Pre-Release 日: 2021 年 10 月 19 日 | リリース日: 2021 年 11 月 19 日および 12 月 3 日
Safety、QualityOne Client Application、RegulatoryOne、Veeva Claims の各アプリケーションのリリース日はそれぞれ異なることがあります。
Vault 21R3 のご紹介です。以下の新機能をご覧ください。新機能の有効化に関する情報については、21R3 Release Impact Assessment をご覧ください。開発者向け機能 (API、VQL など) については、Developer Portal をご覧ください。
ドキュメントでの作業
保護された PDF レンディション
今回のリリースでは、Vault ユーザは、変更を制限するセーフガードが適用されている PDF 表示可能レンディションをダウンロードできるようになります。Vault は PDF 表示可能レンディションにパスワードを適用し、Vault 管理者は権限を設定することで、ユーザが簡単に情報の編集、コピー、印刷、コメント、またはドキュメントのフォームに記入できないようにします。詳しくは、保護されたレンディションをご覧ください。
Google ドライブの統合
今回のリリースでは、Vault は Google ドライブと直接統合できるようになります。認証後、ユーザは Google ドライブを使用して、ファイルをチェックアウト、編集、チェックできます。また、ユーザは Google ドライブにチェックアウトしたファイルを取り消せます。
チェックアウト手順は標準チェックアウトと同様ですが、チェックアウト済みファイルのダウンロード先に Google ドライブを使用します。チェックアウト済みファイルが .docx、.xlsx、または .pptx の場合、Vault は適切な Google Workspace Editor (Google ドキュメント、シートまたはスライド) を使用することで、編集のためファイルを新規ブラウザタブで自動で開きます。詳しくは、Google ドライブの統合をご覧ください。
リッチテキスト向け Microsoft Word Merge フィールドのサポート
今回のリリースでは、Vault は Microsoft Word™ の Rich Text の Merge フィールドをサポートしています。Rich Text タイプのフィールドの Merge フィールドトークンは、Vault Rich Text Editor フィールドに指定されているリッチテキストフォーマットを使用して、Microsoft Word™ ドキュメントを解決して表示します。
注釈カラーが 12 色に復元
この機能強化により、注釈に使用できるカラーが 12 色に復元されました。また、外部ビューアのツールバーはアクション UI を使用できるよう更新され、エスケープキーを使用して未保存の注釈を却下できるオプションが追加されました。
Merge フィールドにおける複数列の表の並べ替え
今回のリリースでは、Vault ユーザは Microsoft Word™ ドキュメントの表の Merge フィールドデータをさらに細かく並べ替えられるようになります。複数列の表の並べ替えにより、ユーザはマップ済みトークンの最大 3 フィールドに ORDER BY VQL 句を使用して、単一列ではなく複数列に基づいて表にマージされたデータを並べ替えられます。
オーバーレイ上書きページサイズの公差
この機能により、Vault は PDF 表示可能レンディションのサイズを最も近いサイズのオーバーレイ上書きに一致させられます。ページサイズ公差 0.5 インチは、実際のオーバーレイ上書きページサイズとは異なるドキュメントページのオーバーレイ上書きテンプレートに導入されています。これまでは、ドキュメントサイズがオーバーレイ上書きのサイズと完全に一致しない場合、Vault は基本のオーバーレイテンプレートを適用していました。
これまでのレンディションバージョンの再レンダリング
今回のリリースでは、Vault Owner Actions: Re-render 権限を持つ Vault ユーザは、最新ではないバージョンのレンディションを再レンダリングできます。
固定状態のレンディション設定のマージを防ぐ
今回のリリースでは、管理者はレンディションに対する Prevent Merge Fields in Steady State 設定の有効化を管理できます。この設定を有効化すると、固定状態のドキュメントは、マージがシステムによってトリガーされているか、または手動でアクションを実行しているかに関わらず、Vault フィールドを Microsoft Word ソースドキュメントにマージできなくなります。この設定はこれまで Veeva サポートが管理していました。
EDL: テンプレートからドキュメントを作成
新規ユーザアクションでは、EDL アイテムのドキュメントテンプレートから直接ドキュメントを作成できます。新しく作成されたドキュメントは、EDL アイテムから直接のドキュメントのアップロードまたはプレースホルダーの作成と同様、自動的に EDL アイテムと一致します。
旧バージョンのドキュメント関係を削除
今回のリリースでは、Vault ユーザは関係がバージョン固有である場合、その関係がバインドされているドキュメントバージョンに対して Edit Relationship 権限を持つことで、旧バージョンドキュメント上の関係を削除できるようになりました。これまで、ユーザは前バージョンのドキュメント関係を作成できましたが、削除できませんでした。
バインダーシステムメッセージのエクスポート: 失敗したドキュメントエクスポートを表示
今回のリリースでは、Vault は、Export Binders アクションの実行後にユーザが受け取るシステムメッセージに、ダウンロードしたドキュメントおよびダウンロードに失敗したドキュメントを表示します。これまで、Vault はシステムメッセージ/メールにこの情報を含めていませんでした。
Vault オブジェクト
ドキュメント参照フィールド制限
Vault は、ドキュメントタイプ、サブタイプ、分類、またはその他のドキュメントフィールドの値に基づき、オブジェクトのドキュメント参照フィールドを制限できるようになりました。これにより、ドキュメント参照フィールドの目的に合わせて選択できるドキュメントを絞り込めます。詳しくは、configuring reference constraints をご覧ください。
オンラインでの大容量オブジェクトの変更
これまで、10,000 件超のレコードがある大容量オブジェクトの場合、管理者は Vault を設定モードにする必要があったため、設定を変更するには Vault からエンドユーザをロックアウトしていました。この機能により、エンドユーザをシステムからロックアウトすることなく、オンラインでメタデータの変更を行えます。大容量オブジェクトの変更中、オブジェクト設定ページではこのオブジェクトに対して In Deployment ステータスが表示されます。
関連オブジェクトセクションおよび詳細検索における HVO パフォーマンスの最適化
関連オブジェクトセクションおよび詳細検索ダイアログにある大容量オブジェクトレコードのページネーション時におけるパフォーマンスを改善するため、返されるレコード数を 1,000 に制限しています。ユーザは Show in Tab 機能および対応するフィルタリングサポートを使用して、興味があるデータを入手できるようリストを絞り込めます。
ライフサイクルとワークフロー
ワークフローキャンセルアクション
ワークフローキャンセルアクションにより、ワークフロー管理者はワークフローがキャンセルされた際にトリガーされるアクションを設定できます。これにより、有効なワークフローのキャンセルによって実行されるアクションに関連する複数のシナリオに対するソリューションを提供します。
当社はこれまで、Update Record/Document Field や Send a notification など、一連のデフォルトキャンセルアクションを提供してきました。この機能により、独自のキャンセルアクションを追加する SDK も利用できるようになります。
ワークフローキャンセルコメント
ワークフロー管理者は、ユーザが有効なワークフローをキャンセルした際にはコメントを提供するよう要求できるようになりました。デフォルトでは、ワークフローキャンセル時のコメントは必須ではありません。
キャンセルコメントが必須の場合、ワークフローをキャンセルしたユーザはコメントを記入する必要があります。キャンセルコメントは、ワークフローキャンセル API の一部としてもサポートされています。
キャンセルコメントはワークフロータイムラインビューに表示されます。キャンセルコメントの最大文字数は 500 です。
オブジェクト日付フィールド更新のタスク期日を更新
ワークフロー管理者は、関連するオブジェクトレコード日付フィールドの変更に基づいて有効なタスクの期日を自動的に更新するよう設定できます。これまで、期日はタスク作成時にのみ設定または更新できました。今回のリリースでは、レコードフィールドが更新されると、そのフィールドに依存するすべての有効なタスクの期日も更新されます。
タスク期日を自動的に更新するよう設定すると、そのようなタスクの Update Task Due Date アクションは利用できなくなります。
参加者にメールを送信するアクション
オブジェクトとドキュメントワークフローは、有効なワークフローの参加者にメールを送信する Email Participants アクションをサポートするようになりました。
メールは、未完了または完了済みタスク所有者にグループとして、または個別の参加者に送信できます。
Email Participants アクションは、レガシーワークフローと同じプロファイルレベル権限を使用します。このアクションはアトミックセキュリテもサポートします。
大容量オブジェクトへの Envelope オブジェクト
ドキュメントのワークフロー (以前の複数ドキュメントワークフロー) は、ドキュメントを含めるために Envelope および Envelope Content オブジェクトを使用します。Envelope オブジェクトは Vault の大容量オブジェクトフレームワークに移動して、大量のワークフローをサポートするために必要な拡張性を提供します。
大容量データストアへの移動により、レコードレベルのアクセスコントロールおよび共有設定の削除など、複数の変更が発生します。Envelope または Envelope Content カスタムオブジェクトタブを作成したお客様は、これらのタブへのアクセス管理に注意を払う必要があります。
Envelope レコードは、ドキュメントが有効なワークフローにある場合、ドキュメントへのアクセス権限がある任意のユーザにも表示されます。このようなユーザは、権限のない新規ドキュメントを表示したりアクションを行ったりすることはできませんが、エンベロープビューアへアクセスできます。
参照フィールドを空白に設定する
ワークフロー管理者は、ライフサイクルおよびワークフローの数式表現を使用して、参照フィールドを空白に設定できるようになりました。この機能は、エントリアクション、イベントアクション、およびワークフロー更新フィールド手順で使用できます。
フィールドを空白に設定するには、更新フィールド手順またはアクション設定の表現に「NULL」値を使用します。
オブジェクトライフサイクルまたはワークフローで親フィールドを空白に設定することはできません。
Today("user") 機能のサポート
Today(“user”) 関数は、ライフサイクルまたはワークフローの数式フィールド表現で使用されている際に、元のユーザのタイムゾーンの日付値を提供できるようになりました。
これまで、Vault はライフサイクルとワークフローの式で Today(“user”) 関数はサポートしておらず、常にシステムユーザの日付値を提供していました。
非同期関連オブジェクトの状態を変更するエントリアクション
オブジェクトライフサイクルは、非同期関連レコードの状態を変更する新規エントリアクションをサポートしています。この機能は、大量の関連レコードがあるオブジェクトにのみ使用してください。このアクションは、状態を変更できる関連レコード数に制限なく使用できます。
既存のエントリアクションと同期的に使用でき、最高 1,000 件の関連レコードの状態を変更できます。詳しくは、configuring object lifecycle state entry actions をご覧ください。
不明な参照によるアクション修正
この機能により、ワークフロー管理者は、Vault リフレッシュ後に紛失したレコードを含むエントリアクション、イベントアクション、ユーザアクション、エントリ条件のルールを更新できます。
Vault は Vault リフレッシュ中に特定のオブジェクトレコードのみをコピーします。たとえば、カスタムオブジェクトと一部の標準オブジェクトレコードはコピーされません。そのため、ソース Vault のこれらのレコードがアクションまたはエントリ条件ルールに含まれる場合、リフレッシュした Vault で問題が発生する可能性があります。管理者は、API または Vault Loader を使用してこれらのレコードを Vault に取り込むか、設定をソース Vault に戻す際にこれらのルールを無視して、これらのルールを修正できます。
レガシーワークフローの作成を削除
Vault はすべての Vault のレガシーワークフローの作成またはコピーのサポートを終了します。サポート終了については、過去 2 回の General Release でお知らせしています。すべての Vault のドキュメントの新規ワークフローは、Admin > Configuration > Workflows を使用して作成する必要があります。
既存のレガシーワークフローのサポートは継続しているため、新規バージョンに更新できます。レガシーワークフローの不具合の修正も引き続き行います。既存のレガシーワークフローが 1 つ以上ある場合、ドキュメントライフサイクルの Legacy Workflow タブは表示されたままになります。
既存のレガシーワークフローを使用した Vault リフレッシュの実行は、これまで通り機能します。VPK を使用したレガシーワークフローへの更新は、ターゲットワークフローが移行先 Vault にすでに存在している限りにおいて引き続き使用できます。
単一裁定: 任意のライフサイクルワークフロー
任意のライフサイクルドキュメントワークフローは単一裁定をサポートするようになりました。ユーザはワークフローのすべてのドキュメントに単一裁定を提示できます。
裁定は除外されたドキュメントには適用されません。
指示のトークンを設定
ワークフロー管理者は、ワークフローの開始およびタスク手順の指示でトークンを設定できます。
オブジェクトのワークフローでは、オブジェクトフィールドはトークンとして設定できます。ドキュメントのワークフローでは、ドキュメント名とドキュメント番号フィールドは設定で使用できます。これらのドキュメントトークンは、ワークフローが単一ドキュメントで構成されている場合のみ値を表示します。
この機能は、システムメッセージオブジェクトメッセージテンプレートのリンクなしトークンも追加し、件名行にドキュメントのリストおよびワークフロー名を表示できます。
開始手順ルール: 必須コントロール
以前のリリースでは、開始手順ルールはコントロールの非表示のみを行えました。今回のリリースでは、ワークフロー管理者はコントロールを要求する開始手順ルールを設定できるようになりました。たとえば、この機能により管理者は、通常はオプションである Participant Control を必須にできます。これは、レガシーワークフローで過去にワークフロー参加者ルールを使用したことがあるお客様に便利です。
Read & Understood ワークフロー: 代理ユーザによるタスク完了を制限
今回のリリースでは、代理ユーザは、21R2 General Release でリリースされた新規 Read & Understood ワークフローの Read & Understood ワークフロータスクを完了できません。これは、従来の Read & Understood ワークフロータスクの動作と同じです。
ワークフローバージョン履歴
ワークフロー管理者は、ワークフロー詳細ページでこれまでのバージョンのワークフローを表示できるようになりました。ワークフローバージョンは、以前のワークフローバージョンからの変更を視覚表示して、お客様サービスおよび Veeva サービスを素早く提供できます。旧バージョンで実行している有効なワークフローインスタンスのリストも表示されます。
管理者は最新バージョンに戻れます。旧バージョン手順は参照専用モードで表示されます。旧バージョンで削除されたフィールドまたは状態がある場合、エラーとして表示されます。詳しくは、ワークフロー設定をご覧ください。
ワークフローの参照制限のサポート
ドキュメントのワークフローにあるタスク完了ダイアログは参照制限をサポートするようになりました。
ベストプラクティスとして、コントロール中およびコントロール済みフィールドは裁定またはタスク完了ダイアログのフィールドプロンプトの一部である必要があります。コントロール済みフィールドのみの場合、無効な値を選択すると Vault がエラーを表示することがあります。
ワークフロー開始状態を表示
ワークフロー詳細ページで、ワークフロー管理者は、ユーザアクションを使用してワークフローを開始できるライフサイクル状態のクリック可能リストを表示できます。
コンテンツアクションまたはイベントアクションの非適用フィールドを無視
今回のリリースでは、Vault はドキュメントのコンテンツアクションまたはイベントアクション中にエラーをスローするのではなく、現在のドキュメントのドキュメントタイプに属さないフィールドを無視できるようになりました。
レポート作成とダッシュボード
マルチパスレポートの数式フィールド
この機能を使うと、ユーザはマルチパスレポートで数式フィールドを定義できます。ユーザは、レポートビューで定義されている集計フィールドを含むレポートビューで計算を実行できます。数式フィールドは、条件付きフィールド、レポートフィルタを定義し、結果をグループ化するために使用され、ダッシュボードチャートでも使用されます。今回のリリースには、オブジェクトおよびドキュメントレポートタイプのサポートも含まれます。
レポートの個別カウント
この機能により、ユーザはグループ化されたレポートの個別レコードの合計数を表示できます。レポートの作成または編集時、ユーザは ID フィールドで個別の集計数式を追加して、個別のレコードをカウントできます。マルチパスおよび標準レポートタイプがサポートされています。
レンディションレポート
この機能により、ユーザはドキュメントレンディションのレポートを作成できます。管理者は「レンディション」および「ドキュメントがあるレンディション」レポートタイプを作成して、エンドユーザがドキュメントレンディションおよび関連ドキュメントのレポートを生成できるようにします。また、管理者は「レンディションがあるドキュメント」レポートタイプを作成して、エンドユーザがドキュメントおよび関連レンディションのレポートを作成可能にできます。
マルチダウンオブジェクトレポートの集計をサポート
この機能によりユーザは、主なオブジェクトを直接参照している複数のオブジェクトを含むレポートの主ではないレコードの数量および平均などの集計を実行できます。集計は、レポートにグループ化を追加した後、数量、日付、および ID フィールドに定義できます。
レポートエクスポートのリンクを削除
この機能により、ユーザはエクスポート済みレポートのリンクを削除できます。エクスポートされたレポートは Vault ユーザ以外に提供されことが多く、リンクが混乱を招く可能性があります。ユーザは、レポートビルダーの Advanced Options セクションにあるエクスポートされたレポートからリンクを削除できます。
フィルタと列のエイリアスを有効化するフラグをレポートビルダーページに移動する
この機能は、「フィルタと列のエイリアスを有効化する」フラグをレポート作成者ページからレポートビルダーページに移動します。これによりユーザは、既存レポートだけでなく新規レポートにフラグを有効化できます。このフラグは列およびフィルタの名前を変更するユーザ権限を制御し、ユーザが名前列を削除できるようにします。
レポートフィールド「Public Key」を「API Name」へ名称変更
この機能は、レポートリストページとレポートビューアの「Public Key」フィールドを「API Name」に名称変更します。
レポートあたりの「含む」演算子の制限を緩和
今回のリリースでは、ユーザは contains 演算子を使用して、レポートに最大 3 件のフィルタを定義できます。この機能強化以前は、ユーザはレポートあたり 1 件に制限されていました。
監査
エクスポート機能強化の代理監査
on behalf of を含む監査エントリには、CSV へのエクスポート時に入力される新規フィールドが追加されました。エクスポートの既存の User Name 列では、on behalf of の左側にユーザ名が含まれますが、新しい on behalf of 列では右側にユーザ名が含まれます。これまで、この 2 人のユーザは User Name 列に集約されていました。
たとえば 21R2.2 以前は、両ユーザは User Name 列に表示されました。
今回の 21R2.2 リリースでは、ユーザは 2 つの列に別々に表示されるようになりました。
監査証跡: データ値を変更しない Vault オブジェクト更新の新規エントリ
この機能は、複数の値を持つ選択リスト選択の順序変更を行う際とユーザがデータ変更のないレコードを保存する際に、新規オブジェクト監査証跡エントリを追加します。後のケースはユーザがオブジェクトレコードのデータを変更する際に発生しますが、続いて変更を元に戻してレコードを保存します。この機能は 12 月 3 日のリリースで利用可能になりますが、Pre-Release または Limited Release の Vault ではご利用いただけません。
検索およびフィルタ
オブジェクトタブの検索修飾語
検索修飾語機能は、これまでドキュメント検索のみで利用可能でしたが、オブジェクトレコード検索でも機能するよう拡張されました。オブジェクトタブの検索時、Vault はユーザが入力した文字で始まるオブジェクトフィールド名のリストを提案します。フィールドの選択後、ユーザはフィルタを素早く適用するために値を追加するか、そのオブジェクトの単一テキストフィールドを検索できます。
検索修飾語は、選択リスト、オブジェクト参照、はい/いいえフィールドと連動して、結果をフィルタリングします。ユーザは、検索修飾語を使用して、Text、Long Text、および Rich Text フィールドの単一フィールドの範囲検索を行います。
「含む」演算子に一致するサブストリング
選択リストまたはオブジェクト参照フィールドの検索結果をフィルタリングする際に利用できる「含む」演算子は、文字または数字の一部に一致するようになりました。たとえば、治験に「B-1」が含まれる結果のフィルタリングは、治験「AB-123」と「BB-124」の結果を検索します。
チェックリスト
チェックリスト: 集計チェックリスト更新の自動化
新規バージョンのチェックリストデザインを承認すると、エントリアクションは、そのチェックリストデザインを含むすべての承認済み集計チェックリストデザインの新規の承認バージョンを自動的に作成するようになります。このアクションにより、お客様は集計チェックリストデザインごとにクリックを何度も繰り返して新規バージョンを作成する手順を実行する必要がなくなります。
チェックリスト: 添付文書バージョンを指定
チェックリスト回答者 UI により、Vault ドキュメントを質問回答者に添付する際、エンドユーザはドキュメントバージョンを指定できます。これにより、エンドユーザは最新バージョンではないドキュメントを選択できるようになります。デフォルト選択は、ユーザにアクセス権限がある最新バージョンのままです。
ユーザビリティの更新
アクション UI は標準の Vault UI です
アクション UI が標準の Vault UI の一部になりました。21R2 の間に管理者はアクション UI の有効化または無効化を行うことができました。21R3 のリリースによって、これまでアクション UI を無効化していた Vault は自動的に有効化されます。今後、その有効化または無効化に対するオプションはありません。
アクション UI に関する詳細は、https://go.veeva.com/21R2-ActionUI からご確認ください。
Vault へのメール
この機能により、お客様は Vault が所有するメールアドレスにメールを送信して、Vault でドキュメント、レコード、添付ファイルを自動的に作成できます。受信メールはまず Vault でメールレコードに変換されてから、Platform、Vault アプリ、およびお客様が定義したメールプロセッサによってドキュメント、添付ファイル、およびその他のレコードとして保存されます。
管理者は、関連プロセッサを含めメールアドレスごとに関連付けられた Vault 所有のメールアドレスと設定を定義できます。すべての Vault は、メールを未分類ドキュメントに変換できるメールプロセッサを含められるようになりました。
マイ Vault ページユーザビリティの強化
My Vaults ヘッダーの下にあるドロップダウンリストの My Vaults ページ表示フィルタおよび並べ替えオプションは、ユーザのセッションを通じて維持できるようになりました。また、すべての My Vaults と My Favorites オプションは、アルファベット順に並べ替えられます。
Administration
管理者設定タブの更新
管理者は Vault のさまざまな設定ページにアクセスするため、頻繁に設定タブを使用します。このタブは、管理者が利用できるすべての設定ページへのリンクが表記されたランディングページが表示されるよう更新されました。管理者は設定ページを検索して、よく使用する設定をお気に入りリストへ簡単に追加することもできます。[最近使用済み] セクションは、最近表示したページを最大 5 つまで表示し、管理者が素早くページに戻れるようにします。詳しくは、Navigating the Business Admin and Configuration Tabs をご覧ください。
特殊文字のエスケープシーケンスを標準化する
この機能は、VQL エスケープ文字 () を標準化して、開発者および管理者が条件 VQL を実装することで、すべてのドキュメントフィールド、オブジェクトフィールド、およびその他の VQL エンドポイントでサポートされている特殊文字を参照できるようにします。サポート対象文字のリストには、バックラッシュ (\)、キャリッジリターン (r)、二重引用符 (")、改行 (n)、パーセント記号 (%)、単一引用符 (')、アスタリスク (*)、およびタブ (t) が含まれます。Vault は、自動的に既存の条件 VQL を変換して標準化されたエスケープ文字を使用します。
保存済みビュー管理
新しいビュー管理ツールは、Vault で作成されたビューの改善された表示機能と制御機能を管理者に提供します。新しい「管理済み」プロパティは、ユーザが自分自身で使用するため、または他のユーザと共有するために作成したビューを、管理者が Vault 設定の一部として作成したビューと区別するために追加されました。
「管理済み」ビューおよび「強制」ビューは、タブのデフォルトビューとして設定できます。この機能は、利用頻繁の少ないユーザをフィルタリング済みの結果に誘導する際に便利です。たとえば、一時ユーザが初めてライブラリを表示する際には固定状態のドキュメント表示のみを期待しているため、このフィルタのデフォルトビューをライブラリタブに追加できます。
新規オプションは、タブからビューを作成するのではなく、検索条件に VQL を使用してビューを表示するために追加されました。これらのビューは常に「管理済み」となり、標準ビューと同様に動作します。
新しいビューツールにアクセスすると、管理者が以下のアクションを実行する検索可能リストですべてのビューを使用できます。
- 検索条件および表示詳細を表示
- ビューを表示するユーザを更新
- ビューの所有権を再割り当て
- タブにビューを表示
- ビューを管理予定または管理済みに設定
- VQL を使用して新規ビューを作成
- ビューのリストをエクスポート
- 削除
これまで、ビュー管理権限を持つユーザは、ビューマネージャダイアログで強制とマークされているすべてのビューを表示できました。この権限は、ビューを強制とマークすることだけに制限された「強制とする」に変更されました。Vault のすべてのビューを表示するために、権限セットの管理者セクションに新しい一連の権限を用意しました。これにより、新しいビュー管理ツールにアクセスできる権限を管理者に付与できます。
Vault Configuration および比較レポートの機能強化
Vault は、レポートがキューになると管理者にシステムメッセージを送信し、レポートがダウンロードの準備ができると後続のシステムメッセージを送信するようになりました。詳しくは、Vault 構成レポートおよび Vault 間比較をご覧ください。
すべての Vault で有効化されたドキュメント利用状況の指標
この機能は、すべての Vault でドキュメント利用状況の指標を有効化し、設定から Enable Document Usage Metrics フラグを削除します。これまで、このフラグは固定ドキュメントのユーザアクティビティが Document Usage オブジェクトで追跡されていたかどうかを制御していました。
スケジュールされているデータエクスポートで利用可能な監査データ
今回のリリースでは、スケジュールされているデータエクスポートに監査データを含めることで、お客様は Vault 監査データを企業のデータレイク、データウェアハウス、またはビジネスインテリジェンスレポートサービスに読み込めます。サポートされている監査ログは、System Audit、Login Audit、Document Audit、Object Record Audit、および Domain Audit です。
スケジュールされているデータエクスポート: カスタム Amazon S3 設定をリセット
今回のリリースでは、管理者は Scheduled Data Export Settings のカスタム Amazon S3 設定を削除できます。
Vault 全体のドキュメント移行モードの廃止
21R3 リリース以降、Vault 全体のドキュメント移行モードは廃止されます。
この機能に代わり、Vault REST API の複数のドキュメントの作成、複数のドキュメントの更新、複数のドキュメントレンディションの追加エンドポイントがある X-VaultAPI-MigrationMode API ヘッダーを使用するか、ドキュメント移行モードチェックボックスが選択された Vault Loader を使用してください。これらのメソッドは、API リクエストで作成・更新されたドキュメントのみが移行モード制限に従っており、残りの Vault は完全に操作可能な状態であることを確認することによってリスクとエンドユーザの影響を最小限に抑えます。
形式のない数字を承認する Text() 機能
この機能によりユーザは、形式を定義する必要がない Text() 機能を使用して、数字をテキストに変換できます。形式が指定されていない場合、Vault は数字に定義された現在の小数点以下の桁数をそのまま維持します。
システム管理接続
この機能により、Veeva が所有する標準接続は System Managed とマークされます。新しい参照専用フィールドである System Managed は、すべての接続レコードに表示されます。カスタム Vault Java SDK コードは、システム管理接続を使用できません。
アクセスコントロール
新規ドキュメント所有者の割り当てを許可されたユーザ
これまでのリリースでは、Vault は新規ドキュメント所有者に割り当てられるユーザを制限していませんでした (手動割り当て、一括アクション、または REST API)。
今回のリリースより、管理者は Owner ライフサイクルロールを設定して、新規ドキュメント所有者として割り当てられるユーザを制限できます。この機能は、クリエイターが自動的にドキュメント所有者に割り当てられるドキュメント作成フローには影響しません。
アップグレード時、許可されているユーザはデフォルトで「すべてのユーザとグループ」に設定されており、前回のリリースの動作を保持します。
Vault ユーザの詳細ページ: 関連オブジェクトセクションの [タブに表示] アクション
Vault ユーザの詳細ページ (Admin > Users & Groups) は、関連オブジェクトセクションの Show in Tab アクションを提供できるようになりました。
認証およびセキュリティ
高度なパスワードポリシーオプション
パスワードのセキュリティポリシーは、統合アカウントやエンドユーザアカウントなど、ユーザアカウントのタイプごとに異なるポリシーを適用するさまざまなオプションが用意されています。
- パスワードの最小文字数は 7~40 文字の値に設定できます。
- パスワードの有効期限は、30~720 日間の値に設定できます。
- パスワードの再利用禁止は、過去 20 回まで設定できます。
また、新規属性のアカウントロックアウト期間はデフォルトで永続的 (21R3 以前の設定) になっていますが、オプションで 5~60 分間に設定できます。
Vault File Manager
Vault File Manager: 新規アイコン
今回のリリースでは、Vault File Manager のデスクトップとシステムトレイアイコンを更新しました。今後数回のリリースにわたって Vault のドキュメントファイルアップロードおよびダウンロード機能を拡張するため、このアイコンは特定のアクションで Vault にも表示され、ユーザがデスクトップアプリケーションと Vault のアクションの接続を確立できるようサポートします。
Vault Loader
Vault Loader コマンドラインツールの Zip ファイルの改良
Vault Loader コマンドラインツールの VaultDataLoader.zip ファイルは、ネイティブ macOS アーカイブユーティリティアプリケーションを使用して抽出できるようになりました。詳しくは、Vault Loader コマンドラインツールをご覧ください。
Vault Loader の抽出: CSV エクスポートにあるすべてのレンディションタイプを含める
ドキュメントとドキュメントバージョンを抽出する際、[Include Renditions] オプションを選択すると、Vault Loader CSV データエクスポートに利用可能なすべてのレンディションタイプのメタデータを含められるようになりました。
Vault 比較レポート: VPK の自動作成が有効化されたシステムメッセージの更新
VPK の自動作成が有効化された Vault 間比較レポートシステムメッセージは、レポートに 40 超のパッケージが生成されている場合、パッケージファイルごとのリンクではなく、送信パッケージページへのリンクを表示するようになりました。
Vault Loader: Map フィールドが有効化されたオブジェクト参照の更新
これまでのリリースでは、Vault は Map Fields が有効化されている CSV 入力ファイルの空白のオブジェクト参照値を無視し、これに対応する、Map Fields が無効化されたオブジェクト参照のみを削除していました。今回のリリースでは、Map Fields が有効化された CSV に空欄または空白のオブジェクト参照フィールド値を読み込む際、オブジェクトレコードの対応するオブジェクト参照が削除されます。詳しくはフィールドマッピングをご覧ください。
Vault Java SDK
Spark メッセージ リバース IP ルックアップの変更
送信 Spark メッセージは、Veeva Vault に関連付けられた IP アドレスから送信されるようになりました。この開発者向け機能の詳細は、Vault Developer Release Notes をご覧ください。
Platform データモデルの変更
上記の機能に関する変更については、21R3 Data Model Changes: Platform をご覧ください。データモデルに影響を与える追加機能は、本セクションに記載されています。
言語オプションを Language フィールドに追加する
多言語 Vault のドキュメントにある Language フィールドには、世界中で行われる作業をより適切にサポートするため、さらに 242 言語が追加されました。選択可能な言語の全リストを参照するには、サポート言語についてをご覧ください。
Person オブジェクトの Veeva ID フィールド
この機能は、「Veeva ID」とラベル付けされたテキストフィールドを Person オブジェクトに追加します。このフィールドは、クロス製品統合のため Veeva アプリケーションによってのみ入力できます。
Vault Connections
CTMS/CDMS: 治験関連データ削除のサポート
Vault 間で共有している治験、イベント、およびプロトコールの逸脱や問題をサポートする CTMS および CDMS Vault スイート間の Vault Connection は、削除されたイベント (Subject Visits など)、手順、被験者、およびプロトコールの逸脱を CTMS Vault にアラートするようになりました。この機能は、キャンセルまたは削除された Subject Visit の Site に送信された支払いなど、下流プロセスの問題を避けながら期待を調整するため、Clinical Operations 担当者に臨床試験ステータスに関するインサイトを提供します。
eTMF/RIM 接続: クエリオブジェクトルールを使用した条件付き転送サポート
この機能は、eTMF/RIM 接続の両サイドで使用されている Vault のクエリオブジェクトルールへのサポートを追加します。
CTMS/CDMS: クエリオブジェクトルールをサポート
治験、施設、および被験者情報を共有する CDMS および Clinical Operations Vault の Veeva Vault Connection は、2 つの Vault で共有されているデータのフィルタリングをサポートし、お客様固有の要件に基づいて転送されたデータをフィルタリングできるようになりました。
Clinical Operations
TMF ボット: すべての Vault eTMF のお客様に対して自動的にオン
この機能は、固定状態のドキュメントが 1,500 超ある限りにおいて、まだ展開していないすべてのお客様の Vault でドキュメント分類トレーニングモデルを自動的に作成、トレーニング、展開します。本モデルのトレーニングおよび展開には 48~72 時間かかる場合があります。Vault がモデルを展開すると、お客様のドキュメントインボックス内のドキュメントが TMF ボットによって自動分類されることがあります。トレーニング済みモデルの詳細については、こちらをクリックしてください。
TMF ボット: モデルトレーニングの機能強化
この機能は、TMF ボット: モデルトレーニング機能に以下の更新を行います。
- トレーニングで「評価済み、未選択の施設」参照モデルにマッピングされたドキュメントタイプを常に除外する
- トレーニング済みモデルが定期的にバージョンアップされるドキュメンのトレーニングを受けられるよう、トレーニングウィンドウドキュメント選択方法では「作成日」ではなく「バージョン作成日」を使用する
- バリデーションを支援するため、抽出範囲、自動分類範囲、および自動分類エラー率を「トレーニング概要結果」に入力する
- 2 文字の ISO コードではなく言語ラベルを使用して言語の失敗を更新する
TMF ボット: 本番データのモデルをトレーニングする
この機能により、管理者は、お客様に関連する本番環境データを使用して、Sandbox、QA、または Pre-Release 環境からトレーニングモデルを訓練できます。
TMF ビューア: 参照専用ユーザの分類フィルタリング
この機能により、参照専用権限を持つユーザは、TMF ビューア内の Classification フィルタリングを使用できるようになりました。
TMF 転送の表示を改善
この機能は、Vault の送受信における TMF 転送アクティビティをより詳細に表示します。ユーザは既知の転送ステータスについて、開始時および終了時を表示できるようになります。転送に失敗した可能性がある個別アイテムは、転送を再試行できるよう新しい「再試行失敗」アクションと一緒に表示されます。
拡張された CTMS 被験者指標
この機能は、Vault CTMS で自動的に計算される追加指標を追加します。新しい被験者ステータスには、「同意済み」、「治療開始済み」、「フォローアップ中」、および「フォローアップ喪失」が含まれます。この機能は以下の CTMS 機能に拡張されます: 指標計算、モニタリング指標計算、Vault CDMS/ClinOps 接続、自動被験者マイルストーン、指標レコードの追加、使用されていない指標、CTMS ホームページ登録ステータスグラフ。
モニタリング報告書の質問分岐
この機能は、以前の質問への回答に基づき、CRA が入力する必要のあるモニタリング報告書の質問をコントロールする方法を提供します。複数選択の質問の場合、管理者は後続の質問を編集可能にできる先の質問と回答を指定できます。
動的なモニタリング報告書セクションのレビューステータス
この機能は、レビューコメントダイアログからコメントが作成、編集、削除、再開封、解決されると、モニタリング報告書グリッド行のレビューステータスアイコンを動的に更新します。*「レビュー者コメントのモニタリングを有効化」が有効化されている Vault で自動オンになります。
孤立したモニタリングレビュー者コメントの解決をサポート
この機能により、管理者はモニタリングイベントから削除された未解決のレビューコメントを自動的に解決するモニタリングイベントライフサイクル状態のエントリアクションを設定できます。これまで、これらの「孤立した」レコードは管理者が手動で解決する必要がありました。
必要なモニタリング報告書を表示する質問ロジックの更新
この機能は、Trip Report Question Response オブジェクトの新しい「Highlight Required」フィールドを使用するため、必要なモニタリング報告書の質問要素をハイライトするロジックを更新します。リリース後、現在レビュー中のモニタリングイベントは、短期間の間、不足データをハイライトしないことがあります。
*モニタリングイベントワークフローで必要な訪問報告書の質問アクションの検証を使用している Vault で自動オンになります。
Yuzu: 治験届 PMDA XML 更新
この機能により、「日本の治験届機能を有効化する」を有効化している Vault CTMS のお客様は、日本の PDMA の最新ガイドラインに対応する治験届ドキュメントを作成できます。お客様は、引き続き「初期」バージョンを使用して治験届ドキュメントおよび XML を生成するか、治験届データコレクションを更新して、更新済み PDMA フォーマットを使用して指定された追加データ要素を取得することができます。
治験責任医師の臨床アクティビティ
この機能は、Vault CTMS の治験責任医師に関連するアクティビティ追跡にサポートを追加します。新しい Activity オブジェクトにより、ユーザは、選択した治験責任医師に関して実施されたすべてのアクションおよびインタラクションの詳細を取得できます。
Vault Payments: Site Advance 決済
この機能により、Vault Payments は Site Advance をサポートします。Site Advance として指定すると、料金から借方および貸方の支払い項目が生成されるので、決済を行うお客様は Site Advance を発行して、レポートおよび表示権限の継続費用を追跡できます。
最小支払いしきい値
Vault Payments では、治験組織が支払いしきい値を定義する機能がサポートされるようになりました。最小支払いしきい値は、支払い要求の最小値を指定する支払い要求の生成機能で使用されます。
マイルストーン状態変更の自動化
この機能は、Vault 全体設定を導入して、特定の日付フィールドの母集団に基づいてマイルストーンの状態を自動的に変更します。この機能を開始すると、ライフサイクルの追加アクションを必要とすることなく、実際の完了日の入力時にマイルストーンが完了状態に移行できるようになります。
EDL アイテムの必要度と # Expected を同期
この機能は、ユーザが必要度フィールドを更新する際、EDL の # Expected フィールドへの更新を自動化するオプションを導入します。具体的には、必要度を「不要」に変更すると # Expected は 0 に設定され、必要度を「必要」に変更すると # Expected は 1 に設定されます (現在 0 の場合)。これにより、治験要件に基づいて EDL アイテムを変更する際に必要なクリック数が半分になることを予期しています。
治験実施施設ライフサイクルへの安全な配布の自動フィルタリング
この機能によって、Site Connect のお客様は、安全性情報の配布を自動フィルタリングして、特定のライフサイクル状態の治験実施施設に安全性ドキュメントのみを送信できるようになりました。このフィルタリングは、既存の Distribution Default オブジェクトを使用して、国ごとの基準に基づいて定義できます。
Safety Distribution: SiteVault 施設へのメール通知
この機能により、Site Connect のお客様は SiteVault への配送に加えて、メール経由で安全性情報の配布を直接治験実施施設のユーザに送信するよう指定できます。
Site Connect ドキュメントの物理削除のサポート
この機能により、Site Connect のお客様は Site Connect 経由で送受信したドキュメントを物理削除できるようになりました。これまで、配布タスクレコードは物理削除が完了する前に削除する必要がありました。
エクスペクテッドドキュメントから 1 つ以上のドキュメントを送信する
この機能により、Site Connect のお客様は、複数のドキュメントがエクスペクテッドドキュメントに一致している場合、そのエクスペクテッドドキュメントから 1 つ以上のドキュメントを SiteVault に送信できるようになります。
Site Connect: 手動での治験案内の作成と送信
この機能は、Study Site レコードから直接 Site Connect 治験アグリーメントを手動で作成・送信するサポートを追加します。
Site Connect: Study フィールド更新のドキュメントバージョンアップを防ぐ
この機能強化により、ドキュメントコンテンツが同じ場合、SiteVault から受領したドキュメントは、新規ドキュメントバージョンの作成に進めなくなります。コンテンツが同じ場合、Site Connect はドキュメントの Study、Study Country、Study Site フィールドのみを更新します。
Site Connect: 同じドキュメントバージョンの再送信を許可
Site Connect のお客様は、同じドキュメントバージョンを単一の治験実施施設に再送信するオプションを利用できるようになりました。
Site Connect: 治験実施施設から施設パッケージを送信
この機能により、Site Connect のお客様は Study Site レコードから直接施設パッケージを送信できます。
Site Connect: 一致するドキュメントが関連付けられたエクスペクテッドドキュメントからドキュメントをリクエストする
この機能により、Site Connect のお客様は、すでに一致するドキュメントがある場合でも、エクスペクテッドドキュメントから追加ドキュメントをリクエストできるようになりました。
フィールドデフォルトの前バージョン値トークン
この機能により、Site Connect のお客様および TMF 転送を使用しているお客様は、既存のドキュメントバージョンに定義された値がある受信ドキュメントバージョンのメタデータを自動的に入力するフィールドデフォルトを作成できます。この機能は、Site Connect 経由で受領したプロファイルドキュメントの Person 値および Organization 値の維持に非常に便利です。
Site Connect: 施設パッケージの改善
この機能には、以下のような Site Connect 施設パッケージフローへの多くの機能強化が含まれます。
- Vault が治験実施施設に送信できるドキュメント数制限の引き上げ。
- Preview Package 画面の Name 列のロック。
- 各施設をより簡単に特定できるよう、治験実施施設に属する治験責任医師の名前のプルイン。
Site Connect: Vault Clinical ドキュメントの追加サポート
この機能は、Veeva Site Connect 経由で新規ドキュメントタイプを転送できるようサポートを追加します。Site Connect から転送可能になった新しい Vault Clinical ドキュメント階層には、以下が含まれます。
- 施設署名とイニシャル
- 施設予算
- 施設選択書
- 治験チームメンバーの連絡先リスト
- IP 廃棄証明書
- サンプル保管条件ログ
- 治験責任医師会議資料
- IP 品質クレーム申請書
Site Connect: 治験案内ウェルカムテキスト
Site Connect のお客様は、治験案内を治験実施施設に送信する際、カスタムのウェルカムテキストを含められるようになりました。SiteVault は、治験案内の承認フロー中、ウェルカムテキストを施設に表示します。また、ウェルカムテキストを TMF 転送アグリーメントに設定できます。
Site Connect: 治験案内の却下理由
受領者が Site Connect 治験案内または TMF 転送アグリーメントを却下した場合、却下理由の裁定を要求できるようになりました。
Site Connect: 接続の自動化
この本番専用機能を使用して、Site Connect のお客様は SiteVault への接続を自動的に確立できます。ユーザが USN 値で Organization レコードを作成するたび、Vault はこれらの標準 Site Connect 接続を作成します。
Clinical Operations 機能フラグの更新
これまで Vault サポートでしか使用できなかった本機能は、Vault の設定セクションにアソートされた Clinical Operations 機能フラグを表示します。
Clinical Operations データモデルの変更
詳しくは、21R3 Data Model Changes: Clinical Operations をご覧ください。
Commercial
医師からの問い合わせ専用のユーザインターフェース
この機能は、ユーザが問い合わせに関するすべての情報をまとめて取得できる、医師からの問い合わせ専用の最新かつ設定可能なユーザインターフェースを提供します。症例に関する詳細に加えて、ユーザは、それぞれが 1 つ以上の回答を追跡できる複数のリクエストや、有害事象または製品品質クレームなど HCP がやりとりを行っているその他の関連事項の詳細を取得できます。これを一つの画面で行うことで、MedComms ユーザの速度と効率性が向上します。
医師からの問い合わせメール受信の自動化
専用アドレス宛てにメールで送信された医師からの問い合わせを受信すると、MedComms が取得して自動的に関連レコードを作成します。これはユーザの利便性を向上させるだけでなく、プロセスを自動化することで医療情報チームはすべての作業を MedComms で操作できるようになり、組織における正確性と一貫性が向上します。詳しくは、自動化されたメール受信の設定をご覧ください。
ポジティブ/ネガティブリスト
この機能は、ポジティブ/ネガティブリストに必要なコンテンツをタグ付けする標準フィールドおよび新規オブジェクトを提供し、サービスエンゲージメントによって Veeva CRM に統合されると、ポジティブ/ネガティブリストにあるドキュメント、印刷資料の有効期限や破棄時期を示す MyInsights から最新情報を担当者に提供します。担当者は印刷資料の破棄時期を確認することで、お客様が各国の保健当局の規制 (フランスの「ポジティブリスト」規制要件など) に準拠できるようにします。詳しくは、ポジティブ/ネガティブリストをご覧ください。
eCTD: eCTD バインダーの追加資料情報
この機能は、Promotional Material Document Name と Promotional Material Document Description フィールドを追加します。これらのフィールドを含む eCTD バインダーが生成されると、Vault は販促用資料の値をクリーンな資料のフィールドにコピーします。これによって、Correspondence 書簡、2253 フォームおよび補足フォームで値を使用できるようになります。これらのフィールドは、PromoMats および RIM Vault Connection でも使用できます。
この機能は、販促用資料 ID を特定のドキュメントタイプに設定する際、eCTD バインダー機能を更新して、販促用資料の Material ID フィールド値を Annotated Material、Annotated Label、Annotated Reference ドキュメントタイプの Promotional Material ID ドキュメントフィールドにコピーします。これは、販促用資料 ID 機能を PromoMats および RIM Vault Connection と組み合わせてご使用になられているお客様にのみ必要となるものです。
メディカルアフェアーズ向けの CLM メタデータの機能強化
本リリースでは、データモデルの変更と業界のベストプラクティスを通じて、MedComms がコンテンツ作成の時点でこれまでよりはるかに豊富なメタデータを取得します。これにより、Veeva CRM を使用している MSL は CLM コンテンツの高度な検索機能を手に入れ、よりすばやく効率的に探しているものを見つけ出せます。
CLM コンテンツの単一ドキュメントパブリッシングのためのドキュメントレベル レンディションタイプ セレクタ
ユーザはこの機能を使って、CLM コンテンツ向けの単一ドキュメントマルチチャネルパブリッシングで使用されているドキュメントに別のレンディションタイプを選択できます。ドキュメントタイプレベルでは既存のデフォルトレンディション設定が引き続き適用されますが、この変更を行うことで必要に応じてドキュメントごとのデフォルトを上書きできます。
コンテンツモジュールの階層コピー
本リリースでは、コンテンツモジュールを使って階層、すなわち「ディープ」コピーを有効化できます。階層コピーにより、オブジェクトレコードのコピープロセス中にコンテンツモジュールとすべての関連レコードをコピーできます。また、関連レコードはコピーせずに、コンテンツモジュールだけをコピーできます。
Commercial Data Model Changes
詳しくは、21R3 Data Model Changes: Commercial をご覧ください。
モバイル
アプリ更新の強制
この機能により、Station Manager Mobile アプリケーションが Veeva のモバイルアプリケーションのサポートポリシーを強制できるようになります。アプリのバージョンが 1 つ古い Vault であることが検知されると、報告可能なカウントダウンが開始され、カウントダウンが終了し、アプリがログアウトされるまで、アプリを更新する必要があることを継続的にユーザに通知します。アプリケーションのバージョンが 2 つ古い Vault である場合、アプリにログインできないことがあります。
この機能は次の 2 つのプラットフォームでサポートされています:
アプリ更新の強制: Android
アプリ更新の強制: iOS
Quality
繰り返しチェック: 逸脱
この機能は Vault QMS に、Deviation が他の Deviation の繰り返しであるかどうかをエンドユーザが判断できる、インテリジェントで簡素化されたインターフェースを導入します。この機能が設定されていると、これまで手動で行っていた複雑のプロセスが、Vault によってテキスト検索用語が自動的に提案されるシンプルなユーザアクションに置き換えられます。並べて比較できる新しいユーザインターフェースには、潜在的な Deviation が類似スコアと共に表示されます。分析が完了すると、Vault がその結果を保存し、Related Quality Event レコードを作成することで、繰り返し Deviation レコードが自動的にリンクされます。詳しくは、configuring Recurrence Check をご覧ください。
繰り返しチェック: クレーム
この機能は、Recurrence Check 機能を Complaint にまで拡大します。シンプルなユーザアクションで可能性のある繰り返しをすばやく見つけ出し、見やすいリストビューで表示します。Vault QMS が、後でレポートや傾向レポートを作成できるよう、チェックの結果を自動的に保存します。
MDCC: Change Authorization セクション
本リリースでは、新しい Change Authorization オブジェクトを使って、変更承認向けに DCC に 1 つまたは多数のドキュメントを追加できます。Change Authorization に追加された各ドキュメントは、インパクトアセスメントの一環として、修正が必要または廃版にするとしてタグ付けできます。
Change Authorization レコードが承認されると、修正が必要なドキュメントに対して新しい下書きバージョンが自動的に作成され、DCC のDocuments to be Made Effective セクションに追加されます。同様に、ドキュメントが廃版になった場合は、Change Authorization レコードにより、ドキュメントが DCC のDocuments to be Made Obsolete セクションに自動的に移動されます。この機能が登場する前は、有効なドキュメントの下書きバージョンを手動で作成してから、DCC に追加する必要がありました。
QMS エントリアクション: Create Related Record
本リリースでは、Quality のご使用のお客様は、Vault の設定を通じて、さらに高度な自動化を行えます。Create Related Record のエントリアクションによって、別のレコードのライフサイクルの進行に基づいて、レコード全体またはプレースホルダーレコードを自動的に作成できます。
たとえば、ライフサイクルの中に初回トリアージの手順があり、Deviation にユーザがタスクの一部として「調査が必要」であると示しているものがあるとします。Create Related Record 機能を使うと、Vault が自動的に Investigation レコードを作成し、その調査のワークフローを Quality Teams を介して割り当て、割り当てられたユーザがトリアージタスク完了後にすぐに作業を始められるようにできます。
この初回リリースでは、Create Related Record フレームワークが導入されたことで、デフォルトのフィールド値設定で設定されていない限り、最初に必要なメタデータを「トリガー」レコードまたはそれに関連するレコードから引き出せます。上記の例に続き、Investigation レコードに「主管部門 (Owning Department)」フィールド値が必要な場合、管理者はこの調査の Owning Department を、トリガーする Deviation の Owning Department の値に設定できます。
この分野にはさらなる投資が行われることが予想されますが、この初回リリースには設定できるフィールドのタイプと、この機能を使用して自動作成を設定できるオブジェクトにいくつかの制限が存在します。詳しくは、関連レコードの自動化の設定をご覧ください。
CAPA および SCAR レコードの外部コラボレーションのサポート
本リリースにより、Vault QMS の監査所見と外部回答の連携機能セットが拡張されます。監査所見の対応と同様に外部コラボレーターを招待して、CAPA と SCAR をフォローアップできるようになりました。本リリースでは、管理者は Audit Finding、CAPA または SCAR、またはこの 3 つのプロセスの組み合わせに外部コラボレーターを招待して、外部コラボレーションを活用するために追加プロセスを設定できます。各プロセスには固有の「ようこそ…」や「さようなら…」のメッセージがあり、Vault は外部コラボレーターが最初に招待されたレコードのタイプに基づいて、送信するメッセージをインテリジェントに選択します。使用可能ないずれかのプロセスからのレコードに参加するよう招待された共同編集者は、共同編集に招待されたプロセスに関係なく、すべてのレコードの共同編集の完了時に Vault から削除されます。
この機能を使用するには設定が必要です。現在監査所見と外部回答の連携をご利用のお客様は、これらの新しいプロセスの導入を評価する際、外部コラボレーション者のセキュリティ設定をご確認ください。詳しくは、外部コラボレーションのサポートをご覧ください。
トレーニングの自己登録
この機能を使って学習者は、トレーニング管理者によって自己登録に使用できるよう設定された Training Requirement に自己登録できます。設定すると、学習者は興味を引く Training Requirement を探したり検索したりして、そのコースに自己登録できます。たとえば、学習者が文書管理基準を学びたいとします。学習者は学習者ホームページからコースを探して、自己登録し、完了できます。学習者はいつでもコースの登録を解除できます。詳しくは、自己登録をご覧ください。
トレーニングのジョブ性能の向上
この機能により、Training Assignments の更新ジョブにさまざまな性能および信頼性の向上がもたらされます。
Quality Event の標準サイクルタイム指標
本リリースでは、Vault QMS は Quality Event 向けに、以下のサイクルタイム指標を自動的に取得できるようになりました。
- Cycle Time: 開始から終了までの日数
- Closure Delta from Original Due Date: 元の期日と Quality Event 終了日の誤差の日数
- Closure Delta from Current Due Date: 最新の期日と Quality Event 終了日の誤差の日数
- Extensions Granted: Quality Event 向けに認められている延長要求の日数
レコードから品質のドキュメント生成: ドキュメントテンプレートに基づいたドキュメントのサポート
本リリースでは、QMS 監査および Quality Event の 21R2 のレコードアクションから品質ドキュメントを生成する機能が拡張され、ドキュメントを Vault 内のどのドキュメントテンプレートに基づいてでも生成できるようになりました。QMS Vault の既存のドキュメント生成機能と同様に、ユーザアクションとして手動で使用したり、レコードのライフサイクルの一部として自動的に使用したりできます。ドキュメントテンプレートからドキュメントを生成する場合、Vault は新しく作成されたドキュメントをレコードに、あるいはレコードは新しく作成されたドキュメントに自動的にリンク付けします。これは、ドキュメントとオブジェクトの両方のライフサイクルのエントリ条件と組み合わせて使用されるため、Quality Vault でリスクの低い新しい強力なプロセスをモデル化できます。
Vault は、同じ名前を持つフィールドのレコードのメタデータに基づいて、ドキュメントのメタデータを設定します。これはつまり、レコードのセキュリティを促進するフィールドを生成されたドキュメントに追加して、ビジネスニーズに合わせて生成されたコンテンツに幅広いまたは細かなセキュリティを設定できるということです。
21R2 機能によって有効化される機能に加え、今回の機能強化によって、次のような追加機能を有効化できます。
- Audit または Quality Event から PDF 以外のドキュメントを生成して、作成後の生成物の編集ができる
- 主要な Audit または Quality Event の生成物を生成する際に、ベストプラクティスドキュメントテンプレートに使用する
- 生成したドキュメントで Merge フィールドを使用して、生成されたレコードに基づいてテンプレートの内容をデフォルト化できる
- 関連する Audit Report が確認・承認されるまでは、監査をそれ以上先に進められないようにする
この機能を使用するには設定が必要です。詳しくは、ドキュメント生成の設定をご覧ください。
レコードからの品質ドキュメントの生成: バッチリリース処理のサポート
QMS をご利用のお客様は、CoA、CoC、あるいはその他のドキュメント生成物など、バッチリリース/処分ライフサイクルの一環として、自動的にドキュメントを作成できるようになりました。フォーマット済み出力またはドキュメントテンプレートに基づいてドキュメントを作成する機能は、Audit または Quality Event を使って作業することの多い QMS ユーザにはなじみのあるものでしょう。この機能は今年の初めに導入されたものです。
ドキュメントを作成する際、Vault は新たに作成されたドキュメントを、この作成をトリガーした Disposition レコードにリンク付けし、その Disposition レコードをドキュメントにリンクし直すことで、双方向の関係を構築します。
この機能を使用するには設定が必要です。詳しくは、レコードからの品質ドキュメントの生成をご覧ください。
マルチカントリー有害事象報告
この機能を使うと、ユーザがインシデントの国、苦情の重要度結果、インシデントに関与した製品、および報告決定ルールに基づいて報告価値評価を終了した時点で、Vault によって自動的に有害事象レポートが作成されます。
TGA (オーストラリア) 向け有害事象レポート
今回のデータモデルの更新により、Vault Product Surveillance をご利用のお客様は、TGA (オーストラリア) 向けに有害事象レポートのレコードを作成できるようになりました。詳しくは、Vault Product Surveillance をご覧ください。
VPS: バリデーション結果の保存
この機能を使うと、有害事象レポートのすべてのバリデーションエラーをオブジェクトレコードとして表示できます。Vault が各保健当局のフォームの特定のセクションにダイレクトすることで、エンドユーザは保健当局にレポートを提出する前にバリデーションエラーを簡単に修正できます。
フィルタリング、レポートのための主要な Quality Team Role の表示
本リリースでは、QMS をご利用のお客様は、主要な Quality Team Role の割り当てを User フィールドとして表示し、関連するリスト、検索、ライブラリビューのフィルタ、レポートなどとして使用できるようになりました。
この機能により、特定の Quality Team 有効化オブジェクトのどの User オブジェクト参照フィールドが Quality Team Role のメンバーシップにリンク付けされるかを示すことができるようになりました。リンク付けされたチームロールのメンバーシップが変更されると、Vault はフィールドを更新して、新しいチームロールのメンバーシップを反映させます。この機能が有効化されると、管理者はビジネスチームにとって最も効果的な戦略を更新して、確実に既存レコードのフィールドを既存の割り当て値と一致させられます。
このフィールドのリンク付けは、Maximum Users 値が 1 の Quality Team Role でサポートされています。チームロールにリンク付けされると、そのオブジェクト参照フィールドは、リンク付けされている Quality Team Role を編集する以外の方法で更新されることはありません。この Quality Team Role フィールドのリンク付けを使用するには事前に設定が必要です。Quality Team Role のリンク付けされたフィールドは、即座に適用されるロールメンバーシップの変更とは非同期的に更新されます。したがって、Vault 全体で更新されたチームロールの割り当てがユーザフィールドとして表示されるようになるまで、Quality Team Role メンバーシップの変更を保存してからしばらく待つ必要がある場合があります。詳しくは、リンク付けされたロールフィールドの設定をご覧ください。
Quality スイート Vault の参照オブジェクトの有効化
Quality スイートでは、特定のオブジェクトは「コア」としてみなされ、使用するためには特定のアプリケーションを展開する必要があります。本リリースは、各 Quality スイートのサブアプリケーションに対して、どのオブジェクトを「コア」とみなすかについて、Vault の定義を修正しました。Quality Vault 内でどの Quality Suite アプリケーションが有効化されているかに関係なく、Quality の操作に影響を及ぼす可能性のあるサプライヤーや外部組織のリストを作成するために使用する Organization (qms_organization__qdm)、Context (context__qdm)、または Department (department__v) などのオブジェクトとやり取りできるようになりました。
この変更は自動的に有効化されますが、この機能は既存の権限セットまたはセキュリティプロファイルには影響を及ぼさないため、エンドユーザに影響を及ぼすためには設定が必要です。
プロセスナビゲータ: 固定状態のみのフラグ
Admin Checkbox によって有効化されるこの機能を使うと、プロセスナビゲータを通して表示させる際に、ドキュメントの最新の固定状態バージョンを表示させることができます。この設定は、ユーザが新しいマイナーバージョン、または下書きバージョンの作業手順にアクセスできるものの、最新の有効なバージョンの作業手順だけを使用する必要がある場合に役立ちます。詳しくは、プロセスナビゲータをご覧ください。
DCC バナーが Document Change Control オブジェクトのラベルを使用する
ドキュメントが Document Change Control 下にある場合に表示される DCC バナーはこれまで、「このドキュメントは現在変更管理によって制御されています」としてハードコード化されていました。本リリースより、メッセージの「変更管理」の部分が Document Change Control オブジェクトのラベルに置き換えられます。これは、Vault で Document Change Control オブジェクトの名前が変更されている場合に役立ちます。
Document Change Control からドキュメントを削除する機能の改善
本リリースより、ユーザが Document Change Control の Documents to be Made Effective セクションからドキュメントを削除した場合、その変更管理を参照したドキュメントのすべてのバージョンが削除されます。
これまでのリリ-スでは、ドキュメントの最新バージョンだけが Document Change Control から削除され、変更管理から完全に切り離すにはエンドユーザがドキュメントの各バージョンを削除する必要がありました。今回の機能強化によってこれまでの問題が解消され、ユーザは一回の削除操作で変更管理からドキュメントのすべてのバージョンを削除できるようになりました。
MDCC: 最新ではないバージョンのドキュメントの状態変更を防止する
この機能を使用すると、Vault QualityDocs がさらにベストプラクティスを強制でき、Document Change Control によって最新ではないバージョンのドキュメントで状態が変更されることがなくなります。
ユーザが Document Change Control を介して、最新ではないバージョンのドキュメントの状態を変更しようとすると、アクションが阻止され、Vault にエラーが表示されます。
プロセスナビゲータ: 調整可能な階層の最大数
この機能により、経営陣の承認を得た上で、オブジェクトタイプあたりの階層、および親レコードあたりの子プロセスの最大数を増やせます。詳しくは、階層の管理をご覧ください。
Station Manager: 選択リストベースのドキュメント分類の廃止
本リリースでは、Station Manager では選択リストベースのドキュメント分類の使用が廃止されました。分類と一致するように選択リストを選択するための設定オプションは、Station Configurations では使用できなくなりました。選択リストベースのドキュメント分類を使用している、またはドキュメント分類の有効化を検討しているお客様は、レコードベースの分類を代用することをお勧めします。このようなお客様はまた、ページレイアウトから Station Configurations を削除する必要があります。詳しくは、Station Manager のドキュメント分類をご覧ください。
Station Manager アプリ更新の強制: データモデルの更新
この更新により、将来のリリースをターゲットとした、モバイルアプリケーションの App Update Enforcement 機能をサポートする新しいフィールドが提供されました。この機能では、報告可能なフィールドとして Station Device オブジェクトの Days Left to Upgrade (days_left_to_upgrade__v) フィールドがプロビジョニングされます。お客様はこのフィールドを使用して、ログアウトする前にアップグレードする必要のあるデバイスを追跡できます。
トレーニングジョイン関係の上限の引き上げ
この機能は、Curriculum あたりの Training Requirement の最大数を 100 まで増やせます。Learner Role あたりの Curriculum の最大数は 60 に引き上げられました。
トレーニングオブジェクトの廃止に際したルールの参照
前提条件ルールが定義されている Training Requirement または Curriculum を廃止しようとすると、ルールラベルと名前がエラーメッセージに含まれるようになりました。
Training Content Set のオブジェクトがコピーされなくなりました
本リリースより、Training Content Set オブジェクトが Vault のプロビジョニング中にコピーされなくなりました。
監査証跡において取得されるトレーニングドキュメントエントリアクション
本リリースより、Issue Training Assignment、Create Training Requirement Impact Assessment、または Retire or Assess Impact on Training Requirements のエントリアクションがドキュメントに対して実行されると、ドキュメントの監査証跡が更新されるようになりました。
インポートされた Training Assignments のオブジェクトタイプのバリデーション
Training Assignment をインポートすると、その Training Assignment のオブジェクトタイプが Training Requirement のオブジェクトタイプと一致しているかどうか、アプリケーションによって検証されるようになりました。
第三者トレーニングセクションのボタン許可
Facilitated Training セクション内で実施されるレコードアクション (Add や Delete など) で、ユーザのセキュリティ権限やその他のセキュリティ設定が考慮されるようになりました。
直接割り当てアクション: ドキュメントの訓練可能性のバリデーション
この機能は、Training Requirement の状態タイプが Steady State か Ready for Training のいずれかではない場合、Training Requirement に対する直接割り当てアクションが表示されないようにすることで、実行できないようにするものです。Curriculum への直接割り当てに関しては、状態タイプが Steady State か Ready for Training のどちらでもないドキュメントがある Curriculum 内の Training Requirement に、Training Assignments が割り当てられることはありません。
AICC E-ラーニングコース: URL バリデーションの改善
本リリースでは、AICC E ラーニングコースがアップロードされると、Vault Training によって、file_name (course.au ファイル) のすべての E-Learning Standard の値が設定されます。ただし、値が「servername.com」である場合は除きます。
Training Assignment のエントリアクションの発行に関する更新
Issue Training Assignment アクションがドキュメントに対して実行され、Training Requirement Impact Assessment が存在しない場合、このアクションによって Training Requirement の Training Content Set が更新されるようになりました。これは、Training Requirement に 1 つのドキュメントがある場合にのみ起こります。
Curriculum オブジェクトと Learner Role オブジェクトで Description フィールドを必須に設定する
Curriculum と Learner Role オブジェクトの Description フィールドで、管理者は User must always enter a value (required) 設定を有効化できるようになりました。
直接割り当てのキャンセル: オープンの Training Assignment アクションと Curriculum 廃止のキャンセル
本リリースより、Cancel Open Training Assignment のドキュメントエントリアクションと Curriculum の Retired 状態への変更のいずれでも、Training Assignment レコードのオープンの直接割り当てがキャンセルされるようになりました。
VPS データモデルの変更
本リリースにより、Vault Product Surveillance の機能をサポートするよう一部のデータモデルが変更されました。データモデルの変更をご覧になるには、こちらをクリックしてください。
Quality Data Model Changes
詳しくは、21R3 Data Model Changes: Quality をご覧ください。
Regulatory
IDMP データモデルとアルゴリズムの更新 (IG v2 に準じる)
この機能は、EU IDMP 実装ガイドバージョン 2.0 と 2.1 に対応するために Vault レジストレーションのデータモデルを更新するものです。更新には、ソースレコード、IDMP サブミッションデータレコード、およびデータ集計メカニズムの追加が含まれます。
IDMP データモデルの設定エラー
新しい設定エラーメッセージが IDMP データ集計プロセスに導入され、IDMP データセット内のデータ要件とデータの依存性が通知されるようになりました。この機能は、EMA の IDMP に関する最新のガイダンス、IG V2.1 による Registration データモデルに合わせて行われた更新に対応するもので、最初に 20R2 で導入された設定エラーに続くものです。
IDMP メッセージの生成
この機能は、FHIR (Fast Healthcare Interoperability Resources) 基準に準じて、製品データを IDMP Medicinal Product Element とその子オブジェクトを XML 構造に置き換えることで、個別のメッセージを作成します。サブミッションモードに応じて、メッセージに EMA の PMS (製品管理サービス) データベースに直接送信するための ZIP ファイルに入った XML ファイルと該当する添付ファイルが含まれるか、メッセージ が EMA のゲートウェイを介して eCTD ドシエと共に提出するために Vault ライブラリに XML ファイルとして保存されます。
メッセージ生成は、IDMP 製品データサブミッション向けに個別に完了することも、すべての関連 Registration を Regulatory Objective から一括で完了することもできます。生成したメッセージのエクスポートもいずれかの場所で完了できます。
登録済み詳細の管理の機能強化
本リリースでは、名前ベースのオブジェクトタイプマッピングにより、登録済み詳細の管理に、オブジェクトタイプを組み合わせた登録済み詳細を同じレジストレーションに追加する機能が提供されます。これは、固有デバイス識別 (UDI) およびコンビネーション製品に必要なもので、ソースオブジェクトとターゲットオブジェクトのオブジェクトタイプの社内名が一致している必要があります。また、登録済み詳細の管理では、UDI に対応するための製品レジストレーションの以下の詳細を追加および更新できます: 登録済み臨床試験、登録済み組織、登録済み承認、および登録済み規制テキスト。また、UDI の 21R2 の既存の登録済み詳細に追加された追加標準フィールドをコピーして更新するためのサポートも追加されます。
レジストレーションの作成の機能強化
本リリースでは、名前ベースのオブジェクトタイプマッピングにより、レジストレーションの作成に、オブジェクトタイプを組み合わせた登録済み詳細を同じレジストレーションに追加する機能が提供されます。これは、固有デバイス識別 (UDI) およびコンビネーション製品に必要なものです。ソースオブジェクトとターゲットオブジェクトのオブジェクトタイプの社内名が一致している必要があります。
レジストレーションの作成では、UDI に対応するために、製品レジストレーションに登録済み臨床試験、登録済み組織、登録済み承認、および登録済み規制テキストといった詳細を追加できるようになりました。
また、UDI の 21R2 の既存の登録済み詳細に追加された追加標準フィールドをコピーして更新するためのサポートも追加されます。
英国 EU 離脱: レジストレーションの作成の集中管理の国に英国 (NI) を追加
本リリースより、欧州連合の集中管理手続き向けにレジストレーションを作成する際の国の 1 つとして、アプリケーション英国 (北アイルランド) (XI) がデフォルトで含まれるようになります。この変更は英国 EU 離脱を受けて行われるものです。
規制およびデバイスの EUDAMED UDI XML 生成
UDI の本初回リリースにより、Vault Registrations のユーザは、EU 市販デバイス製品レジストレーションから UDI データサブミッションを生成できるようになります。生成される XML ファイルは EUDAMED にアップロードして、EU 規制デバイスおよび旧指令デバイスに登録できます。
Active Dossier Item オブジェクトに WHO が国として追加
本リリースより、世界保健機関 (WHO) が国として Active Dossier Item オブジェクトに追加され、提出されたコンテンツの適切な追跡を行えるようになりました。
非 eCTD のパブリッシングによるドシエ形式サポート
本リリースでは、非 eCTD のサブミッションをパブリッシングすると、パブリッシング済みの出力フォルダ構造が、非 eCTD ドシエ形式値を考慮するようになりました。
空欄セクションインジケーター
本リリースでは、削除操作の結果、Submissions Archive でどの eCTD および EAEU サブミッションセクションが空欄になっているかを計算できます。Submissions Archive ビューアのユーザには、削除操作の結果、どのセクションが空欄になっているかを示すアイコンが表示されます。
サブミッションが新たなパブリッシングまたはインポートされると、システムが影響を受けるアプリケーションを再計算します。この機能は、管理者が有効化するもので、実行すると取り消しできません。
アクティブドシエにおけるファイル形式アイコンの表示
本リリースでは、アクティブドシエビューアおよび編集にアクティブドシエ内で追跡されたドキュメントのファイル形式を示すアイコンが表示されます。
現在のユーザのコンテンツプランフィルタ
今回の機能強化によって、コンテンツプラン階層ビューアのユーザ列をフィルタリングできる現在のユーザ値が新たに加わりました。これを使って、ログイン済みユーザによってコンテンツプランをフィルタリングして、コンテンツプランビューで保存できます。
コンテンツプラン作成ロジックの更新
本リリースにより、コンテンツプランアイテムテンプレートで Region と Lead Market Country を指定できるようになりました。コンテンツプランを作成する際、Vault はサブミッションの関連 Application レコードの Lead Market Country フィールドを読み取り、テンプレートで指定されている場合は、一致する Region と Lead Market Country に応じてコンテンツプランアイテムを作成します。
サブミッションコンテンツプランからのアクティブドシエの調達
今回の更新により、アクティブドシエでサブミッションコンテンツプランに目を通して、アクティブドシエテンプレートと一致するドキュメントの候補を収集することもできるようになります。この手法により、Submissions Archive ドキュメントにソースドキュメント関係がない場合、アクティブドシエに追加するドキュメントを増やすことができます。アクティブドシエ内のこれらのドキュメントの現在のステータスを再計算するためにコンテンツプランリーフライフサイクル情報が使用されます。
コンテンツプランへのコピーのエラーバリデーションの更新
本リリースでは、コンテンツプランへのコピー機能が新しくなりました。ユーザは、関連付けられたサブミッションまたはレポートレベルコンテンツプランがなくても、コンテンツプランをサブミッションコンテンツプランおよびレポートレベルコンテンツプランにコピーできるようになります。これまではエラーバリデーションがこのアクションの妨げとなっていました。
Content Plan トークンの拡張
この機能により、Content Plan トークンのトークン化サポートが拡張され、サブミッション関係トークンテキストおよびオブジェクト参照フィールドをトークン化できるようになります。例えば、トークン ${submission_pharmaceutical_product__rim.xml_drug_product__v} がコンテンツプランまたはコンテンツプランアイテムレコードの名前、タイトル、またはパブリッシュ済み出力先に適用される場合、このフィールドのトークンは、参照されるサブミッション製品レコードの XML Drug Product フィールドの値を使って解決されます。これまでは、Content Plan トークンは、サブミッション関係の名前、または一致するドキュメントの名前またはタイトルのみを解決するものでした。
一致するドキュメントのトークン化サポートは、2 つの新しい標準ドキュメントテキストフィールド eCTD Title (ectd_title__v) および Output Name (output_name__v) にも拡張されます。
臨床および非臨床試験コンテンツプランセクション作成の変更
本リリースでは、コンテンツプランニングに使用される Submission Clinical Study または Submission Nonclinical Study において、Study Type および/または Study Subtype が空欄となっている場合、Vault は定義済みの Study Type/Study Subtype を持つセクションごとにこの治験を繰り返さず、定義済みの Study Type/Study Subtype を持つ Content Plan セクションに対してこの治験を作成しなくなります。
ラベルコンセプト管理
本リリースにより、ラベル表示変更管理をより確実にサポートするために、Vault RIM データモデルが拡張されています。この機能には、Labeling Concept と Labeling Deviation.という 2 つの新しいオブジェクトが追加されています。影響を受けるラベル表示ドキュメントと特定し、プランニングを促進し、ラベル表示による影響による変更を管理できるよう、新しいフィールドが既存オブジェクトに追加されます。
キーワードのリンク付けおよび追加リンクタイプのサポート
本リリースより、キーワードおよび代替テキストおよび関連するハイパーリンクのターゲットのライブラリを定義できるようになりました。ユーザはドキュメントに「リンクを推奨」して、自動的に Vault リンク注釈を作成できます。Submissions Publishing および RLCP Publishing が、パブリッシュ済み出力内の推奨リンクを解決します。
さらに、Permalink をターゲットとする Vault リンク注釈および以前のバージョンから引き継がれた Vault リンク注釈が、Submissions Publishing および RLCP Publishing でサポートされるようになりました。
オーバーレイによるパブリッシュ
本リリースより、パブリッシング要素でオーバーレイを定義して、サブミッションコンテンツプランおよびレポートレベルコンテンツプラン (RLCP) 向けに個別のコンテンツプランアイテムに適用できるようになりました。さらに、デフォルトのオーバーレイを定義できます。RLCP またはサブミッションのパブリッシングアクティビティ中、Vault は定義済みのオーバーレイをパブリッシュ済み出力に適用します。
EAEU インポートおよび R.022 XML の表示
この機能を使用して、RIM Submissions Archive で EAEU (ユーラシア経済連合) 電子形式をインポートして表示できます。EAEU サブミッションは、サブミッションのドシエ形式が EAEU に設定されている場合、現在の Submission Archive のインポート方法を使ってサポートされます。
ZIP のインポートをサブミッションレベルで処理する
管理者が有効化すると、この機能を使って、ファイルコンテンツがアプリケーションのサブミッションレベルで作成されたように Submission Archive のインポートを介してすべての ZIP ファイルをインポートして、処理できます。
サブミッションのルートにある参照されていないファイルは、参照されていないファイルのフォルダにグループ化されます。
すべてのインポート方法のメール通知による情報警告
本リリースより、ユーザに新しい形式でサブミッション後のインポートのメール通知が届くようになりました。これには、インポートのバリデーションプロセスからの情報メッセージを使った新しいグループ化が含まれます。
通信文書の動的順序
今回の更新により、[Show Correspondence in Viewer] および [Enable Dynamic Ordering within Submissions Archive Viewer] という Submissions Archive 設定が有効化されている場合、Correspondence ドキュメントも Submission レコードの Actual Submission Date に基づいて順序付けできるようになりました。
RIM における 2253 フォーム生成の廃止
現在の Veeva では、PromoMats および RIM Vault Connection を介して、PromoMats および RIM へのクロスリンクからフォームを生成することを推奨しているため、FDA 2253 フォームの有効化機能は RIM Submissions Vault での有効化には使用できなくなりました。この機能は、現在有効化されている場合にサポートされます。
US FDA v4.2 バリデーション条件のサポート
今回のリリースで、RIM Submissions Publishing Vault は US FDA eCTD バリデーション条件 v4.2 をサポートするようになりました。
21R3 RIM データモデルの変更
詳しくは、21R3 Data Model Changes: Regulatory をご覧ください。
Safety
Safety の機能は、2021 年 11 月 24 日および 2021 年 12 月 10 日の暫定公開を予定しています。
受信トレイアイテムのフォローアップ管理者チェックボックス
受信トレイアイテムをプロモーションすると、以前の症例バージョンのすべてのデータを受信トレイアイテムの新しいデータと比較するフォローアップ症例の作成オプションが重複検出によって表示されるようになりました。受信トレイアイテムに基づいて、更新する情報をユーザが選択できます。症例をプロモーションすると、以前の症例バージョンと受信トレイアイテムのデータがマージされます。この機能は、API を介して受信した受信トレイアイテムのパススルー フィールドと、以前の症例バージョンのフィールドに対するアトミック セキュリティをサポートします。
詳細:
- 有効化: 受信トレイアイテムのフォローアップ症例比較を有効にする
- 管理者ヘルプ: フォローアップ症例比較の設定
- ユーザヘルプ: 症例へのプロモーション: 受信トレイアイテムのフォローアップ
受信トレイアイテムのフォローアップ: 事前計算自動オン
Vault Safety では、受信トレイアイテムをフォローアップ症例バージョンにマージするときに、フィールドの自動計算値、組み合わせ製品の構成要素、および治験製品が事前に計算され、表示されるようになりました。この動作により、新しいフォローアップ症例バージョンでは自動的に計算されるフィールドの値が必ず最新の状態になります。この機能には Case Promotion Settings ページも導入され、そこで受信トレイアイテムのフォローアップに対する設定を管理者が有効にして構成できます。
注: 受信トレイアイテムのフォローアップが有効であれば、Vault ではこの機能が自動的にオンになります。
詳細:
- ユーザヘルプ: 症例へのプロモーション: 自動計算
Vault Safety 受信トレイアイテムへのメール設定
この機能により、Vault が所有するメールアドレスへメールを送信すると、そのメールと添付ファイルを収めた受信トレイアイテムが自動的に作成されます。Vault Intake ユーザは、メールから新しい受信トレイアイテムが作成されると通知を受け取るので、症例の取り込みを開始できます。
お客様は、Vault 所有のメールアドレスへの自動転送ルールを設定することによって既存のメール アドレスを引き続き使用できます。また、Vault ユーザは、Vault所有のメールアドレスへメールを直接送信することもできます。管理者は、メールアドレスごとに、それに関連付けた Vault 所有のメールアドレスと設定を最大で 50 件定義できます。
詳細:
- 管理者ヘルプ: Vault Safety 受信トレイアイテムへのメールを設定する
- ユーザヘルプ: 受信トレイアイテムの手動取り込み
受信トレイアイテム取り込み機能強化自動オン
いくつかの新しい機能強化により、受信トレイアイテムの手動取り込みと検証が容易になりました。まず、受信トレイグリッドで新しい情報の日付がわかるので、新しい受信トレイアイテムを日付順に並べ替えることで、日付が古い症例を容易に識別できるようになりました。2 番目として、インポートした長いテキストのフィールド (症例の説明など) が広くなり、ソース ペインを使用した検証が容易になりました。3 番目として、インポートしたデータまたは抽出したデータの検証から得られたデータアイテムと区別するために、手動入力では受信トレイアイテムセクションに Verify ボタンではなく Save ボタンが表示されるようになりました。4 番目として、すべての必須フィールドは黄色で強調表示され、空白のままにするオプションは用意されません。
注: 受信トレイアイテムを使用する Vault では、この機能が自動的にオンになります。
詳細:
- ユーザヘルプ: 受信トレイアイテムのインポート: E2B インポートソースデータペイン
- ユーザヘルプ: Inbox Item Field Reference
自動予測可能ロールアップ変更自動オン
Case-level Expectedness フィールドを明示的に上書きしていない限り、症例評価の予測可能を上書きしても、Case-level Expectedness フィールドを主要症例評価と一致するように更新する処理が妨げられないように更新されたロジックが、この機能強化によって用意されます。
詳細:
- ユーザヘルプ: 症例の予測可能を手動で上書きする
国内症例処理設定
Vault Safety は、地域固有のフィールドを備えた統合インターフェースで国内症例の処理に対応するようになりました。特定の言語で症例を表示することもできます。たとえば、日本国内の症例の場合、症例画面でのデータ入力には、グローバルなフィールドのほかに PMDA 固有のフィールドも用意されています。取り込み段階からメディカルレビュー段階まで、選択した言語で症例の処理を完了できます。
詳細:
- 有効化: 国内症例の処理を有効にする
- ユーザヘルプ: 国内症例を準備する
MedDRA 同義語設定
Vault Safety では、企業固有の MedDRA コーディング設定をキャプチャし、MedDRA 同義語リストを使用して迅速なコーディングが可能になりました。手動による同義語の作成と編集のほか、外部ソースから同義語を一括でインポートすることもできます。
報告された用語から MedDRA フィールドを Vault Safety で自動的にコーディングすると、まず有効な MedDRA 同義語との完全一致が検索されます。完全一致が見つからない場合、一致する用語が MedDRA 辞書で検索されます。
詳細:
- 管理者ヘルプ: MedDRA 同義語の管理
- ユーザヘルプ: MedDRA 用語の自動コーディング
最も保守的な製品と評価の地域ルールによる評価設定
最も保守的な症例製品と症例評価に対するルールを評価するためのレポート作成ルールセットを、Vault Safety 管理者が必要に応じて設定できるようになりました。疑義のある症例製品や相互作用がある症例製品が特定のレポート作成先に複数登録されている場合に、この機能が特に有用です。最も保守的な症例製品と症例評価の判断では、関連する症例製品の重大度、予測可能、関連度、薬剤ロールがすべて考慮されます。ルールセットを別途設定していない限り、主要な症例製品と症例評価に基づき、FDA や EMA ICSR などの既存のルールセットによってルールが評価されます。
また、Vault Safety では、この機能をルールセットで設定している場合、主要な症例有害事象で指定した国ではなく、プライマリレポーターの症例連絡先で指定した国を使用して「AE in Jurisdiction」ルールパラメータが評価されます。これは ICH ガイドラインに則った措置です。
詳細:
- 有効化: 最も保守的な製品と評価の地域ルールによる評価を有効にする
- 管理者ヘルプ: レポート作成ルールの製品選択を設定する
マスクした分布に設定可能なフィールドマスク処理 (空白、性別、国) に対する例外設定
Vault Safety で患者コンテンツ保護をオンにすると、空白のフィールド、報告者の国、親の性別、患者の性別に対し、設定可能なフィールドマスク処理を使用できるようになりました。これらの PII/マスク可能な E2B フィールドのうち、どのフィールドでマスク解除し、残りのフィールドはマスクしたままにするかを事前に設定できます。また、ICSR レポートの生成では、生年月日フィールドをマスクしていても、年齢フィールドと年齢グループフィールドの値があれば、それが自動的に入力されます。これは、E2B フォームや PDF フォームなどのすべての ICSR 出力で可能です。
詳細:
FDA E2B(R2) における ISO 8859-1 検証自動オン
Vault Safety では、FDA E2B(R2) 提出文書に使用している文字エンコードが検証され、UTF エンコードの文字が自動的に ISO 8859-1 の同等文字に置き換えられます。同等文字がない場合はスペースに置き換えられます。FDA E2B(R2) は ISO 8859-1 規格に準拠していますが、UTF エンコードした文字は使用できません。UTF エンコードした文字を使用した症例を E2B(R2) 形式で FDA の CBER または CDER に送信しようとすると、検証エラーまたは警告が発生します。
詳細:
- ユーザヘルプ: 症例と提出の検証: FDA の文字エンコード検証
MHRA E2B(R2) 投与形態コード自動オン
Vault Safety の投与形態辞書に、約 400 種類の MHRA 投与形態とそれぞれのコード (1 ~ 3 桁) が網羅されました。エンコード済みの MHRA 投与形態を収めた E2B(R2) ファイルを Vault Safety でインポートすると、そのデータが新しい投与形態辞書エントリにマッピングされます。
詳細:
- ユーザヘルプ: 症例データの入力: 投与情報セクション
日本の PMDA ゲートウェイサブミッション設定
Vault Safety は、日本の医薬品医療機器総合機構 (PMDA) への PMDA E2B (R3) ファイル形式による ICSR 提出で AS2 ゲートウェイサブミッション設定をサポートするようになりました。
詳細:
- 管理者ヘルプ: PMDA ゲートウェイの設定
- ユーザヘルプ: 基準当局への ICSR 提出: PMDA
日本の PMDA への提出ルール設定
PMDA への ICSR 提出向けに新しい標準レポートルールセットが用意されました。症例にある日本の製品または治験の登録が PMDA に報告可能であるとシステム側で判断されると、レポート作成ルールに基づいて PMDA へのサブミッションレコードのスケジュールが設定されます。
詳細:
PMDA E2B(R3) 提出物検証自動オン
この機能により、全面的な PMDA E2B(R3) 適合のためのルールが導入され、20R3 に用意されている ICH E2B(R3) 検証機能が拡張されます。PMDA E2B(R3) ファイル形式による ICSR 送信をレポート送信先向けに生成または再生成すると、この検証が自動的に実施されます。「規制適合性の評価」のユーザアクションを使用して、症例レベルで PMDA E2B(R3) の地域検証をトリガーできます。その検証結果レコードは、評価済みの各検証基準に基づき、合格、不合格、または警告として作成されます。
注: 20R3 で導入された E2B の症例と提出の検証機能が Vault に設定済みであれば、この機能は自動的にオンになっています。それを設定していない場合は、この機能を使用するための設定が必要です。「レポート作成義務の再評価」のユーザアクションが有効になって症例のローカル処理で提出レコードが自動的に再評価されるように、PMDA 提出ルール機能を設定することも必要です。
詳細:
- ユーザヘルプ: 症例およびサブミッションのバリデーション
日本向け PMDA E2B(R3) エクスポート自動オン
Vault Safety で、症例ごとに PMDA E2B(R3) のエクスポートファイルを作成できるようになりました。
詳細:
- 有効化: PMDA E2B(R3) のエクスポートを有効にする
- ユーザヘルプ: PMDA E2B(R3) マッピング
定期レポートにおける PT 集計のサポート
この機能には、症例ごとに基本語 (PT) の一意のインスタンスのみをカウントして定期レポートで表にまとめるオプションが用意されています。この機能を有効にすると、同じ PT でコード化された複数の症例有害事象が症例にある場合、レポートではそれらが複数の PT イベントではなく単一の PT イベントとしてカウントされます。この機能は、臨床試験で見られた重篤な有害事象の DSUR と PBRER の累積表、臨床試験で見られた重篤な有害反応の DSUR 添付資料:累積サマリーテーブル 、市販後調査情報から得られた医薬品副作用の PBRER サマリー表、PSUR サマリー表、および市販後調査情報から得られた ADR の PADER サマリーに影響します。この機能を有効にしていない場合は、症例の有害事象が引き続き個別にカウントされます。
詳細:
- ユーザヘルプ: Create DSUR Aggregate Reports
- ユーザヘルプ: PADER 集積レポートの作成
- ユーザヘルプ: PBRER 集積レポートの作成
- ユーザヘルプ: PSUR 集積レポートの作成
定期レポートでの予測可能 (RSI) 設定
Vault Safety では、定期レポートで SUSAR を正確に特定するために、特定の期間に発生する有害事象の予測可能を評価できるようになりました。期間ごとに承認日付の範囲を製品データシートで指定できます。DSUR、PBRER、または PSUR を生成するときに、定期レポートで期間での予測可能の評価対象とする、製品データシートのバージョンを選択できます。
詳細:
- 有効化: 集積レポートで予測可能を有効にする
- 管理者ヘルプ: 製品データシートの管理: 集計レポートでの予測可能
- ユーザヘルプ: 想定しない期間を DSUR レポートで指定する
- ユーザヘルプ: 想定しない期間を PBRER レポートで指定する
- ユーザヘルプ: 想定しない期間を PSUR レポートで指定する
Safety のお客様向け Intake JSON API
Vault Safety で、Safety Intake AI のエンドポイントからの呼び出しを取り込んで、JSON ファイルから受信トレイアイテムを受信できるようになりました。
POST /api/{バージョン}/app/safety/ai/intake
Vault では、このエンドポイントからの呼び出しを受信する Safety.AI が不要になりました。Safety.AI がない Vault では構造化データ (JSON) のみを処理できます。非構造化データは無視されます。
詳細:
- Vault Developer ポータル: Intake JSON REST API
- Vault Developer ポータル: Intake JSON の構文
JSON オブジェクトを使用した Intake API
リクエスト本文でテキストとして渡した JSON が Intake JSON エンドポイントで受け入れられるようになりました。
この機能をサポートするために、既存の本文リクエストのほか、次の 2 つの新しい本文リクエストを使用できます。
multipart/form-data:intake_formパラメータで JSON テキスト以外のドキュメントを渡すときに、intake_json パラメータで JSON テキストを渡しますapplication/json: JSON テキストのみを Raw コンテンツとして渡します
詳細:
- Vault Developer ポータル: Intake JSON REST API
アップロードされたメールからの症例自動取り込み設定
Vault Safety の自動化で、EML メールファイルと MSG メールファイルから症例情報が自動的に抽出されるようになりました。Intake ユーザは、表示可能な PDF レンディションを使用して、メールファイルと添付ファイルからの抽出プロセスを開始できます。抽出プロセスが正常に完了すると、メールと添付ファイルにリンクされた単一の受信トレイアイテムが作成されます。
注: Safety Automation のお客様が「受信トレイアイテムの作成」アクションを設定済みで、ドキュメントタイプで添付ファイルを有効にしている場合は、この機能が自動的にオンになっています。
SiteVault
SiteVault セットアップアシスタント
この機能によって、SiteVault セットアップアシスタントが導入されました。新しい Setup タブを使ってデータとドキュメントをすばやく入力して、電子規制、接続済み治験、および Veeva eConsent などの SiteVault の主要機能を最大限活用できます。本初回リリースによって、1 つのワークフローで SiteVault ユーザを作成し、ユーザプロファイルドキュメントをアップロードし、ユーザを Study に追加できます。
外部ユーザ向けにスケジュールされた治験アクセス
この機能によって、管理者は開始日と終了日を指定して、外部ユーザ向けに治験アクセスのスケジュールを設定できます。外部ユーザは自動的に、スケジュールされた開始日にアクセスを手に入れ、終了日にアクセスを失います。
オリジナルから下書きを作成
この機能では、SiteVault ユーザは、基礎となるオリジナルドキュメントの最新版を使って、ドキュメントの下書きバージョンを作成できます。
治験責任医師と治験割り当ての同期
この機能では、ユーザが Study Assignment に責任医師 (PI) ロールと一緒に有効化された状態で追加され、治験の Principal Investigator フィールドは自動的にその責任医師に更新されます。
1572 向けの保護 PDF
本リリースで登場する保護 PDF レンディション機能は、Vault が署名した 1572 が SiteVault からダウンロードされてから編集されないようにするために使用できます。
ユーザ管理の機能強化
拡張可能な権限セキュリティモデルを使用している Vault の場合、1 つのアクションで、ユーザを完全に無効化できる機能があります。また、責任医師で働くユーザ管理者は、施設全体におけるユーザの権限を一括で更新できます。
Vault セレクタで選択可能な研究機関
この機能では、Vault セレクタを使って自分たちの研究に適したコンテクストを見つけられます。この機能は、拡張可能な権限を使用する SiteVault のみに適用されます。
Veeva eConsent 追加署名者
この機能は、MyVeeva for Patients で、治験参加者に代わって、または関連して、電子的な方法で同意するための署名者の追加をサポートします。
eConsent 以外のドキュメントの患者との共有
この機能を使って、SiteVault ユーザは IRB による承認済みの治験関連ドキュメンを指定して、MyVeeva for Patients を通して治験参加者または署名者と共有できます。
アグリーメントウィザードの製品
接続済み治験アグリーメントを確認する際、Regulatory ユーザに対して、治験の製品を照合するよう求められるようになりました。この機能を使って、治験アグリーメント製品を SiteVault の既存製品を統合するか、既存のものがない場合は新たに製品を作成するかを選ぶことができます。
規制ドキュメントリクエストからの複数ドキュメントワークフローの開始
この機能を使って、規制ドキュメントリクエストページから複数ドキュメントワークフローを開始できます。たとえば、ユーザは治験責任医師の署名を取得するために複数のドキュメントを送信したり、多数のトレーニング資料ドキュメントを 1 つの Read & Understood ワークフローにまとめたりできます。
規制ドキュメントリクエストの改善
今回の改善を受け、SiteVault ユーザは、規制ドキュメントリクエストページのドキュメントセクションのソート、検索、およびページ送りを行うことができるようになりました。さらに、ユーザが返却するドキュメントセクションの最後のアイテムを完了すると、規制ドキュメントリクエストタスクが自動的に終了します。
接続済み治験: 交換可能な組織プロファイルドキュメントの更新
この機能により、ドキュメントが固定状態に達しても、中央臨床検査機関または中央 IRB/IEC の組織プロファイルドキュメントが、スポンサーや CRO の Vault に自動的に転送されなくなりました。
接続済み治験の新しい交換可能なドキュメントタイプ
この機能は、接続済み治験のためにスポンサーまたは CRO の Vault の以下の新規ドキュメントタイプを転送するサポートを追加します。
- 予算
- IP 廃棄
- 署名およびイニシャル
- 試料トラッキング
- スポンサー/CRO の連絡先情報
接続済み治験ドキュメントの削除のサポート
この機能を使って、SiteVault ユーザは、接続済み治験に送信された、または受領したドキュメントを物理削除できるようになりました。
SiteVault データモデルの変更
詳しくは、21R3 Data Model Changes: SiteVault をご覧ください。
QualityOne
インスペクション管理のコントロールチャート
この機能を使って、評価目的のためにコントロールチャートで製品のインスペクションデータを見ることができます。このアセスメントは、生産におけるソースのバリエーションを少なくするなど、製造工程の改善に追加アクションが必要であるかどうかを判断する際に役立ちます。詳しくは、コントロールチャートをご覧ください。
タスクのキャンセルおよび再割り当て - 共有設定の更新
この機能によって、有効な共有設定ロールから削除されるユーザに割り当てられたタスクを自動的にキャンセルするよう QualityOne チームが更新されました。参加者は、設定されたロールの参加者を使用するように設定されているワークフロー参加者グループから削除されます。詳しくは、QualityOne チームの共有設定の変更をご覧ください。
Create Related Record エントリアクションの機能強化
この機能は、管理者が関連子オブジェクトレコードの作成を自動化できるようにすることで、Create Related Record エントリアクションを改善します。管理者は、ソース-子受信関係および関連-子受信関係との間のコンポーネントとそれぞれのフィールドマッピングを設定できます。Create Related Record オブジェクトもユーザアクションとして設定できます。詳しくは、関連-子オブジェクトレコードをご覧ください。
監査プログラムのプランニング
この機能を使って、一定期間中に実施する監査プログラムの開始、計画、および承認を行うことができます。監査プログラムが実行状態になると、Vault は Proposed Audit から Audit を自動作成します。詳しくは、QualityOne 監査プログラムのプランニングをご覧ください。
COA 分析の多言語サポート
この機能を使って、受信インスペクションプロセスの一部として分析証明書 (COA) を多言語で取り込み、処理できます。COA の詳細が、ユーザの Vault ロケールに基づき、Characteristic、Test Result (Attribute Data)、および Test Result Unit フィールドに対して事前にロードされたローカライゼーションと共に表示されます。現在サポートされている言語は英語、フランス語、ドイツ語、スペイン語、イタリア語、およびポルトガル語です。詳しくは、COA 多言語サポートをご覧ください。
注: 現在、この機能を利用できるのは、アーリーアダプタープログラムに登録されているお客様のみです。詳細については、カスタマーサクセスマネージャまでお問い合わせください。
インシデント管理のための OSHA レポート
この機能は、OSHA レポート目的のために、HSE イベントの OSHA 301、OSHA 300、および OSHA 300A ログを生成できるものです。詳しくは、OSHA レポートをご覧ください。
注: 現在、この機能を利用できるのは、アーリーアダプタープログラムに登録されているお客様のみです。詳細については、カスタマーサクセスマネージャまでお問い合わせください。
QualityOne: MDCC: 最新ではないバージョンのドキュメントの状態変更の防止
この機能を使用すると、QualityOne ドキュメント管理がさらにベストプラクティスを強制し、Document Change Control によって最新ではないバージョンのドキュメントで状態が変更されることがないようにします。
ユーザが Document Change Control を介して、最新ではないバージョンのドキュメントの状態を変更しようとすると、アクションが阻止され、Vault にエラーが表示されます。
QualityOne: DCC バナーが Document Change Control オブジェクトのラベルを使用する
ドキュメントが Document Change Control 下にある場合に表示される DCC バナーはこれまで、「このドキュメントは現在変更管理によって制御されています」としてハードコード化されていました。本リリースより、メッセージの「変更管理」の部分が Document Change Control オブジェクトのラベルに置き換えられます。これは、Vault で Document Change Control オブジェクトの名前が変更されている場合に役立ちます。
リスク管理のための設定可能性の改善
本リリースでは、管理者が Risk Event (risk_event__v) オブジェクトの Risk Register (risk_register__v) フィールドに Allow hierarchical copy 属性を設定できる機能が追加されました。
Formulation オブジェクトの設定可能性の改善
本リリースでは、管理者が Formulation (formulation__v) オブジェクトの Formulation ID (name__v) フィールドに Values must be unique 属性を設定できる機能が追加されました。
データモデルの変更
詳しくは、21R3 Data Model Changes: QualityOne をご覧ください。
RegulatoryOne
Formulation Composition Viewer
この機能によって、レギュラトリーユーザは処方組成物を見て、化学成分が市場の要件を満たしているかどうかを判断して、組織が使用する組成物の承認をすばやく行うことが可能になります。詳しくは、Formulation Composition Viewer をご覧ください。
関連トークンのオブジェクトフィールドマッピング
管理者は、ソースとターゲットオブジェクトの間のフィールドマッピングを定義し、それらのフィールドマッピングを特定の関連トークンにリンク付けできます。Vault がこれらの関連トークンの解決方法に基づいて新しいレコードを生成すると、それらのフィールドにはソースレコードの値に基づいて生成されたレコードで自動入力されます。詳しくは、関連トークンのオブジェクトフィールドマッピングをご覧ください。
反復的な関連トークン
この機能を使って管理者は反復的な関連トークンを作成できます。ユーザがこれらの関連トークンに基づいてレコード生成をトリガーすると、Vault は同じオブジェクトの関連レコードを反復的にチェックして、レコード作成に含めます。また、管理者は、階層のすべての反復レコードで関連トークンが解決された後に、関連トークンの VQL 条件を適用するかどうかを指定できます。詳しくは、反復的な関連トークンをご覧ください。
レジストレーション項目の分割
この機能を使って、管理者が設定する分割ルールに基づいて関連レジストレーション項目を作成できます。管理者は分割ルールを関連トークンおよびオブジェクトフィールドマッピングとリンク付けします。これによって、Vault が関連トークンの解決時にどのように関連 Registration Item レコードを作成するかが決定されます。たとえば、EU の項目を加盟国にも登録する必要がある場合、EU のレジストレーション項目を「分割」して、各加盟国向けに関連レジストレーション項目を生成できます。詳しくは、レジストレーション項目の分割をご覧ください。
Formulation オブジェクトの設定可能性の改善
本リリースでは、管理者が Formulation (formulation__v) オブジェクトの Formulation ID (name__v) フィールドに Values must be unique 属性を設定できる機能が追加されました。
Organization オブジェクトの設定可能性の改善
本リリースでは、管理者が Organization (organization__v) オブジェクトの Name (name__v) フィールドに、System manages field value 属性を使ってシステム管理命名を有効化できる機能が追加されました。
データモデルの変更
詳しくは、21R3 Data Model Changes: RegulatoryOne をご覧ください。
Veeva Claims
プロジェクトのクレームの選択的作成
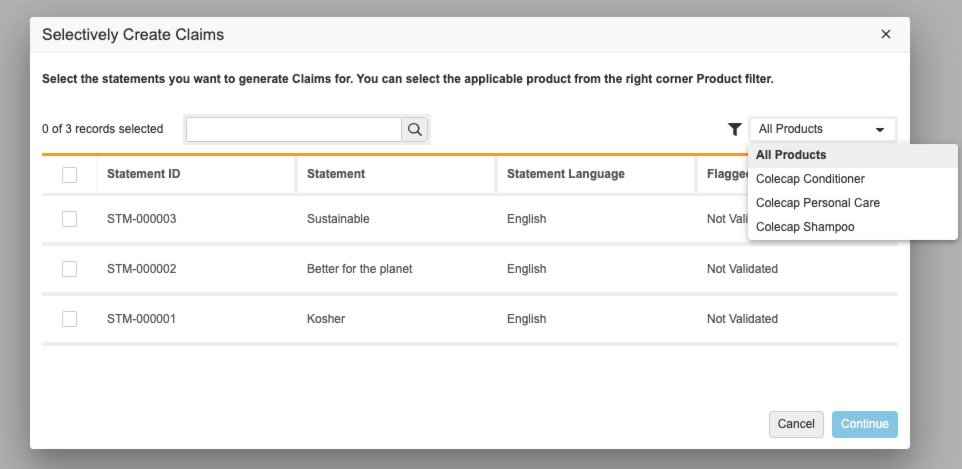
この機能は、新しいユーザアクションを使って、どのステートメントを 1 つまたはすべての製品に適用するかを選択的に管理できるようにすることで、管理者が好きなステートメントと製品に対して複数の Claims を作成できるようにするものです。
この機能によって、どのステートメントと製品の組み合わせで Claims を作成するかを指定できない場合に生成される、不要な Claims によって引き起こされる余分なクリックを減らします。設定が完了すると、プロジェクトに少なくとも 1 つのステートメントと 1 つの製品が存在する場合、ユーザは Project レコード上で新しいアクションを表示できるようになります。アクションをクリックした後に、All または特定の製品に対する新しいダイアログの Project で、ステートメント を選択できます。選択後に Continue をクリックすると、新しい Claim レコードが作成され、既存の Project とリンク付けされます。Claim がすでにいずれかのステートメント-製品の組み合わせに存在する場合、システムは重複するレコードを生成しませんが、既存の Claim レコードを Project にリンク付けします。詳しくは、プロジェクトからのクレームの選択的作成の設定をご覧ください。
ローカル適応でのステートメントテキストの検索
この機能により、ローカル適応ライブラリでステートメントテキストに基づいて検索およびフィルタリングできます。ユーザはまた、ステートメントへの参照をグローバルステートメントから関連するいずれかのステートメントに変更することで、ローカル適応でデフォルトのステートメントテキストを変更できます。詳しくは、ローカル適応でのステートメントテキストの検索をご覧ください。
翻訳者ビューの機能強化
Translator View は、Pack Copy オブジェクトのオブジェクトページレイアウトセクションです。翻訳者はここで、国別の言語向けに複数のローカル要素を入力できます。これまでは、翻訳者がすべての未入力の翻訳の入力を終えると、完成した要素列がビューから消えるため、翻訳を確認するために各要素に移動する必要がありました。今回の機能強化により、すべての定義済みライフサイクル状態で、完成した翻訳が一貫して表示されるため、ユーザは完成したすべての翻訳を確認できます。詳しくは、パックコピー要素の翻訳をご覧ください。
データモデルの変更
詳しくは、21R3 Data Model Changes: Veeva Claims をご覧ください。
Enablement Details
有効化オプションについての詳細は以下をご覧ください。
| 有効化 | 説明 | 自動オン | 自動で有効化済みで、機能を使用する前の設定は不要。場合によっては、新しい機能を使用するために別の機能を有効化したり設定したりしなければならないことがあります。 | 管理者チェックボックス | 管理者は、管理者チェックボックスを使用して機能をオンにする必要があります。一部の「Auto-on」機能には、その機能を非表示にするチェックボックス設定がありますが、非表示にしても「Auto-on」と表示されます。 | 設定 | 機能を使用可能にする (有効化する) には、まず管理者が設定を行う必要があります (管理者チェックボックスとは別に)。たとえば、ユーザがテンプレートからドキュメントを作成できるようにするには、事前に管理者がドキュメントテンプレートを追加する必要があります。 | サポート | サポートがオン/オフのオプションを管理。 |
|---|