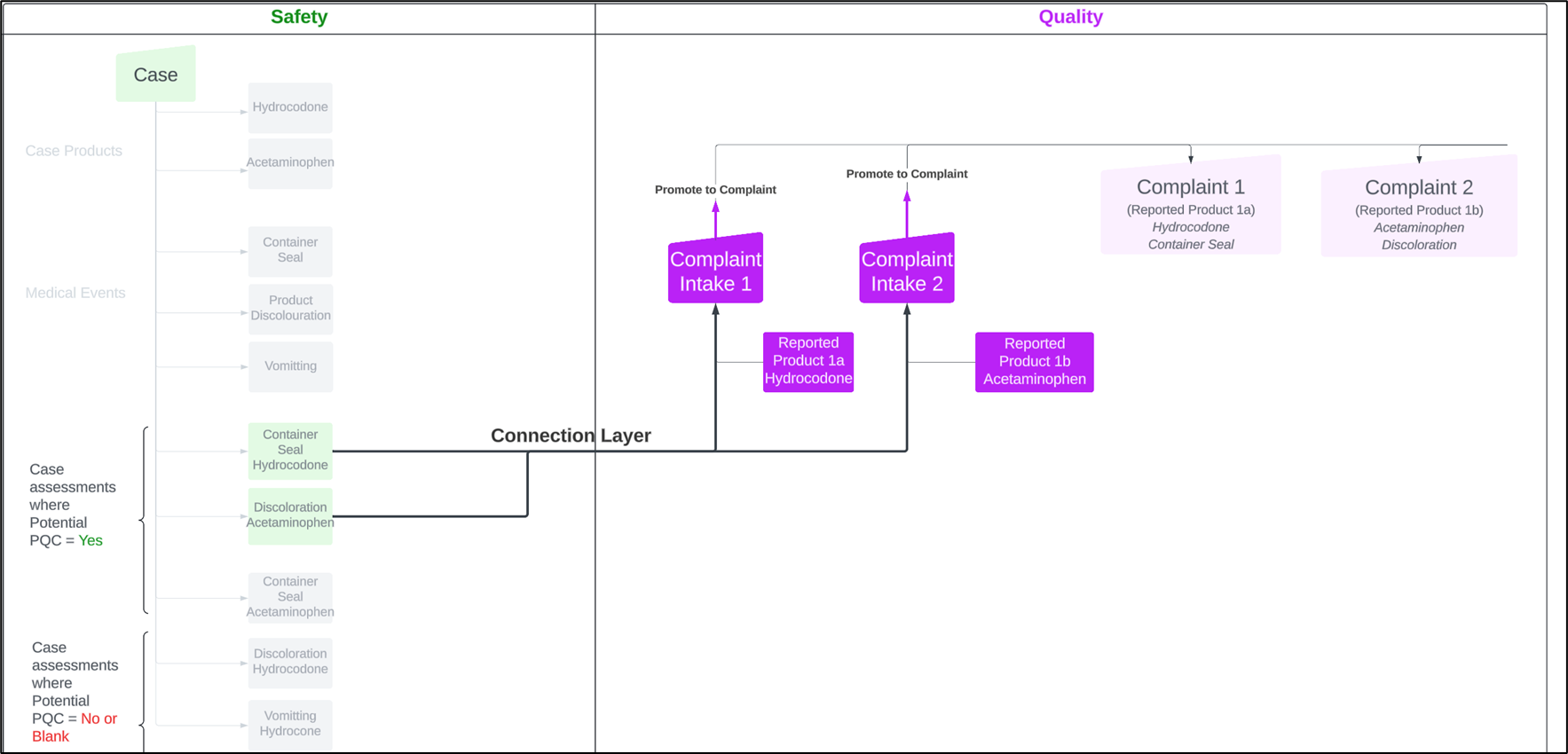
This release introduces a validated Vault-to-Vault (V2V) Connection between Vault Safety and Vault QMS that automatically sends PQC data associated with a Case Assessment in Vault Safety to the Vault QMS application, where it can be triaged using the Complaint Intake process and promoted to a QMS Complaint record as needed. The Quality-Safety Connection eliminates the manual email communication associated with relaying PQC information from Safety to Quality personnel and minimizes the potential for missed Quality Complaint data and investigations, leading to a more compliant operation.
The diagram below depicts the data elements sent from Vault Safety to Vault Quality via this connection.
Use cases supported in this release of the Quality-Safety Connection include:
- Creation of Complaint Intake records in Vault QMS for each Case Assessment record in a Case where Case Assessment records are flagged by Safety personnel as a potential PQC.
- Creation of Complaint Intake records in Vault QMS for each Case Assessment record flagged by Safety personnel indicating a potential PQC in a new version of an existing Case where new medical events have been identified for either new products or products already associated with the previous case version.
- Creation of Related Complaint Intake records for Complaint Intakes created from subsequent Safety Case Versions. The Related Complaint Intake record associates Complaint Intakes that come from Safety Cases containing the same case number but different versions.
Future releases of this connection will address:
- Sending information from Vault QMS back to Vault Safety to address certain outcomes from investigations performed by Quality.
- Sending information from Vault QMS to Vault Safety regarding Complaints that originated in Vault QMS and have potential AE information.
- Enabling Quality and Safety to request information about the AE and Complaint processes in the other application.
- Enabling Quality to send information to Safety that is used in forms submitted to health authorities regarding medical device AEs.
Learn more about other new Quality and Safety features below.
RIM-Clinical Operations Connection
RIM-Clinical Operations Connection: Respect Archived Studies on Clinical CrossLinks
When a source document in Vault Submissions is associated with an archived Clinical Study in Vault eTMF, the archived study is not populated on the Study field of the CrossLinked document. This update respects eTMF’s study association logic and prevents errors when saving the CrossLinked document.
Learn more about other new Regulatory and Clinical Operations features below.
RIM-Clinical Operations Connection: CrossLink Creation Update
When CrossLink documents are created by the connection, it first creates them in a Draft state and then promotes them to a Steady state (such as Approved). If there are any entry criteria on the document’s Approved state that aren’t met from the data in the source document (for example, the document date is blank), a processing error will occur. To resolve this, remove the entry criteria causing the failure.
Learn more about other new Regulatory and Clinical Operations features below.
Safety-Clinical Operations Connection
Safety-Clinical Operations Connection: New Integration Field Rule
The Safety-Clinical Connection now includes a field rule for the recently added Study Type (clinical_study_type__v) field on the Study object. This rule is inactive by default in all Vaults.
Learn more about configuring the Safety-Clinical Operations Vault Connection. Learn more about other new Safety and Clinical Operations features below.
Safety-Clinical Operations Connection: Update to Send to Vault Clinical Criteria
With this release, the Send to Vault Clinical user action is only visible when the Patient Content Protection field of the Transmission is populated.
Learn more about the Safety-Clinical Operations Vault Connection. Learn more about other new Safety and Clinical Operations features below.
Safety-EDC Connection
Safety-EDC Connection: Support Custom Mappings to Standard Safety Fields
With this release, the Safety-EDC connection supports custom field mapping to standard fields. Admins can create and map custom fields across safety-related form types in EDC to standard Safety fields. When transferring SAEs without custom fields, Vault looks for the applicable standard field, optimizing operational efficiency and data integrity.
Learn more about mapping custom fields. Learn more about other new Safety features below.
Safety-EDC Connection: Unexpected Pregnancy Support
With this release the Safety-EDC Connection supports the transfer of unexpected pregnancy events from EDC to Vault Safety. Vault EDC now allows multiple form types, including pregnancy forms, to generate Inbox Items in Vault Safety and customers can create Safety Inbox Items from multiple SAE form variants.
Learn more about mapping custom fields. Learn more about other new Safety features below.
Clinical Operations
In addition to the below release notes, the CTMS, eTMF, Veeva Site Connect, Study Startup, Payments, and Study Training Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Clinical Operations application family:
- Clinical Operations-EDC Connection: Enhanced Review Summaries for EDC Log Events
- Clinical Operations-EDC Connection: Improved User Exception Message Processing
- Clinical Operations-EDC Connection: Originating Site
- Clinical Operations-EDC Connection: Visit Filtering
- RIM-Clinical Operations Connection: Respect Archived Studies on Clinical CrossLinks
- Safety-Clinical Operations Connection: New Integration Field Rule
- Safety-Clinical Operations Connection: Update to Send to Vault Clinical Criteria
All Clinical Operations Applications
Milestones for Complex Trials
This feature enables Clinical teams to specify which Milestones within the Study’s Milestone Master Set will be generated for each Subject Group, offering more granular tracking of study progress and enhancing Complex Trial support. A Subject Group Milestone Schedule (subject_group_milestone_schedule__v) object defines which Milestone Types and Dependencies should be released for each Subject Group on a given Study.
Vault automatically creates Milestones and Milestone Dependencies for a Subject Group when the Subject Group, Study Country Subject Group, or Study Site Subject Group progresses through lifecycle states with the Apply Milestone Template on State Change entry action, eliminating the need for users to manually create Subject Group specific Milestones. The system creates both the Milestones and Milestone Dependencies from the selected Milestone Master Set on the Study. Milestone Dependencies are created between Milestones within the same Subject Group, unless specific Milestone Rollup behavior has been defined in the Subject Group Milestone Schedule to indicate that Subject Group Milestones should roll up to non-Subject Group Milestones.
Clinical teams can now report on Subject Group-specific cycle times. The existing cycle time job, Calculate Milestone Cycle Time Records, has been updated to incorporate Subject Group Milestones.
API Support: Global Directory Merge
Maintaining accurate and duplicate-free Global Directory data is critical for ensuring high data quality, impactful reporting, and efficient filtering for clinical operations. Duplicate records in Person, Contact Information, Organization, and Location data pose a common challenge that requires significant manual effort to address (e.g. re-associating all child records of the duplicate to the main record). Customers have requested a simpler and more efficient way to clean these records.
The new Global Directory Merge via API feature builds on the existing Merge Records API, introduced by the Platform team in 24R1, by extending its functionality to Clinical Operations, specifically Global Directory objects (Person, Contact Information, Organization, and Location). This enhancement enables administrators to select a main record and a duplicate record for merging via API. Then the system automatically updates all inbound references, including attachments, to point to the main record, ensuring data consistency while the duplicate record is deleted. Tailored safeguards and restrictions are also in place to prevent merging in specific Clinical Operations scenarios where records are tied to controlled data, ensuring compliance and preserving data integrity.
This feature greatly improves data management by enabling clinical operations teams to maintain cleaner and more reliable Global Directory data. By reducing duplicates, organizations can achieve better reporting accuracy, streamlined processes, and more impactful insights.
Study Site Addresses
With this feature, Clinical Operations users can manage study site addresses used within a clinical study, such as IP shipment locations, patient visit locations, or IRB/Lab locations.
Study Site Addresses is a functional replacement for Study Site Locations in Clinical Operations Vaults today. The Study Site Addresses feature has several benefits compared to Study Site Locations:
| Study Site Locations | Study Site Addresses |
|---|---|
| A Study Site Location cannot exist without a Location, as it is a child of the Location object. | A Study Site Address can exist without a Location, as the Location is a reference field. |
| Study Site Location requires the tightly knit process of adding Locations to the Global Directory before users can create Study Site Locations. | Study Site Address does not require a closely knit process for the Global Directory; you can handle this as a separate process. |
| The tie to the Global Directory hinders the timeliness of data entry. | You can add data immediately without impact from the Global Directory. |
| Physical address fields are lookups to Locations and are thus unchangeable, resulting in study-specific Global Directory Locations. | Physical address fields can default from Locations or be made study-specific to account for Study Site variations to keep Global Directory Locations clean. |
| Our best practice limits Study Site Locations to Locations related to the Study Site’s Organization. | Study teams can capture meaningful addresses regardless of the hierarchy of the Global Directory. |
When creating a Study Site Address, a user specifies the address type from a standard list: IP Shipment, Non-IP Shipment, Patient Visit Location, Finance Location, Pharmacy, Laboratory, IRB / EC, Other.
From a Sponsor/CRO perspective:
- You can create Study Site Addresses from a configurable, related section on the Study Site record page.
- You can create Study Site Addresses from a Location in the Global Directory by selecting a Location, populating remaining fields, and clicking Save. Study Site Address details are pulled from the Location, eliminating manual entry.
- You can add Study Site Addresses to the Global Directory by promoting the Address to a Location using the Create Location from Address action. The Location’s details are populated with those from the Study Site Address.
Study Person Security Expired Job
This feature introduces the new standard daily job Revoke Access from Study Persons with End Date that removes security access for Study Person records that have an End Date earlier than the current date. When the job runs, Vault sets the Grant Access to Related Records (create_urs_v) field to _No/False for any Study Person whose End Date is before the job run time. When this change occurs, Vault deletes the associated User Role Setup records for that Study Person record.
The Revoke Access from Study Persons with End Date job is provisioned as inactive. The scheduled run time may be adjusted but the daily schedule is not editable.
Excluded States for Milestone & EDL Creation
A new Milestone setting is available for excluding Milestone creation for specified Study, Study Country, and Study Site lifecycle states. When you enable the Prevent Milestone Creation in Excluded Lifecycle States setting, you can select excluded lifecycle states for Studies, Study Countries, and Study Sites. If you select a lifecycle state, milestones and expected documents will not be released for records that are in those prohibited states.
CTMS
Source Data Review (SDR) Tracking
In clinical trials, there is a growing industry shift from Source Data Verification (SDV) to Source Data Review (SDR) in alignment with ICH E6 guidelines promoting a risk-based approach. This shift emphasizes a more holistic review of source documents, ensuring protocol and GCP compliance, rather than focusing solely on the accuracy of data entered into Case Report Forms (CRFs) against the original source records. By prioritizing SDR over exhaustive SDV, organizations can enhance data reliability while reducing effort.
Historically, implementing SDR processes in Vault CTMS required custom configuration, adding additional burdens on implementation teams and increasing long-term maintenance costs.
To empower customers adhering to the latest industry standards and best practices, we introduce a suite of new features for SDR in Vault CTMS. These new capabilities include:
- Definition of SDR Plan for a Study: Users can now define study-specific SDR requirements such as the Target SDR Rate (%), Risk Level, Start Date, and End Date, ensuring that each study is tailored to its unique needs.
- Automatic Subject SDR Selection: Based on the approved SDR plan, the system automatically selects subjects for SDR, reducing manual effort and ensuring consistent subject selection aligned with the plan.
- Tracking of SDR Item Completion and Seeding into Monitoring Events: SDR item completion is tracked and seeded into monitoring events, enabling Clinical Research Associates (CRAs) to efficiently assess SDR progress during monitoring visits.
- Managing SDR Plan Updates: As the SDR plan evolves during the trial lifecycle (e.g., changes in risk levels or SDR rates), the system automatically adjusts subject selection based on the updated plan, ensuring ongoing compliance and accuracy.
By introducing these features, we not only reduce the need for customizations and lower maintenance costs but also enable organizations to scale SDR processes across multiple studies efficiently. This leads to improved data quality and enhanced operational efficiency.
Seed Metrics: Ignore Transferred Metrics
When a Study uses Transferred Metrics for its Metric Calculation, Metrics records are transferred into Vault from another system, typically using an integration or Vault Loader. Previously, the Seed Metrics Records action created Metrics records regardless of Metric Calculation type, which are not needed in the case of Transferred Metrics studies. Now, when a Study’s Metric Calculation field is set to Transferred Metrics, the Seed Metrics Records action does not create Metric records. Learn more about Creating Enrollment Metrics.
Seed Metrics: Error Handling Improvements
Using the Seed Metrics Records action, Vault can automatically create Enrollment Metrics records before there are subjects in the system, allowing you to plan and track future enrollment numbers. This feature enhances the Seed Metrics Records action to notify users if errors are encountered or if duplicate Metrics records are identified, improving troubleshooting abilities.
Subject Visits Track Reverted to In Progress Updates
To support more granular visit progress tracking, this feature introduces a new standard field Reverted to In Progress on the Subject Visit object. Vault automatically updates this field based on changes to Subject Visit status. Reverted to In Progress is populated with Yes if the Subject Visit status is updated to In Progress from Submitted and No when updated from In Progress to any other status. It is not populated if the Subject Visit status is updated to In Progress from any status other than Submitted. This feature is also used in the Automated Adjustments Excludes Reverted to In Progress Visits feature.
Clinical Operations-Veeva Medical CRM: Improved User Exception Message Processing
With this feature, we are improving User Exception Message (UEM) processing for the Clinical Operations-Veeva Medical CRM Connection, leading to more efficient error identification and remediation, faster processing times, and lower use of Vault resources. A single UEM is created on each Job run per Integration that consolidates all failed items and stores them for re-processing. The system also stores the Integration’s Last Successful Run Time (LSRT) unless a Message Processing Error occurs. On its next run, the Job processes failed items stored in the most recent UEM and items that have a more recent Last Modified Date than LSRT.
Disclosures
Support US Results Disclosure
This new feature streamlines posting US clinical trial results to ClinicalTrials.gov by providing structured data entry, dynamic validation, and advanced tabular functionality. It includes generating XML compliant with the ClinicalTrials.gov schema and automatically creates Results disclosures one year after the study closes.
Integrate with ClinicalTrials.gov: API for Submission Protocol & Results
This new feature allows Vault Disclosures to submit US Disclosures to ClinicalTrials.gov via the Protocol Registration System (PRS) API.
Prepopulate Disclosures from Protocol Object
This new feature introduces a Protocol object to centralize core study data across global registries, prepopulating disclosures and handling regulatory discrepancies.
Payments
Global Procedure Definitions
This feature streamlines procedure management in Vault by allowing for a Vault-level library of global Procedure Definitions that can be used across studies. It also introduces Procedure Templates which allow customers to group Global Procedure Definitions that are frequently performed together for quick application to a Study. By using global Procedure Definitions, customers can perform cross-study comparisons and gain additional insights into procedure costs.
Populate Procedure Definitions
To simplify the process of adding Procedure Definitions to a Study, a new user action on the Study object lifecycle allows customers to quickly and populate procedure definitions by either selecting from approved Procedure Templates or copying from existing Studies. If multiple Studies or Procedure Templates are selected as the source, triggers prevent duplicate Procedure Definitions from being created.
Automated Adjustments Excludes Reverted to In Progress Visits
With this feature we are streamlining Automated Payment Adjustments behavior to eliminate noise from unnecessary adjustment items. When enabled, Vault automatically adjusts Payable Items when there are changes to related payable events (such as Subject Visits) which impact fee matching criteria (such as Subject Visit status). At times, changes to Subject Visit data in EDC, such as opening queries, changes the Subject Visit status back to In Progress from Submitted. Previously, this triggered Automated Adjustment behavior, creating unneeded adjustments and making it difficult for users to find the correct items.
Now, with this feature, Vault does not adjust Payable Items when the Subject Visit status is reverted back to In Progress from Submitted for Visit Fees specifying Submitted status as matching criteria. A new field Reverted to In Progress on Subject Visits is populated with Yes when the visit status is updated to In Progress from Submitted and No when updated from In Progress to any other status. Vault automatically populates this field based on changes to the Subject Visit status. Visit status matching criteria now considers a status of In Progress plus Reverted to In Progress = Yes as matching to a visit status of Submitted.
This feature applies to Visit Fees.
eTMF
Daily TMF Transfer
Sponsors have a regulatory obligation to ensure that clinical trials, including those outsourced to Contract Research Organizations (CROs), are conducted properly, with reliable processes and high-quality data that support accurate results, protect participant safety, and enable informed decision-making. To effectively oversee outsourced clinical trials it is essential for Sponsors to have timely access to both TMF documents and CTMS data.
Traditionally, Sponsors have faced challenges in accessing approved TMF documents during a study. These challenges are often compounded by delays in receiving TMF documents at the end of the study. Alternative solutions, such as having the Sponsor log into a CRO’s Vault, creating custom integrations, or using a hybrid approach where the CRO logs into the Sponsor’s Vault to manage the TMF, are not always optimal, as they require significant collaboration and coordination between the Sponsor and CRO.
To address these challenges, the Daily TMF Transfer feature now enables the Sponsors to receive approved TMF documents daily from a CRO using Vault eTMF. The transfer will still be initiated via a Transfer agreement, but to simplify the process, a single agreement can now handle TMF transfers only, CTMS transfers only, or both TMF and CTMS transfers when applicable. Also, as we treat the Source Vault as the source of truth, we prevent end users from reclassifying or creating new versions of transferred documents in the Target Vault while the transfer agreement is active. This eliminates the risk of discrepancies and ensures synchronization with the Source.
With the introduction of Daily TMF Transfer, Sponsors can access real-time insights from their CRO partners using Vault eTMF, eliminating delays and improving trial oversight. It enhances TMF quality control, streamlines compliance, and fosters more efficient collaboration between Sponsors and CROs, ultimately advancing the success of clinical trials.
Self-Evident Corrections
Self-Evident Corrections (SECs) are a type of Quality Issue that automatically tracks misclassified documents and metadata corrections. A new Self-Evident Corrections application configuration is available to allow customers to determine when SECs should be logged based on a document’s lifecycle state type, the type of issue, and the document fields that should generate SECs.
A standard application configuration record for SEC Metadata Correction and SEC Misfiled are available for use in all Clinical Vaults. When activated, these two standard mappings will generate Quality Issues for changes in Document Date (document_date__v), Person (person__v), and External Document Version (external_document_version__v); and Classification (classification__v), Study (study__v), Study Country (study_country__v), Study Site (study_site__v), and Blinding (blinding__v), respectively. Metadata Correction and Misfiled SECs are generated for documents mapped to a Steady State and Document Workflow: Pre-Approval lifecycle state type.
The Quality Issue object has a new field, SEC Issue (sec_issue__v), that is set to Yes for Self-Evident Corrections. SECs automatically track the document fields that were changed and the issue type set in the Self-Evident Corrections application configuration record.
QIs: Auto-Populate Fields from Documents
Study and Content metadata are now captured from the document when creating a Quality Issue (QI) via a tab or Business Admin. Previously, if a QI was created outside of a user action directly on a document, Vault was not requiring users to enter the Study and Content fields, and if users populated these fields the information was not saved in the QI, leading to missing data when tracking and reporting on QIs.
The Assigned To field has now been defaulted to the document owner, making the process of assigning a QI more efficient, as this is typically the person to whom QIs are assigned.
Additionally, advanced searching on the Assigned To field has now been enabled, allowing users to view more information about the person they are assigning the QI to, including the user’s name, email, and organization.
Archival Hardening
This release enhances the One-Click Archival feature to better support document archival and error logging. One-Click Archival now supports archiving documents under legal hold, while maintaining the legal hold on the document. The feature also ensures that documents that have the archived Study on previous versions of the document, but not the most recent version, are still snapshotted and archived. To better support Vault Connections, CrossLinked documents with a single Study on the latest version will maintain Study related reference fields when a snapshot is created for archival.
One-Click Archival now returns more descriptive error messages for instances where one or more documents are in an active workflow and when the Study, Study Country, and Study Site configuration exceeds the entry criteria limit. The Study ID is also added to the job title Archive Study: <Study ID> in non-production environments.
Filter Obsolete on TMF Homepage Unapproved Documents
TMF Managers rely on the TMF Homepage to get an overview of their studies’ status and identify areas that may require attention in the TMF. The current Unapproved Documents report is misleading because it includes Obsolete documents, which should not be categorized as unapproved.
The updated Unapproved Documents chart on the TMF Homepage now excludes any documents categorized as Superseded or Obsolete from the count. This adjustment ensures the chart accurately reflects the intent to see a count of In Progress documents.
Site Connect
Site Addresses in Site Connect
With this feature, Sponsors/CROs and Site Staff can manage study site addresses to capture specific addresses used within a clinical study, such as the IP shipment location or patient visit locations.
A new Study Site Address base clinical object has been introduced which Sponsors/CROs can use to create and manage study site addresses if they have any application in Clinical Operations. Site staff, however, can only manage study site addresses if a Sponsor/CRO has purchased Site Connect.
When creating a Study Site Address, the Sponsor/CRO specifies the address type. Address type is selected from a standard list: IP Shipment, Non-IP Shipment, Patient Visit Location, Finance Location, Pharmacy, Laboratory, IRB / EC, Other.
From a Sponsor/CRO perspective:
- Study site addresses are created from the Study Site Address subtab under Site Connect tab or from a configurable related section on the Study Site record page.
- Study site addresses can be created from a Location in the global directory by selecting a location, populating remaining fields, and saving. Details are pulled from the location, eliminating manual entry.
- Study site addresses that should be in the global directory can be promoted to a Location by taking the Create Location from Address action a study site address. Details for the location are pre-populated with details from the study site address.
- Sponsor/CROs can configure a workflow to review and approve changes by site staff if desired. By default this workflow automatically accepts changes.
From a Site Staff perspective:
- Site addresses are created and managed in the Contacts & Addresses section of Site Connect.
- Site staff can save an address for future use across studies and Sponsors using their USN, reducing duplicate data entry.
- Site staff can request changes to site addresses which may require approval or be automatically accepted depending on a Sponsors/CROs configuration.This feature is auto-on for all Sponsors/CROs with Site Connect.
Exchange Unblinded Content with Sites
With this feature, Sponsors and CROs can enhance Site Connect security by giving certain Site Connect users the ability to view Unblinded content. A new standard responsibility record, Manage Unblinded Content (manage_unblinded_content__v) can now be assigned to a Study Person, ensuring that only selected site personnel have access to the unblinded content being shared. This added level of security is only available for document exchange, end-of-study media, and protocol links, ensuring that sensitive information is only accessible by those with the appropriate permissions. Site Connect does not support the restriction of Unblinded content for Safety Distribution, Announcements, or Payment Information. If users send Unblinded documents from Clinical Vaults to sites as Safety Distributions, Payment Letters, or Announcements, the documents will be visible to all site users.
Smart Filing of Profile Documents
Before the 24R2 release, Site Connect had the ability to file profile documents (CV, Medical License, and Training Evidence) to any of the three VCD artifacts (PI, Sub-I, and Other) as long as at least one of them was mapped to a Sponsor/CRO classification.
For example, as a Sponsor/CRO I could have the VCD Artifact “Principal Investigator CV” mapped to my generic “Site Staff CVs” classification. If the site then uploaded a Sub-Investigator CV, we would map it to Site Staff CV.
We lost this capability with the 24R2 release and are adding it back with this feature. Now, when a CV, Medical License, or Training Evidence document is added we first try to file it in the artifact selected by the site user (PI, Sub-I, or Other) and if it is not mapped, we move down the list below and try the next artifact, looping back to the top of the list if needed.
- Principal Investigator
- Sub-Investigator
- Other Site Staff
If no mapping is found, the document is uploaded as unclassified in the document inbox.
Remove Reply To Email Inconsistency
With this feature, when an email is received by the site user, the Reply To email is set to vault-emails@veeva.com like all other emails. Today, in some cases, the Reply To email for a requested document task is set to the user who requested the document. This causes inconsistent or undesired behavior for how a site user interacts with these emails because the site user can reply to some emails but not others. This is due to the following cases:
- The Auto-Document Exchange feature does not know who is sending the document and sets the reply to email to vault-emails@veeva.com already, and so the same document sent automatically instead of manually will have a different email behavior.
- Summary emails cannot be replied to even though they include the same tasks that a site user could originally reply to.
- The Sponsor/CRO user requesting documents may not be the same person who should be filing documents (e.g. a TMF manager requesting documents versus a CRA who actually converses with the site).
Auto-Document Exchange Enhancements
In this release, multiple changes were made to the Auto-Document Exchange feature to support larger distribution cases, such as during a migration, and marking Expected Documents based on configured Template Expected Documents. These changes are summarized below:
- The Auto Document Distribution job now only considers documents last modified in the last 24 hours.
- The Auto Document Distribution job now supports receiving large sets of documents (more than 1800 documents) in a day.
- The Template Expected Document object now includes the Auto-Request from Site and Sponsor/CRO Comments fields for Auto-Document Exchange.
Site Connect Hardening
Each release we make updates to Site Connect to ensure it can handle even the largest scenarios our customers may use it for. In this release, we have made multiple important changes that will make Site Connect more robust and performant for both Sponsors and Sites. These changes are summarized below:
- The weekly summary email now enumerates tasks 30 days old or older instead of listing each task.
- The weekly summary email now includes one link per site with all outstanding safety distributions.
- Re-selecting Site Connect User on a Study Person now always sends an invitation.
- Vault now validates that a VeevaID user is active in the Vault when a site user is set up.
- Selecting Resend on a Distribution Task Recipient or Resend to New Study Personnel on a Distribution Task for Safety Distributions schedules those records to be picked up in the nightly job instead of immediately sending the records.
- Site Connect now hides archived documents from site users in Site Home.
- Public Access Links (PALs) now expire in 1 year instead of 2 years.
- Vault jobs and actions now ignore inactive Safety Distribution Defaults when sending safety distributions.
- The Site Connect eSignature workflow now includes an appropriate verdict and capacity.
Study Startup
Multi-Country and Site IRB & EC Submissions
Study Startup supports the association of Study Country or Study Site Milestones that are included within a Central Ethics Committee Submission Milestone through a new field on the Template Milestone and Milestone, Select Specific Country and Site (select_specific_country_and_site__v), and two new objects, Milestone Study Country (milestone_study_country__v) and Milestone Study Site (milestone_study_site__v). When the Select Specific Country and Site field is set to True, the association ensures that country- or site-level Expected Documents are not added to the Milestone until a corresponding Milestone Study Country or Milestone Study Site record has been linked to the submission. Once the record is linked, the system automatically creates Milestone Items, associating the Expected Documents with the Central Submission Milestone for the relevant Study Countries and Study Sites. If these records are deleted, the associated Milestone Items will also be removed. Additionally, this feature includes two application sections that can be added to the Milestone page layouts, making the selection of included Study Countries and Sites more seamless.
Autocomplete Approval Milestones
The system will automatically populate the Actual Finish Date on a submission’s Approval Milestone when the submission’s Application Milestone is Approved or Approved with Conditions. This ensures that the same Actual Finish Date is applied to both the Submission Application and Approval Milestones. For the Actual Finish Date to be populated, either a finish-to-finish dependency must exist between the Application and Approval Milestones, or a Milestone Study Site record must be present on the Submission Milestone.
Standard submission Milestone Types (milestone_type__v) and the standard Submission Decision (submission_decision__clin), with standard values Approved (approved__v) and Approved with Conditions (approved_with_conditions__v), and Actual Finish Date (actual_finish_date__v) fields must be in use.
Feasibility Licensing
Study Startup license tracking in the Study Site License object is extended to feasibility. Admins can view license consumption in Vault Settings. Users will see a warning message if consumption is over the licensed amount.
Study Training
In addition to the features described below, the Study Training application also receives enhancements from the Auto-Create Training Requirements feature described in the Quality: Training section.
Study Training Logs Auto-File to eTMF
With this change, Site Evidence of Training and Study Team Evidence of Training reports can be automatically filed to a specific location in the eTMF.
Before this change, training completion records were manually exported and then uploaded to the appropriate locations in eTMF.
The purpose of this feature is to archive completed training in the eTMF, along with other trial artifacts. This automation increases the efficiency of this requirement and reduces the likelihood of human error.
- Two new reports are available in Study Training:
- Site Evidence of Training
- Trial Team Evidence of Training
- A new Send Training Evidence to eTMF action can be configured as a user or entry action within the Study object lifecycle. The action prompts Vault to run the report and save the report output as a Vault Library document.
- The Study Training to Clinical Operations Vault Connection creates a CrossLinked document for the report in eTMF via the Send Study Training Reports to eTMF job.
- The Study Managers can define which report to send to eTMF.
Cross-Sponsor GCP Certification Transfers
In this change, Vault will allow Learners linked to VeevaID users to complete the GCP training for one Sponsor in Study Training and use that as evidence of completion with other Sponsors so that the Learner does not have to retake the course or submit redundant evidence.
For a Training Requirement marked for GCP transfer, Vault tracks the Learners’ completion across all Vaults and automatically creates an Exemption Request for new assignments when a Learner has already completed the training. As a result, a new Training Assignment is automatically marked as completed, and the completion certificate is transferred from the Vault where the GCP training was originally completed.
Prior to this change, if multiple customer Vaults required a Learner to submit a GCP training, the Learner would have to submit for all Vaults separately.
Updates to Learner Role Dependency
This feature changes the way we automate creation of User Role Setup records through Learner Role Dependency records in Study Training to match how User Role Setup record creation is automated through Study Team Role Dependencies in Clinical Operations.
Previously, the level (Study, Study Country, Study Site) of the Learner Role Dependency would need to exactly match the level of the Learner Role Person in order for the Learner Role Dependency to apply. For example, this resulted in Learner Role Dependencies at the Study level not applying to Study Persons at the Site level and User Role Setup records needing to be created manually.
Now, the level of the Learner Role Dependency only needs to be included in the Learner Role Person in order to apply. This will allow Study Persons at the Site level to match Learner Role Dependencies at the Study level and the relevant User Role Setup records will be created automatically.
Training Matrix Conversion
This feature allows Study Training customers to move from version 1 to version 2 of the Study Training Matrix. Version 2 of the Study Training Matrix introduces system-managed Curricula.
- Studies can be transitioned individually or in bulk, via updates to the Study record’s System-Aided Matrix Management (
system_aided_matrix_management__v) field. When set from “No” (false) to “Yes” (true), Vault begins the transition. During the transition, Vault:- Updates the existing Study record, as opposed to creating a new one.
- Ignores any Curricula that are not in the Ready for Use state. This means that any Curriculum added to a version 1 Matrix that does not have any associated Learner Roles will remain as such in version 2. We recommend ensuring all relevant Curricula are assigned for training in version 1 prior to beginning the transition.
- Vault Admins must ensure the following do not occur during the transition process:
- The Connect to Study Training field on Clinical documents should not be updated from “No” (false) to “Yes” (true).
- Any updates to the Study Training Matrix, including manually creating a Curriculum and associating it with a Study. Vault blocks access once the transition begins, however it is possible for users to open the Study Training Matrix page before the transition and attempt to make updates.
- A Matrix that is running the Finalize Training Matrix job cannot be converted.
- Once converted to version 2, the Study cannot be converted back to version 1, and the System-Aided Matrix Management field can no longer be manually edited.
Training Matrix Curriculum Creation Value
A new Creation Source picklist field supports searching for Curriculum Study Training Matrix records tagged as being created via Matrix Automation (system_automation__v) or Document Entry Action.
Upon upgrade, Curriculum records in Study Training Vaults will be updated accordingly.
Study Training Enhancements
Do not override a value in Ready for Training
This change prevents Vault from overriding an existing value in the Ready for Training field. Prior to this change, the Ready for Training field was auto-populated based on certain logic. For example, when a Study Site record was updated with Ready for Training set to “Yes”, Vault also set the related Study Person record’s Ready for Training field to “Yes”.
After this change, Vault no longer overrides an existing Ready for Training value. For roles that should never be trained in Study Training, an Admin can add configuration to populate the Ready for Training field as “No”, which Vault will not override when a site is Ready for Training.
This logic also applies to non-site Study Persons.
Site License Count Visible in Study Training
With this change, the count of Site Licenses is now visible in Admin > About > Vault Information.
Filter Available in View Mode of Configurable Matrix
With this change, the user can now filter the Study Training Matrix while in view. Previously, this filter was only present in edit mode.
Add Attendance Status Filter to the ILT Page for the Instructor
The Instructor Led Training page includes a filter where Instructors can filter attendees by Present, Absent, or unmarked attendance.
Before this change, there was no filtering option, which made it more time-consuming for the Instructor to change a status individually.
Disable eSignature on Class Roster and Session Roster Objects
In this change, the ability to select the Enable Signatures setting on the Class Roster and Session Roster objects is removed.
Before this change, this option could be selected for both objects. This is not a supported configuration. For example, a Learner can be prevented from receiving new assignments because they have an open Classroom Training Assignment with associated roster records with eSignatures enabled. This change prevents that scenario.
Update Waitlist Records When Moving to Auto-Manage Waitlist
When changing from manually managed to auto-managed classes, Vault automatically moves waitlisted records to available slots on the Class Roster.
Mobile App Task Completion
In this change, Training now uses the mobile application’s native task completion for Training. Previously, this was executed via mobile web flow. Upon completing the workflow, the user is sent back to the Learner Homepage.
Add Location to the Class Information Section of Instructor-Led Training Page
This change adds a class Location to the Class Information section of the ILT page. Previously, this field was not visible when creating a class. Now that it is available, it is also added into the Class Information.
Instructor-Led Training: Page Permissions
This change allows Classroom Training Requirement object field configuration to mirror platform behavior.
Some customers use configuration to hide certain standard fields they do not use, and/or have custom role-based configurations for other fields. Prior to this change, users received unclear errors, or the class creation/editing would simply fail. As a result, customers could not reliably configure classroom training fields to be hidden or read-only for certain users.
Going forward, when users create or edit a Classroom Training Requirement for Instructor-Led Training, Vault respects permissions as follows:
- Standard or custom fields for which the user does not have read access are hidden for that user. Similarly, fields for which the user does not have edit access are visible (greyed out) but read-only for that user.
- When a field is hidden, it does not appear in the Create or Edit Class dialog.
- When a field is required but the user does not have permission, Vault displays an error to them.
OpenData Clinical
OpenData Clinical
OpenData Clinical is a new add-on application for eTMF or CTMS that maintains clean Global Directory data on investigators, sites, and the affiliations between them. This data, which is updated on a daily basis, is connected directly to Veeva Clinical and is also available via Direct Data API.
OpenData Clinical customers select mastered records to use, at which point certain standard fields are locked and controlled by OpenData Clinical. They can then submit Data Change Requests (DCRs) for new investigator or site records, or modifications to existing mastered records. DCRs are delivered to Veeva data curators, who endeavor to review and resolve DCRs within 24 hours.
OpenData Clinical is available in 25R1 for early adopters.
Veeva eCOA
Patient and Site Surveys
Interactive Images in Surveys – PainDETECT
This feature allows sponsor/CRO staff to configure a survey that contains an interactive PainDETECT image question for respondents to complete. Respondents select either the front or back of the body, and can then select specific areas on the body to respond to questions. Only the painDetect interactive image question type is available with this release, but other image-based questions may become available in the future.
The Veeva Library team will configure surveys that include this question type and get the implementation approved for use.
In reporting for the PainDETECT interactive image, the answer is a label that refers to the region selected by the user. Labels are defined by Veeva.
NRS Survey Enhancements
With this enhancement, sponsor/CRO staff can configure the following options for numeric rating scale questions on surveys:
- Labels can be displayed above or below the scale.
- The same label can be used for multiple, consecutive scale numbers. For example, the Moderate label can span number positions 4, 5, and 6.
- Percentages can be added to position labels
- Scale increments can be configured with values other than 1. For example, the scale can list position increments of .5 and show .5, 1, 1.5, 2, 2.5, and 3 as the numbers on it.
- The scale can be configured to descend.
- Line breaks can be manually added to labels.
These changes can enable sponsor/CRO staff to more closely align the survey with the copyright version of the survey.
New Survey Type: Training
This feature adds a new survey type of “Training”. Users can easily identify these surveys as training surveys, and once completed, participants or caregivers can continue to view them through Study Resources. Training data will be excluded from reports and metrics, and is also excluded from compliance calculations. However, the training surveys are available in the Compliance report.
Dynamic Time in Display Names
This feature allows sponsor/CRO staff to include time-based tokens in survey display names. These tokens dynamically update based on the survey’s scheduled timepoint, making it easier to distinguish between repeated surveys in different schedules. The display values are included in the translation files.
Repeating Survey Sections
This feature allows sponsor/CRO staff to configure a section containing a set of questions that repeat. Additional parameters include the minimum and maximum number of sets a respondent can answer. Survey respondents can add, edit, and delete their sets of repeating questions.
When paired with Survey Due reminder notifications, surveys with repeating sections can prompt participants to look back and submit data over a longer period of time.
Studio
Minor Collection Versions
This feature updates the UAT process to create a new version for each round of UAT approval, improving visibility into changes. It also enables sponsor/CRO staff to maintain traceability through each round of UAT.
Participant ID Format
This feature allows sponsors to configure a study-specific format for Participant IDs and enforce that format when the site creates a participant in eCOA.
This setting is not tracked through versioning, and you can turn it on and off at any time during the study. If turned on in the middle of a study, site users will have to update the participant IDs the next time they make a change to the participant’s account details.
Survey and Schedule Toolbar Updates
This feature updates the snippets and help toolbar for ease of use, including making the panel independently scrollable and expandable. You can now view the panel in full-screen mode, making it easier to see large snippets of code. The button was also moved from above the text boxes to the navigation bar on the right side of the page.
New Event Type: Unscheduled
This feature adds a new “Unscheduled Event” event type, enabling sponsor/CRO staff to configure optional surveys for events that do not follow a set schedule. Site staff can add these events to a participant’s schedule when applicable. Unscheduled events cannot be recurring and are intended to collect surveys on an ad hoc basis. Site staff can also remove an unscheduled event if it was added by mistake and no surveys have been completed.
New Schedule Type: Open-Ended
This feature adds a new “Open Ended” schedule type, allowing sponsor/CRO staff to configure eClinRO and training surveys without a due date, keeping them available indefinitely for respondents. If the schedule is ended by an end event, related surveys will no longer be available to start.
Image Localization, Translation, and Height Updates
This feature supports image localizations in eCOA, allowing Sponsor/CRO staff to upload source images, add translations, and assign languages for substitution based on respondent language. Images can be accessed from the Tools menu, and the localizations are associated with the image. These localizations can be shared between surveys using a share code.
Additionally, users can define a maximum heading image height of up to 80% of the screen height to improve image viewing in surveys.
Event-Triggered Group Assignment
This feature allows sponsor/CRO staff to configure a group type that must be assigned at an event, such as a randomization event, rather than at the beginning of the study. Site staff are required to assign a participant to a group in that group type before they can enter a date and time for the event.
In Veeva eCOA, site users see:
The Study Preview is also updated to include the event-triggered assignment, anchor event, protocol expected date, and window.
eCOA Vault
Turn Off In-Person Surveys to Require Participant and Caregiver Login
This feature enables sponsors to turn off the option for participants to use the simplified In-Person flow to require the participant and caregiver to log in to MyVeeva. Respondents can still answer surveys in-clinic, but they will need to log into their accounts to access the surveys.
This setting can be controlled at the following levels:
- Study (Studio setting)
- Country (Vault setting)
- Site (Vault setting)
When this setting is disabled for a site, the button is removed and the site user cannot force the workflow with a manually entered URL.
API
Patient Date of Birth Added to API
This feature enables the participant date of birth in the participant list to be seeded from any credentialed external system using the eCOA MyVeeva External APIs, eliminating the need for site staff to manually enter or update the date of birth in Veeva eCOA. The date of birth can be included in the request payload when creating or updating a participant.
Reporting
Reportable Rule Outputs
Criteria check results are now available for reporting as separate .CSV files. The number of columns per file, and the names of those columns, will flex based on the rule configuration.
Criteria checks executed before 25R1 will not be added to the reporting database.
Multiple Export Jobs
This feature enables eCOA to export daily data sets to up to 10 destinations per study instead of just one. You can still only have one Veeva Clinical Database (CDB) connection per study.
The FTP configuration is moved to a new Export Jobs tab under Study Settings in Studio.
A new authentication option of SSH Keypair is added as part of this update.
Participant-Level Data Added to Study Home
This feature enables Study Home to display the following participant-level data as part of the dashboard:
- Events
- Surveys
- Group assignments
Filters are available for the survey list, and up to 50 are displayed on each page of the table.
Generate Library Manager Screenshot Report
This feature allows Sponsor/CRO staff to generate a screenshot report of a survey in Library Manager. The reports are generated based on the survey version and languages that are selected.
25R1 Feature Audit Events
This feature enables the system to add relevant user actions from 25R1 features as audit events on the MyVeeva audit trail.
Veeva eCOA (Sites)
Survey Transcription
This feature allows site staff to transcribe surveys into the system when a respondent is unable to enter the data electronically and instead provides the answers on paper. Transcribed surveys are included in compliance reporting.
Past Survey List View
This feature enables site staff to see the list of a participant’s past surveys in a list of past surveys. The list of surveys can be filtered by:
- Survey Name
- Status
- Event
- Respondent
- Location
- Date
With these updates, past surveys can no longer be viewed on the Event list.
Downtime Notification for Sites Upgrades
This feature updates eCOA to display a notification indicating the expected duration of downtime for site users when the system is being upgraded. Site users are informed of planned downtime in advance, and are prompted to save any in-progress work shortly before it begins.
Restrict SiteVault Participant List to Show Only Eligible Participants
This feature ensures that when a site staff member adds a participant from SiteVault, only participants with an associated patient record in SiteVault are displayed. This ensures that only participants eligible to receive eConsent forms are added to eCOA.
MyVeeva for Patients
General UI
Disable Ask for Help for eConsent-Only Participants
This feature removes help requests from eConsent-only studies, preventing related requests from being routed to SiteVault and reducing the triage burden for sites with large staff sizes.
As part of this update, the eConsent option is removed from the Ask for Help form, and the Ask for Help option is removed from the More Options list on eConsents.
Simplified In-Person Survey Date of Birth Fields
This feature replaces the date-picker for the Date of Birth field with a three-text-field format on the in-person eCOA survey launch screen, improving usability. The order of the month and day fields will automatically be updated based on the language of the participant.
Translations
25R1 Translations and New Languages
MyVeeva users can now view application text, emails, notifications, and the terms of use and privacy policy in Estonian (Estonia) and Russian (Estonia).
Commercial
In addition to the below release notes, the PromoMats Veeva Connect community offers general release communications, release highlights, and key feature demos.
The PromoMats-Medical Connection: Anchor Persistent ID Support feature, listed in the Vault Connections section also affects the Commercial application family.
PromoMats
MLR: Similarity Scores
PromoMats can now calculate a content similarity score between two documents in order to help define review tiers, leading to more efficient review.
Documents to compare can be added to the Created From relationship via the new Content Similarity panel. For each document comparison, an overall similarity score, combining keyword and image similarity, is calculated and displayed on the Content Similarity panel. Similarity scores are calculated using the first 15 pages of content for larger documents.
Similarity scores can be used to drive tier-based review workflows using the new formula function AppHighestSimilarityScore(Document.id__v).
PromoMats: Support UI Upload Up to 20 GB
PromoMats users can now upload files up to 20GB in size directly into their Vault.
eCTD: Redline Annotations
With this release, redline annotations required for some FDA submissions can be automatically created on eCTD annotated materials during compliance package generation using the Vault link annotations on the promotional piece. Previously, redline annotated versions needed to be created manually and then added to compliance packages.
Communication Objectives
Users can gain insight into content strategy execution by using the new Communication Objective object and linking these records to content. Communication objectives can be added to documents, content modules, or claims. When using Claims Auto-Linking, communication objectives used in the claims are automatically associated with the document.
Enhanced MLR Commenting on Content Modules
To improve the user experience when reviewing content modules, the comments and replies are now displayed with the most recent comments appearing first.
Preview Content Module Enhancements
To enhance the preview experience for Content Modules, we have made several enhancements to the user flow, including updating the record action label and button to Generate Preview.
PromoMats Portal: Disable Download Option
Portal Administrators can now choose to disable the Download option for the Portal homepage document cards and quicklook. This extra control is intended to promote use of Make a Copy.
Enable Read & Understood Workflows for PromoMats
Read & Understood workflows allow you to assign a document to specific users for review. When users complete their review, they can mark the document as Read & Understood and provide an eSignature as proof that they’ve completed the task.
Document Product Registration Check
You can now leverage product and registration hierarchies from Vault RIM to ensure a document includes at least one approved Registration for the Country and any product-related document fields (such as Product or Product Family), prior to moving the document to steady state, using the new formula function AppProductRegistrationCheck. If the Registration is withdrawn, the document can be withdrawn automatically via the Auto Expire Documents (Withdrawn Registrations) job. The Registration integration in the RIM-PromoMats Connection is required and must be active to use this feature.
Additional Controlled Vocabularies
This feature introduces ANSM Classifications and new Health Authority Submission values to the Controlled Vocabulary object.
Standard Document Type Descriptions
With this release, we have added descriptions to the Material (material__v), Component (component__v), and Reference (reference__v) document types. Previously, these document types did not have a description.
eCTD: Additional Error Messages
This feature adds additional error messages for the following scenarios in eCTD package generation:
- A document field violates a reference constraint
- The ZIP file uploaded to the eCTD Submission Package rendition type is password protected
- The configured Form Generation Type is not eCTD
- A linked reference or labeling document is not in steady state, or the link status of the crosslink is Deleted
- The Material Type is blank on Submission Ready Copies
- The compression method used for the submission package is not supported
eCTD: Correspondence Letter Name Update
With this release, the Correspondence Letter generated for Pre-Clearance eCTD packages will be named correspondenceletter. Previously, Vault named the document with the document number.
eCTD: Binder Section Naming Update
With this release, Vault names eCTD binder sections with the publication / dissemination date. Previously, Vault named eCTD binder sections using the binder submission date.
Medical
In addition to the below release notes, the MedComms and MedInquiry Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Medical application family:
- Medical-Safety Connection: Support Case Product Indication, Case Cause of Death
- PromoMats-Medical Connection: Anchor Persistent ID Support
All Medical Applications
Medical: Support UI Upload Up to 20 GB
Medical users can now upload files up to 20GB in size directly into their Vault.
MedComms
Auto-On Auto-Linking: Medical
Auto-Linking is now the default for linking Scientific Statements in MedComms Vaults. When initiated, Auto-Linking runs in the background to provide a better matching experience for Scientific Statements, including non-exact text matches.
Modular Content: HTML Previews for CRM Email Modules
Using CRM Email Modules, you can create variations of Email Assets and assemble them in Veeva CRM to create an Approved Email, providing a more personalized customer experience.This feature allows you to visualize variations of Email Assets with predefined Templates, providing a more streamlined and practical way of reviewing CRM Email Module content.
MedComms Portal: Disable Download Option
Portal Administrators can now choose to disable the Download option for the Portal homepage document cards and quicklook. This extra control is intended to promote use of Make a Copy.
MedInquiry
Match Case Contact to the Original Sender for Emails Forwarded to MedInquiry
When a member of the medical information team manually forwards an email from the Med Info inbox into MedInquiry, the original sender (for example, the healthcare provider who sent the medical information request email to the Med Info inbox) will be populated into the Case Contact field.
Contact Search: HCO & Address Support
This feature introduces a redesigned Case Contact selection dialog to show the most relevant data users need to search and find Case Contacts during inquiry intake. Users are able to use affiliated organizations, location data like city and zip code/postal code, and other reference data to find the right Case Contact. To enable the new Case Contact search experience, customers need to submit a Product Support ticket. This new dialog will be auto-on in a later release (the exact date will be confirmed over the next few months).
Object Type Support for FAQs & Suggested Responses
MedInquiry’s Suggested Responses identifies a Frequently Asked Question (FAQ) and the relevant Response based on Case Request details. This may be done at intake or at time of fulfillment, but it is restricted to the Base object type. Suggested Responses now support other object types on Case Requests.
Telephony Support: Click to Dial
When users have access to telephony functionality, they are now able to dial phone numbers from the Case and Case Contact records to ask the healthcare provider about a case they are working on.
Standard Telephony Integration: Genesys
Genesys Cloud is now a standard telephony integration. When configured, users are able to access Genesys Cloud call and chat controls within MedInquiry.
Telephony Support: Error Notifications
With this release, Vault notifies System Administrators and Vault Owners of errors related to OmniConnect Connections (telephony integrations).
Event Data Model: Support Product Indication, Cause of Death on Event
Adverse Events raised alongside medical inquiries may include information on product indication and cause of death. These data model updates will support standard structured capture of this information, as well as more closely aligning the Medical Adverse Event data model with the Safety Case data model.
Quality
In addition to the below release notes, the QMS, QualityDocs, Training, LIMS, and Validation Management Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Quality application family:
- Quality-RIM Connection: Enhanced Change Control
- Quality-Safety Connection: Transfer PQCs Found in Veeva Safety to Veeva Quality
QualityDocs
Document Control Homepage
This feature introduces a new role-specific homepage that provides Document Control users with centralized visibility and actionability around key, open document control processes including Document Change Control, Periodic Review, and Controlled Copies.
The landing page includes four sections (Document Change Controls, Periodic Reviews, Controlled Copies, and Documents with Upcoming Periodic Reviews), which can be filtered using configurable criteria based on Due Date or the length of time that a record has been open.
The Document Control Homepage Landing Page:
Each of the four sections is paginated and is expandable to a more detailed view, allowing users to easily navigate and focus on the section’s content.
Where there are associated workflows, a Document Control User can access and perform available workflow actions by clicking on the ellipsis. Clicking on the Workflow button displays all workflow tasks.
The Workflows button opens a window displaying all workflow tasks. From here, the Document Control User can view Due Dates and Task Status and can send a Nudge (Vault email and notification) to selected task owners:
Document Control Users can leverage the Document Control Homepage as a one-stop landing page from which they can quickly perform management actions and monitor status to help enable timely completion across key document control processes.
Quality Relationships Panel: Bi-Directional Document Relationships
The existing Quality Relationships panel is expanded to include a new section for Document Relationships. Once enabled via a new Application Setting, this section displays both source and target document relationships, allowing the user to easily view and manage document relationships in a bi-directional manner directly from the document. This feature enables a user with the required permissions to create the relationship only once and the relationship will then display in the Document Relationship section for both the Source and Target documents. Users with required permissions can also delete existing relationships directly from this section on the panel.
Quality Relationships Panel: Document Change Request Section Enhancements
A new Application Setting allows Administrators to enable or disable the Document Change Request (DCR) section of the Quality Relationships panel. This can be helpful to avoid user confusion if DCR functionality is not currently being used by an organization.
This enhancement also removes the options for users to create and delete DCRs directly from the section. Users with the permission to create DCRs are able to do so from a User Action when the User Action is made available through configuration in that document’s lifecycle state.
Process Navigator: User Interface Enhancements
This feature includes an enhancement that makes the link to the Process Navigator Detail page more prominent and accessible from the Process Navigator Landing Page. A second UI enhancement groups User Actions available to Process Owners and Admins under an ellipsis:
Training
Note: All features apply to both Training and Study Training unless otherwise stated.
Manager Homepage: Organization View
With this change, Vault allows Managers to view the training status for their entire organization, consisting of direct reports and indirect reports. Managers can use the Manage Homepage (My Team page) to drill down to the lowest reporting structure level to view overdue, due soon, due later, and completed training, as well as send email reminders (Nudges) from any level.
For example, John is a manager of Steven, who has two reports, Anna and Peter. John can click through this organizational hierarchy to view the training data not only for Steven, but also for Anna and Peter. Prior to this change, John could only view Steven’s training data.
Organizational View is supported via the Manager Position object, which Vault automatically creates and uses to set assignment visibility to direct and indirect Managers, as well as counts within the UI. Vault also assigns any indirect Managers a standard “Viewer” Application Role for impacted assignments.
While this feature is auto-on, Vault may detect circular reporting chains in existing Person records, where one member of a hierarchy’s Manager is populated as someone lower in the overall hierarchy. See Setting Up the My Team Page for more information.
Auto-Create Training Requirements
With this change, Vault can automatically create Vault Document Training Requirements when a document enters the document lifecycle state where the Create Related Training Requirement entry action is configured.
This feature aims to improve the efficiency of Training Administrators’ Vault Document Training Requirement creation process. It also decreases human error and ensures consistency of required fields for newly-created requirements.
- The process is triggered via the Create Related Training Requirement entry action configured on a document lifecycle state. We recommend using the Approved state in both the Draft to Approved and Draft to Effective and document lifecycles.
- The Vault Document Training Requirement object must be configured with default field values for all required fields. When Vault cannot create a requirement for this reason, it also prevents the document from transitioning to the state where the action is configured.
- When the Create Related Training Requirement Action is triggered, Vault:
- Checks if there is an existing Document Training Requirement associated with the document. If not, creates a new Vault Document Training Requirement named according to the document Name. This includes documents already associated with a Classroom Training Requirement.
- Verifies whether the file type is supported. E-Learning files are supported, however there must be at least one Training Completion Criteria record with the Use As Default For field value set accordingly for that e-learning standard.
- Notifies the user of any requirements it could not create.
- When creating requirements for LearnGxP Training Materials document types, Vault automatically populates fields as follows:
- Continuing Education Unit (CEU):
ceu__vdocument field value - LearnGxP Training Material?: Yes
- Estimated Time to Complete (Minutes):
learngxp_course_duration__vdocument field value - LearnGxP Course Code:
learngxp_course_code__vdocument field value - LearnGxP Course Type:
learngxp_course_type__vdocument field value - LearnGxP Functional Area:
learngxp_functional_area__vdocument field value
- Continuing Education Unit (CEU):
For more details and configuration, see Vault Training Automation.
Training Requirement Impact Assessment: Admin Alerts
When configured on the Training Requirement Impact Assessment object page layout, new Impact Alerts inform users of the training and document impact of Training Requirement Impact Assessment decisions. They also serve as a building block for a later release, which will improve the unification of Document Change Control and Training Requirement Impact Assessment processes.
The following Impact Alerts are available for configuration:
- Cancel Open Training Assignments: When “Yes”, the alert displays the number of Training Assignments that will be cancelled. When “No”, the alert displays the number of assignments that will remain open until the previous document version is superseded.
- Doc Revision Due Date: The alert notifies the user if this date is different from the document’s Proposed Effective Date.
- Re-Issue Training: The alert displays the number of Training Assignments that will be created, and whether the decision to re-issue training is different from the Training Impact decision on the Library document.
- Require Updated Quiz: If there is a quiz on the Training Requirement, the alert informs the user that there is a quiz. If there is no quiz on the requirement, the alert directs the user to create one or consult with the Training Administrator.
Reconcile Retraining with TRIA Decisions
In this change, the Update Training Assignment job was modified so that a new Training Requirement Impact Assessment’s Execution Date field is included in the calculation. This feature ensures that Vault assigns all necessary Training Assignments to Learners whose Person records were Ineligible during this period, such as during a leave of absence.
For example, a Learner completes a Training Assignment for document v4.0 on 01-May. Shortly thereafter, the Learner goes on leave and the Training Admin updates their Person record to Ineligible. While the Learner is on leave, the document in this requirement is up-versioned twice, resulting in two TRIA records:
- The TRIA for document v5.0 is executed on 01-Jul, indicating that retraining is required. This sets the Training Requirement’s Do Not Retrain field to No. The ineligible Learner does not receive a new Training Assignment.
- The TRIA for document v6.0 indicates that retraining is not required (Do Not Retrain = Yes).
The Learner returns from leave on 01-Oct, and the Update Training Assignment job assigns a new Training Assignment for document v6.0. This closes the training gap between the Learner’s original v4.0 completion and the currently effective v6.0.
Prior to this change, the Learner would not receive a new assignment for document v6.0, as the job logic referenced the requirement’s Do Not Retrain field to determine whether to assign training. This required Training Admin intervention to manually assign training and close the training gap.
With this change, the job instead compares the last completed assignment’s Assigned Date against the Execution Date of the last TRIA where the verdict was to require re-training: Using the example above, the Learner receives an assignment because their last completed assignment (01-May) was before the 01-Jul Execution Date of the TRIA where the verdict was to re-train.
Auto-Manage Person Job Enhancements
This change consists of enhancements to the Auto-Manage Persons job, which automates the creation and update of Person records based on the related User records. The enhancements will impact Person Training Eligibility, field updates, and copying field values.
Prior to this change, when any User record field was updated, Vault updated the corresponding Person record field as well. This meant that if a Training Admin had previously set a Person’s Training Eligibility to Ineligible, any update to User record fields triggered the Auto-Manage Persons job to run and update the value to Eligible again. Additionally, Vault did not maintain certain User fields on the Person object, such as Organization and Department.
This feature allows the Auto-Manage Person job to instead change the Learner’s eligibility only when the corresponding field on the User record is updated. Additionally, custom fields can now automatically flow from the User to the Person record. These enhancements allow for scenarios such as when a Learner goes on long-term leave and their User record should remain active, or to support sending employee metadata from an HR system to Vault.
This concept applies to all matching __vand __c fields on the User and Person records.
Benefits of this feature include allowing:
- A Person record to be Ineligible while the User record is still set to Active.
- Custom and standard fields on a Person record to update automatically when the related User record’s matching fields change.
- Vault to create Individual Learner Role records when creating a new Person. The fields must be unique in name, label, and type for them to copy over.
- For standard Person fields, Vault allows copying from a similar User field when the only difference is the type, for example Person’s standard
organization__vfield and a customorganization__cUser object field. - Supported fields:
- Department (
department__v) - Employee Type (
employee_type__v) - Job Level (
job_level__v) - Job Title (
job_title__v) - Organization (
organization__v) - Person’s Organization (
persons_organization__v) - Persons’ Site (
persons_site__v) - Sub Department (
sub_departent__v) - Working Location (
working_location__v)
- Department (
- The following copy types are supported: String, Number, Boolean, Date, DateTime, Object, Picklist, RichText, LongText
For more details and feature enablement, see Auto-Managing Person Records.
Enable Learner to Perform Actions on a Training Assignment
In this change, custom Learner actions are now supported from the Learner Homepage’s History tab.
Before this change, Learners could not perform any actions on their own History page. This feature allows for custom configuration on the Training Assignment lifecycle’s Completed state, such as a “Download Certificate of Completion” user action (Download Formatted Output). This enables Learners to download a certificate of completion directly from their History page, instead of engaging with their manager to download the information.
Training Enhancements
Add attendance status filter to the ILT page for the Instructor
The Instructor Led Training page includes a filter where Instructors can filter attendees by Present, Absent, or unmarked attendance.
Before this change, there was no filtering option, which made it more time-consuming for the Instructor to change a status individually.
Disable eSignature on Class Roster and Session Roster objects
In this change, the ability to select the Enable Signatures setting on the Class Roster and Session Roster objects is removed.
Before this change, this option could be selected for both objects. This is not a supported configuration. For example, a Learner can be prevented from receiving new assignments because they have an open Classroom Training Assignment with associated roster records with eSignatures enabled. This change prevents that scenario.
Update Waitlist records when moving to Auto-Manage Waitlist
When changing from manually managed to auto-managed classes, Vault automatically moves waitlisted records to available slots on the Class Roster.
Mobile app task completion
In this change, Training now uses the mobile application’s native task completion for Training. Previously, this was executed via mobile web flow. Upon completing the workflow, the user is sent back to the Learner Homepage.
Add location to the Class Information section of Instructor Led Training page
This change adds a class Location to the Class Information section of the ILT page. Previously, this field was not visible when creating a class. Now that it is available, it is also added into the Class Information.
Instructor-Led Training: Page Permissions
This change allows Classroom Training Requirement object field configuration to mirror platform behavior.
Some customers use configuration to hide certain standard fields they do not use, and/or have custom role-based configurations for other fields. Prior to this change, users received unclear errors, or the class creation/editing would simply fail. As a result, customers could not reliably configure classroom training fields to be hidden or read-only for certain users.
Going forward, when users create or edit a Classroom Training Requirement for Instructor-Led Training, Vault respects permissions as follows:
- Standard or custom fields for which the user does not have read access are hidden for that user. Similarly, fields for which the user does not have edit access are visible (greyed out) but read-only for that user.
- When a field is hidden, it does not appear in the Create or Edit Class dialog.
- When a field is required but the user does not have permission, Vault displays an error to them.
Station Manager
Automated Station Document Assignment
This feature provides an automated transition of Station Document records from the Planned for Station (Draft) state to the Assigned to Station (Effective) state when the related document enters its Steady state. When this occurs, Vault updates the Station Document record’s Planned Documents and Assigned Documents lists accordingly. For example, Document A is in a Draft state and is related to the Station Document record in a Planned for Station state. Once the document transitions to its Steady state, Vault changes the lifecycle state of the Station Document to the Assigned to Station state and Document A becomes available at the assigned Station.
Prior to this change, the lifecycle state of the Station Document records had to be updated manually once the document reached its Steady state, either via a workflow or a user action.
Station Manager: Modernized User Interface
This feature introduces a new, modernized user interface in both the iOS and Android versions of Station Manager. The updates made to fonts, icons, colors, button styling, etc., help to collectively provide a more consistent user experience across Vault applications.
QMS
Quality Event Suggestions
With this release, we are excited to introduce the Quality Event Suggestion feature, designed to enhance efficiency during the triage stage and designed to help streamline prioritization and categorization. Based on a similarity check, the system will provide intelligent suggestions for field values on the parent record and for related records associated with it, after executing the Generate Record Suggestion action set up by an Admin.
Suggestions are generated by analyzing the top 100 similar records. The supported objects are Complaints, Deviations, Nonconformances, and their Quality Event object type equivalents. Only one configuration is valid per object and object type combination.
Generating Suggestions
Users can generate the suggestion through a user action. This will invoke a panel with the Match Criteria that defines the “universe” of potential records to be used to calculate field and related record suggestions. The action can be run multiple times, but if a record suggestion has been already completed or it is ongoing, a warning message is displayed.
Admins can add up to five matching fields (Yes/No, picklists, or object reference or lookup data-type fields), date comparisons in months, and up to five text comparison fields (normal, long, and rIch text-type fields). Users may have the possibility to override the match terms if this is enabled in the user action configuration in the lifecycle state.
Field Suggestions
The purpose of a field suggestion is to suggest what field values are most typically referenced for a specific field in similar records. As a user, you will be able to locate a badge next to the section containing the fields with the suggestions and next to the fields themselves; after clicking on it, you can also view the related statistics behind the calculation with the Show More button.
When you click the badge, you can choose to either Accept or Dismiss the suggestion. If you accept, the record enters edit mode, the suggested value is populated in the field, and you need to click the Save button to ensure that the suggestion is saved.
In both cases of accepting or dismissing the selection, the badge then disappears from the record. Admin can specify up to five (5) active fields to capture the suggestions. The types supported are Object Reference, Picklist (single-value), and Yes/No.
Related Record Suggestions
The purpose of a related record suggestion is to suggest to users what related records are most typically referenced in similar records. As a user you will be able to locate a badge next to the related object section with the suggestions; after clicking on it, a list of potential related records and the reference count of the instances found during the search is displayed.
After selecting the related record suggestion and clicking the Accept button, the record is added to the section. If multiple related records are available in the suggestion, the badge will remain visible until you open it again and address the remaining suggestions.
Admins can select up to three (3) active related objects that have a join parent inbound relationship to the primary object, and choose which field of the related record to be displayed to give more context.
Audit Room: Inspector Portal
As part of our ongoing efforts to enhance the Audit Room feature, this release introduces the Inspector Portal. This user-friendly interface is designed specifically for Inspectors, allowing them to easily view Inspection records and associated Inspection Requests. Recognizing that Inspectors may be unfamiliar with the system, the portal simplifies navigation and access to essential information.
Admins are now able to control the information displayed to Inspectors.
Additionally, we are introducing a Deactivate Inspector user or entry action, which will facilitate the deactivation of the User record linked to the Inspector’s Person record on the inspection. Lastly, the Audit Room feature now supports both standard and custom object types on the Audit object.
Learn more about configuring Audit Room and using the Inspector Portal.
Audit Room: Auto-Assignment
With this release, use of the Audit Room capability of Vault QMS has become faster and easier. Vault now can automatically assign fulfillers to new Inspection Requests. As the audit response team sets up, members of the Fulfiller role on the Inspection record can be granted Inspection Request Auto Assignment records, which allows the team to split up how they will own and respond to inquiries during planning and setup for the audit itself. These default assignments are made per audit and can be reassigned manually during an audit as needed.
Setting up automatic assignments for Inspection Requests does take a small amount of configuration before it is available to your business teams. You can learn more about setting up and using Automatic Assignments for Inspection Requests in Vault Help.
Reason for Change: Support for Custom Objects
The Reason for Change feature became available in the 24R3 release. Veeva introduced the feature to address requirements from health authorities to capture why organizations update certain data fields on completed records. This release enhances the feature so that it can be used with any custom object, and expands the supported QMS objects to include the following objects:
- APQR
- APQR Item
- Audit
- Audit Program
- Finding
- Product Return
- Root Cause
- Root Cause Analysis
See Configuring Reason for Change for the full list of standard supported QMS objects.
End User Experience Updates
When a user edits fields managed by a Reason for Change configuration and saves the record, Vault QMS opens a dialog like the one shown below to collect a reason for change. The user must select one of the valid reasons defined by an organization in the Reason for Change picklist. If the selected reason is configured to require supporting information, the Comments field is mandatory.
When the user clicks the Save button in the Reason for Change dialog, the changes to the record’s fields are saved, and Vault QMS creates an entry in a new Reason for Change History section as shown below.
If your organization previously implemented this feature and used the Reason for Change related object section, you must replace it with the Reason for Change History section to ensure users can see all the reasons for changes made to a record. The Reason for Change History section has the added benefit of displaying the labels of modified fields, which are more recognizable to users than just the internal names of the modified fields.
The Reason for Change record includes what was changed, why the changes were made, who made the changes, and when the changes were made. The 25R1 release also includes a new field that allows users to see and navigate to the record to which the Reason for Change record applies.
Note: Vault will no longer prevent users with the appropriate permission from deleting QMS records because they have related Reason for Change records.
System Admin Updates
The examples below show how System Admins define a Reason for Change configuration for an object and object type (standard and custom objects). An object and object type can only have one Reason for Change configuration. Each Reason for Change configuration can define up to 30 standard or custom fields requiring a reason for change in the lifecycle states specified. System Admins can also require users to enter a free text Comment to support specific reasons in the Reason for Change picklist.
When using Reason for Change for custom objects, customers will be prompted to implement a custom trigger using Java SDK. The trigger is responsible for displaying the Reason for Change dialog box when a user edits a field on the custom object that is controlled by a Reason for Change configuration. The trigger will also create a Reason for Change record and an entry in the Reason for Change History section.
Learn more about providing a Reason for Change. Learn more about the developer-facing functionality of this feature.
External Collaboration: Reassign Task for New Person
Starting with the 25R1 release, users can seamlessly reassign a task to a different external collaborator using a new user action, eliminating the need to cancel in-progress workflows.
Previously, reassigning an external collaborator in a QMS record required canceling the ongoing workflow. This process often disrupted workflows and impacted operational efficiency, depending on system configurations.
With the 25R1 release, the new Reassign External Collaborator feature introduces a background operation for task reassignment, ensuring uninterrupted workflow execution. This applies in the following scenarios:
- When the replacement external collaborator has an existing user account linked to their Person record.
- When the replacement external collaborator is a net-new person without a linked user account.
Admins can enable the Reassign External Collaborator action on any object lifecycle that has external collaboration deployed.
When the action is executed, the current task assigned to the original external collaborator is canceled. A new task then is generated and assigned to the replacement external collaborator.
Learn more about replacing external collaborators.
External Collaboration: Impact Assessments & Change Actions
Vault QMS enables organizations to provide business partners with temporary, short-term, streamlined access to certain records for the purpose of completing specific tasks within quality business processes. This release expands the External Collaboration feature’s supported quality business processes to include Impact Assessments and Change Actions.
Customers can create Organization records to identify external business partner companies and Person records to identify the individuals in these organizations who can receive external collaboration tasks.
Internal users can assign an external individual’s Person record to a Change Action or Impact Assessment.
When the Change Action or Impact Assessment reaches a particular state, Vault can automatically:
-
Provision an External User account for the individual identified in the Person record.
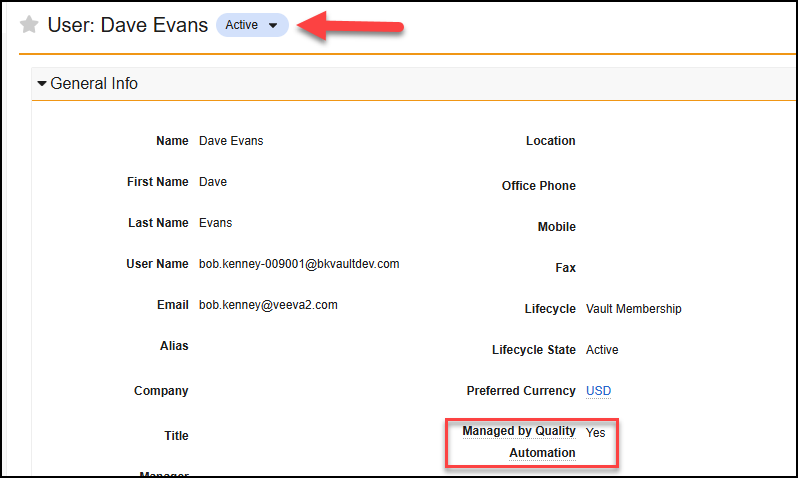
-
Send a specialized email message to the external user that provides information about how to log in and complete the task.
-
Provide the external user with streamlined access to Vault to complete a workflow task.
-
Provide the external user with the minimum required access to the Change Action or Impact Assessment record in order to provide the required information.
When the external collaboration task is completed, Vault can automatically:
-
Send an email message to the external individual indicating that the task is finished and access to Vault is no longer available.

System Admins must configure this feature before use. You can learn more about configuring External Collaboration for Impact Assessments and Change Actions in Vault Help.
Create Record From Template: Support for Impact Assessments
Change Controls and Change Plans often require organizations to conduct similar Impact Assessments over and over again. Manually creating routine Impact Assessments from scratch can prevent organizations from effectively standardizing, which can lead to inconsistencies, errors, and user frustration while performing repetitive tasks.
Beginning in this release, Vault will expand the Create Record from Template action, enabling users to create Impact Assessment records from approved Impact Assessment Templates. Templates enable customers to standardize the values that appear in Impact Assessment record fields.
When Vault creates an Impact Assessment from a template, the values in the template are copied to the corresponding fields in the new Impact Assessment record. Users who create Impact Assessment Templates can decide whether the field values copied from a template should be editable or read-only in the resulting Impact Assessment records.
Impact Assessment Templates are available for use in the standalone Change Control and Change Plan objects, as well as the Quality Event objects for the Change Control object type. Users must create and approve templates to use this feature. Additionally, this feature requires configuration by an Admin. Below are some configuration options to consider when evaluating this feature:
- Admins configure the ability for users to create new Impact Assessment records at specific states in the lifecycle of a parent Change Control or Change Plan.
- Matching fields determine which approved Impact Assessment Templates are available for use in a particular parent Change Control or Change Plan. Admins specify which fields on a Change Control or Change Plan must have matching values for equivalent fields in a template.
- Admins can configure Vault to automatically create Impact Assessments from all matching templates (up to 300 records) when a parent Change Control or Change Plan is created or enters a particular state.
This feature promotes the standardization of common Impact Assessments, reducing the need for repetitive data entry.
Create Related Record: Copy Related Object Data
Prior to this release, the Create Related Record action, configured by Admins, automatically created a new object record when transitioning a source object record through a specified state. For instance, if configured by an Admin, transitioning a Supplier Corrective Action Request (SCAR) in the system would automatically trigger the creation of a related Deviation record.
With this release, we are introducing greater flexibility and control. Admins can now specify which related reference records should be copied to the newly created record during the execution of the Create Related Record action. For example, if a SCAR is linked to multiple batch and material records, these associations can now be replicated in the newly created Deviation record.
In addition to its existing availability as an entry action, the Create Related Record action is now also available as a user action. This allows users to manually trigger related records creation, providing more operational flexibility and aligning with specific workflows as needed.
Note: The Create Related Record user action runs as system in both 24R3.4 and 24R3.5. For 24R3.4, users will require permissions on the Source join objects and related fields and the Target join objects and related fields. For 24R3.5, users will require Read permission on the Source join objects and Create permission on the Target and Target join objects. This release will also remove all field-level permissions checking.
Learn more about Related Record configuration.
PSUR: Periodic Safety Update Report
With this release, we’ve added support for the preparation of Periodic Safety Update Reports (PSURs) for Vault QMS MedTech customers. These reports, consisting of disparate information derived from clinical, commercial, and vigilance data, as well as authored narrative and contextual content, can be difficult to construct, track progress against, and prepare. Now customers can manage that entire process in Vault QMS, yielding efficiencies through template usage, organization, and clear work item tracking.
With EU regulations mandating the preparation and submission of these reports, Vault QMS MedTech organizations can handle these activities now by defining and creating Templates for managing their PSUR preparation activities moving forward. Periodic Reports represent the workspaces for a given PSUR, providing for a description, targeting dates, key metadata, and trackers for individual PSUR Items that need to be, are being, or have been completed. Once the content pieces have been all completed, the final step of the PSUR preparation process is for Vault to automatically generate a concatenated document consisting of all of the component PSUR Items merged into a single PDF, saved to the Vault, and made ready for submission.
Organizations familiar with the Annual Product Quality Report functionalities in Vault QMS should find this to be a somewhat familiar process. For PSUR, Vault QMS will now support the creation of a PSUR workspace (Periodic Report) either from scratch or from a template (Periodic Report Template) that they’ve defined. This workspace will allow for PSUR Items to be identified as being needed and will provide status information for the progress of each PSUR item from authoring through review and approval. PSURs can be created with or without a template to support varying business needs.
The PSUR feature set requires configuration. Learn more about configuring and working with PSURs.
QMS: Download to PDF Enhancements
In this release, we’re expanding on the 24R2 Print Record feature, with a focus on functionality for QMS-specific application controls. The following controls are now supported:
- Action Steps Section
- Auditor Qualification Status Section
- Template Process Steps Section
- Reason for Change Audit Trail Section
These sections now render natively when using the Print Record function on any record in Vault QMS. More application controls are coming in future releases as we further push to provide native printing capabilities across the entire application, so stay tuned for more updates with our upcoming releases.
The following screenshots show examples of how these sections appear in QMS and in a PDF version of the record.
Action Steps Section
Below is a screenshot of a Change Control record showing the Action Steps section, followed by a PDF of the record produced by the Print Record feature with the Action Steps section displayed. This enables users to see Action Steps in a downloaded PDF for a Change Control, Change Plan, or Quality Event Change Control record.
Below is a screenshot of the section control from a System Admin perspective in an object layout.
Auditor Qualification Status Section
Below is a screenshot of an Auditor Profile record showing the Auditor Qualification Status section, followed by a PDF of the record produced by the Print Record feature with the Auditor Qualification Status section displayed.
Below is a screenshot of the section control from a System Admin perspective in an object layout.
Template Process Steps Section
Below is a screenshot of a Risk Assessment record showing the Template Process Steps section, followed by a PDF of the record produced by the Print Record feature with the Template Process Steps section displayed.
Below is a screenshot of the section control from a System Admin perspective in an object layout.
Reason for Change Audit Trail Section
Below is a screenshot of a Reason for Change record showing the Reason for Change Audit Trail section, followed by a PDF of the record produced by the Print Record feature with the Reason for Change Audit Trail section displayed.
Field Actions: Related Event Automation
Continuing our investments into the new Field Corrective Vault, QMS has expanded the Quality Relationship Automation feature to allow Field Corrective Actions and Health Hazard Evaluations to link to Quality Events (either in the Quality Event object or the standalone Event records). Once configured, users may link Event records to Field Corrective Actions or Health Hazard Evaluations via the Related Events section on their records details pages, and the reverse relationships are established automatically for them.
Generate Merged PDF from Record
With this release, we are introducing an enhanced capability to streamline the process of finalizing an Annual Product Quality Review (APQR). It eliminates the need for the intermediary step of generating a binder, enabling users to directly create a single consolidated PDF containing all documents linked in the APQR Item records.
Previously, finalizing an APQR required users to complete the following steps:
- Collect all documents that, when combined, produce a final APQR report.
- Generate a binder and approve it.
- Create a merged PDF from the approved binder to produce the final APQR report.
With this new update, finalizing an APQR now requires the following steps:
- Collect all documents that, when combined, produce a final APQR report.
- Generate a merged PDF directly from the APQR record.
Users will be able to merge up to 100 related records containing the document reference and ultimately also decide whether to include non-steady state documents in the final report.
The feature will be available as a user action to be configured by Admins for APQR and PSUR objects in the desired lifecycle states.
Learn more about configuring Annual Product Quality Reviews.
Execute Root Cause Analysis from Investigation
The addition of the 5 Whys Analysis to Vault QMS provided a flexible and integrated tool to maximize efficiency, consistency, and compliance when users perform a root cause analysis.
In this release, we are enabling customers to launch and link to a Root Cause Analysis record directly from an Investigation record instead of navigating to the parent Quality Event record to perform the analysis. Once root cause analysis is complete, the resulting Root Cause records are linked to both the Investigation and parent Quality Event records.
You can learn more about configuring Root Cause Analysis from Investigation in Vault Help.
5 Whys Analysis: Save Button on Why Card
In this release, we are adding a Save button to the Why? box in the 5 Whys Root Cause Analysis Tool to save any changes made to a record, instead of saves being determined by Vault via clicking outside the panel to trigger a save. This change will make it much more apparent to users which data has been affected by a Save action while working with the 5 Whys Root Cause Analysis Tool’s cards.
The Save action is required on any new or changed card in the Root Cause Analysis Tool before any data is saved in Vault. This change in behavior will be automatically enabled in all Vaults that use the 5 Whys Root Cause Analysis Tool.
Inspection Readiness: Supporting Records
This release deepens our investment into the Audit Room processes within Vault QMS with the first of several components of a new framework for sharing Vault content directly with Inspectors. Audit Room team Fulfillers can now associate records of supported objects to fulfill Inspection Requests, granting Inspectors the ability to see these records directly in the Vault once the Inspection Request is Published. Our focus with this first enhancement in this space is on very tightly controlled shares of specific record information.
Through a shared record’s Viewer lifecycle role, Inspectors benefit from a Layout View and Layout Profile, which can be configured on any shared object to ensure that Inspectors are only ever exposed to a controlled, predetermined view of any given record that is shared during an inspection. In this initial release, shared records include only primary data (fields on the shared record). Shares are for a single record at a time, so Fulfillers can be very specific when sharing content; choosing to share, for example, just a Complaint, or a Complaint and all of its investigations based on the nature or details of the Inspection Request.
Additional controls are given to Fulfillers regarding whether attachments to any Published records are provided to the Inspectors during this share. All shared records associated with a given inspection, regardless of the object or type, are displayed in a single, simple section to make it easy for the Inspector to navigate without additional training on the structure of your Vault.
Below is the list of supported standard objects with this release; records of any type of each of these may be shared with Inspectors to fulfill requests:
- Adverse Event Reports
- Audits
- Annual Product Quality Reviews (APQRs)
- Quality Management Reviews (QMRs)
- Impact Assessments
- Investigations
- Materials
- Products
- Supplier Corrective Action Requests (SCARs)
- Supplier Change Notification (SCN) Impact Assessments
- Qualifications
- Quality Events and standalone Events
Supporting records sharing does require configuration to ensure that the supporting records section is available on Inspection Requests and that appropriate layouts, profiles, and roles are available on the supported shareable objects.
This is the first of several investments in this area, with future plans including support for shares to include inbound relationships automatically and support for sharing custom object records. Learn more about configuring and working with supporting records sharing in the Audit Room.
Surveillance
VPS: Regulatory Updates Enhancements
With this release, Vault Product Surveillance (VPS) has added support for the generation and management of Adverse Event Reports for health authorities that aren’t currently supported with a standardized health authority form, including any reportable event where no configured Report Type is listed for the Country. In these instances, Vault now either properly creates and moves to Draft or properly creates a follow-up for an Adverse Event Report (AER) of type Other with a populated due date, based on whether an AER already exists (and what state it’s in) for that event within the system.
Lastly, given recent FDA guidance, eMDR validations also have been updated to add additional data enforcements. These enforcements cover scenarios like requiring Weight Units when Weights are recorded and requiring all adverse event codes to be in initial MDRs with code entry requirements when Report Submitters are either Blank, Manufacturer, or Importer.
VPS: Japan AE Reporting Custom UI
This release expands our capabilities around MedTech Adverse Event Reporting and introduces the capability for organizations to create and edit a PMDA-submittable Adverse Event. Bespoke UI dedicated to PMDA Form 8 has been introduced to the Vault, allowing users to modify all aspects of such an event’s data from a single page, which is in line with how other regulatory reporting pages work in VPS. Support for attachments to the report is also included. This release, with the help of the related VPS: Japan AE Reporting Data Model and VPS: Japan AE Reporting XML features, enables organizations to manage a PMDA-submittable event from identification as part of the complaint through the generation of the PMDA-submittable XML file.
Learn more about working with Vault Product Surveillance’s Adverse Event Reporting capabilities.
VPS: Japan AE Reporting XML
This release expands our capabilities around MedTech Adverse Event Reporting and introduces the capability for organizations to generate a PMDA-submittable XML representation of an Adverse Event. With this release, you may generate an XML from a completed AER and submit it manually to the PMDA through their web portal.
VPS: Japan AE Reporting Data Model
This feature introduces the data model required for Vault QMS MedTech organizations to be able to support Adverse Event Reporting to Japanese regulatory bodies (PMDA). Details of how this change is beneficial to customers can be found in the VPS: Japan AE Reporting Custom UI feature.
Health Canada: Data Mapping Enhancement
The mappings of where Vault sources and processes data for Health Canada reports has been updated to be more consistent with industry needs and expectations. Field A2 shall now map to the Report Submitter as distinct from the Reporter for the event report. Both the VPS UI and the report will be updated as part of this feature. This feature is automatically enabled and does not require configuration.
Learn more about working with Vault Product Surveillance’s Adverse Event Reporting capabilities.
Batch Release
Batch Release Plan Variation with Parent Plans
Batch Disposition Plan Variation improves the efficiency of managing hundreds or thousands of plans by allowing material-specific child plans to inherit the settings and Check Requirements from a parent plan. This feature is enabled by referencing the parent plan in the new Parent Plan field on a Batch Disposition Plan. A child plan can have only one (1) parent plan; users cannot enter a plan in the Parent Plan field if the selected plan already has a child or parent plan associated. When a setting or field on a child plan is blank, the setting or field value from the parent plan is used instead.
Evaluate Impact Batch field
Currently, a Batch Disposition Item is closed when its related Deviation or Lab Investigation is closed. With the new Impact Batch? field on the Batch - Deviation and Batch - Lab Investigation join objects, a Batch Disposition Item now is closed only when the related Deviation or Lab Investigation is in a Closed state and the Impact Batch? field is False.
Change Control Check for Materials
A batch’s material that is affected by a change will now be monitored when using the Quality Event Type Check Requirement. The change must be approved and implemented prior to batch manufacturing.
Check for Genealogy Batches
Checks can now monitor a batch’s genealogy by creating Batch Disposition Items that are monitored on the Batch Release Execution page. To use this feature, any Check Requirement can have its Include Genealogy? field set to Full or No. Full will create Batch Disposition Items to monitor related records regardless of whether they have been reviewed in a Batch Disposition already.
By Exception Genealogy Check
Checks can now monitor a batch’s genealogy by creating Batch Disposition Items that are monitored on the Batch Release Execution page. To use this feature, any Check Requirement can have its Include Genealogy? field set to By Exception. By Exception will create items for related records that have not been closed on an accepted and closed Batch Disposition.
Implement & Evaluate Change Control Implementation Date
A new Implementation Date field has been added to Change Controls and Change Plans.
Configure Due Date Using Any Batch Date Field
A new UI control has been added to the Batch Disposition Plan so that any Date or DateTime field on the Batch object can be used to calculate Disposition and Check due dates.
Validation Management
Comment Design Enhancements for Dry Run
In 25R1, customers who have configured and enabled Dry Run for Test Scripts will see several enhancements to comments. The Add Comment and Edit Comment forms have been optimized to provide a better user experience. Comments in the Comment panel display the category, groups are updated with simpler labels, and more direct interaction is available for selecting and expanding comments. Resolve and Reject actions for Authors are displayed when users hover over a comment, but only if the user has permissions assigned. This feature is auto-on for customers who have enabled Dry Run for Test Scripts, released in 24R3.
Comment Filter Enhancements for Dry Run
In 25R1, the filters have been updated for the comment panel used for Dry Runs. Admins are now able to specify the default comment states that should be shown in the comment panel when executing dry runs or authoring tests. Dry Run Executors and Test Script Authors can now filter the comment panel by any combination of states that they require. This is an auto-on feature for customers who have enabled Dry Run for Test Scripts released in 24R3.
Improved Navigation to Application Pages
In 25R1, Vault Validation Management will redirect users to the applicable authoring, execution, or review UI when they click the link for a Test Script record from a list view or object reference field. The feature must be enabled in the application settings and configured by an Admin.
Quick Create for Requirements & Specifications
In 25R1, Vault Validation Management will streamline how user requirements, functional requirements, design specifications, and configuration specifications are created by reducing the number of clicks involved to create a new record. Users now can create records directly from an Entity Version without having to click Create from a separate Add dialog, which reduces the number of clicks from six (6) to two (2).
This feature also enhances the Requirement Entity Version control on the Entity Version object to include filters for each column displayed and provides the ability to remove one (1) or more requirements or specifications from the Entity Version.
Responsive Side Panels for Application Pages
In 25R1, the width of the side panels (step navigation, requirement burndown, comments) shown in application pages used within Validation Management (Test Script Authoring, Test Script Execution, Test Script Review, Test Protocol Review), will now adjust in width based upon the resolution of the browser. This optimizes the user experience by giving users more space to see details around requirements when authoring and comments when executing or reviewing test scripts or protocols. This is an auto-on feature.
Template Requirement Refactoring
In 25R1, Vault Validation Management enhances the use of Template Requirements to improve consistency, usability, and functionality. Template Requirements now utilize the existing Validation Requirement object, aligning with the same paradigm for Template Test Protocols and Test Scripts. By leveraging the Validation Requirement object, Template Requirements can now support the Versioning and Validation Team. With this change, we introduce two Admin settings to migrate existing Template Requirements to the Validation Requirement object and to enable the new logic in the system. These settings will help you to transition your Template Requirements to the updated framework at your own pace. However, starting in 25R3, the updated model will become mandatory. While all records will be migrated automatically, configuration will not be migrated.
If you are currently using the Template Requirement feature, please review your current Template Requirement-related configurations and plan for migration and enablement before 25R3.
This feature requires configuration to utilize, and Admins should enable the Refactor Template Requirement feature flag from Admin > Settings > Application Settings > Validation.
Template Test Protocols & Test Scripts
In 25R1, Vault Validation Management is introducing templating capabilities for Test Protocols and Test Scripts. When enabled, Test Protocols and Test Scripts can be created from a Template Test Protocol or Template Test Script record including test steps and additional prompts. With this feature, all existing Test Protocol and Test Script records will be updated with a new Entity Version field value. This feature will help organizations establish a center of excellence for Test Protocols and Test Scripts that can be used globally in their business and have an approved repository of Template Test Protocols and Test Scripts available for use.
This feature requires configuration to utilize.
Test Protocol Execution Review Comments
In 25R1, Reviewers and Approvers for Test Protocols will be able provide feedback on specific steps or prompts as part of their reviews. This drives efficiency by clearly identifying which steps in the Test Scripts require correction, will automatically reject Test Scripts in the Test Protocol and assigns tasks to responsible Executors. Comments and their replies drive collaboration between Executors, Reviewers, and Approvers ensuring that Test Protocols are approved quickly. The feature is not visible to all Validation Management users by default and requires configuration changes to use the feature, including modifications to existing permission sets.
Test Script Execution Review Comments
In 25R1, Reviewers and Approvers for Test Scripts will be able provide feedback on specific steps or prompts as part of their reviews. This drives efficiency by clearly identifying which steps require correction and automatically assigns tasks to responsible executors. Comments and their replies drive collaboration between Executors, Reviewers, and Approvers, ensuring that Test Scripts are approved quickly. The feature is not visible to all Validation Management users by default and requires configuration changes to use the feature, including modifications to existing permission sets.
LIMS
Sample Result Entry: Support for Viewing Attachments
This feature adds additional support to the Sample Result Entry UI by allowing Lab Analysts to upload and view transcription documents, such as the CoA or Stability Report from a third party lab, while entering results.
Sample Result Entry: Support for Multiple Samples
This feature adds additional support to the Sample Result Entry UI for entering result values of multiple Samples at one time (such as transcribing results for a Stability Study).
LIMS: Initiate Lab Investigation
With this feature, authorized users can initiate Lab Investigations from Vault LIMS. During exception review, an authorized user can request a Lab Investigation for any exception. The Lab Investigation is created in QMS for investigation and closure. As the Lab Investigation is processed in QMS, Vault LIMS is aware of the key milestones and can integrate Lab Investigation status into LIMS workflows.
Multiple Test Procedures for a Lab Test
This feature allows users to view multiple documents associated with a Test that’s being executed via Test Execution. Users can access the procedure, work instruction, etc. associated with the Test Definition.
Exception Summary: View Exception Details
This feature allows a user to view record details for all the exceptions that occurred during sample testing. For example, if there is an Out of Specification exception, the user can view which Result was out of spec and click the icons to show a list of related records.
Replicate Samples
This feature allows a user to create one or more Samples from an existing Sample. The resultant Samples will have the same planned Tests and Criteria Evaluations as the copied Sample.
Replicate Tests
This feature allows Samples to have multiple tests, of the same Test Definition, to be automatically assigned. Design Data Admin can use the Number of Replicates field to define how many tests need to be assigned.
Design Data Portability
This feature allows organizations to easily move design data records between Vaults in a fast and efficient way. Design Data records (Sample Plan, Test Definition, and Spec Data) can each be exported to a ZIP file, and then the file is imported to the target Vault. This eliminates the need to build complex loader files to transfer design data.
Modifying Test Inputs
This feature allows Lab Analysts to modify Test Inputs in the method execution screen for one or more Tests to accommodate changes to inputs part way through testing a set of Tests.
Aggregate: View in Test Execution
This feature gives Lab Analysts and Test Reviewers the ability to see aggregate field calculations of completed Tests on the relevant Test’s review and verify screens.
Stability Study: Data Export
Authorized users can now export stability data to an Excel or CSV format. The data is in a table format grouped by Storage Condition and Orientation. For each Condition and Orientation combination, tests and results are exported across timepoints.
Resample from Backup Sample
This feature adds the ability for a user to resample an existing sample with traceability to a backup or reserve sample.
LIMS 25R1 Standard Metrics & KPIs
This feature introduces standard components to the LIMS data model to support the measurement of operational excellence.
Populate KPI-Related Sample Fields Through Aliquot & Pooling Actions
This feature enhances the existing Aliquot and Pooling actions to populate key date fields (such as Delivery Date and Received Date) on the Resultant Lab Samples if there are values on the Source Lab Samples.
Populate Latest Test Approval Date on Spec Execution
This feature adds an entry action within the Lab Test Lifecycle that populates the Latest Test Approval Date (Datetime field) on the related Spec Execution.
Lab Sample: Release & Stability Object Types
This feature introduces standard object types for the Lab Sample object. These object types are inactive by default.
Lab Sample: Set Mode of Creation & Sample Action
This feature details why a Lab Sample was created and what sample action created it. This makes it easy for users to understand the reason for Sample creation.
Resample Actions Now Create Planned Aliquot Sample Actions
Resample, Resample and Keep, and Resample From Backup actions now create planned aliquot actions if the source sample has aliquots associated with it.
Auto Match is Always Set to Yes for Spec Data Sample Action
Moving forward, the Auto Match field value is always set to Yes for Spec Data Sample Action records. This simplifies the Admin experience for setting up a Spec Data record.
Allow One Aliquot for Sample & Target Sample Definition Combinations
This feature prevents Admin errors by ensuring that only one Aliquot action for a given Spec Execution record exists for a given Sample Definition and Target Sample Definition combination.
Test Execution Minor Enhancements
This feature adds usability enhancements to the Test Execution User Interface. The Action menu is displayed when hovering an Input row, and an improved calculation error message is displayed for missing cross-test variables.
Document Viewer: Updated Viewer
The document viewer on Test Execution was updated for a simplified user experience. The Document Info panel is no longer viewable.
Convert Controlling Lookup Fields to Reference Fields
This feature introduces new fields to the standard data model to address an issue where lookup fields were not updating in time to control dependent fields. This includes fixing a bug where new Spec Data versions sometimes referenced old Test Definition versions.
Standard Actions to Create Variation Input & Variation Results
This feature provides standard actions to simplify creation of Test Definition Variation Input and Result records.
Action on Lab Test Definition Result to Create Basic Variables
This feature adds a new action on Lab Test Definition Result to create basic variables based on the existing Test Definition Results. The action is only available on Test Definition Results with Data Entry Method of Calculation.
Actions to Activate & Inactivate All Sublocations
This feature adds new Actions to Activate and Inactivate a Lab Location and all of its sub locations. The Inactivate action will only succeed if there are no object records (for example, Samples, Assets, or Consumables) associated with the Lab Location or any of the sub Locations.
Pulled Lab Sample Must Match Spec Execution Object Type
This feature adds validation to the Pulled Lab Sample field on Spec Data Sample Action so that the Sample’s standard object type must match the current Spec Execution Object Type. This prevents users from selecting an incorrect Lab sample record.
Print Label Workflow Actions with Multi-Record Workflow
This feature adds the ability for a user to run a multi-record workflow where the Print Label action is configured as a workflow step.
Make All Print Actions Workflow Actions
This feature enables Admins to configure existing print label actions (such as Lab Test, Lab Sample, Lab Location, Lab Consumable, and Asset) as workflow steps. This allows label printing to integrate more seamlessly with business processes such as review, approval, and eSignature.
Calculated Result Change Reason: Dependent Value Changed
When a test result is changed, and the test result is used in a calculation (such as an aggregate) that has already been reviewed and requires a change reason for the change, the change reason is set to Dependent value changed.
Calculation & Instrument Override: Simplified Permission
Overriding a Calculation or Instrument Result now requires permission to new Actions and does not require the user to have the Test Execution Override role assigned to their account.
Validate Pulled Lab Samples
This feature ensures users select the correct Lab Sample for the Pulled Lab Sample field by ensuring that the Sample’s Spec Exec and current Spec Execution are the same.
Prevent Changing Object Types on Spec Execution
With this release, Spec Execution object types cannot be changed from Release to Stability or vice versa.
Stability: Prevent Non-Stability Spec Data on Non-Initial Timepoints
This feature adds validation on Study Timepoints so that only Stability Spec Data records can be selected.
Cannot Delete Study Timepoint & Action if Timepoint is Initiated
With this feature, Study Time and Study Action cannot be deleted if the Lab Study Timepoint is Initiated.
Validate Test Definition When Changing Mode of Entry
This feature adds validation to prevent a Test Definition, with Sample Result Entry as Mode of Entry, from having Inputs, Steps, or Summary calculations, such as aggregates.
Consumable Record Must be Part of Family
Only Consumables that are part of a Consumable family record will be available for selection in the Test Execution interface.
Change Analysis Check for Invalid Variables
This feature introduces a new Change Analysis version verification/completion action for Test Definition to check that calculation syntax does not include invalid variables (for example, due to a cancelled Test Definition).
Show Variable Names in Error Messages
Error Messages now display the Result Variable name.
Result Cloning Rules
The following LIMS objects are now cloned moving forward: Calculation Constant, Calculation Constant Value, Calculation Constant Value Version, Result Picklist Option, Result Picklist, Unit of Measure
Sample Action Job: Queue Next Job Instead of Erroring
If the Sample Action job is initiated multiple times within a Spec Execution record, the subsequent jobs now queue instead of ending in an error. The Job Title now contains the record ID of the Spec Execution Sample Action or Spec Execution.
LIMS 25R1 Data Model Standardization
Veeva LIMS adds a number of standard components to support evolving needs around major business processes.
Regulatory
In addition to the below release notes, the RIM Registrations and RIM Submissions, Publishing, and Archive Veeva Connect communities offer general release communications, release highlights, and key feature demos.
The following features listed in the Vault Connections section also affect the Regulatory application family:
- Quality-RIM Connection: Enhanced Change Control
- RIM-Clinical Operations Connection: Respect Archived Studies on Clinical CrossLinks
All RIM Applications
Commitment Extraction
This feature introduces the streamlined creation of Commitment records as text is highlighted within the Document Viewer. This is an extension of the existing Health Authority Question extraction functionality. When Commitments are selected in the panel during Commitment selection mode, Commitment records are created upon highlighting text in the document viewer and their fields are populated based on matching fields from the correspondence document. Once the selection of commitments is complete, the system provides the ability to review the commitments in a dedicated tab. Users can view and edit the records individually or in bulk. This feature requires configuration updates.
Updates to Commitment & HAQ Relationship Deletion Rules
With this release, auto-on updates have been made to the deletion rules of a number of Commitment & HAQ join objects/relationships to facilitate easier & better user experience with deleting extracted HAQs/Commitments. The deletion rules have been updated from Prevent deletion to Cascade deletion on the following objects. This streamlines the deletion of HAQs and Commitments in the panel during the extraction process so that the relationships do not need to be deleted in order to delete the parent record. This will also impact manually created and deleted Commitments and HAQs. The relationships are:
- Commitment Application (
commitment_application__v) - Commitment Submission (
commitment_submission__rim) - HA Meeting Commitment (
ha_meeting_commitment__v) - HA Meeting HAQ (
ha_meeting_haq__v)
Updates to Health Authority Question Extraction
Several auto-on improvements have been made to the Health Authority Question (HAQ) Extraction functionality. These include:
- Updates to the document panel (including the panel name & icon) as well as the RIM Document Type Configuration admin page to accommodate both HAQ & Commitment Extraction.
- Support for auto-population of the HAQ HA Meeting object based on the document’s Health Authority Meeting field when HAQs are extracted
- Update to handling of multi-select Object or Picklist fields on the document that are single select on the HAQ. When there are multiple values on the document, the system will now leave it blank on the extracted HAQ.
- Annotations for extracted HAQs are now created in resolved state instead of unresolved.
- The Question Source and Question Source Document fields have been updated from system managed to non-system managed. This allows these fields to be set on HAQs outside of the extraction functionality.
Data Model to Support eCTD 4.0
To educate and prepare Veeva RIM customers for the future implementation of eCTD 4.0 specifications globally, data model changes to support eCTD 4.0 are now available in all customer Vaults. The eCTD 4.0 data model impacts Veeva Submissions, Submissions Archive, Submissions Publishing, and Registrations.
A new standard Object, Extended eCTD Keyword, has been introduced to support optional eCTD 4.0 metadata not included in eCTD v3.2. The new data will be managed as Object Types on Extended eCTD Keyword:
- Facility
- Component
- Container
- Descriptor
Extended eCTD Keyword relationships, in particular, will impact many aspects of Veeva RIM, including Event, Application, Regulatory Objective, and Submission objects. Submission Wizard, Create Related Records, and Bundling & Splitting bulk actions have been updated to recognize these new relationships. Content Plan creation, updating, and copying processes have been updated to respect new Submission Extended eCTD Keyword tokens and the underlying relationships needed to create repeating Content Plan sections for the keyword Object Types. End user processes will not be impacted by these changes prior to their implementation, though the changes do allow for customers to begin migrating Extended eCTD Keywords in preparation for eCTD 4.0 as their business needs determine it.
Configuration updates are required to begin using the new eCTD 4.0 data model. While eCTD 4.0 will not be mandated by any Health Authority until at least 2026, Veeva strongly recommends customers use this opportunity to familiarize themselves with the data model at this time. More details will be provided around data model adoption requirements as eCTD 4.0 regions are supported in RIM.
The full list of data model changes can be found in the 25R1 RIM Data Model Changes spreadsheet, available for download beginning with the 25R1 General Release of the Data Model Documentation Vault platform feature.
RIM Registrations
IDMP Support for Local Differences in Product Variant Attributes
This feature updates the Product Variant data model and related transactions to support differences in reporting among local markets. A new accelerator automatically creates Active Substance Alias and Inactive Ingredient Alias records to support the management of different naming across markets for the same ingredient. Updates to the IDMP data aggregation algorithm support the updates to the data model as well as additional complex data modeling cases. This results in fewer product variants to manage, while still allowing for all data to be captured for respective markets.
An illustration of how this feature will impact creation and maintenance of Product Variants for differences in dosage form expression is included below as an example:
A migration tool is available in RIM Maintenance to support creating new alias records for existing Active Substance and Inactive Ingredient records, which will be needed to support XEVMPD, IDMP, and UPD. An additional RIM Maintenance migration tool is available for Manufactured Dosage Form, which will create one Product Manufactured Dosage Form record from the Manufactured Dosage Form field on the Product record. This will be used to support IDMP and UPD.
Splitting Support for Enhanced Change Management
The Splitting wizard has been updated to support enhanced change management. Enhanced change management provides the ability to track product changes at a more granular level than an Activity using Change Items, and the ability to split Activity Change Items helps Regulatory manage changes at a level that makes sense from a regulatory perspective (for example, a change control where Quality has grouped many changes together which may fall under different regulatory submission categories).
Customers wanting to use Enhanced Change Management must follow the enablement steps for the Create Related Records Support for Enhanced Change Management feature. No additional configuration is required for Bundling and Splitting.
Support EUDAMED UDI Submissions for Master UDI-DI
Master UDI-DI supports capturing an identifier of a group of highly individualized products/devices. The driver for this update to the existing regulation is contact lenses. This feature supports the use case of managing UDI submissions for contact lenses, spectacle lenses, and spectacle frames. Since contact lenses are available in so many variants due to the high number of clinical parameters that characterize them, a UDI-DI would need to be assigned to each of those variants of lenses. This results in a proliferation of UDI-DIs to be assigned to similar contact lenses, which overwhelms EUDAMED and is disproportionate compared to the safety risk associated with contact lenses.
In response, Master UDI-DI will be used to group contact lenses that have the same clinical and design parameter combinations including at least base curve and diameter. This reduces the number of data records in EUDAMED and prevents assignment of different identifiers to very similar contact lenses.
For customers that have activated the Identifier Type = master_udidi__v on the Data Carrier object, the validation upon generating a submission will work when the master_udidi__v identifier type is used.
Bundling & Splitting: Validation Enhancements
This feature introduces dynamic validation when bundling Regulatory Objectives or bundling Activities. When copying Event details to Regulatory Objectives and/or Submissions, Veeva RIM validates against relevant Application data based on their Related Change Type.
Potential use cases include:
- When bundling Activities, the corresponding local Regulatory Objective and Submission may not yet exist. If this is the first time copying Event details to a local context, validation ensures the details make sense for that Application.
- Bundling Regulatory Objectives may also group Activities together against a Regulatory Objective that did not originate from the same Event. This leads to further propagation of Event relationships to local records that have not yet been validated against the related Application data.
- When bundling Regulatory Objectives across Applications to support worksharing or grouping, the existing Regulatory Objective and Submission relationships may have an Application Source that is not on the primary Application.
Enablement: Admin Setting. Enable admin setting for “Enable dynamic validation for Create Related Records and Bundling”.
Bundling & Splitting: Support for eCTD 4.0
The Bundling and Splitting wizards have been updated to support eCTD 4.0. eCTD 4.0 support includes the ability to manage Extended eCTD Keyword details on Submissions and Regulatory Objectives, which is also extended to the relationships created and updated through the Bundling and Splitting wizards.
Customers wanting to use eCTD 4.0 must follow the enablement steps for the Create Related Records: Support for eCTD 4.0 feature, which includes enabling the Enable Application Relationships application setting. No additional configuration is required for Bundling and Splitting.
Create Related Records: Optional Preview
This feature will make Create Related Records preview optional, where it is currently enabled. Users will now be able to skip directly to record creation on the last step if they do not wish to preview data.
This will be Auto-On when the Preview is enabled. Admin setting to Disable Confirmation Pop-up when Skipping Review.
Create Related Records: Validation Enhancements
This feature allows customers who are not currently using Create and Manage Event Details to take advantage of the enhanced Create Related Records validation logic introduced in 24R3. Veeva RIM will use pre-existing validation logic when the Related Change Type is blank, but customers will still benefit from the updates to inactive and multi-match handling as long as the Enable Application Relationships application setting is enabled.
This update is Auto-On when Enable Application Relationships application setting is enabled.
Create Related Records: Support for eCTD 4.0
Create Related Records can now propagate Extended eCTD Keywords from Events to Submissions, Regulatory Objectives, and Applications to support eCTD 4.0 submissions.
Customers wishing to support eCTD 4.0 in Create Related Records will need to complete the necessary configuration for eCTD 4.0, including adding an Extended eCTD Keywords section to the Event page layouts.
Create Related Records: Support for Application Relationships with Lifecycles
In certain scenarios, the Create Related Records wizard attempts to activate Application relationships that are inactive. Prior to this feature, if the object had a lifecycle state type that made that relationship inactive, this operation would fail. Now, Veeva RIM transitions these records to the Initial lifecycle state type.
Enhanced Admin Settings for Medicinal Product Accelerator
This feature introduces a new Admin setting allowing users to configure the Medicinal Product accelerator by Registration type. This allows for the automated creation of Medicinal Product and Medicinal Product Registration records for animal health products.
Note: This functionality is not currently intended for investigational products and will be supported in a future release.
Create Event Details: Shelf Life or Condition Filtering Enhancements
Create Event Details now limits the values users can select for Shelf Life or Condition as well as Storage Condition based on the object type. For example, users can only select Bulk Ingredient shelf life values applicable to the selected Inactive Ingredient(s).
Registration Verification Workflow Behavior Harmonization
Previously, when the Registration object lifecycle’s Atomic Security for the Start Registration Data Verification workflow action was configured to Hide, Veeva RIM executed the workflow for single-record verification, and did not execute the workflow (with CSV errors) for multiple-record verification. With this fix, when Atomic Security for the Start Registration Data Verification action is set to Hide, Veeva RIM does not execute either workflow, and the error is apparent in the CSV. This change harmonizes the behavior for the two types of registration verification workflows.
RIM Submissions
Submissions: Increase Mini-Browser Window Size
When opening documents using the pop-out button in Veeva Submissions, such as from the Content Plan Viewer, the default width of the mini-browser window has been increased to also display the Document Information Pane and Action Menu. Users will no longer have to resize the mini-browser to initiate document actions or to view or edit document details.
Drag & Drop from Desktop to Content Plan Items With No Document Type
Users can now drag and drop documents from a desktop directly onto a Content Plan Item (CPI), even if the CPI does not have a full Document Type assigned. Previously, the drag and drop option would fail and an error message would be displayed. With this enhancement, users will instead be prompted with a dialog to classify documents now and populate document metadata.
Additionally, the toast for tracking document upload & match progress is updated to better distinguish between documents that are newly created vs. existing documents that are matched as a result of Duplicate Content Detection being enabled in the system. Newly created documents are denoted by the existing icon ( ) while existing documents that are matched use a new icon (
) while existing documents that are matched use a new icon ( ).
).
Drag & Drop to Reorder Content Plans & Content Plan Items
Content Plan (CP) and Content Plan Item (CPI) records can now be reordered via drag and drop functionality. The new drag and drop option provides an easier, more visual option with fewer clicks than the current Move Up/Down, Move to Top/Bottom, and Set Exact Position user actions. Records can only be reordered one at a time, though all descendant CPs, CPIs, and Matched Documents are included in the move to the target location. Drag and drop cannot be used to reparent records, because the target location must be within the same Content Plan section and in the same window.
This feature improves the usability of all types of Content Plans. Drag and drop is a faster and more intuitive solution to rearrange the Content Plan structure, such as after splitting an overmatched CPI, to reorder repeating sections created with tokens, or to prepare for a Section Merge at publishing.
Compare & Merge Submission Content Plans
This feature enables users to select two Submission Content Plans and run a comparison from the source Content Plan to the target Content Plan. The action is initiated from the action menu of the target Submission record and the user is prompted to select the source Submission from which they wish to Compare & Merge. The user can review the changes from the source Content Plan that are proposed to merge into the target Content Plan using the existing Comparison Viewer user interface. Changes are then committed to the target Content Plan based on the user’s selection at the time of comparison completion. This feature requires configuration updates.
Content Plan Creation Support for ASMF/APIMF
This feature supports the creation of Submission Content Plans for markets that distinguish between the Open/Applicant’s Part and the Restricted Part for the Active Substance section in the CTD structure. This is applicable, for example, for EU ASMFs and WHO APIMFs.
A new Information Classification picklist is introduced with the following picklist entries: ASMF Applicant Part, ASMF Restricted Part, APIMF Open Part, APIMF Restricted Part. This feature requires configuration updates.
Learn more about configuring Content Plan creation for ASMF and APIMF.
Multi-Record Workflows From Content Plan Viewer
Content Plan users can now initiate multi-record object workflows directly from the Content Plan Viewer. Similar to the initiation of document workflows when the Content Plan Viewer includes matched documents, object workflows can be initiated on single or multiple (up to 100) Content Plan or Content Plan Item (CPI) records. Workflows may only be initiated on like objects. For example, a group of Content Plan records can be selected, or a group of Content Plan Item records can be included in one Workflow.
The existing Start Workflow action is relabeled to Start Document Workflow and users will see a new Start Object Workflow action. If records have not been filtered to a single object prior to initiating the action, users will be prompted to first select the object to send on the workflow. The available workflows will then depend on the workflows configured for the object records’ lifecycle states and the user’s permissions.
RIM Publishing
Extract Health Authority Certificates from Publishing Gateway Profiles
The US FDA Async ESG and EU EMA ESUB Gateway Profiles will contain a new Download Certificates action. The action downloads the following certificates (if uploaded) in a ZIP file on active Gateway Profiles:
- Health Authority Certificate (in .p7b)
- Sponsor Private Certificate (in .pfx)
- Sponsor Public Certificate (in .p7b), which can be used to send and verify with Health Authorities.
This feature is auto-on.
Australia eCTD v3.2
With this release, Submission Publishing supports publishing and validating Australian dossiers based on the Australian eCTD guidance v3. and validation v3.2. This update includes the generation of the new warning.xml. The warnings.xml is managed via a new Submission Validation Warnings Object and a new User Action. The User Action to generate the warnings.xml is available when:
- Enable Continuous Publishing = No and there is a published output
- The Regional DTD/XSD Version field is set to TGA 3.2 (Veeva RIM UUID: 8c9ce80f-ca66-4598-8db7-4c1dfe77ea42)
- The Submission contains at least one Priority Rule failure (as defined by the Health Authority)
A new object Submission Validation Warnings was created to track Priority Rule failures for a given submission. The object is system managed and the only editable field is the Justification field. Users must enter a justification for each Priority Rule that fails before generating the warnings.xml. Upon running the User Action the XML will be generated and validation rules 2.10 and 2.11 will be executed against the submission. Running on-demand publishing after a warnings.xml file has been generated will remove the warnings.xml file from Submissions Archive This feature requires configuration updates. These changes can be provided to customer support teams via VPK packages or via the Support Portal. Additionally, a configuration change is required to add the Submission Validation Warnings object as a related object section to the Submission object to allow users to populate the Justification for failed Priority Rules. This feature requires configuration updates.
Ukraine eCTD v1.2
With this release, Submission Publishing supports publishing and validating Ukraine dossiers based on the Ukraine eCTD guidance v1.2 and validation v1.0. This feature requires configuration updates. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
Health Canada eCTD Validation Criteria Version 5.3
To comply with Health Canada Validation v5.3 for eCTD submissions, the following rules were added or updated:
- F09 - Updated description
- G22 - Added new file extensions
- H08 - Changed valid values version v5 to v6
- H21 - Added new rule
- I05 and I09 - Added Master file (MF XML)
This feature requires configuration updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
The system will include valid-values version 6 XMLs in util folders on subsequent Publishes post May 31, 2025. Until then, only valid-values version 5.0 will be included.
Health Canada Non-eCTD Validation Criteria Version 5.3
To comply with Health Canada Validation v5.3 for non-eCTD submissions, the following rules were added or updated:
- A08 - Added new extension
- I08 - Added letter “q” for biocides
- I09 - Added MF RT
This feature requires configuration updates to the Validation Criteria. These changes can be provided to customer support teams via VPK packages or via the Support Portal.
South Africa (ZA) eCTD Version 3.1 Document Matrix Updates
To comply with ZA Validation per Document Matrix v2.3 for eCTD submissions, the following rules were updated:
- 4.8.1 - Checks for Errors (E, document must exist) and Excluded Errors (XE, document is prohibited)
- 4.8.2 - Checks for Warnings (W, document is expected) and Excluded Warnings (XW, document is not expected)
- 4.8.3 - Checks for Possible (P, document is required in certain circumstances)
- 4.8.4 - Checks for a total count of E,W,P docs
Allow Dosage Form Envelope Attribute for ZA Submissions to be Overridden
This feature will allow users to override the Dosage Form when updating Submission Administrative Information. The overridden value is carried forward to future Submissions in the Application.
Allow Product Name Envelope Attribute for WHO Submissions to be Overridden
This feature will allow users to override the Product Name when updating Submission Administrative Information.The overridden value is carried forward to future Submissions in the Application.
New Validation Rule for Password-Protected ZIP Files
A new publishing validation rule is available to check for password-protected ZIP files in published output. Currently, the check for encryption on ZIP files is not included in any Health Authority validation criteria, though encrypted ZIP files may result in issues upon dispatch to some Health Authorities. The new validation rule, RIM104, can be configured and added to any existing or new Validation Criteria set via configuration. This feature requires configuration updates.
New Validation Rule for Placeholder Files
A new publishing validation rule is available to check for Placeholder documents in published output. The validation rule will perform a series of checks to try and determine the cause of the placeholder. Such checks include:
- Matched Document(s) Ready For Publishing field does not equal Yes
- Published Output Location is empty and operation is not Delete
- Matched Document Count is less than the Expected Steady State Count
- There is no Viewable Rendition for the document when the Source for Published Document is Viewable Rendition
- There is no manual rendition when the Source for Published Document is set to something that is not Viewable Rendition or Source Document
If any of the above checks fail a detailed result will be displayed explaining the failure. If a Placeholder is generated and the above checks pass a generic failure message will be displayed indicating a Placeholder exists. This feature requires configuration updates.
New Validation Rules for PDF Bookmarks
Updated Criteria ID values for EU eCTD Publishing Validation Criteria v8.1 (Rule16.BP8 and Rule16.BP9, respectively) have been added. The rule updates ensure the Navigation tab settings are appropriate based on whether or not bookmarks exist in the PDF. In addition to EU, the updated Criteria ID values for Rule16.BP8 and Rule16.BP9 are used for CH, TH, and UA validation. It is recommended customers update the Criteria ID field for the respective rules. This feature requires configuration updates.
Updated Validation Rules for Fillable Forms in US Submissions
The validation rules 5030 and 5 have been updated to check fillable forms 2253 and 3674 for US Submissions. If the form is fillable the system will attempt to compare the application number and type on the form with the application number and type of the regional.xml envelope.
Increase Table of Contents Max Row Limit
System generated Table of Contents (TOC) maximum row limit has increased from 2,500 to 10,000 rows. A row in a TOC can include a Content Plan, Matched Document, Bookmark, or combination of the above. Exceeding the 10,000 limit will result in a publishing failure with an error message indicating the maximum rows have been exceeded.
Remove Control Characters from Published Output Location Upon Publishing
ASCII control characters (except for spaces) will be automatically removed from the Published Output Location (POL) field upon publishing, ensuring the folder or file path remains valid. Previously, if a POL contained a control character (such as Tab or a carriage return), folder or file paths could have errors upon ZIP file export or Gateway dispatch. If a POL is manually updated to include a control character while publishing of the Content Plan Item is underway, the most recently completed action (manual or System) will take precedence.
Activate Validation Results Archival Job on All Vaults
The Validation Results Archival Job has been made Active on all RIM Vaults. The job runs daily to clean up Submissions that have an Actual Submission Date with Open Validation Results greater than 90 days old. The job will create an attachment ZIP file of all open results.
This is a follow up to the 24R3 announcement, Validation Results Archival Job Activation in 25R1
Add User-Facing Exceptions for Common Errors
Additional user facing exceptions were added to the Publishing Progress Indicator (PPI) to aid in troubleshooting publishing failures. Previously these failures would have resulted in Publishing failing with no helpful information in the PPI CSV. Additional messages cover the following cases:
- Incorrectly populating the Report Level Content Plan field on a Content Plan or Content Plan Item in a Submission Content Plan
- Workflow action changing state of a Content Plan or Content Plan Item at the same time a publishing job is performing work on that Content Plan or Content Plan Item
- Failing section merge due to Continuous Publishing on Content Plan or Content Plan Items being set to No
- Adding a Submission Archive document to a Content Plan Item
RIM Publishing Scalability Enhancement
Backend improvements to the publishing framework will allow for expanded scalability and improved overall performance. All Publishing Jobs (Initial, Continuous, On-demand) will be processed through the same continuous queue framework rather than processed via separate Jobs. End users will see no change in the publishing process. Admins will no longer see a separate On-demand tasks in Admin > Operations > Job Status.
Use System User to Export Published Submissions for Gateway Transmission
When exporting a Submission Binder to be sent to Health Authorities outside of Veeva RIM, the export will be completed as ‘System’ instead of the individual User. This change to System will override any User-specific permissions that may result in an incomplete export.
RIM Submissions Archive
Australia (AU) eCTD v3.2
With this release Submissions Archive is supporting importing, viewing, and exporting of Australian eCTD v3.2 submissions.
Ukraine (UA) eCTD v1.2
Submissions Archive supports import, viewing, and export of Ukrainian submissions according to the UA eCTD 1.2 specification. The UA Document Type Definition (DTD) has remained as version 1.0 (between the pilot and 1.2 specifications,) as such, any imports or re-imports of pilot submissions that follow the UA eCTD 1.0 specification will display according to UA eCTD 1.2 expectations. UA 1.0 pilot submissions imported before the release of this feature are unaffected, and will continue to display according to the UA eCTD 1.0 specification.
Singapore (SG) eCTD v1.0
With this release Submissions Archive is supporting importing, viewing, and exporting of Singapore eCTD v1.0 submissions.
Submissions Archive Viewer: Mini-Browser To Front
This enhancement returns the mini-browser to the forefront if it has been obscured by other windows or browser windows, or by other system applications. Selecting the pop-out button will bring forward the mini-browser window with a single click.
Improved Notification for Imports with Long File Names
RIM Submissions Archive notifies users when their import is prevented where at least one file name in the submission package exceeds the 255 byte limit for file names. The Import dialog error notification reads: “One or more filenames in this import package exceed the byte limit for import.” This error may be encountered when submission packages have file names where characters require multiple bytes in UTF-8 encoding, such as Chinese, Japanese, Korean, or Cyrillic characters— where a single character may be equal to more than one byte.
Job Title Update: Submissions Archive Cleanup
The system-managed job that removes temporary files generated during the Submissions Archive import process is renamed to Submissions Archive Cleanup. This is not a functional change, and is aimed at improving clarity for the Job Title visible to Admins in Operations > Job Status.
RIM Registrations, RIM Submissions
GCP Dispatch to Respect User-Selected Document Sets
This feature enhances the dispatch logic. The Document Set(s) selected by the user in the action dialog are respected during the actual dispatch comparison process for each target submission. If the target Activity has Document Set(s) other than the one selected by the user, the additional Document Set(s) are excluded from the dispatch as expected. A new field is added to the Dispatch Message object to track the Document Set(s) included in the dispatch/comparison. Document Set(s) tracked on the Dispatch Message are also respected when & if the user chooses to Regenerate Comparison.
Comparison Viewer Changes Saved Across Users
This feature saves selections in the Comparison Viewer across multiple users instead of saving them per individual user to facilitate collaboration. The Comparison can only be reviewed by one user at a time, but each successive user that accesses the Comparison will be able to see the selections, deselections, and manually mapped documents from the users who previously accessed that comparison. Changes are synchronized to the target Content Plan based on the selections that are present in the Comparison viewer at the time the Comparison review is completed.
Global Content Plan Updates for Region & Country Handling
Global Content Plans can now include Content Plans and Content Plan Items that are tagged with the global Submission’s Lead Market Country value, which is typically Global. When dispatching the structure, Region and Country filtering will continue to exclude the Global Content Plans and Content Plan Items. However, dispatching documents only will include documents matched to Global Content Plan Items and attempt to map them into target Submission structures. This feature better supports the Global Content Plan dispatch of Module 1 structure and documents to target Submission Content Plans.
RIM Registrations, RIM Submissions, RIM Submissions Archive
Active Dossier: Product Information & Registrations Tracking
This new feature provides a more granular view of the Active Dossier based on the Registration and select Product Information details. New Active Dossier Item Detail join relationships have been added for Registration, Product Variant, Manufacturer, and Inactive Ingredient, which the system automatically creates as part of the Active Dossier population process based on matching between the related Registrations, Submission, and document metadata. These relationships are surfaced in the Active Dossier Viewer and are editable in the Active Dossier Editor. This feature requires configuration updates.
Active Dossier Generation from Dispatch: Respect Enable Active Dossier Generation
With this release the Active Dossier generation from Global Content Plan Dispatch is updated to respect the Enable Active Dossier Generation (enable_active_dossier_generation__v) field on the Application so that Active Dossier records during dispatch are not created for Applications where the field is set to No.
Active Dossier Generation from Dispatch: Update to Pending Current Visibility
With this release the country status icon is set to Dispatched for Active Dossier Item Details (ADIDs) generated from Global Content Plan Dispatch in the Pending Current status. This allows these records to be visualized in the Country Overview layout without needing pending confirmation. Previously, no country icon was set for these records created in Pending Current and the pending confirmation had to be completed in order for the country status icon to display.
RIM Submissions, RIM Submissions Archive
Active Dossier Editor Enhancements: Inline Editing, Move & Edit Product Information Fields
With this release, enhancements have been made to the Active Dossier Editor.
- Support for inline editing
- A new Move action allows for sections to be moved to a new section in the Active Dossier
- Users must have the Application > Object > Merge Records permission to fully utilize the Move action
- The system managed Product Information fields (Product, Product Variant, Product Manufacturer, Active Substance, Active Substance Manufacturer, Inactive Ingredient, and Inactive Ingredient Manufacturer) are editable in the Editor. This simplifies corrections to Active Dossier records with incorrect Product Information metadata due to incorrect Submission relationship metadata at the time of initial Active Dossier population.
Increase Active Dossier Viewer Limit
With this release the limit on the number of Active Dossier Item Detail (ADID) rows displayed in the Active Dossier Viewer’s Item Detail layout is updated from 500 to 2,000. This means that the limit on the number of ADID rows displayed in the Active Dossier Editor is also increased to 2,000. There is no change to the Country Overview layout’s limits.
Update Active Dossier Translation Document During Population from Submission
With this release, the Translation Document field is refreshed for existing Active Dossier Item Details (ADIDs) during Active Dossier population from the Submission. Previously, the Translation Document field was only set when the ADID is first created during population and not on update. This allows the Translation Document to be appropriately set for Active Dossier records initially created from Global Content Plan dispatch where the translation is not initially present, updated when the population is run from the submission where the translation is included.
Active Dossier Country Hovercard to Respect Global Filters
This feature updates the country overview hovercard display in the Active Dossier Viewer’s Country Overview for the scenario when there are multiple Active Dossier Item Details (ADIDs) for a given Active Dossier Item (ADI) and Country. In the case that there are multiple ADIDs that meet the global filter criteria (including the Active Dossier status filter), the hovercard displays the first ADID that would be displayed in the Item Detail layout that meets the global filters.
Move Saved View Selector in Active Dossier Viewer
With this release the location of the Saved View selector in the Active Dossier Viewer has changed to be in a more logical position next to the Save View button in the header. There is no change to the saved view functionality.
Pop-Out Icon Added to Active Dossier Viewer Country Hovercard
With this release, a pop-out icon has been added to the header of the country hovercard in the Active Dossier Country Overview which opens the selected Active Dossier Item Detail record in the pop-out window. This allows more direct access to the Active Dossier Item Detail data.
Safety
The Safety 25R1 release, including all Platform features, is scheduled for tentative availability on April 10, 2025 & April 18, 2025. For the latest central dictionary updates for your Safety Vault, see Safety Central Dictionary Updates.
Note: In 25R1, Platform introduces the Record List: Flexible First Column, feature. As a result, while the Name field continues to appear first for most object grids, Safety users may see a different first column for the following objects:
- Case Test Result
- Person
- Product Registration
- Study Site
- Substance
- Watchlist
As needed, users can customize the order of grids so that the Name column appears first.
Several features listed in the Vault Connections section also affect the Safety application family.
Safety
Email to Inbox Item: Email Attachment Classification
With this release, Admins can now efficiently manage the handling of email and attachment classifications from their PV email inboxes. This feature introduces configuration options that offer precise control over email classification. Based on the configuration, Vault categorizes email documents as Case > Source > Adverse Event Report and classifies any non-E2B attachment as Case > Source > Non Re-Transmittable Document. This feature enhances efficiency by automating the document classification process, reducing manual effort and the risk of errors. It also improves compliance by ensuring that emails and attachments are properly categorized based on predefined rules, providing better organization and faster retrieval of documents for review.
Learn More
- Enablement: Enable Sender-Based Email Attachment Handling
- User Help: Email to Inbox Item
Document Intake Panel
This release provides Intake users with a fast and streamlined process to extract data from unstructured and semi-structured source documents. Users can easily highlight specific words within the document and generate Inbox Items mapped to the source document field values. All annotations will be mapped to designated Inbox Item fields, enabling quick intake and efficient case creation.
If you’re interested in discussing whether getting early access to this feature is appropriate for your business needs, contact your Veeva Representative.
Learn More
- User Help: Document Intake Panel
Duplicate Detection Search Updates
This release introduces several updates to enhance the duplicate detection in Safety:
- Case Identifier matching: Vault now prioritizes WWUID matching for more reliable identification of duplicates.
- Patient matching: Vault excludes keywords such as “unknown” and “privacy” from Patient ID matching, reducing false positives in potential match results.
- Adverse Event field matching: Vault no longer considers partially matching reported and coded Adverse Event terms when detecting duplicates.
- Updated filtering criteria: When a Study on an imported Inbox Item does not match a Study from the Study library, the filtering of potential matches will now be based on the source Study Type.
Learn More
- User Help: Promote to Case
Case Lifecycle State Change Error Handling
This release introduces enhanced handling for Case lifecycle state changes in Safety. Failures during lifecycle state entry actions are now logged in the System Alerts field on the Case, while the lifecycle state remains unchanged to prevent unintended transitions. These improvements enhance visibility, simplify issue resolution, and ensure a more reliable Case promotion workflow.
Learn More
- User Help: Case Processing Overview
Regions for Countries
Safety now includes standard regions for grouping countries. This feature includes the data structure, which Business Admins can revise. Future enhancements will leverage this data structure.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable Regions for Countries
- Admin Help: Manage Regions
Case Access Group Security: Country Update for Inbox Items and Cases
Safety now prevents users with specific country assignments on their User Access Group Assignment records from seeing Inbox Items and Cases without a Country value. This security change enhances the protection of sensitive information.
Learn More
- Admin Help: Manage Case Access Group Security
Case Follow-Up Compare with Summary
With this release, Safety enhances the Inbox Item to Case Compare page to improve usability and efficiency during follow-up scenarios. The updates include a summary of new and modified information, clarified filtering, and visual indicators for special fields, such as auto-calculated and MedDRA-coded fields, to help users identify changes more quickly. This feature allows customers to focus on the key differences between new information from the Inbox Item and the matched Case to make more informed decisions during follow-up processing.
This feature is controlled by the Enable Follow-up Compare with Summary application setting, which was released in 24R2. If you opt out of this feature by clearing the application setting checkbox, your 25R1 Vault functionality will reflect this choice. However, this application setting will be deprecated in 25R2 and the feature will be added to all Vaults. Ensure you have reviewed any applicable business procedures that may be impacted by this change.
Learn More
- User Help: Perform Inbox Item to Case Compare
Online Questionnaires for Follow-Ups Updates
With this release, Safety extends the ability to generate documents for manual method follow-up correspondences from configured document templates. Admins can also upload translations to send online questionnaire emails in the reporter’s language.
Learn More
- Enablement: Enable Online Questionnaires for Follow-Ups
- Admin Help: Set Up Follow-Up Online Questionnaires
- User Help: Send a Follow-Up Online Questionnaire to Case Contacts
Follow-Ups to Domestic Cases from Global Inbox Items: Japan Update
In the 24R3 release, Safety introduced Follow-Ups to Domestic Cases from Global Inbox Items. With this release, the feature prevents merging non-PMDA domestic Inbox Items to in-flight or follow-up PMDA domestic Cases. This update reduces the risk of error during case processing for PMDA-reportable cases.
Learn More
- User Help: Perform Inbox Item to Case Compare
Extend Company Product Match Verification to All Suspect Products
With this release, Safety extends product match coding verification on Inbox Items to all Company Products with a Drug Role evaluated as suspect. Depending on your configuration, Vault evaluates Company Products with a Drug Role of Suspect, Interacting, and Drug Not Administered as suspect. Previously this feature evaluated the primary Company Product only. By extending this feature to all Company Products, Vault offers more comprehensive Product match coding verification for imported Inbox Items.
Learn More
- Enablement: Enable Company Product Match Verification
- Admin Help: Configure Company Product Match Verification
- User Help: Inbox Item Study and Product Matching
Case Processing Operational Metrics
With this release, Safety facilitates the creation of operational reports and dashboards, providing more accurate Case processing time metrics by introducing:
- New standard Case lifecycle states to isolate Case idle time from active handling time
- Standard Report Types for Case, Inbox Item, and Inbox Item with Case objects to simplify custom report creation
Learn More
- Enablement: Enable Case Volume Metrics
- User Help: View Case Volume Metrics
Recalculate Due Date Keyboard Shortcut
With this release, Safety has introduced a keyboard shortcut to Recalculate Due Date on a Case, enhancing efficiency for users who prefer keyboard navigation.
Learn More
Rules Based Narrative Generation
With this release, Safety introduces the ability for Admins to configure rules for narrative generation. Admins can create Narrative Outline templates composed of Narrative Statements, each with their own Narrative Statement Options, where they can add the conditional rules under which Vault executes the associated statement and includes it within a generated narrative. Admins can use Vault Expressions to define conditions. Narrative Statement Options incorporate related Case data and new functions for formatting statements. Admins can order statements according to their alignment and sequence. Additionally, any changes to the Case between major versions are automatically summarized at the end of the generated narrative.
If you’re interested in discussing whether getting early access to this feature is appropriate for your business needs, contact your Veeva Representative.
Learn More
- Enablement: Enable Rules Based Narrative Generation
- Admin Help: Configure Rules Based Narratives
- User Help: Generate a Case Narrative
Blind Protect Device Constituent for Unblinded Combination Study Product
With this release, in Vaults configured to Isolate Blinded Clinical Trial Information, if the Blinding Type of any non-device constituent is Unblinded, Vault updates the Blinding Type of all related constituents to Unblinded. Vault also applies this Blinding Type to associated child and related records such as Case Assessments, Case Assessment Results, and localized records. Additionally, Vault moves these constituents to the Unblinded lifecycle state. This feature maintains consistency across all related records.
This feature is Auto-on in Vaults with Clinical Trials: Isolate Blinded Product Information enabled.
Learn More
- User Help: Isolate Blinded Clinical Trial Information
Blind Isolation: Simplified Study Product Unblinding
With this release, Vaults configured to Isolate Blinded Clinical Trial Information now feature a simplified Study Product unblinding process. A new action, which can be configured to run manually or automatically within a workflow, creates unblinded records for all blinded Study Products on the Case. This enhancement makes it easier to manage complex studies with multiple blinded products.
Learn More
- Enablement: Enable Blind Isolation: Simplified Study Product Unblinding
- User Help: Isolate Blinded Clinical Trial Information
Expectedness for Investigational-Marketed Study Products
With this release, Safety supports using postmarket datasheets when evaluating expectedness in marketed products involved in clinical trial studies. Admins can configure datasheet selection in this scenario at the agency level, offering control, flexibility, and more precise evaluations.
Learn More
- Enablement: Enable Expectedness for Investigational-Marketed Study Products
- Admin Help: Manage Organizations
- User Help: Expectedness Evaluations for Clinical Trial Study Cases
Inactivate Products and Studies
With this release, Safety enables setting Products and Studies to the Deprecated lifecycle state so the Safety Rule Engine no longer considers them when determining the reportability of a Case. When a Product or Study is deprecated, Vault deprecates all related Product Registrations or Study Registrations as well. Where deprecated Products or Studies appear on or are added to Inbox Items and Cases, Vault adds a Deprecated tag beside the applicable field, indicating to the user the status of the record. Previously, Products and Studies were transitioned to the Inactive lifecycle state, which caused issues in follow-up situations. This new Product and Study capability facilitates more precise downstream ICSR reporting.
Learn More
- Enablement: Enable Inactivate Products and Studies
- Admin Help:
- User Help: Auto-Code and Browse Products
Korea Drug Dictionary Browser and Search for Korean External Products
Safety now includes a central Korea Drug Dictionary, available when processing Domestic Cases and Localized Cases for Korea. This feature allows users to lookup and code Korean Ministry of Food and Drug Safety (MFDS) Standard Drug Codes for External Products registered in Korea.
Learn More
- Enablement: Enable the Korea Drug Dictionary
- User Help: Code Korea Drug Dictionary Products
Destination-Specific Case Document Management Improvements
With this release, to fulfill the document retransmission requirements per destination health authority, Safety offers more flexibility and control over literature article and attachment inclusion on E2B reports. Admins can now specify document content inclusion behavior based on attachment type and whether a document has previously been submitted to the destination on the Transmission Profile. Then, using the new Destination Specific Document Inclusion object, Vault automatically determines which attachments shall be retransmitted with each Submission or Distribution. When working with Localized Cases, the new Generate Localized Case Documents action generates necessary Localized Case Document records for all documents with an applicable document type from a related global Case. These improvements provide greater control over document generation and transmission, ensuring more efficient management of attachments.
Learn More
- Enablement: Enable Destination-Specific Case Document Management Improvements
- Admin Help: Manage Transmission Profiles
- User Help:
Case-Level File Format Validation for Local Destinations
With this release, Safety enhances Case-level file format validations when reporting to the PMDA, ensuring all applicable ICH validations are applied. When regulatory conformance is evaluated at the Case level, Vault validates ICH conformance rules for adherence to the ICH file format for the local destination. Additionally, when users run ICH file format validation at the regional Submission level, regional requirements are taken into account.
Learn More
- User Help: Case and Submission Validation
PMDA MHLW Paper Forms
To support manual submissions to the PMDA and provide an easy-to-read format of PMDA E2B XMLs for quality control and site distribution, Safety introduces the generation of PMDA MHLW Paper Forms. Vault generates a report type with the required standard forms based on the PMDA Reporting Category. The available paper form report types are Adverse Event/Infectious Disease Individual Report (Forms 1 and 2), Research Report (Forms 3 and 4), and Safety Measure Report (Forms 5 and 6). Vault maps all required data to the applicable forms and fields, which are generated in Microsoft Word format.
Learn More
- User Help: PMDA MHLW Paper Form Generation Data Mapping
PMDA: Reportable Case Product Registration Generation Updates
With this release, when processing PMDA-reportable Cases, the Retrieve Reportable Case Product Registrations action on the Localized Case now clears all existing Case Product Registrations and generates new records using the latest Case Products on the Case.
This feature was released in 24R3.2 without documentation.
Learn More
PMDA Post-Market Aggregate Report: Consider Product Registrations & Calculate Expectedness
With this release, the NUPR Line Listing Form 7-2 tabulation of the PMDA Post-Market Aggregate Report can be generated for specific product registrations. Additionally, the tabulation indicates if the Expectedness changed for a given event over the course of the report interval.
Learn More
- Enablement: Enable PMDA Post-Market Aggregate Report: Consider Product Registrations & Calculate Expectedness
- Admin Help: Configure Aggregate Reporting Families
- User Help: Create PMDA Post-Market Aggregate Reports
PMDA E2B(R3): Substance Validation Criteria Updates
With this release, Safety introduces Validation Criteria for the G.k.2.3.r.1 Substance / Specified Substance Name data element in PMDA E2B(R3) files. Vault generates a validation warning when the exported code for Case Product Substances related to Case Products with a Japan Product Code Type of J-Drug Code has fewer than seven (7) digits.
This feature was released in 24R3.2 without documentation.
Learn More
- User Help: E2B Generation: PMDA E2B(R3) Mapping
NMPA E2B(R3)
Safety now supports the generation of files in the NMPA E2B(R3) format for early adopter testing of submissions to the National Medical Products Administration (NMPA). This feature includes all of the regional data elements required for compliance with the NMPA.
Learn More
- Enablement: Enable NMPA E2B(R3)
- Admin Help: Standard Agency ICSR Submissions
- User Help: E2B Generation: NMPA E2B(R3) Mapping
Auxiliary Medicinal Product Support
With this release, Safety enhances the Development Safety Update Report (DSUR) and the Safety Rule Engine to meet new regulatory requirements from the EMA regarding Auxiliary Medicinal Products (AxMPs):
- DSUR: This feature introduces a report-level Submission Format picklist for Annual Safety Report formatting, allowing users to choose between the standard DSUR and the CTIS Annual Safety Report. When a user selects CTIS Annual Safety Report for this field value, Vault generates DSUR aggregate reports with the following criteria:
- Unauthorized AxMPs included in the DSUR SAR line listings.
- Subject IDs hidden in the DSUR SAR line listings and the DSUR list of patient deaths.
This feature also includes formatting updates to align data in rows when a suspect drug has multiple values of Daily Dose Route Formulation and Dates of Treatment, Treatment Duration.
- Safety Rule Engine: This feature, using the new reporting rule parameter, Auxiliary Medicinal Product Status, together with the existing Study Product Role parameter, allows Business Admins to configure the Rule Engine to consider AxMPs as eligible for reporting.
Learn More
- Enablement: Enable Auxiliary Medicinal Product Support
- User Help:
Updated Cross Reporting Logic for Non-Company Sponsored Studies
Safety supports additional requirements around the reporting approach taken for non-company Products. Vault now uses the following reporting rule engine logic for Company Sponsored and non-Company Sponsored Studies:
- Company Studies or Non-Company Studies with Registrations: Vault evaluates reporting for each registration destination.
- Company Studies: Vault evaluates general reporting and cross reporting.
- Non-Company Sponsored Studies with No Registrations: Vault does not evaluate reporting for such Studies.
- Non-Company Sponsored Studies with Registrations: Vault evaluates general reporting only based on the registrations. Vault does not evaluate cross reporting for such Studies.
These changes ensure that the reporting approach aligns with each Study’s sponsorship and registration status for more compliant downstream ICSR reporting and avoidance of over-Submission in certain scenarios.
Learn More
- Admin Help: Manage Studies
- User Help: Cross Reporting
Auto-Submit & Localization Transmission Handling
Vault provides the following changes to better streamline the configuration of Localized Case processing:
- A Regenerate Email Transmissions When Submitting to Gateway application setting that improves the Localized Submission process by automatically regenerating Transmission documents for Auto-Submit Email Transmissions.
- A Change Localized Case State on Creation application setting and a System Processing State state type to the Localized Case Lifecycle. These provide a standard method of hiding Localized Cases from users until they are globally approved. Previously, a custom lifecycle state and entry actions were required to address this.
- A new Calculate Japan Reportability action for Japan Localized Cases, that combines the generation of Case Product Registrations, the evaluation of Reporting Obligations, and the creation of Local Reporting Detail records.
Learn More
- Admin Help: Safety Application Settings & Configuration Options
- User Help:
Enhanced One Last Time Reporting for Transmission Output Templates
With this release, Vault considers transmissions created from Transmission Output Templates when evaluating One Last Reporting.
Learn More
- User Help: One Last Time Reporting
AS2 Connection: ACK Processor
With this release, users can upload AS2 acknowledgment (ACK) messages received from a Partner or Health Authority outside of the standard AS2 communication methods (AS2 Connections). Vault then matches each uploaded ACK to its corresponding Transmission record, ensuring that Transmission messages are generated accurately. Vault also updates the Transmission record’s lifecycle state to reflect the status indicated by the received ACK. This feature provides greater flexibility and ensures smoother tracking and management of Transmission records.
Learn More
- User Help: Resolve Gateway Issues
AS2 Connection: ACK on MDN URL Setting
With this release, Vault introduces a new AS2 Connection setting to accommodate AS2 Partners who send their ACK message using the same URL as their Message Disposition Notification (MDN).
Learn More
- Enablement: Enable AS2 Connection: ACK on MDN URL Setting
- Admin Help: Configure Custom AS2 Connections
AS2 Connection: Uncompressed Request Setting
With this release, Vault replaces the Compress Before Sign field on AS2 Connections with an AS2 Compression Settings field. This new field includes the option of sending AS2 messages uncompressed for agencies and partners that specify this requirement.
Although this feature is Auto-on, some components require additional configuration.
Learn More
- Enablement: Enable AS2 Connection: ACK on MDN URL Setting
- Admin Help: Configure Custom AS2 Connections
Validation Query Builder: Expression Engine for Intake Validations
With this release, Safety introduces the Expression Engine for Sender-Based Inbound Validations. Initially launched in 23R3 for Case Validation, the Expression Engine can now be used instead of JSON or SDK rules for intake validations. This simplifies the creation and maintenance of validation criteria, reducing reliance on technical services and aligning intake with Submission validations.
Learn More
Standard Configuration Only Security Profile: Safety Objects
With this release, Safety now includes a new standard security profile called Configuration Only to support roles that can manage configuration without access to data and documents. The Configuration Only security profile contains a standard permission set called Configuration Only Actions.
Add Product Quality Complaints Event Type to Inbox Item
With this release, users can now classify medical events as Product Quality Complaints (PQCs) on Inbox Items. This allows for the proper documenting of PQCs within Safety. If the Quality-Safety Connection is active in your Vault, the connection automatically transfers these PQCs to the Quality Vault.
Learn More
- User Help: Inbox Item Field Reference
Safety Workbench
Note: Safety Workbench is available for Early Adopters only. To learn more, contact your Veeva Representative.
Standardized Workbench Views
With this release, Safety Workbench introduces standardized Workbench Views, which cannot be edited. Standard views are maintained and validated by Safety Workbench. Admins can copy these standard Workbench Views and customize them.
Upload to Library
With this release, Safety Workbench introduces the ability to upload Workbench Reports directly to the Vault Library. Admins can configure Vault to automatically save aggregate reports or let users decide when to save ad-hoc reports to the Vault Library. Users can easily navigate to the uploaded Workbench Report document or download the PDF rendition directly from the Workbench Report. This feature streamlines report management, ensuring regulatory compliance and providing consistent, accessible report formats.
Learn More
- Enablement: Enable Uploading to Vault Library
- User Help:
Localization for Dates & Times
With this release, Safety Workbench introduces a standardized approach to handling timezones, ensuring consistent date and time representation across ad-hoc and periodic reports for collaborating users. Users can specify a timezone for Workbench Reports, ensuring consistent alignment with regulatory requirements across regions. If no timezone is specified for a report, Vault defaults to the timezone of the user who ran the report for date and time processing. This enhancement guarantees that the set of qualified cases for analysis and reporting remains consistent, supporting accurate and reliable global safety reporting for health authorities.
Learn More
Multi-Value Picklists and Updated Filter Logic
With this release, Safety Workbench introduces the following enhancements to filtering capabilities:
- Multi-Value Picklist Filters: Admins and users can now apply filters to multi-value picklist fields for Workbench Views and Workbench Reports, respectively.
- Optional Filters with Advanced Logic: Admins can now use optional filters with advanced logic to streamline and tailor data queries.
- Restricted Operators: When configuring filters, Admins can now define a specific filter operator and restrict users from editing this field, providing greater control over filtering options.
These enhancements simplify filter configuration while providing Admins with more control and allowing users to perform more efficient and user-friendly data analysis.
Learn More
- Admin Help: Add Safety View Filters in Safety Workbench
- User Help: Use Workbench Report Filters
Workbench Dashboard Tabs
With this release, Safety Workbench introduces tabs on Workbench Dashboards. Users and Admins can create Workbench Dashboards to include up to 12 tabs, each holding up to nine (9) components. Users can navigate between tabs to view specific sets of dashboard components, applying shared filters across all tabs for consistent and comprehensive case data analysis. This feature enhances usability by allowing Workbench Dashboards to scale to dozens of components without impacting performance while providing a structured way to group visualizations together.
Learn More
- User Help: Manage Workbench Dashboards
Share Filters in Workbench Dashboards
With this release, Safety Workbench introduces the ability to save and share data analysis results from Workbench Dashboards. For the relevant results, users can save their Workbench Dashboard filters (including Case series) and share them with other users. This feature improves team collaboration and efficiency by ensuring users can share the most current data analysis results.
Learn More
- User Help: Use Workbench Dashboard Filters
Workbench Report Sets
With this release, Safety Workbench introduces Workbench Report Sets to streamline the management of related reports. A Workbench Report Set allows users to generate multiple Workbench Reports that share a common purpose. Users can also quickly re-run and download all reports in the Workbench Report Set with consistent filters applied. As part of this release, standard Workbench Report Set Definitions are provisioned for PADERs, allowing users to efficiently create report sets for this periodic report. This feature improves usability for teams managing complex reporting requirements by ensuring all related reports in a set always run with the same filters, saving time and reducing errors.
Learn More
- User Help: Manage Workbench Report Sets
Use Registrations from Submissions for Workbench PADERs
This release improves Workbench Periodic Adverse Drug Experience Report (PADER) reporting by generating a Cases Submitted under other NDA’s report instead of the Non-Primary Suspect Product Report PADER tabulation to capture all Cases containing a suspect Product but where the report was filed to another new drug application (NDA). With this release, Safety Workbench includes Cases in the appropriate reports using the Product Registration that triggers the Transmission. This feature ensures tighter FDA compliance and greater accuracy for categorizing Cases with multiple products into reports.
Learn More
- Admin Help: Configure Workbench PADER Aggregate Reports
- User Help:
Workbench PADER for Combination Products
With this release, Safety Workbench introduces the following new Workbench PADERs for Cases that contain combination products with a malfunctioned device constituent:
- Malfunction Line Listing
- Malfunction Tabulation
- Line Listing Summary for Combination Products
These reports are compliant with FDA regulations for combination products per 21 CFR 4.102.
Learn More
- Admin Help: Configure Workbench PADER Aggregate Reports
- User Help:
Workbench SUSAR Reports
With this release, Safety Workbench introduces the SUSAR (Suspected Unexpected Serious Adverse Reaction) Line Listing report which is available in masked and unmasked versions. Admins can customize these reports by copying the standard Workbench Views and modifying the copy to meet specific organizational needs.
Learn More
- Admin Help: Configure Workbench SAR Reports
- User Help:
Safety Signal
Note: Safety Signal is available for Early Adopters only. To learn more, contact your Veeva Representative.
Signal Case Series
With this release, Safety Signal introduces Signal Case Series, which preserves a static list of Case versions and related data sets for signal analysis. Admins can enable Signal Case Series for specific Signal Views to capture and save Case data when running Signal Calculations. Users can analyze this data through Workbench Dashboards, ensuring consistency and accuracy in signal detection and analysis. To leverage Safety Workbench for data analysis, you must have a Safety Workbench license.
Learn More
- Enablement: Enable Signal Case Series
- Admin Help: Configure Safety Views for Signal Case Series
- User Help:
Signal Alert Rule Engine
With this release, Safety Signal introduces the Signal Alert Rule Engine to identify Statistical Data records that meet predefined thresholds. This feature allows Admins to create flexible alert rules for different groups of Products using standard operators and mathematical expressions. The alert criteria support custom business logic and industry-standard rules, for example, when the Product-Event Combination (PEC) is unlisted and when EB05 is greater than or equal to two (2). When a Statistical Data record meets the alert criteria, Safety Signal flags it and allows users to manually review the data. This feature enhances signal detection in compliance with industry standards and custom business criteria. Signal alerts can also run against high volumes of Statistical Data records and support faster signal detection workflows.
Learn More
- Enablement: Enable the Signal Alert Rule Engine
- Admin Help:
- User Help: Run & Review Signal Alerts
Schedule Signal Calculations & Alerts
With this release, Safety Signal automates signal detection on a schedule configured by Admins. The scheduled signal detection process involves running Signal Calculations, creating Statistical Data, and evaluating business rules to flag Product-Event Combinations (PECs) that require review. This enhancement supports routine, automated signal surveillance aligned with each product’s specific surveillance plan, improving efficiency and compliance with safety monitoring practices.
Learn More
- Enablement: Enable Scheduling Signal Calculations & Alerts
- Admin Help: Configure Scheduled Signal Calculations & Alerts
- User Help: Scheduled Signal Calculations & Alerts
SafetyDocs
PVA Scheduling
With this release, SafetyDocs introduces automated scheduling for pharmacovigilance agreements (PVAs), simplifying the management of recurring PVA obligations to ensure timely compliance and reduce manual workload.
Admins can leverage standard PVA management schedules or customize them to align with their organization’s specific requirements. When creating a PVA Obligation record, users can select a predefined schedule, enabling SafetyDocs to automatically generate PVA Activity records at the appropriate intervals.
Learn More
- Enablement: Enable PVA Scheduling
- Admin Help: Configure Schedule Records
- User Help: Manage Pharmacovigilance Agreements
Highlight Literature Abstract Terms
With this release, SafetyDocs introduces the ability to automatically highlight key terms in literature abstracts, enhancing efficiency in literature review workflows. This feature streamlines the identification of ICSRs and emerging safety signals by allowing users to quickly review highlighted terms in an abstract in order to determine whether a full-text review is necessary. While SafetyDocs comes preloaded with a list of standard highlighted terms, Admins can additionally configure their Vault to highlight predefined terms based on their organization’s specific patient, seriousness, and company-specific term needs.
Learn More
- Enablement:
- User Help: Manage the Literature Review Process
Literature Integration to PubMed
With this release, SafetyDocs enhances its literature monitoring capabilities by integrating directly with PubMed to support automated literature searches and article intake. This feature improves review efficiency by:
- Allowing Admins to schedule daily automated PubMed literature searches
- Providing users with the ability to manually test PubMed database searches from within SafetyDocs
- Creating new Literature Review and Literature Article records and populating them with abstracts and other relevant data
- Performing duplicate detection across both PubMed and MEDLINE
Learn More
- Enablement: Enable Literature Integration to PubMed
- Admin Help: Create Literature Search Groups
- User Help: Manage the Literature Review Process
EMA MLM RIS Literature File Support
With this release, SafetyDocs supports the intake of literature references from EMA MLM RIS (European Medicines Agency Medical Literature Monitoring Research Information Systems) files. This feature introduces the EMA Substance Group field on the Product Family object, allowing Admins to configure substance groups such that Vault will only import EMA MLM RIS articles whose substance group tag matches a substance group configured in their Vault.
Learn More
- Enablement: Enable Importing Literature References from Databases
- User Help:
Risk Management Enhancements
With this release, SafetyDocs has made several enhancements to Risk Management:
- For Local Risk Measures, Admins can configure the Target Date field on the RMP Risk Measure Tracking object as required or optional
- Modifies the Copy to New Version and Create Local Implementations actions so that no documents will be created or upversioned
- Introduces a new document type for local implementation plans (classified under Risk Management > Risk Measure > Local Implementation Plan)
- Makes the System manages field value (read-only) attribute editable for the Name field on the RMP Core Implementation Strategy and RMP Local Implementation Strategy objects
These enhancements provide additional flexibility and better align with Risk Management processes.
Although this feature is Auto-on, the System manages field value (read-only) attribute requires additional configuration.
Learn More
- Enablement: Enable Risk Management
- User Help:
PSMF Annex H Report Type Standardization
With this release, SafetyDocs updates the PSMF Annex H Report Type, ensuring easy adoption of industry best practices by:
- Automatically grouping Product Registration records with the same Product Family field values
- Removing filtering such that all Product Registration records show, regardless of their Marketing Status field value
Learn More
- User Help: Generate PSMF Documents from Vault Reports
QualityOne
QualityOne
Print Record for Teams
This feature enhances the Platform Print Record functionality in QualityOne. With this update, the PDF file output of the Print Record functionality now includes a Team section if it appears on object records, along with Team Role assignments such as Reviewers and Approvers. This enhancement ensures that important Team Role information is included and clearly visible in the PDF output, making the printed document more consistent with the on-screen view and improving overall clarity and completeness.
Learn more about working with Teams.
Related Record Automation Enhancement for Entry & User Actions
This feature improves the performance of the Create Related Record action, both for user and entry actions. With this release, the action runs synchronously when there are no Related Child Record Setup records in the corresponding Related Record Setup, and asynchronously when Related Child Record Setup records are present. This enhancement boosts the efficiency and scalability of record creation, ensuring better performance during large-scale operations.
Learn more about the Create Related Record action.
Note: Contact your Veeva Representative to enable this feature. We strongly recommend enabling this feature before 25R2 in Vaults that use Related Record Automation.
QMS (QualityOne)
Supplier Notifications
This feature enables Vault to process and store correspondence initiated by external suppliers via email to proactively notify your organization of critical information such as planned changes or possible deviations, without needing to grant external suppliers access to Vault. This allows for streamlined collaboration with external suppliers, efficient tracking and triaging of supplier-initiated notifications, and the ability to trigger downstream processes and actions stemming from the supplier notification.
When a known supplier sends an email to a designated address, the Supplier Notification Email Processor processes the email. Vault then creates a Supplier Notification record that includes details from the email, such as the supplier name, contact information, email body, and any attachments. As you receive additional messages from the same supplier, Vault gathers all related emails and attachments into the original Supplier Notification record for easy reference. If configured by your Admin, you can also create and initiate related processes and action items directly from the Supplier Notification record to further enhance collaboration with external suppliers.
Learn more about working with Supplier Notifications.
Inspections: CoA Setup Panel
This feature provides a way for Business Admins to create CoA matching variants from the Doc Info page of a CoA file. Working alongside a CoA file allows Business Admins to create matching variants more efficiently, reducing the need to navigate to other Vault pages.
Learn more about using the CoA setup panel.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Information Panel UI Enhancements
This feature offers enhancements to improve the overall usability of the HACCP Flow Diagram’s Information panel. These enhancements ensure users have the information they need to perform hazard analysis from the HACCP Flow Diagram.
Learn more about working with the HACCP Flow Diagram and performing hazard analysis.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Edit PRP & Control Measure Descriptions within Diagram
With this release, users can edit the descriptions of PRPs and Control Measures directly in the Information panel. Previously, these descriptions were displayed but not editable from the Information panel. This feature eliminates the need to leave the HACCP Flow Diagram, update the description on the object record, and refresh the diagram to review the latest description.
Pagination in the Hazard Analyses Section
With this release, the Hazard Analyses section has pagination to allow users to review and complete all Process Hazard Analyses directly in the Information panel. Previously, the Information panel displayed up to 15 Process Hazard Analysis records with a link to view more. This feature eliminates the need to leave the HACCP Flow Diagram to complete the Process Hazard Analyses not displayed in the Information panel if a Process Step or Process Step Group has more than 15 associated Process Hazard Analyses.
Display Ingredients as Clickable Links
With this release, ingredients listed in the Information panel display as clickable links, allowing the user to open a HACCP Plan Ingredient record in a small browser window to review and update the details. Previously, the ingredient list in the HACCP Plan Ingredients section of the Information panel was a read-only bulleted list and the user did not have a direct link to the HACCP Plan Ingredient records to review and update the information.
Append Brief Description to Hazard Name
With this release, users can append a brief description to a Hazard’s name to display in the Hazards subsection of the Information panel. Previously, Vault only displayed the Hazard’s name, which is often very general. Now when users update the Brief Description field on a Process Hazard Analysis - Hazard record, the description displays next to the associated Hazard’s name in the same token that users can hover over to view the full description. This feature enables users to have more context about hazards when performing hazard analysis.
HACCP Flow Diagram: Material & Process Hazard Analysis in the Diagram
This feature allows the user to perform material and process hazard analysis for all types of steps and Process Step Groups. When creating Process Hazard Analysis records from the HACCP Flow Diagram and from object records, users can specify if the hazard analysis is of the Material or Process type. When users create a Process Hazard Analysis from the HACCP Flow Diagram, the Hazard Analysis Type defaults to Process for Process Steps, Logistics Steps, and Process Step Groups, and Material for Material Steps. The Information panel displays a different set of fields based on the type of hazard analysis specified; if the Hazard Analysis Type is not specified, fields for a Process type hazard analysis display. Additionally, the Scenario Options (scenario_options__v) field now appears for both types of hazard analysis.
Learn more about performing hazard analysis and working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Enhanced Configurability for the Information Panel
Previously, Admins were limited in the fields they could configure to display in the Information panel. This feature increases the fields Admins can configure to display in the Potential Hazard Description subection from three (3) fields to eight (8), and adds the ability to configure five (5) fields to display in the Hazard Assessment subsection in addition to the three (3) existing configurable fields for Likelihood, Local Likelihood, and Severity, providing enhanced flexibility to add and remove fields to suit your organization’s business needs.
Learn more about configuring and working with the HACCP Flow Diagram.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Flow Diagram: Display Reasons for Incompleteness
With this release, when the Completeness Check (![]() ) icon is enabled on the HACCP Flow Diagram, users can hover over the incompleteness indicator (
) icon is enabled on the HACCP Flow Diagram, users can hover over the incompleteness indicator (![]() ) icon on Process Steps and Process Step Groups on the diagram canvas, and on Process Hazard Analyses in the Information panel, to see the reasons for hazard analysis incompleteness. Users can update the reasons for incompleteness in the Hazard Analysis Complete Reason field on HACCP Plan Process Step, HACCP Plan Process Step Group, and Process Hazard Analysis records and from the HACCP Flow Diagram.
) icon on Process Steps and Process Step Groups on the diagram canvas, and on Process Hazard Analyses in the Information panel, to see the reasons for hazard analysis incompleteness. Users can update the reasons for incompleteness in the Hazard Analysis Complete Reason field on HACCP Plan Process Step, HACCP Plan Process Step Group, and Process Hazard Analysis records and from the HACCP Flow Diagram.
Learn more about assessing hazard analysis completeness.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Add Offset to the Generated HACCP Flow Diagram Rendition
This feature introduces additional padding to the top of generated HACCP Flow Diagram rendition documents. This allows adequate whitespace for Business Admins to add supported metadata to the rendition document without overlapping with content from the HACCP Flow Diagram.
Learn more about generating HACCP documents.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Plan: Object Type Support for Dynamic Criteria VQL
This feature introduces support for dynamic criteria VQL configured at the object-type level for specific application controls and transactional HACCP objects. With this update, record filtering also considers criteria VQL defined for object types on the HACCP Plan object and related transactional objects, and translated master data displays to users with a Vault language setting other than English.
Learn more about configuring and working with HACCP Management.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
HACCP Plan Translation Enhancements
This feature offers the following incremental usability improvements in configuring HACCP Plan Translation and in working with translated HACCP plans.
Learn more about configuring HACCP Plan Translation and translating HACCP Plans.
Note: This feature is currently available only to Early Adopters. Contact your Veeva Representative for more information.
Support for Editing Translated Controlling Fields in Related Object Sections
This feature lets users edit the value of translated controlling fields directly from related object sections when their dependent fields have values (for instance, in object relationships where a controlling field is configured, or if there is criteria VQL constraining the available records to select). This feature saves time by eliminating the need to navigate to the related object record to edit translated controlling field values if their dependent field is not blank.
Support for Translation File Validation
Prior to this release, when importing a translation file, Vault did not check if the HACCP Plan data in the JSON file corresponded to the target HACCP Plan. With this release, Vault validates the HACCP Plan value on uploaded JSON files to ensure the exported content for translation and the content to be imported belong to the same HACCP Plan.
Support for Material Reference Data
This feature extends support for the translated reference data that can be reused across translated HACCP Plans to the Material (material__v) object.
Support for Inactive Reference Data
In Vaults with HACCP Plan Translation enabled, when users create HACCP reference records for objects that have a translation companion object, Vault generates translation copies of that record for all active Vault-supported languages. When users delete master data records, Vault deletes their translation companion records. With this release, Vault also updates the status of translation companion records to match the status of their master data record every time the master data record’s status changes from Active to Inactive, and vice versa.
QMS (QualityOne) & HSE
Audit Report Printable View
This feature provides a way to generate a printable view of an audit report that includes data from an Audit record and related records, checklists, and evidence collected from auditors, such as images, that are stored as attachments on Audit Findings. Users can run an action to initiate the printable view, and download and print a PDF file of the printable view from a browser. Admins can configure the information that displays on the Audit Report Printable View based on specific business needs, and configure different layouts for the audit report for internal or external consumption.
Learn more about configuring and working with Audit Report Printable View.
Document Generation Actions: Enhance Population Logic of Required Document Fields
This feature enhances the Generate Document from Template Output, Generate Document from Formatted Output, and Generate Document From Report actions to more accurately populate required fields on the generated document. Previously, Vault prioritized standard fields (for instance, prioritizing title__v over title__c) when populating required document fields. With this release, Vault first searches for fields that exactly match the field name and namespace suffix, for example, (__c) or (__v). If no exact match is found, Vault prioritizes standard fields (__v) before attempting to find a matching custom field (__c).
Learn more about generating documents.
Audit Checklist Mobile for Android: Save Progress to Vault
This feature allows users of the Android version of QualityOne Audit Checklist Mobile to save progress on a partially completed Audit Checklist from mobile to Vault. Users can choose to continue editing the checklist in Vault or on mobile.
Learn more about the Save Progress to Vault feature.
The QualityOne Audit Checklist Mobile 25R1 release is scheduled for tentative availability on April 22, 2025.
Audit Checklist Mobile for Android Enhancements
This feature introduces UI enhancements to the Android version of the QualityOne Audit Checklist Mobile application. With this release, a requiredness indicator (*) now displays next to required questions, comments, attachments, and Audit Finding fields on Audit Checklists. Additionally, field labels for Audit Findings now display the same on mobile as in Vault.
Learn more about using QualityOne Audit Checklist Mobile.
The QualityOne Audit Checklist Mobile 25R1 release is scheduled for tentative availability on April 22, 2025.
RegulatoryOne
The RegulatoryOne 25R1 release, including all Platform features, is scheduled for tentative availability on April 22, 2025.
Compliance Management
Formulation Questionnaires: Change PAL Expiration
This feature allows Admins to increase the public access links (PALs) expiration duration Vault generates for FQ Checklists, giving suppliers more time to respond to questionnaires.
Learn more about configuring the PAL expiration on the Send PALs action.
Import Formulation Questionnaires Enhancement: Lifecycle Selection
This feature enhances formulation questionnaire import attachments and enables users to pick the file’s lifecycle when required fields have not been configured by an Admin, so that users can proceed with the import and upload the document into their Vault Library to update at a later time.
Learn more about importing formulation questionnaire attachments.
Permission Updates for Import Questionnaire & Automatic Quantitative Assessments
This feature updates the permissions needed to execute and complete the Import Questionnaire and Create Quantitative Assessments actions.
Registration & Dossier Management
Customize Action Enhancement: Populate Matching Fields
This feature enhances the Customize action by copying matching fields from source EDLs to target EDLs, allowing users to auto-match documents that are different from the source dossier.
Learn more about configuring the Customize action.
Create Binder Enhancement: Support for Optional & Supporting Documents
This feature enables users to exclude certain Requirements from generated Dossier Binders created using the Create Binder and Create and Export Binder actions. The feature also enhances the existing Requirement Hierarchy Viewer to provide users with more flexibility to filter requirements.
Learn more about Dossier Binders.
25R1 Registration & Dossier Management Enhancements
This feature enables users to expand or collapse all nodes displayed in the Requirement Hierarchy Viewer through a simple click of a button. This feature also improves the scrolling experience by freezing the header row of the viewer.
Learn more about the Requirement Hierarchy Viewer.
Veeva Claims
The Veeva Claims 25R1 release, including all Platform features, is scheduled for tentative availability on April 22, 2025.
Veeva Claims
Default Classification for Uploaded Substantiation Documents
This feature classifies documents users upload to Vault using the Upload button on Substantiation records based on an Admin-specified document type so that the uploaded documents are immediately available in the document library.
Learn more about configuring a default document type for simplified substantiation.
25R1 Claims Enhancements
This feature enables users with non-English Vault language settings to view picklist values for the Packaging Level, Pack Type and Panel fields in their specified language setting.